Abstract
Introduction
We aimed to develop a comprehensive risk assessment tool for Alzheimer's disease (AD), vascular dementia (VaD), and any dementia, that will be applicable in high and low resource settings.
Method
Risk factors which can easily be assessed in most settings, and their effect sizes, were identified from an umbrella review, or estimated using meta‐analysis where new data were available.
Results
Seventeen risk/protective factors met criteria for the algorithm to estimate risk for any dementia including age, sex, education, hypertension, midlife obesity, midlife high cholesterol, diabetes, insufficient physical activity, depression, traumatic brain injury, atrial fibrillation, smoking, social engagement, cognitive engagement, fish consumption (diet), stroke, and insomnia. A version for AD excluded atrial fibrillation and insomnia due to insufficient evidence and included pesticide exposure. There was insufficient evidence for a VaD risk score.
Discussion
Validation of the tool on external datasets is planned. The assessment tool will assist with implementing risk reduction guidelines.
Keywords: assessment, dementia, development, questionnaire, risk and protective factors, tool
1. INTRODUCTION
Clinicians, policy makers, and researchers need reliable and valid tools to assess risk factors for dementia, to implement brain health programs, and evaluate population‐level dementia risk. However, authorities differ in their proposed list of risk factors. The World Health Organization (WHO) guidelines 1 did not find sufficient evidence to recommend interventions for hearing loss or social engagement, but did include recommendations related to diet, whereas the Lancet Commission 2 recommended addressing hearing loss in midlife but did not make dietary recommendations. These differences reflect variations in methodologies and sources of data (i.e., clinical trials vs. observational studies). There is no individual cohort study or data source that includes all the risk factors that have been identified for dementia, and none that reflects global ethnic diversity. It is therefore likely that compared to risk tools developed on a single population, tools that are developed from meta‐analyses of the extant literature will provide a more reliable and generalizable assessment, in addition to allowing for inclusion of a larger number of risk factors. 3
1.1. Purpose of the present study
We developed a new risk assessment tool for dementia and Alzheimer's disease (AD) for use by clinicians, researchers, policy makers, and the general public, with the purpose of identifying risk factors and monitoring risk reduction efforts in high and low resource settings. First, we developed numerical risk scores for AD, any dementia and vascular dementia (VaD) using an evidence‐based medicine approach that draws on publications from observational studies. Due to limited data on VaD we did not proceed with a risk score for this. Second, we developed a tool to assess the risk factors included in the risk score. The final tool, called Assessment for Cognitive Health and Dementia Risk Reduction or “CogDrisk”, comprises the questionnaire and scoring algorithms.
2. METHODS
2.1. Selection of risk/protective factors for potential inclusion in the risk tool
We conducted a comprehensive systematic review of meta‐analyses from observational studies and identified 33 potential risk and protective factors for dementia and its subtypes. 4 Criteria for inclusion of risk factors in the assessment tool included: systematic review‐level evidence reporting risk ratios (RRs) for the risk factors for dementia outcomes; risk factors must be assessable in a wide range of settings; and pharmacological risk factors with consistent supportive evidence from both cohort studies and clinical trials. 5 , 6 Recent reports were also considered. 2 , 1
2.2. Selection and computation of risk ratios for individual risk factors
Risk ratios were drawn from our umbrella review 4 where possible. Measures of the risk factors, age group, number and recency of studies, and inclusion and exclusion criteria were evaluated before selecting effect sizes. Effect sizes available by age group and sex were preferentially selected. Risk/protective factors with a single effect size were selected if there was only one systematic review and the individual studies from the meta‐analysis could not be further categorized into mid/late‐life or by sex. In cases in which multiple systematic reviews conducted meta‐analysis of the same risk factor, the RR was recalculated by pooling the odds ratios/hazard ratios/relative RRs from the original cohort studies using the StatsDirect software 7 (see supporting information for details).
2.3. Defining risk factors and selection of items for inclusion in assessment tool
Evidence‐based definitions of risk factors were used (e.g., the WHO guidelines for body mass index categories), and validated clinical cutoffs (e.g., Kivipelto and Solomon 8 ). A questionnaire was collated using self‐reported items from the same scales as used in the original cohort studies from which RRs were drawn, where possible. Otherwise, a validated instrument was used (Table SA1 in supporting information).
