Abstract
Cultures of Mycobacterium sp. strain PYR-1 were dosed with anthracene or phenanthrene and after 14 days of incubation had degraded 92 and 90% of the added anthracene and phenanthrene, respectively. The metabolites were extracted and identified by UV-visible light absorption, high-pressure liquid chromatography retention times, mass spectrometry, 1H and 13C nuclear magnetic resonance spectrometry, and comparison to authentic compounds and literature data. Neutral-pH ethyl acetate extracts from anthracene-incubated cells showed four metabolites, identified as cis-1,2-dihydroxy-1,2-dihydroanthracene, 6,7-benzocoumarin, 1-methoxy-2-hydroxyanthracene, and 9,10-anthraquinone. A novel anthracene ring fission product was isolated from acidified culture media and was identified as 3-(2-carboxyvinyl)naphthalene-2-carboxylic acid. 6,7-Benzocoumarin was also found in that extract. When Mycobacterium sp. strain PYR-1 was grown in the presence of phenanthrene, three neutral metabolites were identified as cis- and trans-9,10-dihydroxy-9,10-dihydrophenanthrene and cis-3,4-dihydroxy-3,4-dihydrophenanthrene. Phenanthrene ring fission products, isolated from acid extracts, were identified as 2,2′-diphenic acid, 1-hydroxynaphthoic acid, and phthalic acid. The data point to the existence, next to already known routes for both gram-negative and gram-positive bacteria, of alternative pathways that might be due to the presence of different dioxygenases or to a relaxed specificity of the same dioxygenase for initial attack on polycyclic aromatic hydrocarbons.
Anthracene and phenanthrene are tricyclic aromatic hydrocarbons that are found in high concentrations in polycyclic aromatic hydrocarbon (PAH)-contaminated sediments, surface soils, and waste sites. These hydrophobic contaminants are widely distributed in the environment, occurring as natural constituents of fossil fuels and their anthropogenic pyrolysis products (6, 24, 55). Unlike the higher-molecular-weight PAHs, phenanthrene and anthracene do not pose a risk to human health, since they exhibit no genotoxic or carcinogenic effects. However, they have been shown to be toxic to fish and algae (45, 46).
Anthracene and phenanthrene are considered prototypic PAHs and serve as signature compounds to detect PAH contamination, since their chemical structures are found in carcinogenic PAHs, such as benzo[a]pyrene and benz[a]anthracene. They have also been used as model PAHs to determine factors that affect the bioavailability, biodegradation potential, and rate of microbial degradation of PAHs in the environment (5, 6, 24, 48).
A variety of bacterial species have been isolated that have the ability to utilize anthracene or phenanthrene as the sole source of carbon and energy (6, 33, 48). The initial reactions in the degradation of anthracene and phenanthrene are catalyzed by multicomponent dioxygenases that incorporate both atoms of molecular oxygen into the PAH nucleus to produce cis-dihydrodiols (1, 22). Genes involved in PAH metabolism and its regulation have been described for Pseudomonas, Sphingomonas, and Nocardioides species (36, 42, 43, 56–58).
Pseudomonas spp. and Sphingomonas yanoikuyae B1 initially oxidize anthracene in the 1,2 position to form (+)-(1R,2S)-cis-1,2-dihydroxy-1,2-dihydroanthracene, which is subsequently converted to 1,2-dihydroxyanthracene, which is further metabolized to 2-hydroxy-3-naphthoic acid, salicylate, and catechol by enzymes of the naphthalene pathway (1, 11, 12, 22).
Phenanthrene degradation by Pseudomonas species proceeds by two different pathways (1, 11, 12, 22, 32, 36). One is via dioxygenation at the C-3 and C-4 ring positions, to form (+)-(3S,4R)-cis-3,4-dihydroxy-3,4-dihydrophenanthrene (phenanthrene cis-3,4-dihydrodiol). This dihydroxylated intermediate is further metabolized to 1-hydroxy-2-naphthoic acid, with subsequent degradation either through salicylate and catechol or through phthalate and protocatechuate, depending upon the bacterial species. The other pathway involves dioxygenation at the C-1 and C-2 positions to form (+)-(1R,2S)-cis-1,2-dihydroxy-1,2-dihydrophenanthrene (phenanthrene cis-1,2-dihydrodiol). The initial product of enzymatic attack in the 9,10 position, trans-9,10-dihydroxy-9,10-dihydrophenanthrene (phenanthrene trans-9,10-dihydrodiol), has been reported to be produced by Streptomyces flavovirens but not by Pseudomonas spp. (45).
Various Mycobacterium, Nocardia, and Rhodococcus species have the ability to degrade PAHs containing more than two rings (4, 7, 9, 10, 13–17, 23, 25, 26, 34, 35, 40, 41, 44, 49, 51, 53, 54). However, detailed metabolite structure elucidation and degradation pathways for anthracene and phenanthrene catabolism by this group of microorganisms are not well known (4, 16, 41, 50). Mycobacterium species metabolize phenanthrene at different sites of the molecule, presumably via both dioxygenase and monooxygenase attacks on the aromatic nucleus. To our knowledge, the catabolic pathway of anthracene degradation by Mycobacterium species has not been investigated.
Mycobacterium sp. strain PYR-1, which was originally isolated in our laboratory from oil-contaminated estuarine sediment, is capable of mineralizing naphthalene, pyrene, 1-nitropyrene, fluoranthene, phenanthrene, anthracene, and benzo[a]pyrene (7, 17, 19, 21, 26, 27, 39, 52). Biodegradation pathways have been elucidated for the metabolism of naphthalene, pyrene, 1-nitropyrene, and fluoranthene by Mycobacterium sp. strain PYR-1 (6, 20, 21, 26–30). We now propose metabolic pathways for the degradation of anthracene and phenanthrene by Mycobacterium sp. strain PYR-1, based on the identification of initial ring oxidation and ring cleavage products.
MATERIALS AND METHODS
Chemicals.
[9,10-14C]anthracene (58 mCi/mmol) with a radiochemical purity of >98% was purchased from Chemsyn Science Laboratories (Lenexa, Kans.). Unlabeled anthracene (97% pure) was purchased from Aldrich Chemical Co. (Milwaukee, Wis.). Unlabeled phenanthrene and [9,10-14C]phenanthrene (10.9 mCi/mmol) with a radiochemical purity of >99% were purchased from Sigma Chemical Co. (St. Louis, Mo.). Bacteriological media and reagents were purchased from BD Biosciences, Difco Laboratories (Detroit, Mich.). Nuclear magnetic resonance (NMR) solvents were purchased from Isotec, Inc. (Miamisburg, Ohio). Other solvents were purchased from J. T. Baker, Inc. (Phillipsburg, N.J.) and were of the highest purity available.
Culture conditions.
