Abstract
Aim:
This meta-analysis aimed to evaluate the association of HIF-1α expression with clinicopathological features and overall survival (OS) of patients with digestive system malignancies.
Background:
Numerous studies have demonstrated that hypoxia-inducible factor-1α (HIF-1α) is abnormally expressed in various solid tumors. However, the clinicopathological features and prognostic value of HIF-1α expression in patients with digestive system malignancies remain controversial.
Methods:
A literature search in PubMed, Web of Science, and Scopus databases was performed to identify all relevant studies published in English until 15 October 2020. The pooled effect was calculated to evaluate the association between HIF-1α expression and clinicopathological features and overall survival in cancer patients. Pooled odds ratios (ORs) or hazard ratios (HRs) with a 95% confidence interval (CI) were calculated using fixed- or random-effects model based on between-study heterogeneity.
Results
A total of 44 eligible studies with 5,964 patients were included. The pooled results indicated a positive association of HIF-1α overexpression with poor overall survival (OS) (HR=1.990, 95% CI: 1.615-2.453, p<0.001) and disease-free survival (DFS) (HR=1.90, 95% CI: 1.084-3.329, p=0.043). Meta-analysis results showed that HIF-1α level expression was significantly associated with positive lymph node metastasis (OR=1.869, 95% CI: 1.488-2.248, p<0.001), distance metastasis (OR=2.604, 95% CI: 1.500-4.519, p<0.001), tumor stage (OR=1.801, 95% CI: 1.437-2.257, p<0.001) and tumor size (OR=1.392. 95% CI: 1.068-1.815, p=0.014).
Conclusion:
This meta-data suggest that HIF-1α expression might serve as an independent prognostic marker and a promising therapeutic target in patients with digestive system malignancies.
Key Words: HIF-1α, Neoplasms, Digestive system, Prognosis
Introduction
The term “digestive system cancer” generally refers to those cancers that affect the gastrointestinal tract (GI tract) and accessory organs of digestion. This type of cancer is responsible for more cancer-related deaths than any other type (1, 2). The most commonly diagnosed digestive system malignancies include esophageal cancer, gastric cancer (GC), liver cancers, pancreatic cancer, and colorectal cancer (CRC) (3). They are reported to account for over 26% of newly diagnosed cases globally and 35% of all cancer-related deaths (4). Despite great advancements in cancer prevention and treatment during the past decades, the 5-year survival for patients with these malignancies has not been significantly improved. The lack of effective biomarkers as potential screening tools for early detection of cancer can be considered as a main reason. The identification of various prognostic and predictive biomarkers for patients with digestive system malignancies might provide to be essential information on the probability of response to a particular therapy.
Various factors including unhealthy lifestyle, genetic legions, comorbid conditions, and medications related to cancer treatment might affect digestive system malignancies in their different aspects such as progression, recurrence, and mortality (5, 6). Increasing the knowledge about the molecular mechanisms involved in these processes may lead to the identification of potential traditional protein- or genome-based markers with high predictive value for tumor behavior, then subsequent clinical management and optimal treatment of cancer patients (7). Hence, special efforts by researchers are required to identify clinically applicable biomarkers for patients affected with these kinds of cancer.
A common feature of most solid tumors, generated by abnormal microvasculature in rapidly proliferating tissues, is called hypoxia (8). Hypoxia promotes the expression of HIF-1α, a key transcription factor that regulates the expression of different genes related to various aspects of cancer biology, such as cell proliferation, angiogenesis, and glucose metabolism (8, 9). Moreover, in hypoxic conditions, intratumor cytokines, growth factors, and other signaling molecules stimulate HIF-1α expression and activity in tumor cells by different molecular mechanisms such as PI3K or MAPK (9). Mounting evidence has shown that HIF-1α activation induces cancer progression; hence, various clinical studies have demonstrated the association between overexpression of HIF-1α and mortality rates in many human cancer types (10, 11). Different studies have indicated the significant connection between high level expression of HIF-1α and poor OS and DSF in patients with digestive system malignancies such as esophageal cancer (EsoC) (12-14), colorectal cancer (CRC) (14-16), gastric cancer (GC) (17-19), pancreatic adenocarcinoma (PDAC) (20), and hepatocellular carcinoma (HCC) (10, 21, 22). However, to clarify to what extent HIF-1α expression level might be of prognostic significance in digestive system cancers, a comprehensive meta-analysis of previous studies is needed. Therefore, we conducted a systematic review of published studies to evaluate the potential prognostic value of HIF-1α expression in digestive system malignancies.
Methods
Literature search procedures
Searches were performed on the Web of Science, PubMed, Scopus, and Google Scholar to identify related studies in the English language published up to 15 October 2020. Search terms, used both individually and/or in various combinations, comprised HIF-1α, tumor, malignant, cancer, neoplasm, carcinoma, adenocarcinoma, hepatocellular, liver, gastric, stomach, gastro, esophageal, colon, colorectal, rectal, and pancreatic. Moreover, the references list in each selected article was checked to optimize sensitivity.
Selection criteria
The current meta-analysis investigated the significance of HIF-1α in digestive system malignancies, including esophageal, gastric, liver, pancreas, and colorectal cancers. To meet the inclusion criteria for this research, the studies had to have: 1) provided pathological evidence to confirm digestive system malignancies, 2) examined the association between HIF-1α and clinic-pathological parameters of various types of digestive system cancers, 3) reported or provided data about disease-free survival (DFS) or overall survival (OS) rates, 4) evaluated HIF-1α expression in either tissue or serum/plasma, and 6) if studies included patients with different cancers, there must be a subgroup analysis of digestive system cancers. Articles were excluded if they: 1) focused on animals or cells to compare HIF-1α with non-human subjects, 2) were reviews, letters, case reports, editorials, or commentaries, 3) were duplicate publications, or 4) lacked key information to calculate hazard rations (HRs) with 95% confidence intervals (CIs). In the case of overlapping patients in more than one study, only the most complete study was enrolled.
Data extraction and methodological assessment
The following information was extracted from each included study: first author’s surname, year of publication, country of origin, tumor type, tumor size, sample size, HIF-1α detection assay, tumor stage, lymph node metastasis (LNM), distance metastasis (DM), prognostic outcomes of interest, and HR with its 95% CI.
The quality of included studies was assessed independently by two authors (MHA and HMM) using the Newcastle-Ottawa scale (NOS) (23). All studies were scored (from 0 to 9) in terms of patient selection, study comparability, and outcome assessment. Any discrepancy was resolved by team consensus.
Statistical analysis
High and low HIF-1α expression rates were defined according to the arbitrary cut-off values provided by the literature. The odds ratios (ORs) and corresponding 95% CIs were applied to evaluate the association between HIF-1α expression and clinicopathological features. HRs in combination with the corresponding 95% CIs of identified studies were used to estimate the effect of HIF-1α expression on survival outcomes. HRs with 95% CIs were directly acquired from the articles or calculated indirectly using Kaplan–Meier curves according to the methods described by Parmar et al. (24), Williamson et al. (25), and Tierney et al. (26). As a rule, a pooled HR > 1 was assumed a poor prognosis for HIF-1α overexpression and considered statistically significant if the 95% CI did not cross one. Heterogeneity across the studies was quantified using the χ2-based Q test and I2 index. I2 > 50% or Q test p < 0.05 reflected significant heterogeneity across studies. In case of significant heterogeneity, the random effect model was adopted; otherwise, a fixed effect model was employed. Potential sources of heterogeneity were explored by performing subgroup, metaregression, sensitivity, and Galbraith plot analyses (27). Begg’s funnel plots and Egger’s linear regression test were also conducted to judge the probability of publication bias. All analyses were performed using the Comprehensive Meta-Analysis software. A p-value<0.05 was considered as statistically significant.
