Abstract
Background:
Melanoma brain metastasis (MBM) prognosis has been dismal historically. However, breakthroughs in targeted and immunotherapies have improved long-term survival in advanced melanoma patients. As such, MBM presentation, prognosis and multimodality CNS-directed treatment use were reassessed.
Methods:
This retrospective study evaluated patients treated at Memorial Sloan Kettering Cancer Center between 2010–2019 with MBM. Kaplan-Meier methodology was used to describe overall survival (OS). Recursive partitioning analysis (RPA) and time-dependent multivariable Cox modeling were used to assess prognostic variables and associate CNS-directed treatments with OS.
Results:
Four hundred and twenty-five patients with 2,488 MBM were included. Median OS from MBM diagnosis was 8.9 months (95%CI: 7.9–11.3). Patients diagnosed with MBM between 2015–2019 experienced longer OS compared to those diagnosed between 2010–2014 (13.0 months [95%CI: 10.47–17.06] versus 7.0 months [95%CI: 6.1–8.3]; p=0.0003). Prognostic multivariable modeling significantly associated shortened OS independently with leptomeningeal dissemination (p<0.0001), increasing number of BM at diagnosis (p<0.0001), earlier MBM diagnosis year (p=0.0008), higher serum LDH (p<0.0001), immunotherapy treatment prior to MBM (p=0.003), and extracranial disease presence (p=0.02). Utilization of different CNS-directed treatment modalities was associated with presenting symptoms, diagnosis year, number and size of BM, and presence of extracranial disease. Multivariable analysis demonstrated improved survival for patients that underwent craniotomy (p=0.01).
Conclusions:
MBM prognosis has improved within the last 5 years, coinciding with approval of PD1 immune checkpoint blockade and combined BRAF/MEK targeting. Improving survival reflects and may influence the willingness to use aggressive multimodality treatment for MBM.
Keywords: melanoma, brain metastases, immunotherapy, targeted therapy, survival
Precis:
Survival of patients with melanoma brain metastases has significantly improved in the last 5 years. Factors currently associated with prognosis include the number of BM, immunotherapy prior to MBM diagnosis, presence of extracranial or leptomeningeal disease, serum LDH, and utilization of CNS-directed treatment.
Lay Summary:
Historically, melanoma brain metastases (MBM) have carried a poor survival prognosis of 4–6 months. However, the introduction of immunotherapy and targeted precision medicines has altered the survival curve for advanced melanoma. In this large, single-institution contemporary cohort we demonstrate a significant increase in survival of MBM patients to 13 months within the last 5 years of the study. Worse MBM prognosis was significantly associated with the number of metastases at diagnosis, prior exposure to immunotherapy, spread of disease to the leptomeningeal compartment, serum lactate dehydrogenase (LDH) elevation, and presence of extracranial disease. The current age of systemic treatments has also been accompanied by shifts in the utilization of CNS-directed therapies.
Introduction:
Melanoma is one of the primary causes of malignant metastases to the central nervous system (CNS), accounting for 6–12% of all metastatic brain tumors1–3. Survival after diagnosis of melanoma brain metastasis (MBM) has historically been dismal with overall survival of 4–6 months4–8. However, over the recent decade, numerous advances have been made in targeted therapy for melanoma, such as BRAF and MEK inhibition, as well as with immunotherapy with the approval of checkpoint inhibitors ipilimumab, nivolumab, and pembrolizumab9. These advances have resulted in significant improvements in the overall survival of patients with metastatic melanoma10–18. Furthermore, patients with MBM have also been found to respond to these therapies19–24. In the COMBI-MB trial, 58% of BRAF-V600E positive MBM responded to combination dabrafenib and trametinib23 and combination of nivolumab and ipilimumab has resulted in intracranial response in 46–56% of patients with MBM22,24. While clinical trials have begun to include more patients with MBM, little data exists to assess how the current treatments have changed the overall prognosis of MBM diagnosis, affected CNS-directed local treatment algorithms with surgery and radiation, or enumerated factors that may inform the survival of patients with MBM. Notably, the most recent iteration of the Graded Prognostic Assessment called the Melanoma-molGPA demonstrated the prognostic value of clinical features, including age, Karnofsky performance status (KPS), the number of CNS metastases, presence of extracranial metastases, and BRAF status in a cohort identified through 2015, however leptomeningeal disease and the contribution of immunotherapy were not investigated25. This large retrospective, single-institution study describes the presentation, treatments, and survival of MBM patients in the contemporary immunotherapy and precision medicine era.
Methods:
This study was approved by the Memorial Sloan Kettering Cancer Center (MSK) Institutional Review Board. Patients (n=440) were identified by an institutional database search for all patients, regardless of treatments, with a diagnosis of cutaneous melanoma, no other systemic malignancy, and brain metastases diagnosed from January 2010 until January 2019. Patients were excluded from the analysis if they had primary leptomeningeal disease without parenchymal metastases at time of MBM diagnosis (n=4) or if medical records had incomplete clinical documentation for the parameters enumerated below, represented a single encounter without any follow up, or were without baseline or follow up imaging (n=11). Retrospective chart review was conducted to identify demographics including age at diagnosis of MBM, number, size and location of BM at diagnosis, CNS symptoms at diagnosis, presence of metastasis-associated hemorrhage, serum lactate dehydrogenase (LDH) at MBM diagnosis, presence or absence of extracranial disease on the CT chest-abdomen-pelvis most contemporaneous to time of BM diagnosis, diagnosis of leptomeningeal disease and/or hydrocephalus during treatment, overall survival, systemic and CNS-directed treatments prior to and following BM diagnosis (chemotherapy, immunotherapy, targeted BRAF/MEK therapy, stereotactic and/or whole brain radiation, and surgery including craniotomy and/or cerebral-spinal fluid [CSF] diversion), and presence of progressive systemic and/or CNS disease at time of death (if known). Radiographic findings were based on radiologist interpretations of MRI and CT studies; these were further reviewed when quantitative or qualitative features of interest (size, number, location, hydrocephalus) were not commented upon. Presence of hemorrhage in metastases was based on radiology reports. Dominant metastasis was defined as largest metastasis present on imaging and size was determined based on largest axial/coronal/sagittal diameter.
Statistical Analysis
Descriptive statistics such as frequencies, means, and standard deviations were used to characterize the cohort under study. Overall survival (OS) was defined as the time from MBM diagnosis until date of death or date of last follow up for those who were censored. Recursive partitioning analysis (RPA) was used for exploration and visualization of empirically identified cutoffs for the association of number of brain metastases, MBM diagnosis year, and size of largest MBM with OS. Univariable and multivariable Cox modeling was used to associate variables of interest with OS. Variables significant in the univariable models were brought forward for evaluation in the multivariable analysis. Leptomeningeal disease and all treatments following diagnosis of MBM were treated as time-dependent variables in the Cox models. The time-dependent Cox models associating leptomeningeal disease with overall survival were stratified by variables of interest and the heterogeneity was tested with nested models using the likelihood ratio test. Kaplan-Meier methodology was used to display survival curves. Cumulative incidence of leptomeningeal disease following BM was calculated using competing risks methodology and Gray’s test was used to compare cumulative incidence curves stratified by pre-BM immunotherapy. The Kruskal-Wallis test was used to investigate the association between presenting MBM symptoms with dominant MBM size and number of MBMs. Fisher’s test was used to explore the association of presenting MBM symptoms with dominant MBM location. The Wilcoxon two sample test was used to investigate the association between pre-BM diagnosis immunotherapy and number of BM at diagnosis. A cause-specific, time-dependent Cox Model was used to model the association of variables of interest with post-MBM treatments. All p-values were two-sided with a level of statistical significance less than 0.05. In order to summarize our work, we emphasized ‘statistically significant’ findings i.e. those with p-values below the threshold of 0.05. Without a power calculation we lack information about the magnitude of the association(s) that could be detected with high probability for our study design. We have additionally presented estimates of the association and their confidence intervals and suggest these results add value to the interpretation, both for findings with p<.05 as well as those with larger p-values. All statistical analyses were performed in SAS (version 9.4, Cary, NC) and R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results:
Demographics, Survival, and BM Presentation
Four hundred-twenty-five patients were diagnosed with a total of 2,488 MBM at MSK between 2010 and 2019 (Table 1). Mean age was 59.3 years and there was a predominance of males (72% male, 28% female). There were 324 deaths over the study duration. Median overall survival from the diagnosis of brain metastasis was 8.9 months (95% CI: 7.9–11.3) (Figure 1). Median follow up was 22.5 months for survivors. The 3-year OS for the cohort was 19.4% (95% CI: 15.5–24.1) and 5-year OS: 13.6% (95% CI: 10.0–18.6). Forty-nine percent of patients (n=206) had BRAF mutation identified by immunohistochemistry, mass spectrometry, and/or targeted sequencing, while 43% were BRAF wild-type, and 8% unknown. Eighty-eight percent of patients had extracranial disease present at diagnosis of MBM, 10% had brain metastases only without evidence of extracranial disease and 2% were unknown. The median number of parenchymal metastases at BM diagnosis was 3 (IQR: 1–6, range: 1 - >50). In 90% of patients, the dominant/largest BM was located in the supratentorial compared to 10% in the infratentorial compartment. Median size of the dominant BM was 1.8cm (IQR: 0.9–2.9cm, range 0.2 – 8.8cm). Fifty-eight percent of patients had radiographic hemorrhage present at BM diagnosis, while 72% had hemorrhage present by last follow-up. Serum LDH at MBM diagnosis was above normal limits in 23%, within normal limits for 32%, and unknown for 45% of patients. Supplemental Figure 1 demonstrates the cumulative incidence of LMD diagnosis after diagnosis of MBM. This demonstrated a 1-year incidence of 12.3% (95%CI: 9.1–15.5) and a plateau in LMD diagnosis by ~3-years post-MBM diagnosis with an overall incidence of 15.3% (95% CI: 11.7–18.9) for MBM patients at 3-years.
