ABSTRACT
Impaired activity of the trophoblasts is a major contributor to the progression of pregnancy pathologies including preeclampsia (PE). This research probed the function of enhancer of zeste homolog 2 (EZH2) in activity of trophoblast cells and its correlation with growth arrest and DNA damage inducible alpha (GADD45A). EZH2 was predicted to be downregulated in placental tissues in PE according to a gene chip analysis, and reduced expression of EZH2 was detected in the placental tissues of patients with PE. Overexpression of EZH2 augmented proliferation and invasiveness of two trophoblast cell lines HTR-8/SVneo and JEG3 cells. EZH2 catalyzed trimethylation of lysine 27 on histone 3 (H3K27me3) in GADD45A promoter to suppress its transcription. GADD45A silencing increased the activity of the trophoblast cell lines and inactivated the p38/mitogen-activated protein kinase (MAPK) signaling pathway. Rescue experiments confirmed that either inhibition of GADD45A or p38 restored the proliferation, migration, and invasiveness of the trophoblast cell lines suppressed by EZH2 silencing. In conclusion, this work suggests that EZH2 enhances activity of trophoblast cell lines by suppressing GADD45A-mediated p38/MAPK signaling pathway.
KEYWORDS: Preeclampsia, trophoblast, EZH2, GADD45A, p38/MAPK
Graphical abstract
Highlights
EZH2 expression is reduced in the placental tissues of patients with PE.
EZH2 overexpression enhances proliferation and invasiveness of trophoblast cell lines.
EZH2 modifies H3K27me3 in the GADD45A promoter to suppress its transcription.
GADD45A silencing promotes activity of trophoblast cell lines by suppressing MAPK.
p38 inhibition blocks the suppressive role of sh-EZH2 in the activity of trophoblast cells.
Introduction
Preeclampsia (PE), mainly characterized by hypertension and maternal proteinuria, has been the leading contributor to morbidity and mortality in mothers, fetus and neonates in the world [1–3]. The onset and progression of PE are unpredictable [4]. Specific nursing care adhered to care protocols based on scientific evidence is required for women with PE or eclampsia [5]. However, the care of women with PE is largely limited owing to the shortage in cost-effective screening tests and interventions that can effectively prevent or treat all variations in presentation, onset, and severity of PE [6]. Due to the elusive pathogenesis and pathophysiology of PE and the limited effective clinical treatment strategies, the morbidity rate of PE is rising [7,8]. Averting adverse maternal outcome takes the first priority for PE management, including prevention and treatment of eclampsia, control of severe hypertension, and timely diagnosis and management of pulmonary edema [9].
During pregnancy, trophoblasts proliferate, migrate to and invade the decidua to help establish the uteroplacental circulation [10]. Impairment in these processes is a major contributor to PE pathogenesis [11]. Maintaining the activity of trophoblast cells is crucial to prevent development of abnormal placentation and PE, which is to date uncurable by medical interventions.
Histone modifications, which represent a major type of epigenetic mechanisms that modify gene function without changing the DNA sequence itself, are closely correlated with differentiation, migration and invasiveness of trophoblasts [12]. Histone modifications lead to active or repressive transcriptional states [13]. Trimethylation of lysine 27 on histone 3 (H3K27me3), a marker of repressive transcription, was found to be positive in most of insufficient extravillous trophoblasts in human placental villi via immunohistochemistry [14]. Enhancer of zeste homolog 2 (EZH2) is a lysine methyltransferase that suppresses expression of target genes by catalyzing H3K27me3 [15]. EZH2-mediated repression of angiopoietin like 4 has been correlated with increased migration and invasiveness of trophoblast cells [16]. However, the direct function of EZH2 in the activity of trophoblast cells requires more investigations.