Results
Literature search
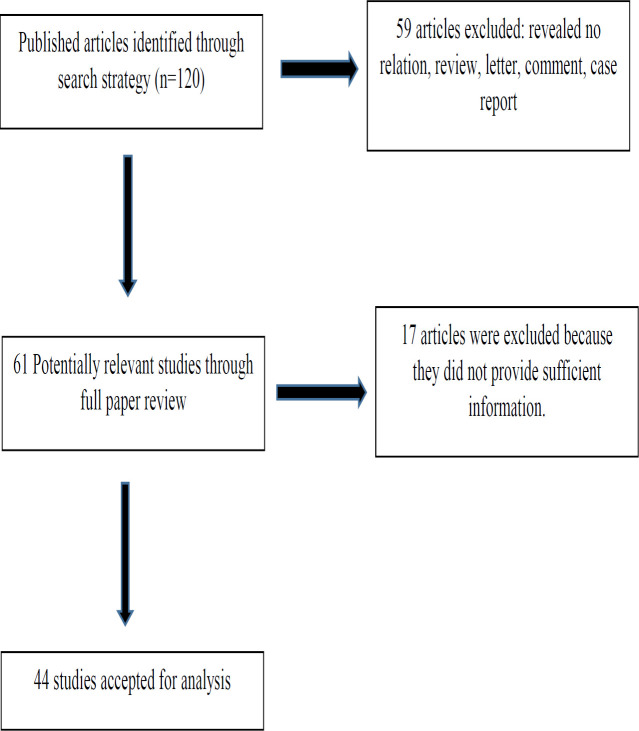
A flow diagram of the study selection process is provided in Figure 1. In our initial searches, 120 potentially relevant articles were retrieved according to the predefined search strategy. In the first screening, 31 duplicate records were excluded, and in subsequent screening steps, 28 additional articles were excluded, because they were conference records, irrelevant to our topic, or non-original papers. A more detailed review resulted in the exclusion of another 17 studies due to insufficient information. Consequently, 44 eligible papers comprising 5,964 patients were included in the meta-analysis for quantitative analysis. The majority of studies were performed in Asia (17 from China, 15 from Japan, one from Korea, and one from Turkey); the remaining studies were from the UK, Germany, Greece, the USA, and Australia.
Figure 1.
Flowchart of study selection process
Study characteristics
The general characteristics of the selected studies are summarized in Table 1. All studies were published between 2003 and 2019. The cancer types evaluated in this meta-analysis were: gastric cancer (n=13) (17, 19, 28-38), CRC (n=16) (16, 39-53), EsoC (n=9) (13, 14, 54-60), HCC (n=5) (10, 21, 61-63), and PDAC (n=1) (20). The studies investigated the association between HIF-1α expression and prognosis index, including OS and DFS. The cut off values for HIF-1α expression level varied throughout the studies.
Table 1.
Basic characteristics of the included studies
| Author/ year |
Age (High/ Low) |
Country | Tumor type | Tumor size (High/Low) | Sample size | Male (High/Low) Female (High/Low) |
TNM Stage (High/Low) |
HIF-1α Expression | Survival analysis | Hr (95% CI) |
Method | Sample type | NOS score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High | Low | ||||||||||||||||||||
| Total | LNM | DM | Total | LNM | DM | ||||||||||||||||
| Jiang 2019 |
≥61(39-24) <61(43-18) |
China | GC | ≥6cm (50/33) <6cm(32/9) |
124 | 63/33 19/9 |
I/II (28/24) III/IV(54/18) |
82 | 59 | - | 42 | 24 | - | OS | 4.60 (2.45-8.66) |
IHC/ RT PCR |
T | 8 | |||
| Zhang 2018 | ≥60(113/116) <60(106/93) |
China | GC | ≥5cm (100/85) <5cm(119/124) |
428 | 155/149 64/60 |
I/II(95/119) III(144/90) |
219 | 151 | 32 | 209 | 98 | 14 | OS/ DFS |
OS: 0.65(0.50-0.85) DFS:0.67(0.52-0.88) |
IHC | T | 8 | |||
| Wang 2018 | ≥55(87/89) <55(136/101) |
China | HCC | ≥5cm (89/52) <5cm(126/133) |
419 | 157/66 151/39 |
I (91/98) II/III (132/92) |
223 | - | - | 190 | - | - | OS | 2.10 (1.35-3.26) |
IHC | T | 8 | |||
| Dai 2018 |
≥50(23/40) <50(16/11) |
China | HCC | ≥5cm(19/21) <5cm(19/30) |
90 | 35/49 4/2 |
- | 39 | - | - | 51 | - | - | OS | 3.109 (1.576-6.131) | IHC | T | 8 | |||
| Saka 2017 |
≥50(68/65) <50(23/30) |
Turkey | CRC | ≥5cm (44/55) <5cm(47/40) |
186 | 57/43 34/52 |
I/II (29/33) III/IV (62/62) |
91 | 53 | 44 | 95 | 56 | 52 | OS | - | IHC | T | 8 | |||
| Chen 2014 |
≥60(117/105) <60(100/124) |
China | GC | ≥5cm (122/131) <5cm (95/98) |
446 | 172/176 45/53 |
I/II(61/71) III/IV(156/158) |
217 | 173 | 63 | 229 | 176 | 25 | OS/ DFS |
OS: 3.53(2.66-4.66) DFS: 2.77(2.14-2.6) |
IHC | T | 8 | |||
| Lu 2013 |
≥49.82(25/18) <49.82(11/14) |
China | GC | ≤5cm (9/15) >5cm (27/17) |
68 | 21/22 15/10 |
I/II(10/16) III/IV(26/16) |
36 | 27 | 9 | 32 | 20 | 6 | *OS | 1.93 (0.65-5.75) | IHC | T | 8 | |||
| Yang 2013 |
≥50(52/40) <50(20/14) |
China | HCC | ≥5cm(40/26) <5cm(32/28) |
126 | 5/105 2/14 |
NR | 72 | - | - | 54 | - | - | OS/DFS | 2.82 (1.63-4.90) | IHC | T | 8 | |||
| Shimomura 2013 |
≥61(10/25) ≤60(10/19) |
Japan | CRC | ≥3cm(10/22) <3cm(13/32) |
64 | 11/31 9/13 |
16/25 | 20 | - | - | 44 | - | - | OS/DFS | OS: 1.34 (0.62-2.88) DFS: 2.09 (1.09-4) |
IHC | T | 8 | |||
| Xie 2013 |
≤60(12/17) >60(16/15) |
China | CRC | ≤2cm (6/18) >2cm (22/14) |
60 | 21/19 7/13 |
I/II(13/13) III/IV(15/19) |
28 | 16 | - | 32 | 11 | - | OS | 1.550.60-4.01) | IHC | T | 8 | |||
| Isobe 2013 |
≥65(48/30) <65(36/14) |
Japan | GC | NR | 128 | 56/35 28/9 |
NR | 84 | 50 | 20 | 44 | 12 | 2 | OS | 6.92(1.24-130.4) | IHC | T | 8 | |||
| Xiang 2012 | ≤50(19/17) >50(11/22) |
China | HCC | ≤5cm (18/15) >5cm (12/24) |
69 | 27/34 3/5 |
I/II(15/23) III/IV(15/16) |
30 | 21 | - | 39 | 23 | - | OS | 2.02 (1.12-3.66) |
IHC | T | 8 | |||
| Ogawa 2011 | ≥60(7/7) <60(4/4) |
Japan | EsoC | NR | 37 | 10/14 1/0 |
NR | 11 | 9 | 4 | 14 | 8 | 1 | RFS | 0.071 (0.015-0.34) | IHC | T | 8 | |||
| Shioya 2011 | NR | Japan | CRC | NR | 50 | 38 | NR | 21 | 17 | - | 29 | 18 | - | RFS | 4.13 (1.52-11.24) | IHC | T | 8 | |||
| Munipalle 2011 | >70(13/5) <70(6/12) |
UK | EsoCl | NR | 36 | 8/7 11/10 |
I/II (4/3) III/IV (17/12) |
19 | 14 | 8 | 17 | 13 | 4 | OS | 0.92 (0.67-1.31) | IHC | T | 8 | |||
| Ogane 2010 | ≤62(15/31) >62(34/16) |
Japan | EsoC | NR | 96 | 60/29 5/2 |
NR | 65 | 35 | - | 31 | 9 | - | OS/DFS | OS:2.92(1.16-7.32) DFS:3.12(1.28-8.63) |
IHC | T | 8 | |||
| Saigusa 2010 | ≥65 | Japan | CRC | NR | 52 | 42/10 | NR | - | - | - | - | - | - | OS | OS : 4.36(0.05-0.91) | IHC/RT-PCR | T | 8 | |||
| Kwon 2010 | - | Korea | CRC | ≥5cm (42/56) <5cm (21/29) |