Table 1.
Melanoma brain metastasis (MBM) cohort characteristics.
| Variable | Level | N | % | Mean | Median | Min | Max |
|---|---|---|---|---|---|---|---|
| Age at Melanoma Diagnosis | continuous | 425 | 100 | 56.6 | 58.8 | 15.2 | 91.8 |
| Age at BM Diagnosis | continuous | 425 | 100 | 59.3 | 61.3 | 18.9 | 92.4 |
| Number of BM at Diagnosis | continuous | 425 | 100 | 5.9 | 3 | 1 | >50 |
| Dominant BM size (cm) | continuous | 425 | 100 | 2.1 | 1.8 | 0.2 | 8.8 |
| Serum LDH value | continuous | 233 | 55 | 389.1 | 221 | 110 | 4970 |
| M | 304 | 72 | |||||
| Unknown | 35 | 8 | |||||
| Unknown | 11 | 3 | |||||
| Unknown | 192 | 45 | |||||
| Yes | 248 | 58 | |||||
| Yes | 306 | 72 | |||||
| Subcortical | 34 | 8 | |||||
| Infratentorial | 44 | 10 | |||||
| Yes | 43 | 10 | |||||
| Cumulative Incidence of LMD (95% CI) at 1 year | -- | 12.3% (95%CI:9.1–15.5) | |||||
| Cumulative Incidence of LMD (95% CI) at 3 years | -- | 15.33% (95% CI:11.7–18.9) | |||||
| Yes | 199 | 47 | |||||
| Yes | 59 | 14 | |||||
| Unknown | 2 | 0 | |||||
| Yes | 108 | 25 | |||||
| Both | 9 | 2 | |||||
| Both | 48 | 11 | |||||
Figure 1.
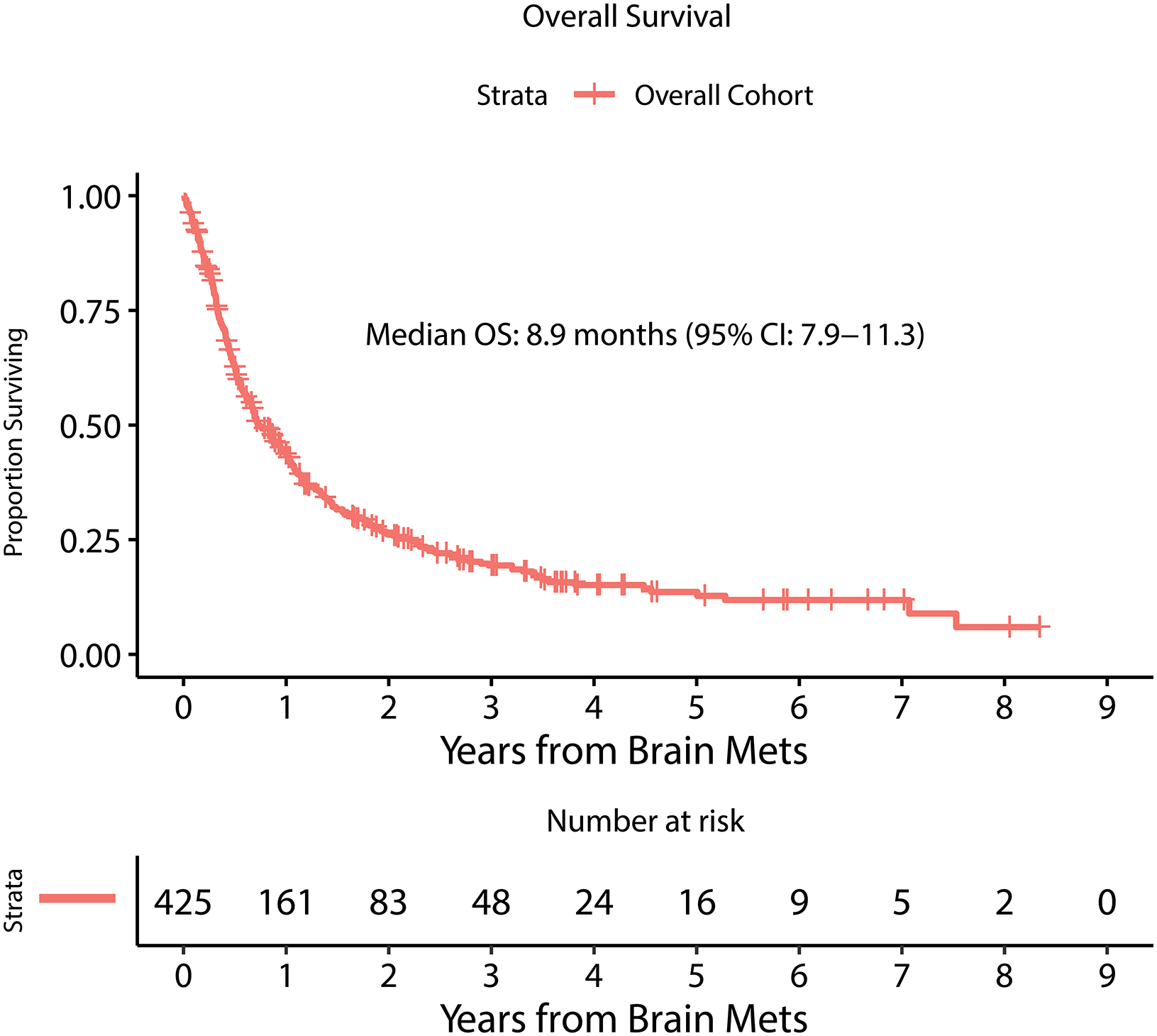
Kaplan Meier estimate of overall survival from time of MBM diagnosis.
The plurality of patients was asymptomatic at time of BM diagnosis (39%), while 20% had focal motor or sensory complaints. Seizure was the initial presenting symptom in 8% of cases, and 33% presented with headache, mental status change, or other neurologic complaint without focal deficit or seizure. Presenting symptom differed significantly in relation to dominant metastasis size (Supplementary Table 1). Asymptomatic patients had a median dominant BM size of 1.0cm compared with 3.1cm for patients presenting with headache, 2.2cm for motor/sensory deficit and 2.6cm for seizure (p <0.0001). Dominant BM location was also significantly associated with presenting symptom (p=0.01, Supplementary Table 2). Headache was the most common presentation for cerebellar/pontine BMs, accounting for 34% of those cases. Seizures and motor/sensory deficits occurred more frequently in patients with frontal and parietal BMs compared to other locations. The number of BM present at diagnosis was not significantly associated with presenting symptom.
Prognostic Factors
RPA was performed to explore the cut-point most associated with OS for each of the following variables individually: number of MBM at diagnosis, year of MBM diagnosis, and size of largest MBM (Figure 2). This demonstrated that <5 versus ≥5 BMs was most associated with overall survival. Median OS for <5 BMs was 12.5 months (95%CI: 10.5–16.0) compared to a median OS for ≥5 BM of 5.5 months (95%CI: 4.2 – 6.8). This also demonstrated that 2010–2014 year of MBM diagnosis versus 2015–2019 was most associated with overall survival. Median OS for 2010–2014 MBM year of diagnosis was 7.0 months (95%CI: 6.1–8.3) compared to a median OS for 2015–2019 of 13.0 months (95%CI: 10.5–17.1). Multivariable hazard ratios (HR) did not demonstrate a significant difference in the risk of systemic (HR=1.46 95%CI [0.89 – 2.39]; p=0.14) or CNS progression (HR=1.37, 95%CI [0.89 – 2.12]; p=0.15) at time of death between the years 2010–2014 and 2015–2019. No cut-point was identified for dominant BM size. Receipt of immunotherapy prior to MBM was not associated with any significant difference in number of BM at diagnosis.
Figure 2.