Growth arrest and DNA damage inducible alpha (GADD45A) belongs to the GADD45 family of genes that are important sensors of physiological and environmental stimuli such as genotoxic damage, oxidative stress, and hypoxia [17]. Activation of GADD45A upon PE-associated stresses has been linked to impaired activity of the trophoblasts and the disease progression through the activation of downstream p38/mitogen-activated protein kinase (MAPK) signaling pathway [17–19]. It has been reported that there is H3K27me3 modification site at the GADD45A promoter [20]. We therefore hypothesized that EZH2 possibly regulates GADD45A and the p38/MAPK signaling through epigenetic regulation to mediate the activity of trophoblast cells. This study was carried out to validate the interaction between EZH2 and the GADD45A/p38/MAPK axis. Considering their convenience, reliability, stability, and frequent application in relevant researches [21,22], two commercially acquired trophoblast cell lines HTR-8/SVneo and JEG3were used in this work to analyze the role of the GADD45A/p38/MAPK axis in proliferation, migration, and invasiveness of trophoblasts.
Materials and methods
Sample collection
Placental tissues were harvested from women (normal, n = 30; PE, n = 30) underwent cesarean section from June 2018 to June 2020 at Suzhou Hospital of Integrated Traditional Chinese and Western Medicine. PE was confirmed by an over 140/90 mmHg blood pressure or mild hypertension and the presence of proteinuria (urine protein > 3 g/24 h). The clinical characteristics of included women are shown in Table 1. The placentas were rinsed with phosphate-buffered saline (PBS). Tissue samples (1 cm × 1 cm × 1 cm) in the calcified area of the surface of the maternal placenta were collected, frozen, and stored at −80°C until further use. All included individuals without unhealthy lifestyle were free of a history of chronic hypertension, heart disease, diabetes, or liver or kidney diseases. This research got the ratification of the Ethics Committee of Suzhou Hospital of Integrated Traditional Chinese and Western Medicine (Approval No. 20180405) and adhered to the guidelines in Declaration of Helsinki. Each respondent signed a written confirmed consent.
Table 1.
Clinical features of patients with PE and the healthy controls
| Clinical parameters | Normal (n = 30) | PE (n = 30) | P values |
|---|---|---|---|
| Maternal age (year) | 33.14 ± 3.56 | 32.63 ± 5.18 | > 0.05 |
| Maternal weight (kg) | 71.65 ± 7.83 | 73.22 ± 8.35 | > 0.05 |
| Smoking | 0 | 0 | > 0.05 |
| Gestational age (week) | 38.83 ± 2.59 | 35.63 ± 2.18 | < 0.05 |
| Systolic blood pressure (mm Hg) | 112.39 ± 6.56 | 165.53 ± 10.68 | < 0.001 |
| Diastolic blood pressure (mm Hg) | 74.68 ± 6.23 | 109.83 ± 9.54 | < 0.001 |
| Proteinuria (g/day) | not detected | 4.26 ± 1.31 | < 0.01 |
Note: PE, preeclampsia.
Cells
Human trophoblast cell lines HTR-8/SVneo (CRL-3271) and JEG3 (HTB-36) were provided by American Type Culture Collection (Manassas, VA, USA). The HTR-8/SVneo and JEG3 cells were incubated in Roswell Park Memorial Institute (RPMI)-1640 (Gibco Company, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. The culture condition was 37°C with 5% CO2.
Cell transfection
Exponentially growing HTR-8/SVneo and JEG3 cells (5 × 106) were seeded in 24-well plates. After 6 h when the cell confluence reached 70%-80%, the cells were transfected with overexpression plasmid-negative control (oe-NC); oe-EZH2, short hairpin (sh) RNA-NC (sh-NC), sh-EZH2 or sh-GADD45A (Vectorbuilder, Guangzhou, Guangdong, China). All transfections were conducted adhering to the instructions of the Lipofectamine 2000 kit (Thermo Scientific Pierce, Rockford, IL, USA). Transfected cells were incubated in RPMI-1640 at constant 37°C with 5% CO2 until further use.