311 | 109/62 87/53 |
I/II (100/73) III/IV (96/42) |
63 | - | - | 85 | - | - | *OS/DFS | OS:2.43(1.39-4.21) DFS:1.71(0.98-2.99) |
IHC | T | 8 | |||
| Baba 2010 |
≥70(53/223) <70(89/366) |
USA | CRC | NR | 731 | 41/220 101/369 |
I/II (42/180) III/IV (74/234) |
142 | - | - | 589 | - | - | OS | 1.50 (1.16-1.94) |
IHC | T | 8 | |||
| Qiu 2010 |
≤60(58/41) >60(52/37) |
China | GC | ≤5cm (36/52) >5cm (74/23) |
188 | 72/55 38/23 |
I/II(41/56) III/IV(69/22) |
110 | 82 | - | 74 | 44 | - | OS | 2.26(1.47-3.48) | IHC | T | 8 | |||
| Dai 2009 |
≤52(26/28) >52(16/40) |
China | HCC | ≤5cn (21/35) >5cm (28/38) |
110 | 38/57 4/11 |
I/II(18/40) III/IV(24/28) |
42 | - | - | 68 | - | - | OS/DFS | OS: 0.47(0.25-0.89) DFS: 0.44(0.25- 0.8) |
IHC/ RT-PCR | T | 8 | |||
| Chen 2009 |
<60(17/13) ≥60(8/16) |
China | EsoC | NR | 54 | 15/23 10/6 |
I/II(5/17) III/IV(20/12) |
25 | 16 | - | 29 | 12 | - | *OS | 1.71(0.57-5.13) | IHC | T | 8 | |||
| Cao 2009 |
≥60(24/23) <60(15/9) |
China | CRC | ≥5cm (22/18) <5cm (1714) |
71 | 24/19 15/13 |
I/II(15/27) III/IV(24/5) |
39 | 22 | 10 | 32 | 5 | 1 | OS | 2.69(1.15-6.30) | IHC | T | 8 | |||
| Jubb 2009 | NR | Australia | CRC | NR | 164 | NR | NR | 95 | - | - | 60 | - | - | OS | 1.61(1.01-2.57) | IHC | T | 8 | |||
| Rajaganesh2009 | NR | UK | CRC | NR | 55 | NR | NR | 25 | - | - | 30 | - | - | *DFS | 6.8(3.11-14.85 | IHC | T | 8 | |||
| Schemitz 2009 | NR | German | CRC | NR | 135 | NR | I/II (9/38) III/IV(24/48) |
34 | 22 | 13 | 90 | 43 | 16 | OS | 1.76(0.99-3.22) | IHC | T | 8 | |||
| Rasheed 2009 | NR | UK | CRC | NR | NR | 32/24 16/18 |
I/II(258/32) III/IV(23/10) |
48 | 23 | - | 42 | 10 | - | OS/DFS | 4.11(1.37-12.35) 4.47 (1.68-11.89) |
IHC | T | 8 | |||
| Oh 2008 | ≥60(9/47) <60(9/49) |
Korea | GC | ≥4cm (14/53) <4cm (4/43) |
114 | 10/57 8/39 |
I/II(74/60) III/IV(11/34) |
18 | 15 | - | 96 | 58 | - | OS | 4.08(1.88-8.88) | IHC | T | 8 | |||
| Kolev 2008 | ≥60(49/26) <60(46/31) |
Japan | GC | ≥5cm (48/24) <5cm (47/33) |
152 | 72/38 23/19 |
I/II(62/42) III/IV(35/15) |
95 | 50 | - | 57 | 21 | - | OS/ DFS |
0.88(0.48-1.62) 1.02(0.5-2.07) |
IHC | T | 8 | |||
| Tzao 2008 | ≤70(36/24) >70(16/9) |
China | EsoC | NR | 85 | 47/33 5/0 |
I/II(25/23) III/IV(27/10) |
52 | 29 | 13 | 33 | 12 | 4 | OS | 1.77(1.05-2.97) | IHC | T | 8 | |||
| Ma 2007 | NR | China | GC | NR | 118 | NR | NR | 58 | 50 | 47 | 60 | 33 | 8 | OS | 7.51(4.30-13.11) | IHC | T | 8 | |||
| Griffiths 2007 | NR | UK | GC | NR | 80 | NR | I/II(41/44) III/IV(51/37) |
93 | 64 | 2 | 83 | 60 | 2 | OS | 1.10(0.8-1.4) | IHC | T | 8 | |||
| SUN 2007 | ≥60(19/16) <60(7/16) |
USA | PDAC | ≥2cm (25/25) <2cm (1/7) |
58 | 16/21 10/11 |
I/II(10/26) III/IV(16/6) |
26 | 12 | - | 32 | 6 | - | OS | 2.22(1.00-4.99) | IHC | T | 8 | |||
| Sumiyoshi 2006 | NR | Japan | GC | NR | 216 | 56/92 29/39 |
I/II (45/80) III/IV(40/60) |
85 | 49 | 5 | 131 | 61 | 4 | OS | 2.19(1.39-3.47) | IHC | T | 8 | |||
| Zhang 2007 | >63(18/8) ≤63(16/10) |
China | EsoC | NR | 50 | 27/10 7/6 |
NR | 34 | 31 | 7 | 16 | 10 | 0 | *OS | 4.01(1.69-9.51) | IHC | T | 8 | |||
| Urano 2006 | NR | Japan | GC | NR | 146 | NR | I/II(46/25) III/IV(43/32) |
83 | 36 | - | 55 | 28 | - | *OS | 0.96(0.42-2.20) | IHC | T | 8 | |||
| Lu 2006 | NR | China | CRC | NR | 30 | NR | NR | 19 | 12 | - | 11 | 0 | - | *OS | 9.54(1.18-70.66) | IHC | T | 8 | |||
| Theodoropoulos 2006 | ≤68(24/20) >68(20/28) |
Greece | CRC | >3cm (36/39) <3cm (8/9) |
92 | 24/31 20/17 |
NR | 44 | 30 | - | 48 | 21 | - | OS/DFS | OS:3.65(1.52-8.81) DFS: 3.46(1.32-9.8) |
IHC | Tissue | 8 | |||
| Mizokami 2006 | ≥65(20/46) <65(29/31) |
Japan | GC | ≥3cm (39/42) <3cm (10/35) |
126 | 35/50 14/27 |
NR | 49 | 25 | - | 77 | 26 | - | OS | 2.09 (1.08-4.06) |
IHC | Tissue | 8 | |||
| Katsuta 2005 | NR | Japan | EsoC | NR | 48 | 24/11 10/3 |
I (16/9) II/III/IV(18/5) |
34 | 15 | 4 | 14 | 2 | 1 | *OS | 1.19(0.4-5.39) | IHC / RT-PCR |
Tissue | 8 | |||
| Yoshimura 2004 | NR | Japan | CRC | NR | NR | 19/32 20/16 |
I/II(12/16) III/IV(27/32) |
39 | 14 | - | 48 | 20 | - | *OS | 2.56(1.19-5.50) | IHC | Tissue | 8 | |||
| Kimura 2004 | ≤60(10/19) ≥61(22/31) |
Japan | EsoC | NR | 82 | 31/41 1/9 |
I/II(9/21) III/IV(23/29) |
32 | 20 | - | 50 | 30 | - | *OS | 1.59(0.86-2.97) | IHC/ RT.PCR |
Tissue | 7 | |||
| Kurokawa 2003 | <60(26/18) ≥60(64/22) |
Japan | EsoC | >4.5cm (47/23) <4.5cm (43/17) |
130 | 79/34 11/6 |
I/II(64/17) III/IV(26/23) |
90 | 38 | 11 | 40 | 28 | 11 | OS | 1.54(0.84-2.84) | IHC | Tissue | 8 | |||
| Kuwai 2003 | NR | Japan | CRC | ≥5cm (39/21) <5cm (42/37) |
139 | NR | NR | 81 | 46 | 21 | 58 | 24 | 5 | *OS | 1.53 (0.46-5.13) | IHC | Tissue | 7 | |||
Abbreviations: Hr: Hazard ratio, CI: Confidence interval, NOS: Newcastle-Ottawa Scale, UK: united kingdom, USA, Unites states of America, GC: Gastric Cancer, HCC: Hepatocellular carcinoma, CRC: Colorectal cancer, EsoC; Esophageal cancer, PDAC: Pancreatic ductal adenocarcinoma, OS: overall survival, DFS: Disease free survival, LNM: lymph node metastasis, DM: distant metastasis. IHC: immunohistochemistry, RT-PCR: real time-PCR, T: Tissue, NR: not reported, Note: The dashes represent no data.
