We appreciate the insightful correspondence from de Nonneville and colleagues (1) regarding our article “NDRG1 in Aggressive Breast Cancer Progression and Brain Metastasis.” Through their in silico analysis of a very large independent clinical cohort of patients with breast cancer (n = 8982), de Nonneville et al. (1) provided data that further validate our clinical findings that NDRG1 is a predictor of poor outcome in patients with aggressive breast cancer. Analysis of the independent cohort also demonstrated that NDRG1 is associated with features of aggressive breast cancer such as enrichment of tumor stemness, tumor invasion and metastasis signatures, further corroborating our preclinical observations. Importantly, de Nonneville et al. (1) expanded their analysis to include breast tumor subtypes considered less aggressive, such as estrogen receptor (ER)+/HER2− tumors; they found that NDRG1 also positively correlates with aggressiveness and poor survival outcome in patients with these tumors. We further stratified the MD Anderson cohort of breast cancer patients we reported in our work (see our article, Figure 6) to assess the prognostic significance of NDRG1 in an independent collection of ER+/HER2− tumors immunostained for NDRG1. We observed a tendency for a worse overall survival in NDRG1-high tumors compared with NDRG1-low tumors in this group (hazard ratio = 2.78, 95% confidence interval = 0.68 to 11.32; 2-sided log-rank test P = .08; Figure 1), consistent with the findings from de Nonneville et al. (1).
Figure 1.
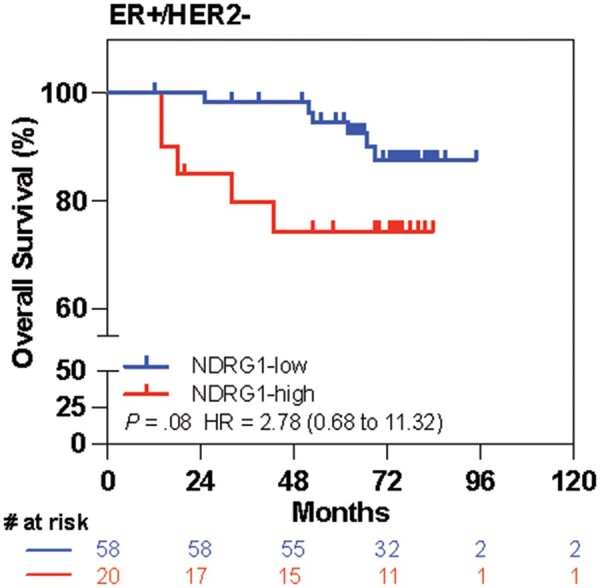
NDRG1 expression correlation with overall survival in ER+/HER2− patients. Kaplan-Meier estimate of overall survival according to NDRG1 expression in ER+/HER2− patients. P value from 2-sided log-rank test, and hazard ratio (HR) with 95% confidence interval (P = .08; HR = 2.78, 95% CI = 0.68 to 11.32) is shown. CI = confidence interval; ER = estrogen receptor.
We would like to emphasize that our suggestion that “NDRG1 has a context-dependent function in breast cancer” was based on the current literature; we did not perform functional experiments using less aggressive ER+ cell lines. Liu et al. (2) demonstrated that overexpressing NDRG1 reduced cellular invasion, adhesion, and anoikis resistance in MCF-7, an ER+/HER2− cell line. Similarly, Godbole et al. (3) found that silencing NDRG1 increased migration in T47D cells, another ER+/HER2− breast cancer cell line.
In summary, we thank de Nonneville and colleagues for a very informative analysis that provides further confirmation of our findings that NDRG1 is a tumor promoter and predictor of poor outcome in aggressive breast cancer subtypes, and for expanding the analysis to less aggressive ER+/HER2− breast tumors. Clearly, these new findings support the need for designing experiments to establish the role of NDRG1 in progression, metastasis, and therapy response in preclinical models of ER+ breast cancer.
Funding
This study was supported in part by the following grants: American Cancer Society Research Scholar grant (RSG-19–126–01 to BGD), Susan G. Komen Career Catalyst Research Grant (CCR16377813 to BGD), Startup and Institutional Research Grants from MD Anderson, The Morgan Welch Inflammatory Breast Cancer Boot Walk Fund (to ESV), and the NCI/NIH Cancer Center Support (Core) Grant P30 CA016672 and the NCI’s Research Specialist Award 1 R50 CA243707-01A1 to The University of Texas MD Anderson Cancer Center.
Notes
Role of the funder: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of this response or decision to submit it for publication.
Disclosures: The authors have no disclosures.
Author contributions: Conceptualization: ESV, BGD. Data curation: ESV, LH, BGD. Resources: LH, BGD. Writing—original draft: ESV, BGD. Writing—review and editing: ESV, XH, LH, BGD.
Data Availability
The data that support the findings are available from the corresponding author (bgdebeb@mdanderson.org) upon reasonable request.
Contributor Information
Emilly S Villodre, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA; MD Anderson Morgan Welch Inflammatory Breast Cancer Clinic and Research Program, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Xiaoding Hu, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA; MD Anderson Morgan Welch Inflammatory Breast Cancer Clinic and Research Program, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Lei Huo, MD Anderson Morgan Welch Inflammatory Breast Cancer Clinic and Research Program, The University of Texas MD Anderson Cancer Center, Houston, TX, USA; Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Bisrat G Debeb, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA; MD Anderson Morgan Welch Inflammatory Breast Cancer Clinic and Research Program, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
References
- 1. De Nonneville A, Finett P, Mamessier E, Bertucci F. RE: NDRG1 in Aggressive Breast Cancer Progression and Brain Metastasis. J Natl Cancer Inst. 2022;114(7):djac031. https://doi.org/10.1093/jnci/djac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu W, Xing F, Iiizumi-Gairani M, et al. N-myc downstream regulated gene 1 modulates Wnt-beta-catenin signalling and pleiotropically suppresses metastasis. EMBO Mol Med. 2012;4(2):93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Godbole M, Togar T, Patel K, et al. Up-regulation of the kinase gene SGK1 by progesterone activates the AP-1-NDRG1 axis in both PR-positive and -negative breast cancer cells. J Biol Chem. 2018;293(50):19263–19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings are available from the corresponding author (bgdebeb@mdanderson.org) upon reasonable request.


