Abstract
The idea of producing vaccines in plants originated in the late 1980s. Initially, it was contemplated that this notion could facilitate the concept of edible vaccines, making them more cost effective and easily accessible. Initial studies on edible vaccines focussed on the use of a variety of different transgenic plant host species for the production of vaccine antigens. However, adequate expression levels of antigens, the difficulties predicted with administration of consistent doses, and regulatory rules required for growth of transgenic plants gave way to the development of vaccine candidates that could be purified and administered parenterally. The field has subsequently advanced with improved expression techniques including a shift from using transgenic to transient expression of antigens, refinement of purification protocols, a deeper understanding of the biological processes and a wealth of evidence of immunogenicity and efficacy of plant-produced vaccine candidates, all contributing to the successful practice of what is now known as biopharming or plant molecular farming. The establishment of this technology has resulted in the development of many different types of vaccine candidates including subunit vaccines and various different types of nanoparticle vaccines targeting a wide variety of bacterial and viral diseases. This has brought further acceptance of plants as a suitable platform for vaccine production and in this review, we discuss the most recent advances in the production of vaccines in plants for human use.
Key Points
| Plants are becoming an accepted platform for the development of vaccine production. | |
| A wide variety of human vaccine targets have been explored with promising outcomes. | |
| Both influenza and SARS-CoV-2 have provided ideal opportunities to prove the applicability of the platform with encouraging results of plant-produced vaccines from phase III trials and authorisation for human use in Canada. |
Introduction
The origins of vaccines are longstanding, with the concept of vaccination of humans to induce immunity against smallpox going as far back as 1000 CE (common era) [1]. Even from this early date, despite the lack of knowledge that viruses and bacteria were agents of disease, records indicate that the composition of the vaccine used for inoculation was smallpox material. With the germ theory of disease, the discovery of viruses and bacteria as agents of disease [2, 3] as well as great strides made in the development in the understanding of immunology, many more viral and bacterial vaccines against human diseases have been developed, all requiring the presence of the disease-causing pathogen. Source material has remained largely the same over the years, as live-attenuated viruses (vaccines for measles, mumps, rubella and rotavirus), inactivated viruses (inactivated polio vaccine or whole-cell pertussis vaccine), inactivated toxins (for example tetanus or diphtheria) or segments/parts of the pathogen (subunit vaccines, for example hepatitis B virus), varying only slightly in composition or presentation [4].
Up until the mid-1980s, preparation of human vaccines required the handling of live virus cultured in mammalian cells or, in the case of some influenza vaccines, in embryonated chicken eggs [5]. In 1986 however, the first human vaccine was produced that did not consist of pathogenic material: a vaccine against hepatitis B virus (HBV) using recombinant DNA technology expressing the HBV surface antigen (sAg) to make virus-like particles (VLPs) was developed in yeast cells [6] and approved for human use [7]. The success of this vaccine has since paved the way for further development of a wide range of recombinant vaccines, albeit using similar expression platforms to those used prior to the mid-1980s. There are now numerous different types of recombinant human vaccine candidates developed which have been licensed for commercialisation. Examples include VLPs, DNA vaccines, viral vector vaccines, as well as mRNA vaccines, the latter two being new candidates recently licensed for emergency use in humans during the COVID-19 pandemic [4].
Conventional Platforms Used for Vaccine Production
Cell culture fermentations and eggs are the conventional platforms used for the production of any of the currently licensed vaccines, whether live attenuated, inactivated or recombinant. Most influenza vaccines are currently made in eggs, with the bulk of the remaining human vaccines being made in mammalian cells [8] and some such as human papillomavirus (HPV) [9] and HBV in insect and/or yeast cells [10].
The prolonged process of making influenza vaccines in eggs, which ranges from 30 to 52 weeks [5], provides numerous challenges including insufficient time (< 6 months) to investigate a variant virus fully to prepare a well-matched vaccine before it is required for dissemination. The current system employed involves an immense collaboration between the WHO, the Global Influenza Surveillance and Response System (GISR) as well as global regulatory authorities, public health laboratories and vaccine manufacturers; this does not allow for anticipation of the recommended composition of a vaccine if a variant emerges subsequent to when a final decision has been made on a particular vaccine composition [5]. Moreover, some viruses do not grow well in eggs, resulting in variable production levels, and there are sometimes egg adaptive changes in the haemagglutinin (HA) antigen which can modify the antigenic profile of the viruses, consequently decreasing vaccine efficacy [5]. Production in eggs usually requires a large supply (millions) of embryonated chicken eggs, and the process is time consuming, in some cases taking up to a year [5, 11]. There is also a risk to human health in the instance of allergies to eggs due to the potential of trace components of the eggs in the vaccine [4].
Production of vaccines in cell culture attracts high running costs with the use of expensive media, the requirement for cleaning in between batch production as well as in cases where single-use equipment is utilised [8, 11]. Moreover, in the case of those vaccines requiring handling of live virus, the cost is escalated by the requirement for high biosafety level containment and skilled personnel. Other potential safety issues include the failure of complete inactivation of viruses, leading to reversion to virulence as well as possible contamination of vaccines with undetected viruses/bacteria [12].
Bacteria are generally undesirable as vaccine production hosts as they lack the capability for carrying out post-translational modifications required for vaccine proteins such as N-glycosylation and are a potential source of endotoxins [13]. Yeasts are a better host for vaccine candidates and although they carry out protein modifications that are markedly different to those of higher eukaryotes which could potentially influence immunogenicity, the development of glycoengineered strains capable of carrying out human glycosylation has overcome this hurdle [14]. Mammalian cells are currently the most predominantly used expression platform for biopharmaceuticals [8] with well-established infrastructure, protocols and regulatory processes [15], but one of the disadvantages of this platform is their predisposition to supporting human pathogen replication. In addition, in the event of rapid response vaccine requirements for pandemics or emerging diseases, the fermentation production method requires time for expression strain development and manufacturing and commissioning of new equipment [8, 16].
In recent years, plants have become increasingly more attractive and acceptable as an expression platform for the production of vaccines and there has been much progress in developing the field, particularly in that of transient expression [17–19]. There are several reasons which make expression in plants a more attractive option over the conventional platforms [4]. These include the lack of ability of plants to host human pathogens as well as the fact that production from gene to product is much faster than any other eukaryotic system on a comparable scale, taking approximately 3 months in plants compared with > 6 months in mammalian cells [16]. In the event of rapid response vaccine requirements for pandemics or emerging diseases, this is very appealing. Most recently, the plant platform has been significantly affirmed as a suitable vaccine production platform with the announcement by Health Canada that it has authorised the use of a plant-produced virus-like particle vaccine against COVID-19 in humans; the vaccine, COVIFENZ®, is made by Medicago Inc. (Québec) [20].
Plant Expression Platform
Vaccine production in plants entails the delivery of genes encoding disease-specific antigens into plant host cells [12]. Vaccine antigens can be produced from stable transgenic plants—the original method used for production—or by transient expression [19]. While transgenic plants are generated either using a direct biolistic method or by introduction of engineered plant bacterial or viral vectors using A. tumefaciens, which results in stable integration of heterologous genes into the chromosome [12], the transient expression method relies on Agrobacterium-mediated transfer of vectors engineered to mediate transfer of heterologous genes into host cells, and their subsequent episomal expression in the host cells [21].
In 1989, a report on the expression of immunoglobulin molecules in transgenic plants [22] marked the beginning of the use of plants as a potential expression platform for pharmaceutical production. Plant-produced vaccines created much interest due to the idea that vaccines could be edible [23, 24]. It was initially reasoned that the lack of requirement for purification and any pre-administration treatment would reduce costs in addition to targeting mucosal immunity. Initially, edible vaccine development included the testing of many different edible species of transgenic plants including potato, rice, banana, tomato, spinach, maize, lettuce and carrots [12, 25, 26]. Many of the vaccines developed were produced by chloroplast transformation [11]. Examples of earlier vaccines tested included those against cholera, lyme disease, anthrax, tetanus, plague and rotavirus [12, 27–30]. However, the generation of transgenic plants is a lengthy process, with yields potentially decreasing over time as a result of transgene silencing [11]. Major improvements in viral vector-mediated technology [31–35] and the fact that the process facilitates rapid expression of newly identified vaccine candidates have shifted efforts more towards the transient expression method for their production [17, 19, 36]; most of the recently described candidates are produced using this type of expression.
Types of Plant-Produced Vaccines
The types of plant-made vaccines produced more recently broadly fall into the categories of virus-based nanoparticles (VNPs), recombinant immune complexes (RICs) as well as subunit vaccines. There are several types of VNPs; these include virus-like particles (VLPs) [37], VLP-display particles as well as pseudovirions (PSVs).
Virus-Based Nanoparticles (VNPs)
It is well documented that many viral capsid proteins, when expressed in plants, can self-assemble into VLPs which lack any infectious nucleic acid but are structurally and visually similar to their wild-type counterparts (Fig. 1a) [38]. Their size and repetitive geometry facilitate mimicking of the native virus, making them ideally immunogenic to the human immune system [39]. However, those representing non-enveloped viruses such as HPV (extensively reviewed in [40]) are easier to make than the more complex enveloped viruses [38] such as influenza virus VLPs, which are reviewed in more depth later. Some VLPs such as rotavirus require the co-expression of multiple capsid proteins [41] and recently, the expression and processing of a polyprotein in planta has been shown to yield poliovirus VLPs [42].