Forty studies investigated the association of HIF-1α expression with OS, ten with DFS, thirty-four with lymph node metastasis (LNM), seventeen with distance metastasis (DM), twenty-nine with TNM stage, and nineteen studies with tumor size. According to the NOS scoring system, all included studies were awarded five or more stars and were considered as being of good quality.
The relationship between HIF-1α expression and OS in digestive system cancers
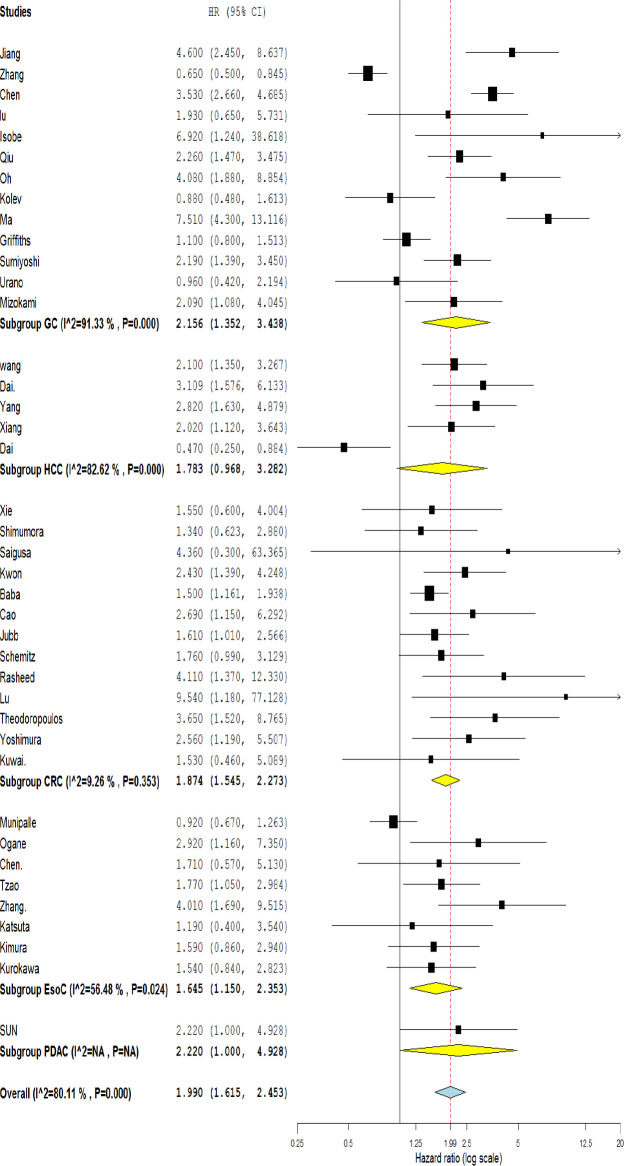
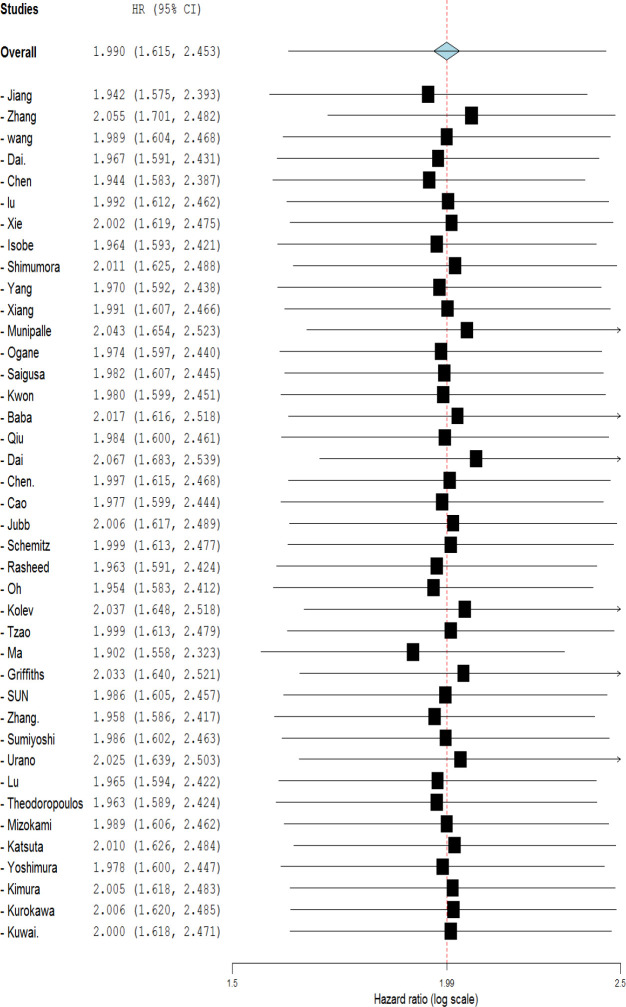
The data of forty eligible studies was summarized to assess the association between HIF-1α expression level and OS. Due to the significant heterogeneity of the reports (I2 = 80.11%, p < 0.001), a random effect model was applied to evaluate pooled HR.
