Abstract
The development and success of RNA-based vaccines targeting SARS-CoV-2 has awakened new interest in utilizing RNA vaccines against cancer, particularly in the emerging use of self-replicating RNA (srRNA) viral vaccine platforms. These vaccines are based on different single-stranded RNA viruses, which encode RNA for target antigens in addition to replication genes that are capable of massively amplifying RNA messages after infection. The encoded replicase genes also stimulate innate immunity, making srRNA vectors ideal candidates for anti-tumor vaccination. In this review, we summarize different types of srRNA platforms that have emerged and review evidence for their efficacy in provoking anti-tumor immunity to different antigens. These srRNA platforms encompass the use of naked RNA, DNA-launched replicons, viral replicon particles (VRP), and most recently, synthetic srRNA replicon particles. Across these platforms, studies have demonstrated srRNA vaccine platforms to be potent inducers of anti-tumor immunity, which can be enhanced by homologous vaccine boosting and combining with chemotherapies, radiation, and immune checkpoint inhibition. As such, while this remains an active area of research, the past and present trajectory of srRNA vaccine development suggests immense potential for this platform in producing effective cancer vaccines.
Subject terms: Tumour immunology, Drug development
Introduction
Despite decades of advances, cancer remains the leading cause of death worldwide, diagnosed in nearly 40% of all adults in the United States, with a projected 15.8% fatality rate among those cases [1, 2]. For more than 50 years, standard treatment for most cancers has involved chemotherapy, radiation, and surgery. Multiple new therapies have evolved over the past several decades, including small molecule inhibitors, monoclonal antibodies, and the recent development of immune checkpoint inhibitor (ICI) antibodies. However, while these ICI antibodies have demonstrated long-term survival gains in a fraction of patients and demonstrated the potential of immunotherapy, they are only effective for a subset of patients in a restricted number of cancers. As such, there has been a renewed interest in the development of cancer vaccines, which would allow for engagement of adaptive immunity against critical tumor antigens [3]. Effective cancer vaccines would generate anti-tumor responses against immunosuppressive cancers and stimulate immune cell infiltration into tumors, which could enable primary tumor regression and, more critically, the elimination of metastatic and dormant tumor cells. Unlike other treatments, vaccination is not limited by tumor accessibility and vaccines used to date have had excellent safety profiles, with minimal toxicity observed across different clinical trials [4–6]. However, there have been few cancer vaccine approvals to date, with almost all larger trials of anti-cancer vaccines being ended due to a lack of observable effect [7]. While many types of vaccine vectors exist, the recent success of mRNA vaccines against SARS-CoV-2 (COVID-19 mortality and morbidity) has generated renewed interest in RNA-based vaccines due to their rapid production, potency, and outstanding safety profile. Among the different options for RNA-based viruses, the utilization of self-replicating RNA (srRNA) vaccines based on single-stranded RNA viruses represent a highly attractive alternative to mRNA-based vaccines. This is due to several critical features of these viruses in comparison to non-replicating mRNA vectors, including their ability to amplify transgene expression and augmented induction of augment innate immune responses, both of which can enhance adaptive responses. In this review, we will describe the different types of srRNA vaccines and their components, review the pre-clinical use of srRNA vaccines, detailing the factors involved in their efficacy and use, and highlight critical unknowns in this field.
Structure and production of self-replicating RNA based vaccines
Multiple types of single-stranded RNA viruses have been utilized for self-replicating vaccine platforms, encompassing both positive-strand viruses (alphaviruses and flaviviruses) and negative-strand viruses (Measles viruses and rhabdoviruses) [3, 8]. In the case of negative-strand viruses, reverse genetics and packaging cell lines need to be used to generate engineered replicons, while positive-stranded viruses can be generated using in vitro transcription (IVT) methods, as well as using intermediate DNA vectors.
To generate srRNA vectors from these viruses, the non-structural replicase genes are left intact while the target antigen(s) are substituted for structural genes. These replicons contain the non-structural protein genes encoding the viral replicase complex, the 5’- and 3’-end cis-active replication sequences, along with a native subgenomic promoter that directs expression of the encoded antigen (heterologous gene insert) [9]. Vaccine replicons lack the ability to form virions that would allow for cell-to-cell infection but can generate multiple RNA copies in the cytoplasm, resulting in elevated antigen expression [10]. The majority of studies to date have utilized a single mRNA transcript containing the replicase non-structural genes, along with the target antigen expressed in cis from a subgenomic promoter. An alternative strategy relies upon a two-helper system whereby the replicase and target antigen are encoded on separate mRNAs that are co-infected into target cells (Fig. 1). The helper system provides a modestly enhanced safety profile but it has the added complications of requiring the production of two different vectors and co-infection of the target cells to generate amplification of the mRNA containing the encoded antigen (Fig. 1) [11]. Critically, in both systems, the replication of RNA can stimulate innate immune responses through double-stranded RNA (dsRNA) intermediates and long RNAs, providing an immunologic adjuvant effect for cancer vaccines [8]. The dsRNA that is produced can also induce apoptosis, which will additionally stimulate innate immune responses and allow for antigen cross-priming [12]. While mRNA vaccines have needed to limit the induction of innate immune responses to prevent suppression of RNA translation of the target antigen, RNA amplification from srRNA vectors allows for sufficient antigen expression despite immune activation [8, 13]. As such, innate responses against the targeted antigen are strongly stimulated, although the extent to which immune responses against non-coding replicase proteins are also induced is unknown. The ratio of responses against target antigen versus replicase proteins will likely differ based on the type of viral replicase utilized due to differences in expression or immunodominance. These unknowns notwithstanding, all of the different srRNA platforms have demonstrated a conserved capacity to elicit potent antigen-specific immunity against their encoded transgene.
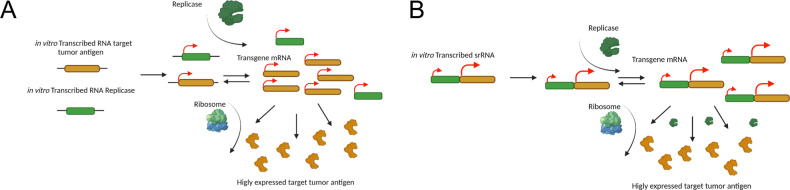
Fig. 1. Schematic representation of both cis and trans delivered self-replicating RNA (srRNA) vaccines.
A In cis delivery of the srRNA vaccine, both replicase gene and transgene target antigen insert exist on the same nucleic structure. B In trans delivery, the RNA gene for the target tumor antigen gets administered separately from the RNA gene for the Replicase machinery. In both panels, viral RNA Replicase genes and transgene antigen RNA insert are represented in transcript form (green and yellow, respectively). Replicase as an expressed protein (green) amplifies the transgene antigen insert by making multiple copies of the mRNA. Host cell ribosomes translate the amplified mRNA, resulting in the expression of high volumes of target tumor antigen (yellow pacman proteins). Both representations occur in the cytoplasm of the cell. Only a naked nucleic acid delivery of the srRNA vaccine is displayed.
