Abstract
Membrane fusion during the entry of herpesviruses is carried out by the viral fusogen gB that is activated by its partner protein gH in some manner. The fusogenic activity of gB is controlled by its cytoplasmic (or intraviral) domain (gBCTD) and, according to the current model, the gBCTD is a trimeric, inhibitory clamp that restrains gB in the prefusion conformation. But how the gBCTD clamp is released by gH is unclear. Here, we identified two new regulatory elements within gB and gH from the prototypical herpes simplex virus 1: a surface pocket within the gBCTD and residue V831 within the gH cytoplasmic tail. Mutagenesis and structural modeling suggest that gH V831 interacts with the gB pocket. The gB pocket is located above the interface between adjacent protomers, and we hypothesize that insertion of the gH V831 wedge into the pocket serves to push the protomers apart, which releases the inhibitory clamp. In this manner, gH activates the fusogenic activity of gB. Both gB and gH are conserved across all herpesviruses, and this activation mechanism could be used by other gB homologs. Our proposed mechanism emphasizes a central role for the cytoplasmic regions in regulating the activity of a viral fusogen.
Author summary
Herpes simplex virus 1 (HSV-1) establishes lifelong infections in over a half of people and causes diseases ranging from oral or genital sores to blindness and brain inflammation. No vaccines or curative treatments are currently available. To infect cells, HSV-1 must first penetrate them by merging its lipid envelope with the membrane of the target cell. This process requires the collective actions of several viral and cellular proteins, notably, viral glycoproteins B and H (gB and gH). gH is thought to activate the fusogenic function of gB, but how the two proteins interact is unclear. Here, using mutational analysis, we have identified two new functional elements within the cytoplasmic regions of gB and gH: a surface pocket in gB and a single residue in gH, both of which are important for membrane fusion. Based on structural modeling, we propose that the gB pocket is the binding site for the gH residue, and that their interaction activates gB to cause membrane fusion. These findings extend our knowledge of the HSV-1 membrane fusion mechanism. Mechanistic understanding of HSV-1 entry is essential for understanding its pathogenesis and developing new strategies to prevent infections.
Introduction
Membrane fusion during the entry of enveloped viruses is carried out by viral fusogens, which are proteins displayed on the viral surface that bring the opposing viral and host membranes so close that they merge. To do so, these proteins must refold from the high-energy prefusion conformation into the low-energy postfusion conformation. The energy released upon refolding is thought to overcome the large kinetic barrier associated with membrane fusion (reviewed in [1]). To ensure proper spatial and temporal deployment of viral fusogens, their activity is regulated by environmental signals, such as proton concentration, or interactions with other viral and cellular proteins.
Some of the most complex membrane fusion mechanisms are found in herpesviruses–a family of double-stranded-DNA, enveloped viruses that infect most animal species for life. Their entry requires, at a minimum, three conserved glycoproteins, gB, gH, and gL. gB is a transmembrane glycoprotein composed of an ectodomain, a transmembrane helix, and a cytoplasmic domain that functions as a membrane fusogen. By analogy with other viral fusogens, the refolding of gB from the prefusion to the postfusion conformation is thought to provide the energy for membrane fusion. Indeed, the structures of the prefusion [2,3] and the postfusion forms [4–8] of gB from several herpesviruses suggest large conformational changes that accompany refolding.
gB is a class III fusogen, along with the Vesicular Stomatitis Virus G, baculovirus gp64, and thogotovirus Gp (reviewed in [9]). Like other class III fusogens, gB exists as a trimer. Yet, uniquely, gB is not a stand-alone fusogen activated by exposure to low pH. Instead, gB must be activated by the conserved heterodimeric complex composed of two viral glycoproteins, gH and gL. gH is a transmembrane glycoprotein composed of an ectodomain, a transmembrane helix, and a short cytoplasmic tail. gL is a soluble glycoprotein that binds gH and is required for its proper folding, trafficking to the cell surface, and function [10,11].
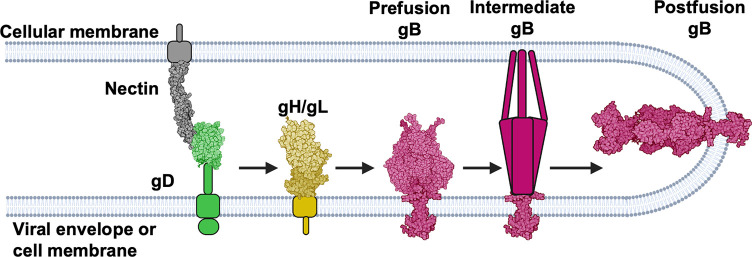
The gH/gL heterodimer occupies a central place in the herpesvirus entry and membrane fusion processes because it interacts with several key participants. On the one hand, gH/gL either interacts with the host cell receptors directly or engages viral receptor-binding accessory proteins, depending on the herpesvirus (reviewed in [12, 13]). On the other hand, it binds and activates gB, the fusogen (reviewed in [12,13]). According to the prevalent model [14], interaction with the host cell receptor triggers a cascade of events in which gH/gL transmits the activating signal from the host cell receptor to gB. For example, in the prototypical herpesvirus herpes simplex virus 1 (HSV-1) (reviewed in [15]), which establishes lifelong infections in over a half of people ([16] and reviewed in [17]) and causes oral or genital sores (reviewed in [18]) as well as encephalitis (reviewed in [19–21]), binding of the viral receptor-binding protein, gD, to one of its cognate cellular receptors–nectin-1, herpesvirus entry mediator (HVEM), or 3-OS-modified heparan sulfate ([22, 23] and reviewed in [24])–causes conformational changes in gD that enable it to activate the gH/gL complex [25,26] that, in turn, activates the fusogenic activity of gB [2,5,14,27] (Fig 1). But how gB is activated by gH/gL is unknown.
Fig 1. HSV-1 fusion pathway model.
gD (2C36 [28]) binds a receptor (3U83 [29]) on the target cell and activates gH/gL; gH/gL (3M1C [27]) triggers gB (6Z9M [2] and 5V2S [5]) to refold and cause fusion. gD has been suggested to be a dimer [28] but is shown here as a monomer for clarity. Figure created with BioRender.com.
While the gB ectodomain undergoes refolding and interacts with the target cell membrane [30], the cytoplasmic domain of gB (gBCTD) is also important for fusion, just as the cytoplasmic domains of fusogens in viruses such as the Human Immunodeficiency Virus [31] and paramyxoviruses [32]. The gBCTD is thought to inhibit the fusogenic activity of gB because most known gBCTD mutations–C-terminal truncations, point mutations, and insertions–are hyperfusogenic, i.e., they increase fusion [33–47]. The crystal structure of the full-length HSV-1 gB [5] revealed that the gBCTD is a trimer stabilized by multiple protein/protein and protein/membrane interactions, and that the majority of the hyperfusogenic gBCTD mutations would be predicted to disrupt these stabilizing interactions [5]. Therefore, we have previously proposed that the gBCTD acts as an inhibitory clamp that stabilizes gB in its prefusion form by restricting conformational rearrangements of the gB ectodomain.
In addition to gBCTD, the cytoplasmic tail of gH (gHCT) is also important for fusion, but instead of inhibiting fusion, it activates it. gHCT truncations decrease fusion in a manner proportional to the length of the truncated sequence [44] such that the shorter the remaining gHCT length, the lower the fusion. Given the apparent inhibitory function of the gBCTD and the activating function of the gHCT, previously, we hypothesized that gH may activate gB by using gHCT to bind the inhibitory gBCTD clamp and disrupt it in a wedge-like manner [5]. However, the respective binding sites on gBCTD and gHCT are unknown.
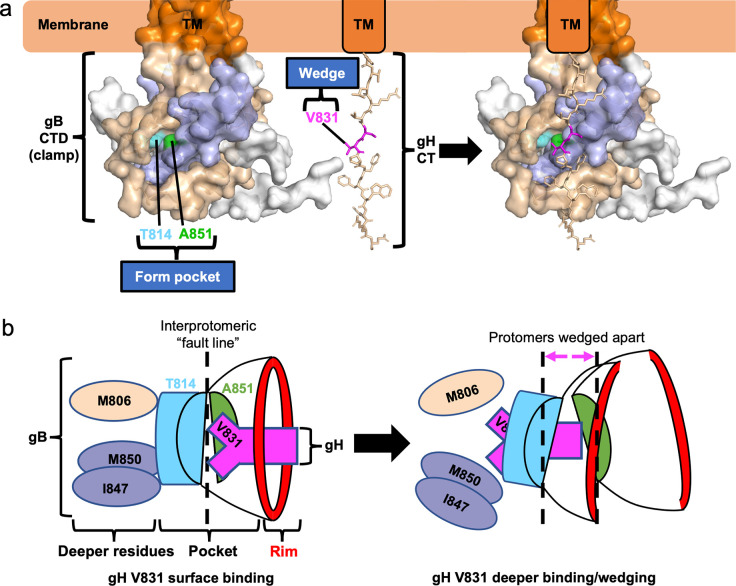
Here, we identified a previously uncharacterized functional site important for fusion within the HSV-1 gBCTD, composed of a surface pocket between adjacent protomers. Mutations of residues A851 and T814 located at the bottom of a surface pocket reduce fusion whereas mutations of residues lining the pocket rim are hyperfusogenic, which suggests that the pocket and the rim have important yet opposite roles in fusion. Moreover, we identified gHCT residue V831 as the most functionally important residue within the gHCT. When the gHCT is modelled as an extended polypeptide, V831 ends up approximately the same distance from the membrane as the gBCTD pocket, making interactions at these respective sites plausible if gH and gB were to come into proximity. We hypothesize that gHCT residue V831 serves as the wedge that inserts into the newly identified gBCTD pocket. The gB pocket is located above the interface between adjacent protomers, which is akin to a “fault line” within the gBCTD trimer, and we hypothesize that insertion of the gH V831 wedge into the pocket serves to push the protomers apart, which releases the inhibitory clamp. This action would destabilize the inhibitory gBCTD clamp, causing it to release its hold on the gB ectodomain. We hypothesize that in this manner, gH activates the fusogenic activity of gB. The proposed gH-gB triggering mechanism extends our understanding of the regulatory cascade that coordinates HSV-1 entry and may inform new therapeutic approaches aiming at blocking HSV-1 glycoprotein interactions. Both gB and gH are conserved across all herpesviruses, and this activation mechanism could be used by other gB homologs. Our proposed mechanism emphasizes a central role for the cytoplasmic regions in regulating the activity of a viral fusogen.