RESEARCH IN CONTEXT
Systematic review: Implementation of brain health programs requires clear guidance on risk factors for dementia that can be validly assessed in low resource settings across different populations. Although there are at least three dementia risk assessments translated into tools, a significant amount of evidence has been published since they were developed. We drew evidence from recent reviews and meta‐analyses for dementia and major subtypes to identify risk factors with sufficient evidence to include in a low‐cost risk assessment tool.
Interpretation: We identified 13 risk factors for any dementia, and 11 factors for Alzheimer's disease (AD), that had consistent evidence from reviews, included risk ratios (RRs), and were validly assessed via self‐report. The RRs were used to develop a scoring algorithm, and risk assessment tools (Assessment for Cognitive Health and Dementia Risk Reduction [CogDrisk] and CogDrisk‐AD) were developed using validated instruments or questions drawn from the original reports.
Future directions: Validation of the CogDrisk on five external cohort studies across different populations is under way.
3. RESULTS
3.1. Number of risk factors selected for each outcome
Figure 1 depicts the steps involved in identifying risk factors for any dementia and shows that of 26 factors identified in the initial review, 4 13 met criteria for inclusion in the CogDrisk tool for any dementia. We further added two risk factors to the risk score for any dementia (i.e., hypertension and stroke) that were not identified in the initial review as well as age and sex, making a total of 17 risk factors (Figure 1, Table 1). Table 1 shows the risk factors and the RRs for the outcome of any dementia that are used in the CogDrisk tool.
FIGURE 1.
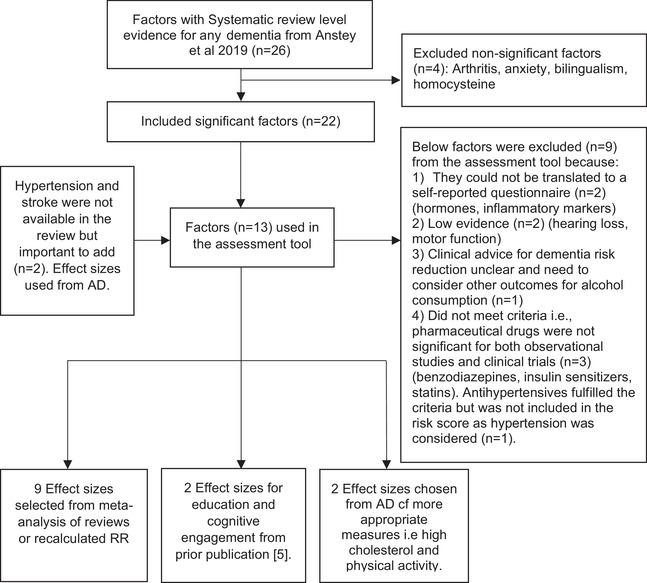
Flowchart for selecting risk/protective factors for any dementia. AD, Alzheimer's disease; RR, risk ratio
TABLE 1.