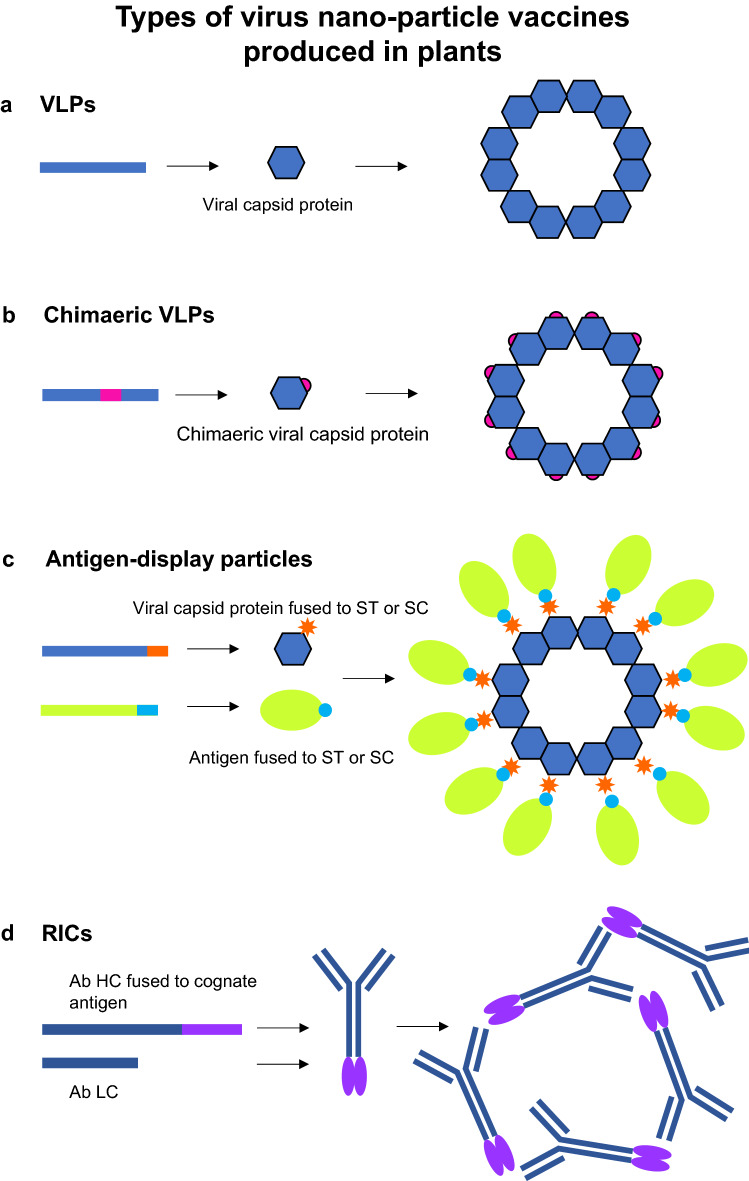
Fig. 1.
Types of virus nano-particle vaccines produced in plants. a VLPs are assembled by expression of a viral capsid protein. b Chimaeric VLPs are similarly assembled by expression of a modified viral capsid protein encoded by a capsid sequence containing an inserted sequence encoding a heterologous epitope/antigen. c Antigen-display particles are assembled by expression of a particle capsid protein sequence fused to ST or SC. Antigens fused to SC or ST are expressed and added to the ST- or SC-fused particles for their display. d RICs are assembled by co-expression of an Ab HC fused at the C terminus with its cognate antigen sequence, and the corresponding Ab LC sequence. Purification of the fused Abs results in Ab-Ag complexes forming. Ab antibody, HC heavy chain, LC light chain, RIC recombinant immune complex, SC SpyCatcher, ST SpyTag, VLP virus-like particle
VNPs can also be made into chimaeric particles (Fig. 1b) which are used as display vehicles for immunogenic epitopes. Epitopes can be substituted into surface-exposed loops of capsid proteins [43] or even added into loops for display on a particle surface [41]. Plant viruses are the most desirable carriers of immunogens as they can be produced in large quantities in plants, are very stable and easily purified. In addition, they do not infect humans, thereby abrogating any potential safety issues [19]. There are numerous examples of plant viruses used including tobacco mosaic virus (TMV), cowpea mosaic virus (CPMV) and potato virus X (PVX) [19, 44]. HBV core and surface antigens have been fairly widely used as carriers for immunogenic epitopes on HBV particles assembled in plants [45]. A more recent development to overcome the crowding effect of displayed antigens/epitopes is Tandem Core Technology [46]. This involves the expression of a Hepatitis B core antigen (HBcAg) dimer encoding an antigen/epitope cloned into only one of the two HBcAg genes positioned in tandem to each other.
Some research has been carried out on the exploitation of TMV VLPs as delivery vehicles of antigenic peptides displayed on their outer surface. These are ideally suited to being taken up by dendritic cells and processed to stimulate strong humoral and cellular responses [47]. Transient expression of the TMV coat protein (CP) in plant cells results in its self-assembly to form rod-shaped virus-like particles. There are three surface-exposed locations on the CP into which peptides can be inserted by genetic fusion for display on the particle surface. Apart from several examples of animal vaccines developed using this technology [47], there are some human vaccine examples. Staczek et al. [48] developed a TMV vaccine displaying a Pseudomonas aeruginosa outer membrane protein F peptide. Vaccinated mice that were subsequently challenged with P. aeruginosa had reduced lesion numbers and disease severity. More recently, TMV-conjugated particles have been produced in plants to target the bacterial pathogen Francisella tularensis [49]. F. tularensis proteins OmpA, DNAk and Tul4 were conjugated to surface-exposed lysine residues of TMV particles resulting in a multi-conjugate vaccine. A proof-of-concept study in mice showed the vaccine stimulated a strong humoral response and protected them against high doses of F. tularensis.
Viruses have also been used for conjugation to immunogenic epitopes (Fig. 1c). One of the more recent technologies is the SpyTag/SpyCatcher technology [50] which allows conjugation of SpyTag peptides displayed on particles to couple with SpyCatcher peptides fused to immunogens (or vice-versa). To date, we have only identified one example of a plant-produced SpyTag/SpyCatcher vaccine candidate targeting West Nile virus [51].
Another novel VNP development has been that of the production of pseudovirions (PSVs) in plants. These comprise VLPs containing heterologous RNA or DNA which encode vaccine antigens of interest once they have entered target cells. Although no PSVs targeting human pathogens have currently been made, one group has reported the production of HPV PSVs encoding alkaline phosphatase in N. benthamiana. These were able to be used in a HPV PSV neutralisation assay [52]. A similar strategy was employed by Zhou et al. [53], who used TMV as a carrier particle of RNA encoding a marker gene.
Recombinant Immune Complexes (RICs)
Immune complexes (ICs) are antigen-antibody complexes. There has been renewed interest in the use of ICs as prophylactic vaccines as advances have been made in the use of therapeutic antibodies for treatment and immunoregulatory responses have become better understood. ICs have been shown to trigger responses both in innate and adaptive immune systems such as cross presentation, CD8+ and CD4+ cell activation, antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity [54]. A development in the generation of ICs, is that of recombinant ICs (RICs) which consist of a modified monoclonal antibody (mAb) such that its cognate antigen is fused to the C-terminus of the heavy chain (Fig. 1d). This modification allows for the molecule to bind to the antigen fused to a similar molecule, forming an IC that can be recognised by the immune system. Phoolcharoen et al. [55] reported the production of an RIC against Ebola in plants. More recently, Kim et al. [56] took this further by expressing a hybrid RIC designed to act against both Ebola and dengue viruses. An improvement on this concept was recently reported by Diamos et al. [57], who made RICs with both N- and C-terminus fusions of antigens to broaden the immune response in mice against the Zika virus.
Subunit Vaccines
Subunit vaccines generally consist of a purified component of a pathogen that is non-infective, specifically selected for its ability to induce an immune response. These are often expressed recombinantly and are considered to be safe, and often require the addition of strong adjuvants or multiple doses to elicit a robust immune response, such as the FDA-approved Recombivax HB® hepatitis B vaccine (Merck and Co Inc, Whitehouse St, NJ, USA) which is made in yeast [58]. This could be ascribed to the possibility that these antigens do not display the immunogenic epitopes in their native conformation [59] and as subunit vaccine antigens are generally small peptides, they are susceptible to proteolytic degradation which, as a result, also limits the extent of the immune response stimulated [60]. Progress has been made on the development of several plant-produced subunit vaccines recently, including those against polio [61], West Nile fever [62, 63], rabies [64], anthrax [65, 66] and COVID-19 [67, 68], which are all discussed below in more detail.
Advantages of Plant-Produced Vaccines
Plants offer several potential benefits compared with the traditional cell-based expression systems. Their cultivation is simple as there is no need for a sterile environment and they are relatively inexpensive, requiring only conventional fertilisers for growth; in comparison, cell culture systems require costly media, and usually require high level biosafety facilities. The platform is infinitely scalable, with the cost of expansion much lower than that required for expansion of more conventional fermentation expression platforms [41]. Plants can easily be scaled-up in a greenhouse or vertical farm that may allow for yields in the multi-tonne scale [69]. An additional valuable advantage is that plants are inherently safe because no human pathogens replicate in plants, abrogating the risk of viral, prion or bacterial endotoxin contamination [17, 70].
Transient expression allows for the rapid generation of products which can be achieved ~ 8 weeks from obtaining the genetic sequence [69] and, as the plants used are not genetically modified, there are potentially less regulatory requirements for commercial production [16]. This rapid production and the ease of manipulation of the plant production system allows for swift responses which are very attractive, particularly for epidemic or pandemic situations [71]. Moreover, plants can perform post-translation modifications (PTM) to yield glycosylated products similar to their mammalian counterparts with the use of transgenically engineered plants [72]; proper folding and processing of proteins can be achieved by transient co-expression of chaperones or other enzymes [73].
Plant-Produced Human Vaccines
The first plant-produced vaccine that was approved by the United States Department of Agriculture (USDA) for commercial use was a veterinary vaccine in 2006 against Newcastle disease virus (NDV) which affects chickens [74]. However, although the discovery that vaccines could be produced using a plant platform materialised early on, the development of vaccine production in plants has been slow and no plant-produced human vaccines have been licensed to date. The concept underwent a lapse due to lack of regulatory frameworks and an unproven track record in the industry [18]. Most early studies of vaccines have focussed on optimising expression and increasing yields as well as developing purification protocols rather than targeting commercial competitiveness, as requirements for clinical trials are challenging and are fraught with more stringent regulatory requirements [75]. However, over time, the consolidation of protocols, establishment of GMP-compliant plant production facilities, drafting of regulatory frameworks as well as techno-economic analyses [76–78] demonstrating feasibility and commercial applicability have established the production of vaccines in plants as a significantly more accepted enterprise [18]. A number have been tested in clinical trials [79–81] and the first plant-produced vaccine for human use against COVID-19 was recently authorised by Health Canada [20] after the successful outcome of phase III clinical trials [81]. Below, we discuss some of the more recent developments in the last 5 years (2018–2022) of plant-produced human vaccine research against various disease targets (Table 1).
Table 1.
