Abstract
Objective:
Dysregulated children experience significant impairment in regulating their affect, behavior, and cognitions and are at risk for numerous adverse sequelae. The unclear phenomenology of their symptoms presents a barrier to evidence-based diagnosis and treatment.
Method:
This study examines the cognitive, behavioral, and psychophysiological mechanisms of dysregulation using the Research Domain Criteria constructs of cognitive control and frustrative non-reward among a mixed clinical and community sample of 294 children ages 7–17.
Results:
Results showed that dysregulated children’s caregivers viewed them as having many more problems with everyday executive function than children with moderate or low levels of psychiatric symptoms; however, during standardized assessments of more complex cognitive control tasks, dysregulated children’s performance only differed from children with low symptoms on tests of cognitive flexibility. In addition, when frustrated, dysregulated children performed more poorly on a Go/No-Go task and demonstrated less autonomic flexibility as indexed by low respiratory sinus arrhythmia and pre-ejection period scores.
Conclusion:
The findings of this study suggest that autonomic inflexibility and impaired cognitive function in the context of frustration may be mechanisms underlying childhood dysregulation.
Keywords: youth, emotion regulation, frustration, cognition, psychophysiology
Introduction
Approximately 7% of youth referred for psychiatric treatment exhibit concurrent and impairing mood, behavior and attention problems.1 This phenotype—commonly referred to as dysregulation— constitutes a formidable clinical and public health challenge. Dysregulated children face enduring impairment throughout adolescence and adulthood and are at increased risk for numerous deleterious outcomes, including substance abuse, suicidality, psychiatric hospitalization, persistent psychopathology, and personality disorders.2–5
In clinical practice, dysregulation is often diagnosed as comorbid oppositional defiant, attention-deficit/hyperactivity, and mood/anxiety disorders and treated using interventions developed for specific problem areas (e.g., aggression, hyperactivity) which have limited data to support their efficacy among children with severe problems in multiple domains. 6,7 Some maintain that this complex constellation of symptoms ought to be conceptualized and treated more parsimoniously as a single syndrome. However, the characterization of such a syndrome has been challenging and controversial, beginning in the late 1990s when a perspective that dysregulated children exhibit a developmentally specific, ultradian-cycling presentation of bipolar disorder was disseminated.8 Since, evidence from epidemiological,9 genetic,10 behavioral,11,12 and neurobiological13 studies have supported a conceptualization of many of these children as chronically irritable, which was first operationalized as severe mood dysregulation (SMD).14 This later led to the development of disruptive mood dysregulation disorder (DMDD), a novel mood disorder in the Diagnostic and Statistical Manual for Mental Disorders – Fifth Edition (DSM-5) intended to capture chronic and co-occurring mood and aggression symptoms.15 However, concerns about DMDD’s evidence base, reliability, and overlap with other conduct disorders have been raised 16 and contributed to the decision to categorize the symptoms associated with dysregulation as a behavioral disorder –oppositional defiant disorder (ODD) with a chronic anger/irritability specifier – in the recent eleventh revision of the International Classification of Diseases (ICD-11).17
To advance evidence-based diagnosis and treatment of dysregulated children, it is necessary to better understand the phenomenology of their illness. Given the substantial phenotypic overlap between dysregulation and other psychological syndromes, the current study seeks to move beyond symptomology and examine the underlying mechanisms of childhood dysregulation using the National Institute of Mental Health’s Research Domain Criteria (RDoC) framework.18 The RDoC promotes the study of psychological and biological processes or constructs that underpin human (dys)function using a multi-modal approach that integrates sources of information, such as neural circuits, physiology, behavior, and self-report.
In particular, the current study focuses on two RDoC constructs that have previously been implicated in dysregulation and chronic irritability: frustrative non-reward and cognitive control. Frustrative non-reward refers to the affective experience elicited by the absence of an anticipated reward,19 whereas cognitive control describes the modulation of cognitions and emotions in the service of goal-directed behavior and encompasses aspects of executive functioning, such as goal selection, response inhibition, and performance monitoring. Dysregulated children are hypothesized to experience impairment in these processes, such that lowered emotional thresholds for frustration diminishes their cognitive and inhibitory control abilities and leads to more frequent and severe behavioral manifestations of frustration (e.g., temper tantrums, aggression). Indeed, a substantial body of neuroimaging evidence lends support for these deficits. Specifically, children with SMD or high levels of irritability show differences in activation in the prefrontal cortex 20,21 as well as brain regions associated with error monitoring, response inhibition, reward-based learning 13,22 and emotion regulation and processing. 22,23
Despite these important findings, current research pertaining to mechanisms of childhood dysregulation has several limitations. First, most studies have relied on comparisons between homogeneous diagnostic groups (e.g., children with SMD or bipolar disorder) and healthy controls, which have not well represented the diversity of emotional-behavioral symptoms present in clinics and the community. More recently, some groups have used dimensional measurements to examine affective, behavioral, and cognitive features of irritability.20, 21, 23 However, irritability represents only one aspect of dysregulated children’s clinical presentation and does not fully capture the complex combination of mood, behavior, and attention symptoms characteristic of this phenotype. Moreover, these studies have largely focused on identifying neural correlates of irritability using fMRI approaches, and therefore, have consisted of relatively small sample sizes.13, 20, 21, 22, 23
To develop a more complete understanding of dysregulation, it is necessary to integrate different sources of information. At present, dimensions including cognition and physiology remain understudied with inconsistent findings. For example, with regard to cognitive functioning, it has been suggested that dysregulated children may uniquely struggle with inattention and efficient processing as compared to healthy controls and children with ADHD alone.24 However, other research has found that dysregulated children cannot be distinguished from children with ADHD on various cognitive processes, including sustained attention, interference control, planning and flexibility, and psychomotor control.25 From a physiological perspective, it has been hypothesized that differences in autonomic arousal may underlie dysregulated children’s symptoms. Indeed, studies have shown that they have higher physiological responses both at rest 26 and in response to emotional stimuli, 27 suggesting that they may experience greater baseline arousal and reactivity to stress than even children with other internalizing or externalizing symptoms. However, the metrics of physiology and arousal used in these prior studies (e.g., heartrate, skin conductance) have primarily focused on either sympathetic or parasympathetic functioning in isolation and have not fully captured the complexity of dysregulated children’s autonomic functioning. 28 Moreover, very little research has examined the role of heartrate variability (HRV). Measuring the fluctuation in time between consecutive heartbeats, HRV is an important indicator of the autonomic nervous system’s flexibility in response to environmental demands.29 Decreased HRV has been widely implicated in both internalizing and externalizing problems in children.30 While one study has suggested that this may be similar for irritable youth, HRV has not been examined in dysregulated children specifically.31
The current study seeks to address these gaps in the literature and further refine the relations between dysregulation, frustrative non-reward, and cognitive control processes, using an RDoC-informed approach that utilizes empirical phenotypes in a large, symptomatically heterogeneous sample in conjunction with multimodal assessment methods, including behavior, physiology, and self-reports. We expect that dysregulated children will show the most severe impairment in both frustrative non-reward and cognitive control processes. Additionally, we expect that these processes will interact, such that cognitive control abilities will moderate responses to frustration.
Method
Participants
Participants were 294 youth aged 7–17 years (M=10.94, SD=2.41; 67% boys). All children were accompanied by at least one caregiver (86% biological mothers). In addition, because the larger study also aimed to examine genetic relatedness among family members, 48% of children had a sibling in the study. As detailed in Table 1, nearly 94 percent of children identified as White, which is consistent with the racial distribution of the catchment area and the mean socio-economic status was 63.52 (SD=26.11) on the Hollingshead scale, which corresponds with middle-class status. 32 To obtain a sample that represented a continuum of children’s emotional-behavioral health, families were recruited through a university-based outpatient psychiatry clinic as well as advertisements in the community. Forty one percent of children met diagnostic criteria for at least one current psychiatric disorder as determined by the Kiddie Schedule for Affective Disorders and Schizophrenia. 33 Two children were excluded due to meeting criteria for bipolar disorder.
