Introduction
Enhanced recovery has sparked excitement in the surgical community primarily because it works, but also because it is an innovative approach to delivering standardized, evidence-based care. Adoption of enhanced recovery pathways (ERPs) has been associated with reducing surgical complications, improving patient experience, and decreasing length of stay (LOS) and associated hospital costs without increasing readmission rates.1–3 To successfully implement ERPs and achieve improvements, the entire perioperative team must function as a coordinated and collaborative group, breaking down silos among preoperative, operating room, recovery room, and inpatient units, creating transdisciplinary collaboration across perioperative disciplines (surgery, anesthesiology, nursing, pharmacy, physical therapy, and others).
The Agency for Healthcare Research and Quality (AHRQ), in partnership with the American College of Surgeons (ACS) and the Johns Hopkins Medicine Armstrong Institute for Patient Safety and Quality (AI) at Johns Hopkins, has developed the Safety Program for Improving Surgical Care and Recovery (ISCR), which is national effort to disseminate best practices in perioperative care to more than 750 hospitals across multiple procedure areas over the next five years. The program will integrate evidence-based processes central to enhanced recovery as well as surgical site infection (SSI), venous thromboembolic events (VTE) and catheter associated urinary tract infections (CAUTI) with socioadaptive interventions to meaningfully improve surgical outcomes, patient experience, and perioperative safety culture. Evidence-based clinical pathways will serve as the foundation for these efforts. To assist hospitals with transforming their perioperative care, the ISCR program will also include a registry for hospitals to track their progress in adhering to the clinical pathway and for benchmarking, patient engagement and education materials, change management and leadership training, as well as tools to facilitate local pathway adaptation, implementation, and program sustainability.
The objective of this manuscript is to provide a comprehensive review of the evidence supporting the surgical components of the ISCR colorectal (CR) pathway. The anesthesiology components were reviewed in parallel and are being reported separately. The objectives of this review are to evaluate the evidence supporting colorectal (CR) pathways and to develop an evidence-based CR protocol to help hospitals participating in the ISCR program implement evidence-based practices.
Methods
A review protocol was developed with input from stakeholders (eDocument1). Two researchers reviewed current CR ERPs from several major US health systems and sought expert feedback to identify individual components for the CR ISCR protocol in each perioperative phase of care (preoperative through postoperative) (Table 1).
Table 1.
Colorectal Protocol for the AHRQ Safety Program for Improving Surgical Care and Recovery - Surgical Components
| Component |
|---|
| Preoperative |
| Patient education |
| Immediate preoperative |
| Bowel preparation |
| Preoperative (home) bathing |
| Preoperative VTE chemoprophylaxis |
| Intraoperative |
| Skin preparation |
| Surgical technique (laparoscopic vs. open) |
| Minimize drains |
| Postoperative |
| Early mobilization |
| Early alimentation |
| Early urinary bladder catheter removal |
| Early IV fluid discontinuation |
| Postoperative VTE prophylaxis |
| Glucose management |
VTE, venous thromboembolic event
Individual literature reviews for each protocol component were performed in PubMed for English-language articles published before December 2016. Specific search terms are provided (eTable 1). First, each search targeted CR surgery, and if no CR surgery literature was identified, the search was broadened to surgical procedures in general. To be included, studies had to report on the specific protocol components. Studies were excluded if they: 1) did not report clinical outcomes, 2) included fewer than 10 patients, 3) were non-English language, or 4) were non-systematic reviews.
Given the large amount of evidence within this field, we used a hierarchical method of inclusion based on study design. If we identified a well-designed systematic review (SR) or meta-analysis (MA), then we included it along with additional randomized controlled trials (RCTs) or observational studies published after the SR/MA, when possible. Data extraction was completed including: sample size, surgical procedure category, comparator (varied by component), and main outcomes of interest (varied by component). Results are described narratively.
Results
Preoperative
Patient Education
Rationale
Detailed preoperative patient education is theorized to set expectations for the patient regarding surgery, which in turn allows the patient to become a partner in their recovery.
Evidence
No randomized or observational studies in CR surgery have isolated the effect of detailed patient education on outcomes. Two MAs including 11 RCTs evaluated the effect of ERP implementation on outcomes and included patient education as a component of ERPs.1,2 Both MAs concluded that ERP implementation was associated with a reduction in morbidity and LOS.1,2 No studies in CR surgery have evaluated the optimal medium for education materials. Options employed in the RCTs above included verbal information provided by the provider, information booklets, and informational videos.
Summary
There is no direct evidence to support patient education as a component of the CR ISCR protocol, however, patient education is recommended as it can only be beneficial and is endorsed by guidelines (Tables 2 and 3).
Table 2.