Results
The A851V gBCTD mutant is hypofusogenic
Unlike the very common hyperfusogenic mutations, hypofusogenic gBCTD mutations, i.e., those that decrease fusion, are rare in HSV-1 and HSV-2 and all result in very low surface expression levels, implying a defect in protein folding [34]. Indeed, within the structure of the trimeric HSV-1 gBCTD, these mutations map to the hydrophobic core and have been proposed to cause misfolding by eliminating interactions critical for basal trimer stability [5]. No “true” hypofusogenic mutations in the HSV-1 gBCTD that decrease fusion without affecting protein expression have yet been reported. Interestingly, the HSV-1 gBCTD mutant A851V decreased viral entry [33]. Importantly, we found that the A851V mutation had no effect on the cell surface expression of gB (Figs 2A and S2) or gH/gL (Figs S1 and 3A), as measured by flow cytometry. Therefore, we hypothesized that A851V reduces viral entry by reducing gB fusogenicity.
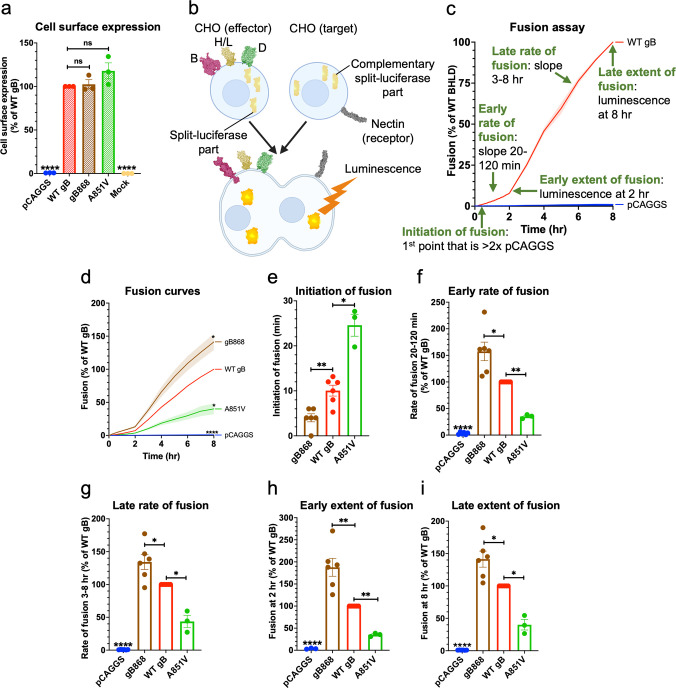
Fig 2. The gBCTD mutant A851V reduced the rate and extent of fusion.
a) gB A851V and gB868 cell surface expression measured by flow cytometry. R68 primary antibody. Columns show mean. Error bars are SEM. b) Split-luciferase cell-cell fusion assay experimental setup. Cells expressing HSV-1 glycoproteins gD (2C36 [28]), gH/gL (3M1C [27]), and gB (6Z9M [2], and 5V2S [5]) fuse with cells expressing a nectin-1 receptor (3U83 [29]). Reconstitution of luciferase reports on fusion. Created with BioRender.com. c) Fusion of cells transfected with WT HSV-1 gB, gH, gL, gD compared to pCAGGS. The initiation of fusion is defined as the first reading at which luminescence is greater than twice that of the pCAGGS negative control. Early and late rates of fusion are the slopes of the fusion curves between 20–120 minutes and 3–8 hours post addition of target cells to effector cells, respectively. Early and late extent of fusion is defined as luminescence at 2 and 8 hours post addition of target cells to effector cells, respectively. This represents the total amount of fusion that has occurred over that time. d) Fusion of A851V over 8 hr by the split-luciferase fusion assay. *: p < 0.05 compared to WT gB at 8 hr. gB868 was used as a hyperfusogenic positive control [48]. Curve indicates mean values. Shaded area represents SEM. e-i) Initiation of fusion, early and late rates and extents of fusion of A851V. Columns show mean. Error bars are SEM. ns: not significant, *: p < 0.05, **: p < 0.01, ****: p <0.0001 in all panels. Data in all panels are from 3–6 independent experiments.
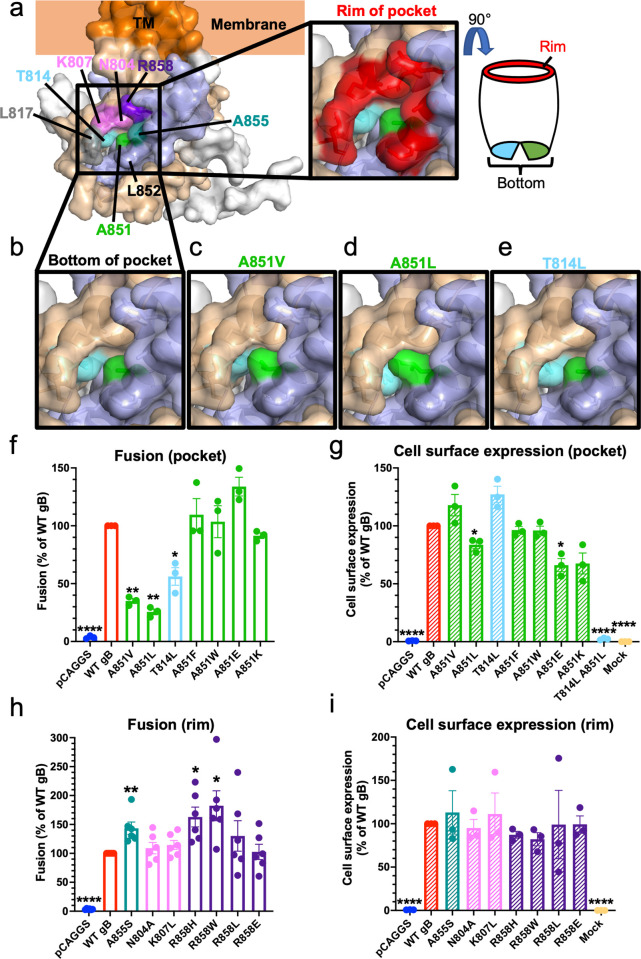
Fig 3. Fusogenicity and structural effects of mutants in the newly identified gBCTD pocket and rim.
a) gBCTD crystal structure and the structure of the pocket on the gBCTD. The pocket is formed at the junction of opposing gBCTD protomers, which are colored in wheat and light blue, with the third protomer in white. The residues that form the pocket on the gBCTD are highlighted in colors (left panel). The residues of the outer rim of the pocket are indicated in red (right panel). b) The bottom of the pocket is made up of T814 and A851, in sky blue and green, respectively. They do not contact each other, leaving a space between them at the bottom of the pocket. c-e) Pocket mutations of T814 and A851 are hypofusogenic and were modeled in PyMOL. All three hypofusogenic mutations of T814 and A851 were predicted to fill the gBCTD pocket as well as the space between T814 and A851 at the bottom of the pocket. f) Fusogenicity of T814 and A851 pocket mutants at 2 hr. A851V data is the same as shown in Fig 2. Fusion trends were the same at 8 hr. g) Cell surface expression of pocket mutants by flow cytometry. A851V data is the same as shown in Fig 2. h) Fusogenicity of mutations of the pocket rim at 2 hr. Fusion trends were the same at 8 hr. i) Cell surface expression of rim mutants. Columns show mean and error bars are SEM in all panels. *: p < 0.05, **: p < 0.01, ****: p < 0.0001 in all panels. Data in all panels are from three to six independent experiments.
To test the hypothesis that the gB A851V mutant was hypofusogenic, we tested its fusogenicity directly. The fusogenic properties of viral fusogens are typically characterized by monitoring cell-cell fusion of uninfected cells expressing viral glycoproteins and host receptors. Here, we used a split-luciferase cell-cell fusion assay reported previously [49], in which the effector and the target cells are transfected with the complementary parts of Renilla luciferase. Upon fusion of effector cells with the target cells, functional luciferase forms, and the resulting luminescence is used to quantify fusion (Fig 2B). This assay can measure not only early and late extent of fusion, but also early and late rate of fusion, and fusion initiation (Fig 2C).
We found that A851V mutation reduced not only the extent of fusion, but also early and late fusion rates while delaying the initiation of fusion (Fig 2D–2I). The known hyperfusogenic truncation mutant gB868 was used as a control and had increased extent and rate of fusion as well as earlier fusion initiation (Fig 2D–2I). The A851V fusion defect manifested within minutes and was sustained over the entire time course, reaching only ~40% of WT gB fusion by 8 hours. The fusion defect at both early and late steps in fusion suggests that A851V mutation impairs an early, rate-limiting step of fusion. Therefore, A851V is a true hypofusogenic mutation–one that impairs fusogenicity despite being properly folded–the first of its kind reported within the HSV-1 gBCTD.
Mutational analysis of the surface pocket containing A851
A851 is located at the bottom of a surface-exposed pocket that contains another residue, T814 (Fig 3A and 3B). The outer rim of the pocket is formed by residues L817, K807, N804, R858, A855, and L852 (Fig 3A). The pocket is located at the junction of neighboring protomers such that residues N804, K807, T814, and L817 belong to one protomer, and residues A851, L852, A855 and R858 belong to the neighboring protomer (Fig 3A). Thus, the gBCTD trimer contains three symmetry-related pockets.
