Abstract
Pain and depression are complex disorders that frequently co-occur, resulting in diminished quality of life. The habenula is an epithalamic structure considered to play a pivotal role in the neurocircuitry of both pain and depression. The habenula can be divided into two major areas, the lateral and medial habenula, that can be further subdivided, resulting in 6 main subregions. Here, we investigated habenula activation patterns in a rat model of neuropathic pain with accompanying depressive-like behaviour. Wistar rats received active surgery for the development of neuropathic pain (chronic constriction injury of the sciatic nerve; CCI), sham surgery (surgical control), or no surgery (behavioural control). All animals were evaluated for mechanical nociceptive threshold using the paw pressure test and depressive-like behaviour using the forced swimming test, followed by evaluation of the immunoreactivity to cFos—a marker of neuronal activity—in the habenula and subregions. The Open Field Test was used to evaluate locomotor activity. Animals with peripheral neuropathy (CCI) showed decreased mechanical nociceptive threshold and increased depressive-like behaviour compared to control groups. The CCI group presented decreased cFos immunoreactivity in the total habenula, total lateral habenula and lateral habenula subregions, compared to controls. No difference was found in cFos immunoreactivity in the total medial habenula, however when evaluating the subregions of the medial habenula, we observed distinct activation patterns, with increase cFos immunoreactivity in the superior subregion and decrease in the central subregion. Taken together, our data suggest an involvement of the habenula in neuropathic pain and accompanying depressive-like behaviour.
Introduction
Neuropathic pain is a disorder with a prevalence of 7–10% [1] that results in great suffering in patients and a significant burden to the healthcare system [2]. Similarly, depression affects approximately 6% of adults worldwide [3]. Interestingly, patients with debilitating pain often present with depressive symptoms [4–6], while individuals who are depressed also demonstrate an exacerbated pain perception [7, 8]. Hence, pain-associated depression refers to a complex disorder in which persistent pain and major depressive disorder co-occur, displaying synergic symptoms [9, 10]. Because of this synergism, pain and depression treatments rely on similar mechanisms [11].
It has been proposed that the neuroanatomical bases of pain and depression involve similar brain areas, such as the thalamus, amygdala and habenula [12–16]. The thalamus is a key area in the ascending pain pathway (i.e. spinothalamic tract) and also thought to be critically involved in depressive symptoms [14, 17]. Distinct amygdala subregions have been shown to be involved in the expression of depressive-like behaviours in rodent models of neuropathic pain [15]. Specifically, the anterior and posterior portions of the basolateral nucleus of the amygdala (BLA) and the central portion of the central nucleus of the amygdala (CeA) are involved in the neurocircuitry underlying neuropathic pain and the pharmacological inactivation of these areas reverses hyperalgesia, allodynia and depressive-like behavior in animals with peripheral neuropathy [15].
The habenula (Hb), an epithalamic structure of the limbic system, plays a key role in the endocrine system, reward, addiction, pain, and depressive behaviours [12, 13, 18]. The Hb can be divided into lateral (LHb) and medial (MHb) parts, based on cell type and connectivity pattern [19, 20]. The LHb can be further subdivided into lateral (LHbL) and medial (LHbM) regions, while the MHb can be parcellated into superior (MHbS), inferior (MHbI), central (MHbC), and lateral (MHbL) regions [20–23]. The LHb sends several outputs to the raphe nuclei and ventral tegmental area [24], which are strongly related to analgesia [25] and have been proposed to play a prominent role in pain processing [26, 27], and in depressive-like behaviours in models of neuropathic pain [15, 28, 29]. Increased LHb activity is associated with depressive behaviours [30, 31] via increased GABAergic neurotransmission, resulting in inhibition of the dopaminergic and serotonergic systems [32, 33], which are involved in mechanisms of pain and depressive symptoms [28]. Furthermore, pharmacological inhibition of the lateral habenula improves depressive-like behaviour in a rat model of depression [16].
The MHb has been implicated in stress, depression, memory processing, and nicotine withdrawal syndrome [30, 34–36] and has a potential role in pain control [37]. Kim and Chang (2005) suggested that MHb may mediate LHb activity by “boutons en passant” synapses from the MHb to the LHb [38]. The MHb is believed to receive inputs from different areas within the limbic system and projects to the interpeduncular nucleus (IPN), which in turn projects to specific areas of the limbic system, thought to be involved in both pain and depressive behaviours [19, 39], including the serotonergic raphe nuclei [40, 41]. The IPN receives input from the MHbS via substance P and from the MHbI via acetylcholine [42–45]. High levels of mu opioid receptor (MOR) can be found in cholinergic neurons in the MHb [46, 47] and are also distributed along the MHb-IPN pathway, co-localizing with substance P [48]. Interestingly, elevated expression of substance P was observed in the MHb-IPN connection in animals presenting depressive-like behaviours, and MHb lesions were sufficient to suppress these behaviours [49].