Summary of Improving Surgical Care and Recovery Colorectal Protocol Components, Associated Outcomes, and Support from the Literature and/or Guidelines
| Intervention | Outcome(s) | Studies | Population | Evidence | Guidelines |
|---|---|---|---|---|---|
| Preoperative | |||||
| Patient education | ↓ LOS; ↓ complications | 2 MAs (indirect evidence) | CR surgery | +* | √† |
| Immediate preoperative | |||||
| Bowel preparation (PO antibiotic and MBP) | ↓ SSI | 1 MA | CR surgery | +‡ | √§ |
| Preoperative (home) bathing | ↓ SSI | 1 MA, 1 SR | All surgery | −‖ | √§ |
| Preoperative VTE prophylaxis | ↓ VTE | CR surgery | +/−¶ | √† | |
| Intraoperative | |||||
| Skin preparation | ↓ SSI | 2 MAs | Clean and clean-contaminated surgery | +‡ | √† |
| (Laparoscopic) surgical technique | ↓ LOS; ↓ complications; faster return of bowel function | 4 MAs | CR surgery | +‡ | √† |
| Minimization of tubes/drains | - anastomotic dehiscence; -SSI; - reoperation; -mortality | 4 MAs, 1 SR | CR surgery | +‡ | √† |
| Postoperative | |||||
| Early mobilization | ↓/− LOS; +/− faster return of bowel function | 1 SR | Abdominal surgery | +/−¶ | √† |
| Early alimentation | ↓ LOS; ↓ complications; −/↓ mortality | 4 MAs, 1 SR | Abdominal surgery | +‡ | √† |
| Early urinary bladder catheter removal | ↓ UTI | 2 MAs, 1 SR | CR surgery | +‡ | √† |
| Early IV fluid discontinuation | ↓ LOS; ↓ complications | 1 MA | Abdominal and CR surgery | +‡ | √† |
| Postoperative VTE prophylaxis | ↓ VTE | 1 MA | All and CR surgery | +‡ | √† |
| Glucose management | ↓ SSI | 1 MA | All and CR surgery | +‡ | √† |
Designates a component where evidence was indirect, but supported given practice
Designates a component where all guidelines supported a given practice
Designates a component where all evidence supported a given practice
Designates a component where some, but not all, guidelines supported a given practice
Designates a component where evidence showed no effect of a given practice
Designates a component where evidence was mixed (some showing benefit, some showing no effect) regarding a given practice
LOS, length of stay; SSI, surgical site infection; VTE, venous thromboembolism; UTI, urinary tract infection; SR, systematic review; MA, meta-analysis; CR, colorectal
Table 3.
Summary of Guidelines Supporting Improving Surgical Care and Recovery Protocol Components
| Intervention | Guideline | Year | Recommendation |
|---|---|---|---|
| Preoperative | |||
| Patient education | ERAS Society40 | 2013 | Routine preoperative patient education recommended as it can only be beneficial. |
| Immediate Preoperative | |||
| Bowel preparation (PO antibiotic and MBP) | ACS/SIS SSI Guidelines42 | 2016 | Combination mechanical and PO antibiotic bowel preparation recommended for elective colorectal surgery. |
| ERAS Society40 | 2013 | Recommends against routine use of mechanical bowel preparation alone. | |
| SHEA/IDSA Practice Recommendation43 | 2013 | Mechanical bowel preparation alone is not recommended. Reduction in SSI has been shown with combined mechanical and PO antibiotic bowel preparation. | |
| Preoperative (home) bathing | ACS/SIS SSI Guidelines42 | 2016 | Chlorhexidine bathing reduces skin surface pathogen counts, but has not been shown to reduce SSI. |
| HICPAC[personal communication with Dr. Bratzler] | Update pending | Bathing with soap or an antiseptic agent is recommended the night before the operative day. | |
| Preoperative VTE prophylaxis | American College of Chest Physicians44 | 2012 | Preoperative administration of VTE chemoprophylaxis is recommended. |
| American Society of Clinical Oncology45 | 2013 | VTE chemoprophylaxis with low-dose unfractionated heparin or low molecular weight heparin is recommended for patients undergoing major cancer surgery beginning preoperatively and continuting until POD 7–10. | |
| European Society of Medical Oncology46 | 2011 | Postop VTE prophylaxis options include compression stockings and chemoprophylaxis with low-dose unfractionated heparin and low molecular weight heparin. | |
| Intraoperative | |||
| Skin preparation | ACS/SIS SSI Guidelines42 | 2016 | Preparation with an alcohol-containing agent is recommended. No superior agent (chlorhexidine vs. iodine) when combined with alcohol. If alcohol cannot be included in the preparation, chlorhexidine should be used instead of iodine unless contraindications exist. |
| ERAS Society40 | 2013 | Chlorhexidine-alcohol is recommended over iodine alone for skin preparation. | |
| SHEA/IDSA Practice Recommendation43 | 2013 | Skin preparation with an alcohol-containing agent is recommended unless contraindications exist. | |
| (Laparoscopic) surgical technique | ERAS Society40 | 2013 | Laparoscopic surgery is recommended if the expertise is available. |
| Minimization of tubes/drains | ERAS Society40 | 2013 | Routine postoperative nasogastric drainage and abdominal drainage are not recommended. |
| Postoperative | |||
| Early mobilization | ERAS Society40 | 2013 | Prompt postoperative mobilization is recommended as prolonged bedrest has been shown to be harmful. |
| Early alimentation | ERAS Society40 | 2013 | Patients should be encouraged to take normal food as soon as possible after surgery. |
| Early urinary bladder catheter removal | ERAS Society40 | 2013 | Urinary catheter removal is recommended between POD 1–2, even in the presence of a thoracic epidural. |
| HICPAC47 | 2009 | Urinary catheter removal within 24 hours of surgery is recommended. | |
| Early IV fluid discontinuation | ERAS Society40 | 2013 | Early initiation of PO fluid intake is recommended, as is early discontinuation of IV fluids if patient is tolerating PO. |
| Postoperative VTE prophylaxis | ASCRS Guidelines48 | 2006 | Chemical thromboprophylaxis is recommended for all patients undergoing CR surgery, and addition of mechanical thromboprophylaxis is recommended in high-risk patients. Patients with cancer should receive posthospital prophylaxis with LMWH. |
| ERAS Society40 | 2013 | Combined chemical and mechanical thromboprophylaxis is recommended for all patients. Extended chemical prophylaxis for 28 days is recommended for patients with cancer. | |
| NICE Guidelines49 | 2010 | At-risk patients should receive combined mechanical and chemical thromboprophylaxis. | |
| Glucose management | ACS/SIS SSI Guidelines42 | 2016 | Blood glucose between 110–150 mg/dL is recommended for all patients regardless of diabetic status to reduce SSI. |
| ERAS Society40 | 2013 | Hyperglyemia increases the risk of SSI and should be avoided. |
MBP, mechanical bowel preparation; VTE, venous thromboembolism; ERAS, Enhanced Recovery after Surgery; ACS, American College of Surgeons; SIS, Surgical Infection Society; SHEA, Society for Healthcare Epidemiology of America; IDSA, Infectious Diseases Society of America; HICPAC, Healthcare Infection Control Practices Advisory Committee; ASCRS, American Society of Colon and Rectal Surgeons; NICE, National Institute for Health and Care Excellence
Immediate Preoperative
Bowel Preparation
Rationale
The use of bowel preparations (mechanical alone, per os (PO) antibiotics alone, or a combination of both) has been proposed to reduce the risk of SSI following CR surgery, but may also cause physiologic derangements leading to prolonged recovery.