We first investigated the functional significance of the pocket. A larger hydrophobic side chain of valine in the A851V mutant would reduce the size of the pocket (Fig 3C). To further probe the role of A851, we mutated it to a leucine, which has a larger hydrophobic side chain than valine and would be expected to reduce the pocket size further (Fig 3D). The other pocket residue, T814, was also mutated to a leucine, keeping up with the strategy of introducing a larger hydrophobic side chain (Fig 3E). Just as A851V, both A851L and T814L mutations were hypofusogenic (35%, 26%, and 56% of WT gB fusion at 2 hrs, respectively) (Fig 3F). T814L had a WT-level of gB cell surface expression whereas the cell surface expression of the A851L mutant was slightly reduced (Figs 3G and S2). The mutations had no effect on gH/gL cell surface expression (Figs S1 and 3A).
To understand the structural basis of the observed fusion phenotypes, we examined the effect of mutations on the local structure of the gBCTD. At the bottom of the pocket, T814 and A851 do not directly contact one another (Fig 3B). A851V, A851L, or T814L mutations are all predicted to fill the gBCTD pocket (Fig 3C–3E), yet none are expected to destabilize the gBCTD trimer. Given that all three mutations reduce fusion, the pocket appears important for fusion.
To further test this hypothesis, we designed more drastic gB pocket mutations A851F, A851W, and the double mutant T814L/A851L, to occlude the pocket fully, expecting them to decrease fusion to an even greater extent. To investigate the effect of charged residues at this position, we also designed A851E and A851K mutations. The T814L/A851L double mutant was not expressed on the cell surface (Fig 3G), possibly, due to protein misfolding caused by a steric clash. A851F and A851W mutants were expressed on the cell surface at WT levels whereas A851E and A851K had slightly reduced cell surface levels (Figs 3G and S2). A851F, A851W, and A851K had no effect on gH/gL cell surface expression and A851E had slightly increased gH/gL cell surface expression (Figs S1 and 3A). Surprisingly, A851F, A851W, A851K, and A851E had no statistically significant effect on fusion (Fig 3F). This was unexpected considering that all substitutions were predicted to completely fill the pocket (S4A–S4F Fig).
Further structural modeling revealed that A851F, A851W, and A851K mutations may cause steric clashes with the surrounding residues (S4G–S4K Fig), which could destabilize the gBCTD trimer. gBCTD trimer destabilization correlates with a hyperfusogenic phenotype [5]. Therefore, we hypothesized that while filling of the pocket would be expected to reduce fusion, this effect would be counteracted by the hyperfusogenic effect of trimer destabilization, yielding the observed WT fusion levels for each mutant. The charges introduced in A851E and A851K could also be causing a similar counter-effect. Collectively, our findings show that the newly identified surface pocket containing residues A851 and T814 is important for fusion (Fig 3A).
Mutational analysis of the pocket rim
We next mutated residues lining the pocket rim (Fig 3A). Mutations were designed to alter side chain polarity, charge, or size (N804A, K807L, A855S, R858W, R858L, R858E) [50] while avoiding large structural changes that could destabilize the gBCTD trimer because such mutations would be expected to have a hyperfusogenic phenotype [5]. Mutations were first modeled in PyMOL [51] and analyzed for significant changes to the surrounding gBCTD structure. Previously reported hyperfusogenic mutations L817H [35] and L817P [36] were not tested here. L852 was not mutated because its mutation L852A was reported to abrogate cell surface expression [34].
Mutations of the rim residues were either hyperfusogenic or had no effect on fusion (Fig 3H). All had similar cell surface expression as WT gB (Figs 3I and S2) and did not affect gH/gL cell surface expression (Figs S1 and 3A). A855S, R858H (also tested in [37,42,44,48]), and R858W were markedly hyperfusogenic. The phenotype of A855S is consistent with the hyperfusogenic phenotype of A855V reported by others [35,40]. The phenotypes of R858H and R858W are consistent with the hyperfusogenic phenotype of R858C reported by others [33]. N804A, K807L, R858L, and R858E had no effect on fusion. According to structural modeling, most mutations are predicted to expose the pocket opening (L817P, N804A, K807L) or the protomeric interface (R858C, R858H, R858L, R858E) whereas some mutations are not (A855S, A855V, and R858W). R858 crosses the protomeric interface between adjacent protomers but lies in the upper rim of the pocket and does not cover the pocket (Fig 3A). Therefore, many of its mutations are predicted to expose the protomeric interface without altering the exposure of the pocket opening directly. Many mutations also neutralize a positive charge in the upper portion of the rim (K807L, R858C, R858H, R858W, and R858L). While the predicted structural effects are diverse, the mutations in the rim of the gBCTD pocket are all either hyperfusogenic or fusion-neutral, contrasting with the hypofusogenicity of the pocket mutants. The hyperfusogenic rim mutants are predicted to either expose the pocket or the protomeric interface to a greater extent or neutralize a positive charge, except for A855S and A855V. This suggests a regulatory role for the rim in the fusogenicity of gB.
gH V831 is the most important gHCT residue for fusion
Mutations that reduce the size of the A851/T814 pocket in gBCTD without destabilizing the trimer–A851V, A851L, and T814L –reduce fusion. Therefore, the size of the pocket is important for fusion, and we hypothesized that it could function as a binding site. Previously, we showed that gHCT has an activating role in fusion because its truncations progressively reduce fusion [44], and proposed that gH may activate gB by using gHCT to disrupt the inhibitory gBCTD trimer in a wedge-like manner [5]. Therefore, we hypothesized that the gBCTD pocket was the binding site for the gHCT.
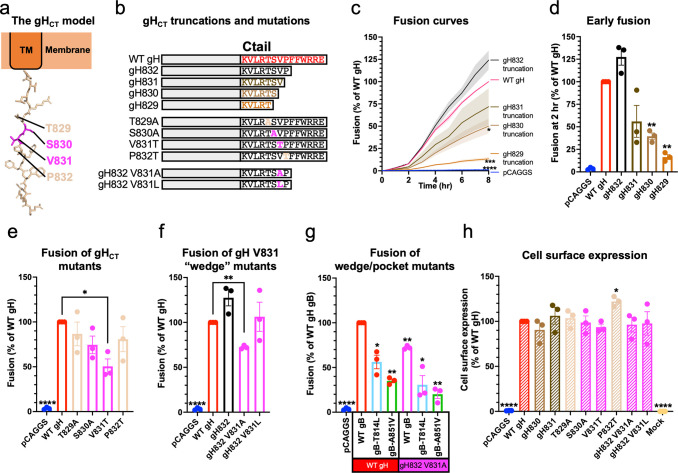
To identify the gHCT residues that could interact with the gBCTD pocket, we first narrowed down residues within the 14-residue gHCT necessary for fusion. In our previous work, we showed that the gH832 truncation mutant lacking 6 residues of the gHCT (Fig 4A and 4B) had WT-level fusion whereas the gH829 mutant lacking 9 residues had a significantly reduced fusion [44]. To narrow down residues most important for fusion, we tested serial truncations of gH829, gH830, gH831, and gH832 (Fig 4B). gH832 was slightly hyperfusogenic (Fig 4C) whereas gH831, gH830, and gH829 were hypofusogenic, with fusion extent proportional to the length of the remaining gHCT (Fig 4C and 4D). Cell surface expression of the gH truncation mutants was similar to the WT gH expression (Figs 4H and S3B).
Fig 4. gH V831 is the most important gHCT residue for fusion.
a) Structural modeling of the gHCT. b) Summary of gHCT truncations and mutations tested to determine which gHCT residues are the most important for fusion and probe their mechanism of action. c) Kinetics of fusion of gHCT truncations over 8 hr. Statistical significance shown is based on comparisons to WT gH fusion at 8 hr. Curves are the mean. Shaded area is SEM. d) Fusion of gHCT truncations at 2 hr. e) Fusion of mutations of gH829-832 residues at 2 hr to probe the function of the residues. Fusion trends were the same at 8 hr. f) Fusion of V831 mutations designed to make the putative gH wedge smaller (V831A gH832) or larger (V831L gH832), at 2 hr. Fusion trends were the same at 8 hr. g) Fusion of mutations creating a smaller wedge (V831A gH832) combined with mutations creating smaller pockets (gB T814L, gB A851V), at 2 hr. Fusion trends were the same at 8 hr. h) Cell surface expression of the gHCT truncations and mutations. Expression of gH829 and gH832 was determined previously to be the same as WT gH expression [44]. LP11 primary antibody. *: p < 0.05, **: p < 0.01, ***: p < 0.001, ****: p < 0.0001 in all panels. Bars indicate the mean and error bars are SEM. Statistical comparisons are to WT gH and gB. All panels represent averages of three independent experiments.
To further probe the functional importance of residues T829, S830, V831, and P832, we reversed their polarity [50] by either making them more hydrophobic (T829A, S830A, and P832T) or more hydrophilic (V831T), in the context of the full-length gH (Fig 4B). Only V831T was hypofusogenic in a statistically significant manner (Fig 4E). There were no significant differences in cell surface expression relative to WT gH, except for P832T, which had slightly increased expression (Figs 4H and S3B). Collectively, these data implicated V831 as the most important gHCT residue for fusion among those tested.
The gHCT is predicted to be unstructured and contains residues that are disfavored in α-helices, such as proline and valine [52,53]. When the gHCT was modelled as an extended polypeptide, gH V831 ended up approximately the same distance from the membrane as the gBCTD binding pocket (Fig 5A). This means that when gH and gB come into proximity, gH V831 could, in principle, interact with the gBCTD binding pocket (Fig 5A). Therefore, we propose that V831 is the gHCT “wedge” and that it binds the gBCTD pocket containing T814 and A851 (Fig 5A).