Risk factor categories and risk ratios for any dementia
| Risk/protective factor, source of effect size | Measure and categories | Effect size (relative risk ratios) | Beta weight | Points |
|---|---|---|---|---|
| Age and sex 9 | ||||
| Age for males | ||||
| 60–64 years | Reference | |||
| 65–69 years | 1.24 | 6 | ||
| 70–74 years | 1.95 | 8 | ||
| 75–79 years | 2.62 | 13 | ||
| 80–84 years | 3.40 | 17 | ||
| 85–89 years | 3.92 | 20 | ||
| >90 years | 4.42 | 22 | ||
| Age for females | ||||
| 60–64 years | Reference | |||
| 65–69 years | 0.72 | 4 | ||
| 70–74 years | 1.39 | 7 | ||
| 75–79 years | 2.19 | 11 | ||
| 80–84 years | 2.98 | 15 | ||
| 85–89 years | 3.74 | 19 | ||
| >90 years | 4.53 | 23 | ||
| Education 13 | Number of years | |||
| Highest category (>11 years) | Reference | Reference | ||
| Highest vs. middle (8–11 years) | 1.52 (0.92–2.50) | 0.42 | 2 | |
| Highest vs. lowest (<8 years) | 2.23 (1.43–3.50) | 0.8 | 4 | |
| Midlife obesity (< = 65 years) 4 , a | BMI categorized according to WHO guidelines | |||
| Normal (18.5–24.9) | Reference | Reference | ||
| Overweight (25–29.9) | 1.34 (1.08–1.66) | 0.29 | 1 | |
| Underweight (<18.5) | 1.36 (1.07–1.73) | 0.31 | 2 | |
| Obese (≥30) | 1.72 (1.45–2.04) | 0.54 | 3 | |
| High cholesterol (<60 years) a | Cholesterol <6.5 mmol/liter | Reference | Reference | |
| Cholesterol >6.5 mmol/liter | 1.71(1.39–2.11) b | 0.54 | 3 | |
| Diabetes 4 , a | History of diabetes | |||
| No diabetes | Reference | Reference | ||
| Diabetes (males) | 1.61(1.42–1.83) | 0.48 | 2 | |
| Diabetes (females) | 1.68 (1.64–1.71) | 0.52 | 3 | |
| Stroke 4 , a | Stroke diagnosis based on ICD | |||
| No stroke | Reference | Reference | ||
| History of stroke (yes) | 1.60 (1.22–2.09) b | 0.47 | 2 | |
| TBI 4 | History of TBI (with and without loss of consciousness) | |||
| No prior TBI | Reference | Reference | ||
| Prior TBI | 1.63 (1.33–2.00) | 0.49 | 2 | |
| Hypertension (>65 years) 4 | All combined high SBP, DBP, and hypertension | 1.31 (1.01–1.07) b | 0.27 | 1 |
| Atrial fibrillation (>65 years) 4 , a | History of atrial fibrillation | |||
| No atrial fibrillation | Reference | Reference | ||
| Atrial fibrillation without stroke | 1.42 (1.17–1.72) | 0.49 | 2 | |
| Insomnia 4 | Clinical diagnosis of insomnia | |||
| No insomnia | Reference | Reference | ||
| Insomnia | 1.53 (1.07–2.18) | 0.43 | 2 | |
| Depression 4 | Centre for Epidemiological Studies Depression (CES‐D) scale | |||
| No depression (CES‐D < = 20) | Reference | Reference | ||
| Depression (CES‐D > 20) | 1.98 (1.50–2.63) | 0.68 | 3 | |
| Physical inactivity 4 | International guidelines for physical activity | |||
| Inactive | Reference | Reference | ||
| Physically active measured as >150 min/week of moderate to vigorous activity | 0.60 (0.51–0.71) b | −0.51 | −3 | |
| Cognitive engagement 13 | Lowest | Reference | Reference | |
| Middle | 0.43 (0.33–0.56) | −0.97 | −5 | |
| Highest | 0.38 (0.24–0.59) | −0.84 | −4 | |
| Social engagement 4 | Loneliness | |||
| Not lonely | Reference | Reference | ||
| Lonely | 1.58 (1.19, 2.09) | 0.46 | 2 | |
| Diet 4 | Fish, 1 serving/week | |||
| Less than 1 serving fish/week | Reference | Reference | ||
| 1 serving/week | 0.95 (0.90–0.99) | −0.05 | −0.25 | |
| Smoking 4 | Never smoked | Reference | Reference | |
| Current smoker | 1.30 (1.18–1.45) | 0.26 | 1 | |
| Former smoker | 1.01 (0.96–1.06) | 0 | 0 |
Effect sizes recalculated, see supporting information for details.
Effect size for AD was also used for any dementia due to classification of exposures being most relevant for risk assessment (e.g., for physical activity the effect size is for adherence to national guidelines whereas for any dementia the available effect size was for “high” and hence not translatable). Hypertension and stroke are other examples where despite the lack of clear effect sizes for any dementia in the review, there is strong evidence in the literature. 14 , 15 This might indicate that existing meta‐analyses may not capture all the relevant literature available highlighting the need to add these risk factors to the risk model.
