Abstract
Background
Prostate cancer (PC) screening guidelines have changed over the last decade to reduce overdiagnosis and overtreatment of low-grade disease. We sought to examine and attempt to explain how changes in screening strategies have impacted temporal trends in Gleason grade group (GG) PC at diagnosis and radical prostatectomy pathology.
Methods
Using the Surveillance, Epidemiology, and End Results Registry database, we identified 438 432 men with newly diagnosed PC during 2010-2018. Temporal trends in incidence of GG at biopsy, radical prostatectomy pathology, prostate-specific antigen (PSA) level, and metastasis at diagnosis were examined. The National Health Interview Survey database was examined to evaluate trends in PSA-screening rates, and a literature review evaluating magnetic resonance imaging and biomarkers utilization during this period was performed.
Results
Between 2010 and 2018, the incidence of low-grade PC (GG1) decreased from 52 to 26 cases per 100 000 (P < .001). The incidence of GG1 as a proportion of all PC decreased from 47% to 32%, and the proportion of GG1 at radical prostatectomy pathology decreased from 32% to 10% (P < .001). However, metastases at diagnosis increased from 3.0% to 5.2% (P < .001). During 2010-2013, PSA screening rates in men aged 50-74 years declined from 39 to 32 per 100 men and remained stable. Utilization rates of magnetic resonance imaging and biomarkers modestly increased from 7.2% in 2012 to 17% in 2019 and 1.3% in 2012 to 13% in 2019, respectively.
Conclusions
We found a significant decrease in the diagnosis and treatment of GG1 PC between 2010 and 2018. Changes in PSA screening practices appear as the primary contributor. Public health efforts should be directed toward addressing the increase in the diagnoses of metastatic PC.
Prostate-specific antigen (PSA) screening has transformed the diagnosis and management of prostate cancer (PC) but has long been controversial. The adoption of PSA screening in the 1990s precipitated a significant decline in metastases at diagnosis and cancer-specific mortality (1). However, increased screening also led to overdiagnosis and overtreatment of low-grade tumors, Gleason grade group (GG)–1 PC (2). Although GG1 has an indolent behavior, its diagnosis leads to patient anxiety and potential biopsy-related harm. Most concerning, during 1990-2013, more than half of men diagnosed with these low-grade tumors were subject to treatment with radiotherapy or surgery, which can result in posttreatment harms such as erectile dysfunction, bowel dysfunction, or urinary incontinence (3-5).
During the past 15 years, there have been 3 major watersheds in PSA screening. First, in 2008, the US Preventive Services Task Force (USPSTF) downgraded screening to a grade D rating for men aged older than 75 years, highlighting that the benefit from treatment of screen-detected cancers was small to none in this demographic (6). Second, in 2009, conflicting evidence was published from 2 randomized clinical trials: the European Randomized Study of Screening for Prostate Cancer and the Prostate, Lung, Colorectal, and Ovarian (PLCO) (7,8). The European Randomized Study of Screening for Prostate Cancer showed a moderate cancer-specific mortality benefit of PSA screening, whereas PLCO did not demonstrate this benefit. Resultantly, in 2012, the USPSTF recommended against screening all men, concluding that the benefits of PSA screening did not outweigh the risks (9).
An improved understanding of the benefits and harms of PSA screening and the natural history of PC led to further changes in screening recommendations. Following emerging data, accepting that the long-term benefit of screening may be underappreciated and the adoption of active surveillance for low-risk disease, the USPSTF issued a grade C recommendation in 2018, advocating for shared decision making when deciding to screen men aged 55-69 years (4,10,11).
Several other changes over recent years may have influenced the diagnosis of GG1, including the wider use of prebiopsy magnetic resonance imaging (MRI), introduction of biomarkers, and increased obesity in the male population. Guidelines now recommend MRI or biomarkers prior to prostate biopsy following an elevated PSA (12,13), potentially reducing diagnosis of indolent disease. Obesity may affect grade distribution because of evidence that is associated with a lower incidence of low-grade but a higher incidence of GG2-5 disease (14,15).
We sought to examine nationally representative tumor registry data to elucidate whether this myriad of changes in screening has led to variations in the incidence of PC by grade. We also sought to determine whether we could disentangle the causes of any such changes in terms of PSA screening, MRI, biomarkers, or obesity.
Methods
Data Source
Using the Surveillance, Epidemiology, and End Results (SEER) data registry, representing cancer incidence rates in approximately 48% of the US population, we identified 438 432 men (aged 18 years and older) with biopsy-proven adenocarcinoma of the prostate from 2010 through 2018. Temporal trends in incidence of GG at diagnosis and radical prostatectomy (RP) pathology were examined relative to annual incident cancers. Within this cohort, 45 216 (10%) had Gleason scores with a primary and/or secondary pattern of less than 3 or had a Gleason sum inconsistent with patterns reported (ie, 4 + 5 = 6). These patients were excluded from the analysis, resulting in 393 216 men (see Figure 1). This study was approved by the institutional review board of Weill Cornell Medicine and SEER custom data group.
Figure 1.
Patient selection and flow diagram for data capture from Surveillance, Epidemiology, and End Results–18 registry to evaluate incidence rates of prostate cancer from 2010 until 2018. PSA = prostate-specific antigen.
We identified rates of PC screening from 54 047 men who completed the National Health Interview Survey, a representative survey of men used to track PSA screening rates in the United States, conducted in 2010, 2013, 2015, and 2018. A separate literature review of the PubMed database for the period from January 2010 to November 2021 was performed to identify studies reporting rates of prebiopsy MRI and biomarker utilization in men at risk of PC, as well as the prevalence of obesity rates in the United States. The text search query included the keywords “MRI prostate,” “prostate biomarkers,” “utilization rates,” and “adult obesity rates US.” Given the limited evidence disclosed in the scientific literature on biomarker utilization rates, we evaluated public company reports when available.