Fig 5. A model for gBCTD/gHCT interactions and fusion triggering.
a) gBCTD residues T814 (sky blue) and A851 (green) form a gH-binding pocket on the surface of the gBCTD trimer. gH V831 (magenta) acts as a wedge. Modeling shows that the gH V831 wedge and the gBCTD pocket are equidistant from the membrane. The V831 wedge binds between gB T814 and A851 in the gB pocket and pushes the gBCTD protomers (wheat and light blue) apart to destabilize gB and trigger fusogenic refolding of gB into the postfusion conformation. b) gH V831 (magenta) acts as a wedge that initially binds between gBCTD residues T814 (sky blue) and A851 (green) in the pocket on the surface of the gBCTD trimer. gH V831 then binds to deeper hydrophobic residues of the gBCTD (colored by protomer), forming favorable hydrophobic interactions. This causes the protomers of gB to be pushed apart as gH V831 enters deeper into the gBCTD, destabilizing the gBCTD clamp and triggering gB to refold and cause fusion. The interprotomeric “fault line” refers to the boundary between the wheat and blue protomers, which widens as the protomers are wedged apart.
We next investigated the side chain requirement at the gH residue 831. A hydrophobic side chain appears to be required at this location because threonine could not effectively substitute for valine despite a comparable side chain size (Fig 4B and 4E). We then asked whether a smaller (alanine) or a larger (leucine) hydrophobic side chain could support efficient fusion. Previously, we showed that V831A mutation was hypofusogenic [54]. Thus, a smaller hydrophobic side chain of alanine could not substitute for a valine. Interestingly, the hypofusogenic phenotype of V831A was observed only in the context of a truncated gH832 but not full-length gH [54], suggesting that residues 833–838 somehow compensated for the fusion defect of the V831A mutation. To test the effect of a larger hydrophobic side chain at position 831, we generated the V831L mutation in the context of the gH832 truncation (gH832 V831L) to prevent potential compensation by residues 833–838 (Fig 4B). gH832 V831L had WT fusion level (Fig 4F) whereas gH832 V831A was hypofusogenic, in accordance with our previous finding [54]. Neither gH832 V831A nor gH832 V831L mutations had any effect on cell surface expression (Figs 4H and S3B). We conclude that valine is minimally required at position 831 to maintain WT levels of fusion.
We then tested the possibility that, in the context of a smaller gBCTD pocket (gB A851V or T814L), a smaller gHCT wedge (gH832 V831A) could support fusion by being able to fit into a smaller pocket. We found that the small pocket/small wedge combinations did not restore fusion to WT levels but, instead, reduced fusion in an additive manner (Fig 4G). Therefore, we hypothesize that both the gBCTD pocket and the gHCT wedge have to be of a certain size to function efficiently.
Discussion
Identification of a new functional pocket in the gBCTD
Using mutational analysis, we have identified a new functional region of the gBCTD composed of a surface-exposed pocket and its rim (Fig 3A). The bottom of the pocket is formed by residues T814 and A851 and the rim is formed by residues N804, K807, L817, L852, A855, and R858. The pocket is located at the junction of two gB protomers, and the pocket and the rim residues are evenly distributed between the neighboring protomers, with N804, K807, T814, and L817 located in one protomer and A851, L852, A855, and R858 in the neighboring protomer. Thus, there are three such pockets within the gBCTD trimer.
Mutations of the gBCTD pocket and rim had opposite effects on fusion. Pocket mutations A851V, A851L, and T814L, which partially filled the pocket, presumably, without otherwise perturbing the surrounding gBCTD structure, significantly decreased fusion, i.e., were hypofusogenic. These three mutations are unusual because, to the best of our knowledge, they are the first hypofusogenic HSV-1 gBCTD mutations that perturbed protein function rather than caused protein misfolding. The hyperfusogenic rim mutations are also unusual because they differed from most other hyperfusogenic mutations in both their location and the presumed mechanism of action. Most hyperfusogenic mutations in the gBCTD [33–45] map to either the protomeric interfaces or the membrane-binding regions and are thus predicted to destabilize the membrane-dependent gBCTD trimer [5]. In contrast, the hyperfusogenic rim mutations, which include mutations of residues L817, A855, and R858 reported here and elsewhere [33, 35–37, 40, 42, 44, 48], are located on the surface and would not be predicted to disrupt the gBCTD trimer. The gBCTD pocket is also located too far from the membrane to participate in membrane interactions. Therefore, the rim mutations increase fusion by a different mechanism, potentially, by exposing the pocket (see below). Collectively, our mutational analysis and structural modeling uncovered a new fusogenic site within the gBCTD trimer composed of two distinct functional regions: the pocket and its rim.
The gBCTD pocket is a putative binding site for the gHCT wedge, residue V831
Surface pockets often function as binding sites, and mutations that fill surface pockets typically disrupt protein function by blocking binding to protein partners. For example, mutagenesis of a putative chaperone-binding pocket of the p53 protein decreased its expression, indicating that the binding pocket was important for binding the chaperone that stabilizes p53 [55]. Introducing an amino acid with a bulkier, branched side chain into a pocket on Rabies virus P protein resulted in decreased binding by STAT proteins, suggesting that the mutated pocket constitutes the STAT-binding site [56]. Furthermore, in enzymes, active sites, which bind and convert substrates, are typically found in surface pockets, and pocket-filling mutations can either block binding of the substrate to the active site [57] or obstruct its access through a tunnel or channel [58,59].
Mutations that filled the gBCTD pocket decreased fusion, suggesting that the size of the pocket is important for fusion, so we hypothesized that the gBCTD pocket serves as a binding site for a protein partner and that the binding event activates fusion. Previously, we speculated that gH may activate gB by using gHCT to disrupt the inhibitory gBCTD trimer in a wedge-like manner [5]. Therefore, we hypothesized that the newly identified gBCTD pocket was the binding site for the gHCT. Using mutational analysis, we identified gHCT residue V831 as the single most critical residue for fusion among those tested. Our structural analysis predicted that if the 14-residue gHCT adopted an extended conformation, gH V831 and the gBCTD pocket would end up roughly equidistant from the membrane, putting them into ideal positions for reciprocal interaction (Fig 5A). Based on these results, we propose that V831 binds in the gBCTD pocket (Fig 5A).
We hypothesize that to trigger fusion, the gH V831 side chain binds the gBCTD pocket and inserts deeply enough to push the gB protomers apart like a wedge or a crowbar. This destabilizes the gBCTD clamp, which releases its inhibitory hold on the gB ectodomain, allowing the latter to refold from the prefusion into the postfusion conformation. This hypothesis is supported by the location of the gBCTD pocket right above the interface between adjacent protomers, which is akin to a “fault line” within the gBCTD trimer, a prime location for pushing the protomers apart. Just as the size of the gBCTD pocket is critical for fusion (with a smaller pocket decreasing fusion), so is the size of the sidechain at gH residue 831. A smaller alanine (V831A) decreased fusion whereas a larger leucine (V831L) preserved WT-level fusion. Thus, there appears to be a minimum requirement for the size of the side chain at gH residue 831 for WT-levels of fusion. An alanine would not be able to insert deeply enough between the gB protomers and would be less effective at destabilizing the gBCTD, which explains why V831A mutant is hypofusogenic. Interestingly, V831T mutant was also hypofusogenic. The side chain of threonine is similar in size to valine yet is more hydrophilic, which suggests that in addition to size, hydrophobicity of the gH residue 831 is important for fusion.
Implicit in our insertion model is the assumption that the gH V831 residue first interacts with T814 and A851 as it inserts into the pocket and then may interact with nearby residues located within the gBCTD core as it wedges in deeper to push the protomers apart (Fig 5B). The residues that lie underneath the gBCTD pocket, M806, I847, and M850, are hydrophobic, so a hydrophobic residue would be a more effective wedge because it could form more favorable interactions with these residues (Fig 5B). This helps explain why a slightly larger and hydrophobic leucine (V831L) is an efficient substitute for the native valine whereas a similarly sized yet hydrophilic threonine (V831T) is not. The observation that the residues underneath the gBCTD pocket are hydrophobic further supports our model that the pocket is the binding site for the gHCT wedge, residue V831.
Role of the rim of the gB pocket in fusion
In contrast to the hypofusogenic mutations of the gBCTD pocket, all mutations of the pocket rim were either hyperfusogenic or fusion-neutral. This included both mutations made in this work (R858W, R858L, R858E, A855S), and those reported previously (R858H [37,42], R858C [33], L817H [35], L817P [36], A855V [35,40]). Being located on the surface, none of these mutations would be predicted to disrupt the gBCTD trimer, in contrast to the majority of the known hyperfusogenic mutations [5]. Therefore, the pocket rim mutations enhance fusion by a different mechanism.
The rim is the entryway into the gBCTD pocket, and some of the hyperfusogenic rim mutations (R858C and R858H) appear to expose the entryway into the pocket to some extent. This may increase fusion by facilitating access of the gHCT wedge to the gBCTD pocket. Some hyperfusogenic rim mutations neutralize the positive charge in the upper portion of the pocket (R858C, R858H, and R858W). Neutralization of a positive charge in the upper portion of the pocket rim could facilitate access of the gHCT wedge to the pocket. Congruent with this idea is that the gHCT is mostly uncharged, notably, residue V831. Some mutants that are predicted to expose the entryway into the pocket (N804A, R858E) or neutralize a positive charge or both (K807L, R858L) were fusion-neutral. It is possible that the effects of these mutations on fusion are more modest than for other mutants, and as a result, statistically significant differences from WT gB could not be detected by our assay.
The A855S and A855V mutations do not change the charge or the access to the pocket. Nonetheless, we hypothesize that by analogy with the other rim mutations listed above, A855S and A855V mutations somehow facilitate gHCT interactions with the gBCTD pocket. The hyperfusogenic phenotype of L817P and L817H mutants is difficult to explain due to insufficient structural data; L817 is the last resolved residue before a disordered loop and its conformation is likely dynamic and difficult to predict.