Cultures of Mycobacterium sp. strain PYR-1 were grown in 125-ml Erlenmeyer flasks containing 30 ml of minimal basal salt medium supplemented with 0.38-g/ml concentrations of peptone, yeast extract, and soluble starch. A 15-μl aliquot of phenanthrene or anthracene in N, N-dimethylformamide (12 mg/ml) was added to each flask for enzyme induction. The cultures (A500, 0.38) were incubated for 6 days in the dark at 24°C with shaking at 150 rpm. Phenanthrene or anthracene was dissolved in N, N-dimethylformamide and added to the cultures, making the final concentrations 0.15 and 0.075 mM, respectively. The phenanthrene-dosed cultures were further incubated for 6 h, and those with anthracene were incubated for 24 h.
The contents of each flask were extracted and dried as previously reported (26). The residues were dissolved in 3 ml of methanol and concentrated to approximately 100 μl, using a model SS21 Savant Speed-vac system (Savant Instruments, Holbrook, N.Y.) for analysis by reversed-phase high-pressure liquid chromatography (HPLC).
Radiolabel experiments.
Experiments to determine the degree of mineralization of anthracene and phenanthrene, as evidenced by CO2 evolution, were carried out in 250-ml biometer flasks. A CO2 trap, consisting of 20 ml of 70% ethylene glycol and 30% monoethanolamine, was added to the side arm of each flask. PAH-induced Mycobacterium cells were added to 50 ml of minimal basal salt medium with nutrients. The optical density of the cells at 500 nm was determined immediately using a Beckman DU-7 spectrophotometer (Beckman Instrument Co., Berkeley, Calif.) with a cell-free control as the background. Fifty micrograms of unlabeled anthracene or phenanthrene and 1.0 μCi of either labeled anthracene or phenanthrene were added to each of three flasks. Each flask was immediately sampled for CO2 production by removing 1.0 ml of the trapping solution. This sample was added to 14 ml of Ultima Gold liquid scintillation fluid (Packard Instruments, Downers Grove, Ill.) and counted in a Packard Tri-Carb 2000A scintillation analyzer. A 2.0-ml aliquot of the aqueous portion was removed from each flask, and its optical density was determined. Each sample was extracted with 3 equal volumes of ethyl acetate, acidified to pH 2.5, and extracted three more times. Flasks were also sampled and extracted at 6, 24, 48, 72, 168, 192, and 312 h.
Each of the radiolabeled extracts from both experiments was dissolved in a small amount of methanol and analyzed by HPLC.
Physical and chemical analysis.
Anthracene, phenanthrene, and their metabolites were separated by HPLC using a Hewlett-Packard model 1050 pump system (Hewlett-Packard, Palo Alto, Calif.) with a Hewlett-Packard diode array model 1040A detector at 254 nm and a 4.6- by 250-mm 5-μm C18 Inertsil ODS-3 column (MetaChem Technologies, Torrance, Calif.) at a flow rate of 1 ml/min. UV absorbance spectra were obtained online. The compounds were eluted using a linear gradient of 40 to 95% methanol/water over 40 min. For collection of larger amounts of metabolites, a Beckman model 100A dual pump system equipped with a Beckman model 160 absorbance detector (Beckman Instruments, Inc., Fullerton, Calif.), a Waters 486 tunable UV absorbance detector (Waters Corp., Milford, Mass.), and an Inertsil ODS-3 10.0- by 250-mm column (MetaChem) were used. The mobile phase was the same as that described above but with a flow rate of 5 ml/min.
Probe mass spectra were obtained on a TSQ 700 triple-quadrupole mass spectrometer (Finnigan Corp., San Jose, Calif.) using a direct exposure probe and electron ionization (EI). Gas chromatography-mass spectrometry (MS) analyses were performed on a model 4500 quadrupole mass spectrometer (Finnigan Corp.) and model 3400 (Varian, Inc., Sunnyvale, Calif.) gas chromatograph. Chromatography was achieved on a DB-1 fused silica capillary column (J & W Scientific, Folsom, Calif.).
Further MS experiments were performed using a Platform single-quadrupole instrument (Micromass, Manchester, United Kingdom) equipped with an atmospheric pressure chemical ionization (APCI) interface. The total liquid chromatography (LC) column effluent was delivered into the atmospheric pressure ion source through a heated nebulizer probe (450°C), using nitrogen as the probe and bath gas (275 liters/h) with an ion source temperature of 150°C. Positive or negative ion spectra were acquired in full-scan mode (m/z of 100 to 400, 1.0-s cycle time) in series with a UV detector at 254 nm. At low cone voltage (15 to 20 V), the positive and negative ion mass spectra of the PAH metabolites predominantly consisted of protonated and deprotonated molecules, respectively. When further fragmentation was required, a higher cone voltage was used (60 V). PAH metabolite sample extracts, dissolved in starting mobile phase and prepared as described above, were injected into the LC-MS system.
NMR spectra were recorded at 500.13 MHz (1H) and 125.77 MHz (13C) on a Bruker AM500 spectrometer (Bruker Instruments, Billerica, Mass.). The metabolites were dissolved in 0.5 ml of deuterated acetone (99.96 atom% 2H), except where otherwise noted. 1H chemical shifts are reported on the δ scale (parts per million) by assigning the residual solvent peak to 2.04 ppm. Typical 1H data acquisition parameters were as follows: data size, 32,000; sweep width, 7,042 Hz; filter width, 8,900 Hz; acquisition time, 2.33 s; flip angle, 90°; relaxation delay, 0 s; temperature, 298.5 K. For spectra recorded under quantitative conditions, a 10- to 20-s relaxation delay was used. For measurement of coupling constants, the free induction decay was zero-filled to 64,000, resulting in a final data point resolution of 0.215 Hz per point. Coupling constants reported are first order. Those that were non-first order and those of overlapping resonances were omitted. Assignments were made from homonuclear decoupling experiments, nuclear Overhauser effect (NOE) experiments, integration, analysis of substituent effects, and comparison to spectra of authentic compounds. A 13C NMR spectrum was obtained for one metabolite (data not shown). The sample was dissolved in 0.5 ml of deuterated methanol (99.96 atom% 2H), and the residual methanol resonance was assigned as 49.0 ppm.
RESULTS
Mineralization of anthracene and phenanthrene.
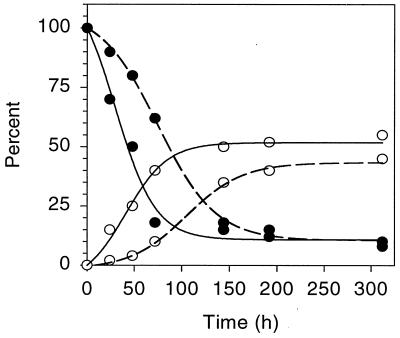
Mycobacterium sp. strain PYR-1, when grown in the presence of anthracene and phenanthrene for 14 days, degraded 92 and 90% of these tricyclic PAHs, respectively. Both PAHs were mineralized; the amounts of CO2 evolved are shown in Fig. 1. The percentages of [14C]anthracene and [14C]phenanthrene evolved as 14CO2 were 45 and 52%, respectively, after 6 days. A lag period of 48 h was observed before significant mineralization of anthracene occurred (Fig. 1).