The generation of srRNA vaccines can take one of three forms: (1) the use of a DNA intermediate, (2) the generation of viral replicon particles (VRPs), or (3) the generation of synthetic srRNA replicons (Fig. 2). In the use of a DNA intermediate (1), the srRNA vector is encoded into a DNA construct that is used as a vaccine, with RNA being transcribed from the DNA template after cellular transduction. The ease of generation and stability of DNA are advantages of this approach, but success is limited by the inability to effectively transduce cells with DNA in vivo [14]. To optimize transduction, the generation of VRPs (2) was explored as a more efficient alternative to generate antigen-specific responses from srRNA replicons. In this process, in vitro transcribed (IVT) RNA containing viral structural proteins is co-transfected into cells along with RNA encoding srRNA replicons. This process can also utilize transfection of DNA intermediates encoding structural genes and srRNA antigen encoding replicons [9, 15–17]. This approach exploits viral features, such as the use of Alphaviral envelopes. These VRPs are highly lymphotropic, which may allow them to generate and sustain broad immune responses [18, 19]. Additionally, certain VRPs can directly activate innate immune responses (e.g., TLR2 activation by the hemagglutination protein of measles virus or TLR4 activation by VSV G protein [20, 21]). However, this approach also generates immune responses against the VRPs themselves, which may alter responses to different encoded antigens or interfere with future use of a specific srRNA VRP vaccine. Finally, the ability to generate positive strand srRNA replicons by IVT, along with the advent of different lipid formulations to encapsulate RNA, has led to the emergence of synthetic srRNA replicon vaccines. While optimization of different lipid formulations to encapsulate the self-replicating RNAs is an area of ongoing research, this completely cell-free in vitro approach is highly scalable, is efficient, and may offer the advantage of not generating immunity against structural VRP antigens [22–24]. These different srRNA vaccine designs are illustrated in Fig. 2 and studies utilizing them are listed in Table 1.
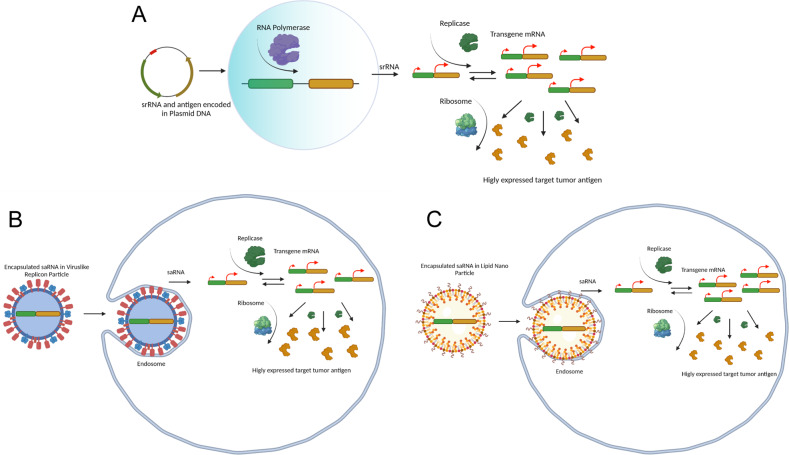
Fig. 2. Different self-replicating RNA (srRNA) vaccine platforms.
A DNA plasmid delivery of Replicase gene (green) and transgene antigen (yellow) enters the cell and travels to the nucleus (cyan circle) where host RNA polymerase (purple pacman) transcribes the full construct into mRNA. The mRNA exits the nucleus and, in the cytoplasm, Replicase gets translated by a host ribosome (blue/green) into an expressed protein (green) where it then amplifies the transgene antigen mRNA. The amplified antigen mRNA gets translated by the host ribosome and results in high volumes of expressed tumor antigen (yellow proteins). B Virus-like Replicon Particle (VRP) with encapsulated srRNA cellular envelope proteins bind to host cell surface membrane proteins, where it is then endocytosed into the cell. The VRP is broken down and releases the srRNA vaccine (replicase and transgene tumor antigen insert) into the cytosol, where it is amplified and expressed into high volume protein tumor antigen. C Positively charged Lipid nano-particle (LNP) with encapsulated srRNA is endocytosed by the negatively charged membrane into the cell where the srRNA escapes from the endosome into the cytosol and is amplified and expressed into high volumes of tumor antigen proteins.
Table 1.
Published pre-clinical studies utilizing self-replicating RNA vectors as a vaccine platform in the treatment of cancers.
| Virus | Gene Target | Target Species | Vector | Cancer | Finding | Reference | Reference |
|---|---|---|---|---|---|---|---|
| SFV | LacZ | Bacteria | RNA | Colorectal Cancer | Protection against pulmonary metastases with 1ug of RNA, associated with apoptosis | Ying H et al., Nat. Med. 1999 | 25 |
| SFV | LacZ | Bacteria | VRP | Colorectal Cancer | Anti-tumor effect driven by CD8 + T cells with antigen spread | Granot et al., MT 2013 | 47 |
| VEE | Neoepitopes | Mouse | LNP replicons | Colorectal Cancer | Anti-tumor effect that synergizes with ICI and enables epitope spreading | Maine et al., MT 2021 | 63 |
| VEE | CEA/IL-12 | Human/Mouse | VRP | Colorectal Cancer | VRP-IL-12+VRP-CEA enhanced CEA-specific T cell and antibody responses | T. Osada et al., Cancer Immunology, Immun. 2012 | 58 |
| SFV | P1A | Mouse | VRP | Mastocytoma | Anti-tumor immunity in vivo | Ni et al., CDP, 2004 | 39 |
| SFV | P1A/IL-12 | Mouse | VRP | Mastocytoma | Tumor regression with activation of immune memory with rSFV-P1A + rSFV-IL-12 combo treatment | P. Colmenero et al., Int. Journal of Cancer 2002 | 57 |
| VEE | E7 | Virus | VRP | Cervical Cancer | Anti-tumor protection for E6/E7 expressing cells | Daemen et al., Gene Therapy, 2000 | 42 |
| VEE | E7 | Virus | VRP | Cervical Cancer | Anti-tumor immunity in vivo (CD8 + Dependent) | Velders et al., Cancer Res., 2001 | 46 |
| SFV | E6 + E7 | Virus | VRP | Cervical Cancer | Enhanced in vivo protection against tumor challenge with fusion E6/E7 | Daemen et al., GT 2002 | 41 |
| SFV | E6 + E7 | Virus | VRP | Cervical Cancer | In vivo protection against established tumors (long term) | Daemen et al., Vaccine 2003 | 40 |
| SFV | E6 + E7 | Virus | VRP | Cervical Cancer | Efficacy of VRP tattoo delivery | van de wall et al., Vaccines 2015 | 44 |
| SFV | E6 + E7 | Virus | VRP | Cervical Cancer | Superior anti-tumor responses from IM and IV administration routes | Daemen et al., Antiviral Therapy 2004 | 43 |
| SFV | E6 + E7 | Virus | VRP | Cervical Cancer | VRP allows for prime-boosting of responses in comparison to Ad vectors | Riezebos-Brilman et al., Gene Therapy 2007 | 38 |
| SFV | E6 + E7 | Virus | VRP | Cervical Cancer | Enhanced anti-tumor effect with radiation | Draghiciu et al., IJC 2014 | 65 |
| SFV | E6 + E7 | Virus | VRP | Cervical Cancer | Enhanced anti-tumor effect with radiation and sunitinib | Draghiciu et al., Oncoimmunology 2015 | 64 |
| SFV | E6 + E7 | Virus | DNA encoding srRNA (DREP) | Cervical Cancer | Anti-tumor immunity in vivo | Van de Wal et al., Oncoimmunology 2018 | 31 |
| SFV | TRP1 | Human/Mouse | DNA encoding srRNA (DREP) | Melanoma | Importance of PKR/OAS and CD8/CD4 cells to response | Leitner et al., Nat. Med 2003 | 28 |
| SFV | TRP1 | Human/Mouse | DNA encoding srRNA (DREP) | Melanoma | Importance of apoptosis in anti-tumor responses from DREP | Leitner et al., Vaccine 2004 | 29 |
| SFV | TRP1/gp100 | Human/Mouse | DNA encoding srRNA (DREP) | Melanoma | Importance of interferon in anti-tumor responses from DREP | Leitner et al., Vaccine 2006 | 30 |
| VEE | Tyr | Human/Mouse | VRP | Melanoma | VRP responses superior to DNA vaccine and enhanced by heterologous prime-boost | Goldberg et al., CCR 2005 | 52 |