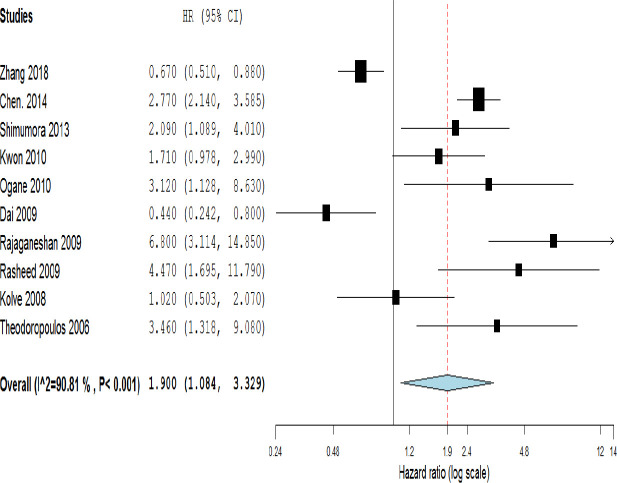
Meta-results showed a significant association between the high expression of HIF-1α and poor OS (HR=1.990, 95% CI: 1.615-2.453, p<0.001) (Figure 2). Moreover, the combined data from ten studies reporting HR for DFS indicated a significant relationship between the high level of HIF-1α and poor outcome (HR=1.90, 95% CI: 1.08-3.33, p=0.043 and I2= 90.81% p<0.001) (Figure 3). To explore the source of heterogeneity, subgroup analyses were performed according to sample size, ethnicity, and cancer type. Subgroup analyses based on sample size revealed a significant correlation between high HIF-1α expression and patients’ overall survival in studies with both more and less than 100 cases (HR=1.918, 95% CI: 1.381-2.665, p=0.000; and HR=2.008, 95% CI: 1.578-2.554, p=0.001; respectively) (Table 2). Ethnicity-based subgroup analysis also indicated a significant association between the expression of HIF-1α and poor OS in Asians (HR=2.010, 95% CI: 1.590-2.541, p=0.000) and Caucasians (HR=1.854, 95% CI: 1.171-2.936, p=0.008) (Table 2). According to subgroup analysis based on cancer type, there was a significant association between HIF-1α expression and poor OS in GC (HR=2.156, 95% CI: 1.352-3.438, p<0.000), CRC (HR=1.874, 95% CI: 1.545-2.273, p<0.001), and EsoC (HR=1.645, 95% CI: 1.150-2.353, p=0.024); however, no significant association was seen regarding the HCC (HR=1.783, 95% CI: 0.968-3.282, p=0.063) (Figure 2). Meta‐regression was performed to find any evidence of covariates affecting OS. The results showed that neither sample size, ethnicity, nor cancer type, alone or in combination, significantly affected OS (Table 2).
Figure 2.
Forest plot showing the association between OS and HIF-1α expression in overall and based on different cancer types. (GC: Gastric Cancer, HCC: Hepatocellular carcinoma, CRC: Colorectal cancer, EsoC; Esophageal cancer, PDAC: Pancreatic ductal adenocarcinoma.)
Figure 3.
Forest plot showing the association between HIF-1α expression and DFS in different cancer types
Table 2.
Stratified analyses of pooled hazard ratios for overall survival
| Stratified analysis | No. of studies |
No. of patients |
Test of association | Test of heterogeneity | P- Valueb | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pooled HR (95% CI) | p-value | I2 (%) | P-valuea | Model | |||||
| Overall survival (OS) | 40 | 5636 | 1.99 (1.62-2.45) | <0.001 | 80.11 | <0.001 | R | - | |
| Disease free survival (DFS) | 10 | 1970 | 1.90 (1.08-3.33) | 0.043 | 90.81 | <0.001 | R | - | |
| Sample size | >100 | 19 | 4637 | 1.92 (1.38- 2.66) | <0.001 | 88.4 | <0.001 | R | 0.685 |
| <100 | 21 | 1327 | 2.01 (1.58- 2.55) | <0.001 | 55.7 | <0.001 | R | ||
| Ethnicity | Asian | 36 | 4613 | 2.01 (1.59- 2.54) | <0.001 | 81.17 | <0.001 | R | 0.818 |
| Caucasian | 6 | 1351 | 1.85 (1.17- 2.94) | 0.008 | 71.03 | 0.004 | R | ||
| Cancer type | CRC | 13 | 2140 | 1.94 (1.58-2.39) | <0.001 | 9.26 | 0.325 | F | 0.910 |
| EsoC | 8 | 618 | 1.64 (1.15-2.35) | 0.006 | 56.48 | 0.024 | R | ||
| GC | 13 | 2334 | 2.16 (1.35-3.44) | <0.001 | 91.33 | <0.001 | R | ||
| HCC | 5 | 814 | 1.78 (0.97-3.28) | 0.063 | 87.61 | <0.001 | R | ||
| PDAC | 1 | 58 | - | - | - | - | - | ||
a P-Value for heterogeneity within each subgroup. b P-Value for heterogeneity between subgroups with meta-regression analysis CRC: Colorectal cancer, EsoC; Esophageal cancer, GC: Gastric Cancer, HCC: Hepatocellular carcinoma, PDAC: Pancreatic ductal adenocarcinoma, R: random effect model, F: fixed effects model
Association of HIF-1α expression with clinicopathological characteristics
A meta-analysis was performed to assess the correlation between HIF-1α expression level and tumor clinicopathological characteristics. The pooled ORs and 95% CIs of all characteristics including tumor size, stage of tumor, LNM, and DM as well as age and sex are presented in Table 3. High expression of HIF-1α showed a significant association with tumor size (OR=1.392. 95% CI: 1.068-1.815, p=0.014, Random effect), stage (OR=1.801, 95% CI: 1.437-2.257, p<0.001, Random effect), LNM (OR=1.869, 95% CI: 1.488-2.248, p<0.001, Random effect), and DM (OR=2.604, 95% CI: 1.500-4.519, p<0.001, Random effect). However, no significant difference was observed between HIF-1α expression and age (OR=0.925, 95% CI: 0.777-1.101, p=0.3, Random effect) or sex (OR=1.69, 95% CI: 0.822-1.391, p=0.659, Random effect).
Table 3.
Meta-analysis of the association between HIF-1α expression and clinicopathological characteristics
| Stratified analysis | No. of studies |
No. of patients |
Test of association | Test of heterogeneity | |||
|---|---|---|---|---|---|---|---|
| Pooled OR (95% CI) | p-value | I2 (%) | P-value | Model | |||
| Gender (male vs. female) | 32 | 4995 | 0.94 (0.70-1.25) | 0.659 | 72.72 | <0.001 | R |
| Age (≥55 vs. <55) | 28 | 4472 | 0.93 (0.78-1.10) | 0.382 | 35.9 | 0.03 | R |
| Tumor size (large vs. small) | 21 | 3571 | 1.39 (1.07-1.82) | 0.022 | 68.65 | <0.001 | R |
| LNM (yes vs. no) | 33 | 3822 | 1.87 (1.49-2.35) | <0.001 | 58.62 | <0.001 | R |
| DM(yes vs. no) | 16 | 2353 | 2.60 (1.50-4.52) | <0.001 | 76.38 | <0.001 | R |
| Tumor stage (III+IV vs. I+II) | 28 | 4462 | 1.80 (1.44-2.26) | <0.001 | 61.65 | <0.001 | R |
Sensitivity analysis and publication bias
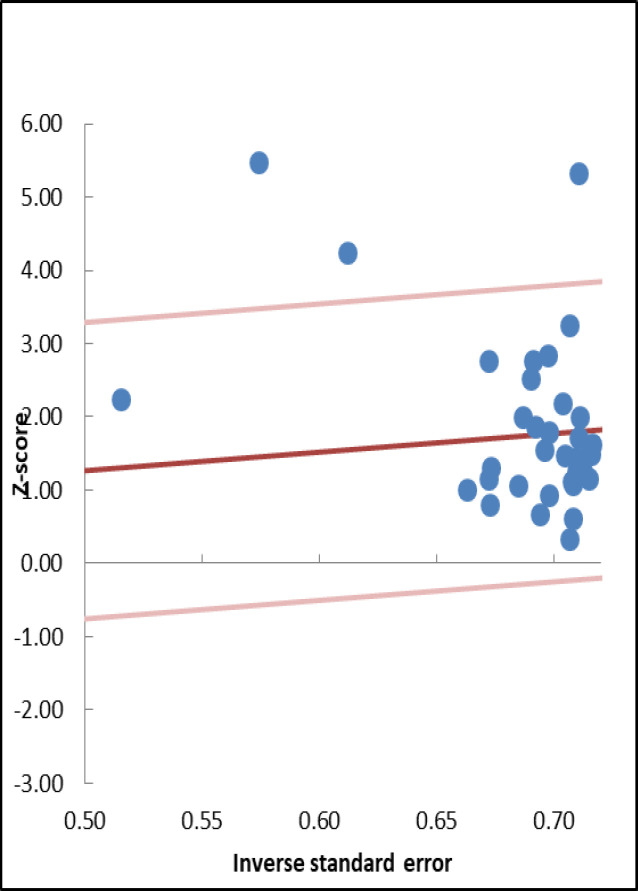
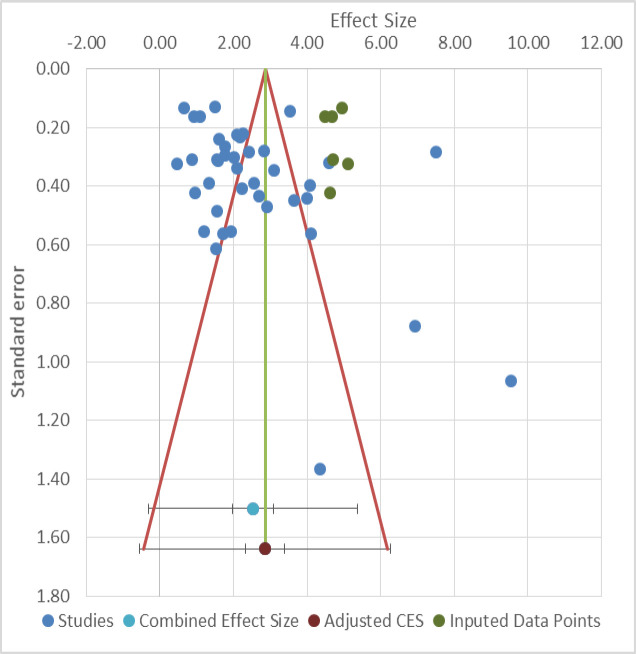
Galbraith plot detected three studies as the outliers with possible contributions to heterogeneity (Figure 4) (17, 29, 30). Sensitivity analysis was done to evaluate the robustness of the results. No single study was found to significantly change the direction of the HRs and ORs (Figure 5). Begg's funnel plot and Egger's test revealed a significant publication bias across the included studies (Egger's test, p=0.024) (Figure 6).