Evidence
We identified 5 MAs of bowel preparation for CR surgery, including one of combined mechanical and PO antibiotic bowel preparation versus mechanical bowel preparation (MBP) alone or versus IV antibiotics alone.4–8 This study of 7 RCTs found that patients who received combined PO antibiotic and MBP had lower total SSI and incisional SSI compared to patients who received MBP and systemic antibiotics alone (total: 7.2% versus 16.0%, p<0.001; incisional: 4.6% versus 12.1%, p<0.001).8 Three MAs of MBP alone versus no MBP showed neither benefit nor harm to the use of MBP with regard to anastomotic leak, SSI, reoperation, or mortality.5–7 One MA of RCTs found that SSI was lower without MBP, though the number needed to harm was high at 333 patients.4
Summary
Despite the possibility that combined bowel preparations cause physiologic derangements in the preoperative period, combined PO antibiotic and MBP is recommended in the ISCR protocol because of the evidence that this practice decreases SSI (Table 2).
Preoperative (Home) Bathing
Rationale
Preoperative at-home bathing has been proposed to decrease both skin surface pathogen counts and SSI following CR surgery.
Evidence
We identified 1 MA and 1 SR of studies evaluating the effect of preoperative antiseptic bathing versus non-antiseptic bathing or no bathing in all surgery.9,10 The MA included 8 RCTs and 8 quasiexperimental studies and concluded that there was no difference in SSI among any of the intervention arms (antiseptic bathing with chlorhexidine, non-antiseptic bathing, no bathing).9 Similarly, the SR concluded that there was no difference in SSI rates between antiseptic versus non-antiseptic preoperative showering.10 Both reviews noted that many included studies had suboptimal rates of patient compliance with recommended bathing protocols.9,10
Evidence
RCTs and quasiexperimental studies have not shown that routine preoperative (at-home) bathing/showering with chlorhexidine reduces SSI, however, routine preoperative bathing with an antiseptic or non-antiseptic agent is supported by current guidelines and recommended in the ISCR protocol (Tables 2 and 3).
Preoperative VTE Prophylaxis
Rationale
Preoperative VTE chemoprophylaxis (versus postoperative alone) may reduce VTE events in the perioperative period.
Evidence
We identified one RCT and one large observational study of preoperative VTE chemoprophylaxis versus postoperative VTE chemoprophylaxis alone. The RCT included patients undergoing CR surgery and failed to show a decrease in early postoperative VTE, 30-day VTE or mortality with the administration of preoperative chemical VTE prophylaxis.11 The observational study, in contrast, showed that in patients undergoing major surgery, preoperative chemoprophylaxis lowered rates of DVT (1.3% versus 0.2%; 95% CI 0.7–1.4%) and PE (1.0% versus 0.4%; 95% CI 0.2–1%). Neither study demonstrated increased bleeding risk with the administration of preoperative VTE chemoprophylaxis.11,12
Summary
There is mixed evidence that preoperative VTE chemoprophylaxis should be given for CR surgery to reduce VTE events (Table 2). This practice is supported by multiple society guidelines (Table 3) and is recommended in the ISCR protocol.
Intraoperative
Skin Preparation
Rationale
Skin preparation prior to surgery with antiseptic agents is thought to decrease SSI.
Evidence
We identified 2 MAs evaluating the efficacy of various antiseptic agents in preventing SSI following clean or clean and clean-contaminated surgery.13,14 One MA included 4 RCTs and concluded that chlorhexidine + alcohol significantly reduced the risk of SSI compared to aqueous iodine.13 The second MA included 10 RCTs and concluded that chlorhexidine + alcohol was likely the most effective treatment (compared to iodophor + alcohol), but acknowledged that all effect estimates were judged to be low or very low quality.14
Summary
Evidence from 2 MAs supports the use of chlorhexidine + alcohol over iodine alone, and showed little difference between chlorhexidine + alcohol and iodine + alcohol for skin preparation prior to surgery (Table 2).
Surgical Technique
Rationale
Minimally invasive (laparoscopic) surgical technique is believed to decrease postoperative pain, speed recovery, and shorten LOS.
Evidence
We identified 4 MAs comparing laparoscopic to open surgical approach for CR surgery within the setting of ERPs.15–18 We did not query studies of robotic versus open technique. The most recent MA included 4 RCTs and 6 clinical controlled trials and concluded that laparoscopic surgical approach was associated with shorter LOS (weighted mean difference (WMD) −1.65 days, p<0.001), shorter time to return of bowel function, decreased postoperative complications, decreased readmissions, and decreased mortality.15 Additional MAs found similar benefits to laparoscopic surgery for LOS and complications, but some failed to show reduced readmission or mortality.16–18
Summary
Evidence from 4 MAs concludes that laparoscopic approach (versus open) is associated with improved outcomes in the setting of ERPs. If surgeon expertise is available and there are no patient contraindications, a laparoscopic surgical approach is recommended (Table 2) in the ISCR protocol.