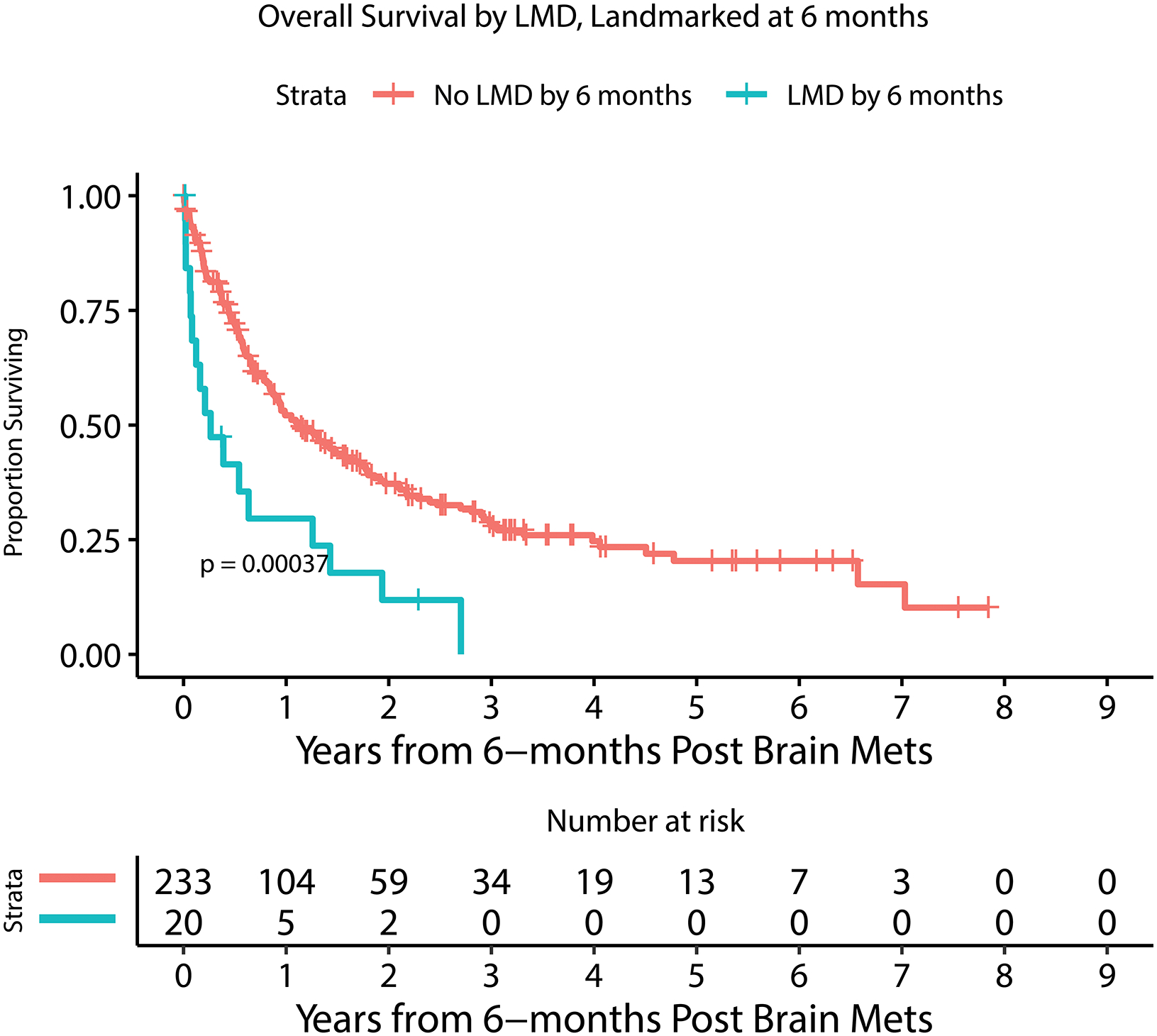
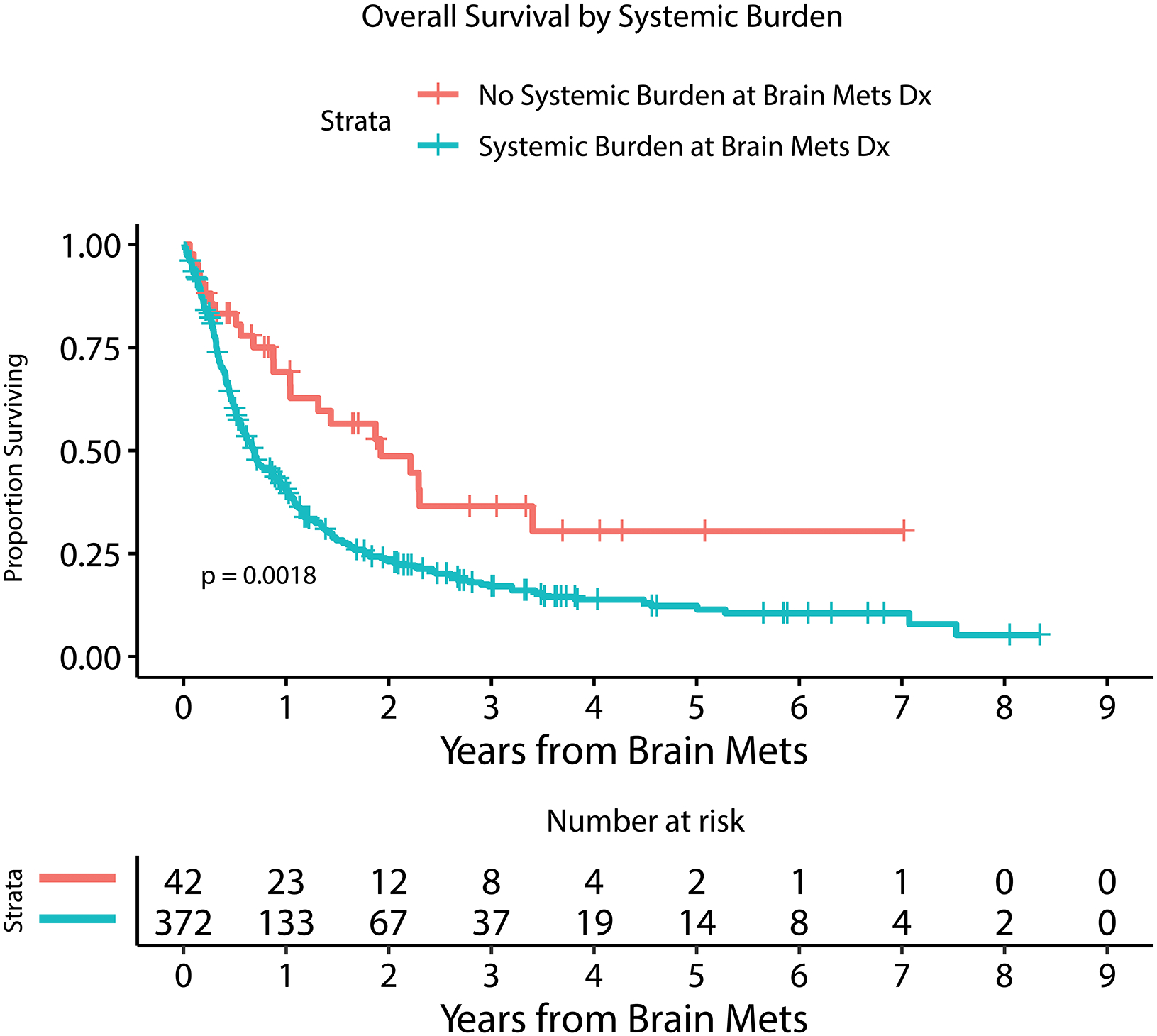
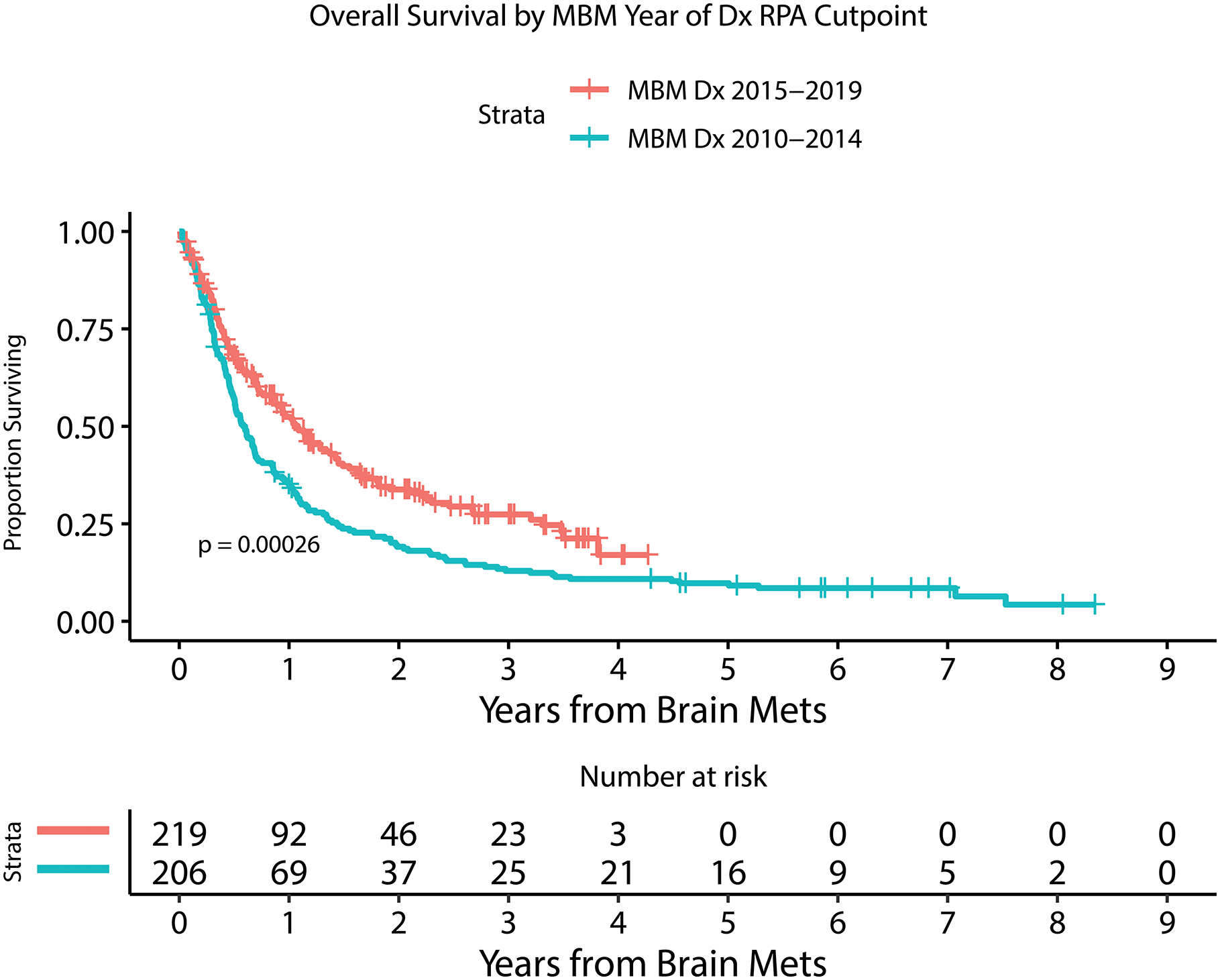
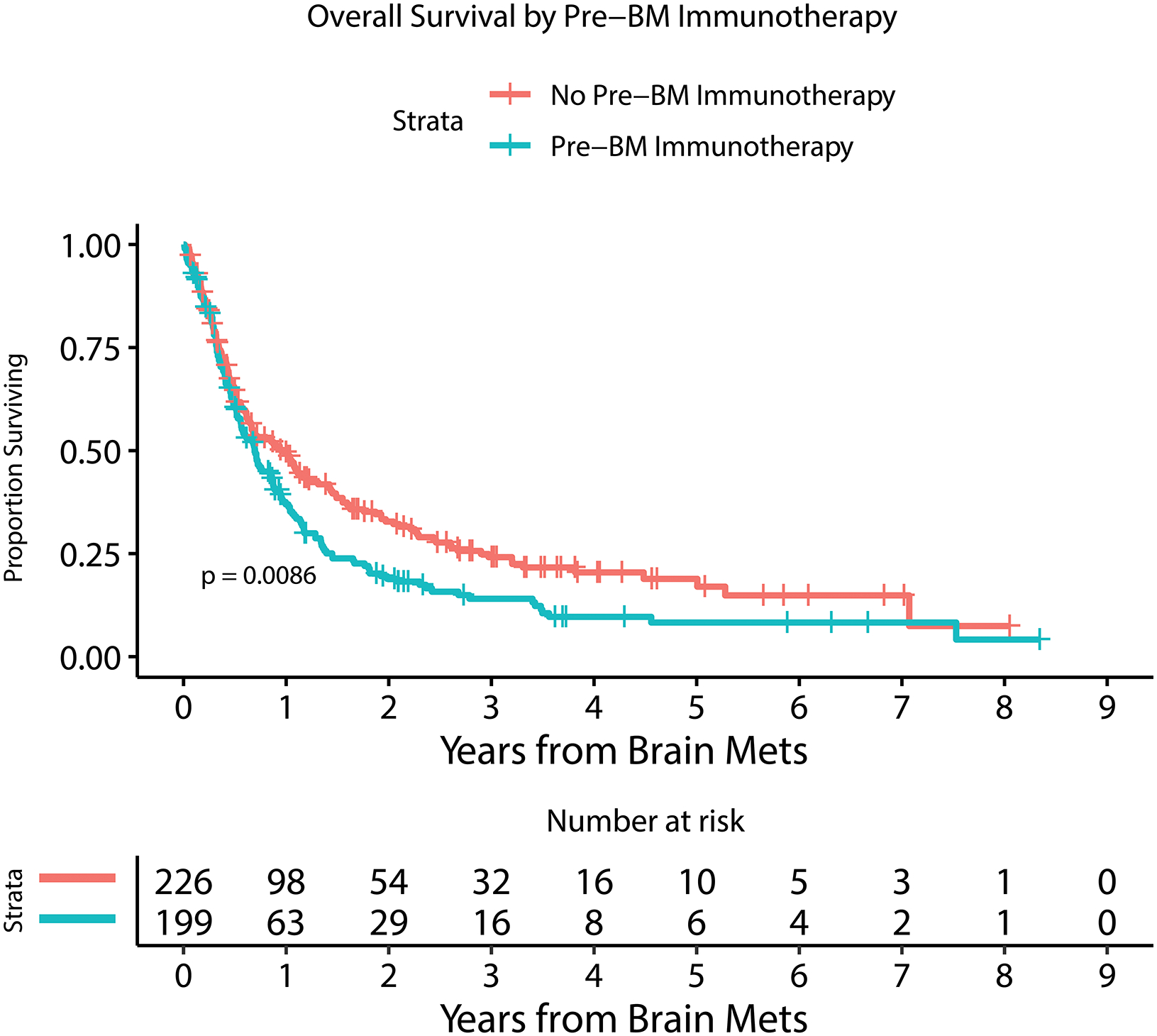
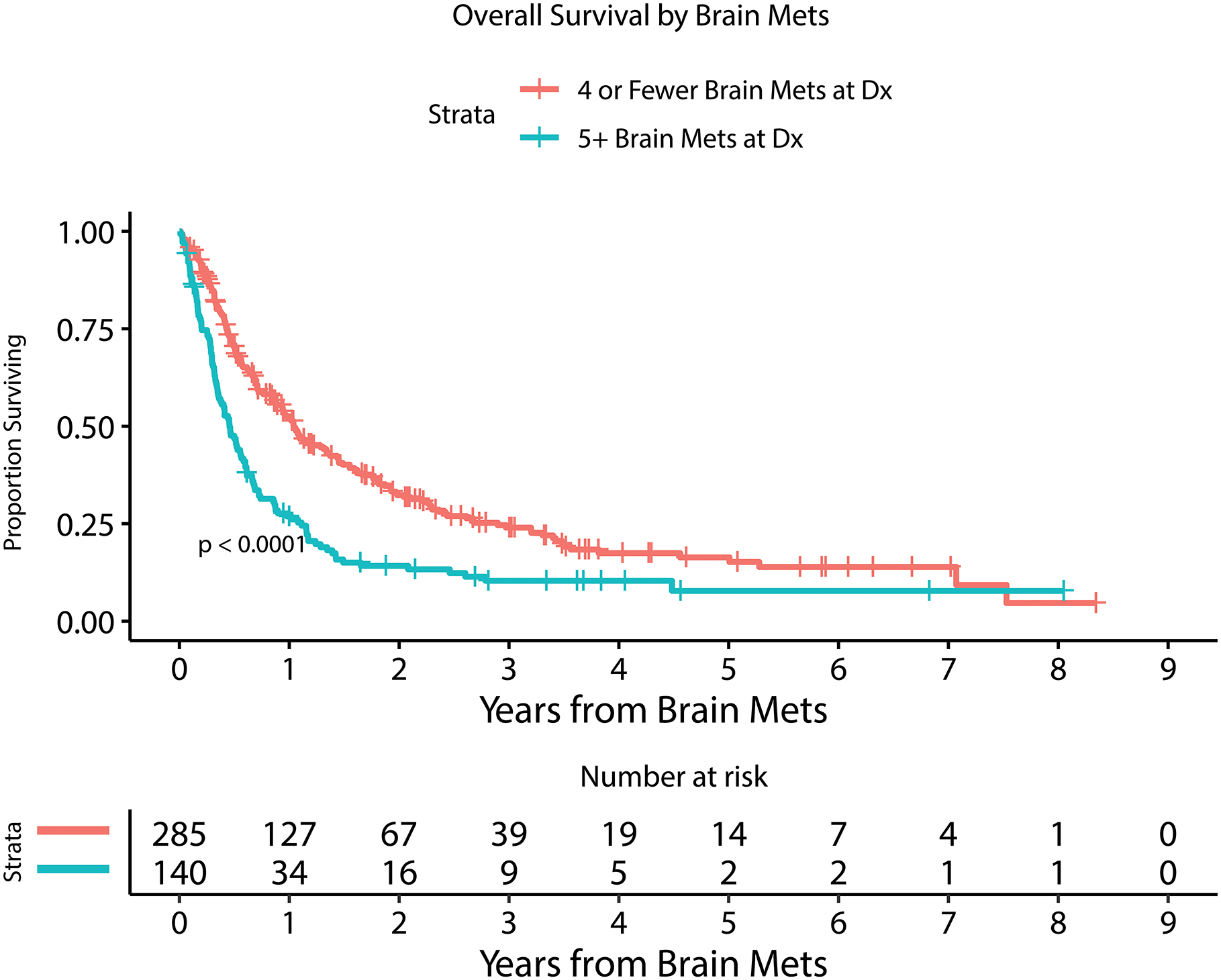
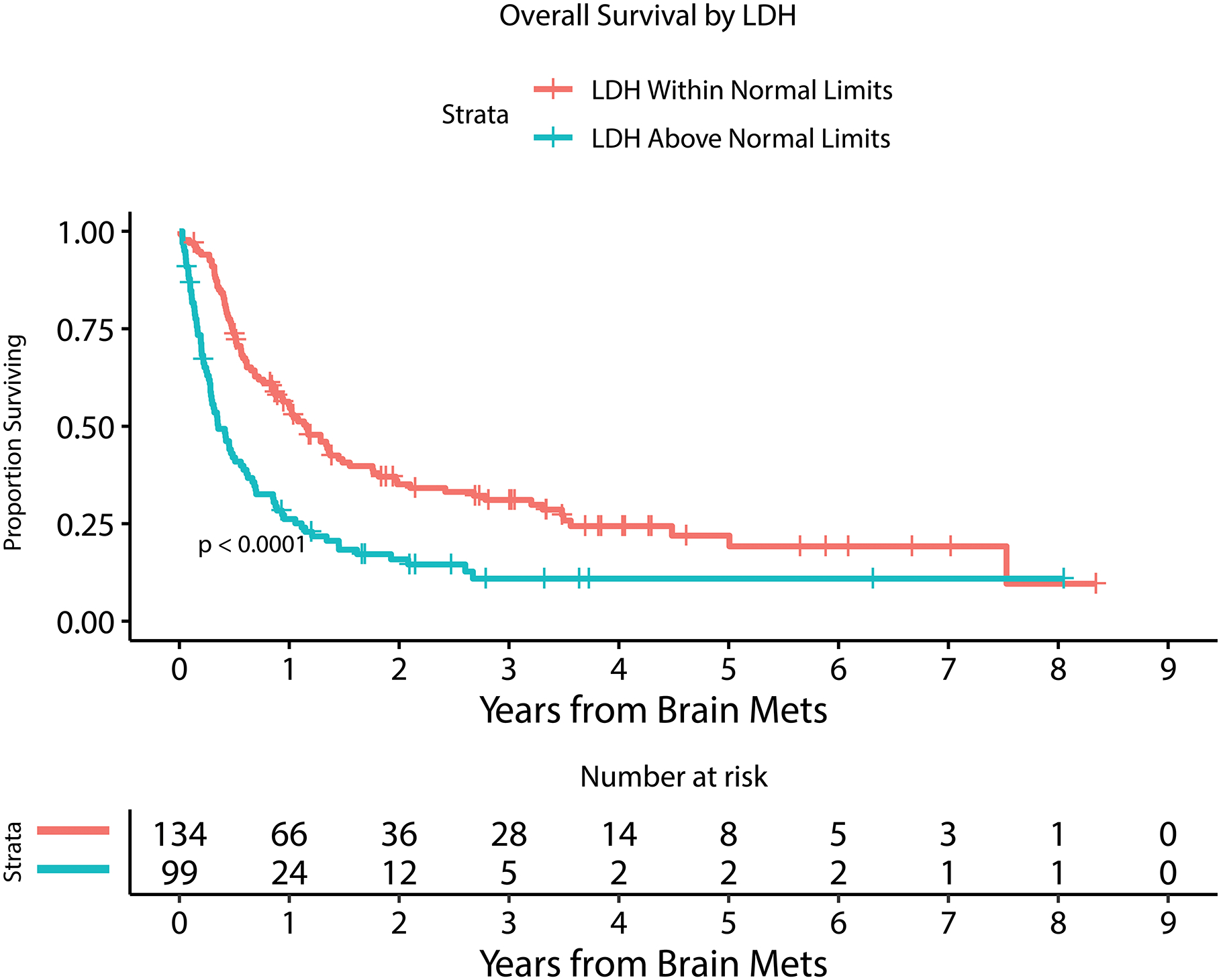
Kaplan Meier estimate of overall survival for patients with or without LMD by 6 months after MBM diagnosis (A), with or without extracranial systemic burden (B), with MBM diagnosis between 2010–2014 or 2015–2019 (C), with or without immunotherapy prior to MBM diagnosis (D), with <5 or ≥ 5 BMs at MBM diagnosis (E), and with serum LDH above or within normal limits (F).