Table 1:
Demographic Characteristics
| Characteristic | n | % | |
|---|---|---|---|
|
| |||
| Sex | |||
| Male | 196 | 66.7 | |
| Female | 98 | 33.3 | |
| Age | |||
|
| |||
| 7 | 20 | 6.8 | |
|
| |||
| 8 | 28 | 9.5 | |
|
| |||
| 9 | 38 | 12.9 | |
|
| |||
| 10 | 56 | 19.0 | |
|
| |||
| 11 | 33 | 11.2 | |
|
| |||
| 12 | 44 | 15.0 | |
|
| |||
| 13 | 26 | 8.8 | |
|
| |||
| 14 | 23 | 7.8 | |
|
| |||
| 15 | 11 | 3.7 | |
|
| |||
| 16 | 8 | 2.7 | |
|
| |||
| 17 | 5 | 1.7 | |
|
| |||
| Race | |||
| American Indian or Alaska | 5 | 1.7 | |
| Native | |||
| Asian or Asian American | 8 | 2.7 | |
| Black or African American | 6 | 2.0 | |
| White | 275 | 93.5 | |
| Hawaiian or Other Pacific | 0 | 0.0 | |
| Islander | |||
| Ethnicity | |||
|
| |||
| Hispanic or Latinx | 13 | 4.4 | |
|
| |||
| Not Hispanic or Latinx | 281 | 95.6 | |
| Hollingshead SES score | |||
| 0–9 (lowest) | 14 | 4.8 | |
| 10–19 | 8 | 2.7 | |
| 20–29 | 7 | 2.4 | |
| 30–39 | 1 | 0.3 | |
| 40–49 | 9 | 3.1 | |
| 50–59 (e.g., | 20 | 6.8 | |
| 60–69 | 46 | 15.6 | |
| 70–79 | 47 | 15.9 | |
| 80–89 | 56 | 19.0 | |
| 90–100 (highest) | 56 | 19.0 | |
| Psychiatric Diagnoses | |||
| MDD | 15 | 5.1 | |
| Dysthymia | 7 | 2.4 | |
| Separation Anxiety | 17 | 5.8 | |
| Social Phobia | 21 | 7.1 | |
| GAD | 51 | 17.3 | |
| OCD | 9 | 3.1 | |
| PTSD | 8 | 2.7 | |
| ADHD | 103 | 35.0 | |
| Conduct | 2 | 0.7 | |
| ODD | 77 | 26.2 | |
| Substance Abuse | 0 | 0 | |
| Any diagnosis | 120 | 40.8 | |
Note. ADHD = Attention Deficit/Hyperactivity Disorder; GAD=Generalized Anxiety Disorder; MDD= Major Depressive Episode; PTSD= Post-traumatic Stress Disorder; OCD=Obsessive Compulsive Disorder; ODD=Oppositional Defiant Disorder
Procedures
The University of Vermont Institutional Review Board approved all study procedures. Data collection proceeded through two cross-sectional family studies that used similar recruitment and data collection procedures (see Table S1, available online). Prior to participation, families were given a detailed verbal explanation of the study and completed written consent and assent forms. Each family member met separately with trained research assistants in a private laboratory setting. In both studies, caregivers completed interviews and online questionnaires, while children participated in computerized behavioral tasks and online questionnaires. In addition, in one of the studies (N=158), children also completed an assessment of their executive functioning skills. Research Electronic Data Capture (REDCap) was used to collect and manage study data.34
Measures
Dysregulation profile.
Child Behavior Checklist/ 6–18 (CBCL).
The CBCL is a 113-item caregiver-report questionnaire that assesses children’s emotional, behavioral, and social problems during the previous six months. Items are rated on a three-point scale and comprise eight factor-analytically derived syndrome scales that are consistent across age, informant, and culture with test-retest reliabilities ranging from 0.74 to 0.95 and Cronbach alphas ranging from 0.79 to 0.97.35 4,36 Dysregulation was characterized by the Dysregulation Profile (DP), a widely used and reliable measure of dysregulation formed of items from the Anxious/Depressed (AD), Attention Problems (AT), and Aggressive Behavior (AG) syndrome scales.37 Given that prior research has demonstrated measurement invariance in the DP across parents 38, paternal report was used for 24 participants for whom maternal report was unavailable.
Cognitive control.
Behavioral Rating Inventory of Executive Function (BRIEF).
The BRIEF is a standardized rating scale of children’s executive function that has analogous parent and youth self-report versions, which are empirically validated for children ages 5–18 and 11–18, respectively. Items comprise eight clinical subscales and three broad indices, each with Cronbach alphas ranging from 0.94 to 0.96. 39 In this study, the Global Executive Composite (GEC; measure of overall executive function ability), Metacognition Index (MI; measure of independent task initiation, working memory, planning, organization, and self-monitoring), and Behavioral Regulation Index (BRI; measure of ability to control impulses, change tasks, adapt to new situations, and modulate emotions) were examined. In addition, within the BRI, the Emotional Control (EC) subscale, which measures executive regulation of emotional expression was also included. For all scales, higher t-scores indicate less developed executive function skills.
Stop Signal Task (SST).
The SST is a widely used measure of attentional, inhibitory, and self-monitoring processes.40 In this paradigm, Xs and Os were displayed on the screen for one second and preceded by a 500ms fixation screen. Participants were instructed to press the corresponding key as quickly as possible (go trials) except for on occasional trials during which a tone was presented (stop trials). The delay of the tone was adjusted in accordance with participants’ performance to maintain an overall rate of successful inhibitions of 50% (+/−15%). Stop signal reaction time (SSRT) represents the speed of inhibiting (in milliseconds) calculated by subtracting the mean delay of the stop signal from the mean choice reaction time on go trials. Lower SSRT values are indicative of greater cognitive control.
Delis-Kaplan Executive Function System (D-KEFS).
The Trail-Making and Tower Tests of the D-KEFS were used to assess various domains of executive function.41 The Trail-Making Test consists of four conditions: Visual Scanning (VS), Number Sequencing (NS), Letter Sequencing (LS), Number-Letter Switching (N-LS). The N-LS condition constitutes the most cognitively demanding condition and primary measure of executive function. This condition assesses cognitive flexibility by requiring participants to switch quickly and accurately between sequentially connecting numbers and letters. The VS, NS, and LS conditions each assess more basic, requisite cognitive processes involved in the N-LS condition. The Tower Test measures problem solving, planning and organization, as well as inhibitory control and rule following in children.41,42 In the task, participants attempted to build a series of nine increasingly difficult towers using as few moves as possible within an allotted amount of time from which a total achievement score (TAS) was calculated. For both tests, scores were based on performance accuracy and efficiency and scaled according to age. 42
Frustrative non-reward.
Go/No-Go Task.
Emotion and behavior regulation were assessed using a computerized, frustration-induction Go/No-Go paradigm.43 Participants were instructed to press a controller button as quickly as possible each time a letter appeared on the screen (go signal), but to withhold the button press if the same letter appeared twice in a row (no-go signal). Additionally, they were told that by earning “a lot of points,” they would receive “the big prize” (a $10 coupon to a toy or bookstore), but that too few points would result in the “small prize” (a small box of crayons).
The task consisted of three blocks: baseline, frustration-induction, and recovery. If the participant failed to quickly press the button on a prepotent go trial or withhold the button press on a no-go trial, a red bar appeared on the screen, indicating an error. Additionally, the total number of points accrued was displayed every 5– 25 trials. During the baseline block, the stimulus duration was adjusted, such that participants steadily gained points. In the frustration-induction block, the stimulus duration was accelerated resulting in a consistent loss of points until few or no points remained. Finally, the recovery block was identical to the baseline block, and participants regained their points and earned the “big prize.” To achieve a comparable level of difficulty across participants, the stimulus duration was adjusted dynamically, such that the rate of incorrect no-go trials was held constant at 50% (+/−10%). Following each block, participants rated their level of frustration on a visual analog scale from 1 (not at all frustrated) to 5 (extremely frustrated). Performance accuracy was computed separately for each block by averaging accuracy across go and no-go trials.