Minimize Drains/Tubes
Rationale
Minimization of drains (intraperitoneal abdominal and nasogastric (NGT)) after CR surgery has been promoted to speed recovery without increasing complications.
Evidence
We identified 4 MAs and 1 SR in CR surgery of outcomes with versus without drains/NGTs.19–23 3 MAs including RCTs of CR patients with both peritoneal and pelvic drains failed to show a statistically significant difference in anastomotic dehiscence, SSI, reoperation, or mortality with drain use.20,21,23 In contrast, one MA (3 RCTs and 5 non-RCTs) of pelvic drains and concluded that drain use was associated with a decreased risk of anastomotic dehiscence, however, MA of the RCTs alone revealed no difference.19
We found 1 MA of 7 RCTs of prophylactic NGT use after CR surgery, which concluded that NGT use was associated with higher rates of respiratory complications and more pharyngolargyngitis.22 While the MA demonstrated that NGT use was associated with less vomiting and less frequent NGT replacement, there was no difference in LOS or return of bowel function with routine prophylactic NGT use.22
Summary
Evidence from 3 MAs supports avoidance of routine peritoneal drainage, but there may be a role for prophylactic drainage for patients with a pelvic anastomosis (Table 2). Evidence from 1 MA fails to support significant clinical benefit from routine NGT use (Table 2).
Postoperative
Early Mobilization
Rationale
Early mobilization has been proposed to reduce LOS and complications like VTE and ileus.
Evidence
We identified 1 SR including 3 RCTs and 1 observational study of patients undergoing abdominal surgery evaluating the effect of early mobilization protocols.24 Most early mobilization protocols entailed supervised, mandatory exercises performed at 12–24 hours postoperative versus delayed ambulation or activity totally at the patient’s discretion.24 No studies showed a difference in overall complications. In early mobilization cohorts, one study demonstrated shorter LOS and one study showed improved GI function.24 The authors concluded that overall, study methodology was poor and there was no evidence to support any specific early mobilization protocol; however, they concluded that bed rest may be harmful.24
Summary
Evidence from one SR did not support any specific postoperative mobilization protocol, but there is evidence that prolonged bedrest is harmful (Table 2). Based on expert consensus, it is recommended in the ISCR protocol that patients be mobilized (out of bed to a chair) at least once POD0 and ambulate BID POD1 and thereafter.
Early Alimentation
Rationale
Early postoperative alimentation is proposed to speed gastrointestinal recovery after CR surgery and contribute to shorter LOS without increasing complications.
Evidence
We found 4 MAs and 1 SR comparing outcomes following early versus traditional feeding after abdominal surgery.25–29 The MA of patients undergoing elective CR surgery included 7 RCTs and found that early feeding was associated with reduced LOS, reduced complications, and no difference in anastomotic dehiscence, SSI, emesis, NGT reinsertion, or mortality.28 Two additional MAs in gastrointestinal surgery reported mixed results, with one supporting reduced complications, and the other supporting decreased mortality, but no additional clinical benefit.25,27 Of note, early feeding was defined different in each RCT, but typically entailed introduction of a diet within 24 hours.
Summary
Early postoperative alimentation is recommended in the ISCR protocol as RCTs support an association with reduced LOS and reduced complications (Table 2).
Early Urinary Bladder Catheter Removal
Rationale
Presence of a urinary bladder catheter is a risk factor for urinary tract infection (UTI), and one strategy for reducing CAUTI is prompt removal or avoiding their use. Early removal for mid- to low-rectal surgery can be associated with urinary retention.
Evidence
We found two MAs, one retrospective cohort study, and an SR in rectal surgery on interventions to reduce duration of catheter use.30–33 Both MAs demonstrated that interventions to reduce the use or duration of urinary bladder catheters reduce rates of CAUTI, with the best evidence supporting “stop orders” in the electronic health record (CAUTI rates reduced by 53%, p<0.001).30,31 In the setting of CR ERPs, the cohort study showed that early catheter discontinuation was associated with decreased LOS, though of note, early catheter discontinuation was defined differently for colon (24 hours) versus rectal procedures (72 hours).32 The SR (RCTs and observational studies) gave special consideration to urinary catheter management in patients undergoing rectal resections, where early catheter removal (POD 1 versus POD 5) decreased UTI (20% versus 42%) at the cost of increased urinary retention (31% versus 10%).33 Overall, the SR author concluded that in patients undergoing mid- to low rectal resection, evidence supported consideration of catheterization through POD 3–5 due to the increased incidence of urinary retention in this population.33
Summary
Evidence from 2 MAs, 1 SR and 1 retrospective observational cohort study supports routine early urinary bladder catheter removal for colon surgery or upper rectal surgery (Table 2). For mid- to low rectal surgery, evidence from 2 RCTs summarized in a SR supports consideration of routine drainage through POD3–5 based on the higher risk of urinary retention.
Early IV Fluid Discontinuation
Rationale
Early postoperative discontinuation of intravenous (IV) fluid in patients who are euvolemic and tolerating enteral intake is thought to speed return of bowel function and minimizes postoperative complications.