Statistical Analysis
We calculated age-adjusted incidence rates by groups (younger than 50, 50-54, 55-59, 60-64, 65-69, 70 years and older), GG classification (1–5) on prostate biopsy as compared with GG in RP specimens, and metastasis at presentation using SEER*Stat software version 8.3.9.2 (National Cancer Institute, Bethesda, MD, USA). The incidence rates were age standardized to the 2000 US population and expressed per 100 000 men. Differences in proportions of PC by GG and metastasis at diagnosis were tested using the Cochran-Armitage trend test. Differences in patient-level PSA values across years were tested using linear regression, and the Kendall Tau-b correlation was used to detect trends in age-adjusted incidence rates. Statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). A 2-sided P value of less than .05 was considered statistically significant. Because of the large sample size, 95% confidence intervals (CI) for rates were extremely narrow, less than 1, and so are omitted except for subgroups.
Results
Changes in PC Incidence by Grade
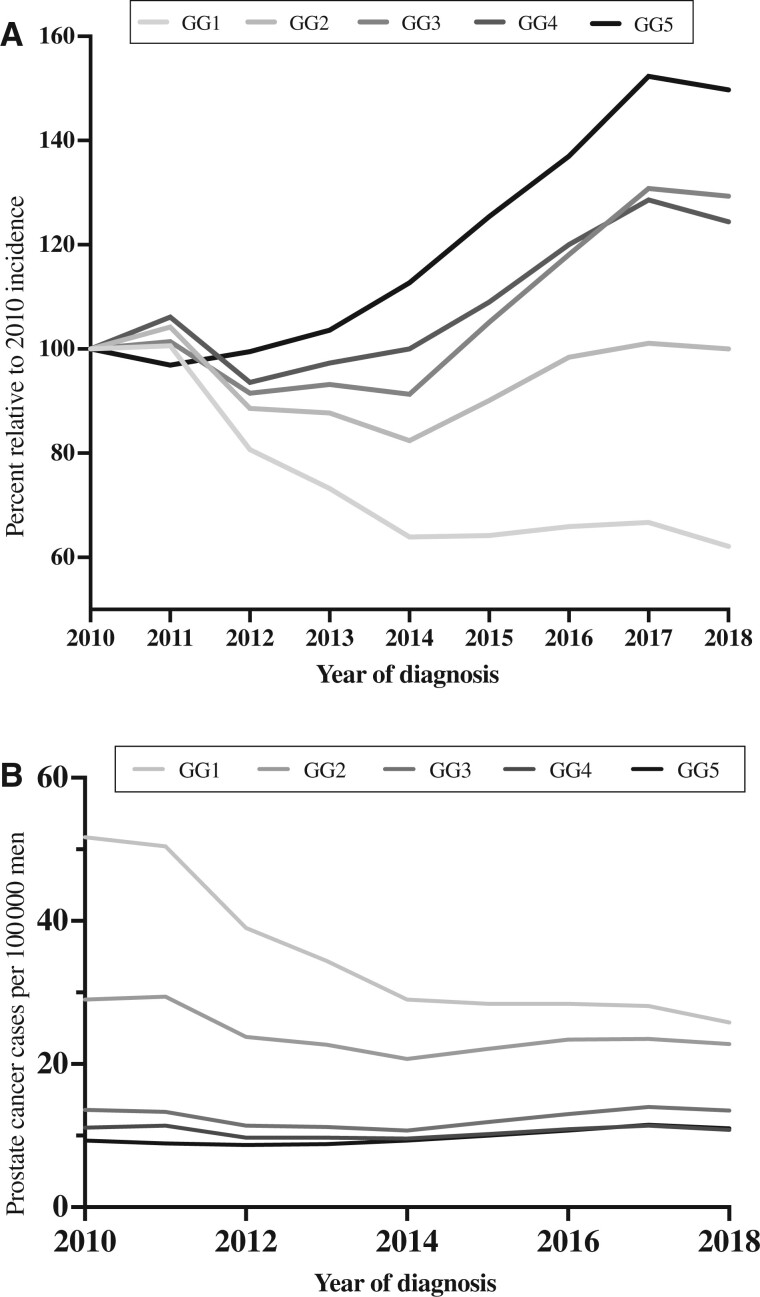
Between 2010 and 2018, there was a decline in the incidence of GG1 (P < .001; Figure 2, A) as a proportion of all PCs from 47% (22 628 of 48 467) cases in 2010 to 32% (14 043 of 44 377) cases in 2018 and a significant decrease in GG1 at RP surgical pathology (P < .001) from 32% (5726 of 18 155) cases in 2010 to 10% (1402 of 14 134) cases in 2018. The overall age-adjusted incidence rate of GG1 disease declined from 52 to 26 cases per 100 000 men over the same interim (Figure 2, B) and occurred across all age groups (Table 1).
Figure 2.
Age-adjusted incidence rates of prostate cancer in the United States during the last decade. A) Incidence rates of prostate cancer are shown relative to 2010, stratified by Gleason grade groups (GG) followed by (B) temporal trends of the overall age-adjusted incidence rates of prostate cancer from 2010 until 2018, stratified by GG at diagnosis per 100 000 men.
Table 1.