FIG. 1.
Degradation (●) and mineralization (○) of anthracene (dashed line) and phenanthrene (solid line) by Mycobacterium sp. strain PYR-1.
Identification of phenanthrene degradation products.
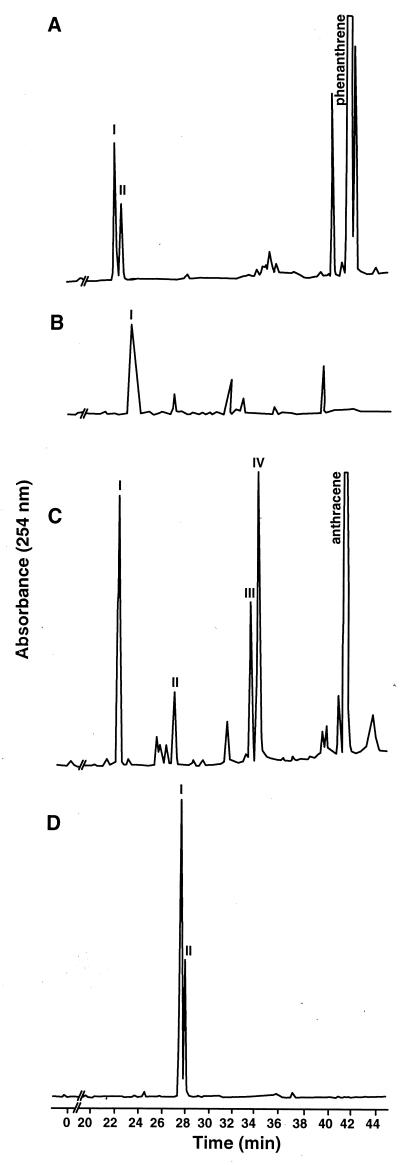
HPLC analysis of the neutral extract from phenanthrene incubations produced two metabolites as shown in Fig. 2A. The EI mass spectrum of compound I, eluting at 21.6 min with λmax of 202 and 278 nm, contained a base peak at an m/z of 212, the molecular ion [M+.]. Fragment ions at m/z values of 194 [M-18]+, 166 [M-18-28]+, and 165 [M-18-29]+ are characteristic of a dihydrodiol. A fragment ion at an m/z of 181 possibly indicated the loss of CH2-OH from the molecular ion. The mass and 1H NMR spectra are consistent with those previously reported for cis-9,10-dihydroxy-9,10-dihydrophenanthrene (phenanthrene cis-9,10-dihydrodiol). The NMR assignments and coupling constants are as follows: 7.57 (H1,8; J1,2 =7.5 Hz, J1,3 = 1.5 Hz), 7.31 (H2,7; J2,3 = 7.5 Hz, J2,4 = 1.5 Hz), 7.37 (H3,6; J3,4 = 8.0 Hz), 7.84 (H4,5), 4.75 (H9,10). The chemical shift of the H9,10 resonance showed that the compound was the cis- rather than the trans-isomer, because the resonance is at 4.60 ppm in the trans-isomer (37).
FIG. 2.
HPLC elution profile of metabolites produced during the growth of Mycobacterium sp. strain PYR-1 in the presence of phenanthrene and anthracene. (A) Ethyl acetate-extractable metabolites from phenanthrene-grown cultures. (B) Ethyl acetate-extractable metabolites from the acidified aqueous phase of phenanthrene-grown cultures. (C) Ethyl acetate-extractable metabolites from anthracene-grown cultures. (D) Ethyl acetate-extractable metabolites from the acidified aqueous phase of anthracene-grown cultures.
The EI mass spectrum of compound II (22.1 min, λmax = 202, 260, and 268 nm) had a molecular ion at an m/z of 212 and characteristic dihydrodiol fragment ions at m/z values of 194, 166, and 165. Additional fragment ions at m/z values of 168 and 140 were present at lower intensities than in the mass spectrum of metabolite I, indicating that II was a different dihydrodiol. The aromatic region of the 1H NMR spectrum of the metabolite contained 10 resonances belonging to one compound, with four of the resonances exhibiting large upfield shifts. NOE and homonuclear decoupling experiments proved that the substitution was at C-3 and C-4. The small coupling constant (J3,4 = 5.6 Hz) is consistent with a cis conformation. Compound II was identified as cis-3,4-dihydroxy-3,4-dihydrophenanthrene (phenanthrene cis-3,4-dihydrodiol). NMR assignments and coupling constants were as follows: 6.51 (H1; J1,2 = 9.6 Hz, J1,3 = 2.8 Hz), 5.97 (H2; J2,3 = 1.7 Hz, J2,4 = 1.4 Hz), 4.63 (H3; J3,4 = 5.2 Hz), 5.28 (H4), 8.24 (H5; J5,6 = 8.7 Hz), 7.54 (H6; J6,7 = 7.7 Hz, J6,8 = 1.4 Hz), 7.45 (H7; J7,8 = 8.3 Hz, J7,9 = 1.2 Hz), 7.85 (H8), 7.83 (H9; J9,10 = 8.3 Hz), and 7.31 (H10). A second compound apparent in the NMR spectrum of compound II was identified as trans-9,10-dihydroxy-9,10-dihydrophenanthrene (phenanthrene trans-9,10-dihydrodiol) by comparing its chemical shifts and coupling constants—7.70 (H1,8; J1,2 = 6.7 Hz, J1,3 = 2.2 Hz), 7.35 (H2,3,6,7; J2,4 = 1.7 Hz, J3,4 = 7.1 Hz), 7.79 (H4,5), and 4.60 (H9,10)—to those in the literature. The chemical shift of H9,10 was the basis for the trans determination (3, 37, 45, 47).
The phenanthrene cis-3,4-and 9,10-dihydrodiols were also detected by online HPLC with APCI/MS, which gave negative ion mass spectra with [M-H-H2O]− at an m/z of 193 and LC retention times of 14.3 and 15.0 min, respectively. The amounts of both the cis-3,4-and 9,10-dihydrodiols increased following 4 to 8 h of incubation. However, between 8 and 32 h of incubation, the compounds were totally degraded.
One acid-extractable metabolite eluting at 23.3 min with a λmax of 214 was detected by HPLC analysis (Fig. 2B). The EI mass spectrum of the metabolite consisted of a molecular ion at an m/z of 242 and characteristic fragment ions at m/z values of 197 and 153 resulting from consecutive losses of CO2H and CO2. The 1H NMR spectrum—7.72 (H1,8; J1,2 = 7.5 Hz, J1,3 = 1.7 Hz), 7.38 (H2,7; J2,3 = 7.5 Hz, J2,4 = 1.7 Hz), 7.40 (H3,6; J3,4 = 7.5 Hz), 7.05 (H4,5)—was the same as that of authentic 2,2′-diphenic acid (3). The metabolite began to accumulate at 8 h after incubation, and the concentration remained essentially unchanged after 96 h.
The parent compound, phenanthrene, was eluted at 41.9 min.