Evidence
There is no literature isolating the effect of early IV fluid discontinuation following abdominal or CR surgery. We identified 1 MA of 9 RCTs comparing standard, restrictive, and liberal fluid administration in the perioperative period following major elective open abdominal surgery.34 The authors opted to compare “balanced” versus “imbalanced” fluid administration, with “balanced” defined as between 1.75–2.75 L of crystalloid/day, and “imbalanced” as any volume of crystalloid less than or greater than this amount.34 Patients who received “balanced” fluid administration had fewer complications (RR 0.59, 95% CI 0.44–0.81) and shorter LOS (WMD −3.44, 95%CI −6.33 to −0.54).34
Summary
Evidence from RCTs supports “balanced” fluid administration in the perioperative period (Table 2). Based on expert consensus, it is recommended in the ISCR protocol that maintenance IV fluid be discontinued POD1 unless the patient has difficulty taking PO and/or evidence of kidney injury.
Postoperative VTE Prophylaxis
Rationale
Timely administration of VTE chemoprophylaxis is thought to reduce VTE events. Extended VTE chemoprophylaxis is thought to be beneficial for CR cancer patients as they are at increased risk of VTE events.
Evidence
We identified 1MA of RCTs comparing combined mechanical and chemoprophylaxis to either modality alone after any surgery and 1 observational study evaluating the timing of VTE chemoprophylaxis and outcomes after CR surgery.35,36 The MA concluded that combination mechanical and chemical VTE prophylaxis was most effective in preventing VTE events.35 The observational study concluded that patients who received VTE chemoprophylaxis within 24 hours after surgery had lower mortality, clinical VTE events, and composite adverse events compared to patients who did not receive VTE chemoprophylaxis.36 Looking at the role of extended chemoprophylaxis in the cancer population, we identified 1 MA of RCTs and non-RCTs of prolonged VTE chemoprophylaxis (1 month after surgery) compared to in-hospital VTE chemoprophylaxis alone in patients undergoing major abdominal surgery.37 Patients receiving prolonged VTE chemoprophylaxis were less likely to have a confirmed VTE event (OR 0.41, 95%CI 0.26–0.63) than those who received in-hospital VTE chemoprophylaxis alone.37
Summary
Evidence from RCTs supports the use of combined mechanical and chemoprophylaxis for the duration of hospitalization in all patients to prevent VTE events (Table 2). Extended VTE chemoprophylaxis until 28 days total is recommended for patients undergoing surgery for colorectal cancer in the ISCR protocol. Multiple guidelines support these practices (Table 3).
Glucose Management
Rationale
Blood glucose control in the perioperative period may decrease the risk of SSI.
Evidence
We identified 1 MA of 15 RCTs comparing intensive glucose management (<150 mg/dL) versus conventional glucose management (220 mg/dL or less), and one large observation study that examined blood glucose levels and SSI in bariatric and CR surgery patients.38,39 The MA determined there was significant benefit to an intensive protocol, resulting in decreased odds of SSI (OR 0.43, 95% CI 0.29–0.64), with no difference in stroke or mortality. One adverse outcome associated with intensive glucose management was increased odds of hypoglycemia.38 Of note, these results were consistent among patients with and without diabetes.38 The retrospective study concluded that CR patients (both diabetic and non-diabetic) with perioperative hyperglycemia had increased odds of infection, reoperation and mortality.36 Additionally, there was a dose-response relationship between blood glucose control and SSI.36
Summary
Perioperative blood glucose control is recommended for all patients in the ISCR protocol regardless of diabetic status to prevent SSI (Table 2). Current guidelines recommended a target range of 110–150 mg/dL (Table 3).
Discussion
The benefits of CR ERPs are well-documented and include improved patient outcomes, reduced LOS, reduced morbidity, and no change in readmission rates. This report expands on guidelines endorsed by the ERAS Society and the American Society for Enhanced Recovery and includes additional best practices for preventable harms.40,41 Protocol elements were supported in the literature, though the contribution of publication bias favoring the publication of positive findings cannot be discounted.
Conclusions
We identified 23 overall components (eTable 2) for the ISCR CR protocol, including the 12 surgical components in this review, supported by the literature, existing guidelines, and/or expert consensus that should be delivered consistently for optimal surgical care of the CR patient. Structural limitations at individual hospitals (e.g., formulary, hospital policy, and technical expertise) will require local adaptation of these recommendations for successful implementation. The ISCR CR protocol components span the preoperative, immediate preoperative, intraoperative, and postoperative phases of care and will require transdisciplinary collaboration between surgeons, anesthesia providers, nurses, hospital leadership, and patients. Hospitals participating in the AHRQ Safety Program for ISCR will be supported in expeditiously and sustainably translating this evidence base into practice over the next few years with the goal of moving the needle on surgical outcomes in the United States. Importantly, as we unite to improve patient care for this work, such collaborations will extend to other areas with anticipated improvement in clinical outcomes, patient experience, and workplace culture.
Supplementary Material
Goal of our evidence review is find the highest level evidence for each component of the clinical pathways
General Overview
STEPS
PROTOCOL COMPONENTS. Identify the critical components of the Optimal Surgical Recovery (OSR) protocol(s). These components will form the general foundation for the searches. Topics include — colorectal (CR) surgery, emergency general surgery, orthopedic (hip/knee), gynecology (hysterectomy), and bariatric.
SEARCH. For each component, perform a literature search that is procedure-specific. Search should be limited to English only. Keep track of the search terms. Initial searches can be for the specific component or for ERAS— this may vary by procedure so adjust as you see appropriate. We will also run our search terms by a librarian — as time permits. Also, you may need to search for broad surgical procedures. Examples of terms for ERAS: “fast track”, “enhanced recovery”, “clinical pathway”, “critical pathway”, “multimodal perioperative” and “perioperative protocol”. (Don’t limit searches by study design).