Figure 4.
Galbraith plots of the association between HIF-1α expression and OS in different cancer types
Figure 5.
The sensitivity analysis for the meta-analysis of OS in tumor patients
Figure 6.
Funnel plot analysis of potential publication bias for meta-analysis
Discussion
The current meta-results indicate that high expression of HIF-1α is associated with poor prognosis in patients with digestive system malignancies. Subgroup analysis with regard to cancer type showed a positive correlation between HIF-1α expression and poor OS in EsoC, GC, and CRC. However, no correlation was observed between HIF-1α expression and poor OS in HCC. More studies are needed to elucidate the role of HIF-1α in HCC. Additionally, subgroup analyses according to ethnicity and sample size showed that HIF-1α expression was related to worse OS. Elevated HIF-1α expression was also positively associated with four clinicopathological characteristics, namely LNM, DM, tumor size, and clinical stage of tumor. This could confirm the fact that HIF-1α overexpression plays a critical role in the biological behavior of different solid tumors. Pooled data demonstrated that high HIF-1α expression can act as a significant prognostic factor for survival outcomes and can provide a new reference point for predicting the metastasis and progression of cancer.
It is well known that the HIF-1α transcription factor upregulates and promotes the expression of many genes that are critical for cellular function (64). A possible explanation for this strong relationship between HIF-1α overexpression and tumor clinicopathologic factors could be the direct regulatory effect of HIF-1α on the vascular endothelial growth factor (VEGF) gene which is responsible for tumor angiogenesis (65). Angiogenesis is essential for the process of solid tumor formation, invasion, and metastatic spread. Moreover, HIF-1α may play a central role in tumorigenesis by upregulating different signaling pathways such as Myc and PI3K/AKT/mTOR that are involved in tumor proliferation, differentiation, migration, and invasion (66). Recent studies have confirmed that the overexpression of HIF-1α is associated with the aggressive phenotype of tumors.
According to the current results and in line with those of other studies, the relationship between HIF-1α expression and worse outcomes suggest HIF-1α as a target for therapeutic uses. HIF-1α target therapy may increase the survival of patients with advanced GI malignancies undergoing chemotherapy or radiotherapy. The data further suggests an important role for HIF-1α in GI cancer progression and poor OS in Asians and Caucasians. Moreover, HIF-1α expression is related to poor OS in both genders; hence, it may be a potential therapeutic target for cancer stratification in both genders.
The current meta-analysis had some limitations. First, all included studies were published in English, which may be a source of limited generalizability and selection bias. Second, the considerable heterogeneity might affect the study results. However, to minimize the effect of heterogeneity, a random effect model was applied. Third, HRs in a few of the selected studies were extracted from the Kaplan-Meier curve, which might not reflect true values. Finally, there is no standard threshold or definite cut-off value for HIF-1α expression in digestive system malignancies.
The current meta-analysis indicates that overexpression of HIF-1α is associated with poor prognosis in patients with digestive system malignancies and might be a novel prognostic factor for patient survival. The data also demonstrates that elevated HIF-1α is correlated with clinicopathological features such as LNM, DM, advanced TNM stage, and larger tumor size in digestive system cancers. HIF-1α has the potential to serve as a tumor marker for predicting the prognosis of digestive system malignancies.
Conflict of interests
The authors declare that they have no conflict of interest.
Acknowledgment
The authors would like to express their deep appreciation to the Clinical Research Development Unit, Ghaem Hospital, Mashhad University of Medical Sciences, for facilitating the data analysis. This work was supported by Mashhad University of Medical Sciences [Research Project No.981791, as part of a MD dissertation].
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin D, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–53. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Haringsma J, van Heel NCM, Kuipers EJ. Are we making progress in diagnosing and preventing gastrointestinal cancers? Therap Adv Gastroenterol. 2010;3:213–20. doi: 10.1177/1756283X10372984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, et al. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology. 2020;159:335–49. doi: 10.1053/j.gastro.2020.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammadi M, Mianabadi F, Mehrad‐Majd H. Circulating visfatin levels and cancers risk: A systematic review and meta‐analysis. J Cell Physiol. 2019;234:5011–22. doi: 10.1002/jcp.27302. [DOI] [PubMed] [Google Scholar]
- 6.Arjmand M-H, Moradi A, Akbari A, Mehrad-Majd H. Clinical significance of circulating omentin levels in various malignant tumors: Evidence from a systematic review and meta-analysis. Cytokine. 2020;125:154869. doi: 10.1016/j.cyto.2019.154869. [DOI] [PubMed] [Google Scholar]
- 7.Banin Hirata BK, Oda JMM, Losi Guembarovski R, Ariza CB, Oliveira CECd, Watanabe MAE. Molecular Markers for Breast Cancer: Prediction on Tumor Behavior. Dis Markers. 2014;2014:513158. doi: 10.1155/2014/513158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 9.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 10.Dai C-X, Gao Q, Qiu S-J, Ju M-J, Cai M-Y, Xu Y-F, et al. Hypoxia-inducible factor-1 alpha, in association with inflammation, angiogenesis and MYC, is a critical prognostic factor in patients with HCC after surgery. BMC Cancer. 2009;9:418. doi: 10.1186/1471-2407-9-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wigerup C, Pahlman S, Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol Ther. 2016;164:152–69. doi: 10.1016/j.pharmthera.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Ping W, Sun W, Zu Y, Chen W, Fu X. Clinicopathological and prognostic significance of hypoxia-inducible factor-1α in esophageal squamous cell carcinoma: a meta-analysis. Tumor Biol. 2014;35:4401–9. doi: 10.1007/s13277-013-1579-0. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa K, Chiba I, Morioka T, Shimoji H, Tamaki W, Takamatsu R, et al. Clinical significance of HIF-1α expression in patients with esophageal cancer treated with concurrent chemoradiotherapy. Anticancer Res. 2011;31:2351–9. [PubMed] [Google Scholar]