More drastic gB A851 pocket mutations may offset the pocket-filling effect with trimer destabilization
Having determined that the gBCTD pocket-reducing mutations A851V, A851L, and T814L all reduced fusion, we had anticipated that more drastic mutations would reduce fusion further. Therefore, we designed mutations A851F and A851W to completely fill the gBCTD pocket and A851E and A851K to both fill the pocket and introduce charge. However, A851F, A851W, A851K, and A851E were fusion-neutral. Structural analysis suggested that due to their large side chains, both phenylalanine and tryptophan would clash with nearby residues causing significant steric strain. Since the gBCTD pocket spans neighboring protomers, we hypothesize that in addition to filling the gBCTD pocket, A851F or A851W also destabilize the gBCTD trimer. Previous mutations predicted to disrupt the gBCTD trimer were hyperfusogenic and were proposed to be more easily triggered by gH/gL due to the gBCTD destabilization, putting the gBCTD on a “hair-trigger” [5]. However, these mutants still required gH/gL, suggesting that the inhibitory effect of the gBCTD clamp in these mutants was weakened but not abrogated. We propose that A851F and A851W have a similar effect. As a result, the hypofusogenic phenotype expected of the pocket-filling mutation is counterbalanced by the hyperfusogenic effect of gBCTD destabilization, resulting in the observed WT-level fusion phenotype. A851K would likewise be predicted to destabilize the gBCTD trimer due to steric strain. Additionally, A851K and A851E could destabilize the gBCTD by the introduction of a charge.
Potential conservation of gH-gB triggering mechanism in other herpesviruses
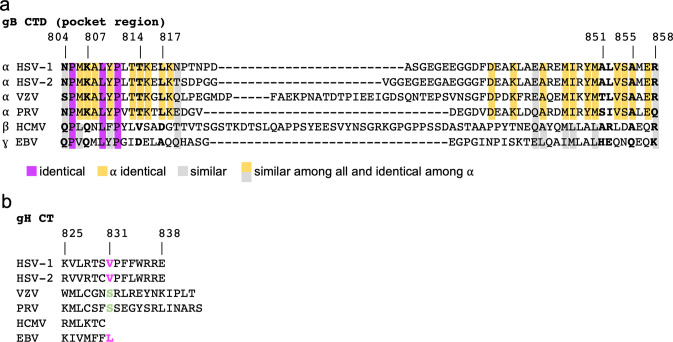
The gBCTD sequences are conserved across alphaherpesviruses and, to a lesser extent, among herpesviruses [5]. Therefore, the gBCTD homologs may share a conserved structural fold and the surface pocket observed in HSV-1 gBCTD. Indeed, regions containing the pocket and rim residues (HSV-1 gB 804–819 and 849–858) are more highly conserved (Fig 6A), with most pocket and rim residues being identical across alphaherpesviruses and similar across herpesviruses examined here (Fig 6A). While residue at the position 851 (HSV-1 gB) is less well conserved, three out of six aligned sequences have an alanine (Fig 6A). Thus, the sequence analysis supports the hypothesis that the proposed HSV-1 gHCT V831 wedge/gBCTD pocket mechanism of gB triggering by gH could be conserved in other herpesviruses.
Fig 6. Conservation of gBCTD and gHCT sequences across selected herpesviruses.
a) Alignment of gBCTD sequences of selected herpesviruses in the region that includes all HSV-1 pocket and rim residues [60]. Chosen sequences include all human alphaherpesviruses (HSV-1, HSV-2, VZV), a closely related non-human alphaherpesvirus (PRV), and important human beta- and gammaherpesviruses (HCMV, EBV). Equivalent residues to the HSV-1 pocket and rim residues are bolded and labeled with HSV-1 residue numbers. Identical and similar residues across herpesviruses are colored. b) Alignment of gHCT sequences of selected herpesviruses [60]. Equivalent residues to the HSV-1 V831 residue is bolded and colored. Pink indicates similar to HSV-1. Green is different from HSV-1.
On the gHCT side, position 831 (HSV-1 gH) has a conserved valine in HSV-2 and a similar leucine in Epstein-Barr Virus (EBV) (Fig 6B). However, in both Varicella Zoster Virus (VZV) and Pseudorabies Virus (PRV), the equivalent position is occupied by serine. A previous study found that HSV-1 gH/gL can trigger PRV gB effectively but not the other way around [61]. This suggests that PRV gH/gL is less effective at activating the fusogenic ability of gB than HSV-1 gH/gL. We hypothesize that the serine in the PRV gHCT−and perhaps also in VZV–is a less effective wedge than the valine in HSV-1 gH, similarly to the threonine in the HSV-1 gH V831T mutant. Surprisingly, the 6-residue long gHCT in human cytomegalovirus (HCMV) is much shorter than its counterparts in other herpesviruses. Future studies will examine the gH-gB triggering mechanism across herpesviruses.
Open questions
According to our model of gB triggering, residue V831 within the gHCT acts as a wedge that binds in the gBCTD pocket and pushes the gB protomers apart. This destabilizes the gBCTD trimer, relieving its inhibitory hold on the ectodomain and allowing fusogenic refolding. However, a few unanswered questions remain. First, we do not yet have direct evidence of an interaction between the gBCTD pocket and the gHCT wedge. Recently, we reported that gH/gL and gB interact through multiple domains, independently of gD [62]. Disrupting the interactions within the cytoplasmic regions did not reduce overall gH-gB interaction, presumably due to the remaining interactions between the ectodomains and the TMDs. Such extensive interactions between gH and gB present a challenge for future investigations of a direct interaction between the gBCTD pocket and the gHCT wedge. Second, it remains to be elucidated what changes occur in gH that cause gH V831 to perform our proposed wedging action in the gBCTD pocket. We hypothesize that pre-existing interactions between the gBCTD and the gHCT [62] position the gH V831 wedge near the gBCTD pocket. Binding of gD to one of its cognate receptors activates gH/gL, causing it to undergo a conformational change that would push the wedge deeper into the gBCTD pocket, triggering the fusogenic refolding of gB. Third, it is unknown how the signal from the destabilized gBCTD trimer is transmitted to the gB ectodomain on the other side of the membrane. A recent study suggested that a conserved regulatory helix in the gB ectodomain proximal to the membrane may be involved in the transduction of the triggering signal from the cytoplasmic domain to the ectodomain [63]. Further studies are needed to decipher how gH/gL interacts with and activates gB and how the gB structure changes in response to this interaction.
Materials and methods
Cells and plasmids
CHO cells were received as a gift from J. M. Coffin and grown in Ham’s F-12 medium supplemented with 10% fetal bovine serum (FBS), 100 IU penicillin, and 100 μg/ml streptomycin at 37° C in the presence of 5% CO2, except as noted otherwise. Plasmids pPEP98, pPEP99, pPEP100, and pPEP101 contain the full-length HSV-1 (strain KOS) gB, gD, gH, and gL genes, respectively, in a pCAGGS vector and were gifts from P. G. Spear [64]. Plasmids RLuc1-7 and RLuc8-11 (carrying the Renilla split luciferase genes) and pBG38 (carrying the nectin-1 gene) were gifts from G. H. Cohen and R. J. Eisenberg [49, 65]. Plasmids pJLS11 (gB868) [48], pJLS8 (gB R858H) [48], pJLS15 (gH832) [54], pJLS16 (gH832 V831A) [54], and pHR26 (gH829) [44] were generated previously in our lab.
gBCTD mutagenesis
Point mutations in the cytoplasmic domain of the full-length gB gene were generated in pPEP98 background by using PCR and Gibson assembly [66]. pPEP98 was cut at PmlI and MfeI sites to generate the backbone for the assembly. Two DNA fragments were created by PCR for the mutants using the primers listed in S1 Table. The digested backbone and two DNA inserts generated by PCR were assembled using the Gibson Assembly Master Mix from New England Biolabs. The T814L/A851L double mutant was generated using the same strategy, by making a plasmid with the T814L mutation first and then repeating the steps to introduce the A851L mutation. The A851E mutant was generated by QuikChange PCR [67] using the primers listed in S1 Table followed by ligation with T4 ligase. The resulting plasmids were pZP6 (A851V), pZP22 (N804A), pZP23 (K807L), pZP7 (T814L), pZP21 (A851L), pZP25 (A851K), pZP25 (A855S), pZP60 (R858W) pZP24 (R858L), pZP27 (R858E), pZP57 (A851F), pZP58 (A851W), pZP61 (T814L/A851L), pZP2 (A851E).
gHCT mutagenesis
Point mutations in and truncations of the gHCT were generated in the pPEP100 background by using PCR and Gibson assembly [66]. pPEP100 was cut at MfeI and XhoI sites to generate the backbone for the assembly. Two DNA fragments were created by PCR for the mutants using the primers listed in S2 Table. The digested backbone and two DNA inserts generated by PCR were assembled using the Gibson Assembly Master Mix. gH832 V831L was constructed by digesting pJLS15 with MfeI and XhoI to obtain the backbone for the assembly, PCR of the insertion fragment using the primers listed in S2 Table, and assembly using the Gibson Assembly Master Mix. The resulting plasmids were pZP32 (gH830), pZP33 (gH831), pZP28 (T829A), pZP29 (S830A), pZP30 (V831T), pZP31 (P832T), pZP62 (gH832 V831L).
Cell-cell fusion assay
Cell-cell fusion of gB and gH mutants was measured using a split-luciferase assay [49]. Chinese hamster ovary (CHO) cells [68] were seeded into 96-well plates at 50,000 cells per well, three wells per condition, for effector cells and 6-well plates at 200,000 cells per well for target cells. The next day, effector cells were transfected per well with 125 ng gB (pPEP98 or gB mutant) and 41.7 ng each of split luciferase (RLuc1-7), gH (pPEP100 or gH mutant), gL (pPEP101), and gD (pPEP99) using 0.58 μl JetPrime (Polyplus, Illkirch-Graffenstaden, France) in 10 μl JetPrime buffer. For the pCAGGS negative control condition, 250 ng of pCAGGS empty vector was transfected in place of the gB, gH, gL and gD plasmids. CHO cells lack HSV-1 receptors, so no fusion can occur until receptor-bearing target cells are introduced [69]. Each well of target cells was transfected with 1 μg of the complementary part of the split luciferase (RLuc8-11) and 1 μg of the HSV-1 receptor nectin-1 (pBG38) with 4 μl of JetPrime in 200 μl of JetPrime buffer. On day 3, the tissue culture media was removed from the 96-well plate wells and replaced with 40 μl per well of fusion medium (Ham’s F12 with 10% FBS, Penicillin/Streptomycin, 50 mM HEPES), with 1:500 Enduren luciferase substrate (Promega, Madison, WI) added. The Enduren concentration becomes 1:1000 once the target cells are added. Cells were incubated for 1 hr at 37° C. Meanwhile, target cells were detached by incubating with 1 ml per well of Versene (Fisher Scientific, Waltham, MA). Target cells were collected, spun down, and resuspended in 500 μl of fusion medium per well. 40 μl of target cells were added to each well of effector cells. The plate was immediately placed in a BioTek plate reader. Luminescence measurements were taken every 1–2 minutes for 2 hrs followed by measurements every hour until hour 8. Either gB868 or gB R858H was always included as a hyperfusogenic positive control to ensure that the assay was working as expected. The average hyperfusogenic positive control signal was higher than that of the WT condition in all experiments. Luminescence values were then averaged for the three wells in each condition, normalized to the WT signal at 8 hrs, and expressed as a percentage of WT. Reported values are averages of three biological replicates.