Two other ring fission products were detected by gas chromatography-mass spectrometry analysis of the acidified aqueous-phase extracts. Gas chromatography-mass spectrometry analysis of methyl esters of the acid-extractable material gave retention times and EI mass spectra consistent with 1-hydroxynaphthoic acid (9.55 min, M+· = m/z of 216) and phthalic acid (4.15 min, M+· = m/z of 194).
Identification of anthracene degradation products.
HPLC analysis of the neutral extract from anthracene incubation (Fig. 2C) produced four metabolites that were eluted at 22.9, 27.6, 34.2, and 34.9 min. The EI mass spectrum of compound I (retention time = 22.9 min, λmax = 204, 252, 296, and 306 nm) from the neutral extract of anthracene consisted of a molecular ion at an m/z of 212 and characteristic fragment ions at m/z values of 194, 166, and 165 resulting from losses of H2O and then either CO or HCO. The fragment ions at m/z values of 168 and 140 indicate substitution on the first aromatic ring. The 1H NMR assignments and coupling constants are as follows: 4.75 (H1; J1,2 = 4.7 Hz), 4.35 (H2; J2,3 = 4.5 Hz), 6.09 (H3; J3,4 = 9.7 Hz), 6.69 (H4), 7.82 (H5,8), 7.44 (H6,7), 7.94 (H9), and 7.58 (H10). The H1 and H2 resonances of the 1H NMR spectrum had chemical shifts that were characteristic of a dihydrodiol. NOE experiments were performed to make resonance assignments by irradiating H1 and H4 to produce enhancements at H9 and H10, respectively. The final NOE experiments were performed on the two singlets at 7.94 and 7.58 ppm (H9,10). They each produced an enhancement of the multiplet at 7.82 ppm (H5,8). Homonuclear decoupling experiments showed that multiplet to be coupled to the one at 7.44 ppm (H6,7). Further decoupling experiments were used to assign H2 and H3. The metabolite was identified as cis-1,2-dihydroxy-1,2-dihydroanthracene (anthracene cis-1,2-dihydrodiol) by MS and 1H NMR spectrometry and by comparison to data previously published (1, 8, 22, 46).
Compound II was eluted at 27.6 min and had λmax values of 202, 232, 274, 284, and 328 nm. Its EI mass spectrum consisted of a molecular ion at an m/z of 196 and fragment ions at m/z values of 168 and 140 resulting from consecutive losses of CO. The 1H NMR assignments and coupling constants were 6.47 (H3; J3,4 = 9.7 Hz), 8.12 (H4; J3,4 = 9.7 Hz), 8.03 (H5; J5,6 = 8.4 Hz), 7.54 (H6; J6,7 = 7.7 Hz, J6,8 = 1.3 Hz), 7.62 (H7; J7,8 = 8.6 Hz, J7,9 = 1.3 Hz), 8.00 (H8), 7.79 (H9), 8.27 (H10). Homonuclear decoupling and NOE experiments allowed resonance assignments and showed substitutions at C-1 and C-2. Comparison of the H3 and H4 chemical shifts of compound II to those reported for coumarin (38) indicated that the compound was 6,7-benzocoumarin. After 72 h of incubation, 6,7-benzocoumarin could not be detected, suggesting that it is a transient intermediate and a substrate for ring fission enzymes.
Compound III was eluted at 34.2 min with λmax values of 204 and 266 nm. The EI mass spectrum of compound III had an apparent molecular ion at an m/z of 224 and strong fragment ions at m/z values of 209, 181, and 152 that may be attributed to sequential losses of CH3 and CO or to the loss of C3H4O2. The 1H NMR chemical shifts and coupling constants were assigned as follows: 4.00 (CH3), 7.78 (H3; J3,4 = 9.0 Hz), 7.26 (H4), 8.05 (H5; J5,6 = 8.2 Hz, J5,7 = 1.5 Hz), 7.46 (H6; J6,7 = 7.3 Hz, J6,8 = 1.5 Hz), 7.41 (H7; J7,8 = 8.2 Hz), 8.00 (H8), 8.53 (H9), and 8.45 (H10). The sharp singlet at 4.00 ppm in the NMR spectrum was characteristic of a methoxyl group. When irradiated, the singlet produced an NOE to the aromatic singlet at 8.53 ppm (H9), indicating that the methoxyl group was attached to C-1. Other proton assignments were made from NOE and homonuclear decoupling experiments. When the metabolite was dissolved in deuterated methylene chloride, the exchangeable hydroxyl proton was observed at 5.91 ppm. Metabolite III was identified as 1-methoxy-2-hydroxyanthracene. The concentration of 1-methoxy-2-hydroxyanthracene remained constant during the incubation period, suggesting that it is a dead-end metabolite.
Compound IV (retention time = 34.9 min, λmax = 204, 260, and 334 nm) had an EI molecular ion at an m/z of 208 and fragment ions at m/z values of 180 and 152, representing successive losses of CO. The 1H NMR spectrum consisted of two resonances at 8.29 and 7.94 ppm, consistent with that of authentic 9,10-anthraquinone (46), and compound IV was identified as such.
Anthracene was eluted at 42.4 min.
Negative- and positive-ion APCI mass spectra and HPLC retention times were consistent with the formation of anthracene cis-1,2-dihydrodiol ([M-H-H2O]−, m/z = 193, retention time = 7.3 min), 9,10-anthraquinone ([M−⋅], m/z = 208, retention time = 27.7 min), and 6,7-benzocoumarin ([M+H]+, m/z = 197, retention time = 17.8 min).
The acid extract from the aqueous phase yielded two ring fission metabolites (Fig. 3D), eluting at 27.1 and 27.9 min. Compound I was identified as 6,7-benzocoumarin by comparison of its NMR spectrum to that of 6,7-benzocoumarin collected from the neutral extract.