Abbreviations: AD, Alzheimer's disease; BMI, body mass index; DBP, diastolic blood pressure; ICD, International Classification of Diseases; SBP, systolic blood pressure; TBI, traumatic brain injury; WHO, World Health Organization.
Results for AD are shown in supporting infomation (Figure SA1 and Table SA1). Of the 33 risk factors for AD, 13 met criteria for inclusion in CogDrisk tool for AD (CogDrisk‐AD). We further added one risk factor of social engagement to the list of risk factors for AD, and age and sex, making a total of 16. Figure SA2 in supporting information shows that of the eight risk factors identified for VaD, only four were suitable for inclusion in a tool. Table SA2 in supporting information shows the risk factors included in the CogDrisk compared to risk factors included in the Australian National University's Alzheimer's Disease Risk Index (ANU‐ADRI); Cardiovascular Risk Factors, Aging, and Dementia (CAIDE); and Lifestyle for Brain Health (LIBRA) tools. The main differences between the CogDrisk tool and CogDrisk‐AD are the omission of atrial fibrillation and insomnia from CogDrisk‐AD due to insufficient evidence that they increase the risk of AD, and the inclusion of pesticide exposure in CogDrisk‐AD. The sex‐based weights are also slightly different because of the higher risk of AD among women. 9 A copy of the questionnaire is included in supporting information.
3.2. Construction of the risk algorithm and risk score
Risk algorithms were developed for any dementia to allow for the computation of risk scores for individuals (supporting information Part B). RRs were converted to points that were added to form a risk score. 10 Conditional equations were specified for risk factors that only had an effect in midlife (high cholesterol, obesity and overweight). Sex was included as a conditional factor where RRs were available for males and females. Sex‐specific beta coefficients of age were estimated using recent global prevalence estimates. 9 The final risk factors and weights for inclusion in the risk algorithm are shown in Table 1. A similar process was followed for AD (see Figure SA1 and Table SA1). The CogDrisk dementia score ranges from –4.25 to 45 for late‐life adults and from –8.25 to 28 for midlife adults, with a higher score indicating higher risk. A constant will be added to bring the range from 0 to 49.25 for late‐life adults and 0 to 36.52 for midlife adults. The weights for risk factors for AD are included in supporting information. The CogDrisk‐AD ranges from –3.4 to 43 for late‐life adults and from –8.4 to 26 for midlife adults. After adding a constant, that is 0 to 46.4 for late‐life adults and 0 to 34.4 for midlife adults.
3.3. Creation of the CogDrisk assessment tool
The CogDrisk assessment tool includes ≈90 questions and takes 30 to 40 minutes to complete (see supporting information).
4. DISCUSSION
To our knowledge, CogDrisk includes the largest number of modifiable risk factors for dementia of any existing dementia risk tool, and it also incorporates age group and sex differences. A more comprehensive assessment has greater capacity to identify risk factors relevant to more individuals, enabling preventive advice to be given for a larger group. The actual questionnaire includes items for both AD and any dementia to provide options for use in research or clinical settings. The weights associated with risk factors can also be used in population‐level research to estimate population‐attributable risk using administrative and registry data.
Prior risk assessment tools include fewer risk factors: the CAIDE 11 score assesses 7 risk factors, the LIBRA index 12 assesses 11, and the ANU‐ADRI 13 assesses 15. CogDrisk incorporates all the risk factors included in CAIDE and LIBRA except for coronary heart disease and renal dysfunction, which is only included in the LIBRA scale. In addition, the CogDrisk for any dementia includes social engagement and traumatic brain injury, which have only previously been included in the ANU‐ADRI. Importantly, the CogDrisk tool includes stroke and atrial fibrillation, which have not been included in any previous tool. The LIBRA tool does not include age, sex, or education because it focuses solely on modifiable risk factors. Some authors have argued that a limitation of risk tools is that age and sex account for a large proportion of the predictive power. However, risk reduction advice and interventions differ by age so retention of these variables is useful for developing preventive advice and programs.