- INCLUSION/EXCLUSION terms and Screening. Develop these terms for each protocol component — inclusion: specific procedure, perioperative period, component topic, reports outcomes, not case report, > ten sample size. Not necessary to track the reasons for exclusion at the title and abstract level.
-
For the full text article screen, track reasons for includes and excludes. Includes: 1. SR/MA, 2. RCTs, 3. Prospective/case controlled observational studies, 4. Retrospective observational studies. Excludes: 1. Not on the specific procedure, 2. Lack of postop outcome, 3. Not primary data, 4. Non SR, etc.Hierarchy of the selecting includes:
- First identify well-done recent SR/Mas (within the past 5 years, if possible. If you have multiple SR/MAs then pick the most recent or the better quality ones. Well-done studies are:
- Was a specific question(s) defined that the SR/MA set out to answer? Yes
- Provided inclusion/exclusion terms and the search terms? Yes
- Did the studies they included make clinical sense to do so? Yes (this is often a fail)
- They did not pool RCT and observation data together unless state a strong justification. Yes
- Was a quality assessment of the studies performed? (doesn’t really matter which tool). Yes
- Of note, if there is a well-done SR/MAs cross reference with search results looking for additional studies — ones performed after the SR/MA or ones that simply weren’t included. Include RCTs and observational studies performed after the SR/MA.
- If you use primarily observational studies (find none or just a few RCTs) then limit to the highest study design. For example, limit by sample size (n>100)/matched cohort/multi-institutional, etc. Need to keep track of any specific decisions that change the inclusion/exclusions at this point.
-
- DATA ABSTRACTION
- Evidence tables for RCTs. This can be done later, but It will be helpful to develop these and include: Article author name and year of publication, study design, multi- or single institution, sample size (f/u rate if relevant), surgical procedure(s), details of the component of interest, outcomes measured and findings (f/u time period for some outcomes).
- Evidence tables for observational studies. Include: author name and year of publication, study design, multi or single institution, sample size (f/u rate if relevant), surgical procedure(s), details of the component of interest, outcomes measured and findings (f/u time period for some outcomes).
REFERENCE MINING. Check the references of the better studies for articles we may have missed. Then those identified from this step need to be screened.
Flow example
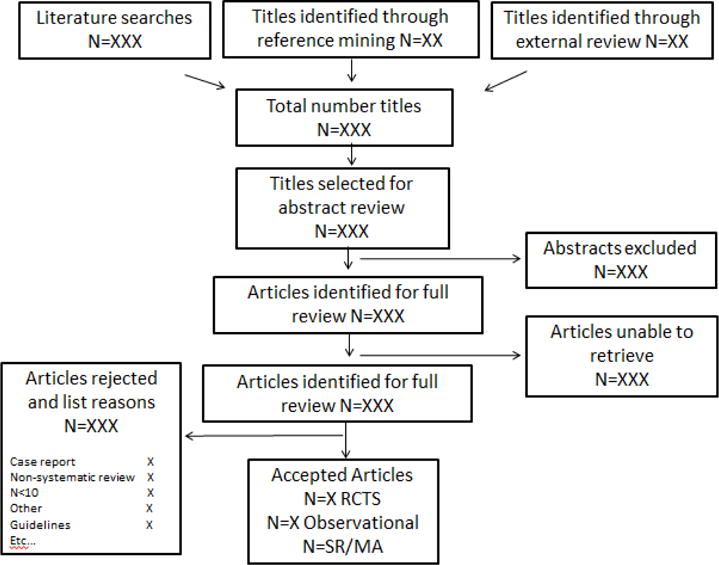
Acknowledgments
Support: This review was funded under contract number HHSP233201500020I from the AHRQ, US Department of Health and Human Services. The study authors received salary support (Drs Ban, Ko and Wick) or a consultant fee (Dr Gibbons) from the Agency for Healthcare Research and Quality (AHRQ) for completion of this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Disclosure outside the scope of this work: Dr Ban received honorarium for speaking at the American Society for Enhanced Recovery conference and honorarium for invited submission to Selected Readings in General Surgery.
Disclaimer: The opinions expressed in this document are those of the authors and do not reflect the official position of AHRQ or the US Department of Health and Human Services.