Flow cytometry
Cell surface expression of gB and gH mutants was measured using flow cytometry. CHO cells were seeded at 250,000 cells per well in 6-well plates. The next day, each well was transfected with 2 μg gB (pPEP98 or gB mutant), or pCAGGS, or 1 μg each of gH (pPEP100 or gH mutant) plus 1 μg gL (pPEP101) using 4 μl of JetPrime in 200 μl JetPrime buffer. For conditions testing gH/gL expression in the presence of gB mutants, the transfected DNA was 0.4 μg each of WT gH and gL, and 1.2 μg of gB (WT or mutant). One well per experiment was left untransfected as a ‘mock’ control. On day 3, the cells were detached with 1 ml per well of Versene and collected using FACS medium (PBS with 3% FBS). Cells were washed with FACS media and incubated for 1 hr on ice with 250 μl of primary antibody (R68 for gB and pCAGGS, LP11 for gH and pCAGGS, anti-c-myc rabbit (A14, Santa Cruz Biotechnology, Dallas, TX) or mouse (9E10, Santa Cruz Biotechnology) antibody as a non-targeting negative control for the Mock condition) at a 1:500 dilution in FACS medium. Cells were washed three times and incubated for 1 hr on ice in the dark with 250 μl secondary FITC-conjugated anti-rabbit antibody (for R68 and rabbit anti-myc; MPBio, Santa Ana, CA) or Alexa Fluor 488 anti-mouse antibody (for LP11 and mouse anti-myc; Invitrogen, Waltham, MA) at a 1:250 dilution in FACS medium. Cells were washed three times and resuspended in 500 μl of FACS medium. Cell fluorescence was measured by flow cytometry. Gating of live cells was performed based on FSC and SSC using FlowJo software. gB+ and gH+ cells were gated using the pCAGGS condition as a negative control, using a cutoff of 5% gB+ or gH+ pCAGGS cells to capture the vast majority of true positives while minimizing false positives. Total cell surface expression of the transfected population was obtained by calculating the product of % gB+ or gH+ cells and the mean fluorescence intensity of the gB+ or gH+ cells. Total cell surface expression was then normalized to the WT gB or WT gH condition, expressed as a percentage. The values represent the average of three independent experiments. To generate flow cytometry histograms, the live cells gated using FSC and SSC were used, and their fluorescence was plotted vs cell count. R68 (polyclonal anti-HSV-1 gB) was a gift from G. H. Cohen and R. J. Eisenberg. LP11 (monoclonal anti-HSV-1 gH/gL) was a gift from H. Browne.
Statistics
Statistical analysis was performed for each experiment on the normalized values using GraphPad PRISM 9 software. Unpaired t-test with Welch’s correction was used to compare conditions to each other as indicated.
Structural analysis
The crystal structure of full-length HSV-1 gB, PDB 5V2S [5] was used for in silico structural analysis. Predicted effects of gBCTD mutants on the structure were analyzed using PyMOL software ([51] Version 2.5.1 Schrödinger, LLC.). Mutations were introduced one by one. For each rotamer, PyMol provides information about its probability, the degree of structural strain, and the extent of predicted clashing with surrounding residues. The first two parameters are shown as number values whereas the extent of predicted clashing is shown as red disks, with larger disks representing more clashing. Clashing is also apparent on visual inspection based on proximity to surrounding atoms. The rotamers were selected based on a combination of the highest probability, lowest strain, and lowest clashing. Although this strategy should increase the accuracy of modeling, the true rotamer cannot be predicted with high confidence. The area where the mutation was introduced was then “cleaned” in a 5-Å radius using the ‘clean’ function in PyMOL, which analyzes the selected area and shifts residues into positions that are predicted to be more favorable to minimize energy and strain. The resulting structure after cleaning is more likely to reflect the actual structure of the mutant. The “cleaned” model of the mutant was then visually compared to the WT structure or overlayed onto the WT structure. Predicted changes to salt bridges and H-bonds were also analyzed. The distance of the gBCTD pocket from the membrane is known from the crystal structure of the HSV-1 gB (5V2S [5]). The gHCT was modelled as an unstructured peptide in PyMOL. To compare the distance of gH V831 from the membrane to the distance of the gBCTD pocket from the membrane, the gHCT−modelled as an unstructured peptide in PyMol–was lined up such that its first residue (K825) would begin just after the end of the gB transmembrane domain (R796), which represents the border between the membrane and cytoplasm. Visual inspection determined that the positions of the gBCTD pocket and gH V831 coincided and, therefore, were approximately the same distance from the membrane by this analysis. The electrostatic surface potential of the gHCT was calculated using the PyMOL ABPS Tools v. 2.1.5 plugin.
Supporting information
Cell surface expression of gH/gL was tested in the presence of co-transfected gB mutants. LP11 primary antibody. Data are the average of three independent biological replicates.
(TIF)
Cell surface expression of gB constructs tested in this work. Mutants tested in separate experiments are shown on separate graphs. The data represent live cells in each condition that were gated using SSC and FSC. FITC signal represents relative levels of gB expression on the cell surface. R68 primary antibody. Data in all panels are from a representative biological replicate.
(TIF)
a) Cell surface expression of WT gH/gL in the presence of gB mutants tested in this work. b) Cell surface expression of gH/gL constructs tested in this work. Conditions tested in separate experiments are shown on separate graphs. The data represent live cells in each condition that were gated using SSC and FSC. FITC signal represents relative levels of gH/gL expression on the cell surface. LP11 primary antibody. Data in all panels are from a representative biological replicate.
(TIF)
a-b) gBCTD crystal structure and the structure of the pocket on the gBCTD. c-f) gB A851F, A851W, A851K, and A851E mutations were modeled in PyMol and are predicted to fill the pocket. g) WT gBCTD in cartoon representation to visualize predicted effects of mutations on the surrounding structure. h-k) A851F, A851W, A851K, and A851E mutations (green) after energy minimization overlayed onto WT gB (wheat) to show predicted shifts in nearby residues. The large F and W introduced are predicted to cause N804 and K807 to be pushed apart. A851K is predicted to push T814 downwards. A851E is not predicted to cause significant shifts in the nearby residues.
(TIF)
(DOCX)
(DOCX)
Acknowledgments
We thank Stephen Kwok and Allen Parmelee from the Tufts Flow Cytometry Core and Adam Hilterbrand for flow cytometry training. We thank Roselyn Eisenberg and Gary Cohen (U. Pennsylvania) for the gift of the antibodies and split luciferase plasmids. We thank Doina Atanasiu (U. Pennsylvania) for advice regarding the split luciferase fusion assay. We thank John Coffin, Marta Gaglia, and Karl Munger for helpful discussions and Andrea Rebolledo Viveros for comments on the manuscript. PyMOL software was installed and maintained by SBGrid [70].