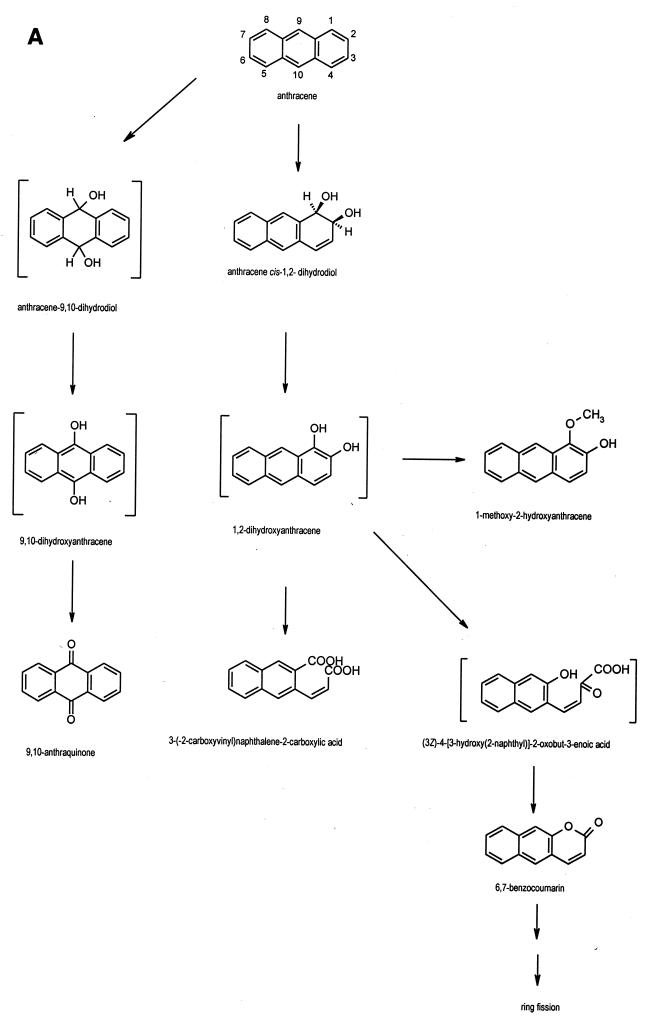
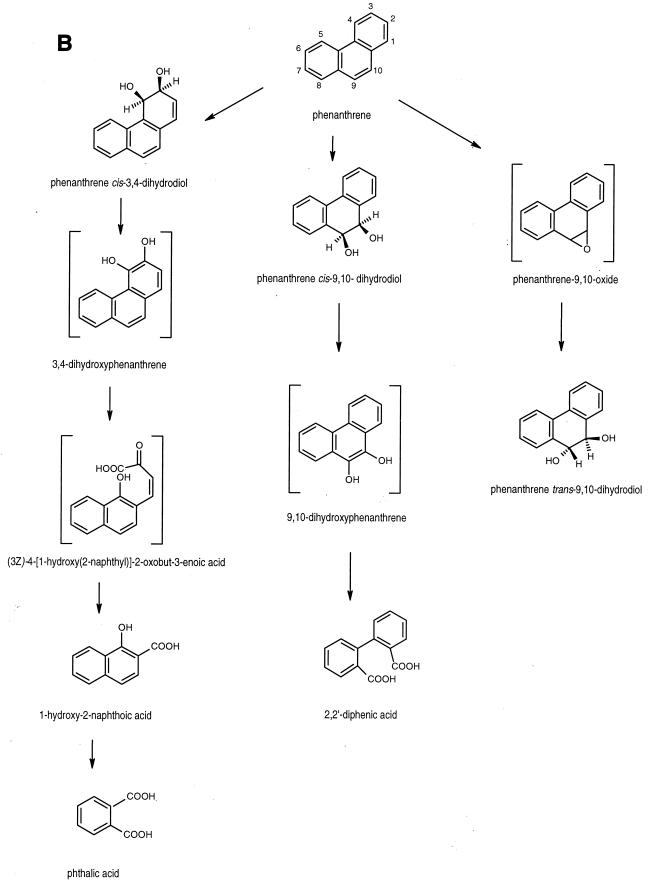
FIG. 3.
Proposed pathways for the degradation of anthracene (A) and phenanthrene (B) by Mycobacterium sp. strain PYR-1.
Compound II was dissolved in deuterated methanol for NMR analysis. Only eight aromatic resonances were present in the 1H NMR spectrum (6.56 [H3; J3,4 = 15.9 Hz], 8.09 [H4], 7.84 [H5], 7.44 [H6,7], 7.81 [H8], 7.96 [H9], 8.10 [H10]) of compound II (λmax = 266 nm), with a coupling pattern consistent with either substitution or ring cleavage at the C-1 and C-2 positions. Assignments of proton resonances 3 through 10 were made from decoupling and NOE measurements. The proton-decoupled 13C NMR spectrum of the metabolite had 12 aromatic resonances; 4 of the resonances were from quaternary carbons. The resonances at 170.32 and 177.59 ppm were consistent with the chemical shifts of carbons in carboxylic acid groups.
Compound II was eluted with a retention time of 26.2 min using APCI/MS to produce diagnostic fragment ions in addition to molecular species. The negative-ion mass spectrum acquired at 25 V contained ions corresponding to the deprotonated molecule (M-H)− at an m/z of 241. Other diagnostic ions present were (M-CO2-H)− at an m/z of 197 and (M-2CO2-H)− at an m/z of 153, indicating sequential losses of the acid moieties. Based on the NMR and APCI/MS data, compound II was identified as 3-(2-carboxyvinyl)naphthalene-2-carboxylic acid. This compound was formed after 8 h of incubation and remained constant throughout the incubation period. A minor metabolite that eluted at 20.8 min had an identical mass spectrum, suggesting the presence of an isomer of compound II also having two acid moieties. The isomer was not characterized by NMR due to quantity limitations.
DISCUSSION
The results show that Mycobacterium sp. strain PYR-1 extensively metabolized anthracene and phenanthrene. The isolation and characterization of the major initial oxidation and ring fission products indicated multiple routes of enzymatic attack. The degradation pathways of anthracene and phenanthrene by Mycobacterium sp. strain PYR-1 are proposed in Fig. 3.
Mycobacterium sp. strain PYR-1 oxidized anthracene to anthracene cis-1,2-dihydrodiol in a reaction similar to those previously reported for anthracene degradation by Pseudomonas and Sphingomonas species (1, 22). The enzymatic attack in the C-1 and C-2 positions of the anthracene moiety was similar to the naphthalene dioxygenase pathways previously reported in Mycobacterium sp. strain PYR-1 (28). The resulting anthracene cis-dihydrodiol was dehydrogenated to 1,2-dihydroxyanthracene. The accumulation of 1-methoxy-2-hydroxyanthracene provided further evidence for the dioxygenation of anthracene by Mycobacterium sp. strain PYR-1. This is a novel metabolite for anthracene biodegradation studies; however, methylation of a dihydroxylated PAH intermediate was found previously in Mycobacterium sp. strain PYR-1 with the formation of 8-hydroxy-7-methoxyfluoranthene during the metabolism of fluoranthene (30). Kinetic studies indicate that these methoxylated derivatives are dead-end metabolites. The isolation of 6,7-benzocoumarin suggests that (3Z)-4-[3-hydroxy(2-naphthyl)]-2-oxobut-3-enoic acid was formed as a ring fission product of 1,2-dihydroxyanthracene. Previously, scholars reported the identification of 6,7-benzocoumarin in the degradation of anthracene by S. yanoikuyae B1 (31). A novel ring fission product, 3-(2-carboxyvinyl)naphthalene-2-carboxylic acid, was also identified in the present investigation. ortho-Ring cleavage of 1,2-dihydroxyanthracene could lead to the formation of 3-(2-carboxyvinyl)naphthalene-2-carboxylic acid. A minor amount of an isomer of this ortho-ring cleavage product was also detected in cultures of Mycobacterium sp. strain PYR-1, which suggests that dioxygenation could also occur in the C-2 and C-3 positions of anthracene. The detection of these ortho-cleavage ring fission products is analogous to evidence in a recent report on naphthalene degradation in Bacillus thermoleovorans (2). An alternate route of enzymatic attack by Mycobacterium sp. strain PYR-1 is in the C-9 and C-10 positions of anthracene. The presence of the dead-end product 9,10-anthraquinone could be explained by the formation and nonenzymatic oxidation of 9,10-dihydroxyanthracene (Fig. 3).