- 14.Ogane N, Yasuda M, Shimizu M, Miyazawa M, Kamoshida S, Ueda A, et al. Clinicopathological implications of expressions of hypoxia-related molecules in esophageal superficial squamous cell carcinoma. Ann Diagn Pathol. 2010;14:23–9. doi: 10.1016/j.anndiagpath.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Kuwai T, Kitadai Y, Tanaka S, Onogawa S, Matsutani N, Kaio E, et al. Expression of hypoxia‐inducible factor‐1α is associated with tumor vascularization in human colorectal carcinoma. Int J Cancer. 2003;105:176–81. doi: 10.1002/ijc.11068. [DOI] [PubMed] [Google Scholar]
- 16.Kwon HC, Kim SH, Oh SY, Lee S, Kwon KA, Lee JH, et al. Clinicopathological significance of nuclear factor‐kappa B, HIF‐1 alpha, and vascular endothelial growth factor expression in stage III colorectal cancer. Cancer Sci. 2010;101:1557–61. doi: 10.1111/j.1349-7006.2010.01553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma J, Zhang L, Ru G-Q, Zhao Z-S, Xu W-J. Upregulation of hypoxia inducible factor 1α mRNA is associated with elevated vascular endothelial growth factor expression and excessive angiogenesis and predicts a poor prognosis in gastric carcinoma. World J Gastroenterol. 2007;13:1680. doi: 10.3748/wjg.v13.i11.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu X, Lin S, Zheng J, Guo R, Li H, You C. Clinicopathological significance of hypoxia-inducible factor-1 alpha polymorphisms in cancers: evidence from a meta-analysis. Tumor Biol. 2013;34:2477–87. doi: 10.1007/s13277-013-0971-0. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Wu Y, Lin Y-H, Guo S, Ning P-F, Zheng Z-C, et al. Prognostic value of hypoxia-inducible factor-1 alpha and prolyl 4-hydroxylase beta polypeptide overexpression in gastric cancer. World J Gastroenterol. 2018;24:2381. doi: 10.3748/wjg.v24.i22.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun H-C, Qiu Z-J, Liu J, Sun J, Jiang T, Huang K-J, et al. Expression of hypoxia-inducible factor-1 alpha and associated proteins in pancreatic ductal adenocarcinoma and their impact on prognosis. Int J Cancer. 2007;30:1359–67. [PubMed] [Google Scholar]
- 21.Xiang Z-L, Zeng Z-C, Fan J, Tang Z-Y, He J, Zeng H-Y, et al. The expression of HIF-1α in primary hepatocellular carcinoma and its correlation with radiotherapy response and clinical outcome. Mol Biol Rep. 2012;39:2021–9. doi: 10.1007/s11033-011-0949-1. [DOI] [PubMed] [Google Scholar]
- 22.Yang SL, Liu LP, Jiang JX, Xiong ZF, He QJ, Wu C. The correlation of expression levels of HIF-1α and HIF-2α in hepatocellular carcinoma with capsular invasion, portal vein tumor thrombi and patients' clinical outcome. Jpn J Clin Oncol. 2014;44:159–67. doi: 10.1093/jjco/hyt194. [DOI] [PubMed] [Google Scholar]
- 23.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 24.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta‐analysis with time‐to‐event outcomes. Stat Med. 2002;21:3337–51. doi: 10.1002/sim.1303. [DOI] [PubMed] [Google Scholar]
- 26.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med. 1988;7:889–94. doi: 10.1002/sim.4780070807. [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Shi Y, Yuan J, Han Y, Qin R, Wu Q, et al. HIF-1 alpha overexpression correlates with poor overall survival and disease-free survival in gastric cancer patients post-gastrectomy. PLoS One. 2014;9:e90678. doi: 10.1371/journal.pone.0090678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu X-X, Chen Y-T, Feng B, Mao X-B, Yu B, Chu X-Y. Expression and clinical significance of CD73 and hypoxia-inducible factor-1α in gastric carcinoma. World J Gastroenterol. 2013;19:1912. doi: 10.3748/wjg.v19.i12.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isobe T, Aoyagi K, Koufuji K, Shirouzu K, Kawahara A, Taira T, et al. Clinicopathological significance of hypoxia-inducible factor-1 alpha (HIF-1α) expression in gastric cancer. J Cancer Res Clin Oncol. 2013;18:293–304. doi: 10.1007/s10147-012-0378-8. [DOI] [PubMed] [Google Scholar]
- 31.Qiu M-z, Han B, Luo H-y, Zhou Z-w, Wang Z-q, Wang F-h, et al. Expressions of hypoxia-inducible factor-1α and hexokinase-II in gastric adenocarcinoma: the impact on prognosis and correlation to clinicopathologic features. Tumor Biol. 2011;32:159–66. doi: 10.1007/s13277-010-0109-6. [DOI] [PubMed] [Google Scholar]
- 32.Oh SY, Kwon H-C, Kim S-H, Jang JS, Kim MC, Kim KH, et al. Clinicopathologic significance of HIF-1α, p53, and VEGF expression and preoperative serum VEGF level in gastric cancer. BMC Cancer. 2008;8:123. doi: 10.1186/1471-2407-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.KolevY U, TakagiY S. Lactate Dehydrogenase-5 (LDH-5) expression in human gastric cancer: association with hypoxia-Inducible factor (HIF-1a) pathway, angiogenic factors production and poor prognosis. Ann Surg Oncol. 2008;15:2336–44. doi: 10.1245/s10434-008-9955-5. [DOI] [PubMed] [Google Scholar]
- 34.Griffiths EA, Pritchard S, Valentine HR, Whitchelo N, Bishop P, Ebert M, et al. Hypoxia-inducible factor-1 α expression in the gastric carcinogenesis sequence and its prognostic role in gastric and gastro-oesophageal adenocarcinomas. Br J Cancer. 2007;96:95–103. doi: 10.1038/sj.bjc.6603524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sumiyoshi Y, Kakeji Y, Egashira A, Mizokami K, Orita H, Maehara Y. Overexpression of hypoxia-inducible factor 1α and p53 is a marker for an unfavorable prognosis in gastric cancer. Clin Cancer Res. 2006;12:5112–7. doi: 10.1158/1078-0432.CCR-05-2382. [DOI] [PubMed] [Google Scholar]
- 36.Urano N, Fujiwara Y, Doki Y, Tsujie M, Yamamoto H, Miyata H, et al. Overexpression of hypoxia-inducible factor-1 alpha in gastric adenocarcinoma. Gastric Cancer. 2006;9:44–9. doi: 10.1007/s10120-005-0356-1. [DOI] [PubMed] [Google Scholar]
- 37.Mizokami K, Kakeji Y, Oda S, Irie K, Yonemura T, Konishi F, et al. Clinicopathologic significance of hypoxia‐inducible factor 1α overexpression in gastric carcinomas. J Surg Oncol. 2006;94:149–54. doi: 10.1002/jso.20568. [DOI] [PubMed] [Google Scholar]
- 38.Jiang X, Zhang S, Yin Z, Sheng Y, Yan Q, Sun R, et al. The correlation between NEDD4L and HIF-1α levels as a gastric cancer prognostic marker. Int J Med Sci. 2019;16:1517–24. doi: 10.7150/ijms.34646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saka B, Ekinci O, Dursun A, Akyurek N. Clinicopathologic and prognostic significance of immunohistochemical expression of HIF-1α, CXCR4 and CA9 in colorectal carcinoma. Pathol Res Pract. 2017;213:783–92. doi: 10.1016/j.prp.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Shimomura M, Hinoi T, Kuroda S, Adachi T, Kawaguchi Y, Sasada T, et al. Overexpression of hypoxia inducible factor-1 alpha is an independent risk factor for recurrence after curative resection of colorectal liver metastases. Ann Surg Oncol. 2013;20:527–36. doi: 10.1245/s10434-013-2945-2. [DOI] [PubMed] [Google Scholar]
- 41.Xie YQ, Fu D, He ZH, Tan QD. Prognostic value of Annexin A3 in human colorectal cancer and its correlation with hypoxia‑inducible factor‑1α. Oncol Lett. 2013;6:1631–5. doi: 10.3892/ol.2013.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shioya M, Takahashi T, Ishikawa H, Sakurai H, Ebara T, Suzuki Y, et al. Expression of hypoxia-inducible factor 1α predicts clinical outcome after preoperative hyperthermo-chemoradiotherapy for locally advanced rectal cancer. J Radiat Res. 2011;52:821–7. doi: 10.1269/jrr.11117. [DOI] [PubMed] [Google Scholar]
