Abstract
Physicochemical and biological water quality, including the microcystin concentration, was investigated from spring to autumn 1999 in the Daechung Reservoir, Korea. The dominant genus in the cyanobacterial blooming season was Microcystis. The microcystin concentration in particulate form increased dramatically from August up to a level of 200 ng liter−1 in early October and thereafter tended to decrease. The microcystin concentration in dissolved form was about 28% of that of the particulate form. The microcystins detected using a protein phosphatase (PP) inhibition assay were highly correlated with those microcystins detected by a high-performance liquid chromatograph (r = 0.973; P < 0.01). Therefore, the effectiveness of a PP inhibition assay for microcystin detection in a high number of water samples was confirmed as easy, quick, and convenient. The microcystin concentration was highly correlated with the phytoplankton number (r = 0.650; P < 0.01) and chlorophyll-a concentration (r = 0.591; P < 0.01). When the microcystin concentration exceeded about 100 ng liter−1, the ratio of particulate to dissolved total nitrogen (TN) or total phosphorus (TP) converged at a value of 0.6. Furthermore, the microcystin concentration was lower than 50 ng liter−1 at a particulate N/P ratio below 8, whereas the microcystin concentration varied quite substantially from 50 to 240 ng liter−1 at a particulate N/P ratio of >8. Therefore, it seems that the microcystin concentration in water can be estimated and indirectly monitored by analyzing the following: the phytoplankton number and chlorophyll-a concentration, the ratio of the particulate and the dissolved forms of N and P, and the particulate N/P ratio when the dominant genus is toxigenic Microcystis.
Blooms of the cyanobacterium Microcystis aeruginosa are ubiquitous phenomena in eutrophic lakes and reservoirs in many countries of the world. Many strains of Microcystis are known to produce cyanobacterial hepatotoxins called microcystins (MC). These toxins are soluble peptides and are lethal to many kinds of aquatic organisms (2, 23, 28). MC are found in strains of the genera Microcystis, Oscillatoria, Anabaena, and Nostoc (26). To date, at least 69 MC have been structurally characterized (9).
The Daechung reservoir located in the middle of South Korea was formed by the construction of a multipurpose dam in 1980 to conserve water resources for drinking, agricultural, and industrial use and for electric power supply. Since the end of the 1980s, the reservoir has shown some eutrophic phenomena, such as cyanobacterial blooms, in the summer and a deterioration in water quality. With the appearance of cyanobacterial blooms, the production of cyanobacterial toxins, particularly MC, becomes a threat to human health and natural resources (10, 20, 22). Therefore, the ability to detect and predict MC in water resources is very important.
Normally, a high-performance liquid chromatography (HPLC) analysis is used for the detection and qualification of MC in water (7, 8, 9). However, this method has certain weaknesses in that it usually requires a concentration process and is only feasible in a laboratory equipped with an HPLC system. Recently, a protein phosphatase (PP) inhibition assay was introduced for detecting MC in water and algal samples. The PP inhibition assay for MC consists of measuring the release of acid-soluble 32P from 32P-labeled glycogen phosphorylase (16) or a colorimetric assay utilizing the ability of PP-1 to dephosphorylate p-nitrophenyl phosphate (1, 27). Although some research has been carried out to compare the results from the HPLC and PP inhibition assays (30), a clear relationship between these methods has not yet been established.
MC production by cyanobacteria results from cyanobacterial blooms caused by an abundance of nutrients and favorable conditions for cyanobacterial growth. Changing environmental factors in a water system will have an impact on the MC concentration. Chlorophyll-a would appear to be useful for an initial estimate of a MC concentration in field situations dominated by potentially MC-producing genera (6, 15). The relationships between MC concentration and the N and P concentrations in water have already been studied (11, 12, 24, 25, 29). However, the development of proper parameters, including the ratios of particulate N to P and particulate to dissolved N or P, to estimate MC concentrations is still needed to improve the ability to manage water quality.
Accordingly, this study monitored the changes in the MC concentration in water and algal samples taken every week during the period of cyanobacterial blooms. In addition, methods for detecting MC were evaluated for ease and convenience, along with an indirect monitoring method for estimating MC concentrations in eutrophic waters.
MATERIALS AND METHODS
Sampling and field survey.
The Daechung Reservoir is located on the upper part of the Geum River in the central region of South Korea. This reservoir is a large branch-type lake with a 72-m-high dam and a gross storage capacity of 1,490 Mm3. The reservoir is mainly subject to agricultural runoff. The sampling site was located on the shore in the vicinity of the Daechung Reservoir dam. The depth of the sampling site was about 20 m. The sampling was conducted weekly from the same site from 27 April to 12 October 1999. In total, the sampling was conducted 25 times from spring to autumn. The water temperature and Secchi depth of the sampling site were measured with portable instruments (YSI model 95; Secchi disk). The samples for water analysis were collected at a depth of 0 to 0.1 m using a Van Dorn water sampler (WILDCO Instruments) and stored in 20-liter polyethylene bottles at 4°C until the laboratory analysis was done. The samples for plankton identification and enumeration were preserved in Lugol's solution.