In this study, we aimed to investigate the activation pattern of the LHb and MHb and its subareas (LHbL, LHbM, MHbS, MHbI, MHbC, and MHbL) in a preclinical rat model of neuropathic pain accompanied by depressive-like behaviour.
Materials and methods
Animals
Seventeen male Wistar rats (200–250 g) were used in this study. All animals were maintained in regular rat cages (2–3 rats/box) with wood shavings and free access to water and rat chow pellets under a 12h light/dark cycle and controlled temperature (22±2°C). Before the experimental procedures, animals were allowed to habituate to the animal facility for one week. The protocols used in this project were approved by the Ethics Committee on the Use of Animals for Research of the Hospital Sírio-Libanês (Brazil, CEUA# 2014/07) and were conducted and reported in accordance with the ARRIVE guidelines (http://www.nc3rs.org.uk/arrive-guidelines).
Experimental design
After habituation to the animal facility, animals were habituated to the paw pressure test (PPT) apparatus (10 minutes). On the next day, baseline measures of mechanical nociceptive thresholds were obtained for all animals, followed by random allocation into three groups: I. naive (n = 6, no surgery); II. false-operated (FOP, n = 6, sham surgery); and III. chronic constriction injury (CCI, n = 5; active surgery) and surgery. After 13 days, animals were habituated to the forced swimming test (FST). On the last day (day 14 after surgery) all animals were evaluated in the Open Field Test (OFT), PPT and FST. Ninety minutes after behavioural tests, the animals were transcardially perfused, and brains were recovered for histological analysis. Fig 1A illustrates the study timeline.
Fig 1. Methods of study.
A. Experimental design. After habituation to the animal facility, Wistar rats habituated to the paw pressure test (PPT) and, on the following day, were evaluated for baseline measures of mechanical nociceptive threshold. Animals were then randomly allocated into three groups (i.e. naive [no surgery], false-operated, and chronic constriction injury [active surgery]), followed by the assigned surgery. Thirteen days after the baseline measure, animals were habituated to the Forced Swimming Test (FST). On the following day, animals were tested in the Open Field Test (OFT) and final measures of the PPT and FST were taken. B. Photomicrography of a Nissl-stained coronal slice, showing the lateral and medial habenula and its subdivisions. Abbreviations: LHbL: Lateral subdivision of the lateral habenula. LHbM: Medial subdivision of the lateral habenula. MHbS: Superior subdivision of the medial habenula. MHbI: Inferior subdivision of the medial habenula. MHbL: Lateral subdivision of the medial habenula. MHbC: Central subdivision of the medial habenula. sm: stria medullaris.
Peripheral neuropathy surgery
The CCI model was established as previously described [15, 50, 51]. Briefly, rats were anaesthetised with Isoflurane (4–5% induction, 2–3% maintenance), the right sciatic nerve was exposed, and four ligatures (1–1.5 mm apart) were loosely tied around the nerve using 4.0 Catgut chromic sutures. FOP rats were anaesthetised, and the right sciatic nerve was exposed, but there was no constriction of the nerve. Naive rats received no surgery.
Evaluation of nociceptive threshold–Paw pressure test
The mechanical nociceptive threshold was determined using a PPT apparatus (EEF-440, Insight, SP, Brazil), as previously described [52]. Briefly, the hind paw of the animal was placed into the apparatus, and the force (in grams) required to induce a paw withdrawal response represented the mechanical nociceptive threshold. All animals were habituated to the apparatus before testing, by handling the animals and simulating the test without applying paw pressure. The PPT was conducted on all animals at baseline and last time point. A significant reduction in mechanical nociceptive thresholds represented neuropathic pain.
Evaluation of depressive-like behaviour–Forced swimming test
Depressive-like behaviour was determined using the FST, as previously described [53]. A cylindrical tank (30 cm diameter × 60 cm height) was filled with 30 cm high lukewarm water (24±1°C), and animals were gently placed on the water. All animals were habituated to the FST for 15 minutes one day before testing. On the last day, all animals were tested in the FST for 5 minutes. Immobility time (in seconds) was determined by measuring the time during which no additional activity was observed other than the movements necessary to keep the head above the water surface. Increased time spent immobile characterized depressive-like behaviour.