Examples of recently developed human vaccines in plants (within the last 5 years)
| Year reported | Target pathogen | Type of vaccine | Antigen | Plant host | Animal trials | Human trials | References |
|---|---|---|---|---|---|---|---|
| 2019 | Hepatitis B virus (HBV) | VLP | HBcAg | N. benthamiana and L. sativa | Mice—prime oral boost with lettuce stimulated predominant Th1 response with Th2 after boost | ND | [88] |
| 2021 | Hepatitis E virus (HEV) | Chimaeric VLPs | HBcAg-ORF2 AA551-AA607 immunogenic epitope | N. benthamiana | ND | ND | [89] |
| 2020 | Poliovirus (PV) | Subunit | VP1, VP2, VP3, VP4 | N. tabacum | Mice—SC prime and oral boost with lyophilised leaf material stimulated a humoral response | ND | [61] |
| 2021 | Dengue virus (DENV) | VLPs | C-pRM-E + NSP truncated (lacking NS5) | N. benthamiana | Mice—prime-boost elicited anti-DENV1 antibodies | ND | [90] |
| 2019 | DENV | Antigen-display VLPs | HBcAg-DENV EDIII | N. benthamiana | Mice—animals showed some seroconversion | ND | [91] |
| 2018 | West Nile virus (WNV) | Soluble protein | WNV EDIII | N. benthamiana | Mice—potent humoral and cellular response and protection against WNV challenge | ND | [63] |
| 2021 | WNV | Antigen display VLPS | Phage AP205-WNV EDIII | N. benthamiana | Mice—potent humoral response | ND | [51] |
| 2020 | Zika virus (ZIKV) | RIC | ZIKV mAb and cognate Ag | N. benthamiana | Mice—strong specific antibody titers correlating with Zika neutralisation | ND | [57] |
| 2018 | Yellow fever virus (YFV) | Subunit | E alone & E fused to lichenase | N. benthamiana | Mice & monkeys—strong NAbs stimulated | ND | [96] |
| 2021 | Rotavirus (RV) | VLPs | VP2, VP6, VP7 | N. benthamiana | Rats—stimulated robust Nabs | Adults, toddlers and infants—IgG response and NAbs in infants | [98, 99] |
| 2020 | Norovirus (NoV) | Not specified | Not specified | N. benthamiana | ND | Adults >18 years—results not yet available | [138] |
| 2022 | NoV | VLPs | GI & GIII.4 capsid proteins | N. benthamiana | Rabbits—stimulated strong specific antibody and blocking antibody titers | ND | [102] |
| 2021 | Rabies virus (RABV) | Subunit | RG2 protein | N. benthamiana | Mice—stimulated strong specific antibody titers | ND | [64] |
| 2022 | Anthrax | Subunit | Bacillus anthracis pp-PA83 | N. benthamiana |
Mice & rabbits—strong NAbs stimulated Rabbits—protection against B. anthracis challenge |
Adults >18 years— seroconverted | [66] |
| 2018 | Malaria | VLPs | Pfs25 protein | N. benthamiana | Mice—strong humoral response and transmission blocking activity | Adults >18 years—good antibody responses but low transmission reducing activity | [105, 106] |
| 2019 | Human papillomavirus (HPV) | Chimaeric VLPs | HPV-16 L1-L2 | N. benthamiana | Mice—stimulated HPV-16 L1 NAbs and cross-protection against HPV-58 and -18 | ND | [43] |
| 2018/2021 | Influenza virus | VLPs | H5N1 of 4 different circulating strains—quadrivalent vaccine | N. benthamiana | Mice—strong immunity and protection against challenge | Adults >18 years—strong HI antibody titers and CD4+ T-cell response | [117, 119, 126] |
| 2021 | Influenza virus | VLPs | H5N1 quadrivalent vaccine | N. benthamiana | ND | ND | [120] |
| 2021 | SARS-CoV-2 | VLPs | Modified S1 glycoprotein | N. benthamiana | ND | ND | [125] |
| 2021 | SARS-CoV-2 | Antigen display VLPs | CoV-RBD121 | N. benthamiana | Mice—strong NAbs stimulated | Approval for Phase I | [128] |
| 2021 | SARS-CoV-2 | VLPs | E, M & S1 | N. benthamiana | ND | ND | [130] |
| 2020 | SARS-CoV-2 | Soluble protein | S1, RBD & N | N. benthamiana | Mice—antigen-specific immunogenicity | ND | [131] |
| 2022 | SARS-CoV-2 | Soluble protein | Truncated S1 | N. benthamiana | Mice—low cellular and humoral immunogenicity | ND | [132] |
| 2021 | SARS-CoV-2 | Subunit | RBD fused to human IgG1-Fc | N. benthamiana | Mice & monkeys—strong NAbs stimulated | ND | [67, 68] |
EdIII envelope protein domain III, HBcAg hepatitis B core antigen, mAb monoclonal antibody, NAbs neutralising antibodies, ND not done, NSP non-structural protein, RBD receptor-binding domain, RIC recombinant immune complex, SC subcutaneous, VLP virus-like particle, WNV West Nile virus
Hepatitis B Virus (HBV)
The first human vaccine candidate to be produced in plants was for hepatitis B virus (HBV) in transgenic tobacco (N. tabacum) [82]. The expression of HBV surface antigens (HBsAg) resulted in the assembly of particles resembling those from human-derived serum and produced recombinantly in yeast, heralding the potential for plant-made vaccines. Despite successful production of HBV VLPs in both potato and lettuce, their testing in phase I clinical trials [83, 84], the production of HBV VLPs transiently in tobacco [85] and increased levels of expression [86], commercialisation of this vaccine has still not been pursued. In the last 5 years, more recent studies have looked at the use of HBcAg expression in plants as a therapeutic vaccine [87]. Pyrski et al. [88] have recently reported the parenteral immunisation of mice with HBcAg produced transiently in N. benthamiana with an oral boost of HBcAg-expressing lettuce. The low dose elicited a specific high titre antibody response with a predominant Th1 response and evidence of a Th2 response after the oral boost.
Hepatitis E Virus (HEV)
The ORF2 AA551-AA607 immunogenic epitope of the hepatitis E capsid protein was inserted into one of the HBcAg surface loops using the Tandem Core Technology [46] and expressed transiently in N. benthamiana. Chimaeric VLPs ranging in size from 28 to 38 nm in diameter were visualised by electron microscopy, which had ‘knobbly’ surfaces, most likely representing the epitopes being displayed on the HBcAg surface spikes. Although no animal immunology has been carried out yet, the chimaeric VLPs showed they could bind both to an anti-HBcAg mAb as well as swine serum containing anti-HEV IgG, suggesting a possible bivalent vaccine function for the candidate [89].
Poliovirus
Marsian et al. [42] have reported the development of poliovirus VLPs in N. benthamiana by co-expression of a mutated capsid sequence of poliovirus serotype 3 (PV3) and the poliovirus 3CD protease which cleaves the intermediate capsid sequence P1 into three capsid proteins (VP0, VP1 and VP3), which assemble into VLPs. These were shown to retain the stability and potency of the currently used inactivated polio vaccine (IPV). The vaccine was shown to protect immunised transgenic mice expressing the human poliovirus receptor after challenge with the wild-type Saukett strain of PV3. More recently, poliovirus Sabin subunit capsid-forming proteins (VP1, VP2, VP3 and VP4) were produced individually in transgenic N. tabacum plants. Immunisation of mice with four subcutaneous injections 2 weeks apart and four subsequent oral immunisations with suspensions of lyophilised transgenic leaf material containing all four proteins induced local humoral immune responses, exhibiting potential for further application [61].
Flaviviruses
Ponndorf et al. [90] have recently reported the successful production of dengue virus serotype 1 (DENV1) VLPs in N. benthamiana. Co-expression of constructs encoding the DENV1 structural polyprotein C-pRM-E together with a construct encoding an NS5-truncated non-structural protein (NSP) domain of DENV1 resulted in particles ranging in size from 25 to 40 nm. Immunisation of mice with a prime-boost regimen of 10 µg VLPs per dose was shown to elicit anti-DENV1-specific antibodies.
Pang et al. [91] utilised the Tandem Core Technology previously developed using HBcAg display [46]. A consensus sequence representing four DENV serotypes of envelope protein domain III (EDIII) was inserted into one of the immunodominant c/e1 surface loop regions encoding a tandem HBcAg. VLPs displaying EDIII epitopes were transiently assembled in N. benthamiana after Agrobacterium-mediated infiltration and mice immunised with the VLPs showed some seroconversion.
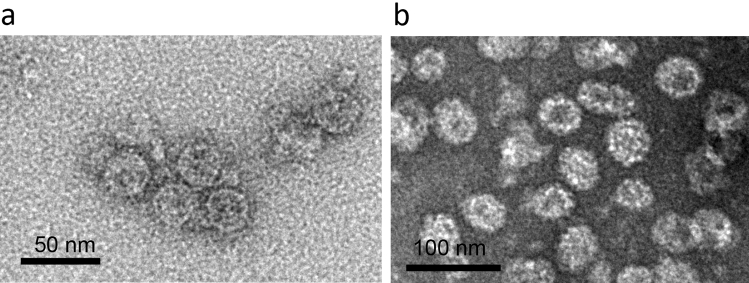
Up until 2015, there have been a number of developments of subunit and VLP-based vaccine candidates produced in plants against West Nile virus (WNV), reviewed in detail by Chen [92]. Subsequently, He et al. [62] showed that soluble WNV EDIII expressed transiently in N. benthamiana induced a potent immune response in mice and was potentially protective in mice challenged with WNV [63]. The potential efficacy of EDIII was taken further by two different studies testing the immunogenicity of displaying EDIII on particles. He et al. [93] transiently expressed a construct encoding HBcAg fused to WNV EDIII which resulted in the assembly of VLPs resembling wild-type particles. Results from immunisation of mice demonstrated high levels of immunogenicity with potent anti-IgG-specific titers, equivalent to levels required for protection. Stander et al. [51] developed an antigen display vaccine for WNV. This vaccine candidate relied on the use of the SpyTag-SpyCatcher (ST/SC) technology [50]. The immunogenic WNV EDIII fused to SC was expressed transiently in N. benthamiana. Phage AP205 coat protein genetically fused to ST was similarly expressed in N. benthamiana and assembled into particles (Fig. 2a). Co-purification of these from N. benthamiana leaves resulted in AP205 particles displaying conjugated EDIII. Mice immunised with the AP205:EDIII particles elicited potent IgG responses.
Fig. 2.