Physicochemical water quality analysis.
A 500-ml portion of each water sample was centrifuged for 10 min at 15,000 × g (Sorvall model RC5C). The supernatant was then used to analyze the levels of dissolved N and P. The pellets, including any particulate material, were washed with distilled water followed by centrifugation and stored at −65°C for further analysis. The particulate C, which was mainly composed of cellular C, was determined with a total organic carbon analyzer (Shimadzu model 5000A). The total N (TN) and P (TP) were determined after persulfate oxidation to nitrate (3) and orthophosphate (17), respectively. The nitrate was determined with a Szechrome NB reagent (33), and the orthophosphate was determined by the phosphomolybdate method (18).
Biological water quality analysis.
Chlorophyll-a was extracted using a chloroform-methanol mixture (2:1 [vol/vol]) and measured with a fluorometer (Turner model 450) (31). The phycocyanin was analyzed using the whole-cell absorption spectrum method (19). The cell pellet was extracted twice in 80% acetone and then resuspended in a 0.2 M sodium acetate buffer (pH 5.5). The absorbance was measured at 625, 678, and 725 nm. The phytoplankton and zooplankton were enumerated with a hemocytometer (Fuchs-Rosenthal Ultra Plane; Hausser Scientific) under a phase-contrast microscope (MICROPHOT-FXA; Nikon).
MC analysis by HPLC.
Algal cells from natural samples were collected and concentrated using a Whatman GF/C filter. Those filter papers containing algal cells were lyophilized and stored at −20°C until the chemical analysis was performed. The analytical procedure, as previously reported by Harada et al. (8), was as follows. The lyophilized algal cells were extracted three times with 50 ml of 5% (vol/vol) acetic acid for 30 min. The extract was then centrifuged at 9,300 × g, and the supernatant was passed through a C18 cartridge (Sep-Pak; Waters Assoc.). The cartridge containing MC was first rinsed with 10 ml of water, followed by 10 ml of 10% (vol/vol) methanol in water. The MC adsorbed on the cartridge were finally eluted with 10 ml of methanol. The eluate was then evaporated under reduced pressure below 40°C, and the residue, dissolved in methanol, was subjected to an HPLC analysis (CLASS-LC10; Shimadzu). An HPLC equipped with a constant-flow pump was used with a variable-wavelength UV detector operated at 238 nm. The separation was performed with a Nucleosil C18 column (5 μm; 150-by 4.6-mm inside diameter) with a mobile-phase, methanol–0.05 M phosphate buffer (57:43; pH 3.0) at a flow rate of 1.0 ml min−1. The MC were identified based on their UV spectra and retention times (14) and by spiking the sample with a purified standard of MC-LR, -RR, and -YR (Sigma, St. Louis, Mo.). In addition, the MC peaks were isolated and identified according to their mass spectra. The recoveries of MC-RR and -LR were 93.2% ± 3.1% and 92.7% ± 2.9% 3 h after the MC were spiked within a range of 20 to 200 ng liter−1. Each analysis was performed in triplicate.
MC analysis by PP inhibition assay.
An aliquot (10 ml) of the natural sample was filtered with a Whatman GF/C filter. The filtrate was then directly subjected to a PP inhibition assay. The algal cells were lyophilized, extracted, and purified as described above. After concentration, the residue was dissolved in 10 ml of distilled water and subjected to the PP inhibition assay. The PP activity was determined with some modifications as previously described by Lambert et al. (13). The 32P-labeled phosphorylase-a was prepared using a commercial kit from Gibco BRL (Gaithersburg, Md.). To determine the PP inhibition activity of the algal cells, 100 ml of each water sample was filtered with a Whatman GF/C filter. The filter papers were frozen at −70°C, extracted with 5% acetic acid, purified with Sep-Pak cartridges, and then diluted with a buffer (pH 7.6) containing 20 mM imidazole-HCl, 0.1 mM EDTA, 1 mg liter−1 bovine serum albumin and 0.1% (vol/vol) β-mercaptoethanol.
The dephosphorylation was conducted by mixing 20 μl of each water sample with 20 μl of PP-1 and 20 μl of the 32P-labeled phosphorylase-a. The samples were then incubated for 30 min at 30°C, and the reaction was stopped by the addition of 180 μl of 20% trichloroacetic acid. The samples were kept on ice for 10 min and centrifuged at 15,000 × g for 3 min, and then the supernatants were collected. The PP activity of the sample was taken as the radioactivity released in the supernatant as determined by a liquid scintillation counter (Beckman model 6000A).