HTR-8/SVneo cells stably transfected with sh-EZH2 were seeded in six-well plates (1 × 103 cells per well). Later, each well was filled with 2 mL RPMI-1640, and cells were treated with 20 µM SB203580 (HY-10256, MedChemExpress, Monmouth Junction, NJ, USA) for 1 h. Cells treated with equal volume of dimethyl sulfoxide (DMSO) for 1 h were set to control. After that, the medium was refreshed and the cells were collected 48 h later.
Quantitative PCR (qPCR) analysis
Total RNA was extracted according to the instructions of the TRIzol Total RNA isolation reagent (Thermo Fisher Scientific) and reverse-transcribed to cDNA using the PrimeScript RT Master Mix (Takara Holdings Inc., Kyoto, Japan). The qPCR analysis was conducted following the instruction manual of the SYBR select master mix (Thermo Fisher Scientific) and the CFX96 real-time PCR system (Bio-Rad Inc., Hercules, CA, USA). Primers listed in Table 2 were synthesized by Genewiz BioTech Co., Ltd. (Suzhou, Jiangsu, China), in which glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as the endogenous loading. Relative gene expression was evaluated by the 2−ΔΔCt method [23].
Table 2.
Primers for RT-qPCR
| Primer | Sequences (5’-3’) |
|---|---|
| EZH2 | F: GACCTCTGTCTTACTTGTGGAGC |
| R: CGTCAGATGGTGCCAGCAATAG | |
| GADD45A | F: CTGGAGGAAGTGCTCAGCAAAG |
| R: AGAGCCACATCTCTGTCGTCGT | |
| GAPDH | F: GTCTCCTCTGACTTCAACAGCG |
| R: ACCACCCTGTTGCTGTAGCCAA |
Note: EZH2, enhancer of zeste homolog 2; GADD45A, growth arrest and DNA damage inducible alpha; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; F: forward; R: reverse.
Western blot (WB) analysis
Cells were lysed in the radio-immunoprecipitation assay lysis buffer (Solarbio Science & Technology Co., Ltd., Beijing, China) to extract total protein. After protein quantification using a bicinchoninic acid kit (Sigma-Aldrich Chemical Company, St Louis, MO, USA), the protein sample was run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Millipore Corp., Billerica, MA, USA). The membranes were blocked with 5% nonfat milk at 22°C for 2 h and hybridized with the antibodies to GAPDH (1:10,000, ab181602, Abcam Inc., Cambridge, MA, USA), EZH2 (1:1,000, GTX82503, GeneTex, CA, USA), GADD45A (1:1,000, PA5-88248, Thermo Fisher Scientific), H3K27me3 (1:1,000, ab192985, Abcam), p38 (1:2,000, GTX110720, GeneTex) and p-p38 (1:2,000, GTX133460, GeneTex) at 4°C for 16 h, and then with goat anti-rabbit immunoglobulin G (IgG, 1:2,000, ab205718, Abcam) at 22°C for 2 h. The blot bands were developed using the ECL reagent (Beyotime Biotechnology Co., Ltd., Shanghai, China) and imaged utilizing the BIO-RAD imaging system (Bio-Rad). Protein level relative to the endogenous loading GAPDH was evaluated using Image J [24].
Immunohistochemistry (IHC)
The collected placental tissues were embedded and sectioned. After dewaxing and rehydration, the sections were treated with 3% H2O2 at 22°C for 15 min and blocked with normal goat serum at 22°C for 15 min. Thereafter, the sections were reacted with 50 μL the primary antibodies against EZH2 (1:50, GTX82503, GeneTex), cytokeratin 7 (CK7, 1:100, ab183344, Abcam), and GADD45A (1:100, PA5-88248, Thermo Fisher Scientific) overnight at 4°C, and then with IgG (1:2,000, ab205718, Abcam) at 37°C for 15 min. After that, 40 μL horseradish peroxidase-labeled streptavidin-working solution was added for 15 min. After color development using 3,3’-diaminobenzidine, the sections were counter-stained with hematoxylin for 30s, dehydrated, and sealed for observation. Positive cells were counted under the microscope (Olympus Optical Co., Ltd., Tokyo, Japan) [25].