References
- 1.Zhuang CL, Ye XZ, Zhang XD, et al. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum. 2013;56(5):667–678. doi: 10.1097/DCR.0b013e3182812842. [DOI] [PubMed] [Google Scholar]
- 2.Greco M, Capretti G, Beretta L, et al. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014;38(6):1531–1541. doi: 10.1007/s00268-013-2416-8. [DOI] [PubMed] [Google Scholar]
- 3.Thiele RH, Rea KM, Turrentine FE, et al. Standardization of care: impact of an enhanced recovery protocol on length of stay, complications, and direct costs after colorectal surgery. J Am Coll Surg. 2015;220(4):430–443. doi: 10.1016/j.jamcollsurg.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 4.Slim K, Vicaut E, Launay-Savary MV, et al. Updated systematic review and meta-analysis of randomized clinical trials on the role of mechanical bowel preparation before colorectal surgery. Ann Surg. 2009;249(2):203–209. doi: 10.1097/SLA.0b013e318193425a. [DOI] [PubMed] [Google Scholar]
- 5.Dahabreh IJ, Steele DW, Shah N, Trikalinos TA. Oral Mechanical Bowel Preparation for Colorectal Surgery: Systematic Review and Meta-Analysis. Dis Colon Rectum. 2015;58(7):698–707. doi: 10.1097/DCR.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 6.Cao F, Li J, Li F. Mechanical bowel preparation for elective colorectal surgery: updated systematic review and meta-analysis. Int J Colorectal Dis. 2012;27(6):803–810. doi: 10.1007/s00384-011-1361-y. [DOI] [PubMed] [Google Scholar]
- 7.Pineda CE, Shelton AA, Hernandez-Boussard T, Morton JM, Welton ML. Mechanical bowel preparation in intestinal surgery: a meta-analysis and review of the literature. J Gastrointest Surg. 2008;12(11):2037–2044. doi: 10.1007/s11605-008-0594-8. [DOI] [PubMed] [Google Scholar]
- 8.Chen M, Song X, Chen LZ, Lin ZD, Zhang XL. Comparing Mechanical Bowel Preparation With Both Oral and Systemic Antibiotics Versus Mechanical Bowel Preparation and Systemic Antibiotics Alone for the Prevention of Surgical Site Infection After Elective Colorectal Surgery: A Meta-Analysis of Randomized Controlled Clinical Trials. Dis Colon Rectum. 2016;59(1):70–78. doi: 10.1097/DCR.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 9.Chlebicki MP, Safdar N, O’Horo JC, Maki DG. Preoperative chlorhexidine shower or bath for prevention of surgical site infection: a meta-analysis. American journal of infection control. 2013;41(2):167–173. doi: 10.1016/j.ajic.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database Syst Rev. 2015;2:CD004985. doi: 10.1002/14651858.CD004985.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaghiyan KN, Sax HC, Miraflor E, et al. Timing of Chemical Thromboprophylaxis and Deep Vein Thrombosis in Major Colorectal Surgery: A Randomized Clinical Trial. Ann Surg. 2016;264(4):632–639. doi: 10.1097/SLA.0000000000001856. [DOI] [PubMed] [Google Scholar]
- 12.Selby LV, Sovel M, Sjoberg DD, et al. Preoperative Chemoprophylaxis is Safe in Major Oncology Operations and Effective at Preventing Venous Thromboembolism. J Am Coll Surg. 2016;222(2):129–137. doi: 10.1016/j.jamcollsurg.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maiwald M, Chan ES. The forgotten role of alcohol: a systematic review and meta-analysis of the clinical efficacy and perceived role of chlorhexidine in skin antisepsis. PloS one. 2012;7(9):e44277. doi: 10.1371/journal.pone.0044277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumville JC, McFarlane E, Edwards P, et al. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev. 2015;4:CD003949. doi: 10.1002/14651858.CD003949.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao JH, Sun JX, Huang XZ, et al. Meta-analysis of the laparoscopic versus open colorectal surgery within fast track surgery. Int J Colorectal Dis. 2016;31(3):613–622. doi: 10.1007/s00384-015-2493-2. [DOI] [PubMed] [Google Scholar]
- 16.Spanjersberg WR, van Sambeeck JD, Bremers A, et al. Systematic review and meta-analysis for laparoscopic versus open colon surgery with or without an ERAS programme. Surg Endosc. 2015;29(12):3443–3453. doi: 10.1007/s00464-015-4148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li MZ, Xiao LB, Wu WH, et al. Meta-analysis of laparoscopic versus open colorectal surgery within fast-track perioperative care. Dis Colon Rectum. 2012;55(7):821–827. doi: 10.1097/DCR.0b013e31824bd31e. [DOI] [PubMed] [Google Scholar]
- 18.Zhuang CL, Huang DD, Chen FF, et al. Laparoscopic versus open colorectal surgery within enhanced recovery after surgery programs: a systematic review and meta-analysis of randomized controlled trials. Surg Endosc. 2015;29(8):2091–2100. doi: 10.1007/s00464-014-3922-y. [DOI] [PubMed] [Google Scholar]
- 19.Rondelli F, Bugiantella W, Vedovati MC, et al. To drain or not to drain extraperitoneal colorectal anastomosis? A systematic review and meta-analysis. Colorectal Dis. 2014;16(2):O35–42. doi: 10.1111/codi.12491. [DOI] [PubMed] [Google Scholar]
- 20.Petrowsky H, Demartines N, Rousson V, Clavien PA. Evidence-based value of prophylactic drainage in gastrointestinal surgery: a systematic review and meta-analyses. Ann Surg. 2004;240(6):1074–1084. doi: 10.1097/01.sla.0000146149.17411.c5. discussion 1084–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karliczek A, Jesus EC, Matos D, et al. Drainage or nondrainage in elective colorectal anastomosis: a systematic review and meta-analysis. Colorectal Dis. 2006;8(4):259–265. doi: 10.1111/j.1463-1318.2006.00999.x. [DOI] [PubMed] [Google Scholar]
- 22.Rao W, Zhang X, Zhang J, et al. The role of nasogastric tube in decompression after elective colon and rectum surgery: a meta-analysis. Int J Colorectal Dis. 2011;26(4):423–429. doi: 10.1007/s00384-010-1093-4. [DOI] [PubMed] [Google Scholar]