Examples of antigen-display particles and VLPs. a ST-fused phage AP205 particles displaying SC-fused WNV EdIII produced in N. benthamiana. b HPV VLPs produced in N. benthamina by expression of HPV L1 capsid protein. EdIII envelope protein domain III, HPV human papillomavirus, SC SpyCatcher, ST SpyTag, VLP virus-like particle, WNV West Nile virus
Similar to the WNV HBcAg-EDIII VLP, Yang et al. [94] showed that Zika virus (ZV) EDIII fused to HBcAg resulted in great VLPs when expressed transiently in N. benthamiana and elicited potent humoral and cellular responses in mice at levels exceeding those correlating with protective immunity against several Zika strains tested. A recently reported advancement has been the co-delivery of such particles with RICs. Based on previous research carried out to develop an Ebola virus subunit vaccine consisting of an Ebola glycoprotein GP1 fused to the C terminus of a cognate mAb heavy chain (HC), co-expression of matching light chain (LC) in N. benthamiana using a geminiviral vector was shown to form immunoglobulins. C1q binding assays and purification and analysis confirmed assembly of ICs in planta and immunisation of mice showed some immunogenicity [55]. Further work confirmed that co-administration of the RICs with adjuvant elicited a more robust immune response and protected mice challenged with Ebola virus [95]. More recently, the RIC platform was enhanced to accommodate a wider variety of antigens. Diamos et al. [57] showed that fusion of Zika EDIII to either the N or the C terminus of the HC of a Zika mAb showed immunogenicity in mice, which was enhanced further when co-delivered with HBcAg displaying Zika EDIII.
Currently, all yellow fever virus (YFV) vaccines licensed for use are live attenuated virus vaccines produced in eggs; for example, YF-Vax® (Sanofi-Pasteur, Lyon) and 17DD-YFV (Bio-Manguinhos, Rio de Janeiro). Recently, Tottey et al. [96] developed YFV vaccine candidates through transient expression of the envelope protein alone (YFE) or fused to the bacterial enzyme lichenase (YFE-LicKM) in N. benthamiana. High virus-neutralising titers were observed following immunisation in mice for both YFE and YFE-LicKM with 71% and 88% of animals surviving lethal YFV challenge, respectively. In addition, the YFE and YFE-LicKM vaccine candidates induce high virus-neutralising antibody responses in Macaque monkeys.
Rotavirus
Currently, available vaccines prequalified for clinical use by the World Health Organization against rotavirus comprise only live-attenuated virus preparations for oral use: RotaTeq® (Merck, Kenilworth), Rotarix™ (GlaxoSmithKline Biologicals, Rixensart), Rotavac® (Bharat Biotech, Hyderabad) and ROTASIIL™ (Serum Institute of India, Hadapsar) [97]. Kurokawa et al. [98], however, have developed a monovalent Ro-VLP vaccine composed of rotavirus surface proteins VP7, VP6 and VP2 of the rotavirus G1 genotype as well as the rotavirus non-structural helper protein NSP4. Agrobacterium-mediated transient expression in N. benthamiana yielded VLPs that were structurally similar to the triple-layered rotavirus particles. Rats immunised intramuscularly (IM) with two doses, 3 weeks apart, of aluminium hydroxide adjuvanted Ro-VLPs (1, 5 or 30 µg), elicited robust neutralising antibodies. Non-adjuvanted Ro-VLPs, at doses of 5 µg or 30 µg, also induced potent neutralising antibodies. The safety and immunogenicity of the vaccine was subsequently evaluated in a phase I trial (NCT03507738) in adults (aged 18–35 years), toddlers (12–24 months) and infants (6–10 weeks) [99] and was well tolerated with few mild adverse events in all cohorts. Anti-G1P IgG responses and neutralising antibodies were elicited in infants regardless of dose levels (7 µg or 21 µg adjuvanted).
Norovirus
Diamos and Mason [100] reported the assembly of human norovirus VLPs by transient expression of the GI or GIII.4 capsid protein in N. benthamiana using a geminiviral vector. The VLPs were purified up to 90% but no immunogenicity or efficacy of the vaccine candidate has been reported to date by this group. A noteworthy development by another group has reported the co-administration of insect cell-produced rotavirus VP6 with transiently plant-produced GI.3 and GII.4 VLPs in mice, which generated norovirus-specific antibodies against the vaccine as well as heterologous norovirus genotypes [101]. Norovirus VLPs alone did not elicit any response and it was concluded that the rotavirus VP6 had an adjuvant effect as doses as low as 1 µg were effective in stimulating specific antibodies. A bivalent VLP norovirus vaccine candidate comprising the GI.4 and GII.4 antigens (rNV-2v) has been produced transiently in N. benthamiana [102]. Results obtained from preclinical toxicity studies in rabbits illustrated the safety and tolerability of the vaccine. In addition, strong rNV-2v-specific antibody responses were observed in both male and female rabbits in the absence of adjuvants with very high levels of blocking antibodies.
Rabies virus (RABV)
Since 1967, the standard rabies vaccine used has consisted of the attenuated-type human diploid cell vaccine [103], with several other attenuated and inactivated RABV vaccines approved for human use. Due to the cost associated with the production of these vaccines in animal cell culture, Mammadova et al. [64] recently investigated the efficacy of a subunit RABV vaccine produced in N. benthamiana. Following transient expression of a truncated form of the G protein (RG2), a yield of ~ 32 mg/kg fresh leaf weight of purified RG2 protein was obtained and high RG2-specific antibody titers were observed in mice following IM immunisation with two doses of 5 µg RG2 with Alhydrogel®.
Bacillus anthracis (Anthrax)
Currently, there is only one anthrax vaccine approved for use in the US, namely BioThrax® (Emergent BioSolutions, MD, USA), which is prepared from cell-free filtrates of microaerophilic cultures of an avirulent, nonencapsulated strain of Bacillus anthracis and formulated with an aluminum hydroxide adjuvant. Recombinant subunit vaccine research against anthrax has been focused on the protective antigen (PA) which is the principal virulence factor of B. anthracis. In 2013, a full-length PA (pp-PA83) prophylactic vaccine candidate transiently produced in N. benthamiana was shown to elicit high neutralising antibody titers in mice and rabbits when administered with Alhydrogel® [65]. Additionally, rabbits immunised with the pp-PA83 vaccine (with Alhydrogel®) were protected from a lethal aerosolised B. anthracis challenge. Subsequently, very recently, a phase I trial (NCT02239172) in healthy adults (18–49 years) was conducted with pp-PA83, named PA83-FhCMB vaccine candidate [66]. There were 30 participants across four dose-escalating vaccine groups, 12.5 µg (n = 5), 25 µg (n = 5), 50 µg (n = 10) and 100 µg (n = 10), that received three IM doses of PA83-FhCMB with Alhydrogel®. The plant-made vaccine was found to be safe and well tolerated in all groups with no observed serious adverse events. In addition, all participants seroconverted by day 56, with the highest geometric mean titre occurring by day 84 following the third dose. The authors concluded that a fourth booster dose of the PA83-FhCMB vaccine at 6 months could be beneficial since an increase in the geometric mean concentration was observed for individuals who received the BioThrax® vaccine following a fourth booster dose.
Malaria
In the past 15 years, a plant-based malaria transmission blocking vaccine (TBV) against the Plasmodium falciparum protein, Pfs25, has been in development. The TBV comprises the Pfs25 protein fused to the Alfalfa mosaic virus coat protein, and transient expression in N. benthamiana using a TMV-based hybrid vector resulted in the formation of a chimaeric non-enveloped VLP (Pfs25-CP VLP) [104]. Approximately 20–30% of the Pfs25 protein was incorporated onto the VLP surface with a diameter of ~ 19 nm [105]. Mice immunised IM with two doses of Pfs25-CP VLPs at either 1.0 or 0.1 mg (equivalent to Pfs25) with Alhydrogel® had high IgG responses with 100% transmission blocking (TB) activity that was maintained through day 168 post-immunisation. The immunogenicity study was repeated in mice and similar IgG responses were observed and maintained for 5 months. As observed in the first immunogenicity study, a TB activity of 100% following the booster dose was obtained and maintained at 98–99% through day 168. A third mouse immunogenicity study with a single Pfs25-CP VLP IM dose at either 0.2, 1.0, 5.0 or 25 mg (equivalent to Pfs25) with Alhydrogel® was performed and high Pfs25-specific IgG titers were observed and maintained for the 6-month test period. For doses ≥ 5.0 mg, 100% TB activity was observed, while 99.5% and 98.8% was observed for doses of 1.0 and 0.2 mg, respectively. Remarkably, for doses ≥ 1.0 mg, the TB activity was maintained at ~ 94% for 6 months post-vaccination.
These results highlighted the potential of Pfs25-CP VLP as a malaria TBV and subsequently Chichester et al. [106] produced a candidate vaccine named Pfs25 VLP-FhCMB in N. benthamiana under Good Manufacturing Practice guidelines for a phase I trial (NTC02013687) in adults (aged 18–50 years). The Pfs25 VLP-FhCMB (with Alhydrogel®) dose groups consisted of 2 µg (n = 6), 10 µg (n = 6), 30 µg (n = 16) and 100 µg (n = 16) total protein that was administered IM three times. The plant-made Pfs25 VLP-FhCMB vaccine was shown to be safe in healthy adults with no vaccine-related serious adverse events or dose-limiting or dose-related toxicity; this highlighted the safety and tolerability of the vaccine. Good antibody responses were observed in individuals immunised with vaccine doses > 30 µg, however the transmission-reducing activity of the generated antibodies was weak. The authors suggested that the low transmission reducing activity might be overcome with the use of alternative adjuvant formulations.
Human Papillomavirus (HPV)
Although there are currently very efficacious HPV VLPs on the market made in both yeast (Gardasil®, Merck, Whitehouse Station) and insect cells (Cervarix™ ,GlaxoSmithKline Biologicals, Rixensart) [107], there has been ongoing research since 2003 into the production of plant-produced HPV VLPs by transient expression of L1 capsid protein in plants (Fig. 2b) (extensively reviewed elsewhere [40]). Further developments on this include the development of chimaeric HPV-16 VLPs consisting of substituted L2 peptides into four different regions on the DE surface loop of HPV-16 L1 or at its C-terminal region [43]. The former resulted in complete assembly of VLPs while for the latter, assembly was partial, resulting in pentavalent capsomeres. Immunogenicity studies on mice immunised with a cocktail of these resulted in elicitation of neutralising antibodies to HPV-16 as well as cross-neutralisation with HPV-58 and -18.
Influenza Virus
Prior to the emergence of the COVID-19 pandemic, the influenza vaccine developed by Medicago Inc. (Québec, Canada) was the vaccine most likely to put plant-produced vaccines on the commercial map. In 2008, D’Aoust et al. [108] showed that individual expression of the HA protein of two strains (H5/N1 and H1/N1) of influenza A expressed transiently in N. benthamiana assembled into VLPs. These consisted of pleomorphic enveloped particles of ~ 100 nm in diameter, studded with trimeric HA spikes. Latterly, cryo-electron microscopy and tomography have confirmed the ovoid or discoid structure of the VLPs bearing HA trimers evenly distributed at their surface or presented on their outer diameter, respectively [109]. In addition, the VLPs are morphologically stable for at least 12 months at 4 °C. Immunisation of mice with H5 VLPs demonstrated immunogenicity (in the form of HI antibodies) against homologous virus and complete protection with a heterologous H5/N1 challenge.