Evaluation of locomotor activity–Open Field Test
The OFT was used to evaluate locomotor behaviour as a control for possible locomotor impairment that could confound the results of the remaining behavioural tests. The PFT apparatus consists of a 60x60x50 dark grey Formica box. No habituation to the test is required. During the test, each animal was placed in the centre of the apparatus and allowed to freely explore for 5 min. The behaviour was video-recorded and the total distance travelled during the test was evaluated by a blind observer. After the end of the test, the open field was cleaned with 5% ethanol and subsequently dried with a cloth
Histological analysis–Immunohistochemistry for cFos and Nissl-staining
The immunohistochemistry (IHC) protocol was performed as previously described [15, 51]. Briefly, brains were frozen cut in sequential 30μm-thick slices. Brain slices were then washed in buffer and incubated overnight at 4°C with rabbit anti- cFos primary antibody (1:20000; Ab-5, Calbiochem, CA, USA) followed by incubation with biotinylated secondary antibody (1:200; donkey anti-rabbit IgG, Jackson ImmunoResearch, PA, USA) and avidin-biotin complex (1:100; ABC Elite kit, Vector Laboratories, CA, USA). The antibody complex was visualized by exposure to a chromogen solution containing 0.05% diaminobenzidine tetrahydrochloride (DAB, Sigma-Aldrich, MO, USA) and 0.01% hydrogen peroxide in the buffer. Images were captured using a light microscope (E1000, Nikon, NY, USA), and cFos immunoreactivity (cFos-IR) of the Hb was evaluated by a blinded observer at 10x magnification. Adjoining Nissl-stained sections provided the histological landmarks for the accurate identification and delineation of the LHb (LHbL and LHbM) and of the MHb (MHbS, MHbI, MHbC, MHbL) regions (Bregmas: -3.00 mm to -4.36 mm of the Paxinos and Watson Atlas [54]; Fig 1B).
Statistical analyses
Data are presented as the mean ± standard error of the mean (SEM). Statistical analyses were conducted using GraphPad Prism software (version 5.0; GraphPad Software Inc., CA, USA). Normal distribution was confirmed for all variables using the Kolmogorov-Smirnov test. Mechanical nociceptive thresholds were analyzed with two-way repeated measures analysis of variance (ANOVA), followed by Tukey’s post-hoc test. Immobility time in the FST and total distance travelled in the OFT were evaluated with one-way ANOVA, followed by Tukey’s post-hoc test, where applicable. cFos-IR was normalized by defining the naive group as 100%, and analyzed using one-way ANOVA, followed by Tukey’s post-hoc test. For all tests, statistical significance was set at p<0.05. Power analysis was performed to assess the power of this study, as previously described [55]. Considering the mechanical nociceptive threshold in the PPT the primary outcome measure, and power (1-β) set at 0.80 (i.e. 80% power) and α = 0.05 (i.e. significance level of p<0.05), the analysis resulted in a minimum sample size of 4.2 animals per/group.
Results
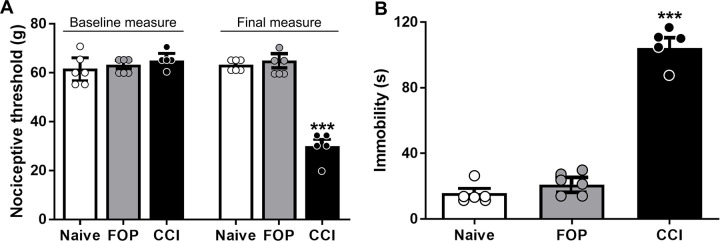
The CCI group showed a significant decrease in the mechanical nociceptive threshold at the final measurement (F(2,14) = 29.92, p<0.001, Fig 2A, Table 1) and a significant increase in immobility time in the FST (F(2,12) = 114.8, p<0.001, Fig 2B, Table 1), compared to control groups. No differences between groups was observed in the total distance travelled in the OFT (F(2,14) = 0.998, p>0.05, Table 1).
Fig 2. Behavioural results.
A. Mechanical Nociceptive Threshold (g) in the paw pressure test before randomization (baseline measure) and after 14 days (final measure). A significant reduction in mechanical nociceptive thresholds represents neuropathic pain. B. Immobility time (s) in the Forced Swimming Test, evaluated 14 days after group allocation. Increased time spent immobile characterized depressive-like behaviour. Values are presented as mean ± SEM. ***p < 0.001. Abbreviations: FOP: false-operated, CCI: chronic constriction injury.
Table 1. Mean and standard deviation—behaviour and habenula c-fos immunoreactivity.