Age-adjusted incidence rate of GG1 prostate cancer from 2010 to 2018 by age subgroups score per 100 000 mena
| Rate (95% confidence interval) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age groups, y | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 |
| <50 | 3.2 | 3.1 | 2.7 | 2.2 | 1.8 | 1.7 | 1.4 | 1.3 | 1.3 |
| (3 to 3.4) | (2.9 to 3.3) | (2.6 to 2.9) | (2 to 2.4) | (1.6 to 1.9) | (1.6 to 1.9) | (1.3 to 1.6) | (1.2 to 1.5) | (1.2 to 1.5) | |
| 50-54 | 71 | 71 | 57 | 50 | 44 | 43 | 40 | 41 | 39 |
| (67.6 to 73.6) | (67.6 to 73.6) | (54.3 to 59.7) | (47.7 to 52.7) | (41.9 to 46.6) | (41.1 to 45.9) | (38.1 to 42.7) | (38.3 to 43) | (36.8 to 41.5) | |
| 55-59 | 148 | 150 | 114 | 100 | 91 | 89 | 87 | 88 | 78 |
| (142.9 to 152.3) | (145.8 to 155.2) | (110.4 to 118.5) | (96.6 to 104.1) | (87.2 to 94.2) | (85.2 to 92.2) | (83.3 to 90.1) | (84.8 to 91.7) | (74.9 to 81.4) | |
| 60-64 | 233 | 223 | 176 | 158 | 132 | 129 | 133 | 134 | 122 |
| (226.7 to 239.6) | (216.8 to 229.1) | (170.4 to 181.3) | (152.4 to 162.7) | (127.2 to 136.5) | (124.4 to 133.4) | (128.4 to 137.5) | (129.6 to 138.7) | (117.5 to 126) | |
| 65-69 | 331 | 324 | 251 | 222 | 187 | 185 | 190 | 187 | 166 |
| (321.4 to 339.8) | (315 to 332.8) | (243.4 to 258.4) | (215 to 228.9) | (180.8 to 193.2) | (179.4 to 191.4) | (183.6 to 195.6) | (181.2 to 193) | (160.5 to 171.5) | |
| ≥70 | 189 | 181 | 135 | 120 | 98 | 95 | 97 | 94 | 89 |
| (183.9 to 193.8) | (176.4 to 186) | (131.2 to 139.3) | (116.5 to 124) | (94.8 to 101.5) | (91.8 to 98.3) | (94 to 100.5) | (90.9 to 97.1) | (85.6 to 91.4) | |
| Overall | 52 | 50 | 39 | 34 | 29 | 28 | 28 | 28 | 26 |
| (51 to 52.3) | (49.7 to 51) | (38.4 to 39.6) | (33.8 to 34.9) | (28.5 to 29.5) | (27.9 to 28.8) | (27.9 to 28.8) | (27.6 to 28.5) | (25.3 to 26.2) | |
Rates are per 100 000 and age-adjusted to the 2000 US standard population (19 age groups—Census P25-1130) standard. GG = Gleason grade group.
The incidence of high-grade disease (GG2-5) varied during the study period. Initially, per 100 000 men, there was a decline in age-adjusted incidence from 63 cases in 2010 to 50 cases in 2014, followed by an increase of 58 cases in 2018. The incidence rate of GG2 per 100 000 patients increased from 21 in 2014 to 23 in 2018, GG3 from 11 in 2014 to 14, GG4 increased from 10 in 2014 to 11 in 2018, and GG5 increased from 9.3 in 2010 to 11 cases in 2018 (Table 2).
Table 2.
Overall age-adjusted incidence ratea of prostate cancer from 2010 to 2018 by Gleason grade group score per 100 000 mena,b
| Year | GG1 | GG2 | GG3 | GG4 | GG5 |
|---|---|---|---|---|---|
| 2010 | 52 | 29 | 14 | 11 | 9.3 |
| 2011 | 50 | 29 | 13 | 11 | 8.9 |
| 2012 | 39 | 24 | 11 | 10 | 8.7 |
| 2013 | 34 | 23 | 11 | 10 | 8.8 |
| 2014 | 29 | 21 | 11 | 10 | 9.3 |
| 2015 | 28 | 22 | 12 | 10 | 10 |
| 2016 | 28 | 23 | 13 | 11 | 11 |
| 2017 | 28 | 24 | 14 | 11 | 12 |
| 2018 | 26 | 23 | 14 | 11 | 11 |
Rates are per 100 000 and age-adjusted to the 2000 US standard population (19 age groups—Census P25-1130) standard. GG = Gleason grade group.
95% confidence intervals are all plus or minus 1 or less and are not shown.
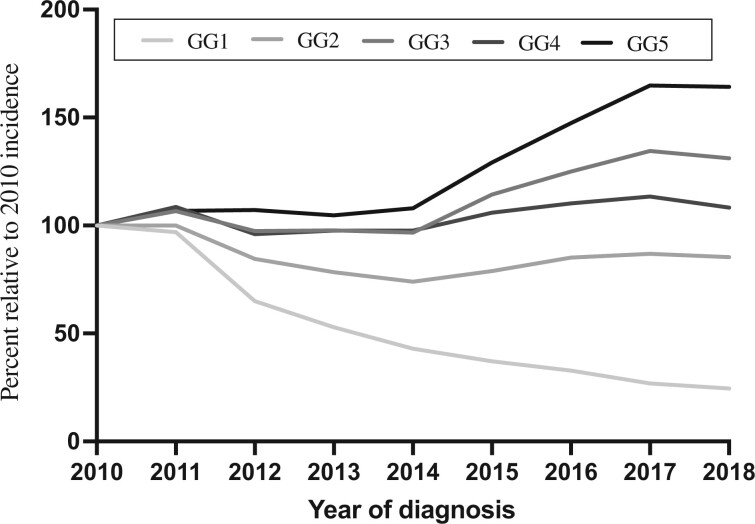
Regarding RP pathology, there was an increase in GG2-5 as a proportion of all cases during 2010-2018, with a notable doubling in the proportion of the highest grade (GG5). Specifically, GG2 increased from 45% to 49%, GG3 from 14% to 24%, GG4 from 4% to 6%, and GG5 from 5% to 11% (Figure 3).