Further studies showed that VLPs could be made similarly for other influenza HA strains including H2, H3, H6 and H9 as well as influenza B HA [110]. Immunisation of ferrets—the target animal model for influenza [111]—with the VLPs showed good protective responses against heterologous challenges, fulfilling the criteria of the European Committee of Medicinal Products (CHMP) for human use [112]. A phase I preclinical and safety trial in humans (NCT00984945) of H5N1 VLPs demonstrated promising immunogenicity and a good safety profile [113]. This encouraged Medicago Inc. to develop a VLP platform using tobacco plants as the production host after demonstrating that they could produce more than 10 million doses of influenza H1N1 VLP vaccines in 1 month, as part of a DARPA Blue Angel program in 2012 [114]. Landry et al. [115] showed in a phase I trial (NCT01302990) that unadjuvanted H1N1 and H5N1 plant-produced VLPs (5-, 13- or 28-µg doses) elicited T-cell responses in humans that were durable and cross-reactive. Pillet et al. [116] further illustrated in a phase I–II clinical trial (NCT01991587) that a quadrivalent VLP (QVLP) vaccine comprising influenza A H1N1 and H3N2, and influenza B Yamagata and Victoria lineage HA VLPs (3, 9 or 15 µg) was safe, met the HI antibody titers of the European licensure criteria and also induced and sustained a substantial CD4+ T-cell response which was cross-reactive against a different H3N2 strain and B lineage. A phase II dose-ranging trial of humans (NCT01991561) immunised with H5N1 VLPs co-administered with GLA-SE adjuvant showed that lower doses (3.5 or 7.5 µg) than those previously tested could be used whilst still meeting the licensure criteria for HI antibody responses as well as producing a sustained polyfunctional and cross-reactive CD4+ T-cell response [117]. These results bode well for dose-sparing requirements during a pandemic.
However, a further two phase II trials (NCT02233816 and NCT02236052) reported by Pillet et al. [118] to test and compare the effect of doses on older adult populations confirmed that a 30-µg dose of QVLPs provided the most consistent humoral and cellular immunity in the two age groups tested (18–49 years and ≥ 50 years) and this dosage was agreed upon as the best compromise for all age groups for further clinical trials. During the 2017–2018 and 2018–2019 influenza seasons, two randomised phase III trials (NCT03301051 and NCT03739112) were carried out by Medicago Inc. to test safety, immunogenicity and efficacy of a QVLP vaccine (non-adjuvanted 30-µg dose) in adults (18–64 years) and older adults (≥ 65 years), respectively [119]. These trials are now complete, both studies reporting elicitation of strong CD4+ T-cell responses to the QVLP vaccine.
In 2020, British American Tobacco plc (UK) and Kentucky Bioprocessing Inc (USA) (BAT/KBP) announced the approval for registration of a phase I trial (NCT04439695) to test the immunogenicity and safety of a quadrivalent influenza vaccine produced in plants (KBP-V001). Details of the vaccine candidate are not published, but it is reported to make use of the same plant-based technology platform as described for SARS-CoV-2 in the following section [120].
SARS-CoV-2
The COVID-19 pandemic starting in 2019, triggered considerable efforts to develop vaccines against its causative agent, SARS-CoV-2. It proffered an ideal opportunity for the biopharming community to prove that plants are a model platform for vaccine production under pandemic circumstances [121–124]. The platform’s ability to develop a candidate in a short time frame and rapidly scale up the production of vaccine candidates should fulfil all the requirements imposed by the pandemic and there have been several reports on COVID-19 vaccine developments.
Having completed their phase III influenza trials, Medicago Inc. were able to rapidly adapt their technology to develop a VLP vaccine similar to that reported for influenza, using a modified spike (S) glycoprotein from SARS-CoV-2 strain hCoV-19/USA/CA2/2020 [125]. The transmembrane (TM) and cytoplasmic tail (CT) regions of SARS-CoV-2 S were substituted with those of the influenza HA strain previously used [108], which increased VLP assembly and budding. In addition, several substitutions in S were made to increase stability at the S1/S2 cleavage site and stabilise the protein in a pre-fusion conformation. The coronavirus-like particle (CoVLP) vaccine was tested in a phase I randomised trial of 180 adults (NCT04450004) who were immunised IM with two doses ranging from 3 to 15 µg either alone or adjuvanted with ASO3 or CpG1018. Interim data 21 days after the second boost reflected neutralising antibody titers as well as IFN-γ and IL-4 cellular responses which were not affected by different doses, but which increased significantly with either of the adjuvants used. However, ASO3 was more effective than CpG1018 in enhancing the immune response as well as dose sparing. The safety profile was similar to that reported for Medicago’s plant-produced influenza QVLP vaccine [126], with adverse events (AEs) more prevalent in patients who received adjuvanted doses and those receiving CoVLP alone showing few AEs.
Medicago Inc. subsequently followed up with a phase II/III trial to assess the immunogenicity, safety and efficacy of the CoVLP vaccine formulation and dosing regimen (NCT04636697). Most encouragingly, results show that the vaccine had 78.8% efficacy against moderate-to-severe disease and a 74% efficacy against seronegative participants [81]. Moreover, the vaccine elicited 69.5% efficacy against COVID-19 caused by five different variants including those of delta, gamma, alpha, lambda and mu. In February 2022, it was officially approved for human use in Canada by Canada Health and is to be marketed under the name of COVIFENZ® (Medicago Inc., Québec) [20, 127].
Kentucky Bioprocessing Inc, the US biotech subsidiary of BAT, together with several others have also developed a SARS-CoV-2 vaccine candidate (CoV-RBD121-NP) by using a previously developed virus-like particle-type nano-particle (VLP-type NP) vaccine platform [47] to produce a TMV-like NP displaying a chemically conjugated SARS-CoV-2 antigen [128]. The antigen—CoV-RBD121—comprised amino acids 331–632 of the SARS-CoV-2 spike glycoprotein receptor-binding domain (RBD) fused to a human IgG1 Fc domain to enhance stability of the RBD as well as facilitate ease of purification. CoV-RBD121 was transiently expressed in N. benthamiana plants, purified and then chemically conjugated to modified TMV particles, also produced in plants by infection of N. benthamiana with virions using a previously established method [129]. Immunogenicity of CoV-RBD121-NP was tested in mice immunised subcutaneously with two doses 2 weeks apart; two different vaccine doses were tested (15 or 45 µg), with or without 7909 CpG adjuvant. Overall, antibody responses were strong irrespective of whether formulated with or without 7909 CpG. Although neutralising antibodies were stimulated, titers were much higher for the higher dose of 45 µg used, irrespective of the presence or absence of the adjuvant. Most importantly, characterisation of the NPs and stability assays showed that this vaccine candidate was stable for up to 12 months at 2–8 °C as well as 22–28 °C, fulfilling an important criterion for worldwide distribution requirements for vaccines. In addition, the pilot batch of CoV-RBD121-NP was manufactured within 28 days of receiving the SARS-Cov-2 RBD sequence showing that the platform can adapt rapidly to vaccine target demands in the event of a pandemic [128]. BAT/KBP has recently been given approval to commence with a phase I clinical trial to test its safety and immunogenicity (NCT04473690).
In 2021, Siriwattananon et al. [68] reported the production of a SARS-CoV-2 subunit vaccine by transient expression in N. benthamiana; the vaccine comprised SARS-CoV-2 RBD fused to the human IgG1 Fc fragment. The plant-made RBD-Fc fusion protein with alum elicited high neutralising antibody titers in mice and cynomolgus macaques following two IM doses of 10 µg and 25–50 µg, respectively. The vaccine candidate was evaluated further with attention to adjuvant formulation effects on the vaccine immunogenicity [67]. Four commercial adjuvants—Alhydrogel®, monophosphoryl lipid A from Salmonella Minnesota R595 (mPLA-SMA), AddaVax (MF59) and polyinosinic-polycytidylic acid (poly[I:C])—were combined with the plant-produced RBD-Fc fusion protein and the difference in immunogenicity evaluated in mice. Following two IM doses of 10 µg, high levels of total IgG and neutralising antibodies were observed for each adjuvant formulation group. Besides total IgG antibodies, mPLA-SM and poly(I:C) resulted in significantly enhanced levels of IgG2a subtype responses, indicating a more balanced Th2/Th1 immune response compared with those induced by Alhydrogel® and AddaVax. Additionally, a significant increase in the frequency of interferon-γ secreting cells was observed for the different adjuvant formulation groups compared with the control; however, no significant difference was observed between the adjuvanted groups. In 2021, this group, in conjunction with Baiya Phytopharm Co., Ltd, tested this vaccine (Baiya SARS-CoV-2 Vax 1) for safety, reactogenicity and toxicity in a phase I clinical trial (NCT04953078). The vaccine is reportedly safe [80], although results have not been published to date.
There are several recent reports on more developmental stage vaccine candidates for SARS-CoV-2. Peyret et al. [130] have provided preliminary evidence of SARS-CoV-2 VLPs upon transient co-expression of the E, M and S proteins, although yields appear to be low and have prevented quantification or further characterisation at this stage. Mamedov et al. [131] have pre-published data on the expression of SARS-CoV-2 S, RBD and N proteins in N. benthamiana using transient expression. Purified proteins used to immunise mice demonstrated immunogenicity as well as binding of RBD to the SARS-CoV-2 receptor angiotensin converting enzyme 2 (ACE2). In 2022, Margolin et al. [132] reported the increased transient expression of a soluble form of SARS-CoV-2 S1 by co-expression with the human chaperone calreticulin in plants. This has been previously shown to increase virus glycoprotein yields in plants [133]. Finally, iBio Inc., a plant-based manufacturing company in the US [134], have used their proprietary plant-based protein production platform (FastPharming® system) to make a SARS-CoV-2 vaccine based on the spike protein, and recently announced the development of a second-generation vaccine targeting SARS-CoV-2 N protein, although no details have been provided [135].