Based on rigorous chemical structure determination for the identification of cis-3,4- and 9,10-dihydrodiols, trans-9,10-dihydrodiol, and ring fission products formed from phenanthrene by Mycobacterium sp. strain PYR-1, at least three pathways are evident in the degradation of phenanthrene. As shown in an earlier study on pyrene catabolism (20), phenanthrene was metabolized by Mycobacterium sp. strain PYR-1 with initial attack in the K region to form the cis- and trans-9,10-dihydrodiols. Furthermore, the formation of 2,2′-diphenic acid during phenanthrene degradation is analogous to the formation of 4,5-phenanthrene dicarboxylic acid during pyrene degradation (10, 20, 44). Therefore, it is likely that the same mono- and dioxygenases and ortho-cleavage enzymes are involved in initial K region attack and subsequent ring fission of the dihydroxylated intermediates of phenanthrene and pyrene. Interestingly, dioxygenase attack also occurred at the C-3 and C-4 positions of phenanthrene to form a cis-3,4-dihydrodiol. This was followed by dehydrogenation to form 3,4-dihydroxyphenanthrene and then by meta-cleavage to form 1-hydroxy-2-naphthoic acid. This degradation pathway is similar to the phthalate pathway in other bacterial strains (32, 42). The isolation of phenanthrene trans-9,10-dihydrodiol indicates a monooxygenase attack on the phenanthrene nucleus to form phenanthrene 9,10-epoxide, followed by epoxide hydrolase to form the trans-dihydrodiol. This mode of enzymatic attack is similar to what was found in previous studies on the naphthalene and pyrene degradation pathways (20, 28). This work confirms and extends the catabolic pathways previously proposed for phenanthrene degradation by Mycobacterium species. We note that Mycobacterium sp. strains BG1 and BB1 degrade phenanthrene via 1-hydroxy-2-naphthoic acid and then via the meta-cleavage of protocatechuate (4, 16); however, no data have been provided for the initial oxidation reactions.
Rehmann et al. (41) showed that Mycobacterium sp. strain KR2 metabolizes phenanthrene in the 3,4 and 9,10 positions to form cis-3,4- and 9,10-dihydrodiols. 1-Hydroxy-2-naphthoic acid, phthalic acid, and 2-carboxybenzaldehyde were isolated from the culture filtrate, suggesting that ring fission pathways in Mycobacterium sp. strain KR-2 are similar to those previously reported for gram-negative bacteria. The degradation pattern of Mycobacterium sp. strain PYR-1 was similar to that of Mycobacterium sp. strain KR-2, except that phenanthrene trans-9,10-dihydrodiol was not detected using KR-2.
Recently, Tongpim and Pickard (50) reported that Mycobacterium sp. strain S1 grown on commercial anthracene, which contained phenanthrene as an impurity, formed phenanthrene trans-9,10-dihydrodiol. NMR analysis was not conducted on the metabolite to rigorously confirm that the phenanthrene 9,10-dihydrodiol was the trans and not the cis-isomer, although the trans-isomer assignment was supported by cytochrome P450 inhibitor experiments. In the present investigation, we show that both the cis and trans-9,10-dihydrodiols were produced by Mycobacterium sp. strain PYR-1.
Data from this investigation and previous studies of the degradation of PAHs by Mycobacterium sp. strain PYR-1 suggest that both dioxygenases and monooxygenases catalyze the initial attack on the aromatic ring. Since positional isomers of cis-dihydrodiols are formed, it may also be suggested that several dioxygenases are present in Mycobacterium sp. strain PYR-1. The broad range of PAHs that are degraded by Mycobacterium sp. strain PYR-1 may also indicate a relaxed specificity of the same dioxygenase for initial attack on PAHs. It is interesting that the ortho- and meta-ring fission pathways have similarities to, but also differences from, those known for other bacterial strains. The identification of ortho-ring cleavage intermediates from the degradation of dihydroxylated metabolites of anthracene, phenanthrene, and pyrene (20) indicates alternative enzymatic routes in the degradation of PAHs by Mycobacterium sp. strain PYR-1. Since, to date, all evidence on the catabolic pathways has been based on the identification of initial aromatic ring oxidation and ring fission products, it would be speculative to conclude what the oxygenation mechanisms in Mycobacterium sp. strain PYR-1 are without biochemical and molecular genetic analysis of the biodegradation pathways.
ACKNOWLEDGMENTS
We thank John B. Sutherland and Thomas M. Heinze for critical review of the manuscript. We also thank Pat Fleischer for clerical assistance and Mona I. Churchwell for technical assistance.
Part of this work was supported by Cooperative Agreement CR820773 from the U.S. Environmental Protection Agency.