Figure 3.
Incidence rates of radical prostatectomy surgical pathology during the last decade relative to 2010, stratified by Gleason grade groups (GG).
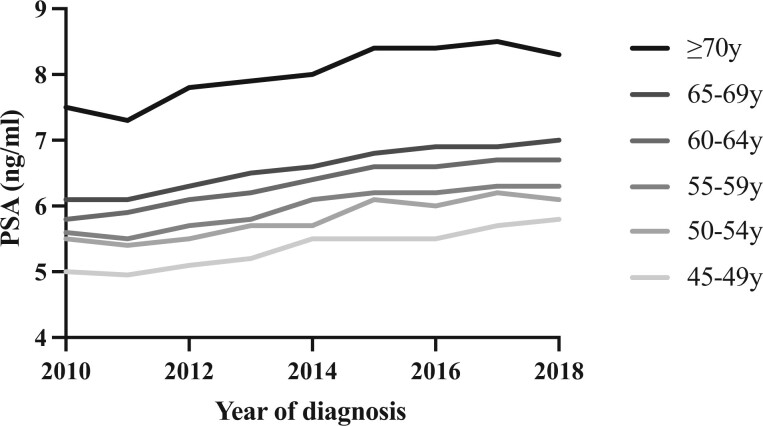
When evaluating the incidence of metastases at diagnosis, there was an increase over time (P < .001) from 3% (1462 of 48 467) annual diagnoses in 2010 to 5.2% (2298 of 44 377) annual diagnoses in 2018. Median PSA at diagnosis was 6.2 ng/mL in 2010 and 7.1 ng/mL in 2018 (P < .001, for change over time) and occurred across all age groups (Figure 4).
Figure 4.
Median PSA at diagnosis of prostate cancer from 2010 until 2018, stratified by age group cohorts. PSA = prostate-specific antigen.
Changes in PSA Screening
During 2010-2013, there was a decline in the proportion of men who underwent PSA testing during the previous year, from 39% to 32%, respectively. This was seen across all age groups (Table 3) (16,17). In relative terms, screening rates decreased by approximately 20%. In comparison, when observing the incidence of PC by GG, there was a similar decline of approximately 30% of all GG1 diagnoses from 2010 to 2013.
Table 3.
Percentage of men aged 40 years or older who had a prostate-specific antigen test within the past year by age at the time of screening, 2005-2018a
| Cohort age, yb | Year | Adults, % | Annual percent change | Average annual percent change |
|---|---|---|---|---|
| 40-54 | 2005 | 16 | ||
| 40-54 | 2008 | 19 | 6.17 (2005-2008) | |
| 40-54 | 2010 | 15 | 20.1 | |
| 40-54 | 2013 | 12 | 22.29 | |
| 40-54 | 2015 | 13 | 2.84 (2013-2015) | 2.30 (2013-2018) |
| 40-54 | 2018 | 13 | 1.94 (2015-2018) | 2.30 (2013-2018) |
| 55-69 | 2005 | 45 | ||
| 55-69 | 2008 | 48 | 2.39 (2005-2008) | |
| 55-69 | 2010 | 46 | −1.96 (2008-2010) | |
| 55-69 | 2013 | 38 | 18.3 | |
| 55-69 | 2015 | 39 | 0.73 (2013-2015) | 0.40 (2013-2018) |
| 55-69 | 2018 | 39 | 0.18 (2015-2018) | 0.40 (2013-2018) |
| 70 | 2005 | 53 | ||
| ≥70 | 2008 | 56 | 2.15 (2005-2008) | |
| ≥70 | 2010 | 52 | −3.81 (2008-2010) | |
| ≥70 | 2013 | 46 | 10.83 | |
| ≥70 | 2015 | 43 | −3.40 (2013-2015) | −0.74 (2013-2018) |
| ≥70 | 2018 | 45 | 1.08 (2015-2018) | −0.74 (2013-2018) |
Source: Centers for Disease Control and Prevention, National Center for Health Statistics. National Health Interview Survey.
Data are age-adjusted to the 2000 US standard population (19 age groups—Census P25-1130) standard.
Although screening rates steadily declined between 2010 and 2018 for men aged 55-69 years from 46% to 39%, respectively, and in men older than 70 years, from 52% to 45%, respectively (17), it is worth noting that the recent 5-year trend for PSA screening (2013-2018) remains stable with an annual average percentage change of 0.40. Furthermore, recent studies have shown that when evaluating PSA screening in insured men between 2016 and 2019, rates increased from 32.5 to 36.5 tests per 100 person-years, a relative increase of 13% (95% CI = 1.1% to 24%). These increasing trends were also observed among men aged 40-54 years and 70-89 years, outside of the recommended screening age group (18).