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay
Viability of cells was examined using the MTT kit (C0009, Beyotime) adhering to the manufacturer’s protocol. Well-growing cells were seeded in 96-well plates at 1 × 103 cells per well. Cells were counted at the 0, 24, 48, and 72 h. Collected cells were treated with 20 μL of MTT solution and further incubated at 37°C with 5% CO2 for 4 h. Thereafter, 150 μL DMSO was added to each well to dissolve the crystal. The optical density at 490 nm was determined using the microplate reader (ELx808, Biotek, USA) [25].
5-ethynyl-2’-deoxyuridine (EdU) labeling assay
An EdU labeling kit (RiboBio) was applied to evaluate cell proliferation. In short, transfected HTR-8/SVneo and JEG3 cells were loaded in 24-well plates (5 × 105 cells/well). After 24 h, 50 μM EdU was added according to the kit’s instructions for 2 h of incubation. The cells were washed, fixed, penetrated with 0.5% Triton X-100, reacted with 200 μL of 1× Apollo reaction mixture avoiding light exposure for 30 min, and stained with 200 μL 1× Hoechst 33342 solution avoiding light exposure at 22°C for 30 min. Positively stained cells in five random fields of views were counted under the fluorescence microscope (FSX100, Olympus). The EdU-positive (proliferating) cells were stained in red and total cells were stained in blue [26].
Scratch test
The transfected trophoblast cells were cultured in 6-well plates at 1 × 106 cells per well. After complete adherence of cells to the bottom, a 2-mm cell scraper was used to scratch in the middle of each well. The medium and floating cells were washed with PBS. The cell culture was continued in renewed medium at 37°C with 4% CO2 for 48 h. The width of the scratches at the 0 and 48 h was observed under the microscope (IX51, Olympus) and analyzed using the cellSens standard imaging software (Olympus). The migration rate was cells was measured as follow: rate = (width at 0 h -width at 48 h)/width at 0 h [27].
Transwell assay
The invasiveness of cells was analyzed by the Transwell assay. In short, 200 μL serum-free suspension of HTR-8/SVneo or JEG3 cells (5 × 104) was loaded on the Transwell apical chambers pre-coated with Matrigel. To the basolateral chambers, 500 μL 10% FBS-contained medium was added. The cells were incubated for 48 h. After that, the invaded cells were fixed and stained with 1% crystal violet for 30 min, and then counted under the microscope [28].
Chromatin immunoprecipitation (ChIP)-qPCR
The cells were warm incubated in 4% formaldehyde for 10 min, and lysed at 4°C for 1 h. The cell lysates were ultrasonicated to 500–800-bp chromatin fragments. Thereafter, the samples were pre-purified with protein A agarose at 4°C and then reacted with anti-EZH2 (1:100, GTX110384, GeneTex) and anti-H3K27me3 (1:200, ab192985, Abcam) at 4°C overnight. The immunoprecipitates were captured using 10% (v/v) protein A agarose. To examine the binding of EZH2 and H3K27me3 with the GADD45A promoter, the enrichment of GADD45A in the immunoprecipitates was examined by qPCR [29].
Statistical analysis
All data analysis was performed using the SPSS22.0 (IBM Corp. Armonk, NY, USA). All cellular experiments were repeated for three times. Measurement data were presented as the mean ± standard deviation. The inter-group differences were determined by the unpaired t test, or by one- or two-way analysis of variance followed by Tukey’s post-hoc test. The log rank test was applied for post-statistical analysis. Significant differences were set at p < 0.05.