MC-RR was used for the preparation of standard curves by PP inhibition assay, because it was most frequently detected in the lakes and rivers of Korea. The inhibition of PP-1 by MC-RR has been shown to have a sigmoid response curve when percent activity of PP-1 is plotted versus log MC-RR concentrations ranging from 10−8 to 10−11 M. The PP-1 activities of all samples, standard MC-RR and sample, were calculated by the equation percent PP-1 activity = 100 − [(sample cpm − blank cpm) × 100%/(control cpm − blank cpm)], where (i) control cpm is the maximum activity of the PP-1 enzyme (100% activity without MC-RR) and (ii) blank cpm is the free [32P]phosphate in the phosphorylase-a solution.
Statistical analysis.
The particulate-to-dissolved TN and TP ratios as a function of the MC concentrations were curve fitted with either exponential-decay or exponential-rise equations using SigmaPlot 5.0 software (SPSS Inc., Chicago, Ill.). Repeated measurements for the variance analysis (P < 0.05) were used to evaluate the significance of these experimental results.
RESULTS
Changes in phytoplankton numbers and chlorophyll-a concentration.
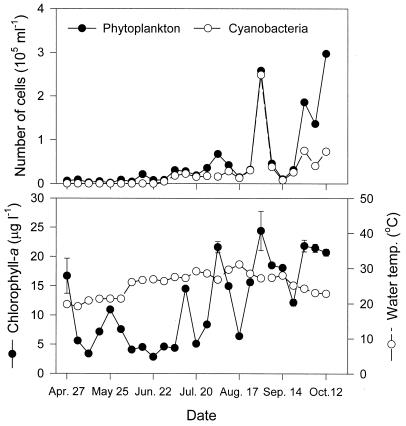
Changes in the phytoplankton density and chlorophyll-a concentration, as an indicator of algal biomass, were investigated for 6 months, from April to October 1999, in the Daechung Reservoir (Fig. 1). The number of phytoplankton increased dramatically up to 2 × 105 to 3 × 105 ml−1 in August and October. In August, the dominant species were cyanobacteria, which were composed of Microcystis spp. (47%), Anabaena spp. (39%), and Oscillatoria spp. (6%). In October, the phytoplankton numbers were still high, but the dominant species changed to diatoms.
FIG. 1.
Number of phytoplankton and cyanobacteria (top) and chlorophyll-a concentration and water temperature (bottom) in Daechung Reservoir. Sampling was carried out at 1-week intervals from 27 April to 12 October 1999. The error bars indicate standard deviation (n = 3).
The chlorophyll-a concentration varied within a range of 3 to 24 μg liter−1 and reflected the number of phytoplankton. The maximum chlorophyll-a concentration was recorded at 24 μg liter−1 on August 31 and was concomitant with a cyanobacterial number of 2.5 × 105 ml−1. The water temperature, which is an important factor in supporting algal growth, varied from 19°C in the spring to 31°C in the summer and gradually decreased in the autumn.
Seasonal variation of MC concentration.
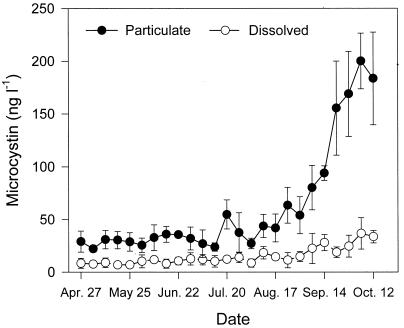
The MC concentrations in water and algal samples were analyzed by a PP inhibition assay. The variation in the MC concentrations in the dissolved and particulate samples is shown in Fig. 2. Measurable MC levels were detected in dissolved and particulate fractions in all the examined samples. In particular, from the beginning of August, the particulate MC concentration increased dramatically up to a level of 200 ng liter−1 in the beginning of October; thereafter, it tended to decrease, judging from the measurements of not only MC concentration but also cyanobacterial numbers, chlorophyll-a concentration, and water temperature. The particulate MC were highly correlated with the dissolved MC in the water body (Y = 5.88X − 24.90; r = 0.891; P < 0.01). The dissolved MC composed about 28% of the particulate form.
FIG. 2.
MC concentration of particulate and dissolved forms in Daechung Reservoir. Sampling was carried out at 1-week intervals from 27 April to 12 October 1999. The error bars indicate standard deviation (n = 3).