| Variable | Naive | FOP | CCI |
|---|---|---|---|
| Paw Pressure Test | 60.83±5.85 | 63.33±4.08 | 30.00±6.12*** |
| Forced Swimming Test | 15.50±5.68 | 19.00±7.75 | 92.80±27.96*** |
| Open Field Test | 731.67±82.80 | 841.67±145.66 | 758.00±183.36 |
| Total Habenula | 100±12.5 | 64.5±30.4 | 55±12* |
| Total Lateral Habenula | 100±8.4 | 45.7±13.9 | 33.2±13.8** |
| Lateral Habenula–Lateral | 100±13.2 | 43.6±16.1 | 33.4±15.3* |
| Lateral Habenula—Medial | 100±7.4 | 49.9±12.8 | 29.4±14.5* |
| Total Medial Habenula | 100±24.8 | 84.5±40.1 | 79.9±15.6 |
| Medial Habenula—Superior | 100±26.5 | 222±60.4 | 454.2±65* |
| Medial Habenula—Central | 100±12.3 | 82.7±22.8 | 53.4±5.4*** |
| Medial Habenula—Lateral | 100±15.7 | 76.5±20.7 | 93.8±8.7 |
| Medial Habenula—Inferior | 100±35.9 | 81.6±8.82 | 44.0±7.34 |
Abbreviations: FOP: false-operated, CCI: chronic constriction injury
*p<0.05
**p<0.01
***p<0.001
A significant reduction in cFos-IR were observed in the CCI group in the total Hb (F(2,14) = 4.696, p<0.05, Fig 3A, Table 1) when compared to naive controls. There was also a significant reduction in cFos-IR in the total LHb (F(2,14) = 7.032, p<0.01, Fig 3B, Table 1), subregions LHbL (F(2,14) = 4.512, p<0.05, Fig 3C, Table 1), LHbM (F(2,14) = 4.248, p<0.05, Fig 3D, Table 1), in the CCI group compared to FOP and naive groups. When evaluating the sub-regions of the MHb, we observed a significant reduction in cFos-IR in the MHbC (F(2,12) = 17.84, p = 0.0011, Fig 3E, Table 1) and significant increase in cFos-IR in the subregion MHbS (F(2,13) = 6.574, p = 0.013, Fig 3F, Table 1), in the CCI group compared to FOP and naive groups. No differences in cFos-IR were observed between groups when evaluating total MHb (F(2,11) = 0.326, p = 0.72, Table 1), subregion MHbL (F(2,14) = 0.611, p = 0.55, Table 1) and subregion MHbI (F(2,13) = 0.087, p = 0.91; Table 1).
Fig 3. cFos immunoreactivity (cFos-IR) pattern in the habenula.
A. Total Habenula (tHb) cFos-IR. B. Total Lateral Habenula (tLHb) cFos-IR. C. cFos-IR in the lateral subregion of the LHb (LHbL). D. cFos-IR in the medial subregion of the LHb (LHbM). E. cFos-IR in the central subregion of the medial habenula (MHbC). F. cFos-IR in the superior subregion of the medial habenula (MHbS). Abbreviations: FOP: false-operated group, CCI: chronic constriction injury group. Values are presented as normalized mean±SEM. *p<0.05, **p<0.01, ***p<0.001.
Discussion
In this study we described the activation pattern of the Hb, and its subregions, in a preclinical neuropathic pain model accompanied by depressive-like behaviour. Clinical and preclinical studies have provided evidence of the involvement of the Hb in pain and depressive behaviours. Using functional magnetic resonance imaging, (fMRI), Shelton and colleagues (2012) showed bilateral Hb activation during noxious stimulation, suggesting the Hb to be involved in the pain processing network [27]. In an examination of transient effects of deep brain stimulation in the Hb, Zhang and colleagues showed that one of the most common transient effects associated with increased voltage was pain [56]. Preclinical studies have demonstrated that the electrical stimulation of the Hb, or intra-nuclear morphine injections have been shown to induce analgesia [57, 58], while lesions restricted to the MHb, to the IPN, or to the fibre bundle connecting these structures, increase pain sensitivity [59]. Furthermore, significant reductions in LHb activation patterns were also observed in animal models of diabetes-induced neuropathic pain [60] and tail pinch intermittent stressor [61]. In line with these findings, in this study we observed a significant reduction in cFos-IR in the LHb and subdivisions in animals with neuropathic pain accompanied by depressive-like behaviours. It is important to highlight that with our methodology, we are able to determine the stimulus-induced nociception at day 14 post surgery, but not to perform an ongoing evaluation of pain throughout the study [62, 63]. Also, the increased time in immobility observed in the CCI group is not a result of impaired locomotor activity, as no differences were observed between groups in the OFT. As both pain and depression are observed simultaneously, it is not possible to depict which component is more relevant for the cFos expression pattern observed in the Hb. A study focusing on pharmacological manipulations (e.g. use of antidepressants) could provide some insight into this aspect.
It has been shown that patients diagnosed with major depressive disorder [64] and in psychiatric disorders that present depressive components [65, 66] present with altered habenula volume. Furthermore, deep brain stimulation of the habenula results in symptom alleviation in depressive patients [67, 68]. It has been shown that the LHb is involved in effort-based decision-making, a key contributor to willingness to exert physical effort in psychiatric conditions [69]. Han and colleagues (2017) showed a down-regulation of cholinergic genes in the Hb of animals exposed to the chronic restraint stress model of depression [70]. Previous studies have also shown increased metabolic activity in the MHb of a genetic rat model of helpless behaviour [30] and rats exposed to the chronic unpredictable mild stress paradigm [49].