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
EEH was supported by grant R01AI164698 from the National Institutes of Health (https://www.nih.gov/) and by Faculty Scholar grant 55108533 from the Howard Hughes Medical Institute (https://www.hhmi.org/). ZP was supported by grants F30AI161795, T32GM731042 and T32GM731043 from the National Institutes of Health (https://www.nih.gov/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Harrison SC. Viral membrane fusion. Nat Struct Mol Biol. 2008;15(7):690–8. Epub 2008/07/04. nsmb.1456 [pii] doi: 10.1038/nsmb.1456 ; PubMed Central PMCID: PMC2517140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vollmer B, Prazak V, Vasishtan D, Jefferys EE, Hernandez-Duran A, Vallbracht M, et al. The prefusion structure of herpes simplex virus glycoprotein B. Sci Adv. 2020;6(39). Epub 2020/09/27. doi: 10.1126/sciadv.abc1726 ; PubMed Central PMCID: PMC7518877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Heim KP, Che Y, Chi X, Qiu X, Han S, et al. Prefusion structure of human cytomegalovirus glycoprotein B and structural basis for membrane fusion. Sci Adv. 2021;7(10). Epub 2021/03/07. doi: 10.1126/sciadv.abf3178 ; PubMed Central PMCID: PMC7935361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. Crystal structure of glycoprotein B from herpes simplex virus 1. Science. 2006;313(5784):217–20. doi: 10.1126/science.1126548 . [DOI] [PubMed] [Google Scholar]
- 5.Cooper RS, Georgieva ER, Borbat PP, Freed JH, Heldwein EE. Structural basis for membrane anchoring and fusion regulation of the herpes simplex virus fusogen gB. Nat Struct Mol Biol. 2018;25(5):416–24. Epub 2018/05/08. doi: 10.1038/s41594-018-0060-6 ; PubMed Central PMCID: PMC5942590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke HG, Heldwein EE. Crystal Structure of the Human Cytomegalovirus Glycoprotein B. PLoS Pathog. 2015;11(10):e1005227. doi: 10.1371/journal.ppat.1005227 ; PubMed Central PMCID: PMC4617298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vallbracht M, Brun D, Tassinari M, Vaney MC, Pehau-Arnaudet G, Guardado-Calvo P, et al. Structure-Function Dissection of Pseudorabies Virus Glycoprotein B Fusion Loops. J Virol. 2018;92(1). Epub 2017/10/20. doi: 10.1128/JVI.01203-17 ; PubMed Central PMCID: PMC5730762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Backovic M, Longnecker R, Jardetzky TS. Structure of a trimeric variant of the Epstein-Barr virus glycoprotein B. Proc Natl Acad Sci U S A. 2009;106(8):2880–5. Epub 2009/02/07. doi: 10.1073/pnas.0810530106 ; PubMed Central PMCID: PMC2650359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baquero E, Albertini AA, Gaudin Y. Recent mechanistic and structural insights on class III viral fusion glycoproteins. Curr Opin Struct Biol. 2015;33:52–60. doi: 10.1016/j.sbi.2015.07.011 . [DOI] [PubMed] [Google Scholar]
- 10.Hutchinson L, Browne H, Wargent V, Davis-Poynter N, Primorac S, Goldsmith K, et al. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J Virol. 1992;66(4):2240–50. Epub 1992/04/01. doi: 10.1128/JVI.66.4.2240-2250.1992 ; PubMed Central PMCID: PMC289017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cairns TM, Friedman LS, Lou H, Whitbeck JC, Shaner MS, Cohen GH, et al. N-terminal mutants of herpes simplex virus type 2 gH are transported without gL but require gL for function. J Virol. 2007;81(10):5102–11. Epub 2007/03/09. doi: 10.1128/JVI.00097-07 ; PubMed Central PMCID: PMC1900195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connolly SA, Jardetzky TS, Longnecker R. The structural basis of herpesvirus entry. Nat Rev Microbiol. 2021;19(2):110–21. Epub 2020/10/23. doi: 10.1038/s41579-020-00448-w ; PubMed Central PMCID: PMC8579738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Del Pino GL, Heldwein EE. Well Put Together-A Guide to Accessorizing with the Herpesvirus gH/gL Complexes. Viruses. 2022;14(2). Epub 2022/02/27. doi: 10.3390/v14020296 ; PubMed Central PMCID: PMC8874593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atanasiu D, Saw WT, Cohen GH, Eisenberg RJ. Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB. J Virol. 2010;84(23):12292–9. doi: 10.1128/JVI.01700-10 ; PubMed Central PMCID: PMC2976417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renner DW, Szpara ML. Impacts of Genome-Wide Analyses on Our Understanding of Human Herpesvirus Diversity and Evolution. J Virol. 2018;92(1). Epub 2017/10/20. doi: 10.1128/JVI.00908-17 ; PubMed Central PMCID: PMC5730764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Looker KJ, Magaret AS, May MT, Turner KM, Vickerman P, Gottlieb SL, et al. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS One. 2015;10(10):e0140765. Epub 2015/10/29. doi: 10.1371/journal.pone.0140765 ; PubMed Central PMCID: PMC4624804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wald A, Corey L. Persistence in the population: epidemiology, transmission. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, et al., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press Copyright Cambridge University Press 2007.; 2007. [Google Scholar]
- 18.Arduino PG, Porter SR. Herpes Simplex Virus Type 1 infection: overview on relevant clinico-pathological features. J Oral Pathol Med. 2008;37(2):107–21. Epub 2008/01/17. doi: 10.1111/j.1600-0714.2007.00586.x . [DOI] [PubMed] [Google Scholar]
- 19.Steiner I, Benninger F. Update on Herpes Virus Infections of the Nervous System. Current Neurology and Neuroscience Reports. 2013;13(12):414. doi: 10.1007/s11910-013-0414-8 [DOI] [PubMed] [Google Scholar]
- 20.Sauerbrei A, Wutzler P. Herpes simplex and varicella-zoster virus infections during pregnancy: current concepts of prevention, diagnosis and therapy. Part 1: herpes simplex virus infections. Med Microbiol Immunol. 2007;196(2):89–94. Epub 2006/12/14. doi: 10.1007/s00430-006-0031-0 . [DOI] [PubMed] [Google Scholar]
- 21.Whitley R, Kimberlin DW, Prober CG. Pathogenesis and disease. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, et al., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press Copyright Cambridge University Press 2007.; 2007. [PubMed] [Google Scholar]
- 22.Carfi A, Willis SH, Whitbeck JC, Krummenacher C, Cohen GH, Eisenberg RJ, et al. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol Cell. 2001;8(1):169–79. doi: 10.1016/s1097-2765(01)00298-2 . [DOI] [PubMed] [Google Scholar]
- 23.Di Giovine P, Settembre EC, Bhargava AK, Luftig MA, Lou H, Cohen GH, et al. Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1. PLoS Pathog. 2011;7(9):e1002277. Epub 2011/10/08. doi: 10.1371/journal.ppat.1002277 ; PubMed Central PMCID: PMC3182920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spear PG. Herpes simplex virus: receptors and ligands for cell entry. Cell Microbiol. 2004;6(5):401–10. doi: 10.1111/j.1462-5822.2004.00389.x . [DOI] [PubMed] [Google Scholar]
- 25.Atanasiu D, Whitbeck JC, Cairns TM, Reilly B, Cohen GH, Eisenberg RJ. Bimolecular complementation reveals that glycoproteins gB and gH/gL of herpes simplex virus interact with each other during cell fusion. Proc Natl Acad Sci U S A. 2007;104(47):18718–23. Epub 2007/11/16. 0707452104 [pii] doi: 10.1073/pnas.0707452104 ; PubMed Central PMCID: PMC2141843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avitabile E, Forghieri C, Campadelli-Fiume G. Complexes between herpes simplex virus glycoproteins gD, gB, and gH detected in cells by complementation of split enhanced green fluorescent protein. J Virol. 2007;81(20):11532–7. Epub 2007/08/03. JVI.01343-07 [pii] doi: 10.1128/JVI.01343-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chowdary TK, Cairns TM, Atanasiu D, Cohen GH, Eisenberg RJ, Heldwein EE. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat Struct Mol Biol. 2010;17(7):882–8. Epub 2010/07/06. nsmb.1837 [pii] doi: 10.1038/nsmb.1837 ; PubMed Central PMCID: PMC2921994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krummenacher C, Supekar VM, Whitbeck JC, Lazear E, Connolly SA, Eisenberg RJ, et al. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J. 2005;24(23):4144–53. doi: 10.1038/sj.emboj.7600875 ; PubMed Central PMCID: PMC1356314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang N, Yan J, Lu G, Guo Z, Fan Z, Wang J, et al. Binding of herpes simplex virus glycoprotein D to nectin-1 exploits host cell adhesion. Nat Commun. 2011;2:577. Epub 2011/12/08. doi: 10.1038/ncomms1571 . [DOI] [PubMed] [Google Scholar]
- 30.Hannah BP, Cairns TM, Bender FC, Whitbeck JC, Lou H, Eisenberg RJ, et al. Herpes simplex virus glycoprotein B associates with target membranes via its fusion loops. J Virol. 2009;83(13):6825–36. Epub 2009/04/17. JVI.00301-09 [pii] doi: 10.1128/JVI.00301-09 ; PubMed Central PMCID: PMC2698560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulligan MJ, Yamshchikov GV, Ritter GD Jr., Gao F, Jin MJ, Nail CD, et al. Cytoplasmic domain truncation enhances fusion activity by the exterior glycoprotein complex of human immunodeficiency virus type 2 in selected cell types. J Virol. 1992;66(6):3971–5. doi: 10.1128/JVI.66.6.3971-3975.1992 ; PubMed Central PMCID: PMC241192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seth S, Vincent A, Compans RW. Mutations in the cytoplasmic domain of a paramyxovirus fusion glycoprotein rescue syncytium formation and eliminate the hemagglutinin-neuraminidase protein requirement for membrane fusion. J Virol. 2003;77(1):167–78. Epub 2002/12/13. doi: 10.1128/jvi.77.1.167-178.2003 ; PubMed Central PMCID: PMC140627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gage PJ, Levine M, Glorioso JC. Syncytium-inducing mutations localize to two discrete regions within the cytoplasmic domain of herpes simplex virus type 1 glycoprotein B. J Virol. 1993;67(4):2191–201. doi: 10.1128/JVI.67.4.2191-2201.1993 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruel N, Zago A, Spear PG. Alanine substitution of conserved residues in the cytoplasmic tail of herpes simplex virus gB can enhance or abolish cell fusion activity and viral entry. Virology. 2006;346(1):229–37. doi: 10.1016/j.virol.2005.11.002 . [DOI] [PubMed] [Google Scholar]