Conclusion
For many years, vaccines have been produced using conventional egg or cell culture platforms, often involving the use of wild-type viral or bacterial material. Vaccine production is a very challenging industrial process with many factors influencing its development and marketing. The length of time entailed from the design and development phase right through to commercialisation can take years and requires considerable financial resources and a substantial skilled workforce. There is a growing demand for the employment of alternative vaccine production strategies, particularly in regions where vaccines are needed the most. These are often in resource-poor countries that do not have the financial resources or suitable facilities for producing vaccines by conventional means. The production of vaccines by plant molecular farming provides an opportunity to fulfil this limitation. It is an easily scalable process, shown to be more cost effective than that of conventional methods. In the last few years, there has been a rapid increase in the development of human vaccine candidates produced in plants, addressing a variety of different targets. Perhaps the most encouraging target is that of SARS-CoV-2, which triggered the swift development of at least two different vaccines against COVID-19 reaching phase III trial stages within 2 years and one (Medicago Inc.’s plant-produced VLP vaccine) already completed. Even more conclusive is the recent announcement by Health Canada of the authorisation of this vaccine (COVIFENZ®) for human use.
There are some limitations of the plant platform for vaccine production. In some cases, yields of vaccines can be insufficient for supplying adequate numbers of doses. Advances are being made, however, by the investigation of co-expression of chaperones or enzymes which encourage proper protein modification and folding, thereby increasing yields. Other limitations include the lack of well-established regulatory criteria and a global paucity of companies having plant pharmaceutical manufacturing capacity—in 2020, there were fewer than five operational large-scale facilities [16].
Despite the current limitations, the latest efficacy success of human trials and Canadian Government approval of Medicago Inc.’s plant-produced VLP vaccine against SARS-CoV-2 demonstrates the flexibility and ability of the plant production platform to be equivalent to conventional vaccine production platforms. Moreover, the current focus on and trend towards making mRNA vaccines has opened an additional avenue for plant-made vaccines. There have been several studies in recent years investigating the engineering and immunogenicity of plant virus nanoparticles encapsidating target RNA against specific diseases [41, 136, 137]. These show great promise, and this flexibility bodes well for the future acceptance and deployment of plant-produced human vaccines.
Acknowledgements
The authors would like to thank Alta van Zyl for use of her HPV image.
Declarations
Conflict of interest
Ann Meyers is a shareholder in a molecular biopharming firm (Cape Bio Pharms Pty Ltd, Cape Town, South Africa). There are no other competing interests.
Funding
This manuscript was not funded.
Ethics approval
Not applicable.
Consent to participate/publish
Not applicable.
Availability of data and material
Any material will be made available on request
Code availability
Not applicable.
Author contributions
AM drafted and edited the manuscript, JS helped with drafting and edited the manuscript, SM edited the manuscript.
References
- 1.Flemming A. The origins of vaccination, in nature milestones in vaccines. In: Fehervari KMZ, João HD editors; 2020. https://www.nature.com/collections/hcajdiajij. Accessed 28 Jan 2022.
- 2.Artenstein AW. The discovery of viruses: advancing science and medicine by challenging dogma. Int J Infect Dis. 2012;16(7):e470–e473. doi: 10.1016/j.ijid.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Blevins SM, Bronze MS. Robert Koch and the ‘golden age’ of bacteriology. Int J Infect Dis. 2010;14(9):e744–e751. doi: 10.1016/j.ijid.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21(2):83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trombetta CM, et al. Challenges in the development of egg-independent vaccines for influenza. Expert Rev Vaccines. 2019;18(7):737–750. doi: 10.1080/14760584.2019.1639503. [DOI] [PubMed] [Google Scholar]
- 6.Valenzuela P, et al. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature. 1982;298(5872):347–350. doi: 10.1038/298347a0. [DOI] [PubMed] [Google Scholar]
- 7.Bucci M. First recombinant vaccine for HBV. Nature Milestones; 2020. https://www.nature.com/articles/d42859-020-00016-5#.
- 8.Huebbers JW, Buyel JF. On the verge of the market—plant factories for the automated and standardized production of biopharmaceuticals. Biotechnol Adv. 2021;46:107681. doi: 10.1016/j.biotechadv.2020.107681. [DOI] [PubMed] [Google Scholar]
- 9.Villa LL, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6(5):271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 10.Fuenmayor J, Gòdia F, Cervera L. Production of virus-like particles for vaccines. New Biotechnol. 2017;39:174–180. doi: 10.1016/j.nbt.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laere E, et al. Plant-based vaccines: production and challenges. J Bot. 2016;2016:4928637. [Google Scholar]
- 12.Kurup VM, Thomas J. Edible vaccines: promises and challenges. Mol Biotechnol. 2020;62(2):79–90. doi: 10.1007/s12033-019-00222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demain AL, Vaishnav P. Production of recombinant proteins by microbes and higher organisms. Biotechnol Adv. 2009;27(3):297–306. doi: 10.1016/j.biotechadv.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Kulagina N, et al. Yeasts as biopharmaceutical production platforms. Front Fungal Biol. 2021;2:733492. doi: 10.3389/ffunb.2021.733492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Flaherty R, et al. Mammalian cell culture for production of recombinant proteins: a review of the critical steps in their biomanufacturing. Biotechnol Adv. 2020;43:107552. doi: 10.1016/j.biotechadv.2020.107552. [DOI] [PubMed] [Google Scholar]
- 16.Tusé D, et al. The emergency response capacity of plant-based biopharmaceutical manufacturing-what it is and what it could be. Front Plant Sci. 2020;11:594019. doi: 10.3389/fpls.2020.594019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lomonossoff GP, D'Aoust MA. Plant-produced biopharmaceuticals: a case of technical developments driving clinical deployment. Science. 2016;353(6305):1237–1240. doi: 10.1126/science.aaf6638. [DOI] [PubMed] [Google Scholar]
- 18.Fischer R, Buyel JF. Molecular farming—the slope of enlightenment. Biotechnol Adv. 2020;40:107519. doi: 10.1016/j.biotechadv.2020.107519. [DOI] [PubMed] [Google Scholar]
- 19.Chung YH, et al. Integrating plant molecular farming and materials research for next-generation vaccines. Nat Rev Mater. 2021;7:372–388. doi: 10.1038/s41578-021-00399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Health Canada. Medicago Covifenz COVID-19 vaccine. 2022 31 March 2022. https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/vaccines/medicago.html. Accessed 5 May 2022.
- 21.Canto T. Transient expression systems in plants: potentialities and constraints. Adv Exp Med Biol. 2016;896:287–301. doi: 10.1007/978-3-319-27216-0_18. [DOI] [PubMed] [Google Scholar]
- 22.Hiatt A, Caffferkey R, Bowdish K. Production of antibodies in transgenic plants. Nature. 1989;342(6245):76–78. doi: 10.1038/342076a0. [DOI] [PubMed] [Google Scholar]
- 23.Langridge WHR. Edible vaccines. Sci Am. 2000;283(3):66–71. doi: 10.1038/scientificamerican0900-66. [DOI] [PubMed] [Google Scholar]
- 24.Arntzen CJ. Edible vaccines. Public Health Rep. 1997;112(3):190–197. [PMC free article] [PubMed] [Google Scholar]
- 25.Rybicki EP. Plant-made vaccines for humans and animals. Plant Biotechnol J. 2010;8(5):620–637. doi: 10.1111/j.1467-7652.2010.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunasekaran B, Gothandam KM. A review on edible vaccines and their prospects. Braz J Med Biol Res. 2020;53(2):e8749. doi: 10.1590/1414-431x20198749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loh H-S, Green BJ, Yusibov V. Using transgenic plants and modified plant viruses for the development of treatments for human diseases. Curr Opin Virol. 2017;26:81–89. doi: 10.1016/j.coviro.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorantala J, et al. Generation of protective immune response against anthrax by oral immunization with protective antigen plant-based vaccine. J Biotechnol. 2014;176:1–10. doi: 10.1016/j.jbiotec.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 29.Czyż M, et al. Freeze-drying of plant tissue containing HBV surface antigen for the oral vaccine against hepatitis B. Biomed Res Int. 2014;2014:485689. doi: 10.1155/2014/485689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh A, et al. Expression of rabies glycoprotein and ricin toxin B chain (RGP-RTB) fusion protein in tomato hairy roots: a step towards oral vaccination for rabies. Mol Biotechnol. 2015;57(4):359–370. doi: 10.1007/s12033-014-9829-y. [DOI] [PubMed] [Google Scholar]
- 31.Peyret H, Brown JKM, Lomonossoff GP. Improving plant transient expression through the rational design of synthetic 5' and 3' untranslated regions. Plant Methods. 2019;15:108. doi: 10.1186/s13007-019-0494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hefferon K. Plant virus expression vectors: a powerhouse for global health. Biomedicines. 2017;5(3):44. doi: 10.3390/biomedicines5030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abrahamian P, Hammond RW, Hammond J. Plant virus-derived vectors: applications in agricultural and medical biotechnology. Ann Rev Virol. 2020;7(1):513–535. doi: 10.1146/annurev-virology-010720-054958. [DOI] [PubMed] [Google Scholar]