Autonomic arousal.
Electrocardiogram and thoracic impedance data were continuously recorded (1000 samples per second) during the Go/No-Go task using the Vrije Universiteit-Ambulatory Monitoring System (VU-AMS) Version 3.5.44 This device was attached to the participant via seven electrodes. The recording was divided into three blocks that temporally corresponded with the blocks of the Go/No-Go task. Potentially erroneous R-peaks were first detected by the computer and then visually inspected with noisy fragments removed from analysis. The corrected recordings were used to calculate pre-ejection period (PEP) and respiratory sinus arrhythmia (RSA).
PEP was used to measure the influence of the sympathetic nervous system (SNS; responsible for mobilizing the body’s resources in response to threat) on cardiac function.45 PEP reflects ventricular contractility and is defined as the interval from the onset of left ventricular depolarization to the opening of the aortic valves.46 Shorter PEP intervals indicate greater activation of the SNS, which has been related to more efficient attention-related processing 47 and less sensitivity to reward.48
RSA was used to measure the influence of the parasympathetic nervous system (PNS; responsible for dampening stress responses and maintaining homeostasis) on cardiac function.45 RSA scores reflect heart rate variability synchronized with respiration and were calculated using the peak-valley method, such that the shortest inter-beat interval during inhalation was subtracted from the longest inter-beat interval during exhalation.44 Higher RSA scores indicate greater activation of the PNS, which serves as an indicator of an individual’s ability to engage flexibly with the demands of their environment and has been associated with increased cognitive capacity.47
Analytic Plan
Main statistical analyses were conducted using MPlus Version 8.1. Consistent with previous studies, phenotypic groups were empirically derived using latent class analysis (LCA) of items from the AD, AT and AG subscales of the CBCL, which were dichotomized such that 0 = not present and 1 = present.4,36 Models estimating one through seven classes were performed iteratively using maximum likelihood estimation with robust standard errors to account for non-normally distributed data. Model fit was evaluated based on commonly accepted fit indices, including Bayesian Information Criteria (BIC), Vuong-Lo-Mendell-Rubin Likelihood Ratio Test (VLMR) and Bootstrap Likelihood Ratio Test (BLRT), in conjunction with substantive meaning and theory.
After determining the best-fitting model, means for distal outcomes for each latent class were estimated using the Bolck, Croon and Hagenaars (BCH) method. This approach was advantageous as it allowed for estimation of distal outcomes accounting for classification uncertainty.50 Next, Wald Chi-Squared tests were conducted to test for differences in distal outcomes across latent classes (See Figure S1 for conceptual model, available online). Variables derived from the same instruments were tested together in a model to account for common method variance and reduce the number of comparisons and associated risk of type 1 error. Finally, interaction effects between the constructs of cognitive control and frustrative non-reward were tested using cross-products of mean-centered caregiver-reported GEC with RSA, PEP, and performance accuracy during each Go/No-Go block.
Age and sex were included as covariates in all measurement models, and familial clusters were nested within models to adjust for non-independence of siblings.
Results
Descriptive statistics for primary study variables and missing data are presented in Table 2. Twenty-five (8.41%) participants were excluded due to missing CBCL data. Missing data percentages varied from 3.35% to 17.47% with a mean of 9.06% and were generally related to participant noncompliance, technological malfunction, excessive child movement or experimenter error. The assumption of missingness completely at random was supported by a non-significant Little’s test.
Table 2:
Descriptive Statistics and Missing Data
| n (% missing)a | Mechanisms of missingness | M | SD | Skewness | SE | Kurtosis | SE | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||||
| BRIEF - Parent | ||||||||||||||||||||||
| EC | 139 (5.44) | Non-compliance, experimenter error | 56.21 | 14.66 | 0.49 | 0.21 | −0.67 | 0.41 | ||||||||||||||
| BRI | 139 (5.44) | 57.79 | 15.71 | 0.57 | 0.21 | −0.49 | 0.41 | |||||||||||||||
| MI | 139 (5.44) | 57.01 | 12.36 | 0.17 | 0.21 | −0.67 | 0.41 | |||||||||||||||
| GEC | 139 (5.44) | 57.97 | 13.97 | 0.30 | 0.21 | 0.71 | 0.41 | |||||||||||||||
| BRIEF - Youth | ||||||||||||||||||||||
| EC | 135 (8.16) | Non-compliance, experimenter error | 51.94 | 11.80 | 0.62 | 0.21 | −0.29 | 0.41 | ||||||||||||||
| BRI | 136 (7.48) | 50.54 | 12.41 | 0.40 | 0.21 | −0.53 | 0.41 | |||||||||||||||
| MI | 136 (7.48) | 50.42 | 11.57 | 0.26 | 0.21 | −0.67 | 0.41 | |||||||||||||||
| GEC | 136 (7.48) | 50.54 | 12.33 | 0.30 | 0.21 | −0.61 | 0.41 | |||||||||||||||
| D-KEFS | ||||||||||||||||||||||
| VS | 139 (5.44) | Non-compliance, experimenter error | 10.34 | 2.93 | −0.92 | 0.21 | 0.93 | 0.41 | ||||||||||||||
| NS | 139 (5.44) | 10.53 | 3.17 | −1.38 | 0.21 | 1.63 | 0.41 | |||||||||||||||
| LS | 139 (5.44) | 10.22 | 3.46 | −1.40 | 0.21 | 1.54 | 0.41 | |||||||||||||||
| N-LS | 138 (6.12) | 9.24 | 3.68 | −1.03 | 0.21 | 0.42 | 0.41 | |||||||||||||||
| TAS | 137 (6.80) | 10.45 | 3.35 | −1.55 | 0.21 | 3.28 | 0.41 | |||||||||||||||
| Stop Signal Task | ||||||||||||||||||||||
| SSRT | 123 (16.32) | Non-compliance, invalid responding | 247.58 | 117.11 | 0.97 | 0.22 | −0.46 | 0.41 | ||||||||||||||
| Self-Reported Frustration (Go/No-Go) | ||||||||||||||||||||||
| Baseline | 260 (3.35) | 2.02 | 0.97 | 1.03 | 0.15 | 0.95 | 0.30 | |||||||||||||||
| Frustration | 259 (3.72) | Non-compliance | 3.28 | 1.27 | −0.11 | 0.15 | −1.08 | 0.30 | ||||||||||||||
| Recovery | 258 (4.09) | 1.93 | 1.03 | 1.25 | 0.15 | 1.31 | 0.30 | |||||||||||||||
| % Accuracy (Go/No-Go) | ||||||||||||||||||||||
| Baseline | 245 (8.92) | Non-compliance, technological malfunction | 67.95 | 6.85 | −2.19 | 0.16 | 10.63 | 0.31 | ||||||||||||||
| Frustration | 245 (8.92) | 56.97 | 4.80 | −0.16 | 0.16 | 2.49 | 0.31 | |||||||||||||||
| Recovery | 243 (9.67) | 69.22 | 6.56 | −1.14 | 0.16 | 6.38 | 0.31 | |||||||||||||||
| RSA (Go/No-Go) | ||||||||||||||||||||||
| Baseline | 227 (15.61) | Non-compliance, technological malfunction, excessive movement | 75.13 | 39.13 | 1.30 | 0.16 | 2.56 | 0.32 | ||||||||||||||
| Frustration | 224 (16.73) | 76.87 | 41.58 | 1.73 | 0.16 | 4.60 | 0.32 | |||||||||||||||
| Recovery | 225 (16.35) | 73.70 | 41.58 | 1.67 | 0.16 | 3.64 | 0.32 | |||||||||||||||
| PEP (Go/No-Go) | ||||||||||||||||||||||
| Baseline | 226 (15.99) | Non-compliance, technological malfunction, excessive movement | 53.91 | 31.04 | 0.34 | 0.16 | <-0.01 | 0.32 | ||||||||||||||
| Frustration | 224 (16.73) | 56.02 | 34.29 | 0.84 | 0.16 | 2.89 | 0.32 | |||||||||||||||
| Recovery | 222 (17.47) | 54.88 | 30.26 | 0.13 | 0.16 | −0.64 | 0.33 | |||||||||||||||
These missing data were treated as missing at random and estimated in MPlus using Maximum Likelihood model.