Although we did not observe a significant difference between groups in cFos-IR of the total MHb, when analyzing its subregions, we noted a distinct activation pattern, with the CCI group presenting increase cFos-IR in the MHbS and reduced cFos-IR in the MHbC. These results suggest heterogeneity in the MHb subregions and highlight the importance of further investigating the role of MHb subareas in depressive behaviours. While the MHbS consists exclusively of densely packed glutamatergic neurons that strongly express interleukin-18, MHbC is composed of neurons that either co-express substance-P and glutamate, or acetylcholine and glutamate [21, 71]. Efferents from the MHb forms the core aspect of the fasciculus retroflexus, with dorsal projections reaching lateral aspects of the IPN, medial projections reaching the ventral aspect of IPN and lateral projections ending on the dorsal aspect of IPN [19]. It is believed that glutamatergic projections from the MHb terminate in the IPN, cholinergic and substance P-ergic projections through the IPN and indirect connections terminate in the VTA, and additional projections from the MHb reaches the raphe nuclei and LHb [38, 72]. These connections suggest a possible modulatory role of both the MHbS and MHbC on serotonin, IPN and LHb function, and of the MHbC on dopamine [72]. This work sheds light on the involvement of the Hb in the neural-network of neuropathic pain and accompanying depressive-like behaviour. Further studies are necessary to better understand the neurobiological mechanisms underlying neuropathic pain and depression.
Acknowledgments
The authors are grateful to Dr. Bruno Gregnanin Pedron and the staff of the Hospital Sírio-Libanês.
Data Availability
All relevant data are within the article.
Funding Statement
This research was supported by grants FAPESP to RCRM (#11/08575-7), ACPC (#18/18695-9), and FVG (#13/20602-5 and #17/10466-8), CAPES to GFA (#88882.366209/2019-01), and Canadian Institutes of Health Research Fellowship to FVG (CIHR - #472484).
References
- 1.Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3: 17002. doi: 10.1038/nrdp.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith BH, Torrance N. Epidemiology of neuropathic pain and its impact on quality of life. Curr Pain Headache Rep. 2012;16: 191–198. doi: 10.1007/s11916-012-0256-0 [DOI] [PubMed] [Google Scholar]
- 3.WHO 2018. World Health Organization 2018. Mental Disorders. Available: http://www.who.int/mediacentre/factsheets/fs396/en
- 4.von Knorring L, Perris C, Eisemann M, Eriksson U, Perris H. Pain as a symptom in depressive disorders. II. Relationship to personality traits as assessed by means of KSP. Pain. 1983;17: 377–384. doi: 10.1016/0304-3959(83)90169-0 [DOI] [PubMed] [Google Scholar]
- 5.Lee YC, Chibnik LB, Lu B, Wasan AD, Edwards RR, Fossel AH, et al. The relationship between disease activity, sleep, psychiatric distress and pain sensitivity in rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther. 2009;11: R160. doi: 10.1186/ar2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agüera-Ortiz L, Failde I, Mico JA, Cervilla J, López-Ibor JJ. Pain as a symptom of depression: prevalence and clinical correlates in patients attending psychiatric clinics. J Affect Disord. 2011;130: 106–112. doi: 10.1016/j.jad.2010.10.022 [DOI] [PubMed] [Google Scholar]
- 7.Dworkin RH, Gitlin MJ. Clinical aspects of depression in chronic pain patients. Clin J Pain. 1991;7: 79–94. doi: 10.1097/00002508-199106000-00004 [DOI] [PubMed] [Google Scholar]
- 8.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163: 2433–2445. doi: 10.1001/archinte.163.20.2433 [DOI] [PubMed] [Google Scholar]
- 9.Lépine J-P, Briley M. The epidemiology of pain in depression. Hum Psychopharmacol. 2004;19 Suppl 1: S3–7. doi: 10.1002/hup.618 [DOI] [PubMed] [Google Scholar]
- 10.Li J-X. Pain and depression comorbidity: a preclinical perspective. Behav Brain Res. 2015;276: 92–98. doi: 10.1016/j.bbr.2014.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campos ACP, Antunes GF, Matsumoto M, Pagano RL, Martinez RCR. Neuroinflammation, Pain and Depression: An Overview of the Main Findings. Front Psychol. 2020;11: 1825. doi: 10.3389/fpsyg.2020.01825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11: 503–513. doi: 10.1038/nrn2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henn FA. Circuits, cells, and synapses: toward a new target for deep brain stimulation in depression. Neuropsychopharmacology. 2012;37: 307–308. doi: 10.1038/npp.2011.