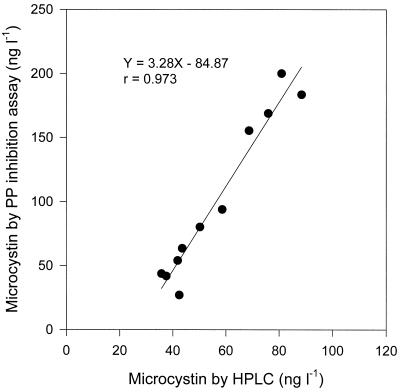
MC analysis by HPLC has low sensitivity. Therefore, the HPLC method is normally used for relatively high concentrations of MC; otherwise, it is necessary to concentrate a water sample to the detectable level for MC. The water samples collected between August and October contained sufficient MC to be analyzed by HPLC. MC concentrations analyzed using HPLC were compared with the results of the PP inhibition assay (Fig. 3). The MC were composed of MC-LR and -RR. MC-RR was the main component, and MC-YR was not detected at all. The MC concentrations detected by the PP inhibition assay were highly correlated with those determined by HPLC (Y = 3.28X − 84.87; r = 0.973; P < 0.01). However, the MC concentrations measured by the HPLC method were only about 70% of those measured by the PP inhibition assay.
FIG. 3.
Relationship between MC measured by HPLC and PP inhibition assay in Daechung Reservoir.
Relationship between MC concentration and algal biomass, water quality, and zooplankton numbers.
Certain physicochemical and biological indicators of water quality that appeared to be related to the MC concentration were analyzed for their statistical relationships (Table 1). The total MC concentration was highly correlated with the chlorophyll-a concentration (r = 0.591; P < 0.01) and phytoplankton density (r = 0.650; P < 0.01). The chlorophyll-a concentration was highly correlated with the number of phytoplankton, cyanobacteria, and Microcystis (P < 0.01) and Anabaena (P < 0.05) organisms.
TABLE 1.
Correlation among water quality parameters related to microcystin concentration in water
| No. | Parameter (unit) | Correlationa
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| 1 | Secchi depth (m) | 1.000 | |||||||||
| 2 | Chlorophyll-a (μg/liter) | −0.484∗ | 1.000 | ||||||||
| 3 | Phycocyanin (μM) | −0.311 | 0.642∗∗ | 1.000 | |||||||
| 4 | Particulate carbon (mg/liter) | −0.416∗ | 0.270 | 0.050 | 1.000 | ||||||
| 5 | Zooplankton (per milliliter) | −0.258 | 0.502∗ | 0.428∗ | −0.016 | 1.000 | |||||
| 6 | Phytoplankton (per milliliter) | −0.439∗ | 0.699∗∗ | 0.601∗∗ | 0.062 | 0.510∗∗ | 1.000 | ||||
| 7 | Cyanobacteria (per milliliter) | −0.351 | 0.611∗∗ | 0.770∗∗ | 0.062 | 0.192 | 0.801∗∗ | 1.000 | |||
| 8 | Microcystis (per milliliter) | −0.290 | 0.581∗∗ | 0.800∗∗ | 0.038 | 0.084 | 0.662∗∗ | 0.944∗∗ | 1.000 | ||
| 9 | Anabaena (per milliliter) | −0.280 | 0.484∗ | 0.730∗∗ | 0.049 | 0.065 | 0.642∗∗ | 0.966∗∗ | 0.911∗∗ | 1.000 | |
| 10 | Microcystin (ng/liter) | −0.370 | 0.591∗∗ | 0.306 | 0.035 | 0.403 | 0.650∗∗ | 0.311 | 0.221 | 0.132 | 1.000 |
∗, P < 0.05; ∗∗, P < 0.01.
The standing crops of zooplankton were within a range of 7 individuals liter−1 on May 25 to 390 individuals liter−1 on October 12 and showed a mean value of 93 individuals liter−1 over the period of investigation. The dominant species of zooplankton were Tintinnopsis cratera, Polyarthra trigla, and Cyclops strenuus. The number of zooplankton as primary consumers in an aquatic ecosystem was correlated with the chlorophyll-a concentration and also the phycocyanin concentration. The phycocyanin concentration showed a high correlation with the number of cyanobacteria and Microcystis and Anabaena organisms.
The Secchi depth, as an easy way to determine the trophic status of a body of water, was within a range of 2.3 m on August 3 to 6.5 m on May 18. The Secchi depth was negatively correlated with all the other water qualities examined, which were either directly or indirectly related with the algal biomass. In particular, the Secchi depth was highly negatively correlated with the chlorophyll-a concentration, phytoplankton density, and particulate C.
Relationship between MC concentrations and nutrients.