Results
Starting paragraph
Dysregulated histone methylation represents one of the major epigenetic changes closely linked to the development of PE. H3K27me3 is a repressive transcription marker positively expressed in most of insufficient extravillous trophoblasts in human placental villi, and our bioinformatics analyses predicted the lysine methyltransferase EZH2, which can catalyze H3K27me3 modification, is poorly expressed in PE. H3K27me3 modification site has also been found at the GADD45A promoter, and GADD45A has reportedly been correlated with impaired activity of trophoblasts via the p38/MAPK signaling. We therefore hypothesized that EZH2 possibly affects GADD45A via H3K27me3 modification and validated this interaction via ChIP-qPCR assay. Also, altered expression of EZH2, GADD45A, and p38 was introduced in two trophoblast cell lines HTR-8/SVneo and JEG3 to investigate their roles in proliferation, migration, and invasiveness of trophoblast cells.
EZH2 is expressed at low levels in the placental tissues of patients with PE
A PE-related dataset GSE147776 was obtained from Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/gds/?term=GSE147776+) to analyze differentially expressed genes (DEGs) between normal women and PE patients. The data were downloaded, and the raw data were re-normalized. DEGs were ranked and then screened by p < 0.05. Dysregulated histone methylation represents one of the major epigenetic changes closely linked to the development of PE [30]. We therefore focused on the histone methyltransferases and demethylases in these DEGs, which are presented in the heatmap in Figure 1(a). According to the |Log foldchange|, we selected the histone methyltransferase EZH2 as the study subject. EZH2 was suggested to be poorly expressed in the placental tissues of PE patients. In line, the subsequent qPCR analysis on clinical placental tissue samples from PE patients (n = 30) and normal women (n = 30) suggested that EZH2 was significantly poorly expressed in the placental tissues of PE (Figure 1(b)). The IHC assay confirmed the positive expression of the pan-trophoblast marker CK7 in the tissues (Figure 1(c)) and further detected decreased expression of EZH2 in the placental tissues of PE versus the normal placental tissues (Figure 1(d)). The clinical characteristics of included PE patients and normal pregnant women are presented in Table 1. It was found that the systolic blood pressure and diastolic blood pressure of patients with PE were increased compared to the normal women. The patients had proteinuria, but the normal patients did not. Moreover, the gestational age of patients was shorter than that of the healthy women.
Figure 1.
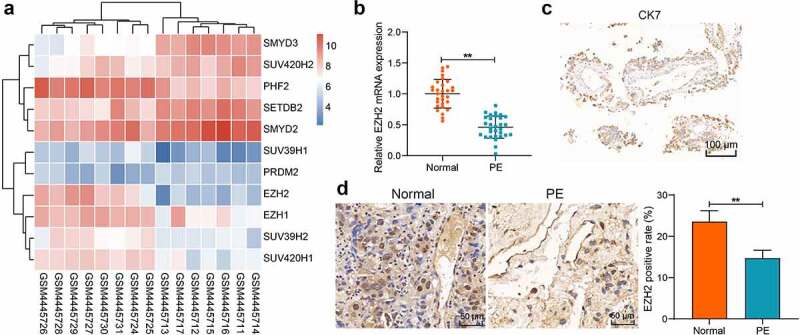
EZH2 expression is reduced in the placental tissues of PE patients. A, a heatmap for differentially expressed histone methyltransferases and demethylases in the GSE147776 dataset; B, EZH2 expression level in the clinical placental tissue samples from PE patients (n = 30) and normal pregnant women (n = 30) examined by qPCR analysis; C, expression of the pan-trophoblast marker CK7 in the clinically collected placental tissues examined by IHC; D, EZH2 expression level in the clinical placental tissue samples from PE patients (n = 30) and normal pregnant women (n = 30) detected by IHC assays. Repetition = 3. **p < 0.01.
EZH2 overexpression induces proliferation and invasiveness of trophoblast cell lines
To examine the function of EZH2 in trophoblast cell lines, EZH2 overexpression was induced in HTR-8/SVneo and JEG3 cells. The qPCR and WB analyses suggested that the EZH2 levels in cells were significantly elevated by oe-EZH2 (Figure 2(a-b)). The MTT assay indicated that the viability of the trophoblast cells was enhanced after EZH2 overexpression (Figure 2(c)). The EdU labeling also suggested that the proliferation activity of the cells was enhanced by oe-EZH2 (Figure 2(d)). The scratch and Transwell assays indicated that the migration ability and invasiveness of the trophoblast cell lines were increased as well after EZH2 overexpression (Figure 2(e-f)). These results indicate that EZH2 can enhance proliferation and migration of trophoblast cells.