| VEE | TRP2/gp100 | Mouse | VRP | Melanoma | Anti-tumor effect mediated by CD8 + /CD4 + T cells and FcGR from antibodies | Avogadri F et al., PLOSOne 2010 | 53 |
| VEE | TRP2 | Mouse | VRP | Melanoma | Anti-tumor synergy with ICIs | Avogadri F et al., CIR 2014 | 63 |
| VEE | PSMA | Human | VRP | Prostate Cancer | Immunogenicity in vivo | Durso et al., CCR 2007. | 48 |
| VEE | STEAP | Mouse | VRP | Prostate Cancer | Anti-tumor immunity in vivo without autoimmunity (CD4 + Dependent) | Garcia-Hernandez et al., Cancer Res. 2007 | 49 |
| VEE | PSCA | Mouse | VRP | Prostate Cancer | Anti-tumor immunity in endogenous model without autoimmunity (CD4 + Dependent) | de la Luz et al., Cancer Res. 2008 | 50 |
| VEE | PSA | Human | VRP | Prostate Cancer | Anti-tumor immunity in vivo | Riabov V et al., Vaccine 2015 | 51 |
| Sindbus virus | ErbB2 | Rat | VRP | Breast Cancer | Enhanced anti-tumor immunity with chemotherapy | Erlap et al., BCR, 2004 | 54 |
| VEE | ErbB2 | Rat | VRP-DC | Breast Cancer | Anti-tumor immunity in vivo dependent on CD4 + T cells | Moran et al., Vaccine 2007 | 55 |
| VEE | ErbB2 | Human | VRP | Breast Cancer | Tumor regression after vaccination | Crosby et al., CCR 2019 | 36 |
Column 1 specifies the specific virus/viral backbone that was used to generate the self-replication aspect of the srRNA vaccine, column 2 identifies the gene used as the target antigen for the study, column 3 describes the species of origin of that target antigen, column 4 specifies the delivery mechanism of the vaccine, column 5 names the type of cancer each study focused on, column 6 briefly describes the main finding of each study, column 7 gives the manuscript reference and column 8 tells the reader which reference it is in this manuscript. Table is grouped by what type of cancer each study focused on.
Self-replicating RNA based vaccines in cancer therapies
‘Naked’ srRNA cancer vaccines
The first efforts to utilize self-replicating RNA vaccines against cancer occurred over 20 years ago using intramuscular vaccination of naked srRNA molecules encoding a LacZ model antigen followed by implantation of a LacZ expressing colorectal carcinoma (CT26.CL25) [25]. This study demonstrated that direct injection of srRNA (doses of 0.1ug to 100ug) could elicit anti-LacZ responses and offer protection against CT26.CL25 metastatic challenge. Critically, this study showed that even injection of high doses of non-replicating LacZ expressing control vaccines (100ug) offered no anti-tumor protection, suggesting a fundamental benefit to using replicating vectors. In vitro studies revealed that replicating vectors only offered a two-fold enhancement of antigen expression, but they elicited a significantly higher level of apoptosis, a difference presumably essential for the immunogenicity seen in vivo. This study established the potential of cancer vaccination using replicating RNA vectors, although issues with RNA generation and delivery led to a pivot towards DNA-launched RNA replicon vaccines.
DNA-launched srRNA Replicon (DREP) cancer vaccines
The generation of srRNA vectors required the use of DNA templates, which had already been established as a cancer vaccine modality [26, 27]. In the first studies, homologous gene gun injection of DNA-launched srRNA Replicons (DREP) encoding a mouse self-antigen expressed in melanoma (Tyrosinase Related Protein 1, TRP-1) proved to be superior to non-replicating DNA vectors in protecting mice from B16-F10 melanoma challenge [28]. Notably, this study demonstrated that DREP vectors could break immunological tolerance against a TRP-1 self-antigen and allow for the induction of TRP-1-specific antibodies. Mice vaccinated with DREP-TRP-1 vectors experienced no vitiligo, a symptom of autoimmunity that is observed with the use of non-replicating DNA vaccines encoding TRP-1. Surprisingly, this study documented no difference in levels of antigen production in the skin between replicating and non-replicating vectors, although this data was not shown. Using RNAse L KO mice, the authors demonstrated that enhanced anti-tumor responses following DREP treatment were dependent upon the recognition of dsRNA intermediates. Additionally, CD8+ T-cell depletion and transfer experiments confirmed the critical nature of CD8+ T-cell effectors in vivo for anti-tumor responses. Secondary studies demonstrated that suppression of apoptosis, through the co-delivery of Bcl-XL expressing plasmids, reduced the anti-tumor efficacy of TRP-1 DREP vaccination [29]. Further studies using Type I interferon receptor (IFNAR) KO mice demonstrated the importance of Type I interferon induction in DREP-induced TRP-1-specific adaptive immune and anti-tumor responses [30]. These mechanistic studies established the importance of the induction of apoptosis and interferon responses in the elicitation of anti-tumor immunity by DREP vaccines. Studies using an alphavirus replicon (based on Semliki Forest Virus or SFV) and targeting human papilloma virus (HPV) E6 and E7 antigens also demonstrated the potent induction of anti-tumor immunity at low DNA doses (0.05 ug) [31]. Notably, this study demonstrated that intradermal delivery by electroporation of higher doses of E6/E7 encoding DREPs (up to 10 ug) offered no advantage in eliciting E6/E7-specific T-cell immunity or tumor control compared to lower doses (.05ug), confirming that srRNA vectors require a low delivery threshold to achieve significant antigen-specific immunity.
Viral replicon particles (VRP) cancer vaccines
The use of srRNA cancer vaccines from naked RNA and DNA encoded intermediates also prompted the development of viral replicon particles (VRP) using helper plasmids [32–34]. These vaccines contain the native structural proteins that could potentially aid in trafficking and/or innate immune stimulation to augment the vaccine efficacy. The disadvantage of this strategy is the elicitation of immunity against structural proteins, as demonstrated by the induction of antibodies that limit secondary transduction in vivo and promote T-cell competition against encoded antigens [35, 36]. Despite this, studies have demonstrated that fully homologous VRP vaccines are capable of boosting adaptive antigen-specific immunity and augmenting anti-tumor responses [35, 37, 38]. In a comparison study utilizing homologous vaccination against P1A (a self-antigen expressed in mastocytoma), VRP particles were found to elicit striking prophylactic and therapeutic anti-tumor immunity, which was superior to DREP vectors encoding the same antigen [39]. Given their potential, different types of VRPs have been widely used and have demonstrated anti-tumor immunity against a variety of foreign and self-antigen targets expressed in cancer, as reviewed below.