- 43.Saigusa S, Tanaka K, Toiyama Y, Yokoe T, Okugawa Y, Koike Y, et al. Clinical significance of CD133 and hypoxia inducible factor-1α gene expression in rectal cancer after preoperative chemoradiotherapy. Clin Oncol. 2011;23:323–32. doi: 10.1016/j.clon.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Baba Y, Nosho K, Shima K, Irahara N, Chan AT, Meyerhardt JA, et al. HIF1A overexpression is associated with poor prognosis in a cohort of 731 colorectal cancers. Am J Pathol. 2010;176:2292–301. doi: 10.2353/ajpath.2010.090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao D, Hou M, Guan Y-s, Jiang M, Yang Y, Gou H-f. Expression of HIF-1alpha and VEGF in colorectal cancer: association with clinical outcomes and prognostic implications. BMC Cancer. 2009;9:432. doi: 10.1186/1471-2407-9-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jubb A, Turley H, Moeller H, Steers G, Han C, Li J, et al. Expression of delta-like ligand 4 (Dll4) and markers of hypoxia in colon cancer. Br J Cancer. 2009;101:1749–57. doi: 10.1038/sj.bjc.6605368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajaganeshan R, Prasad R, Guillou P, Scott N, Poston G, Jayne D. Expression patterns of hypoxic markers at the invasive margin of colorectal cancers and liver metastases. Eur J Surg Oncol. 2009;35:1286–94. doi: 10.1016/j.ejso.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Rasheed S, Harris A, Tekkis P, Turley H, Silver A, McDonald P, et al. Hypoxia-inducible factor-1α and-2α are expressed in most rectal cancers but only hypoxia-inducible factor-1α is associated with prognosis. Br J Cancer. 2009;100:1666–73. doi: 10.1038/sj.bjc.6605026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu X-g, Xing C-g, Feng Y-z, Chen J, Deng C. Clinical significance of immunohistochemical expression of hypoxia-inducible Factor–1α as a prognostic marker in rectal adenocarcinoma. Clin Colorectal Cancer. 2006;5:350–3. doi: 10.3816/ccc.2006.n.005. [DOI] [PubMed] [Google Scholar]
- 50.Theodoropoulos GE, Lazaris AC, Theodoropoulos VE, Papatheodosiou K, Gazouli M, Bramis J, et al. Hypoxia, angiogenesis and apoptosis markers in locally advanced rectal cancer. Int J Colorectal Dis. 2006;21:248–57. doi: 10.1007/s00384-005-0788-4. [DOI] [PubMed] [Google Scholar]
- 51.Yoshimura H, Dhar DK, Kohno H, Kubota H, Fujii T, Ueda S, et al. Prognostic impact of hypoxia-inducible factors 1α and 2α in colorectal cancer patients: correlation with tumor angiogenesis and cyclooxygenase-2 expression. Clin Cancer Res. 2004;10:8554–60. doi: 10.1158/1078-0432.CCR-0946-03. [DOI] [PubMed] [Google Scholar]
- 52.Kuwai T, Kitadai Y, Tanaka S, Kuroda T, Ochiumi T, Matsumura S, et al. Single nucleotide polymorphism in the hypoxia-inducible factor-1α gene in colorectal carcinoma. Oncol Rep. 2004;12:1033–7. [PubMed] [Google Scholar]
- 53.Schmitz KJ, Müller CI, Reis H, Alakus H, Winde G, Baba HA, et al. Combined analysis of hypoxia-inducible factor 1 alpha and metallothionein indicates an aggressive subtype of colorectal carcinoma. Int J Colorectal Dis. 2009;24:1287–96. doi: 10.1007/s00384-009-0753-8. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y, Lu Y, Lu C, Zhang L. Beclin-1 expression is a predictor of clinical outcome in patients with esophageal squamous cell carcinoma and correlated to hypoxia-inducible factor (HIF)-1α expression. Pathol Oncol Res. 2009;15:487. doi: 10.1007/s12253-008-9143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tzao C, Lee S-C, Tung H-J, Hsu H-S, Hsu W-H, Sun G-H, et al. Expression of hypoxia-inducible factor (HIF)-1α and vascular endothelial growth factor (VEGF)-D as outcome predictors in resected esophageal squamous cell carcinoma. Dis Markers. 2008;25:141–8. doi: 10.1155/2008/468323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H, Wang Y, Xu N, Zhu S, Liu B. Expression and clinical significance of HIF-1α, VEGF and Survivin in esophageal squamous cell carcinoma. Chin-Germ J Clin Oncol. 2007;6:339–44. [Google Scholar]
- 57.Katsuta M, Miyashita M, Makino H, Nomura T, Shinji S, Yamashita K, et al. Correlation of hypoxia inducible factor-1α with lymphatic metastasis via vascular endothelial growth factor-C in human esophageal cancer. Exp Mol Pathol. 2005;78:123–30. doi: 10.1016/j.yexmp.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Kimura S, Kitadai Y, Tanaka S, Kuwai T, Hihara J, Yoshida K, et al. Expression of hypoxia-inducible factor (HIF)-1α is associated with vascular endothelial growth factor expression and tumour angiogenesis in human oesophageal squamous cell carcinoma. Eur J Cancer. 2004;40:1904–12. doi: 10.1016/j.ejca.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 59.Kurokawa T, Miyamoto M, Kato K, Cho Y, Kawarada Y, Hida Y, et al. Overexpression of hypoxia-inducible-factor 1 α (HIF-1 α) in oesophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Br J Cancer. 2003;89:1042–7. doi: 10.1038/sj.bjc.6601186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munipalle PC, Viswanath YK, Davis PA, Scoones D. Prognostic value of hypoxia inducible factor 1α in esophageal squamous cell carcinoma. Dis Esophagus. 2011;24:177–81. doi: 10.1111/j.1442-2050.2010.01122.x. [DOI] [PubMed] [Google Scholar]
- 61.Wang D, Zhang X, Lu Y, Wang X, Zhu L. Hypoxia inducible factor 1α in hepatocellular carcinoma with cirrhosis: Association with prognosis. Pathol Res Pract. 2018;214:1987–92. doi: 10.1016/j.prp.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Dai X, Pi G, Yang S-l, Chen GG, Liu L-p, Dong H-H. Association of PD-L1 and HIF-1α coexpression with poor prognosis in hepatocellular carcinoma. Transl Oncol. 2018;11:559–66. doi: 10.1016/j.tranon.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang S-L, Liu L-P, Jiang J-X, Xiong Z-F, He Q-J, Wu C. The correlation of expression levels of HIF-1α and HIF-2α in hepatocellular carcinoma with capsular invasion, portal vein tumor thrombi and patients' clinical outcome. Jpn J Clin Oncol. 2014;44:159–67. doi: 10.1093/jjco/hyt194. [DOI] [PubMed] [Google Scholar]
- 64.Bottaro DP, Liotta LA. Out of air is not out of action. Nature. 2003;423:593–5. doi: 10.1038/423593a. [DOI] [PubMed] [Google Scholar]
- 65.Bao B, Azmi AS, Ali S, Ahmad A, Li Y, Banerjee S, et al. The biological kinship of hypoxia with CSC and EMT and their relationship with deregulated expression of miRNAs and tumor aggressiveness. Biochim Biophys Acta. 2012;1826:272–96. doi: 10.1016/j.bbcan.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lv X, Li J, Zhang C, Hu T, Li S, He S, et al. The role of hypoxia-inducible factors in tumor angiogenesis and cell metabolism. Genes Dis. 2016;4:19–24. doi: 10.1016/j.gendis.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]