REFERENCES
- 1.Akhtar N M, Boyd D R, Thompson M J, Koreeda M, Gibson D T, Mahadevan V, Jerina D M. Absolute stereochemistry of the dihydroanthracene-cis- and trans-1,2-diols produced from anthracene by mammals and bacteria. J Chem Soc Perkin Trans I. 1975;1:2506–2511. [PubMed] [Google Scholar]
- 2.Annweiler E, Richnow H H, Antranikian G, Hebenbrock S, Garms C, Franke S, Franke W, Michaelis W. Naphthalene degradation and incorporation of naphthalene-derived carbon into biomass of the thermophile Bacillus thermoleovorans. Appl Environ Microbiol. 2000;66:518–523. doi: 10.1128/aem.66.2.518-523.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bezalel L, Hadar Y, Fu P P, Freeman J P, Cerniglia C E. Metabolism of phenanthrene by the white rot fungus Pleurotus ostreatus. Appl Environ Microbiol. 1996;62:2547–2553. doi: 10.1128/aem.62.7.2547-2553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boldrin B, Thiem A, Fritsche C. Degradation of phenanthrene, fluorene, fluoranthene, and pyrene by a Mycobacterium sp. Appl Environ Microbiol. 1993;59:1927–1930. doi: 10.1128/aem.59.6.1927-1930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouchez M, Blanchet D, Vandecastelle J P. Degradation of polycyclic aromatic hydrocarbons by pure strains and by defined strain associations: inhibition phenomena and cometabolism. Appl Microbiol Biotechnol. 1995;43:156–164. doi: 10.1007/BF00170638. [DOI] [PubMed] [Google Scholar]
- 6.Cerniglia C E. Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation. 1992;3:351–368. [Google Scholar]
- 7.Cerniglia C E, Heitkamp M A. Polycyclic aromatic hydrocarbon degradation by Mycobacterium. Methods Enzymol. 1990;188:148–153. doi: 10.1016/0076-6879(90)88027-8. [DOI] [PubMed] [Google Scholar]
- 8.Cerniglia C E, Yang S K. Stereoselective metabolism of anthracene and phenanthrene by the fungus Cunninghamella elegans. Appl Environ Microbiol. 1984;47:119–124. doi: 10.1128/aem.47.1.119-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Churchill S A, Harper J P, Churchill P F. Isolation and characterization of a Mycobacterium species capable of degrading three- and four-ring aromatic and aliphatic hydrocarbons. Appl Environ Microbiol. 1999;63:549–552. doi: 10.1128/aem.65.2.549-552.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean-Ross D, Cerniglia C E. Degradation of pyrene by Mycobacterium flavescens. Appl Microbiol Biotechnol. 1996;46:307–312. doi: 10.1007/s002530050822. [DOI] [PubMed] [Google Scholar]
- 11.Evans W C, Fernley H N, Griffiths E. Oxidative metabolism of phenanthrene and anthracene by soil pseudomonads. Biochem J. 1965;95:819–831. doi: 10.1042/bj0950819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernley H N, Griffiths E, Evans W C. Oxidative metabolism of phenanthrene and anthracene by soil bacteria: the initial ring-fission step. Biochem J. 1964;91:15p–16p. doi: 10.1042/bj0950819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fritsche C. Degradation of pyrene at low defined oxygen concentrations by a Mycobacterium sp. Appl Environ Microbiol. 1994;60:1687–1689. doi: 10.1128/aem.60.5.1687-1689.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grosser R J, Warshawsky D, Vestal J R. Endogenous and enhanced mineralization of pyrene, benzo[a]pyrene, and carbazole in soils. Appl Environ Microbiol. 1991;57:3462–3469. doi: 10.1128/aem.57.12.3462-3469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grosser R J, Warshawsky D, Vestal J R. Mineralization of polycyclic and N-heteropolycyclic aromatic compounds in hydrocarbon-contaminated soils. Environ Toxicol Chem. 1995;14:375–382. [Google Scholar]
- 16.Guerin W, Jones G E. Mineralization of phenanthrene by a Mycobacterium sp. Appl Environ Microbiol. 1988;54:937–944. doi: 10.1128/aem.54.4.937-944.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heitkamp M A, Cerniglia C E. Mineralization of polycyclic aromatic hydrocarbons by a bacterium isolated from sediment below an oil field. Appl Environ Microbiol. 1988;54:1612–1614. doi: 10.1128/aem.54.6.1612-1614.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heitkamp M A, Cerniglia C E. Polycyclic aromatic hydrocarbon degradation by a Mycobacterium sp. in microcosms containing sediment and water from a pristine ecosystem. Appl Environ Microbiol. 1989;55:1968–1973. doi: 10.1128/aem.55.8.1968-1973.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heitkamp M A, Franklin W, Cerniglia C E. Microbial metabolism of polycyclic aromatic hydrocarbons: isolation and characterization of a pyrene-degrading bacterium. Appl Environ Microbiol. 1988;54:2549–2555. doi: 10.1128/aem.54.10.2549-2555.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heitkamp M A, Freeman J P, Miller D W, Cerniglia C E. Pyrene degradation by a Mycobacterium sp.: identification of ring oxidation and ring fission products. Appl Environ Microbiol. 1988;54:2556–2565. doi: 10.1128/aem.54.10.2556-2565.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heitkamp M A, Freeman J P, Miller D W, Cerniglia C E. Biodegradation of 1-nitropyrene. Arch Microbiol. 1991;156:223–230. doi: 10.1007/BF00249119. [DOI] [PubMed] [Google Scholar]
- 22.Jerina D M, Selander H, Yagi H, Wells M C, Davey J F, Mahadevan V, Gibson D T. Dihydrodiols from anthracene and phenanthrene. J Am Chem Soc. 1976;98:5988–5996. doi: 10.1021/ja00435a035. [DOI] [PubMed] [Google Scholar]
- 23.Jimenez I Y, Bartha R. Solvent-augmented mineralization of pyrene by a Mycobacterium sp. Appl Environ Microbiol. 1996;62:2311–2316. doi: 10.1128/aem.62.7.2311-2316.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanaly R A, Harayama S. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J Bacteriol. 2000;182:2059–2067. doi: 10.1128/jb.182.8.2059-2067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kästner M, Breuer-Jammali M, Mahro B. Enumeration and characterization of the soil microflora from hydrocarbon-contaminated soil sites able to mineralize polycyclic aromatic hydrocarbons (PAH) Appl Microbiol Biotechnol. 1994;41:267–273. [Google Scholar]
- 26.Kelley I, Cerniglia C E. The metabolism of fluoranthene by a species of Mycobacterium. J Ind Microbiol. 1991;7:19–26. [Google Scholar]
- 27.Kelley I, Cerniglia C E. Degradation of a mixture of high-molecular-weight polycyclic aromatic hydrocarbons by a Mycobacterium strain PYR-1. J Soil Contam. 1995;4:44–91. [Google Scholar]
- 28.Kelley I, Freeman J P, Cerniglia C E. Identification of metabolites from degradation of naphthalene by a Mycobacterium sp. Biodegradation. 1990;1:283–290. doi: 10.1007/BF00119765. [DOI] [PubMed] [Google Scholar]