Figure 2.
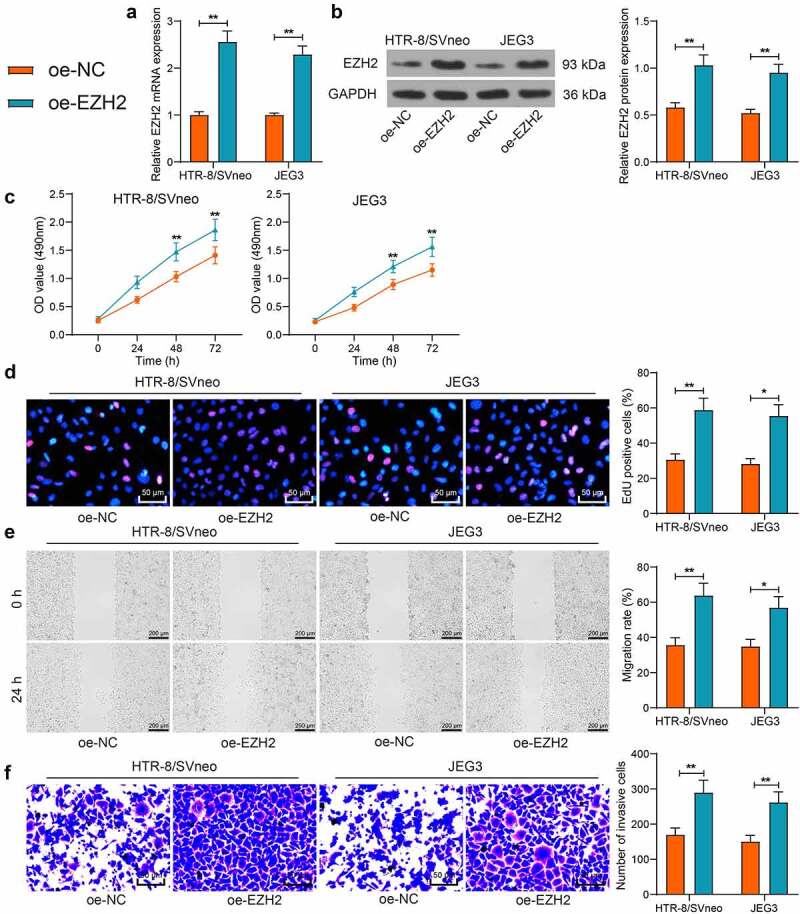
EZH2 overexpression induces proliferation and invasiveness of trophoblast cell lines. A-B, mRNA (a) and protein (b) levels of EZH2 in HTR-8/SVneo and JEG3 cells after oe-EZH2 or oe-NC transfection examined by qPCR and WB assays; C, viability of HTR-8/SVneo and JEG3 cells examined by the MTT assay; D, proliferation activity of HTR-8/SVneo and JEG3 cells determined by the EdU labeling assay; E, migration of HTR-8/SVneo and JEG3 cells examined by the scratch test; F, invasiveness of HTR-8/SVneo and JEG3 cells examined by the transwell assay. Repetition = 3. *p < 0.05; **p < 0.01.