- 34.Peyret H, Lomonossoff GP. When plant virology met Agrobacterium: the rise of the deconstructed clones. Plant Biotechnol J. 2015;13(8):1121–1135. doi: 10.1111/pbi.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto T, et al. Improvement of the transient expression system for production of recombinant proteins in plants. Sci Rep. 2018;8(1):4755. doi: 10.1038/s41598-018-23024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balke I, Zeltins A. Use of plant viruses and virus-like particles for the creation of novel vaccines. Adv Drug Deliv Rev. 2019;145:119–129. doi: 10.1016/j.addr.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Tariq H, et al. Virus-like particles: revolutionary platforms for developing vaccines against emerging infectious diseases. Front Microbiol. 2022;12:790121. doi: 10.3389/fmicb.2021.790121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nooraei S, et al. Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J Nanobiotechnol. 2021;19(1):59. doi: 10.1186/s12951-021-00806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohsen MO, et al. Interaction of viral capsid-derived virus-like particles (VLPs) with the innate immune system. Vaccines (Basel) 2018;6:37. doi: 10.3390/vaccines6030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rybicki EP. Plant-based vaccines against viruses. Virol J. 2014;11(1):205. doi: 10.1186/s12985-014-0205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rybicki EP. Plant molecular farming of virus-like nanoparticles as vaccines and reagents. WIREs Nanomed Nanobiotechnol. 2020;12(2):e1587. doi: 10.1002/wnan.1587. [DOI] [PubMed] [Google Scholar]
- 42.Marsian J, et al. Plant-made polio type 3 stabilized VLPs—a candidate synthetic polio vaccine. Nat Commun. 2017;8(1):245. doi: 10.1038/s41467-017-00090-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chabeda A, et al. Substitution of human papillomavirus type 16 L2 neutralizing epitopes into L1 surface loops: the effect on virus-like particle assembly and immunogenicity. Front Plant Sci. 2019;10:779. doi: 10.3389/fpls.2019.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shukla S, et al. Plant viruses and bacteriophage-based reagents for diagnosis and therapy. Ann Rev Virol. 2020;7(1):559–587. doi: 10.1146/annurev-virology-010720-052252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moradi Vahdat M, et al. Hepatitis B core-based virus-like particles: a platform for vaccine development in plants. Biotechnol Rep. 2021;29:e00605. doi: 10.1016/j.btre.2021.e00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peyret H, et al. Tandem fusion of hepatitis B core antigen allows assembly of virus-like particles in bacteria and plants with enhanced capacity to accommodate foreign proteins. PLoS One. 2015;10(4):e0120751. doi: 10.1371/journal.pone.0120751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCormick AA, Palmer KE. Genetically engineered Tobacco mosaic virus as nanoparticle vaccines. Expert Rev Vaccines. 2008;7(1):33–41. doi: 10.1586/14760584.7.1.33. [DOI] [PubMed] [Google Scholar]
- 48.Staczek J, et al. Immunization with a chimeric tobacco mosaic virus containing an epitope of outer membrane protein F of Pseudomonas aeruginosa provides protection against challenge with P. aeruginosa. Vaccine. 2000;18(21):2266–2274. doi: 10.1016/S0264-410X(99)00571-X. [DOI] [PubMed] [Google Scholar]
- 49.Banik S, et al. Development of a multivalent subunit vaccine against tularemia using tobacco mosaic virus (TMV) based delivery system. PLoS One. 2015;10(6):e0130858. doi: 10.1371/journal.pone.0130858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zakeri B, et al. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc Natl Acad Sci USA. 2012;109(12):E690–E697. doi: 10.1073/pnas.1115485109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stander J, et al. A plant-produced virus-like particle displaying envelope protein domain III elicits an immune response against west Nile virus in mice. Front Plant Sci. 2021;12:1915. doi: 10.3389/fpls.2021.738619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamprecht RL, et al. Production of Human papillomavirus pseudovirions in plants and their use in pseudovirion-based neutralisation assays in mammalian cells. Sci Rep. 2016;6(1):20431. doi: 10.1038/srep20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Y, et al. In planta production of flock house virus transencapsidated RNA and its potential use as a vaccine. Mol Biotechnol. 2015;57(4):325–336. doi: 10.1007/s12033-014-9826-1. [DOI] [PubMed] [Google Scholar]
- 54.Wang X-Y, Wang B, Wen Y-M. From therapeutic antibodies to immune complex vaccines. NPJ Vaccines. 2019;4(1):2. doi: 10.1038/s41541-018-0095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phoolcharoen W, et al. Expression of an immunogenic Ebola immune complex in Nicotiana benthamiana. Plant Biotechnol J. 2011;9(7):807–816. doi: 10.1111/j.1467-7652.2011.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim M-Y, et al. Novel vaccination approach for dengue infection based on recombinant immune complex universal platform. Vaccine. 2015;33(15):1830–1838. doi: 10.1016/j.vaccine.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 57.Diamos AG, et al. Codelivery of improved immune complex and virus-like particle vaccines containing Zika virus envelope domain III synergistically enhances immunogenicity. Vaccine. 2020;38(18):3455–3463. doi: 10.1016/j.vaccine.2020.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.FDA. Vaccines licensed for use in the United States
- 59.Chen Q, Lai H. Plant-derived virus-like particles as vaccines. Hum Vaccin Immunother. 2013;9(1):26–49. doi: 10.4161/hv.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thrane S, et al. Bacterial superglue enables easy development of efficient virus-like particle based vaccines. J Nanobiotechnol. 2016;14(1):30. doi: 10.1186/s12951-016-0181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bolaños-Martínez OC, et al. Expression of immunogenic poliovirus Sabin type 1 VP proteins in transgenic tobacco. J Biotechnol. 2020;322:10–20. doi: 10.1016/j.jbiotec.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 62.He J, et al. A plant-produced antigen elicits potent immune responses against West Nile Virus in Mice. Biomed Res Int. 2014;2014:952865. doi: 10.1155/2014/952865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lai H, et al. A plant-produced vaccine protects mice against lethal West Nile virus infection without enhancing Zika or dengue virus infectivity. Vaccine. 2018;36(14):1846–1852. doi: 10.1016/j.vaccine.2018.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mammadova G, et al. Engineering, production, and immunogenicity studies of a truncated form of rabies virus glycoprotein produced in Nicotiana benthamiana plant. Med Sci. 2022;11(2):478–483. doi: 10.5455/medscience.2021.09.278. [DOI] [Google Scholar]
- 65.Chichester JA, et al. A plant-produced protective antigen vaccine confers protection in rabbits against a lethal aerosolized challenge with Bacillus anthracis Ames spores. Hum Vaccin Immunother. 2013;9(3):544–552. doi: 10.4161/hv.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paolino KM, et al. Safety and immunogenicity of a plant-derived recombinant protective antigen (rPA)-based vaccine against Bacillus anthracis: a Phase 1 dose-escalation study in healthy adults. Vaccine. 2022;40(12):1864–1871. doi: 10.1016/j.vaccine.2022.01.047. [DOI] [PubMed] [Google Scholar]
- 67.Siriwattananon K, et al. Immunogenicity studies of plant-produced SARS-CoV-2 receptor binding domain-based subunit vaccine candidate with different adjuvant formulations. Vaccines. 2021;9(7):744. doi: 10.3390/vaccines9070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siriwattananon K, et al. Plant-produced receptor-binding domain of SARS-CoV-2 elicits potent neutralizing responses in mice and non-human primates. Front Plant Sci. 2021;12:682953. doi: 10.3389/fpls.2021.682953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buyel JF. Plant molecular farming–integration and exploitation of side streams to achieve sustainable biomanufacturing. Front Plant Sci. 2019;9:1893. doi: 10.3389/fpls.2018.01893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moustafa K, Makhzoum A, Trémouillaux-Guiller J. Molecular farming on rescue of pharma industry for next generations. Crit Rev Biotechnol. 2016;36(5):840–850. doi: 10.3109/07388551.2015.1049934. [DOI] [PubMed] [Google Scholar]
- 71.Brisse M, et al. Emerging concepts and technologies in vaccine development. Front Immunol. 2020;11(2578):583077. doi: 10.3389/fimmu.2020.583077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Montero-Morales L, Steinkellner H. Advanced plant-based glycan engineering. Front Bioeng Biotechnol. 2018;6:81. doi: 10.3389/fbioe.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Margolin E, et al. A roadmap for the molecular farming of viral glycoprotein vaccines: engineering glycosylation and glycosylation-directed folding. Front Plant Sci. 2020;11:609207–609207. doi: 10.3389/fpls.2020.609207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vermij P, Waltz E. USDA approves the first plant-based vaccine. Nat Biotechnol. 2006;24(3):234. [Google Scholar]
- 75.Schillberg S, et al. Critical analysis of the commercial potential of plants for the production of recombinant proteins. Front Plant Sci. 2019;10:720. doi: 10.3389/fpls.2019.00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nandi S et al. Techno-economic analysis of a transient plant-based platform for monoclonal antibody production. In: MAbs. Taylor & Francis; 2016. [DOI] [PMC free article] [PubMed]
- 77.McNulty MJ, et al. Techno-economic analysis of a plant-based platform for manufacturing antimicrobial proteins for food safety. Biotechnol Prog. 2019;36:e2896. doi: 10.1002/btpr.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alam A, et al. Technoeconomic modeling of plant-based Griffithsin manufacturing. Front Bioeng Biotechnol. 2018;6:102. doi: 10.3389/fbioe.2018.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maharjan PM, Choe S. Plant-based COVID-19 vaccines: current status, design, and development strategies of candidate vaccines. Vaccines. 2021;9(9):992. doi: 10.3390/vaccines9090992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.CNBC. A Thai start-up is working on a COVID vaccine—using tobacco leaves; 2022.