- 23.Jesus EC, Karliczek A, Matos D, et al. Prophylactic anastomotic drainage for colorectal surgery. Cochrane Database Syst Rev. 2004;4:CD002100. doi: 10.1002/14651858.CD002100.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castelino T, Fiore JF, Jr, Niculiseanu P, et al. The effect of early mobilization protocols on postoperative outcomes following abdominal and thoracic surgery: A systematic review. Surgery. 2016;159(4):991–1003. doi: 10.1016/j.surg.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 25.Osland E, Yunus RM, Khan S, Memon MA. Early versus traditional postoperative feeding in patients undergoing resectional gastrointestinal surgery: a meta-analysis. JPEN J Parenter Enteral Nutr. 2011;35(4):473–487. doi: 10.1177/0148607110385698. [DOI] [PubMed] [Google Scholar]
- 26.Charoenkwan K, Phillipson G, Vutyavanich T. Early versus delayed (traditional) oral fluids and food for reducing complications after major abdominal gynaecologic surgery. Cochrane Database Syst Rev. 2007;4:CD004508. doi: 10.1002/14651858.CD004508.pub3. [DOI] [PubMed] [Google Scholar]
- 27.Lewis SJ, Andersen HK, Thomas S. Early enteral nutrition within 24 h of intestinal surgery versus later commencement of feeding: a systematic review and meta-analysis. J Gastrointest Surg. 2009;13(3):569–575. doi: 10.1007/s11605-008-0592-x. [DOI] [PubMed] [Google Scholar]
- 28.Zhuang CL, Ye XZ, Zhang CJ, et al. Early versus traditional postoperative oral feeding in patients undergoing elective colorectal surgery: a meta-analysis of randomized clinical trials. Dig Surg. 2013;30(3):225–232. doi: 10.1159/000353136. [DOI] [PubMed] [Google Scholar]
- 29.Lewis SJ, Egger M, Sylvester PA, Thomas S. Early enteral feeding versus “nil by mouth” after gastrointestinal surgery: systematic review and meta-analysis of controlled trials. Bmj. 2001;323(7316):773–776. doi: 10.1136/bmj.323.7316.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meddings J, Rogers MA, Macy M, Saint S. Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010;51(5):550–560. doi: 10.1086/655133. [DOI] [PubMed] [Google Scholar]
- 31.Meddings J, Rogers MA, Krein SL, et al. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23(4):277–289. doi: 10.1136/bmjqs-2012-001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aarts MA, Okrainec A, Glicksman A, et al. Adoption of enhanced recovery after surgery (ERAS) strategies for colorectal surgery at academic teaching hospitals and impact on total length of hospital stay. Surg Endosc. 2012;26(2):442–450. doi: 10.1007/s00464-011-1897-5. [DOI] [PubMed] [Google Scholar]
- 33.Hendren S. Urinary catheter management. Clin Colon Rectal Surg. 2013;26(3):178–181. doi: 10.1055/s-0033-1351135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varadhan KK, Lobo DN. A meta-analysis of randomised controlled trials of intravenous fluid therapy in major elective open abdominal surgery: getting the balance right. Proc Nutr Soc. 2010;69(4):488–498. doi: 10.1017/S0029665110001734. [DOI] [PubMed] [Google Scholar]
- 35.Zareba P, Wu C, Agzarian J, et al. Meta-analysis of randomized trials comparing combined compression and anticoagulation with either modality alone for prevention of venous thromboembolism after surgery. Br J Surg. 2014;101(9):1053–1062. doi: 10.1002/bjs.9527. [DOI] [PubMed] [Google Scholar]
- 36.Kwon S, Meissner M, Symons R, et al. Perioperative pharmacologic prophylaxis for venous thromboembolism in colorectal surgery. J Am Coll Surg. 2011;213(5):596–603. 603 e591. doi: 10.1016/j.jamcollsurg.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasmussen MS, Jorgensen LN, Wille-Jorgensen P. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev. 2009;1:CD004318. doi: 10.1002/14651858.CD004318.pub2. [DOI] [PubMed] [Google Scholar]
- 38.de Vries FE, Gans SL, Solomkin JS, et al. Meta-analysis of lower perioperative blood glucose target levels for reduction of surgical-site infection. Br J Surg. 2016 doi: 10.1002/bjs.10424. [DOI] [PubMed] [Google Scholar]
- 39.Kwon S, Thompson R, Dellinger P, et al. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8–14. doi: 10.1097/SLA.0b013e31827b6bbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS((R))) Society recommendations. World J Surg. 2013;37(2):259–284. doi: 10.1007/s00268-012-1772-0. [DOI] [PubMed] [Google Scholar]
- 41.Thiele RH, Raghunathan K, Brudney CS, et al. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on perioperative fluid management within an enhanced recovery pathway for colorectal surgery. Perioper Med (Lond) 2016;5:24. doi: 10.1186/s13741-016-0049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ban KA, Minei JP, Laronga C, et al. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J Am Coll Surg. 2016 doi: 10.1016/j.jamcollsurg.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 43.Anderson DJPK, Berrios-Torres SI, Bratzler DW, et al. Strategies to Prevent Surgical Site Infections in Acute Care Hospitals: 2014 Update. Infect Control Hosp Epidemiol. 2014;35(06):605–627. doi: 10.1086/676022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e227S–277S. doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(17):2189–2204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]
- 46.Mandala M, Falanga A, Roila F, Group EGW Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22(Suppl 6):vi85–92. doi: 10.1093/annonc/mdr392. [DOI] [PubMed] [Google Scholar]
- 47.Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory C Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319–326. doi: 10.1086/651091. [DOI] [PubMed] [Google Scholar]
- 48.Stahl TJ, Gregorcyk SG, Hyman NH, Buie WD, Standards Practice Task Force of The American Society of C. Rectal S. Practice parameters for the prevention of venous thrombosis. Dis Colon Rectum. 2006;49(10):1477–1483. doi: 10.1007/s10350-006-0686-z. [DOI] [PubMed] [Google Scholar]
- 49.Hill J, Treasure T, Guideline Development G Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital: summary of the NICE guideline. Heart. 2010;96(11):879–882. doi: 10.1136/hrt.2010.198275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