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62: 429–437. doi: 10.1016/j.biopsych.2006.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seno MDJ, Assis DV, Gouveia F, Antunes GF, Kuroki M, Oliveira CC, et al. The critical role of amygdala subnuclei in nociceptive and depressive-like behaviors in peripheral neuropathy. Sci Rep. 2018;8: 13608. doi: 10.1038/s41598-018-31962-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winter C, Vollmayr B, Djodari-Irani A, Klein J, Sartorius A. Pharmacological inhibition of the lateral habenula improves depressive-like behavior in an animal model of treatment resistant depression. Behav Brain Res. 2011;216: 463–465. doi: 10.1016/j.bbr.2010.07.034 [DOI] [PubMed] [Google Scholar]
- 17.Vartiainen N, Perchet C, Magnin M, Creac’h C, Convers P, Nighoghossian N, et al. Thalamic pain: anatomical and physiological indices of prediction. Brain. 2016;139: 708–722. doi: 10.1093/brain/awv389 [DOI] [PubMed] [Google Scholar]
- 18.Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470: 535–539. doi: 10.1038/nature09742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187: 19–47. doi: 10.1002/cne.901870103 [DOI] [PubMed] [Google Scholar]
- 20.Gouveia FV, Ibrahim GM. Habenula as a neural substrate for aggressive behavior. Front Psychiatry. 2022;13. doi: 10.3389/fpsyt.2022.817302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andres KH, von Düring M, Veh RW. Subnuclear organization of the rat habenular complexes. J Comp Neurol. 1999;407: 130–150. doi: [DOI] [PubMed] [Google Scholar]
- 22.Hikosaka O, Sesack SR, Lecourtier L, Shepard PD. Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci. 2008;28: 11825–11829. doi: 10.1523/JNEUROSCI.3463-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Namboodiri VMK, Rodriguez-Romaguera J, Stuber GD. The habenula. Curr Biol. 2016;26: R873–R877. doi: 10.1016/j.cub.2016.08.051 [DOI] [PubMed] [Google Scholar]
- 24.Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev. 1982;6: 1–13. doi: 10.1016/0149-7634(82)90003-3 [DOI] [PubMed] [Google Scholar]
- 25.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150: 971–979. doi: 10.1126/science.150.3699.971 [DOI] [PubMed] [Google Scholar]
- 26.Shelton L, Becerra L, Borsook D. Unmasking the mysteries of the habenula in pain and analgesia. Prog Neurobiol. 2012;96: 208–219. doi: 10.1016/j.pneurobio.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shelton L, Pendse G, Maleki N, Moulton EA, Lebel A, Becerra L, et al. Mapping pain activation and connectivity of the human habenula. J Neurophysiol. 2012;107: 2633–2648. doi: 10.1152/jn.00012.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Li Y, Zhang B, Shen X, Zhao H. Why depression and pain often coexist and mutually reinforce: Role of the lateral habenula. Exp Neurol. 2016;284: 106–113. doi: 10.1016/j.expneurol.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 29.Medeiros P, Dos Santos IR, Júnior IM, Palazzo E, da Silva JA, Machado HR, et al. An Adapted Chronic Constriction Injury of the Sciatic Nerve Produces Sensory, Affective, and Cognitive Impairments: A Peripheral Mononeuropathy Model for the Study of Comorbid Neuropsychiatric Disorders Associated with Neuropathic Pain in Rats. Pain Med. 2021;22: 338–351. doi: 10.1093/pm/pnaa206 [DOI] [PubMed] [Google Scholar]
- 30.Shumake J, Edwards E, Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Res. 2003;963: 274–281. doi: 10.1016/s0006-8993(02)04048-9 [DOI] [PubMed] [Google Scholar]
- 31.Yang L-M, Hu B, Xia Y-H, Zhang B-L, Zhao H. Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behav Brain Res. 2008;188: 84–90. doi: 10.1016/j.bbr.2007.10.022 [DOI] [PubMed] [Google Scholar]
- 32.Ferraro G, Montalbano ME, Sardo P, La Grutta V. Lateral habenular influence on dorsal raphe neurons. Brain Res Bull. 1996;41: 47–52. doi: 10.1016/0361-9230(96)00170-0 [DOI] [PubMed] [Google Scholar]
- 33.Brinschwitz K, Dittgen A, Madai VI, Lommel R, Geisler S, Veh RW. Glutamatergic axons from the lateral habenula mainly terminate on GABAergic neurons of the ventral midbrain. Neuroscience. 2010;168: 463–476. doi: 10.1016/j.neuroscience.2010.03.050 [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi Y, Sano Y, Vannoni E, Goto H, Suzuki H, Oba A, et al. Genetic dissection of medial habenula-interpeduncular nucleus pathway function in mice. Front Behav Neurosci. 2013;7: 17. doi: 10.3389/fnbeh.2013.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathuru AS, Jesuthasan S. The medial habenula as a regulator of anxiety in adult zebrafish. Front Neural Circuits. 2013;7: 99. doi: 10.3389/fncir.2013.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molas S, DeGroot SR, Zhao-Shea R, Tapper AR. Anxiety and Nicotine Dependence: Emerging Role of the Habenulo-Interpeduncular Axis. Trends Pharmacol Sci. 2017;38: 169–180. doi: 10.1016/j.tips.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plenge P, Mellerup ET, Wörtwein G. Characterization of epibatidine binding to medial habenula: potential role in analgesia. J Pharmacol Exp Ther. 2002;302: 759–765. doi: 10.1124/jpet.102.033498 [DOI] [PubMed] [Google Scholar]