- 81.Hager KJ, et al. Efficacy and safety of a recombinant plant-based adjuvanted COVID-19 vaccine. N Engl J Med. 2022;386:2084–2096. doi: 10.1056/NEJMoa2201300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mason HS, Lam DM, Arntzen CJ. Expression of hepatitis B surface antigen in transgenic plants. Proc Natl Acad Sci. 1992;89(24):11745–11749. doi: 10.1073/pnas.89.24.11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kapusta J, et al. Oral immunization of human with transgenic lettuce expressing hepatitis B surface antigen. Adv Exp Med Biol. 2001;495:299–303. doi: 10.1007/978-1-4615-0685-0_41. [DOI] [PubMed] [Google Scholar]
- 84.Thanavala Y, et al. Immunogenicity in humans of an edible vaccine for hepatitis B. Proc Natl Acad Sci USA. 2005;102(9):3378–3382. doi: 10.1073/pnas.0409899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang Z, et al. High-yield rapid production of hepatitis B surface antigen in plant leaf by a viral expression system. Plant Biotechnol J. 2008;6(2):202–209. doi: 10.1111/j.1467-7652.2007.00316.x. [DOI] [Google Scholar]
- 86.Pniewski T. The twenty-year story of a plant-based vaccine against hepatitis B: stagnation or promising prospects? Int J Mol Sci. 2013;14(1):1978–1998. doi: 10.3390/ijms14011978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joung YH, et al. The last ten years of advancements in plant-derived recombinant vaccines against hepatitis B. Int J Mol Sci. 2016;17:1715. doi: 10.3390/ijms17101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pyrski M, et al. Parenteral-oral immunization with plant-derived HBcAg as a potential therapeutic vaccine against chronic hepatitis B. Vaccines. 2019;7(4):211. doi: 10.3390/vaccines7040211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zahmanova G, et al. Efficient production of chimeric hepatitis B virus-like particles bearing an epitope of hepatitis E virus capsid by transient expression in Nicotiana benthamiana. Life. 2021;11(1):64. doi: 10.3390/life11010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ponndorf D, et al. Plant-made dengue virus-like particles produced by co-expression of structural and non-structural proteins induce a humoral immune response in mice. Plant Biotechnol J. 2021;19(4):745–756. doi: 10.1111/pbi.13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pang EL, et al. Epitope presentation of dengue viral envelope glycoprotein domain III on hepatitis B core protein virus-like particles produced in Nicotiana benthamiana. Front Plant Sci. 2019;10:455. doi: 10.3389/fpls.2019.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen Q. Plant-made vaccines against West Nile virus are potent, safe, and economically feasible. Biotechnol J. 2015;10(5):671–680. doi: 10.1002/biot.201400428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.He J, et al. Plant-produced antigen displaying virus-like particles evokes potent antibody responses against West Nile Virus in Mice. Vaccines. 2021;9(1):60. doi: 10.3390/vaccines9010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang M, et al. Virus-like particles that display Zika virus envelope protein domain III induce potent neutralizing immune responses in mice. Sci Rep. 2017;7(1):7679. doi: 10.1038/s41598-017-08247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Phoolcharoen W, et al. A nonreplicating subunit vaccine protects mice against lethal Ebola virus challenge. Proc Natl Acad Sci. 2011;108(51):20695–20700. doi: 10.1073/pnas.1117715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tottey S, et al. Plant-produced subunit vaccine candidates against yellow fever induce virus neutralizing antibodies and confer protection against viral challenge in animal models. Am J Trop Med Hyg. 2018;98(2):420–431. doi: 10.4269/ajtmh.16-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Burke RM, et al. Current and new rotavirus vaccines. Curr Opin Infect Dis. 2019;32(5):435–444. doi: 10.1097/QCO.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kurokawa N, et al. Development and characterization of a plant-derived rotavirus-like particle vaccine. Vaccine. 2021;39(35):4979–4987. doi: 10.1016/j.vaccine.2021.07.039. [DOI] [PubMed] [Google Scholar]
- 99.Kurokawa N, et al. Safety and immunogenicity of a plant-derived rotavirus-like particle vaccine in adults, toddlers and infants. Vaccine. 2021;39(39):5513–5523. doi: 10.1016/j.vaccine.2021.08.052. [DOI] [PubMed] [Google Scholar]
- 100.Diamos AG, Mason HS. High-level expression and enrichment of norovirus virus-like particles in plants using modified geminiviral vectors. Protein Expr Purif. 2018;151:86–92. doi: 10.1016/j.pep.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 101.Malm M, et al. Rotavirus VP6 as an adjuvant for bivalent norovirus vaccine produced in Nicotiana benthamiana. Pharmaceutics. 2019;11(5):229. doi: 10.3390/pharmaceutics11050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tusé D, et al. Safety and immunogenicity studies in animal models support clinical development of a bivalent norovirus-like particle vaccine produced in plants. Vaccine. 2022;40(7):977–987. doi: 10.1016/j.vaccine.2022.01.009. [DOI] [PubMed] [Google Scholar]
- 103.Fletcher MA, Hessel L, Plotkin SA. Human diploid cell strains (HDCS) viral vaccines. Dev Biol Stand. 1998;93:97–107. [PubMed] [Google Scholar]
- 104.Musiychuk K, et al. A launch vector for the production of vaccine antigens in plants. Influenza Other Respir Viruses. 2007;1(1):19–25. doi: 10.1111/j.1750-2659.2006.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jones RM, et al. A plant-produced Pfs25 VLP Malaria vaccine candidate induces persistent transmission blocking antibodies against Plasmodium falciparum in immunized mice. PLoS One. 2013;8(11):e79538. doi: 10.1371/journal.pone.0079538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chichester JA, et al. Safety and immunogenicity of a plant-produced Pfs25 virus-like particle as a transmission blocking vaccine against malaria: a phase 1 dose-escalation study in healthy adults. Vaccine. 2018;36(39):5865–5871. doi: 10.1016/j.vaccine.2018.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cheng L, Wang Y, Du J. Human papillomavirus vaccines: an updated review. Vaccines. 2020;8(3):391. doi: 10.3390/vaccines8030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.D’Aoust M-A, et al. Influenza virus-like particles produced by transient expression in Nicotiana benthamiana induce a protective immune response against a lethal viral challenge in mice. Plant Biotechnol J. 2008;6(9):930–940. doi: 10.1111/j.1467-7652.2008.00384.x. [DOI] [PubMed] [Google Scholar]
- 109.Lindsay BJ, et al. Morphological characterization of a plant-made virus-like particle vaccine bearing influenza virus hemagglutinins by electron microscopy. Vaccine. 2018;36(16):2147–2154. doi: 10.1016/j.vaccine.2018.02.106. [DOI] [PubMed] [Google Scholar]
- 110.D’Aoust M-A, Couture M, Ors F, Trépanier S, Lavoie P-O, Dargis M, Vézina L-P. Recombinant influenza virus-like particles (VLPs) produced in transgenic plants expressing hemagglutinin. International Patent application; 2009.
- 111.Matsuoka Y, Lamirande EW, Subbarao K. The ferret model for influenza. Curr Protoc Microbiol. 2009; Chapter 15: p. Unit 15G.2. [DOI] [PubMed]
- 112.D’Aoust M-A, et al. The production of hemagglutinin-based virus-like particles in plants: a rapid, efficient and safe response to pandemic influenza. Plant Biotechnol J. 2010;8(5):607–619. doi: 10.1111/j.1467-7652.2009.00496.x. [DOI] [PubMed] [Google Scholar]
- 113.Landry N, et al. Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza. PLoS One. 2010;5(12):e15559. doi: 10.1371/journal.pone.0015559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.USA News. RT. DARPA’s Blue Angel—Pentagon Prepares Millions of Vaccines Against Future Global Flu. USA News 2012; https://www.rt.com/usa/future-vaccine-darpa-research-255. Accessed 9 May 2022.
- 115.Landry N, et al. Influenza virus-like particle vaccines made in Nicotiana benthamiana elicit durable, poly-functional and cross-reactive T cell responses to influenza HA antigens. Clin Immunol. 2014;154(2):164–177. doi: 10.1016/j.clim.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 116.Pillet S, et al. A plant-derived quadrivalent virus like particle influenza vaccine induces cross-reactive antibody and T cell response in healthy adults. Clin Immunol. 2016;168:72–87. doi: 10.1016/j.clim.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 117.Pillet S, et al. Humoral and cell-mediated immune responses to H5N1 plant-made virus-like particle vaccine are differentially impacted by alum and GLA-SE adjuvants in a Phase 2 clinical trial. NPJ Vaccines. 2018;3:3. doi: 10.1038/s41541-017-0043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pillet S, et al. Immunogenicity and safety of a quadrivalent plant-derived virus like particle influenza vaccine candidate—two randomized phase II clinical trials in 18 to 49 and ≥ 50 years old adults. PLoS One. 2019;14(6):e0216533. doi: 10.1371/journal.pone.0216533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ward BJ, et al. Efficacy, immunogenicity, and safety of a plant-derived, quadrivalent, virus-like particle influenza vaccine in adults (18–64 years) and older adults (≥ 65 years): two multicentre, randomised phase 3 trials. Lancet. 2020;396(10261):1491–1503. doi: 10.1016/S0140-6736(20)32014-6. [DOI] [PubMed] [Google Scholar]
- 120.BAT. Vaccine Development. 12 January 2022. https://www.bat-science.com/groupms/sites/BAT_C6ZJDE.nsf/vwPagesWebLive/DOC67AQU?opendocument. Accessed 12 Jan 2022.
- 121.Capell T, et al. Potential applications of plant biotechnology against SARS-CoV-2. Trends Plant Sci. 2020;25(7):635–643. doi: 10.1016/j.tplants.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rosales-Mendoza S, et al. What does plant-based vaccine technology offer to the fight against COVID-19? Vaccines. 2020;8:183. doi: 10.3390/vaccines8020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lico C, et al. Plant molecular farming as a strategy against COVID-19—the Italian perspective. Front Plant Sci. 2020;11:609910. doi: 10.3389/fpls.2020.609910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McDonald KA, Holtz RB. From farm to finger prick—a perspective on how plants can help in the fight against COVID-19. Front Bioeng Biotechnol. 2020;8:782. doi: 10.3389/fbioe.2020.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ward BJ, et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat Med. 2021;27(6):1071–1078. doi: 10.1038/s41591-021-01370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ward BJ, et al. Phase III: Randomized observer-blind trial to evaluate lot-to-lot consistency of a new plant-derived quadrivalent virus like particle influenza vaccine in adults 18–49 years of age. Vaccine. 2021;39(10):1528–1533. doi: 10.1016/j.vaccine.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 127.Health Canada. Health Canada authorizes Medicago COVID-19 vaccine for adults 18 to 64 years of age. 2022. https://www.canada.ca/en/health-canada/news/2022/02/health-canada-authorizes-medicago-covid-19-vaccine-for-adults-18-to-64-years-of-age.html. Accessed 24 Feb 2022.
- 128.Royal JM, et al. Development of a SARS-CoV-2 vaccine candidate using plant-based manufacturing and a Tobacco mosaic virus-like nano-particle. Vaccines. 2021;9(11):1347. doi: 10.3390/vaccines9111347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Smith ML, et al. Modified tobacco mosaic virus particles as scaffolds for display of protein antigens for vaccine applications. Virology. 2006;348(2):475–488. doi: 10.1016/j.virol.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 130.Peyret H, et al. Producing vaccines against enveloped viruses in plants: making the impossible, difficult. Vaccines. 2021;9(7):780. doi: 10.3390/vaccines9070780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mamedov T et al. Engineering, production and characterization of Spike and Nucleocapsid structural proteins of SARS-CoV-2 in Nicotiana benthamiana as vaccine candidates against COVID-19. bioRxiv. 2020: p. 2020.12.29.424779.
- 132.Margolin E et al. Investigating constraints along the plant secretory pathway to improve production of a SARS-CoV-2 spike vaccine candidate. Front Plant Sci. 2022;12:798822. [DOI] [PMC free article] [PubMed]
- 133.Margolin E, et al. Co-expression of human calreticulin significantly improves the production of HIV gp140 and other viral glycoproteins in plants. Plant Biotechnol J. 2020;18(10):2109–2117. doi: 10.1111/pbi.13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.iBio Inc. https://ibioinc.com/. Accessed 9 May 2022.
- 135.iBio Inc. iBio announces progression of vaccine program for multi-variant COVID-19 disease. 2022. https://ibioinc.com/ibio-announces-progression-of-vaccine-program-for-multi-variant-covid-19-disease/.
- 136.Steinmetz NF, Lim S, Sainsbury F. Protein cages and virus-like particles: from fundamental insight to biomimetic therapeutics. Biomater Sci. 2020;8(10):2771–2777. doi: 10.1039/D0BM00159G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Biddlecome A, et al. Delivery of self-amplifying RNA vaccines in in vitro reconstituted virus-like particles. PLoS One. 2019;14(6):e0215031. doi: 10.1371/journal.pone.0215031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.IconGenetics. Icon Genetics clinical development of its novel norovirus vaccine reaches milestone of complete dosing of the first cohort. https://www.icongenetics.com/icon-genetics-clinical-development-of-its-novel-norovirus-vaccine-reaches-milestone-of-complete-dosing-of-the-first-cohort/. Accessed May 2022.