Changes in Utilization of Prebiopsy MRI of the Prostate
As illustrated in Table 4, adoption of prebiopsy MRI in the United States was slow during our study period (21,23). A study by Gaffney et al. (23) of Medicare beneficiaries showed an increase of prebiopsy MRI utilization from 0.5% in 2008 to 8.2% in 2015, and Liu et al. evaluated health insurance data and reported a modest increase in MRI adoption, from 0.2% in 2009 to 6.5% in 2015 (25). Insurance coverage led to a more rapid uptake of this adjunct test, as highlighted by Kim et al. (21), who found that the age-adjusted rates of prebiopsy MRI increased from 6 per 1000 biopsies performed in 2010 to 74 per 1000 biopsies in 2016. However, overall utilization remained relatively low, as shown by Leapman et al. (19), who demonstrated that only 7.2% of men underwent MRI during 2012-2014, which increased to 16.7% during 2017-2019. As shown in Table 1, the greatest decline in GG1 at diagnosis occurred during 2010-2014, with a reduction in age-adjusted incidence rates from 52 to 29 cases per 100 000. When comparing these changes relative to MRI utilization rates, it is evident that major changes in GG1 migration occurred before the broader adoption of prebiopsy MRI.
Table 4.
Utilization rates of prebiopsy MRI of the prostate as reported in the reviewed literaturea
| Reference | Journal | Study population (pts) | Database source | Study period | Mean age, y | Total patients who underwent prebiopsy MRI, % | Rates of utilization from beginning to end of study period, % |
|---|---|---|---|---|---|---|---|
| Leapman et al. (19) | JAMA Net Open | 65 530 | Blue Cross Blue Shield Axis | 2012-2019 | 58 | na | 7.2-16.7 |
| Abashidze et al. (20) | JAMA Net Open | 794 809 | Medicare Advantage health plans (Optum) | 2011-2017 | 59.8 | 3 | 1.2-3.8 |
| Kim et al. (21) | Eur Urol Focus | 119 202 | OptumLabs Data Warehouse | 2010-2016 | 62.6 | na | 6-83 per 1000 biopsies (age-adjusted) |
| Quinn et al. (22) | Cancer | 50 709 | SEER-Medicare | 2010-2015 | 72.1 | 1.9 | 0.3- 5.2 |
| Gaffney et al. (23) | AJR Am J Roentgenol | 78 243 | SEER-Medicare | 2008-2015 | 72 | na | 0.5-8.2 |
| Rosenkrantz et al. (24) | J Urol | na | Medicare RIFs | 2010-2015 | na | na | 0.1-10.3 |
| Liu et al. (25) | Urology | 241 681 | MarketScan | 2009-2015 | 57.5 | 1.4 | 0.2-6.5 |
MRI = magnetic resonance imaging; na = data not available; pts = patients; RIF = research identifiable files.
Changes in Utilization of Biomarkers
The adoption of biomarkers similarly does not seem to explain temporal changes in GG at diagnosis. First, the introduction of these biomarkers appeared late, with CPT codes assigned and Medicare approval for prostate health index in 2012, 4Kscore in 2015, and ExosomeDx (Exosome Diagnostics, Waltham, MA) in 2017. Publicly available company reports from OPKO (Miami, FL), the manufacturer of the 4Kscore, described an increasing trend in the use of its tests from approximately 58 000 in 2017 to 70 000 tests in 2019 (26,27). In a study evaluating private insurance claims in men with recent PC diagnoses, the proportion of men who underwent biomarker testing prior to biopsy increased from 1.3% (95% CI = 1.1% to 1.4%) between 2012 and 2014 to 12.7% (95% CI = 12.3% to 13.0%) during 2017-2019 (19). Another study evaluating biomarker utilization trends found that the proportion of men who underwent biomarker testing increased from 0.8% in 2012 to 11.3% in 2018 (28). By contrast, the incidence of PC by GG changed dramatically during 2010-2014, with GG1 declining from 52 to 29 cases and GG2 from 29 to 21 cases per 100 000, respectively.
Changes in Obesity Rates in the Male Population
According to data reported in the National Health and Nutrition Examination Survey, the prevalence of obesity has steadily increased over the last 2 decades, from 31% to 42% during 1999-2018, when adjusted for age. It is estimated that body mass index (BMI) increased approximately 20% in the last 2 decades in men aged 55 to 69 years recommended to consider PSA screening (29). These changes appear to develop at a relatively steady pace but over a prolonged period. In comparison, as seen in Table 1, the incidence rates of GG1 halved in 8 years, whereas GG2-5 did not increase.
Discussion
We found that the incidence of GG1 PC, at diagnosis and on RP pathology, has declined sharply over the last decade. Conversely, we demonstrated an upward grade migration of PC at presentation, with increased rates of high-grade (GG2-5) disease and greater PSA at presentation for men of all ages since 2014. To our knowledge, this is the first population-based analysis to demonstrate that the incidence and treatment of GG1 PC have significantly declined during the last decade. Concerning the rising trend in high-grade and advanced disease, our study results are consistent with similar findings shown by others, since the recommendation against PSA-based screening for all men was issued by the USPSTF in 2012 (30–33). This confirms that public health efforts have been effective in reducing the overdiagnosis and overtreatment of GG1 PC; however, there is a trade-off with an increased incidence in higher-grade cancers and metastases at diagnosis.
Multiple hypotheses explain the grade migration phenomenon highlighted in this study. Possible explanations include changes in guidelines for PSA screening, utilization of prebiopsy MRI, and use of biomarkers (25,34). Additionally, modifications in tumor biology because of the increased incidence of male obesity in the United States have been proposed as determinants for the increased incidence of high-grade PC (10,12).