Viral and model antigen vaccines
In the earliest use of VRP cancer vaccines, an alphaviral replicon based on SFV was developed that permitted the expression of HPV E6 and E7 antigens. Vaccination using E6/E7 VRPs stimulated potent E6- and E7-specific T-cell immunity that translated into rejection of E6/E7 tumor challenge and suppressed the growth of established E6/E7 expressing tumors [40–42]. These vaccines proved to be most potent when delivered by intramuscular or intravenous routes (compared to subcutaneous and intradermal) and conferred long-term immunity against tumor challenge [40, 43]. Subsequent studies also determined that administration by tattoo yielded comparable responses to intramuscular injection [44]. While E6 and E7 were foreign antigens in this model, this vaccine was also able to break tolerance in an E6/E7 transgenic mouse, although the magnitude of adaptive responses was significantly reduced in comparison to non-tolerant naïve mice [45]. Using a Venezuelan Equine Encephalitis virus (VEE) VRP vaccine encoding only E7, a different study demonstrated significant protection against HPV E7+ tumor challenge and a strong suppression of E7+ tumor growth [46]. These responses were demonstrated to be dependent upon CD8+ T-cells, as the anti-tumor effect of E7 VRP vaccination was eliminated in CD8 KO mice. Using a Sindbis virus VRP vaccine encoding the model foreign antigen LacZ, another group also demonstrated that srRNA VRP vaccination could elicit a significant anti-tumor response against CT25.CL26 cells dependent upon CD8 + LacZ-specific T-cells [47]. Early mediastinal lymph node expression of encoded VRP genes gave rise to CD8 + T-cell memory responses that allowed for antigen spread to other CT26 epitopes (such as gp70) and permitted anti-tumor immunity against LacZ negative CT26 cells. As antigenic spread is likely to be essential in eliminating metastatic and dormant tumor cells that have diverged from the primary tumor and lost the targeted antigen, these findings are critically important for the future of srRNA vaccines. Additionally, multiple groups have begun to explore the use of srRNA vectors for intratumoral delivery of different genes to evoke anti-tumor immunity, although our review focuses on the use of the vectors to stimulate systemic immunity against tumor (or model tumor) antigens.
Tumor-associated antigen vaccines
Prostate cancer contains a class of tumor-associated antigens (TAA) with more restricted anatomical expression, spawning some of the earliest studies that utilized srRNA VRP vaccine platforms. In 2007, VEE VRPs that encoded Prostate Specific Membrane Antigen (PSMA) were generated and utilized to vaccinate mice with both single dose and homologous boosting regimens [48]. These studies demonstrate PSMA-specific immunity, which can be significantly boosted by multiple PSMA-VRP vaccinations. As the encoded PSMA was of human origin, epitope mapping revealed that the dominant epitopes were non-homologous in mice, suggesting antigen immunodominance of non-self epitopes. To address whether induction of T-cell responses against self-epitopes was possible, the murine form of Six-transmembrane Epithelial Antigen of Prostate (STEAP) was used and revealed the induction of T-cell specific responses against mouse STEAP epitopes [49]. Critically, vaccination significantly improved anti-tumor responses against tumor challenge, without observable prostate autoimmunity. Using CD8 and CD4 KO mice, this study also demonstrated an essential role for both CD8+ and CD4+ T-cells in these anti-tumor responses. In an established tumor model, this vaccine had a predictably more modest effect, but still significantly retarded tumor growth.
Subsequent studies targeting mouse Prostate Stem Cell Antigen (PSCA) using a heterologous DNA prime and VEE VRP boost demonstrated the ability of this regimen to break immune tolerance and induce T-cells specific for PSCA epitopes [50]. This heterologous vaccine strategy was effective in providing protection from tumor challenge and also allowed for protection against emerging prostate cancer using an endogenous TRAMP mouse model. Anti-tumor responses were associated with greater infiltration of CD4+ and CD8+ T-cells, as well as the intratumoral expression of Th1 cytokines (such as IFN-g, GM-CSF, and IL-2). The genetic ablation of CD4 or CD8 T-cells resulted in the loss of protective immunity to tumor challenge, thus demonstrating the critical nature of these T-cell populations in mediating anti-tumor immunity. Finally, Prostate Specific Antigen (PSA), a secreted prostate-specific antigen, has also been tested as an antigen target using srRNA VRPs [51]. This study utilized a human PSA encoding VEE VRP to immunize HLA-DR transgenic mice, demonstrating the induction of PSA-specific T-cell responses, as well as PSA-specific antibodies of multiple isotypes. Infiltration of CD8+ T-cells in a PSA expressing TRAMP tumor model following vaccination was associated with a loss of PSA expression in tumors and suppression of tumor growth in vivo.
VRP vaccines have also been utilized in select studies of melanoma TAAs. In initial studies of melanoma TAA, the potential of VEE VRP vaccines to elicit immunity against tyrosinase (Tyr) was compared to DNA vaccine vectors [52]. As observed in studies targeting STEAP, VRP srRNA vectors elicited significantly enhanced adaptive immunity against both human and mouse tyr. This study also confirmed the ability of VRP homologous vaccination to break immune tolerance and augment transgene-specific immunity and anti-tumor responses, as was demonstrated with E6/E7 VRPs [35, 37]. A subsequent study then evaluated the ability of VEE VRPs to elicit immunity across a variety of melanoma differentiation antigens (Tyr, gp100, and TRP-2) [53]. This study found that the most potent prophylactic anti-tumor responses came from VRPs encoded with TRP-2. TRP-2 VRP vaccination allowed for enhanced survival in the treatment model, reduced pigmentation in tumors, and enhanced immune infiltration. Notably, experiments using T-cell depletion, as well as MHCI and MHCII KO mice, revealed that this protection was only partially dependent on CD8+ T-cells. Depletion of NK and NK T-cells revealed no role for these immune cell types, but the use of FCGR KO mice revealed an unexpected role for antibody-mediated immunity, which was associated with the induction of TRP-2 IgG responses. A lack of effect from C3 KO mice suggests that Antibody-Dependent phagocytosis from macrophages could be mediating this effect, although this was not formally tested. However, this study suggests the importance of B-cell responses to VRP cancer vaccines, highlighting the potential advantages in targeting cellular receptor TAAs. Related to this, several studies have targeted ErbB2, a receptor highly overexpressed in certain types of breast and gastric cancer [54]. In an initial study, an SV VRP encoding rat ErbB2 was generated and utilized to elicit Erb2-specific T-cells [54]. While this study did not report the anti-tumor effect of ErbB2-VRP vaccination alone, it did document an augmented T-cell infiltration in tumors. A subsequent study utilized a VEE VRP encoding rat ErbB2 to transduce dendritic cells (DCs) as an alternate strategy to elicit immunity in vivo [55]. VRP transduced DCs stimulated a significant induction of ErbB2-specific T-cell and B-cell responses after delivery into mice. Notably, delivery of VRP-ErbB2 transduced DCs allowed for long-term survival gains, which were critically dependent upon the presence of CD4+ T-cells. These studies utilized an endogenous mouse model that develops rat ErbB2-driven breast cancer and is tolerant to rat ErbB2. These studies make it clear that VRP vectors can activate DCs to generate anti-tumor immunity, although the benefit of ex vivo DC transduction and in vivo delivery was not directly assessed. A different study utilizing a VEE VRP vector expressing a truncated form of human ErbB2 (HER2TM) demonstrated the induction of HER2-specific T- and B-cell responses after vaccination in a transgenic human HER2 expressing mouse model [36]. These responses were sufficient to elicit strong anti-tumor immunity in vivo, which translated into a Phase I human trial testing this vector [36]. Despite a small number of enrolled patients, this trial demonstrated the safety of the VRP-HER2TM vaccine, as well as its ability to elicit CD8+ T-cell memory responses in patients, which were associated with a significantly enhanced progression-free survival. Additional details of this clinical trial and summation of other srRNA VRP trials can be viewed in a separate review by Morse et al. in this special issue.