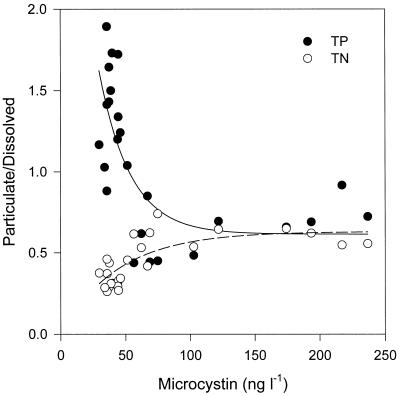
The ratio of particulate to dissolved TP or TN was expressed as a function of the MC concentration in water (Fig. 4). In the case of TP, the ratio of particulate to dissolved TP decreased dramatically with increasing MC concentration up to about 100 ng of MC liter−1. Interestingly, when the MC concentration exceeded about 100 ng liter−1, the ratio of particulate to dissolved TP converged at a value of 0.6.
FIG. 4.
Relationship between MC concentration and particulate-to-dissolved TP and TN in Daechung Reservoir.
In contrast, the ratio of particulate to dissolved TN increased with increasing MC concentration up to about 100 ng of MC liter−1. When the MC concentration exceeded about 100 ng liter−1, the ratio of particulate to dissolved TN converged at a value of 0.6. Therefore, it would appear that the ratio of particulate to dissolved N or P at 0.6 is the threshold value for determining high or low MC concentration. In other words, the MC concentration was lower than 100 ng liter−1 when the ratio of particulate to dissolved TP was high while the particulate-to-dissolved TN ratio was low.
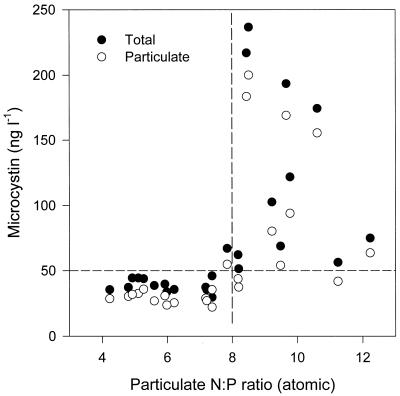
The N/P atomic ratio in particulate form, which was mainly composed of phytoplankton, varied within a range of 4 to 12 in this body of water (Fig. 5). The MC concentration in the water varied with the particulate N/P ratio. That is, at a particulate N/P ratio under 8, the MC concentration was lower than 50 ng liter−1, whereas at a particulate N/P ratio of >8, the MC concentration varied quite substantially, from 50 to 240 ng liter−1. In other words, the level of the MC concentration was also determined to some extent by the particulate N/P ratio.
FIG. 5.
MC concentration as a function of particulate N/P atomic ratio in total (dissolved plus particulate) and particulate form in Daechung Reservoir.
The change of MC in particulate and total forms relative to the N/P ratio also showed a similar pattern. Consequently, an increase in the N concentration in the cells exhibited a potential increase in the MC concentration in the cells and also in the body of water.
DISCUSSION
Trophic state of Daechung Reservoir.
According to Organization for Economic Cooperation and Development guidelines (21), 8 μg of chlorophyll liter−1 is considered the boundary between mesotrophy and eutrophy. The chlorophyll-a concentration exceeded 8 μg liter−1 with a frequency of 56% (14 out of the 25) in the samples examined. The chlorophyll-a concentrations in the samples taken in August, September, and October, except for one sample, were particularly high, within a range of 12 to 24 μg liter−1. From these results, it would appear that the Daechung Reservoir is in a eutrophic state and that this phenomenon is severe in summer and early autumn.
The maximum chlorophyll-a concentration was recorded at 24 μg liter−1 on August 31 and was concomitant with an increase in cyanobacterial numbers (Fig. 1). That is, the chlorophyll-a concentration was highly correlated with phytoplankton numbers (r = 0.699; P < 0.01) and cyanobacterial numbers (r = 0.611; P < 0.01) (Table 1). Therefore, it can be concluded that the chlorophyll-a concentration in the Daechung reservoir is mainly determined by the number of cyanobacteria in the phytoplankton.
Variation of MC concentration.
After August, the MC concentration in particulate form increased dramatically, up to 200 ng liter−1, while the dissolved MC did not vary much (Fig. 2). Water safety guidelines have been proposed with 1 μg of cyanobacterial peptide toxins liter−1 as the maximum concentration in drinking water (4). Recently, this guideline was also proposed by the World Health Organization (32). Therefore, it would appear that the MC concentration in the Daechung Reservoir is much lower than the level that would cause concern. In a previous report (10), MC-RR was identified as the main component of MC variants in Korean reservoirs, and the total content of MC varied from 288 to 2,612 μg g−1, which corresponds to a concentration per water volume of 0.4 to 21.6 μg liter−1.