Table 2 and Figure 2 demonstrate univariable and multivariable (adjusted) analysis for prognostic factors and their association with overall survival. Number of BM at diagnosis (HR=1.03, 95%CI [1.01 – 1.04]; p<0.0001), year of MBM diagnosis (HR=0.92, 95%CI [0.87 – 0.97]; p=0.0008), the diagnosis of leptomeningeal dissemination treated as a time-dependent variable (HR=3.63, 95%CI [2.71 – 4.87]; p<0.0001), serum LDH level above normal limits at diagnosis (HR=2.14, 95%CI [1.58 – 2.88]; p<0.0001), the administration of immunotherapy prior to the diagnosis of BM (HR=1.40, 95%CI [1.12 – 1.75]; p=0.003), and presence of extracranial disease at diagnosis (HR=1.67, 95%CI [1.07 – 2.60]; p=0.02) were all statistically significantly associated with overall survival in a multivariable model. Factors that were not associated with survival included age, gender, dominant met size, presence of hemorrhage at MBM diagnosis, presenting symptom, and BRAF mutation status.
Table 2.
Factors associated with MBM overall survival.
| Variable | Level | N | % | HR | 95% CI | p-value | HR | 95% CI | p-value |
|---|---|---|---|---|---|---|---|---|---|
| Age at MBM Diagnosis | continuous | 425 | 100 | 1.007 | 0.999–1.014 | 0.08 | |||
| Dominant BM size (cm) | continuous | 425 | 100 | 0.99 | 0.91–1.08 | 0.84 | |||
| Year of MBM Diagnosis | continuous | 425 | 100 | 0.92 | 0.87–0.96 | 0.0004 | 0.92 | 0.87–0.97 | 0.0008 |
| Male | 304 | 72 | 1.00 | 0.78–1.27 | 0.98 | ||||
| Other | 14 | 3 | 0.90 | 0.44–1.84 | 0.77 | ||||
| Yes | 248 | 58 | 1.04 | 0.83–1.30 | 0.73 | ||||
| Yes (time-dependent variable) | 62 | 15 | 3.59 | 2.69–4.78 | <0.0001 | 3.63 | 2.71–4.87 | <0.0001 | |
| Mutated | 206 | 48 | 0.98 | 0.78–1.23 | 0.87 | ||||
| Yes | 199 | 47 | 1.34 | 1.08–1.67 | 0.0089 | 1.40 | 1.12–1.75 | 0.003 | |
| Extracranial disease present | 372 | 88 | 1.97 | 1.28–3.04 | 0.002 | 1.67 | 1.07–2.60 | 0.02 |
Variables significant in the unadjusted models were brought forward.
Given that leptomeningeal disease is only rarely present at time of MBM diagnosis, it was assessed as a time-dependent variable, not at a specific time point, and was one of the strongest negative prognostic factors in this cohort. All patients diagnosed with LMD (n=66) had a median OS of 2.3 months (95% CI: 1.8–3.4) from time of LMD diagnosis. The cumulative incidence of developing LMD (accounting for death as a competing event) is demonstrated in Supplementary Figure 1. The association of LMD with overall survival was further investigated by stratifying the cohort by age, year of MBM diagnosis, systemic burden, pre-BM immunotherapy, and pre-BM BRAF targeted treatment for BRAF patients (Supplementary Table 3). In all of these stratification analyses, LMD remained a statistically significant negative prognostic factor for all categories with the exception of patients age 60 or younger, though the p-value for heterogeneity was not statistically significant across age categories. Clinical variables potentially associated with developing LMD were also examined (Supplementary Table 4), with only age at BM diagnosis (HR=0.98 [0.96–0.997], p=0.02) remaining significantly associated in multivariable analysis; patients treated with WBRT prior to LMD were more likely to experience an LMD diagnosis (HR=3.02 [1.71–5.33], p=0.0001). Of note, craniotomy (HR=0.95 [0.54–1.68], p=0.86) and number of BM (HR=1.01 [0.98–1.04], p=0.58) did not significantly associate with LMD diagnosis.
Treatments
Prior to MBM diagnosis, 199 patients (47%) had received immunotherapy, 59 patients (14% of total patients, 29% of BRAF mutated patients) had received BRAF-targeted therapy. By time of last follow up, 326 patients (77%) had ever received immunotherapy and 108 patients (25% of total patients and 52% of BRAF-mutated patients) had undergone BRAF-directed therapy. These treatments were not evaluated in a time-dependent manner and therefore do not fully reflect the at-risk population. After diagnosis of MBM, 39% of patients underwent surgery (craniotomy, ventriculoperitoneal shunt, or both) and 78% underwent either stereotactic radiosurgery (SRS), whole brain radiation therapy (WBRT), or both for treatment of MBM (Table 1 and Supplementary Figure 2). Each type of local therapy was examined as a first local/CNS treatment in relation to age, gender, year of BM diagnosis, extracranial disease, pre-BM immunotherapy, the number of BM, dominant BM size, hemorrhage present at BM diagnosis, and presenting symptom (Table 3).
Table 3.
The association of variables of interest with specific CNS-directed MBM treatments as first treatment.
| First Treatment of Shunt | First Treatment of Craniotomy | First Treatment of SRS | First Treatment of WBRT | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable* | Univariable | Multivariable* | Univariable | Multivariable* | Univariable | Multivariable* | ||||||||||||||||||||||||||||||||
| Variable of Interest | N (%) | N events (%) | Cause-Specific HR | 95% CI | P-value | Cause-Specific HR | 95% CI | P-value | N (%) | N events (%) | Cause-Specific HR | 95% CI | P-value | Cause-Specific HR | 95% CI | P-value | N (%) | N events (%) | Cause-Specific HR | 95% CI | P-value | Cause-Specific HR | 95% CI | P-value | N (%) | N events (%) | Cause-Specific HR | 95% CI | P-value | Cause-Specific HR | 95% CI | P-value | |||||||
| Number of BM | 425 (100) | 10 (100) | 0.73 | 0.48–1.09 | 0.13 | 425 (100) | 137 (100) | 0.87 | 0.81–0.92 | <0.0001 | 0.87 | 0.81–0.93 | <0.0001 | 425 (100) | 138 (100) | 0.97 | 0.94–1.00 | 0.03 | 0.97 | 0.94–1.00 | 0.04 | 425 (100) | 118 (100) | 1.04 | 1.03–1.06 | <0.0001 | 1.04 | 1.03–1.06 | <0.0001 | ||||||||||
| Dominant BM Size | 425 (100) | 10 (100) | 1.71 | 1.32–2.20 | <0.0001 | 1.72 | 1.22–2.42 | 0.002 | 425 (100) | 137 (100) | 1.57 | 1.46–1.70 | <0.0001 | 1.4 | 1.26–1.55 | <0.0001 | 425 (100) | 138 (100) | 0.98 | 0.85–1.13 | 0.80 | 425 (100) | 118 (100) | 0.98 | 0.84–1.14 | 0.78 | |||||||||||||
| Age at BM Diagnosis | 425 (100) | 10 (100) | 0.98 | 0.94–1.02 | 0.26 | 425 (100) | 137 (100) | 0.99 | 0.98–1.00 | 0.24 | 425 (100) | 138 (100) | 1.00 | 0.99–1.01 | 0.99 | 425 (100) | 110 (98) | 1.00 | 0.99–1.01 | 0.97 | |||||||||||||||||||
| Year of BM Diagnosis | 425 (100) | 10 (100) | 1.19 | 0.89–1.60 | 0.25 | 425 (100) | 137 (100) | 1.00 | 0.92–1.07 | 0.88 | 425 (100) | 138 (100) | 1.05 | 0.97–1.13 | 0.25 | 425 (100) | 110 (92) | 0.80 | 0.74–0.87 | <0.0001 | 0.79 | 0.73–0.86 | <0.0001 | ||||||||||||||||
| Gender: | |||||||||||||||||||||||||||||||||||||||
| Female | 121 (28) | 1 (10) | ref | 121 (28) | 38 (28) | ref | 121 (28) | 40 (29) | ref | 121 (28) | 39 (33) | ref | |||||||||||||||||||||||||||
| Male | 304 (72) | 9 (90) | 3.42 | 0.43–27.04 | 0.24 | 304 (72) | 99 (72) | 0.97 | 0.67–1.42 | 0.89 | 304 (72) | 98 (71) | 0.90 | 0.62–1.30 | 0.58 | 304 (72) | 79 (67) | 0.76 | 0.52–1.11 | 0.16 | |||||||||||||||||||
| Hemorrhage at BM Diagnosis: | |||||||||||||||||||||||||||||||||||||||
| No | 177 (42) | 4 (40) | ref | 177 (42) | 35 (26) | ref | ref | 177 (42) | 75 (54) | ref | 177 (42) | 45 (38) | ref | ref | |||||||||||||||||||||||||