Given the immunosuppression present in tumors and difficulties eliciting immune responses against self-antigens, two studies have also explored including immunostimulatory cytokines in srRNA vaccine vectors have also been included to enhance immunity against encoded antigens. Both of these studies have utilized L-12, a pleiotropic immune stimulatory cytokine, as a means to enhance Th1 immune responses against tumor antigens [56]. In the first study, established were treated by peri-tumoral injection with VRP vectors encoding a P1A, IL-12, or a combination of these vectors [57]. These studies demonstrated an enhanced impact for antigen-specific responses against established tumors injected by a combination of P1A and IL-12 encoding vectors. In the second study, VRP-CEA vaccine vectors were utilized to elicit immunity against Carcioembryonic Antigen (CEA), which is frequently overexpressed in colorectal cancers [58]. These vectors were combined with VRP-IL12 encoding vectors to enhance the stimulation of CEA, which was found to significantly enhance T-cell and B-cell responses to CEA, as well as enhance anti-tumor responses against CEA + mouse colorectal cancers. While preliminary, these studies demonstrate the ability of immune-stimulatory cytokines to enhance srRNA vaccines against established tumors, suggesting their potential for future srRNA vaccines strategies.
Collectively the past two decades, have seen a large number of studies confirm the stimulatory capacity of srRNA VRPs as a vaccine platform, in their ability to directly stimulate innate immune responses and elicit both T-cell and B-cell responses against their encoded target antigen(s). Critically, multiple studies have also documented that these vaccines can also overcome immune tolerance against different antigens in vivo. While anti-VRP responses have also been demonstrated, these anti-vector responses do not appear to negate the capacity of VRP vectors to be used in homologous boosting strategies against a single target antigen. While promising, the issue of scalable manufacture may prove a limitation in the deployment of these vectors, as potentially indicated by their limited clinical studies (reviewed in Morse et al. CGT this issue).
Synthetic self-replicating RNA cancer vaccines
The past decade has seen the emergence of novel processes to amplify RNA ex vivo and the development of lipid nanoparticle (LNP) formulation with related synthetic encapsulation methods, which has allowed the packaging of srRNA vectors [22, 59]. These dual revolutions have enabled the scalable development of ‘synthetic’ self-replicating RNA vaccines, first demonstrated in 2012 with srRNAs adsorbed to a cationic nanoemulsion particle formulation. [60]. The following year, it was demonstrated that LNP encapsulated srRNA vectors could elicit immune responses in vivo against viral antigens [61]. These developments prompted subsequent studies demonstrating that synthetic RNA vaccines were highly effective in generating immunity against a variety of pathogen antigens [8, 34]. Their scalability has made them highly attractive for clinical development, with several companies launching these vaccines in clinical trials against infectious diseases. The use of synthetic formulations is a complex and rapidly evolving field, but promises significant benefits in effective encapsulation and delivery of srRNAs (reviewed in Lundstrom et al., CGT this issue). Additionally, recent clinical trials have also used this modality as cancer therapeutics, vaccinating against neoepitopes as part of a prime-boosting strategy (NCT03639714 and NCT03953235) [62].
The clinical use of synthetic srRNA cancer vaccines is supported by a recent pre-clinical study that documented their ability to elicit neoepitope-specific immunity in a well-annotated colorectal model (CT26), which translated into significant anti-tumor responses in vivo [63]. These synthetic srRNA vaccines elicited polyclonal CD4+ and CD8+ T-cell responses and were significantly boosted by homologous vaccination, particularly with longer time intervals between boosts. The ability to elicit antigen-specific T-cell responses for tumor neoepitopes was further demonstrated using human HLA-A*1101 transgenic mice, supporting the translatability of this approach. Finally, the LNP srRNA vaccines elicited antigen-specific polyfunctional T-cell responses in non-human primates without any obvious toxicities. While this study validates the potential of targeting neoepitopes, large differences in immune responsiveness to various epitopes were observed between different platforms and antigen targets. As such, it is likely that any set of antigens within any given srRNA vaccine strategy will need to be optimized empirically. This will be particularly essential in the development of multi-antigen vaccines and may vary based on the model system utilized during vaccine development. In sum, while only a single published study to date has examined the potential of synthetic srRNA replicons in cancer models, this approach appears to elicit comparable responses to srRNA VRP strategies and has appreciable advantages in the rapid and scalable manufacturing process.
Combination of srRNA vaccines with other therapeutic modalities
While many studies have demonstrated the potential of different srRNA vaccine platforms in eliciting anti-tumor immunity, almost all of these studies have documented a suppression of tumor growth or delay of engraftment, neither of which was curative. As such, there is a pressing need to understand how these vaccine platforms could potentially synergize with standard-of-care therapies and newly developed immune-based therapies, such as ICI antibodies. In this vein, several studies have identified that vaccine responses can be improved by combining them with chemotherapy, radiation, and a small molecule inhibitor (sunitinib) [54, 64, 65]. These studies have collectively indicated that forms of immunogenic cell death (ICD) can enhance vaccine efficacy, although it is unclear if these approaches could also hamper the expansion of immune cells elicited by vaccination. Other studies have combined vaccination with different types of ICI antibodies (GITR, CTLA-4, and PD-1), resulting in augmented T-cell responses and improved anti-tumor efficacy using both VRP and synthetic srRNA replicons [63, 66]. Clinical studies exploring this approach (such as NCT03632941) are starting to enroll and should help establish the potential of this combination in allowing for sustained anti-tumor immunity in non-responsive patient populations. Thus, while early studies suggest the potential for srRNA vaccines to augment standard-of-care therapies and be enhanced by ICIs, future studies are needed to define the optimal timing, dosing, and specific combinations of these therapies across different cancers.
Conclusions
A variety of different srRNA vaccine platforms have evolved over the past two decades, which have demonstrated the ability to elicit antigen-specific T- and B-cell immunity against a diverse assortment of self- and non-self-antigens and tumor neoepitopes. Starting with the injection of naked RNA, the use of srRNA revealed a striking ability to elicit immunity in comparison to non-replicating RNAs, particularly when delivered at low RNA doses [25]. Development of more stable DNA-launched srRNA vectors confirmed that the induction of immunity was related to the detection of amplified RNA (with dsRNA intermediates) and the stimulation of apoptosis [28–30]. Generation of immune responses has historically hampered the efficacy of mRNA vaccines due to the suppression of target gene translation, but this has not been the case for srRNA vaccines [8, 24, 33]. The potential to leverage structural protein stimulation of innate immunity and exploit different viral tropisms led to the development of VRP srRNA vaccines. These fully RNA vectors have safety benefits, as they have no risk of integration and comprise a more limited expression cycle, being particularly advantageous in the development of vaccines targeting oncogenic genes. Studies using VRP vectors proved that the platform was sufficient to break immune tolerance and was improved by homologous boosting strategies, despite the induction of anti-vector responses. However, these VRPs have been less amenable to scalable manufacturing, leading to fewer clinical trials and decreasing the utility of these vectors in comparison to other vaccine platforms.