The MC concentrations in dissolved form were about 28% of those in particulate form. In related research, the high percentage of extracellular MC in filtered lake water (>20%) at the end of the bloom period would seem to suggest that the release of MC occurs during the senescence and decomposition periods of Microcystis cells (22). Dissolved MC have seldom been detected in water during cyanobacterial blooms, and if detected, the concentrations have been very low compared to the toxins in the particulate matter (12). In particular, in the season of severe cyanobacterial blooms, most MC were in particulate form (Fig. 2). Based on these results, the removal of particles, including algal cells, from water resources is recommended to effectively reduce the risk of MC in drinking water.
Comparison of methods for MC analysis.
The MC concentrations detected by the PP inhibition assay were highly correlated with those detected by HPLC (Fig. 3). Similarly, in other research, the MC content of field samples from German lakes estimated on the basis of a PP-1 inhibition assay was compared to that obtained by a reversed-phase HPLC, and a good correlation was observed (r = 0.9202; P < 0.0001) (30). Therefore, it is apparent that a PP inhibition assay is a quick, easy, and convenient method to use when screening many water samples for MC. For example, about 300 samples can be analyzed within a day. In contrast, the HPLC assay is also needed to gain more quantitative and qualitative information on the MC in the water body.
The MC concentrations detected by the PP inhibition assay were about 1.4 times higher than those detected by HPLC. This is the opposite of the result of Wirsing et al. (30), who reported that the colorimetric PP-1 assay has a tendency to slightly underestimate the MC content in cyanobacterial samples compared with the reversed-phase HPLC results. In contrast, the hepatotoxicity measured in rat bioassays by other investigators (5) was usually two to three times higher than the maximal toxicity predicted from their MC contents. It is also probable that other substances in either the algae or their environment may cause reactions that are synergistic with or additive to MC.
In this study, MC were detected in all the samples examined from spring to autumn. There are two possibile reasons for this phenomenon. First, MC-producing cyanobacteria existed in all the examined samples during the survey period and produced MC. Second, due to an unknown factor, the PP inhibition analysis used in this work overestimated the MC content in the water and cells.
Indirect monitoring of MC.
MC analysis is a necessary process to determine the safety of drinking water and water resources; however, it is a somewhat complex process when using an HPLC or isotopes and requires much time. Therefore, there is current interest in the development of a simpler and more convenient method for estimating the MC concentration in water. After this process, further analysis with an HPLC or isotopes can be carried out with selected samples to gain more detailed information. This strategy would seem to be more profitable for field samples.
MC production by cyanobacteria during blooms is likely related to the abundance of nutrients and favorable conditions for cyanobacterial growth. Changes in the environmental factors in a water system will have an impact on the composition of both the algal species and the cellular components. As a result, the MC concentration will also change relative to the water quality, nutrient distribution, and cellular components.
First of all, the MC concentration was highly correlated with phytoplankton numbers (r = 0.650; P < 0.01) and chlorophyll-a concentration (r = 0.591; P < 0.01). Similarly, in other research, the seasonal changes in the MC-LR concentrations were found to be positively correlated to the abundance and biomass of the cyanobacterium Microcystis aeruginosa, the total and total dissolved P concentrations, the pH, and chlorophyll-a (11). Therefore, it would seem plausible that an initial estimation of the existence of MC based on phytoplankton numbers and the chlorophyll-a concentration can be used to evaluate the quality of water for drinking.
The MC concentrations at a higher ratio (>0.6) of particulate to dissolved TP were lower than 100 ng liter−1. Interestingly, where the MC concentrations exceeded about 100 ng liter−1, the ratio of particulate to dissolved TN or TP converged at a value of 0.6. Rapala and Sivonen (24) also suggested that the concentrations of dissolved inorganic N and P may regulate the species and strain composition and, hence, the toxicity of a bloom. Rapala et al. (25) suggested that MC concentrations in different cyanobacterial genera respond similarly to extracellular P concentrations: not only cyanobacterial growth but also the amounts of intracellular hepatotoxins increase with the P concentration. The most significant discriminating factors between the different types of blooms were the concentrations of dissolved PO4-P and NO3-N (24). The hepatotoxic Microcystis blooms exhibited the highest concentrations of PO4-P.
In addition, the MC concentration in the water varied with the particulate N/P ratio. That is, at a particulate N/P ratio under 8, the MC concentration was lower than 50 ng liter−1, whereas at a particulate N/P ratio of >8, the MC concentration varied quite substantially, from 50 ng to 240 ng liter−1. The amount of toxin in the particulate material correlated positively with the cyanobacterial biomass as well as with the TN and TP concentrations in the water (12). In other words, the level of the MC concentration was also determined to some extent by the particulate N/P ratio.