To evaluate the potential influence of these different changes, we first considered changes in PSA screening. During 2010-2013, there was a decline of approximately 20% in PSA screening rates. This is plausibly attributed to media coverage of the negative results of the PLCO trial—despite significantly high rates of screening contamination in the control arm—followed by the USPSTF blanket statement in 2012 against PSA screening in all men (9,35,36). These changes appear to be led firstly by primary care physicians as PSA screening rates nearly halved between 2010 and 2012, when looking at this group (37).
Our findings confirmed that the most significant decline in the incidence of GG1 occurred between 2010 and 2014 from 52 to 29 per 100 000 cases, which, accounting for delays in PC reporting, coincided with the 2 screening guidelines modifications in 2008 and 2012 (6,9). Notably, this sharp decline of GG1 during 2010-2014 was present across all age groups, consistent with the latter recommendation against screening for all men in 2011 (Table 2). Although the grade migration phenomenon observed here may be multifactorial, the decreased PSA screening rates in the early years of this study seem to be causally important.
The second hypothesis to explain our findings may be increased use of prebiopsy MRI with avoidance of biopsy for patients without visible lesions (19). American Urological Association guidelines now support obtaining an MRI in biopsy-naïve patients at risk of PC, with a statement released in 2019 (12). The adoption of prebiopsy MRI in the United States is relatively low, with the highest utilization rate of 19% in 2019 (19). As illustrated in Table 4, multiple studies highlight the low proportion of men who underwent prebiopsy MRI during our early study period. MRI may play a role in decreasing the incidence rates of low-grade PC, however, based on the available evidence, it is unlikely to be the main driver for temporal changes in grade migration.
Third, biomarkers used as a reflex test when evaluating patients with elevated PSA may have contributed to avoiding biopsies and less low-grade disease diagnosed. Studies have shown that serum- and urine-based biomarkers optimize the detection of high-grade PC, with the goal of reducing the number of biopsies and associated harms (38,39). For example, de la Calle et al. (40) demonstrated that a screening algorithm limiting biopsy to men with positive biomarker scores (4K score or ExosomeDx) using the manufacturer’s cutoff points would have resulted in avoiding 30%-40% of unnecessary biopsies, while missing high-grade disease in less than 4%-5%, respectively. A variety of biomarkers, such as the 4Kscore, Prostate Health Index, SelectMDx, and ExosomeDx are now included in the National Comprehensive Cancer Network guidelines and American Urological Association guidelines (12,41). However, evidence on contemporary biomarker utilization is limited. Moreover, none of the biomarkers were in wide use during the early 2010s when rates were changing the most, hence, suggesting that biomarkers are not the cause for the steep decline in GG1 incidence rates within our study period.
Finally, there is evidence that high BMI increases the risk of high-grade and advanced PC while concomitantly lowering the risk of indolent disease (42). A recent meta-analysis noted a significant 8% increased risk of advanced or high-grade PC per 5 kg/m2 increase in BMI (relative risk of 1.08, 95% CI = 1.04 to 1.12) (42). Dysregulated sex steroid metabolism, proinflammatory cytokine surge, and a lower propensity to be screened among men with obesity have been proposed as potential causes. In addition, hypervolemia contributes to lower serum concentrations of PSA because of hemodilution, which may lead to delayed cancer diagnosis among obese individuals (43). Although we observed a 20% increase in the rates of obesity among US men over the past 2 decades, this occurred at a steady pace and over a prolonged time, making it unlikely to be a significant cause of the rapid decline in the incidence of GG1 during 2010-2014.
Our findings have implications for public health. Although a reduction in low-grade PC is encouraging, decreased PSA screening at the beginning of the last decade has led to missed opportunities for early detection of aggressive disease. Several studies have shown that the incidence of distant-stage PC continues to increase in men aged older than 50 years during the last decade. Moreover, following a prolonged period of significant decline in PC-specific mortality since 1993, this trend has now stabilized over 5 years (2013-2018) (44–46). Public health efforts should consider implementing risk-stratified screening, such as the use of MRI or biomarkers to avoid biopsy in men at low risk of aggressive cancer, to continue minimizing overdiagnosis of low-grade disease and mitigating the rising trends of high-grade and metastatic PC.
Our findings must be interpreted in the context of the study design. Attribution of causality is problematic in an ecologic study, and the lack of granularity limited our ability to provide a more convincing association of the various trends. Moreover, regional and temporal variations in GG reporting by pathologists may have contributed to the observed trends. Although decreased PSA screening remains the best causal explanation for the observed changes in grade distribution, we cannot discount other explanations. Self-reported surveys of PSA screening, such as the National Health Interview Survey, may be subject to recall bias. Additionally, we were unable to evaluate the effects of the latest grade C recommendation by the USPSTF implemented in 2018, as the SEER registry has not matured beyond that year. Considering recent studies highlighting that PSA screening rates are increasing since 2018 (18), additional research is needed to determine whether the grade and stage migration findings noted in this and prior studies will persist.
The reduction in the incidence of GG1 PC at biopsy and RP pathology is encouraging and suggests that public health efforts have decreased overdiagnosis and overtreatment of indolent PC during the last decade. Changes in PSA testing are the most likely reason for the grade migration observed, as changes in obesity, and use of prebiopsy MRI or markers, were temporally inconsistent with changes in the grade distribution. Future studies should evaluate the downstream effects of evolving changes in practice patterns, such as prebiopsy MRI and markers, on the discrimination of high-grade disease at the population level. Public health efforts should focus on addressing the unintended consequences of changes in screening practices as observed by the increase in the diagnoses of high-grade and metastatic PC.