Latent Class Analysis
Cronbach alphas for the three CBCL syndrome scales were in the good to excellent range (AD=0.85, AT=0.88, AG=0.92). Fit indices for candidate LCA models are provided in Table 3. The BIC was lowest for the four-class solution; however, the VLMR was non-significant, indicating that four classes did not fit the data significantly better than three classes. Thus, a three-class solution was selected for subsequent analyses.
Table 3:
Fit Indices for Latent Class Models
| # of classes | Log Likelihood | BIC | Entropy | VLMR P |
|---|---|---|---|---|
|
| ||||
| 1 | −6823.48 | 13876.32 | n/a | n/a |
| 2 | −5474.80 | 11413.96 | 0.97 | <.001 |
| 3 | −5166.77 | 11032.87 | 0.95 | .0043 |
| 4 | −5040.90 | 11016.12 | 0.94 | .467 |
| 5 | −4934.43 | 11038.15 | 0.96 | .165 |
| 6 | −4842.58 | 11089.43 | 0.96 | .398 |
| 7 | −4776.40 | 11192.05 | 0.97 | .629 |
Note. Bold text indicates the number of classes best fitting the data for each fit index.
As shown in Figure S2, Class 1 (41.33% of the sample) represented a pattern of high probabilities of endorsement for AD, AT, and AG items and was characterized as the dysregulation profile (DP) class. Class 2 (30.51%) demonstrated moderate probabilities of endorsement for AD, AT, and AG items, and was therefore labelled as the moderate symptoms class. Finally, Class 3 (28.16%) was characterized by low probabilities of endorsement and was labelled as the low symptom class. Class membership was related to several demographic factors. Children in the low symptoms class were older (F [2,268] =5.05, p=.007) and had higher SES (F [2,264] =8.41, p<.001) and IQs (F [2,243] =12.64, p<.001) than both the DP and moderate symptoms classes. Additionally, the low symptoms class had a higher proportion of female children than the DP class (χ2=7.19, p<.028).
Relations with RDoC Constructs
Wald Chi-Square statistics and p-values for overall models and pairwise comparisons are presented in Table 4.
Table 4:
Wald Chi-Square Statistics for Difference Tests Between Latent Classes on Distal Outcomes
| Model | Overall (df=2) | DP vs Moderate | DP vs Low | Moderate vs Low | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Wald χ2 | p | Wald χ2 | p | Wald χ2 | p-value | Wald χ2 | p | |||
|
| ||||||||||
| 1 | BRIEF- Parent Version | |||||||||
| EC | 187.01 | <.001 | 54.78 | <.001 | 184.79 | <.001 | 36.54 | <.001 | ||
| BRI | 277.18 | <.001 | 64.13 | <.001 | 249.63 | <.001 | 79.57 | <.001 | ||
| MI | 134.90 | <.001 | 12.60 | <.001 | 116.54 | <.001 | 68.27 | <.001 | ||
| GEC | 206.51 | <.001 | 39.91 | <.001 | 189.08 | <.001 | 85.93 | <.001 | ||
| 2 | BRIEF- Youth Version | |||||||||
| EC | 16.81 | <.001 | 3.75 | .053 | 16.50 | <.001 | 2.68 | .101 | ||
| BRI | 19.40 | <.001 | 2.02 | .155 | 19.38 | <.001 | 8.15 | .004 | ||
| MI | 11.49 | .003 | 0.15 | .702 | 10.15 | .001 | 7.89 | .005 | ||
| GEC | 16.71 | <.001 | 0.98 | .323 | 16.05 | <.001 | 9.38 | .002 | ||
| 3 | D-KEFS | |||||||||
| VS | 7.96 | .018 | 7.21 | .007 | 3.72 | .054 | 0.45 | .504 | ||
| NS | 3.16 | .206 | 0.07 | .785 | 2.72 | .099 | 1.28 | .259 | ||
| LS | 11.62 | .003 | 11.57 | .001 | 4.78 | .029 | 2.15 | .143 | ||
| N-LS | 6.22 | .045 | 0.45 | .501 | 6.19 | .013 | 1.59 | .207 | ||
| TAS (Tower) | 4.08 | .130 | 4.07 | .044 | 1.58 | .209 | 0.44 | .508 | ||
| 4 | Stop Signal Task | |||||||||
| SSRT | 4.62 | .100 | 3.99 | .046 | 3.76 | .053 | 0.33 | 0.564 | ||
| 5 | Self-Reported Frustration (Go/No-Go) | |||||||||
| Baseline | 1.31 | .521 | 0.21 | .650 | 1.13 | .288 | 0.19 | .666 | ||
| Frustration | 3.73 | .155 | 0.06 | .811 | 2.96 | .085 | 3.12 | .078 | ||
| Recovery | 3.39 | .183 | <0.01 | .958 | 2.93 | .087 | 1.78 | .182 | ||
| 6 | % Accuracy (Go/No-Go) | |||||||||
| Baseline | 12.55 | .002 | 0.04 | .847 | 6.98 | .008 | 2.29 | .131 | ||
| Frustration | 10.58 | .005 | 6.41 | .011 | 6.87 | .009 | 0.07 | .786 | ||
| Recovery | 10.69 | .005 | 6.30 | .012 | 9.30 | .002 | 0.64 | .423 | ||
| 7 | RSA (Go/No-Go) | |||||||||
| Baseline | 13.79 | .001 | 10.54 | .001 | 1.47 | .225 | 13.27 | <.001 | ||
| Frustration | 18.77 | <.001 | 9.89 | .002 | 2.87 | .090 | 18.40 | <.001 | ||
| Recovery | 0.86 | .649 | 0.65 | .421 | 0.27 | .602 | 0.02 | .886 | ||
| 8 | PEP (Go/No-Go) | |||||||||
| Baseline | 1.14 | .566 | 0.14 | .711 | 1.14 | .286 | 0.28 | .594 | ||
| Frustration | 2.75 | .253 | 2.75 | .097 | 0.64 | .425 | 0.99 | .319 | ||
| Recovery | 3.30 | .192 | 3.28 | .070 | 0.56 | .454 | 1.02 | .312 | ||
Note. Bold text indicates statistically significant (p <.05) results.
Cognitive control.
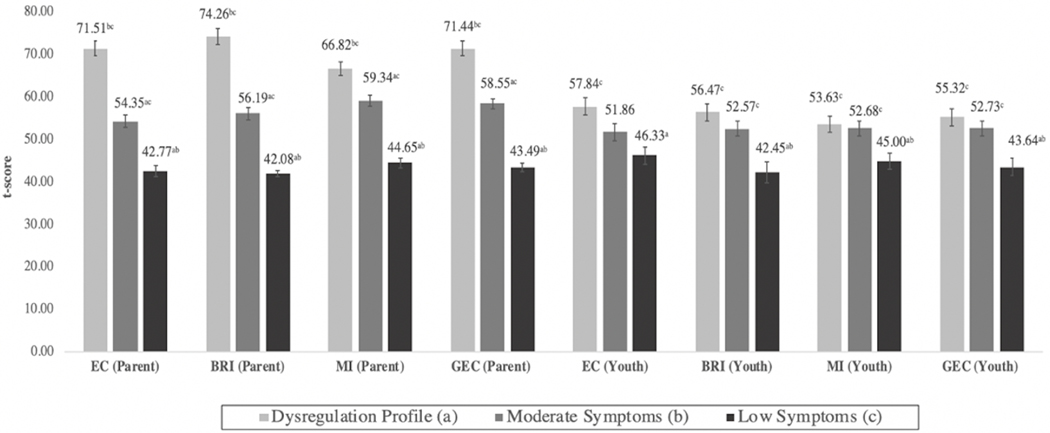
The three latent classes differed with regard to various aspects of cognitive control. In general, ratings on the BRIEF (Figure 1) showed a rank-order association between latent class membership and problems with emotional control, behavioral regulation, metacognition, and global executive function, such that the DP class had the most impairment and the low symptom class had the least. Based on caregivers’ reports, class differences were statistically significant across all executive functioning domains. In contrast, the DP and moderate classes did not differ in their self-reported levels of executive function problems, although both classes consistently rated themselves as more impaired than the low symptom class (with the exception of the moderate class on the emotional control subscale).