| Yes | 248 (58) | 6 (60) | 1.36 | 0.38–4.87 | 0.63 | 248 (58) | 102 (74) | 2.68 | 1.82–3.94 | <0.0001 | 1.68 | 1.09–2.58 | 0.02 | 248 (58) | 63 (46) | 0.85 | 0.61–1.19 | 0.33 | 248 (58) | 73 (62) | 1.56 | 1.08–2.27 | 0.02 | 1.25 | 0.83–1.88 | 0.29 | |||||||||||||
| BRAF: | |||||||||||||||||||||||||||||||||||||||
| WT | 184 (43) | 2 (20) | ref | 184 (43) | 56 (41) | ref | 184 (43) | 72 (52) | ref | ref | 184 (43) | 43 (36) | ref | ||||||||||||||||||||||||||
| Mutated | 206 (48) | 8 (80) | 3.41 | 0.72–16.09 | 0.30 | 206 (48) | 69 (50) | 1.08 | 0.76–1.53 | 0.68 | 206 (48) | 61 (44) | 0.70 | 0.50–0.98 | 0.04 | 0.81 | 0.57–1.16 | 0.25 | 206 (48) | 63 (53) | 1.24 | 0.84–1.82 | 0.28 | ||||||||||||||||
| Pre-BM Immunotherapy: | |||||||||||||||||||||||||||||||||||||||
| No | 226 (53) | 6 (60) | ref | 226 (53) | 87 (64) | ref | ref | 226 (53) | 54 (39) | ref | ref | 226 (53) | 56 (47) | ref | |||||||||||||||||||||||||
| Yes | 199 (47) | 4 (40) | 0.77 | 0.22–2.74 | 0.69 | 199 (47) | 50 (37) | 0.67 | 0.47–0.95 | 0.02 | 0.93 | 0.65–1.35 | 0.71 | 199 (47) | 84 (61) | 1.89 | 1.34–2.66 | 0.0003 | 1.74 | 1.22–2.46 | 0.002 | 199 (47) | 62 (53) | 1.31 | 0.91–1.88 | 0.15 | |||||||||||||
| Systemic Burden at BM Diagnosis: | |||||||||||||||||||||||||||||||||||||||
| No | 42 (10) | 0 | ref | 42 (10) | 29 (22) | ref | ref | 42 (10) | 10 (7) | ref | 42 (10) | 4 (3) | ref | ||||||||||||||||||||||||||
| Yes | 372 (88) | 10 (100) | no est | no est | no est | 372 (88) | 103 (78) | 0.29 | 0.19–0.44 | <0.0001 | 0.43 | 0.28–0.68 | 0.001 | 372 (88) | 125 (93) | 0.85 | 0.45–1.63 | 0.63 | 372 (88) | 111 (97) | 2.08 | 0.77–5.65 | 0.15 | ||||||||||||||||
| Presenting Symptom: | |||||||||||||||||||||||||||||||||||||||
| Asymptomatic | 166 (39) | 2 (20) | ref | ref | 166 (39) | 19 (14) | ref | ref | 166 (39) | 78 (57) | ref | 166 (39) | 48 (41) | ref | ref | ||||||||||||||||||||||||
| Headache | 72 (17) | 4 (40) | 8.25 | 1.47–46.32 | 0.02 | 3.76 | 0.60–23.78 | 0.16 | 72 (17) | 41 (30) | 8.06 | 4.66–13.93 | <0.0001 | 3.69 | 2.05–6.64 | <0.0001 | 72 (17) | 16 (12) | 0.97 | 0.57–1.67 | 0.92 | 72 (17) | 13 (11) | 1.12 | 0.60–2.07 | 0.73 | 1.17 | 0.63–2.18 | 0.61 | ||||||||||
| Motor/sensory | 83 (20) | 2 (20) | 2.67 | 0.37–19.07 | 0.33 | 0.94 | 0.09–9.47 | 0.96 | 83 (20) | 32 (23) | 4.29 | 2.43–7.58 | <0.0001 | 1.93 | 1.03–3.60 | 0.04 | 83 (20) | 20 (14) | 0.74 | 0.45–1.21 | 0.23 | 83 (20) | 29 (25) | 1.60 | 1.01–2.54 | 0.046 | 1.13 | 0.70–1.84 | 0.62 | ||||||||||
| Seizure | 34 (8) | 0 (0) | no est | no est | no est | no est | no est | no est | 34 (8) | 13 (9) | 5.74 | 2.82–11.67 | <0.0001 | 3.23 | 1.52–6.83 | 0.002 | 34 (8) | 8 (6) | 1.17 | 0.56–2.43 | 0.67 | 34 (8) | 11 (9) | 2.17 | 1.12–4.20 | 0.02 | 1.27 | 0.64–2.53 | 0.49 | ||||||||||
| Mental status change | 56 (13) | 2 (20) | 5.17 | 0.71–37.76 | 0.11 | 2.95 | 0.37–23.43 | 0.31 | 56 (13) | 27 (20) | 6.47 | 3.59–11.68 | <0.0001 | 3.65 | 1.94–6.85 | <0.0001 | 56 (13) | 11 (8) | 0.74 | 0.39–1.40 | 0.36 | 56 (13) | 14 (12) | 1.41 | 0.78–2.57 | 0.26 | 1.22 | 0.65–2.28 | 0.54 | ||||||||||
| Other | 14 (3) | 0 (0) | no est | no est | no est | no est | no est | no est | 14 (3) | 5 (4) | 3.56 | 1.33–9.54 | 0.01 | 2.02 | 0.74–5.56 | 0.17 | 14 (3) | 5 (4) | 0.89 | 0.36–2.20 | 0.80 | 14 (3) | 3 (3) | 0.84 | 0.26–2.70 | 0.77 | 1.22 | 0.38–3.95 | 0.74 | ||||||||||
Variables significant in the unadjusted models were brought forward.
In multivariable analysis, patients presenting with headache (HR=3.69 [2.05–6.64], p<0.0001), motor/sensory deficits (HR=1.93 [1.03–3.60], p=0.04), seizure (HR=3.23 [1.52–6.83], p=0.002), or mental status change (HR=3.65 [1.94–6.85], p<0.0001) were significantly more likely to undergo craniotomy as a first treatment compared to patients presenting asymptomatically. Symptomatic presentation was not significantly associated with any other treatment modality. Fewer BMs, with quantity evaluated as a continuous variable, was significantly associated with craniotomy (HR=0.87 [0.81–0.93], p=<0.0001) and SRS (HR=0.97 [0.94–1.00], p<0.04), while higher BM quantity was associated with treatment with WBRT (HR=1.04 [1.03–1.06], p<0.0001). Number of BM was not significantly associated with receiving a shunt. The dominant BM size was significantly associated with first treatment of craniotomy (HR=1.38 [1.26–1.52], p<0.0001) or shunt (HR=1.72 [1.22–2.42], p=0.002). For each centimeter increase in dominant BM size, patients were 72% more likely to receive a shunt and 38% more likely to undergo craniotomy. Dominant BM size was not associated with likelihood of ultimately receiving SRS or WBRT. Presence of hemorrhage at BM diagnosis was also significantly associated with increased likelihood of undergoing craniotomy as first treatment on multivariable analysis (HR=1.68, [1.09–2.58], p=0.02). Presence of extracranial disease was associated with decreased likelihood of craniotomy as a first treatment (HR=0.43 [0.28–0.68], p=0.001). Administration of immunotherapy prior to BM diagnosis was associated with increased likelihood of SRS as first treatment (HR=1.74 [1.22–2.46], p=0.002). Age at BM diagnosis was not significantly associated with any of the CNS-directed treatment modalities. Year of BM diagnosis demonstrated significant association with WBRT (HR=0.79 [0.73–0.86], p<0.0001). With each subsequent year, the likelihood of WBRT decreased 21%.
Table 4 demonstrates the associations of local treatment modalities, performed at any time during disease course, with overall survival. Using multivariable analysis, all factors identified as significant in univariable analysis retained significance except for SRS. Patients undergoing craniotomy experienced improved survival compared to those that had not (HR 0.72, 95% CI: 0.56–0.93, p = 0.01). Patients who underwent shunt (HR 4.24, 95% CI: 2.48–7.24, p < 0.0001) and WBRT (HR 2.65, 95% CI: 2.08–3.38, p < 0.0001) experienced shorter survival than those patients who did not undergo one of these treatments. SRS, while associated with improved survival in univariable analysis (HR 0.59, 95% CI: 0.47–0.74, p<0.0001), did not maintain an association when adjusted in multivariable analysis (HR 0.87, 95% CI: 0.68–1.13, p = 0.30).
Table 4.
Association of local/CNS treatment modalities with overall survival.
| Unadjusted | Adjusted | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment* | Level | N | % | HR | 95% CI | p-value | HR | 95% CI | p-value |
| Shunt | No | 409 | 96 | ref | ref | ||||
| Yes (time-dependent variable) | 16 | 4 | 4.14 | 2.45–6.99 | <0.0001 | 4.24 | 2.48–7.24 | <0.0001 | |
| Craniotomy | No | 269 | 63 | ref | ref | ||||
| Yes (time-dependent variable) | 156 | 37 | 0.68 | 0.53–0.86 | 0.001 | 0.72 | 0.56–0.93 | 0.0099 | |
| SRS | No | 195 | 46 | ref | ref | ||||
| Yes (time-dependent variable) | 230 | 54 | 0.59 | 0.47–0.74 | <0.0001 | 0.87 | 0.68–1.13 | 0.3 | |
| WBRT | No | 274 | 64 | ref | ref | ||||
| Yes (time-dependent variable) | 151 | 36 | 2.96 | 2.37–3.69 | <0.0001 | 2.65 | 2.08–3.38 | <0.0001 | |
Treatments were analyzed as time-dependent variables, performed at any time during the course of disease.