Potentially improving from this design, in vitro RNA amplification and RNA packaging using LNPs have both emerged as scalable modalities that have been used in combination to generate fully synthetic srRNA replicon vaccines. While not yet widely tested, initial studies have documented the ability of these vectors to elicit significant anti-tumor immunity, even against more restrictive neoepitope targets. As with VRP vaccines, these antigen-specific responses can be boosted by homologous vaccination, despite the retention of replicase genes. While it is currently unclear how immunity to non-structural replicase genes may alter overall efficacy, recent studies have not documented any negative impact on encoded transgene immunity. While select in vitro studies have reported modestly amplified transgene expression from srRNA vectors, in vivo measurement of target antigen expression has been lacking. It is also unclear if srRNA vaccines may also benefit from additional innate immune stimulation, potentially through the addition of different immune stimulatory payloads. This concept has been demonstrated in studies using srRNA platforms as a delivery vehicle for cytokine production that enable enhancement of antigen-specific immunity to vaccination [8, 67, 68]; thus, the potential of this approach in enhancing systemic vaccine responses is an area of considerable interest. Finally, as with other vaccines, several studies using different types of srRNA vaccines have demonstrated enhanced anti-tumor efficacy in combination with standard-of-care anti-tumor therapies and ICI antibodies. Given the highly immunosuppressive nature of many tumors, these combinatorial approaches may be critical in generating effective clinical responses, with some clinical trials already underway to explore this approach (NCT03632941).
In conclusion, srRNA vaccines have had a relatively rapid evolution, transitioning from naked srRNA vaccines to fully synthetic srRNA vaccine platforms. Different srRNA vaccines are capable of eliciting potent, antigen-specific immune responses to a wide spectrum of antigens, including viral antigens, tumor-associated self-antigens, and tumor-specific neoepitopes. This class of therapies is likely to benefit from combinations with standard-of-care therapies and immune-enabling ICIs. Moreover, newly developed synthetic srRNA vectors can be easily and cheaply manufactured, thus providing a strong advantage over older viral vaccines. While additional studies of these vectors and comparisons between mRNA and srRNA backbones are needed, the potent induction of immunity, elevated level of antigen expression, low toxicity, and potential scalability of srRNA vectors make them particularly attractive for future use as cancer vaccines.
Author contributions
All authors contributed to the research, writing, review and editing of this manuscript. Authors GPD and ZCH generated Table 1. Author GPD created Figs. 1, 2.
Data availability
The data presented in this review was not generated by the authors, but gathered through existing research/publications. Thus all data is publicly available.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Institute, NC Cancer Statistics. Understanding Cancer [webpage] 2020 9/20/20 [cited 2021 9/21/21]; Available from: https://www.cancer.gov/about-cancer/understanding/statistics.
- 2.Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, et al. SEER Cancer Statistics Review, 1975–2018, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2018/, based on November 2020 SEER data submission, posted to the SEER web site, April 2021.
- 3.Fuller DH, Berglund P. Amplifying RNA vaccine development. N Engl J Med. 2020;382:2469–71. doi: 10.1056/NEJMcibr2009737. [DOI] [PubMed] [Google Scholar]
- 4.Hu Z, Ott PA, Wu CJ. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18:168–82. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins JM, Redman JM, Gulley JL. Combining vaccines and immune checkpoint inhibitors to prime, expand, and facilitate effective tumor immunotherapy. Expert Rev Vaccines. 2018;17:697–705. doi: 10.1080/14760584.2018.1506332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellmann MD, Friedman CF, Wolchok JD. Combinatorial cancer immunotherapies. Adv Immunol. 2016;130:251–77. doi: 10.1016/bs.ai.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019;4:7. doi: 10.1038/s41541-019-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundstrom K. Self-Amplifying RNA viruses as RNA vaccines. Int J Mol Sci. 2020;21:5130. doi: 10.3390/ijms21145130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perri S, Greer CE, Thudium K, Doe B, Legg H, Liu H, et al. An alphavirus replicon particle chimera derived from venezuelan equine encephalitis and sindbis viruses is a potent gene-based vaccine delivery vector. J Virol. 2003;77:10394–403. doi: 10.1128/JVI.77.19.10394-10403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pushko P, Parker M, Ludwig GV, Davis NL, Johnston RE, Smith JF. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: Expression of heterologous genesin vitroand immunization against heterologous pathogensin vivo. Virology. 1997;239:389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 11.Beissert T, Perkovic M, Vogel A, Erbar S, Walzer KC, Hempel T, et al. A trans-amplifying rna vaccine strategy for induction of potent protective immunity. Mol Ther. 2020;28(1):119–28. doi: 10.1016/j.ymthe.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Restifo NP (2000). Building better vaccines: How apoptotic cell death can induce inflammation and activate innate and adaptive immunity. Curr Opin Immunol. 597–603. 10.1016/s0952-7915(00)00148-5. [DOI] [PMC free article] [PubMed]
- 13.Mogler MA, Kamrud KI. RNA-based viral vectors. Expert Rev Vaccines. 2015;14(2):283–312. doi: 10.1586/14760584.2015.979798. [DOI] [PubMed] [Google Scholar]
- 14.Geall AJ, Verma A, Otten GR, Shaw CA, Hekele A, Banerjee K, et al. Nonviral delivery of self-amplifying RNA vaccines. Proc Natl Acad Sci. 2012;109(36):14604–9. doi: 10.1073/pnas.1209367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polo JM, Belli BA, Driver DA, Frolov I, Sherrill S, Hariharan MJ, et al. Stable alphavirus packaging cell lines for Sindbis virus- and Semliki Forest virus-derived vectors. Proc Natl Acad Sci. 1999;96(8):4598–603. doi: 10.1073/pnas.96.8.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liljeström P, Garoff H. A New Generation of Animal Cell Expression Vectors Based on the Semliki Forest Virus Replicon. Nat Biotechnol. 1991;9:1356–61. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- 17.Bredenbeek PJ, Frolov I, Rice CM, Schlesinger S. Sindbis virus expression vectors: Packaging of RNA replicons by using defective helper RNAs. J Virol. 1993;67(11):6439–46. doi: 10.1128/jvi.67.11.6439-6446.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamrud KI, Alterson KD, Andrews C, Copp LD, Lewis WC, Hubby B, et al. Analysis of Venezuelan equine encephalitis replicon particles packaged in different coats. PLoS ONE. 2008;3(7):e2709. doi: 10.1371/journal.pone.0002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macdonald GH, Johnston RE. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J Virol. 2000;74(2):914–22. doi: 10.1128/JVI.74.2.914-922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bieback K, Lien E, Klagge IM, Avota E, Schneider-Schaulies J, Duprex PW, et al. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J Virol JID. 2002;76(17):8729–36. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schabbauer G, Luyendyk J, Crozat K, Jiang Z, Mackman N, Bahram S, et al. TLR4/CD14-mediated PI3K activation is an essential component of interferon-dependent VSV resistance in macrophages. Mol Immunol. 2008;45(10):2790–6. doi: 10.1016/j.molimm.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blakney AK, McKay PF, Yus BI, Aldon Y, Shattock RJ, et al. Inside out: Optimization of lipid nanoparticle formulations for exterior complexation and in vivo delivery of saRNA. Gene Ther. 2019;26(9):363–72. doi: 10.1038/s41434-019-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundstrom K. Latest development on RNA-based drugs and vaccines. Future Sci OA. 2018;4(5):FSO300. doi: 10.4155/fsoa-2017-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundstrom K. Self-amplifying RNA virus vectors: clinical applications in cancer drug delivery. Exp Opin Drug Deliv. 2019;16(10):1027–9. doi: 10.1080/17425247.2019.1653851. [DOI] [PubMed] [Google Scholar]
- 25.Ying H, Zaks TZ, Wang RF, Irvine KR, Kammula US, Marincola FM, et al. Cancer therapy using a self-replicating RNA vaccine. Nat Med. 1999;5(7):823–7. doi: 10.1038/10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber LW, Bowne WB, Wolchok JD, Srinivasan R, Qin J, Moroi Y, et al. Tumor immunity and autoimmunity induced by immunization with homologous DNA. J Clin Invest. 1998;102(6):1258–64. doi: 10.1172/JCI4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SH, Danishmalik SN, Sin JI. DNA vaccines, electroporation and their applications in cancer treatment. Hum Vaccin Immunother. 2015;11(8):1889–900. doi: 10.1080/21645515.2015.1035502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leitner WW, Hwang LN, deVerr MJ, Zhou A, Silverman RH, Williams BRG, et al. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nat Med. 2003;9(1):33–9. doi: 10.1038/nm813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leitner WW, Hwang LN, Bergmann-Leitner ES, Finkelstein S, Frank S, Restifo NP. Apoptosis is essential for the increased efficacy of alphaviral replicase-based DNA vaccines. Vaccine. 2004;22(11-12):1537–44.. doi: 10.1016/j.vaccine.2003.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leitner WW, Bergmann-Leitner ES, Hwang LN, Restifo NP. Type I Interferons are essential for the efficacy of replicase-based DNA vaccines. Vaccine. 2006;24(24):5110–8. doi: 10.1016/j.vaccine.2006.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Wall S, Ljungberg K, Ip PP, Boerma A, Knudsen ML, Nijman HW, et al. Potent therapeutic efficacy of an alphavirus replicon DNA vaccine expressing human papilloma virus E6 and E7 antigens. Oncoimmunology. 2018;7(10):e1487913. doi: 10.1080/2162402X.2018.1487913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tews BA, Meyers G. Self-replicating RNA. Methods Mol Biol. 2017;1499:15–35. doi: 10.1007/978-1-4939-6481-9_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lundstrom K. Replicon RNA viral vectors as vaccines. Vaccines (Basel) 2016;4:39. doi: 10.3390/vaccines4040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ljungberg K, Liljestrom P. Self-replicating alphavirus RNA vaccines. Expert Rev Vaccines. 2015;14:177–94. doi: 10.1586/14760584.2015.965690. [DOI] [PubMed] [Google Scholar]
- 35.de Mare A, Lambeck AJA, Regts J, van Dan GM, Nijamn HW, Snippe H, et al. Viral vector-based prime-boost immunization regimens: a possible involvement of T-cell competition. Gene Ther. 2008;15(6):393–403. doi: 10.1038/sj.gt.3303060. [DOI] [PubMed] [Google Scholar]
- 36.Crosby EJ, Gwin W, Blackwell K, Marcom PK, Chang S, Maecker HT, et al. Vaccine-induced memory CD8(+) T cells provide clinical benefit in HER2 expressing breast cancer: A mouse to human translational study. Clin Cancer Res. 2019;25(9):2725–36. doi: 10.1158/1078-0432.CCR-18-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambeck AJ, Nijaman HW, Hoogeboom BN, Regts J, de Mare A, Wilschut J, et al. Role of T cell competition in the induction of cytotoxic T lymphocyte activity during viral vector-based immunization regimens. Vaccine. 2010;28(26):4275–82. doi: 10.1016/j.vaccine.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 38.Riezebos-Brilman A, Walczak M, Regts J, Rots MG, Kamps G, Dontje B, et al. A comparative study on the immunotherapeutic efficacy of recombinant Semliki Forest virus and adenovirus vector systems in a murine model for cervical cancer. Gene Ther. 2007;14(24):1695–704. doi: 10.1038/sj.gt.3303036. [DOI] [PubMed] [Google Scholar]
- 39.Ni B, Lin Z, Zhou L, Wang L, Jia Z, Zhou W, et al. Induction of P815 tumor immunity by DNA-based recombinant Semliki Forest virus or replicon DNA expressing the P1A gene. Cancer Detect Prev. 2004;28(6):418–25.. doi: 10.1016/j.cdp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Daemen T, Riezebos-Brilman A, Bungener L, Regts J, Dontje B, Wilschut J. Eradication of established HPV16-transformed tumours after immunisation with recombinant Semliki Forest virus expressing a fusion protein of E6 and E7. Vaccine. 2003;21(11–12):1082–8. doi: 10.1016/S0264-410X(02)00558-3. [DOI] [PubMed] [Google Scholar]
- 41.Daemen T, Regts J, Holtrop M, Wilschut J. Immunization strategy against cervical cancer involving an alphavirus vector expressing high levels of a stable fusion protein of human papillomavirus 16 E6 and E7. Gene Ther. 2002;9(2):85–94. doi: 10.1038/sj.gt.3301627. [DOI] [PubMed] [Google Scholar]
- 42.Daemen T, Pries F, Bungener L, Kraak M, Regts J, Wilschut J. Genetic immunization against cervical carcinoma: induction of cytotoxic T lymphocyte activity with a recombinant alphavirus vector expressing human papillomavirus type 16 E6 and E7. Gene Ther. 2000;7(21):1859–66. doi: 10.1038/sj.gt.3301257. [DOI] [PubMed] [Google Scholar]
- 43.Daemen T, Riezebos-Brilman A, Regts J, Dontje B, van der Zee A, Wilschut J. Superior therapeutic efficacy of alphavirus-mediated immunization against human papilloma virus type 16 antigens in a murine tumour model: effects of the route of immunization. Antivir Ther. 2004;9(5):733–42. doi: 10.1177/135965350400900515. [DOI] [PubMed] [Google Scholar]
- 44.van de Wall S, Walczak M, van Rooij N, Hoogeboom BN, Meijerhof T, Nijman HW, et al. Tattoo delivery of a semliki forest virus-based vaccine encoding human papillomavirus E6 and E7. Vaccines (Basel) 2015;3(2):221–38. doi: 10.3390/vaccines3020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riezebos-Brilman A, Regts J, Freyschmidt EJ, Donteje B, Wilschut J, Daemen T. Induction of human papilloma virus E6/E7-specific cytotoxic T-lymphocyte activity in immune-tolerant, E6/E7-transgenic mice. Gene Ther. 2005;12(18):1410–4. doi: 10.1038/sj.gt.3302536. [DOI] [PubMed] [Google Scholar]