Figure 1: Mean Behavioral Rating Inventory of Executive Function (BRIEF) (Parent- and Youth Self-Report) Scores by Latent Class.
Note: Superscripts denote statistically significant (p<0.05) differences between classes with corresponding alphabetical designation. BRI = Behavioral Regulation Index; EC = Emotional Control; GEC = Global Executive Composite Score; MI = Metacognition Index.
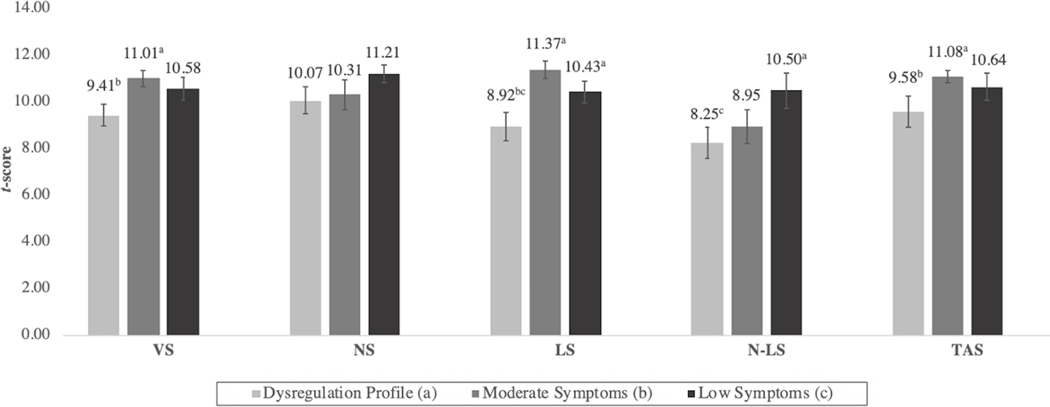
On the D-KEFS (Figure 2), no differences emerged between the moderate and low symptom classes, whereas the DP class scored significantly lower on both some basic and more demanding cognitive tasks. Specifically, on the Trail Making Test, the DP class performed significantly worse than both low and moderate symptom classes on the LS condition, and significantly worse than the moderate class on VS. In the more complex condition, N-LS, the DP class performed more poorly than the low symptoms class, whereas on the Tower Test, the DP class scored significantly lower than the moderate symptom class only.
Figure 2: Mean Delis-Kaplan Executive Function System (D-KEFS) (Trail-Making and Tower Test) Scores by Latent Class.
Note: Differing superscripts denote statistically significant (p<.05) differences between classes with corresponding alphabetical designation. LS = letter sequencing; N-LS = number-letter switching; NS = number sequencing; TAS = total achievement score; VS = visual scanning.
Finally, on the SST, the DP class showed the slowest SSRT (M=295.56, SE= 24.91), which differed significantly from the moderate (M= 218.39, SE= 23.59), but not the low symptom class (M=235.04, SE=16.54). SSRT did not differ between the moderate and low symptom classes.
Frustrative non-reward.
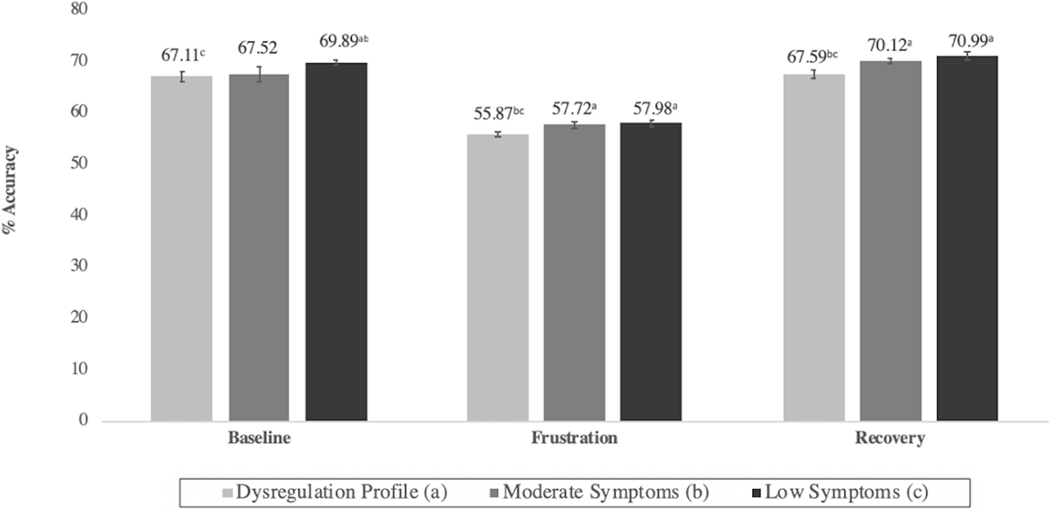
During the Go/No-Go task, children generally reported higher subjective levels of frustration during the frustration-induction block than during baseline or recovery. However, frustration levels did not significantly differ among classes (Figure S3). Follow-up change score analyses showed that frustration levels increased more between baseline and frustration blocks for children in the DP (M=1.41, SE=0.11) and moderate (M=1.46, SE=0.12) classes as compared with the low symptom (M=0.86, SE=0.12) class (χ2= 11.37, p=.001 and χ2 =10.46, p=.001, respectively). Behaviorally, the three classes did not differ in terms of performance accuracy at baseline. However, during the frustration block, the DP class was significantly less accurate than both the moderate and low symptom classes. This pattern persisted during the recovery block, suggesting that frustration had a unique and prolonged influence on the behavior of dysregulated children (Figure 3).
Figure 3: Performance Accuracy During Go/No-Go Task by Latent Class.
Note: Differing superscripts denote statistically significant (p <.05) differences between classes with corresponding alphabetical designation.