It is very important to monitor the water quality of lakes and reservoirs that are sources for drinking water. The toxin microcystin comes from cyanobacteria and is dominant in an environment where cyanobacteria grow well. Therefore, the basic water quality must be routinely examined to provide data so that analyses of the presence and concentration of MC can be conducted, thereby providing information on water safety. In order to gain more detailed, accurate information on the kinds and concentrations of toxins present, a further analysis of MC by using an HPLC assay should be conducted with selected samples identified as containing particularly high toxin concentrations.
ACKNOWLEDGMENTS
This work was supported by the Korean Ministry of Science and Technology (MOST).
We are also grateful to anonymous reviewers for their valuable comments on this study and to Lorne Hwang for her careful reading of the manuscript.
REFERENCES
- 1.An J, Carmichael W W. Use of a colorimetric protein phosphatase inhibition assay and enzyme linked immunosorbent assay for the study of microcystins and nodularins. Toxicon. 1994;32:1495–1507. doi: 10.1016/0041-0101(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 2.Codd G A, Poon G K. Cyanobacterial toxins. In: Rogers L J, Gallon J R, editors. Biochemistry of the algae and cyanobacteria. Oxford, United Kingdom: Clarendon Press; 1988. pp. 283–296. [Google Scholar]
- 3.D'Elia C F, Steudler P A, Corwin N. Determination of total nitrogen in aqueous samples using persulfate digestion. Limnol Oceanogr. 1977;22:760–764. [Google Scholar]
- 4.Falconer I R. Health problems from exposure to cyanobacteria and proposed safety guidelines for drinking and recreational water. In: Codd G A, Jefferies T M, Keevil C W, Potter E, editors. Detection methods for cyanobacterial toxins. Cambridge, United Kingdom: The Royal Society of Chemistry; 1994. pp. 3–10. [Google Scholar]
- 5.Fastner J, Heinze R, Chorus I. Microcystin-content, hepatotoxicity and cytotoxicity of cyanobacteria in some German water bodies. Water Sci Technol. 1995;32:165–170. [Google Scholar]
- 6.Fastner J, Neumann U, Wirsing B, Weckesser J, Wiedner C, Nixdorf B, Chorus I. Microcystins (hepatotoxic heptapeptides) in German fresh water bodies. Environ Toxicol. 1999;14:13–22. [Google Scholar]
- 7.Harada K-I. Chemistry and detection of microcystins. In: Watanabe M F, Harada K-I, Carmichael W W, Fujiki H, editors. Toxic microcystis. New York, N.Y: CRC Press; 1996. pp. 103–148. [Google Scholar]
- 8.Harada K-I, Matsuura K, Suzuki M, Oka H, Watanabe M F, Oishi S, Dahlem A M, Beasley V R, Carmichael W W. Analysis and purification of toxic peptides from cyanobacteria by reversed-phase high-performance liquid chromatography. J Chromatogr. 1988;448:275–283. doi: 10.1016/s0021-9673(01)84589-1. [DOI] [PubMed] [Google Scholar]
- 9.Kaya K. Blooms of toxic cyanobacteria and removal strategies. Proceedings of the 8th Environment Symposium. Pusan, Korea: Inje University; 1999. Microcystin variants and their quantitative analyses; pp. 2–5. [Google Scholar]
- 10.Kim B, Kim H-S, Park H-D, Choi K, Park J-G. Microcystin content of cyanobacterial cells in Korean reservoirs and their toxicity. Korean J Limnol. 1999;32:288–294. [Google Scholar]
- 11.Kotak B G, Lam A K-Y, Prepas E E, Kenefick S L, Hrudey S E. Variability of the hepatotoxin microcystin-LR in hypereutrophic drinking water lakes. J Phycol. 1995;31:248–263. [Google Scholar]
- 12.Lahti K, Rapala J, Färdig M, Niemelä M, Sivonen K. Persistence of cyanobacterial hepatotoxin, microcystin-LR in particulate material and dissolved in lake water. Water Res. 1997;31:1005–1012. [Google Scholar]
- 13.Lambert T W, Boland M P, Holmes C F B, Hrudey S E. Quantitation of the microcystin hepatotoxins in water at environmentally relevant concentrations with the protein phosphatase bioassay. Environ Sci Technol. 1994;28:753–755. doi: 10.1021/es00053a032. [DOI] [PubMed] [Google Scholar]
- 14.Lawton L A, Edwards C, Codd G A. Extraction and high-performance liquid chromatographic method for the determination of microcystins in raw and treated waters. Analyst. 1994;119:1525–1530. doi: 10.1039/an9941901525. [DOI] [PubMed] [Google Scholar]