- 29.Kelley I, Freeman J P, Evans F E, Cerniglia C E. Identification of a carboxylic acid metabolite from the catabolism of fluoranthene by a Mycobacterium sp. Appl Environ Microbiol. 1991;57:636–641. doi: 10.1128/aem.57.3.636-641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelley I, Freeman J P, Evans F E, Cerniglia C E. Identification of metabolites from the degradation of fluoranthene by Mycobacterium sp. strain PYR-1. Appl Environ Microbiol. 1993;59:800–806. doi: 10.1128/aem.59.3.800-806.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim E, Zylstra G J, Freeman J P, Heinze T M, Deck J, Cerniglia C E. Evidence for the role of 2-hydroxychromene-2-carboxylate isomerase in the degradation of anthracene by Sphingomonas yanoikuyae B1. FEMS Microbiol Lett. 1997;153:479–484. doi: 10.1111/j.1574-6968.1997.tb12613.x. [DOI] [PubMed] [Google Scholar]
- 32.Kiyohara H, Nagao K, Nomi R. Degradation of phenanthrene through o-phthalate by an Aeromonas sp. Agric Biol Chem. 1976;40:1075–1082. [Google Scholar]
- 33.Kiyohara H, Nagao K, Yana K. Rapid screen for bacteria degrading water-insoluble, solid hydrocarbons on agar plates. Appl Environ Microbiol. 1982;43:454–457. doi: 10.1128/aem.43.2.454-457.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleespies M, Kroppenstedt R M, Rainey F A, Webb L E, Stackebrandt E. Mycobacterium hodleri, sp. nov., a new member of the fast-growing mycobacteria capable of degrading polycyclic aromatic hydrocarbons. Int J Syst Bacteriol. 1996;46:683–687. doi: 10.1099/00207713-46-3-683. [DOI] [PubMed] [Google Scholar]
- 35.Lloyd-Jones G, Hunter D W F. Characterization of fluoranthene- and pyrene-degrading mycobacterium-like strains by RAPD and SSU sequencing. FEMS Microbiol Lett. 1997;153:51–56. doi: 10.1111/j.1574-6968.1997.tb10462.x. [DOI] [PubMed] [Google Scholar]
- 36.Menn F, Applegate B M, Sayler G S. NAH plasmid-mediated catabolism of anthracene and phenanthrene by naphthoic acids. Appl Environ Microbiol. 1993;59:1938–1942. doi: 10.1128/aem.59.6.1938-1942.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narro L M, Cerniglia C E, van Baalen C, Gibson D T. Metabolism of phenanthrene by the marine cyanobacterium Agmenellum quadruplicatum PR-6. Appl Environ Microbiol. 1992;58:1351–1359. doi: 10.1128/aem.58.4.1351-1359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pouchert C J, Behnke J, editors. The Aldrich library of 13C and 1H FT NMR spectra. 1st ed. Vol. 2. Milwaukee, Wis: Aldrich Chemical Company, Inc.; 1993. p. 1311B. [Google Scholar]
- 39.Rafii F, Butler W R, Cerniglia C E. Differentiation of a rapidly growing, scotochromogenic, polycyclic-aromatic-hydrocarbon-metabolizing strain of Mycobacterium sp. from other known Mycobacterium species. Arch Microbiol. 1992;157:512–520. [Google Scholar]
- 40.Rehmann K, Noll H P, Steinberg C E W, Kettrup A A. Pyrene degradation by Mycobacterium sp. strain KR2. Chemosphere. 1998;36:2977–2992. doi: 10.1016/s0045-6535(97)10240-5. [DOI] [PubMed] [Google Scholar]
- 41.Rehmann K, Steinberg C E W, Kettrup A A. Branched metabolic pathway for phenanthrene degradation in a pyrene-degrading bacterium. Polycycl Aromat Comp. 1996;11:125–130. [Google Scholar]
- 42.Saito A, Iwabuchi T, Harayama S. A novel phenanthrene dioxygenase from Nocardioides sp. strain KP7: expression in Escherichia coli. J Bacteriol. 2000;182:2134–2141. doi: 10.1128/jb.182.8.2134-2141.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanseverino J, Applegate B M, King J H, Sayler G S. Plasmid-mediated mineralization of naphthalene, phenanthrene and anthracene. Appl Environ Microbiol. 1993;59:1931–1937. doi: 10.1128/aem.59.6.1931-1937.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneider J, Grosser R, Jayasimhulu K, Xue W, Warshawsky D. Degradation of pyrene, benz[a]anthracene and benzo[a]pyrene by Mycobacterium sp. strain RJGII-135, isolated from a former coal gasification site. Appl Environ Microbiol. 1996;62:13–19. doi: 10.1128/aem.62.1.13-19.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sutherland J B, Freeman J P, Selby A L, Fu P P, Miller D W, Cerniglia C E. Stereoselective formation of a K-region dihydrodiol from phenanthrene by Streptomyces flavovirens. Arch Microbiol. 1990;154:260–266. doi: 10.1007/BF00248965. [DOI] [PubMed] [Google Scholar]
- 46.Sutherland J B, Selby A L, Freeman J P, Fu P P, Miller D W, Cerniglia C E. Identification of xyloside conjugates formed from anthracene by Rhizoctonia solani. Mycol Res. 1992;96:509–517. [Google Scholar]
- 47.Sutherland J B, Fu P P, Yang S K, Von Tungeln L S, Casillas R P, Crow S A, Cerniglia C E. Enantiomeric composition of the trans-dihydrodiols produced from phenanthrene by fungi. Appl Environ Microbiol. 1993;59:2145–2149. doi: 10.1128/aem.59.7.2145-2149.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sutherland J B, Rafii F, Khan A A, Cerniglia C E. Mechanisms of polycyclic aromatic hydrocarbon degradation. In: Young L Y, Cerniglia C E, editors. Microbial transformation and degradation of toxic organic chemicals. New York, N.Y: Wiley-Liss; 1995. pp. 169–306. [Google Scholar]
- 49.Tiehm A, Fritzsche C. Utilization of solubilized and crystalline mixtures of polycyclic aromatic hydrocarbons by a Mycobacterium sp. Appl Microbiol Biotechnol. 1995;42:964–968. [Google Scholar]
- 50.Tongpim S, Pickard M A. Cometabolic oxidation of phenanthrene to phenanthrene trans-9,10-dihydrodiol by Mycobacterium strain S1 growing on anthracene in the presence of phenanthrene. Can J Microbiol. 1999;45:369–376. [PubMed] [Google Scholar]
- 51.Walter U, Beyer M, Klein J, Rehm H-J. Degradation of pyrene by Rhodococcus sp. UW1. Appl Microbiol Biotechnol. 1991;34:671–676. [Google Scholar]
- 52.Wang R-F, Cao W-W, Cerniglia C E. Phylogenetic analysis of polycyclic aromatic hydrocarbon degrading mycobacteria by 16S rRNA sequencing. FEMS Microbiol Lett. 1995;130:75–80. doi: 10.1016/0378-1097(95)00186-9. [DOI] [PubMed] [Google Scholar]
- 53.Weissenfels W D, Beyer M, Klein J. Degradation of phenanthrene, fluorene and fluoranthene by pure bacterial cultures. Appl Microbiol Biotechnol. 1990;32:479–484. doi: 10.1007/BF00903787. [DOI] [PubMed] [Google Scholar]
- 54.Weissenfels W D, Beyer M, Klein J, Rehm H-J. Microbial metabolism of fluoranthene: isolation and identification of ring fission products. Appl Microbiol Biotechnol. 1991;34:528–535. [Google Scholar]
- 55.Wilson S C, Jones K C. Bioremediation of soil contaminated with polynuclear aromatic hydrocarbons (PAHs): a review. Environ Pollut. 1993;81:229–249. doi: 10.1016/0269-7491(93)90206-4. [DOI] [PubMed] [Google Scholar]
- 56.Yang Y, Chen R F, Shiaris M P. Metabolism of naphthalene, fluorene, and phenanthrene: preliminary characterization of a cloned gene cluster from Pseudomonas putida NCIB 9816. J Bacteriol. 1994;178:2158–2164. doi: 10.1128/jb.176.8.2158-2164.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zylstra G J, Kim E. Aromatic hydrocarbon degradation by Sphingomonas yanoikuyae B1. J Ind Microbiol Biotechnol. 1997;19:408–414. doi: 10.1038/sj.jim.2900475. [DOI] [PubMed] [Google Scholar]
- 58.Zylstra G J, Wang X P, Kim E, Didolkar V A. Cloning and analysis of the genes for polycyclic aromatic hydrocarbon degradation. Ann N Y Acad Sci. 1994;721:386–398. doi: 10.1111/j.1749-6632.1994.tb47410.x. [DOI] [PubMed] [Google Scholar]