A limitation of CogDrisk is the length of the assessment. This is somewhat compensated by the design, which allows for the assessment without involvement of blood or imaging measures. Strengths include the evidence underpinning CogDrisk's development, the inclusion of age‐ and sex‐specific weights for some factors, and that it can be used in low‐resource settings. Future work already in progress will assess the validity of CogDrisk on five external cohort studies from the United States and Sweden that include many of the risk factors. Its predictive accuracy with and without age and sex, and its correlation with biomarkers, will also be examined.
CONFLICTS OF INTEREST
K.J.A. received a speaker honorarium from Nutricia in 2021. There are no other conflicts of interest.
Supporting information
SUPPORTING INFORMATION
SUPPORTING INFORMATION
ACKNOWLEDGMENT
K.J.A. is funded by Australian Research Council Fellowship FL190100011, S.K. is funded by NHMRC GNT1171279, R.E. and R.P. are funded by a NeuRA Discovery Grant. These funding sources had no role in the design, conduct, analysis, interpretation, or decision to publish the project. There is no project‐specific funding.
Anstey KJ, Kootar S, Huque MH, Eramudugolla R, Peters R. Development of the CogDrisk tool to assess risk factors for dementia. Alzheimer's Dement. 2022;14:e12336. 10.1002/dad2.12336
REFERENCES
- 1. WHO. Risk Reduction of Cognitive Decline and Dementia: WHO guidelines Geneva: World Health Organization; 2019. [PubMed]
- 2. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stephan BCM, Pakpahan E, Siervo M, et al. Prediction of dementia risk in low‐income and middle‐income countries (the 10/66 Study): an independent external validation of existing models. Lancet Glob Health. 2020;8:e524‐e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anstey KJ, Ee N, Eramudugolla R, Jagger C, Peters R. A systematic review of meta‐analyses that evaluate risk factors for dementia to evaluate the quantity, quality, and global representativeness of evidence. J Alzheimers Dis. 2019;70:S165‐S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peters R, Dodge H, James S, et al. The epidemiology is promising but the trial evidence is weak. Why pharmacological dementia risk reduction trials haven't lived up to expectations, and where do we go from here? Alzheimers Dementia. 2022;18(3):507‐512. [DOI] [PubMed] [Google Scholar]
- 6. Peters R, Breitner J, James S, et al. Dementia risk reduction: why haven't the pharmacological risk reduction trials worked? An in‐depth exploration of seven established risk factors. Alzheimers Dementia. 2021;7(1):e12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ltd S. StatsDirect statistical software. 2013.
- 8. Kivipelto M, Solomon A. Cholesterol as a risk factor for Alzheimer's disease ‐ epidemiological evidence. Acta Neurol Scand Suppl. 2006;185:50‐57. [DOI] [PubMed] [Google Scholar]
- 9. Huque MH, Eramudugolla R, Chidiac B, et al. Global distribution of sex differences in the incidence and prevalence of any dementia, Alzheimer's disease and vascular dementia: a systematic review and meta‐analysis. Unpublished. 2021. 10.2139/ssrn.3965370 [DOI]
- 10. Austin PC, Lee DS, D'Agostino RB, Fine JP. Developing points‐based risk‐scoring systems in the presence of competing risks. Stat Med. 2016;35:4056‐4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population‐based study. Lancet Neurol. 2006;5:735‐741. [DOI] [PubMed] [Google Scholar]
- 12. Schiepers OJG, Kohler S, Deckers K, et al. Lifestyle for Brain Health (LIBRA): a new model for dementia prevention. Int J Geriatr Psychiatry. 2018;33:167‐175. [DOI] [PubMed] [Google Scholar]
- 13. Anstey KJ, Cherbuin N, Herath PM. Development of a new method for assessing global risk of Alzheimer's disease for use in population health approaches to prevention. Prev Sci. 2013;14:411‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joas E, Bäckman K, Gustafson D, et al. Blood pressure trajectories from midlife to late life in relation to dementia in women followed for 37 years. Hypertension. 2012;59:796‐801. [DOI] [PubMed] [Google Scholar]
- 15. Kuźma E, Lourida I, Moore SF, Levine DA, Ukoumunne OC, Llewellyn DJ. Stroke and dementia risk: a systematic review and meta‐analysis. Alzheimers Dementia. 2018;14:1416‐1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
SUPPORTING INFORMATION