- 38.Kim U, Chang S-Y. Dendritic morphology, local circuitry, and intrinsic electrophysiology of neurons in the rat medial and lateral habenular nuclei of the epithalamus. J Comp Neurol. 2005;483: 236–250. doi: 10.1002/cne.20410 [DOI] [PubMed] [Google Scholar]
- 39.Carlson J, Noguchi K, Ellison G. Nicotine produces selective degeneration in the medial habenula and fasciculus retroflexus. Brain Res. 2001;906: 127–134. doi: 10.1016/s0006-8993(01)02570-7 [DOI] [PubMed] [Google Scholar]
- 40.Groenewegen HJ, Ahlenius S, Haber SN, Kowall NW, Nauta WJ. Cytoarchitecture, fiber connections, and some histochemical aspects of the interpeduncular nucleus in the rat. J Comp Neurol. 1986;249: 65–102. doi: 10.1002/cne.902490107 [DOI] [PubMed] [Google Scholar]
- 41.McLaughlin I, Dani JA, De Biasi M. The medial habenula and interpeduncular nucleus circuitry is critical in addiction, anxiety, and mood regulation. J Neurochem. 2017;142 Suppl 2: 130–143. doi: 10.1111/jnc.14008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sastry BR, Zialkowski SE, Hansen LM, Kavanagh JP, Evoy EM. Acetylcholine release in interpeduncular nucleus following the stimulation of habenula. Brain Res. 1979;164: 334–337. doi: 10.1016/0006-8993(79)90032-5 [DOI] [PubMed] [Google Scholar]
- 43.Houser CR, Crawford GD, Barber RP, Salvaterra PM, Vaughn JE. Organization and morphological characteristics of cholonergic neurons: an immunocytochemical study with a monoclonal antibody to choline acetyltransferase. Brain Res. 1983;266: 97–119. doi: 10.1016/0006-8993(83)91312-4 [DOI] [PubMed] [Google Scholar]
- 44.Hamill GS, Jacobowitz DM. A study of afferent projections to the rat interpeduncular nucleus. Brain Res Bull. 1984;13: 527–539. doi: 10.1016/0361-9230(84)90035-2 [DOI] [PubMed] [Google Scholar]
- 45.Contestabile A, Villani L, Fasolo A, Franzoni MF, Gribaudo L, Oktedalen O, et al. Topography of cholinergic and substance P pathways in the habenulo-interpeduncular system of the rat. An immunocytochemical and microchemical approach. Neuroscience. 1987;21: 253–270. doi: 10.1016/0306-4522(87)90337-x [DOI] [PubMed] [Google Scholar]
- 46.Zastawny RL, George SR, Nguyen T, Cheng R, Tsatsos J, Briones-Urbina R, et al. Cloning, characterization, and distribution of a mu-opioid receptor in rat brain. J Neurochem. 1994;62: 2099–2105. doi: 10.1046/j.1471-4159.1994.62062099.x [DOI] [PubMed] [Google Scholar]
- 47.Bunzow JR, Zhang G, Bouvier C, Saez C, Ronnekleiv OK, Kelly MJ, et al. Characterization and distribution of a cloned rat mu-opioid receptor. J Neurochem. 1995;64: 14–24. doi: 10.1046/j.1471-4159.1995.64010014.x [DOI] [PubMed] [Google Scholar]
- 48.Gardon O, Faget L, Chu Sin Chung P, Matifas A, Massotte D, Kieffer BL. Expression of mu opioid receptor in dorsal diencephalic conduction system: new insights for the medial habenula. Neuroscience. 2014;277: 595–609. doi: 10.1016/j.neuroscience.2014.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu C, Sun Y, Cai X, You T, Zhao H, Li Y, et al. Medial Habenula-Interpeduncular Nucleus Circuit Contributes to Anhedonia-Like Behavior in a Rat Model of Depression. Front Behav Neurosci. 2018;12: 238. doi: 10.3389/fnbeh.2018.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bennett GJ, Xie Y-K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33: 87–107. doi: 10.1016/0304-3959(88)90209-6 [DOI] [PubMed] [Google Scholar]
- 51.Pagano RL, Assis DV, Clara JA, Alves AS, Dale CS, Teixeira MJ, et al. Transdural motor cortex stimulation reverses neuropathic pain in rats: a profile of neuronal activation. Eur J Pain. 2011;15: 268.e1–14. doi: 10.1016/j.ejpain.2010.08.003 [DOI] [PubMed] [Google Scholar]
- 52.Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111: 409–419. Available: https://www.ncbi.nlm.nih.gov/pubmed/13471093 [PubMed] [Google Scholar]
- 53.Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc. 2012;7: 1009–1014. doi: 10.1038/nprot.2012.044 [DOI] [PubMed] [Google Scholar]