- 35.Engel JP, Boyer EP, Goodman JL. Two novel single amino acid syncytial mutations in the carboxy terminus of glycoprotein B of herpes simplex virus type 1 confer a unique pathogenic phenotype. Virology. 1993;192(1):112–20. doi: 10.1006/viro.1993.1013 . [DOI] [PubMed] [Google Scholar]
- 36.Diakidi-Kosta A, Michailidou G, Kontogounis G, Sivropoulou A, Arsenakis M. A single amino acid substitution in the cytoplasmic tail of the glycoprotein B of herpes simplex virus 1 affects both syncytium formation and binding to intracellular heparan sulfate. Virus Res. 2003;93(1):99–108. Epub 2003/05/03. S0168170203000704 [pii]. doi: 10.1016/s0168-1702(03)00070-4 . [DOI] [PubMed] [Google Scholar]
- 37.Cai WH, Gu B, Person S. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J Virol. 1988;62(8):2596–604. doi: 10.1128/JVI.62.8.2596-2604.1988 ; PubMed Central PMCID: PMC253689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan Z, Grantham ML, Smith MS, Anderson ES, Cardelli JA, Muggeridge MI. Truncation of herpes simplex virus type 2 glycoprotein B increases its cell surface expression and activity in cell-cell fusion, but these properties are unrelated. J Virol. 2002;76(18):9271–83. doi: 10.1128/jvi.76.18.9271-9283.2002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walev I, Lingen M, Lazzaro M, Weise K, Falke D. Cyclosporin A resistance of herpes simplex virus-induced "fusion from within" as a phenotypical marker of mutations in the Syn 3 locus of the glycoprotein B gene. Virus Genes. 1994;8(1):83–6. doi: 10.1007/BF01703606 . [DOI] [PubMed] [Google Scholar]
- 40.Haanes EJ, Nelson CM, Soule CL, Goodman JL. The UL45 gene product is required for herpes simplex virus type 1 glycoprotein B-induced fusion. J Virol. 1994;68(9):5825–34. doi: 10.1128/JVI.68.9.5825-5834.1994 ; PubMed Central PMCID: PMC236987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muggeridge MI. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J Gen Virol. 2000;81(Pt 8):2017–27. Epub 2000/07/19. doi: 10.1099/0022-1317-81-8-2017 . [DOI] [PubMed] [Google Scholar]
- 42.Bzik DJ, Fox BA, DeLuca NA, Person S. Nucleotide sequence of a region of the herpes simplex virus type 1 gB glycoprotein gene: mutations affecting rate of virus entry and cell fusion. Virology. 1984;137(1):185–90. doi: 10.1016/0042-6822(84)90022-9 . [DOI] [PubMed] [Google Scholar]
- 43.Muggeridge MI, Grantham ML, Johnson FB. Identification of syncytial mutations in a clinical isolate of herpes simplex virus 2. Virology. 2004;328(2):244–53. Epub 2004/10/07. S0042-6822(04)00497-0 [pii] doi: 10.1016/j.virol.2004.07.027 . [DOI] [PubMed] [Google Scholar]
- 44.Rogalin HB, Heldwein EE. Interplay between the Herpes Simplex Virus 1 gB Cytodomain and the gH Cytotail during Cell-Cell Fusion. J Virol. 2015;89(24):12262–72. doi: 10.1128/JVI.02391-15 ; PubMed Central PMCID: PMC4665236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chowdary TK, Heldwein EE. Syncytial phenotype of C-terminally truncated herpes simplex virus type 1 gB is associated with diminished membrane interactions. J Virol. 2010;84(10):4923–35. doi: 10.1128/JVI.00206-10 ; PubMed Central PMCID: PMC2863819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia NJ, Chen J, Longnecker R. Modulation of Epstein-Barr virus glycoprotein B (gB) fusion activity by the gB cytoplasmic tail domain. MBio. 2013;4(1):e00571–12. doi: 10.1128/mBio.00571-12 ; PubMed Central PMCID: PMC3551549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klupp BG, Nixdorf R, Mettenleiter TC. Pseudorabies virus glycoprotein M inhibits membrane fusion. J Virol. 2000;74(15):6760–8. Epub 2000/07/11. doi: 10.1128/jvi.74.15.6760-6768.2000 ; PubMed Central PMCID: PMC112192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silverman JL, Greene NG, King DS, Heldwein EE. Membrane requirement for folding of the herpes simplex virus 1 gB cytodomain suggests a unique mechanism of fusion regulation. J Virol. 2012;86(15):8171–84. Epub 2012/05/25. doi: 10.1128/JVI.00932-12 ; PubMed Central PMCID: PMC3421659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saw WT, Matsuda Z, Eisenberg RJ, Cohen GH, Atanasiu D. Using a split luciferase assay (SLA) to measure the kinetics of cell-cell fusion mediated by herpes simplex virus glycoproteins. Methods. 2015;90:68–75. Epub 2015/05/30. doi: 10.1016/j.ymeth.2015.05.021 ; PubMed Central PMCID: PMC4655157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pommié C, Levadoux S, Sabatier R, Lefranc G, Lefranc MP. IMGT standardized criteria for statistical analysis of immunoglobulin V-REGION amino acid properties. J Mol Recognit. 2004;17(1):17–32. Epub 2004/02/12. doi: 10.1002/jmr.647 . [DOI] [PubMed] [Google Scholar]
- 51.Delano WL. The PyMOL Molecular Graphics System. http://www.pymol.org. 2002.
- 52.Li SC, Goto NK, Williams KA, Deber CM. Alpha-helical, but not beta-sheet, propensity of proline is determined by peptide environment. Proc Natl Acad Sci U S A. 1996;93(13):6676–81. Epub 1996/06/25. doi: 10.1073/pnas.93.13.6676 ; PubMed Central PMCID: PMC39085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lyu PC, Sherman JC, Chen A, Kallenbach NR. Alpha-helix stabilization by natural and unnatural amino acids with alkyl side chains. Proc Natl Acad Sci U S A. 1991;88(12):5317–20. Epub 1991/06/15. doi: 10.1073/pnas.88.12.5317 ; PubMed Central PMCID: PMC51863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silverman JL, Heldwein EE. Mutations in the cytoplasmic tail of herpes simplex virus 1 gH reduce the fusogenicity of gB in transfected cells. J Virol. 2013;87(18):10139–47. Epub 2013/07/12. doi: 10.1128/JVI.01760-13 ; PubMed Central PMCID: PMC3753984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tong X, Xu D, Mishra RK, Jones RD, Sun L, Schiltz GE, et al. Identification of a druggable protein-protein interaction site between mutant p53 and its stabilizing chaperone DNAJA1. J Biol Chem. 2021;296:100098. Epub 2020/11/20. doi: 10.1074/jbc.RA120.014749 ; PubMed Central PMCID: PMC7948449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiltzer L, Okada K, Yamaoka S, Larrous F, Kuusisto HV, Sugiyama M, et al. Interaction of rabies virus P-protein with STAT proteins is critical to lethal rabies disease. The Journal of infectious diseases. 2014;209(11):1744–53. Epub 2013/12/25. doi: 10.1093/infdis/jit829 . [DOI] [PubMed] [Google Scholar]
- 57.Witkowska D, Cox HL, Hall TC, Wildsmith GC, Machin DC, Webb ME. Analysis of substrate binding in individual active sites of bifunctional human ATIC. Biochim Biophys Acta Proteins Proteom. 2018;1866(2):254–63. Epub 2017/10/19. doi: 10.1016/j.bbapap.2017.10.005 . [DOI] [PubMed] [Google Scholar]
- 58.Sode O, Voth GA. Electron transfer activation of a second water channel for proton transport in [FeFe]-hydrogenase. J Chem Phys. 2014;141(22):22d527. Epub 2014/12/17. doi: 10.1063/1.4902236 . [DOI] [PubMed] [Google Scholar]
- 59.Korasick DA, Christgen SL, Qureshi IA, Becker DF, Tanner JJ. Probing the function of a ligand-modulated dynamic tunnel in bifunctional proline utilization A (PutA). Arch Biochem Biophys. 2021;712:109025. Epub 2021/09/11. doi: 10.1016/j.abb.2021.109025 ; PubMed Central PMCID: PMC8559791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Consortium TU. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2020;49(D1):D480–D9. doi: 10.1093/nar/gkaa1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bohm SW, Backovic M, Klupp BG, Rey FA, Mettenleiter TC, Fuchs W. Functional Characterization of Glycoprotein H Chimeras Composed of Conserved Domains of the Pseudorabies Virus and Herpes Simplex Virus 1 Homologs. J Virol. 2016;90(1):421–32. Epub 2015/10/23. doi: 10.1128/JVI.01985-15 ; PubMed Central PMCID: PMC4702552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pataki Z, Rebolledo Viveros A, Heldwein EE. Herpes simplex virus 1 entry glycoproteins form stable complexes prior to and during membrane fusion. bioRxiv. 2022:2022.05.07.491029. doi: 10.1101/2022.05.07.491029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vallbracht M, Lötzsch H, Klupp BG, Fuchs W, Vollmer B, Grünewald K, et al. In Vitro Viral Evolution Identifies a Critical Residue in the Alphaherpesvirus Fusion Glycoprotein B Ectodomain That Controls gH/gL-Independent Entry. mBio. 2021;12(3). Epub 2021/05/06. doi: 10.1128/mBio.00557-21 ; PubMed Central PMCID: PMC8262866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pertel PE, Fridberg A, Parish ML, Spear PG. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology. 2001;279(1):313–24. Epub 2001/01/09. doi: 10.1006/viro.2000.0713 . [DOI] [PubMed] [Google Scholar]
- 65.Krummenacher C, Baribaud I, Sanzo JF, Cohen GH, Eisenberg RJ. Effects of herpes simplex virus on structure and function of nectin-1/HveC. J Virol. 2002;76(5):2424–33. Epub 2002/02/12. doi: 10.1128/jvi.76.5.2424-2433.2002 ; PubMed Central PMCID: PMC153823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6(5):343–5. Epub 2009/04/14. doi: 10.1038/nmeth.1318 . [DOI] [PubMed] [Google Scholar]
- 67.Liu H, Naismith JH. An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC Biotechnol. 2008;8:91. Epub 2008/12/06. doi: 10.1186/1472-6750-8-91 ; PubMed Central PMCID: PMC2629768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Puck TT, Cieciura SJ, Robinson A. Genetics of somatic mammalian cells. III. Long-term cultivation of euploid cells from human and animal subjects. J Exp Med. 1958;108(6):945–56. Epub 1958/12/01. doi: 10.1084/jem.108.6.945 ; PubMed Central PMCID: PMC2136918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arii J, Uema M, Morimoto T, Sagara H, Akashi H, Ono E, et al. Entry of herpes simplex virus 1 and other alphaherpesviruses via the paired immunoglobulin-like type 2 receptor alpha. J Virol. 2009;83(9):4520–7. doi: 10.1128/JVI.02601-08 ; PubMed Central PMCID: PMC2668467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morin A, Eisenbraun B, Key J, Sanschagrin PC, Timony MA, Ottaviano M, et al. Collaboration gets the most out of software. eLife. 2013;2:e01456. doi: 10.7554/eLife.01456 ; PubMed Central PMCID: PMC3771563. [DOI] [PMC free article] [PubMed] [Google Scholar]