- 15.Lee S J, Jang M-H, Kim H-S, Yoon B-D, Oh H-M. Variation of microcystin content of Microcystis aeruginosa relative to medium N:P ratio and growth stage. J Appl Microbiol. 2000;89:323–329. doi: 10.1046/j.1365-2672.2000.01112.x. [DOI] [PubMed] [Google Scholar]
- 16.MacKintosh C, Beattle K A, Klumpp S, Cohen P, Codd G A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatase 1 and 2A from both mammals and higher plants. FEBS Lett. 1990;264:187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- 17.Menzel D W, Corwin N. The measurement of total phosphorous in seawater based on the liberation of organically bound fractions by persulfate oxidation. Limnol Oceanogr. 1965;10:280–282. [Google Scholar]
- 18.Murphy J, Riley J P. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta. 1962;27:31–36. [Google Scholar]
- 19.Myers J, Graham J-R, Wang R T. On spectral control of pigmentation in Anacystis nidulans (Cyanophyceae) J Phycol. 1978;14:513–518. [Google Scholar]
- 20.Oh H-M, Lee S J, Jang M-H, Yoon B-D. Microcystin production by Microcystis aeruginosa in a phosphorus-limited chemostat. Appl Environ Microbiol. 2000;66:176–179. doi: 10.1128/aem.66.1.176-179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Organization for Economic Cooperation and Development. Eutrophication of waters: monitoring, assessment and control. Paris, France: Organization for Economic Cooperation and Development; 1982. [Google Scholar]
- 22.Park H-D, Kim B, Kim E, Okino T. Hepatotoxic microcystins and neurotoxic anatoxin-a in cyanobacterial blooms from Korean lakes. Environ Toxicol Water Qual. 1998;13:225–234. [Google Scholar]
- 23.Penaloza R, Rojas M, Vila I, Zambrano F. Toxicity of a soluble peptide from Microcystis sp. to zooplankton and fish. Freshwater Biol. 1990;24:233–240. [Google Scholar]
- 24.Rapala J, Sivonen K. Assessment of environmental conditions that favor hepatotoxic and neurotoxic Anabaena spp. strains cultured under light limitation at different temperatures. Microb Ecol. 1998;36:181–192. doi: 10.1007/s002489900105. [DOI] [PubMed] [Google Scholar]
- 25.Rapala J, Sivonen K, Lyra C, Niemelä S I. Variation of microcystins, cyanobacterial hepatotoxins, in Anabaena spp. as a function of growth stimuli. Appl Environ Microbiol. 1997;63:2206–2212. doi: 10.1128/aem.63.6.2206-2212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skulberg O M, Carmichael W W, Codd G A, Skulberg R. Taxonomy of toxic cyanophyceae (cyanobacteria) In: Falconer I R, editor. Algal toxins in seafood and drinking water. London, United Kingdom: Academic Press; 1993. pp. 145–164. [Google Scholar]
- 27.Ward C J, Beattie K A, Lee E Y C, Codd G A. Colorimetric protein phosphatase inhibition assay of laboratory strains and natural blooms of cyanobacteria: comparisons with high-performance liquid chromatographic analysis for microcystins. FEMS Microbiol Lett. 1997;153:465–473. doi: 10.1016/s0378-1097(97)00290-5. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe M F, Harada K-I, Matsuura K, Watanabe M, Suzuki M. Hepatopeptide toxin production during the batch culture of two Microcystis species (Cyanobacteria) J Appl Phycol. 1989;1:161–165. [Google Scholar]
- 29.Wicks R J, Thiel P G. Environmental factors affecting the production of peptide toxins in floating scums of the cyanobacterium Microcystis aeruginosa in a hypertrophic African reservoir. Environ Sci Technol. 1990;24:1413–1418. [Google Scholar]
- 30.Wirsing B, Flury T, Wiedner C, Neumann U, Weckesser J. Estimation of the microcystin content in cyanobacterial field samples from German lakes using the colorimetric protein-phosphatase inhibition assay and RP-HPLC. Environ Toxicol. 1999;14:23–29. [Google Scholar]
- 31.Wood L W. Chloroform-methanol extraction of chlorophyll a. Can J Fish Aquat Sci. 1985;42:38–43. [Google Scholar]
- 32.World Health Organization. Guidelines for drinking-water quality. 2nd ed. 1998. Addendum to vol. 2, Health criteria and other supporting information. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 33.Wynne D, Rhee G-Y. Effects of light intensity and quality on the relative N and P requirement (the optimum N:P ratio) of marine planktonic algae. J Plankton Res. 1986;8:91–103. [Google Scholar]