Funding
This work was supported by the National Institute of Health (R01 CA241758, R01 CA259173-01A1 to JCH), Patient Centered Outcomes Research Institute (CER-2019C1-15682, CER-2019C2-17372 to JCH) a Cancer Center Support Grant to Memorial Sloan Kettering Cancer Center (P30-CA008748), a SPORE grant in Prostate Cancer to Dr H. Scher (P50-CA92629), the Sidney Kimmel Center for Prostate and Urologic Cancers and David H. Koch through the Prostate Cancer Foundation.
Notes
Role of the funders: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures: All authors declare no support from any organization for the submitted work. Competing interests are reported for 3 authors. JCH and LDB receive salary support from the Frederick J. and Theresa Dow Wallace Fund of the New York Community Trust, JCH receives salary support from Prostate Cancer Challenge Award. AV is named on a patent for a statistical method to detect prostate cancer that has been licensed to and commercialized by OPKO Health as the 4Kscore. He receives royalties from sales of the test and has stock options in OPKO Health. There are no other relationships or activities that could appear to have influenced the submitted work.
Author contributions: LDB: Conceptualization, Data Curation; Investigation; Methodology; Visualization; Writing-Original Draft; Writing-Review & Editing. GD: Data Curation; Investigation; Writing-Original Draft; Writing-Review & Editing. XG: Data Curation; Formal analysis, Methodology; Writing-Review & Editing. EC: Data Curation; Writing-Review & Editing. VD: Methodology, Writing-Review & Editing; ES: Methodology, Writing-Review & Editing; HN: Data Curation; Methodology; Writing-Review & Editing. SC: Conceptualization; Methodology, Supervision; Writing-Review & Editing. AV: Conceptualization; Data Curation; Methodology; Supervision; Visualization; Writing-Review & Editing. JCH: Conceptualization; Data Curation; Methodology; Supervision; Visualization; Writing-Review & Editing.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Contributor Information
Leonardo D Borregales, Department of Urology, Weill Cornell Medicine/New York-Presbyterian, New York, NY, USA.
Gina DeMeo, Department of Urology, Weill Cornell Medicine/New York-Presbyterian, New York, NY, USA.
Xiangmei Gu, Department of Healthcare Policy and Research, Weill Cornell Medicine, New York, NY, USA.
Emily Cheng, Department of Urology, Weill Cornell Medicine/New York-Presbyterian, New York, NY, USA.
Vanessa Dudley, Department of Urology, Weill Cornell Medicine/New York-Presbyterian, New York, NY, USA.
Edward M Schaeffer, Department of Urology, Northwestern University, Chicago, IL, USA.
Himanshu Nagar, Department of Radiation Oncology, Weill Cornell Medicine/New York-Presbyterian, New York, NY, USA.
Sigrid Carlsson, Department of Surgery (Urology Service), Memorial Sloan Kettering Cancer Center, New York, NY, USA; Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, NY, USA; Institute of Clinical Sciences, Department of Urology, Sahlgrenska Academy at the University of Gothenburg, Gothenburg, Sweden.
Andrew Vickers, Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Jim C Hu, Department of Urology, Weill Cornell Medicine/New York-Presbyterian, New York, NY, USA.
References
- 1. Welch HG, Albertsen PC. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986-2005. J Natl Cancer Inst. 2009;101(19):1325–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65(6):1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eggener SE, Badani K, Barocas DA, et al. Gleason 6 prostate cancer: translating biology into population health. J Urol. 2015;194(3):626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990-2013. JAMA. 2015;314(1):80–82. [DOI] [PubMed] [Google Scholar]
- 5. Hoffman RM, Mott SL, McDowell BD, Anand ST, Nepple KG. Trends and practices for managing low-risk prostate cancer: a SEER-Medicare study. Prostate Cancer Prostatic Dis. 2022;25(1):100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(3):185–191. [DOI] [PubMed] [Google Scholar]
- 7. Andriole GL, Crawford ED, Grubb RL, et al. ; for the PLCO Project Team. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schröder FH, Hugosson J, Roobol MJ, et al. ; for the ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–1328. [DOI] [PubMed] [Google Scholar]
- 9. Moyer VA; for the U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120–134. [DOI] [PubMed] [Google Scholar]
- 10. Grossman DC, Curry SJ, Owens DK, et al. ; for the US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(18):1901–1913. [DOI] [PubMed] [Google Scholar]
- 11. Shoag JE, Nyame YA, Gulati R, Etzioni R, Hu JC. Reconsidering the trade-offs of prostate cancer screening. N Engl J Med. 2020;382(25):2465–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part I: risk stratification, shared decision making, and care options. J Urol. 2018;199(3):683–690. [DOI] [PubMed] [Google Scholar]
- 13. Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243–262. [DOI] [PubMed] [Google Scholar]
- 14. Lee DJ, Mallin K, Graves AJ, et al. Recent changes in prostate cancer screening practices and epidemiology. J Urol. 2017;198(6):1230–1240. [DOI] [PubMed] [Google Scholar]
- 15. Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378(19):1767–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314(19):2054–2061. [DOI] [PubMed] [Google Scholar]
- 17.National Institutes of Health, National Cancer Institute. Prostate cancer screening; 2021. https://progressreport.cancer.gov/detection/prostate_cancer. Accessed September 10, 2021.