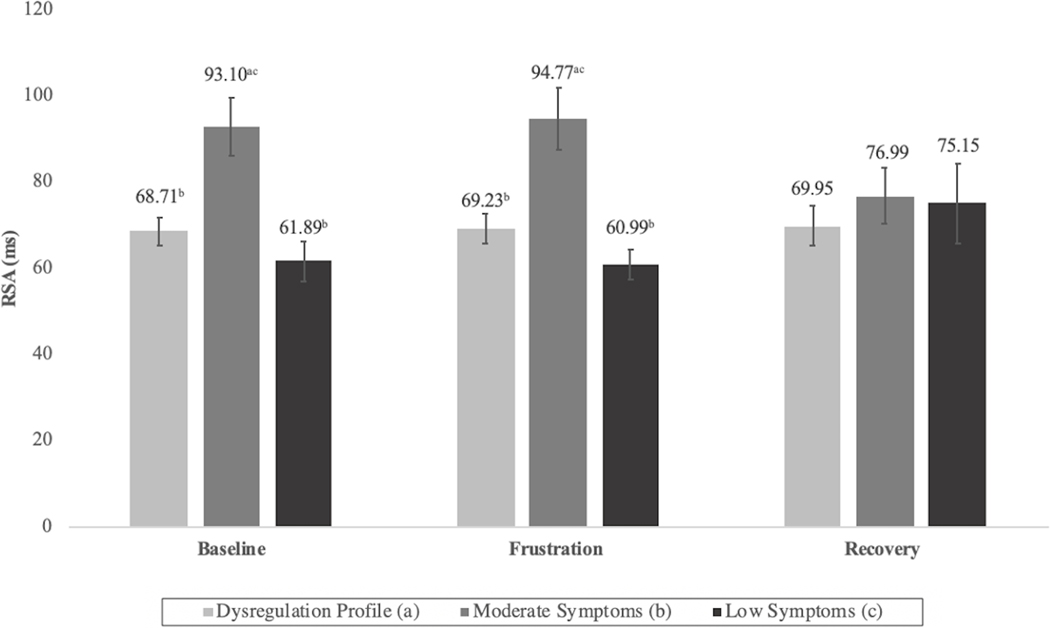
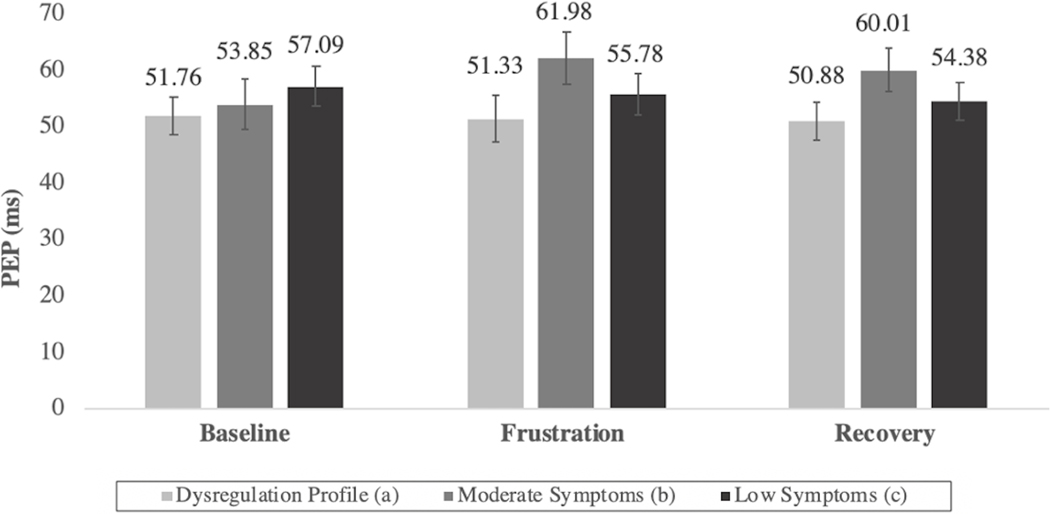
Next, the psychophysiological profiles of the three classes during the Go/No-Go task were examined (Figures 4 and 5). Although group differences in some cases did not reach the threshold for statistical significance, several noteworthy patterns emerged. As compared to the moderate and DP classes, the low symptom class was generally characterized by longer PEP and low-to-moderate RSA values, reflecting co-inhibition of the PNS and SNS, an autonomic pattern associated with adaptive engagement with stressors.51 The moderate class had significantly higher RSA values than both the DP and low symptom classes during the baseline and frustration blocks. Additionally, their PEP values were highest during frustration and recovery. Taken together, this pattern indicates a decrease in physiological arousal via the activation of the PNS and inhibition of the SNS. Finally, the DP class’ autonomic profile was characterized by both low PEP and RSA scores and indicated high levels of SNS activation and PNS inhibition. This physiological profile is associated with increased arousal,51 which was notably consistent throughout all blocks of Go/No-Go task, suggesting that these children’s physiological response did not appear to vary based on interactions with the environment.
Figure 4: Respiratory Sinus Arrhythmia (RSA) Values During Go/No-Go Task by Latent Class.
Note: Differing superscripts denote statistically significant (p <.05) differences between classes with corresponding alphabetical designation.
Figure 5: Pre-Ejection Period (PEP) Values During Go/No-Go Task by Latent Class.
Interactions between Cognitive Control and Frustrative Non-Reward.
Lastly, possible moderating effects of cognitive control on the relations between latent class assignment and frustrative non-reward were tested. Based on the results of earlier models, Go/No-Go performance accuracy, RSA and PEP during the Go/No-Go task were used as indicators of frustrative non-reward and caregiver-reported GEC score was selected as a global measure of cognitive control intended to capture the various domains of executive function that emerged as significantly related to latent class assignment. Results showed that cognitive control did not significantly moderate the relations between latent class assignment and behavioral or physiological responses to frustration. In other words, children’s behavioral and physiological responses to frustration did not depend on their baseline level of cognitive control.
Discussion
This study examined the RDoC constructs of frustrative non-reward and cognitive control as potential mechanisms of childhood dysregulation. Consistent with our hypothesis, dysregulated children were characterized by the most severe impairment in both processes. Importantly, their impairment was separable from that of children with moderate psychiatric symptoms. More specifically, within the construct of cognitive control, dysregulated children were consistently rated by their caregivers (but not themselves) as more impaired than children with moderate or low symptoms across various domains of everyday executive function. However, despite having lower overall IQ scores, dysregulated children’s performance on more complex executive function tasks generally did not differ from children with low levels of psychiatric symptoms, except in the N-LS condition of the D-KEFS Trail-Making Test, which measures cognitive flexibility. This pattern was somewhat consistent with results reported by Peyre and colleagues (2012) which found no association between cognitive control processes and dysregulation among children with ADHD. Notably, in the current study, dysregulated children only performed worse than both moderate and low symptom classes during the frustration-induction and recovery blocks of the Go/No-Go task, although they did not endorse higher levels of subjective frustration. In contrast to previous studies, which have observed increased physiological reactivity to negatively valanced emotion among dysregulated children, 26,27 in the current study, dysregulated children demonstrated a pattern of minimal sympathetic and parasympathetic modulation in response to frustration. Nonetheless, this finding integrates well with previously reported associations between decreased PNS activation and diminished cognitive capacity.47 Inconsistent with our prediction, we did not find evidence for an interaction between the constructs of cognitive control and frustrative non-reward, as children’s level of executive function did not moderate their behavioral or physiological responses to frustration. This suggests that children’s expression of frustration does not depend on their baseline cognitive functioning. Taken together, these findings suggest a model of dysregulation whereby frustration exerts more severe and prolonged impairment of cognitive and behavioral control via a lack of physiological adaptation to situational demands. These findings also align with neuroimaging studies that have shown deficits in performance monitoring, goal selection, and response inhibition in the context of frustration.12,13,22
The examination of HRV during frustration represents a novel contribution of this study. The patterns of co-inhibition and heightened PNS activation observed among the low symptom and moderate symptom classes, respectively, were consistent with previous research; however, studies of HRV among dysregulated children have been limited and relied on more general measures, such as baseline heart rate and change in heart rate. 26 To our knowledge, this is the first study to examine both parasympathetic and sympathetic activation in this population. Our finding that dysregulated children exhibited higher levels of SNS activation and lower levels of PNS activation is consistent with literature that has shown that among children with psychopathology the SNS dominates the PNS and leads to inadequate suppression of emotional arousal and deficits in emotion regulation.52 However, our observation that dysregulated children’s level of physiological arousal remained stable despite reporting similar changes in frustration to other children was unexpected and noteworthy. Speculatively, this may reflect some level of autonomic desensitization to emotional arousal, such that dysregulated children’s chronic experiences with irritability and frustration may lead to physiological habituation to this internal state in a manner akin to the desensitization that can result from ongoing exposure to external stress or adversity. 53
This study has several strengths. First, the use of LCA allowed for the inclusion of a clinically heterogeneous sample and the identification of empirically derived phenotypes. Many prior studies of dysregulation were conducted in the service of differentiating the construct from bipolar disorder and validating DMDD as an alternative diagnosis. As such, these studies have relied on DSM-oriented criteria to formulate homogeneous groups a priori, and thus have not represented the full continuum of emotional-behavioral symptoms. Furthermore, the relatively large sample of children with clinical symptoms and multimodal data methods allowed for the examination of multiple RDoC constructs. Nonetheless, there were several noteworthy limitations of this study. First, these data were collected as part of two larger studies, which required families to attend several multi-hour study sessions. In particular, one study required that either both biological parents or one biological parent and a sibling participate in all sessions with the referred child. These aspects of the study design may have biased the sample towards more traditional and intact families with higher levels of cohesion and planning/organizational skills. Additionally, our sample represented almost entirely White families, and therefore, the generalizability of these findings to other racial groups is unable to be tested. Classification using the LCA was limited by its reliance on parent-report, which may be biased towards more outwardly observable behaviors and influenced by caregivers’ background, fatigue, distractions and social desirability. Furthermore, the LCA method used did not allow for covariates to be included in the comparisons of distal outcomes by latent classes. Finally, this study used cross-sectional and linear analyses. The use of non-linear and intensive longitudinal methods may yield additional insights into the complex relationships between dysregulation, cognitive control, and frustrative non-reward.