- 54.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition. Elsevier; 2006. Available: https://play.google.com/store/books/details?id=0prYfdDbh58C [Google Scholar]
- 55.Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4: 303–306. doi: 10.4103/0976-500X.119726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang C, Lai Y, Zhang Y, Xu X, Sun B, Li D. Deep Brain Stimulation-Induced Transient Effects in the Habenula. Front Psychiatry. 2021;12: 674962. doi: 10.3389/fpsyt.2021.674962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen SR, Melzack R. Morphine injected into the habenula and dorsal posteromedial thalamus produces analgesia in the formalin test. Brain Res. 1985;359: 131–139. doi: 10.1016/0006-8993(85)91420-9 [DOI] [PubMed] [Google Scholar]
- 58.Cohen SR, Melzack R. Habenular stimulation produces analgesia in the formalin test. Neurosci Lett. 1986;70: 165–169. doi: 10.1016/0304-3940(86)90457-x [DOI] [PubMed] [Google Scholar]
- 59.Mészáros J, Gajewska S, Tarchalska-Kryńska B. Habenulo-interpeduncular lesions: the effects on pain sensitivity, morphine analgesia and open-field behavior in rats. Pol J Pharmacol Pharm. 1985;37: 469–477. Available: https://www.ncbi.nlm.nih.gov/pubmed/3909126 [PubMed] [Google Scholar]
- 60.Paulson PE, Wiley JW, Morrow TJ. Concurrent activation of the somatosensory forebrain and deactivation of periaqueductal gray associated with diabetes-induced neuropathic pain. Exp Neurol. 2007;208: 305–313. doi: 10.1016/j.expneurol.2007.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith WJ, Stewart J, Pfaus JG. Tail pinch induces fos immunoreactivity within several regions of the male rat brain: effects of age. Physiol Behav. 1997;61: 717–723. doi: 10.1016/s0031-9384(96)00524-0 [DOI] [PubMed] [Google Scholar]
- 62.Adcock SJJ, Tucker CB. Conditioned place preference reveals ongoing pain in calves 3 weeks after disbudding. Sci Rep. 2020;10: 3849. doi: 10.1038/s41598-020-60260-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sufka KJ. Conditioned place preference paradigm: a novel approach for analgesic drug assessment against chronic pain. Pain. 1994;58: 355–366. doi: 10.1016/0304-3959(94)90130-9 [DOI] [PubMed] [Google Scholar]
- 64.Savitz JB, Nugent AC, Bogers W, Roiser JP, Bain EE, Neumeister A, et al. Habenula volume in bipolar disorder and major depressive disorder: a high-resolution magnetic resonance imaging study. Biol Psychiatry. 2011;69: 336–343. doi: 10.1016/j.biopsych.2010.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Germann J, Gouveia FV, Martinez RCR, Zanetti MV, de Souza Duran FL, Chaim-Avancini TM, et al. Fully Automated Habenula Segmentation Provides Robust and Reliable Volume Estimation Across Large Magnetic Resonance Imaging Datasets, Suggesting Intriguing Developmental Trajectories in Psychiatric Disease. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5: 923–929. doi: 10.1016/j.bpsc.2020.01.004 [DOI] [PubMed] [Google Scholar]
- 66.Lee Y-A, Goto Y. The Habenula in the Link Between ADHD and Mood Disorder. Front Behav Neurosci. 2021;15: 699691. doi: 10.3389/fnbeh.2021.699691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67: e9–e11. doi: 10.1016/j.biopsych.2009.08.027 [DOI] [PubMed] [Google Scholar]
- 68.Germann J, Mameli M, Elias GJB, Loh A, Taha A, Gouveia FV, et al. Deep Brain Stimulation of the Habenula: Systematic Review of the Literature and Clinical Trial Registries. Front Psychiatry. 2021;12: 730931. doi: 10.3389/fpsyt.2021.730931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sevigny JP, Bryant EN, Encarnacion É, Smith DF, Acosta R, Baker PM. Lateral Habenula Inactivation Alters Willingness to Exert Physical Effort Using a Maze Task in Rats. Front Behav Neurosci. 2021;15: 652793. doi: 10.3389/fnbeh.2021.652793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han S, Yang SH, Kim JY, Mo S, Yang E, Song KM, et al. Down-regulation of cholinergic signaling in the habenula induces anhedonia-like behavior. Sci Rep. 2017;7: 900. doi: 10.1038/s41598-017-01088-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aizawa H, Kobayashi M, Tanaka S, Fukai T, Okamoto H. Molecular characterization of the subnuclei in rat habenula. J Comp Neurol. 2012;520: 4051–4066. doi: 10.1002/cne.23167 [DOI] [PubMed] [Google Scholar]
- 72.Viswanath H, Carter AQ, Baldwin PR, Molfese DL, Salas R. The medial habenula: still neglected. Front Hum Neurosci. 2013;7: 931. doi: 10.3389/fnhum.2013.00931 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the article.