Discussion:
In this large, retrospective evaluation of contemporary multimodality management of melanoma brain metastases at a large referral cancer center diagnosed between 2010–2019, we identified progressive improvement in overall survival over historical cohorts including from our own institution, and even within the latter half of the cohort studied. The median survival was 8.9 months (95% CI: 7.6–11.2), with a median OS of 13.0 months among patients diagnosed with MBM in 2015–19. One-year survival is estimated at 35.1% (95% CI: 29.1–42.3) for patients diagnosed with MBM in 2010–2014, and 52.4% (95% CI: 45.9–59.8) for those diagnosed in 2015–2019, with a median follow-up of 1.7 years for survivors of the latter group (log-rank test p = 0.0003 across the entire survival distribution). A prior cohort of melanoma patients at MSK from 1991–2001 found a median overall survival of 5.2 months after diagnosis of melanoma BM5. Other historical large institutional cohorts found similar survival rates of 4.1–4.7 months, and do not reflect the current treatment environment which has changed considerably in recent years4,6,7. A recent large national database study revealed improved survival of patients treated with checkpoint blockade as the first initial treatment after MBM diagnosis (12.4 months versus 5.2 months), but was limited by the fact that the database only included patients presenting with MBM at time of initial melanoma diagnosis and did not have data on location, size, or number of BM26. Our empirically identified cutoff for the most pronounced survival improvement between 2014 and 2015 coincides with the FDA approval of PD-1 blockade with nivolumab and pembrolizumab as well as the subsequent approvals of combination ipilimumab/nivolumab, dabrafenib/trametinib, and vemurafenib/cobimetinib. The improved 3-year survival rates in the tail of our cohort, particularly during the 2015–2019 period, are consistent with the increased proportion of longer-term survivors conveyed by recent targeted and immunotherapy trials compared to historical cohorts12,15,27. This improved median survival for MBM patients, however, remains considerably shorter than the years-long overall survival improvements seen in advanced melanoma in general, suggesting that while immunotherapy and targeted therapies may elicit responses in MBM, these responses may not be as frequent or durable as those in the extracranial compartments12,15. It remains unclear whether the increase in overall survival in the age of targeted- and immuno-therapy is due to improved systemic or CNS disease control. In an attempt to investigate this question, we classified patients as having either progressive systemic or CNS disease at time of death, when the data was available. However, in a multivariable analysis, we did not find statistically significant differences in the risk of systemic or CNS progression at time of death between the years 2010–2014 and 2015–2019. Ultimately, these targeted/immunotherapy agents require further investigation and inclusion of patients with MBM in clinical trials. It is likely that these systemic modalities remain poorly efficacious relative to CNS-penetrant strategies seen in select other brain-metastatic malignancies such as EGFR-mutant and ALK-rearranged lung cancers for example28,29.
In addition to the changes in systemic targeted therapies, our cohort demonstrates additional developments in the treatment algorithm for BM compared with prior decades. The use of WBRT has waned due to data suggesting that WBRT, when compared with SRS, causes significant cognitive decline with no significant increase in overall survival despite similar local and improved distant CNS control30–33. SRS is currently increasingly used for patients with >5 BMs in light of these neurocognitive data, improving survival and given that SRS for 5–10 BMs was found to be non-inferior to SRS treatment for 2–4 BMs34. In the Raizer MSK cohort, approximately 53.5% of patients were treated with WBRT compared to 21.9% treated with SRS5. Our current cohort has now seen a reversal of those numbers with 24% of patients undergoing WBRT, 43% SRS, and 11% both, and indeed year of MBM diagnosis predicted CNS radiation modality. It is possible that the increasing use of SRS in combination with immunotherapy as seen in this cohort could have played a role in improved survival through the hypothesized abscopal effect35. Coupling of targeted therapies with stereotactic radiosurgery has also been shown to improve survival in a retrospective analysis36,37. However, the precise roles for SRS and immunotherapy remain controversial given the CNS and extra-CNS efficacy of the latter, and the risk of symptomatic edema requiring corticosteroid use with the former38. It is possible that increasing use of SRS contributed to the observed survival benefit after 2014, however, our institution was an early adopter of SRS for oligometastatic disease and patients were treated with this modality prior to 2014. Surgery has remained a significant component in the treatment of MBM with 37% of patients undergoing craniotomy, which is comparable to 35.5% in the Raizer cohort. Craniotomy was employed for patients with fewer, larger and symptomatic BM, and its association with improved survival upon multivariable analysis can be attributed both to its efficacy, in line with the established literature demonstrating survival and functional benefits for metastasectomy in both the palliative and local-control settings, and its reservation for selected patients motivated for therapy39. Shunting and WBRT both were associated with worse prognosis and overall survival likely due to their use as palliative, end-of-life treatments.
The factors associated with poorer prognosis in this cohort included pre-BM immunotherapy, number of metastases at MBM diagnosis, serum LDH, presence of extracranial disease burden, and leptomeningeal disease. These factors are consistent with prior reports4–7,40,41. The hazard ratio of 1.67 (95% CI: 1.07–2.59) for patients with extracranial disease in the current cohort is similar to the 2.13 in our institution’s prior report5. Leptomeningeal disease had been identified as a poor prognostic factor in prior cohorts, but in the current cohort it was the strongest factor that remained statistically significant in our multivariable analysis5,6. The presence of ≥5 parenchymal metastases was associated with significantly worse survival in this study. This is comparable, although higher than the 3–4 BM cutoffs previously reported5,6. This increase could perhaps be related to increased evidence for and evident use of early SRS prior to WBRT for oligometastatic disease in the last decade34,42. While other analyses have found an association between BRAF mutation and improved survival, our cohort did not identify a similar relationship. This likely can be attributed to the improved survival of BRAF wild-type patients that have been increasing treated with and responding to immunotherapy25. Given the success of immunotherapy and BRAF/MEK inhibition on controlling systemic disease, and the concept of CNS privilege in particular for macromolecules, one might have expected an increase in the number of patients presenting with melanoma BM and no evidence of extracranial disease at time of BM diagnosis. However, the 10% rate of CNS-only disease is lower than our institution’s prior report (16%), and may also reflect the reported CNS efficacy of the targeted agents as discussed above5. Furthermore, treatment with immunotherapy prior to BM diagnosis did not significantly alter the number of BM present at diagnosis, nor did it significantly alter the timeline of development of LMD once diagnosed with BM. It did, however, portend a worse prognosis after diagnosis of MBM, which is not surprising given this scenario is akin to treatment failure of immunotherapy, which has more limited available salvage options43.
Leptomeningeal disease remains a dismal prognostic factor in our cohort, despite the treatment advances for extracranial and CNS parenchymal control. Prior reports describe overall survival of 1.2–4 months after diagnosis of LMD, and the current cohort falls within this range, with an OS of 2.3 months after LMD diagnosis5,6,44. Our cohort excluded four patients with a diagnosis of primary LMD; defined as LMD without parenchymal BM at MBM diagnosis. Primary LMD may represent a separate clinical entity with particularly poor prognosis that requires separate attention and study. However, many patients are diagnosed with LMD over the course of CNS disease. While most LMD diagnoses were made within the first two years after MBM diagnosis, a plateau around 3 years was seen with approximately 15.3% of MBM patients diagnosed at 3 years. A time-dependent analysis found LMD diagnosis to be a strong negative prognostic factor at any time during the course of disease. The effects of small molecule serine-threonine kinase inhibitor therapy and immunotherapy on LMD remain poorly understood given the broad exclusion of patients with LMD from the larger clinical trials, in general. Intrathecal (IT) administration of immunotherapy has been proposed and in the case of IT IL-2 has been suggested to improve survival45. However, systemic administration of immunotherapies after LMD diagnosis has not been demonstrated to significantly benefit patients with LMD except in case reports46. Only four patients with LMD were treated in the combination nivolumab/ipilimumab immunotherapy trial, with none achieving complete response24. Clearly further investigation is necessary to identify treatments that can reduce LMD development or contribute to its control.
This study assessed prognostic factors in patients diagnosed with MBM in the recent decade after the introduction of precision-targeted and immunotherapies. While all patients diagnosed with melanoma BM were included, this study was not designed to assess whether immunotherapy/precision therapies decreased the rate or shifted the timeline of development of BM in patients with advanced melanoma. While ipilimumab and vemurafenib were first FDA-approved in 2011, we included patients from 2010 onwards due to our institution’s early involvement in the clinical trials for these therapies. Given the heterogeneity of the cohort at diagnosis and its retrospective nature, this study was also not designed to compare the effectiveness of treatment paradigms. In particular, we did not specifically assess the effects of BRAF/MEK inhibition due to its application to only a smaller subset of patients. Furthermore, the focus of this study was to describe the treatment and prognosis of all melanoma BM patients. The study may be biased by its limitation to a single institution, however, is aided by the institution’s early involvement in immunotherapy and targeted therapy trials for the metastatic melanoma population and large patient population. This single-institution study also provided a unique opportunity to compare outcomes with a similarly-sized cohort at the same institution a decade prior5. Going forward, however, it will be valuable to assess these prognostic factors in a validation cohort from other institutions.
Conclusions:
This study demonstrates that the prognosis of melanoma BM has improved compared to historical cohorts and even within the later time period studied herein. The number of BM at diagnosis, systemic disease burden, and presence of leptomeningeal disease are important prognostic indicators and can guide patient counseling. As treatment paradigms continue to evolve, both CNS-directed and systemic trials should be open to and accruing melanoma BM patients in order to understand treatment efficacy on this morbid, difficult-to-treat and increasingly prevalent disease stage, and to continue improving their prognosis.
Supplementary Material
Supplemental Figure 1. Kaplan Meier estimate of overall survival from time of LMD diagnosis (A). Cumulative incidence of LMD over time (dotted lines represent 95% CI) (B). Cumulative incidence of LMD and death events stratified by treatment with immunotherapy prior to MBM diagnosis (C).
Supplemental Figure 2. Timing of CNS-directed intracranial treatments represented over time for all patients in the cohort (A), patients with MBM diagnosis between 2010–2014 (B), and patients with MBM diagnosis between 2015–2019 (C).