In conclusion, despite phenotypic overlap, this study suggests that dysregulation may have different underlying cognitive, behavioral, and physiological mechanisms than more moderate presentations of emotional and behavioral problems and may warrant distinct diagnoses and treatment. While future work, such as longitudinal and neuroimaging studies, is needed to continue refining the characterization of these severely impaired and clinically challenging children, the results of the present study suggest that the simultaneous examination of the dynamic relations between cognitive control and autonomic responses both in and outside the context of frustrative non-reward may be important in advancing proper care for dysregulated children.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of General Medical Sciences (P20GM103644) and the National Institute of Mental Health (NIMH: K08MH082116).
The research was performed with permission from University of Vermont’s Institutional Review Board.
Disclosure: Dr. Potter has received grant or research support from the National Institutes of Health. Dr. Althoff has received grant or research support from NIMH, the National Institute on Drug Abuse, and the Klingenstein Third Generation Foundation. He has served on the editorial board of Child Psychiatry and Human Development and as consulting editor of the Journal of Clinical Child and Adolescent Psychology. He has received honoraria from the Massachusetts General Hospital Psychiatry Academy and Frontline Medical Communications, Inc. He is a partner of WISER Systems, LLC. Drs. Crehan, O’Loughlin, Schreck, Sigmon and Mss. Ametti and Dube have reported no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Merelise R. Ametti, University of Vermont, Burlington..
Eileen T. Crehan, Tufts University, Medford, Massachusetts..
Kerry O’Loughlin, Judge Baker Children’s Center, Boston, Massachusetts..
Meghan C. Schreck, McLean Hospital/Harvard Medical School, Belmont, Massachusetts..
Sarahjane L. Dube, University of Vermont, Burlington..
Alexandra S. Potter, University of Vermont, Burlington..
Stacey C. Sigmon, University of Vermont, Burlington..
Robert R. Althoff, University of Vermont, Burlington..
References
- 1.Holtmann M, Becker A, Banaschewski T, Rothenberger A, Roessner V. Psychometric validity of the strengths and difficulties questionnaire-dysregulation profile. Psychopathology. 2011;44(1):53–59. 10.1159/000318164. [DOI] [PubMed] [Google Scholar]
- 2.Althoff RR, Verhulst FC, Rettew DC, Hudziak JJ, van der Ende J. Adult outcomes of childhood dysregulation: a 14-year follow-up study. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(11):1105–1116. e1101. 10.1016/j.jaac.2010.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biederman J, Petty C, Monuteaux MC, et al. The CBCL-pediatric bipolar disorder profile predicts a subsequent diagnosis of bipolar disorder and associated impairments in ADHD youth growing up: a longitudinal analysis. The Journal of Clinical Psychiatry. 2009;70(5):732. 10.4088/JCP.08m04821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Caluwe E, Decuyper M, De Clercq B. The child behavior checklist dysregulation profile predicts adolescent DSM-5 pathological personality traits 4 years later. Eur Child Adolesc Psychiatry. 2013;22(7):401–411. 10.1007/s00787-013-0379-9. [DOI] [PubMed] [Google Scholar]
- 5.Holtmann M, Buchmann AF, Esser G, Schmidt MH, Banaschewski T, Laucht M. The Child Behavior Checklist-Dysregulation Profile predicts substance use, suicidality, and functional impairment: a longitudinal analysis. Journal of Child Psychology and Psychiatry. 2011;52(2):139147. 10.1111/j.1469-7610.2010.02309.x. [DOI] [PubMed] [Google Scholar]
- 6.Waxmonsky J, Pelham WE, Gnagy E, et al. The efficacy and tolerability of methylphenidate and behavior modification in children with attention-deficit/hyperactivity disorder and severe mood dysregulation. Journal of Child and Adolescent Psychopharmacology. 2008;18(6):573–588. 0.1089/cap.2008.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickstein DP, Towbin KE, Van Der Veen JW, et al. Randomized double-blind placebo-controlled trial of lithium in youths with severe mood dysregulation. Journal of Child and Adolescent Psychopharmacology. 2009;19(1):61–73. 10.1089/cap.2008.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biederman J, Mick E, Faraone SV, Spencer T, Wilens TE, Wozniak J. Pediatric mania: a developmental subtype of bipolar disorder? Biological Psychiatry. 2000;48(6):458–466. 10.1016/S0006-3223(00)00911-2 [DOI] [PubMed] [Google Scholar]
- 9.Brotman MA, Schmajuk M, Rich BA, et al. Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biological Psychiatry. 2006;60(9):991–997. 10.1016/j.biopsych.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 10.Brotman MA, Kassem L, Reising MM, et al. Parental diagnoses in youth with narrow phenotype bipolar disorder or severe mood dysregulation. American Journal of Psychiatry. 2007;164(8):12381241. 10.1176/appi.ajp.2007.06101619 [DOI] [PubMed] [Google Scholar]
- 11.Rich BA, Brotman MA, Dickstein DP, Mitchell DGV, Blair RJR, Leibenluft E. Deficits in Attention to Emotional Stimuli Distinguish Youth with Severe Mood Dysregulation from Youth with Bipolar Disorder. Journal of Abnormal Child Psychology. 2010;38(5):695–706. 10.1007/s10802-010-9395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rich BA, Schmajuk M, Perez-Edgar KE, Fox NA, Pine DS, Leibenluft E. Different psychophysiological and behavioral responses elicited by frustration in pediatric bipolar disorder and severe mood dysregulation. The American Journal of Psychiatry. 2007;164(2):309–317. 10.1176/ajp.2007.164.2.309. [DOI] [PubMed] [Google Scholar]
- 13.Adleman NE, Kayser R, Dickstein D, Blair RJR, Pine D, Leibenluft E. Neural correlates of reversal learning in severe mood dysregulation and pediatric bipolar disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50(11):1173–1185. e1172. 10.1016/j.jaac.2011.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. The American Journal of Psychiatry. 2003;160(3):430–437. 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and statistical manual of mental disorders – fifth edition (DSM-5®). American Psychiatric Pub; 2013. 10.1007/978-1-4899-7996-4 [DOI] [Google Scholar]
- 16.Evans SC, Burke JD, Roberts MC, et al. Irritability in child and adolescent psychopathology: An integrative review for ICD-11. Clinical Psychology Review. 2017;53:29–45. 10.1016/j.cpr.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. International statistical classification of diseases and related health problems. 11th ed. 2019. 10.1016/S0140-6736(19)31205-X [DOI] [Google Scholar]
- 18.Kozak MJ, Cuthbert BN. The NIMH Research Domain Criteria Initiative: Background, Issues, and Pragmatics. Psychophysiology. 2016;53(3):286–297. 10.1111/psyp.12518. [DOI] [PubMed] [Google Scholar]
- 19.Amsel A The role of frustrative non-reward in noncontinuous reward situations. Psychological Bulletin. 1958;55(2):102. 10.1037/h0043125 [DOI] [PubMed] [Google Scholar]