- 46.Velders MP, McElhiney S, Cassetti MC, Eiben GC, Higgins T, Kovacs GR, et al. Eradication of established tumors by vaccination with Venezuelan equine encephalitis virus replicon particles delivering human papillomavirus 16 E7 RNA. Cancer Res. 2001;61(21):7861–7. [PubMed] [Google Scholar]
- 47.Granot T, Yamanashi Y, Meruelo D. Sindbis viral vectors transiently deliver tumor-associated antigens to lymph nodes and elicit diversified antitumor CD8+ T-cell immunity. Mol Ther. 2014;22(1):112–22. doi: 10.1038/mt.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durso RJ, Andjelic S, Gardner JP, Margitich DJ, Donovan GP, Arrigale RR, et al. A novel alphavirus vaccine encoding prostate-specific membrane antigen elicits potent cellular and humoral immune responses. Clin Cancer Res. 2007;13(13):3999–4008. doi: 10.1158/1078-0432.CCR-06-2202. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Hernandez Mde L, Gray A, Hubby B, Kast WM. In vivo effects of vaccination with six-transmembrane epithelial antigen of the prostate: a candidate antigen for treating prostate cancer. Cancer Res. 2007;67(3):1344–51. doi: 10.1158/0008-5472.CAN-06-2996. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Hernandez Mde L, Gray A, Hubby B, Klinger OJ, Kast WM. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer Res. 2008;68(3):861–9. doi: 10.1158/0008-5472.CAN-07-0445. [DOI] [PubMed] [Google Scholar]
- 51.Riabov V, Tretyakova I, Alexander RB, Pushko P, Klyushnenkova EN. Anti-tumor effect of the alphavirus-based virus-like particle vector expressing prostate-specific antigen in a HLA-DR transgenic mouse model of prostate cancer. Vaccine. 2015;33(41):5386–95. doi: 10.1016/j.vaccine.2015.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldberg SM, Bartido SM, Bardner JP, Guevara-Patiño J, Mongomery SC, Perales MA, et al. Comparison of two cancer vaccines targeting tyrosinase: Plasmid DNA and recombinant alphavirus replicon particles. Clin Cancer Res. 2005;11(22):8114–21. doi: 10.1158/1078-0432.CCR-05-1410. [DOI] [PubMed] [Google Scholar]
- 53.Avogadri F, Merghoub T, Maughan MF, Hirschhorn-Cymerman D, Morris J, Ritter E, et al. Alphavirus replicon particles expressing TRP-2 provide potent therapeutic effect on melanoma through activation of humoral and cellular immunity. PLoS One. 2010;5:1–6. doi: 10.1371/journal.pone.0012670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eralp Y, Wang X, Wang JP, Maughan M, Polo JM, Lachman LB. Doxorubicin and paclitaxel enhance the antitumor efficacy of vaccines directed against HER 2/neu in a murine mammary carcinoma model. Breast Cancer Res. 2004;6(4):R275–R283. doi: 10.1186/bcr787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moran TP, Burgents JE, Long B, Ferner I, Jaffe EM, Roland TM, et al. Alphaviral vector-transduced dendritic cells are successful therapeutic vaccines against neu-overexpressing tumors in wild-type mice. Vaccine. 2007;25(36):6604–12. doi: 10.1016/j.vaccine.2007.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng EM, Tsarovsky NW, Sondel PM, Rakhmilevich AL. Interleukin-12 as an in situ cancer vaccine component: A review. Cancer Immunol Immunother. 2022:1–9. [DOI] [PMC free article] [PubMed]
- 57.Colmenero P, Chen M, Castaños-Velez E, Liljeström P, Jondal M. Immunotherapy with recombinant SFV-replicons expressing the P815A tumor antigen or IL-12 induces tumor regression. Int J Cancer. 2002;98(4):554–60.. doi: 10.1002/ijc.10184. [DOI] [PubMed] [Google Scholar]
- 58.Osada T, Berglund P, Morse MA, Hubby B, Lewis W, Niedzwieckl D, et al. Co-delivery of antigen and IL-12 by Venezuelan equine encephalitis virus replicon particles enhances antigen-specific immune responses and antitumor effects. Cancer Immunol Immunother. 2012;61(11):1941–51. doi: 10.1007/s00262-012-1248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20:41. doi: 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geall AJ, Verma A, Otten GR, Shaw CA, Hekele A, Banerjee K, et al. Nonviral delivery of self-amplifying RNA vaccines. Proc Natl Acad Sci USA. 2012;109(36):14604–9. doi: 10.1073/pnas.1209367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hekele A, Bertholet S, Archer J, Gibson DG, Palladino G, Brito LA, et al. Rapidly produced SAM((R)) vaccine against H7N9 influenza is immunogenic in mice. Emerg Microbes Infect. 2013;2(8):e52. doi: 10.1038/emi.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drake CG, Johnson ML, Spira AI, Manji GA, Carbone DP, Henick BS, et al. Personalized viral-based prime/boost immunotherapy targeting patient-specific or shared neoantigens: Immunogenicity, safety, and efficacy results from two ongoing phase I studies. J Clin Oncol. 2020;38(15_suppl):3137–3137. doi: 10.1200/JCO.2020.38.15_suppl.3137. [DOI] [Google Scholar]
- 63.Maine CJ, Richard G, Spasova DS, Miyake-Stoner S, Sparks J, Moise L, et al. Self-replicating RNAs drive protective anti-tumor T Cell responses to neoantigen vaccine targets in a combinatorial approach. Mol Ther. 2021;29(3):1186–98. doi: 10.1016/j.ymthe.2020.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Draghiciu O, Boerma A, Hoogeboom BN, Nijman HS, Daemen J. A rationally designed combined treatment with an alphavirus-based cancer vaccine, sunitinib and low-dose tumor irradiation completely blocks tumor development. Oncoimmunology. 2015;4(10):e1029699. doi: 10.1080/2162402X.2015.1029699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Draghiciu O, Walczak M, Hoogeboom BN, Franken KLMC, Melief KJM, Nijman HS, et al. Therapeutic immunization and local low-dose tumor irradiation, a reinforcing combination. Int J Cancer. 2014;134(4):859–72. doi: 10.1002/ijc.28418. [DOI] [PubMed] [Google Scholar]
- 66.Avogadri F, Zappasodi R, Yang A, Budhu S, Malandro N, Hirschhorn-Cymerman D, et al. Combination of alphavirus replicon particle-based vaccination with immunomodulatory antibodies: therapeutic activity in the B16 melanoma mouse model and immune correlates. Cancer Immunol Res. 2014;2(5):448–58. doi: 10.1158/2326-6066.CIR-13-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Osada T, Berglund P, Morse MA, Hubby B, Lewis W, Niedzwiecki D, et al. Co-delivery of antigen and IL-12 by Venezuelan equine encephalitis virus replicon particles enhances antigen-specific immune responses and antitumor effects. Cancer Immunol, Immunother. 2012;61:1941–1951. doi: 10.1007/s00262-012-1248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colmenero P, Chen M, Castaños-Velez E, Liljeström P, Jondal M. Immunotherapy with recombinant SFV-replicons expressing the P815A tumor antigen or IL-12 induces tumor regression. Int J Cancer. 2002;98:554–560. doi: 10.1002/ijc.10184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this review was not generated by the authors, but gathered through existing research/publications. Thus all data is publicly available.