- 18. Leapman MS, Wang R, Park H, et al. Changes in prostate-specific antigen testing relative to the revised US Preventive Services Task Force recommendation on prostate cancer screening. JAMA Oncol. 2022;8(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leapman MS, Wang R, Park HS, et al. Adoption of new risk stratification technologies within US hospital referral regions and association with prostate cancer management. JAMA Netw Open. 2021;4(10):e2128646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abashidze N, Stecher C, Rosenkrantz AB, Duszak R Jr, Hughes DR. Racial and ethnic disparities in the use of prostate magnetic resonance imaging following an elevated prostate-specific antigen test. JAMA Netw Open. 2021;4(11):e2132388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim SP, Karnes RJ, Mwangi R, et al. Contemporary trends in magnetic resonance imaging at the time of prostate biopsy: results from a large private insurance database. Eur Urol Focus. 2021;7(1):86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quinn TP, Sanda MG, Howard DH, Patil D, Filson CP. Disparities in magnetic resonance imaging of the prostate for traditionally underserved patients with prostate cancer. Cancer. 2021;127(16):2974–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gaffney CD, Cai P, Li D, et al. Increasing utilization of MRI before prostate biopsy in Black and non-Black men: an analysis of the SEER-Medicare cohort. AJR Am J Roentgenol. 2021;217(2):389–394. [DOI] [PubMed] [Google Scholar]
- 24. Rosenkrantz AB, Hemingway J, Hughes DR, Duszak R Jr, Allen B Jr, Weinreb JC. Evolving use of prebiopsy prostate magnetic resonance imaging in the Medicare population. J Urol. 2018;200(1):89–94. [DOI] [PubMed] [Google Scholar]
- 25. Liu W, Patil D, Howard DH, et al. Adoption of prebiopsy magnetic resonance imaging for men undergoing prostate biopsy in the United States. Urology. 2018;117:57–63. [DOI] [PubMed] [Google Scholar]
- 26.OPKO Health. Q4 revenues up 1 percent; 2020. https://www.360dx.com/business-news/opko-health-q4-revenues-1-percent#.YVIDQ6ApCgQ. Accessed January 10, 2021.
- 27.Seeking Alpha. OPKO Health: The importance of the 4kscore test study; 2018. https://seekingalpha.com/article/4157379-opko-health-importance-of-4kscore-test-study. Accessed January 10, 2021.
- 28. Leapman MS, Wang R, Ma S, Gross CP, Ma X. Regional adoption of commercial gene expression testing for prostate cancer. JAMA Oncol. 2021;7(1):52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Overweight & obesity. https://www.cdc.gov/obesity/data/prevalence-maps.html. Accessed January 10, 2021.
- 30. Eapen RS, Herlemann A, Washington SL 3rd, Cooperberg MR. Impact of the United States Preventive Services Task Force ‘D’ recommendation on prostate cancer screening and staging. Curr Opin Urol. 2017;27(3):205–209. [DOI] [PubMed] [Google Scholar]
- 31. Fleshner K, Carlsson SV, Roobol MJ. The effect of the USPSTF PSA screening recommendation on prostate cancer incidence patterns in the USA. Nat Rev Urol. 2017;14(1):26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hu JC, Nguyen P, Mao J, et al. Increase in prostate cancer distant metastases at diagnosis in the United States. JAMA Oncol. 2017;3(5):705–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Houston KA, King J, Li J, Jemal A. Trends in prostate cancer incidence rates and prevalence of prostate specific antigen screening by socioeconomic status and regions in the United States, 2004 to 2013. J Urol. 2018;199(3):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gandaglia G, Briganti A, Fossati N, et al. The problem is not what to do with indolent and harmless prostate cancer-the problem is how to avoid finding these cancers. Eur Urol. 2016;70(4):547–548. [DOI] [PubMed] [Google Scholar]
- 35. Penson DF. The pendulum of prostate cancer screening. JAMA. 2015;314(19):2031–2033. [DOI] [PubMed] [Google Scholar]
- 36. Coghlan A. Prostate screening does more harm than good in US; 2011. https://www.newscientist.com/article/dn21041-prostate-screening-does-more-harm-than-good-in-us/. Accessed July 1, 2022.
- 37. Zavaski ME, Meyer CP, Sammon JD, et al. Differences in prostate-specific antigen testing among urologists and primary care physicians following the 2012 USPSTF recommendations. JAMA Intern Med. 2016;176(4):546–547. [DOI] [PubMed] [Google Scholar]
- 38. Lepor A, Catalona WJ, Loeb S. The prostate health index: its utility in prostate cancer detection. Urol Clin North Am. 2016;43(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Braun K, Sjoberg DD, Vickers AJ, Lilja H, Bjartell AS. A Four-kallikrein panel predicts high-grade cancer on biopsy: independent validation in a community cohort. Eur Urol. 2016;69(3):505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de la Calle CM, Fasulo V, Cowan JE, et al. Clinical utility of 4Kscore(®), ExosomeDx™ and magnetic resonance imaging for the early detection of high grade prostate cancer. J Urol. 2021;205(2):452–460. [DOI] [PubMed] [Google Scholar]
- 41.National Comprehensive Cancer Network Guidelines: Prostate cancer early detection; 2021. https://www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf. Accessed January 10, 2021.
- 42.World Cancer Research Fund. Diet, Nutrition, Physical Activity and Prostate Cancer; 2018. https://www.wcrf.org/wp-content/uploads/2021/02/prostate-cancer-report.pdf. Accessed January 10, 2021.
- 43. Bañez LL, Hamilton RJ, Partin AW, et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA. 2007;298(19):2275–2280. [DOI] [PubMed] [Google Scholar]
- 44. Negoita S, Feuer EJ, Mariotto A, et al. Annual report to the nation on the status of cancer, part II: recent changes in prostate cancer trends and disease characteristics. Cancer. 2018;124(13):2801–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jemal A, Culp MB, Ma J, Islami F, Fedewa SA. Prostate cancer incidence 5 years after US preventive services task force recommendations against screening. J Natl Cancer Inst. 2021;113(1):64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.