- 20.Grabell AS, Li Y, Barker JW, Wakschlag LS, Huppert TJ, Perlman SB. Evidence of Non-Linear Associations between Frustration-Related Prefrontal Cortex Activation and the Normal:Abnormal Spectrum of Irritability in Young Children. Journal of Abnormal Child Psychology. 2018;46(1):137–147. 10.1007/s10802-017-0286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tseng W-L, Deveney CM, Stoddard J, et al. Brain Mechanisms of Attention Orienting Following Frustration: Associations With Irritability and Age in Youths. The American Journal of Psychiatry. 2019;176(1):67–76. 10.1176/appi.ajp.2018.18040491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deveney CM, Connolly ME, Haring CT, et al. Neural mechanisms of frustration in chronically irritable children. The American Journal of Psychiatry. 2013;170(10):1186–1194. 10.1176/appi.ajp.2013.12070917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perlman SB, Jones BM, Wakschlag LS, Axelson D, Birmaher B, Phillips ML. Neural substrates of child irritability in typically developing and psychiatric populations. Developmental Cognitive Neuroscience. 2015;14:71–80. 10.1016/j.dcn.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGough JJ, McCracken JT, Cho AL, et al. A potential electroencephalography and cognitive biosignature for the child behavior checklist-dysregulation profile. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(11):1173–1182. 10.1016/j.jaac.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peyre H, Speranza M, Cortese S, Wohl M, Purper-Ouakil D. Do ADHD children with and without child behavior checklist-dysregulation profile have different clinical characteristics, cognitive features, and treatment outcomes? Journal of Attention Disorders. 2015;19(1):63–71. 10.1177/1087054712452135. [DOI] [PubMed] [Google Scholar]
- 26.Deutz M, Woltering S, Vossen H, Deković M, van Baar A, Prinzie P. Underlying Psychophysiology of Dysregulation: Resting Heart Rate and Heart Rate Reactivity in Relation to Childhood Dysregulation. Journal of the American Academy of Child & Adolescent Psychiatry. 2018. 10.1016/j.jaac.2018.09.434. [DOI] [PubMed] [Google Scholar]
- 27.Tonacci A, Billeci L, Calderoni S, et al. Sympathetic arousal in children with oppositional defiant disorder and its relation to emotional dysregulation. Journal of Affective Disorders. 2019;257:207213. 10.1016/j.jad.2019.07.046. [DOI] [PubMed] [Google Scholar]
- 28.Fanti KA. Editorial: Heart Rate as a Biomarker for Co-Occurring Externalizing and Internalizing Problems. Journal of the American Academy of Child and Adolescent Psychiatry. 2019;58(6):569571. 10.1016/j.jaac.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Shaffer F, Ginsberg JP. An Overview of Heart Rate Variability Metrics and Norms. Frontiers in Public Health. 2017;5:258. 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beauchaine TP, Thayer JF. Heart rate variability as a transdiagnostic biomarker of psychopathology. International Journal of Psychophysiology. 2015;98(2):338–350. 10.1016/j.ijpsycho.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 31.Naim R, Goodwin MS, Dombek K, et al. Cardiovascular reactivity as a measure of irritability in a transdiagnostic sample of youth: Preliminary associations. International Journal of Methods in Psychiatric Research. n/a(n/a):e1890. 10.1002/mpr.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollingshead AB. Four factor index of social status. 1975. [Google Scholar]
- 33.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):980–988. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- 34.Weiss B, Harris V, Catron T, Han SS. Efficacy of the RECAP intervention program for children with concurrent internalizing and externalizing problems. Journal of Consulting and Clinical Psychology. 2003;71(2):364–374. 10.1037/0022-006x.71.2.364 [DOI] [PubMed] [Google Scholar]
- 35.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT.: University of Vermont Research Center for Children, Youth and Families; 2001. [Google Scholar]
- 36.Althoff RR, Rettew DC, Ayer LA, Hudziak JJ. Cross-informant agreement of the Dysregulation Profile of the Child Behavior Checklist. Psychiatry Res. 2010;178(3):550–555. 10.1016/j.psychres.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rescorla LA, Blumenfeld MC, Ivanova MY, Achenbach TM. International Comparisons of the Dysregulation Profile Based on Reports by Parents, Adolescents, and Teachers. Journal of Clinical Child & Adolescent Psychology. 2018:1–15. 10.1080/15374416.2018.1469090. [DOI] [PubMed] [Google Scholar]
- 38.Deutz MH, Geeraerts SB, van Baar AL, Deković M, Prinzie P. The Dysregulation Profile in middle childhood and adolescence across reporters: factor structure, measurement invariance, and links with self-harm and suicidal ideation. European Child & Adolescent Psychiatry. 2016;25(4):431442. 10.1007/s00787-015-0745-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gioia GA, Isquith PK, Guy SC, Kenworthy L. The behavior rating inventory of executive function. Professional manual. Lutz, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- 40.Logan GD, Schachar RJ, Tannock R. Impulsivity and Inhibitory Control. Psychological Science. 1997;8(1):60–64. DOI: 10.1111/j.1467-9280.1997.tb00545.x. [DOI] [Google Scholar]
- 41.Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: an update. Journal of the International Neuropsychological Society. 2004;10(2):301–303. 10.1017/s1355617704102191. [DOI] [PubMed] [Google Scholar]
- 42.Yochim BP, Baldo JV, Kane KD, Delis DC. D-KEFS Tower Test performance in patients with lateral prefrontal cortex lesions: The importance of error monitoring. Journal of Clinical and Experimental Neuropsychology. 2009;31(6):658–663. 10.1080/13803390802448669. [DOI] [PubMed] [Google Scholar]
- 43.Lamm C, Zelazo PD, Lewis MD. Neural correlates of cognitive control in childhood and adolescence: Disentangling the contributions of age and executive function. Neuropsychologia. 2006;44(11):2139–2148. 10.1016/j.neuropsychologia.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 44.de Geus EJ, Willemsen GH, Klaver CH, van Doornen LJ. Ambulatory measurement of respiratory sinus arrhythmia and respiration rate. Biological Psychology. 1995;41(3):205–227. [DOI] [PubMed] [Google Scholar]
- 45.Porges SW. The polyvagal perspective. Biological Psychology. 2007;74(2):116–143. 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, Van Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27(1):1–23. 10.1111/j.14698986.1990.tb02171.x [DOI] [PubMed] [Google Scholar]
- 47.Giuliano RJ, Karns CM, Bell TA, et al. Parasympathetic and sympathetic activity are associated with individual differences in neural indices of selective attention in adults. Psychophysiology. 2018;55(8):e13079. 10.1111/psyp.13079. [DOI] [PubMed] [Google Scholar]
- 48.Brenner SL, Beauchaine TP. Pre-ejection period reactivity and psychiatric comorbidity prospectively predict substance use initiation among middle-schoolers: a pilot study. Psychophysiology. 2011;48(11):1588–1596. 10.1111/j.1469-8986.2011.01230.x. [DOI] [PubMed] [Google Scholar]
- 49.Bolck A, Croon M, Hagenaars J. Estimating latent structure models with categorical variables: Onestep versus three-step estimators. Political Analysis. 2004;12(1):3–27. 10.1093/pan/mph001 [DOI] [Google Scholar]
- 50.Asparouhov T, Muthén B. Auxiliary variables in mixture modeling: Three-step approaches using M plus. Structural Equation Modeling: A Multidisciplinary Journal. 2014;21(3):329–341. 10.1080/10705511.2014.915181 [DOI] [Google Scholar]
- 51.Murray-Close D, Holterman LA, Breslend NL, Sullivan A. Psychophysiology of proactive and reactive relational aggression. Biological Psychology. 2017;130:77–85. 10.1016/j.biopsycho.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Beauchaine TP, Gatzke-Kopp L, Neuhaus E, Chipman J, Reid MJ, Webster-Stratton C. Sympathetic-and parasympathetic-linked cardiac function and prediction of externalizing behavior, emotion regulation, and prosocial behavior among preschoolers treated for ADHD. Journal of Consulting and Clinical Psychology. 2013;81(3):481. 10.1037/a0032302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuras YI, McInnis CM, Thoma MV, et al. Increased alpha-amylase response to an acute psychosocial stress challenge in healthy adults with childhood adversity. Dev Psychobiol. 2017;59(1):91–98. 10.1002/dev.21470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.