Funding:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Conflicts of Interest:
At the time of research execution and publication, all authors are affiliated with Memorial Sloan Kettering Cancer Center. The authors of this research deny any conflicts of interest regarding this study, and make the following disclosures:
NSM: Consulting fees for advisory board participation: AstraZeneca
MAP: Consulting fees for advisory board participation: BMS, Merck, Array BioPharma, Novartis, Incyte, NewLink Genetics, Aduro; Honoraria: BMS, Merck; Institutional Support: RGenix, Infinity, BMS, Merck, Array BioPharma, Novartis, AstraZeneca
References
- 1.Schouten LJ, Rutten J, Huveneers HAM, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698–2705. doi: 10.1002/cncr.10541 [DOI] [PubMed] [Google Scholar]
- 2.Smedby KE, Brandt L, Bäcklund ML, Blomqvist P. Brain metastases admissions in Sweden between 1987 and 2006. Br J Cancer. 2009;101(11):1919–1924. doi: 10.1038/sj.bjc.6605373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22(14):2865–2872. doi: 10.1200/JCO.2004.12.149 [DOI] [PubMed] [Google Scholar]
- 4.Sampson JH, Carter JH, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88(1):11–20. doi: 10.3171/jns.1998.88.1.0011 [DOI] [PubMed] [Google Scholar]
- 5.Raizer JJ, Hwu W-J, Panageas KS, et al. Brain and leptomeningeal metastases from cutaneous melanoma: Survival outcomes based on clinical features. Neuro-Oncol. 2008;10(2):199–207. doi: 10.1215/15228517-2007-058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011;117(8):1687–1696. doi: 10.1002/cncr.25634 [DOI] [PubMed] [Google Scholar]
- 7.Fife KM, Colman MH, Stevens GN, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22(7):1293–1300. doi: 10.1200/JCO.2004.08.140 [DOI] [PubMed] [Google Scholar]
- 8.Frinton E, Tong D, Tan J, et al. Metastatic melanoma: prognostic factors and survival in patients with brain metastases. J Neurooncol. 2017;135(3):507–512. doi: 10.1007/s11060-017-2591-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliva IG, Tawbi H, Davies MA. Melanoma brain metastases: current areas of investigation and future directions. Cancer J Sudbury Mass. 2017;23(1):68–74. doi: 10.1097/PPO.0000000000000237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasquali S, Hadjinicolaou AV, Chiarion Sileni V, Rossi CR, Mocellin S. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev. 2018;2:CD011123. doi: 10.1002/14651858.CD011123.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schadendorf D, Hodi FS, Robert C, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33(17):1889–1894. doi: 10.1200/JCO.2014.56.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381(16):1535–1546. doi: 10.1056/NEJMoa1910836 [DOI] [PubMed] [Google Scholar]
- 13.Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1480–1492. doi: 10.1016/S1470-2045(18)30700-9 [DOI] [PubMed] [Google Scholar]
- 14.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377(14):1345–1356. doi: 10.1056/NEJMoa1709684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robert C, Grob JJ, Stroyakovskiy D, et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N Engl J Med. 2019;381(7):626–636. doi: 10.1056/NEJMoa1904059 [DOI] [PubMed] [Google Scholar]
- 16.Robert C, Karaszewska B, Schachter J, et al. Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib. N Engl J Med. 2015;372(1):30–39. doi: 10.1056/NEJMoa1412690 [DOI] [PubMed] [Google Scholar]
- 17.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–164. doi: 10.1016/S1470-2045(09)70334-1 [DOI] [PubMed] [Google Scholar]
- 18.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. 10.1056/NEJMoa1302369. doi: 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman PB. Changing the standard of care for treating melanoma brain metastases. Lancet Oncol. 2018;19(5):589–591. doi: 10.1016/S1470-2045(18)30187-6 [DOI] [PubMed] [Google Scholar]
- 20.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–465. doi: 10.1016/S1470-2045(12)70090-6 [DOI] [PubMed] [Google Scholar]
- 21.Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17(7):976–983. doi: 10.1016/S1470-2045(16)30053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tawbi HA, Forsyth PA, Algazi A, et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N Engl J Med. 2018;379(8):722–730. doi: 10.1056/NEJMoa1805453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18(7):863–873. doi: 10.1016/S1470-2045(17)30429-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672–681. doi: 10.1016/S1470-2045(18)30139-6 [DOI] [PubMed] [Google Scholar]
- 25.Sperduto PW, Jiang W, Brown PD, et al. Estimating Survival in Melanoma Patients With Brain Metastases: An Update of the Graded Prognostic Assessment for Melanoma Using Molecular Markers (Melanoma-molGPA). Int J Radiat Oncol Biol Phys. 2017;99(4):812–816. doi: 10.1016/j.ijrobp.2017.06.2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iorgulescu JB, Harary M, Zogg CK, et al. Improved Risk-Adjusted Survival for Melanoma Brain Metastases in the Era of Checkpoint Blockade Immunotherapies: Results from a National Cohort. Cancer Immunol Res. 2018;6(9):1039–1045. doi: 10.1158/2326-6066.CIR-18-0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korn EL, Liu P-Y, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(4):527–534. doi: 10.1200/JCO.2007.12.7837 [DOI] [PubMed] [Google Scholar]
- 28.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non–Small-Cell Lung Cancer. N Engl J Med. 2017;377(9):829–838. doi: 10.1056/NEJMoa1704795 [DOI] [PubMed] [Google Scholar]
- 29.Ballard P, Yates JW, Yang Z, et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin Cancer Res. Published online January 1, 2016. doi: 10.1158/1078-0432.CCR-16-0399 [DOI] [PubMed] [Google Scholar]
- 30.Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049–1060. doi: 10.1016/S1470-2045(17)30441-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. doi: 10.1016/S1470-2045(09)70263-3 [DOI] [PubMed] [Google Scholar]
- 32.Brown PD, Jaeckle K, Ballman KV, et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA. 2016;316(4):401–409. doi: 10.1001/jama.2016.9839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(2):134–141. doi: 10.1200/JCO.2010.30.1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387–395. doi: 10.1016/S1470-2045(14)70061-0 [DOI] [PubMed] [Google Scholar]
- 35.Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18(5):313–322. doi: 10.1038/nrc.2018.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knisely JPS, Yu JB, Flanigan J, Sznol M, Kluger HM, Chiang VLS. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg. 2012;117(2):227–233. doi: 10.3171/2012.5.JNS111929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed KA, Abuodeh YA, Echevarria MI, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiosurgery and anti-PD-1 therapy, anti-CTLA-4 therapy, BRAF/MEK inhibitors, BRAF inhibitor, or conventional chemotherapy. Ann Oncol Off J Eur Soc Med Oncol. 2016;27(12):2288–2294. doi: 10.1093/annonc/mdw417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin AM, Cagney DN, Catalano PJ, et al. Immunotherapy and Symptomatic Radiation Necrosis in Patients With Brain Metastases Treated With Stereotactic Radiation. JAMA Oncol. 2018;4(8):1123–1124. doi: 10.1001/jamaoncol.2017.3993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patchell RA, Tibbs PA, Walsh JW, et al. A Randomized Trial of Surgery in the Treatment of Single Metastases to the Brain. N Engl J Med. 1990;322(8):494–500. doi: 10.1056/NEJM199002223220802 [DOI] [PubMed] [Google Scholar]
- 40.Petrelli F, Ardito R, Merelli B, et al. Prognostic and predictive role of elevated lactate dehydrogenase in patients with melanoma treated with immunotherapy and BRAF inhibitors: a systematic review and meta-analysis. Melanoma Res. 2019;29(1):1–12. doi: 10.1097/CMR.0000000000000520 [DOI] [PubMed] [Google Scholar]
- 41.Long GV, Grob J-J, Nathan P, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol. 2016;17(12):1743–1754. doi: 10.1016/S1470-2045(16)30578-2 [DOI] [PubMed] [Google Scholar]
- 42.Mazzola R, Corradini S, Gregucci F, Figlia V, Fiorentino A, Alongi F. Role of Radiosurgery/Stereotactic Radiotherapy in Oligometastatic Disease: Brain Oligometastases. Front Oncol. 2019;9. doi: 10.3389/fonc.2019.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schvartsman G, Ma J, Bassett RL, et al. Incidence, patterns of progression, and outcomes of preexisting and newly discovered brain metastases during treatment with anti-PD-1 in patients with metastatic melanoma. Cancer. 2019;125(23):4193–4202. doi: 10.1002/cncr.32454 [DOI] [PubMed] [Google Scholar]
- 44.Ferguson SD, Bindal S, Bassett RL, et al. Predictors of survival in metastatic melanoma patients with leptomeningeal disease (LMD). J Neurooncol. 2019;142(3):499–509. doi: 10.1007/s11060-019-03121-2 [DOI] [PubMed] [Google Scholar]
- 45.Glitza IC, Rohlfs M, Guha-Thakurta N, et al. Retrospective review of metastatic melanoma patients with leptomeningeal disease treated with intrathecal interleukin-2. ESMO Open. 2018;3(1). doi: 10.1136/esmoopen-2017-000283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smalley KSM, Fedorenko IV, Kenchappa R, Sahebjam S, Forsyth PA. Managing leptomeningeal melanoma metastases in the era of immune and targeted therapy. Int J Cancer. 2016;139(6):1195–1201. doi: 10.1002/ijc.30147 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Kaplan Meier estimate of overall survival from time of LMD diagnosis (A). Cumulative incidence of LMD over time (dotted lines represent 95% CI) (B). Cumulative incidence of LMD and death events stratified by treatment with immunotherapy prior to MBM diagnosis (C).
Supplemental Figure 2. Timing of CNS-directed intracranial treatments represented over time for all patients in the cohort (A), patients with MBM diagnosis between 2010–2014 (B), and patients with MBM diagnosis between 2015–2019 (C).