EZH2 catalyzes H3K27me3 modification in the GADD45A promoter to suppress its transcription
As a histone methyltransferase, EZH2 can regulate H3K27me3 modification, a transcriptional repressive marker, to mediate gene expression. There is H3K27me3 modification site at the GADD45A promoter [20]. Therefore, we surmised that EZH2 possibly regulates GADD45A expression in PE. The GADD45A expression in the placental tissues was measured. Both qPCR and IHC assays identified that the GADD45A expression was noticeably increased in the placenta of PE patients (Figure 3(a-b)). In HTR-8/SVneo and JEG3 cells overexpressing EZH2, the GADD45A expression was significantly reduced (Figure 3(c-d)). To validate the conjecture above, the H3K27me3 protein level in cells was determined. We found that EZH2 upregulation elevated the H3K27me3 levels both in HTR-8/SVneo and JEG3 cells (Figure 3(e)). Moreover, the enrichment of GADD45A promoter by EZH2 and H3K27me3 was detected by the ChIP-qPCR assay. EZH2 overexpression in cells increased the abundance of GADD45A fragments either enriched by anti-EZH2 or anti-H3K27me3 (figure 3(f)). These results indicated that EZH2 can catalyze H3K27me3 modification at the GADD45A promoter to suppress its transcription.
Figure 3.
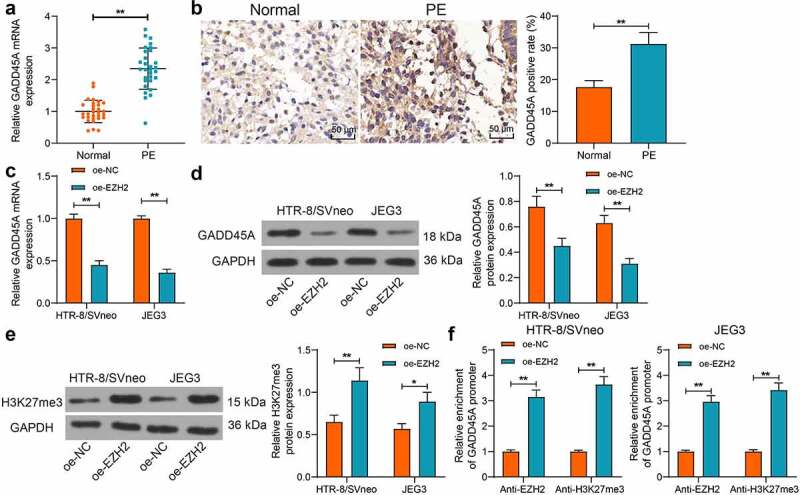
EZH2 catalyzes H3K27me3 modification in GADD45A promoter to suppress its transcription. A-B, GADD45A expression level in the clinical placental tissue samples from PE patients (n = 30) and normal pregnant women (n = 30) detected by qPCR (a) and IHC (b) assays; C-D, mRNA (c) and protein (d) levels of GADD45A in HTR-8/SVneo and JEG3 cells after oe-EZH2 or oe-NC transfection examined by qPCR and WB analyses; E, protein level of H3K27me3 in HTR-8/SVneo and JEG3 cells detected by WB analysis; F, enrichment of GADD45A fragments enriched by anti-EZH2 and anti-H3K27me3 examined by the ChIP-qPCR assay. Repetition = 3. *p < 0.05; **p < 0.01.
Silencing of GADD45A promotes proliferation and invasiveness of trophoblast cell lines possibly by suppressing the MAPK signaling pathway
To determine the function of GADD45A in the activity of trophoblast cell lines, sh-NC or sh-GADD45A was administrated into the HTR-8/SVneo and JEG3 cells, and the successful silencing of GADD45A in cells was detected by qPCR analysis (Figure 4(a)). Thereafter, the MTT assay suggested that the growth and viability of cells was enhanced after GADD45A silencing (Figure 4(b)). Silencing of GADD45A also strengthened the proliferation activity of the HTR-8/SVneo and JEG3 cells (Figure 4(c)). Moreover, the scratch and Transwell assays indicated that the migration and invasiveness of cells were enhanced after GADD45A silencing (Figure 4(d-e)). To examine the molecular mechanism, a Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was performed to analyzed the GADD45A-related genes. The results were visualized using a bubble chart. Seventeen GADD45A-enriched signaling pathways were presented, and GADD45A was analyzed to be closely correlated with cell cycle, cancer progression, and the MAPK signaling pathway (figure 4(f-g)). Moreover, KEGG analysis of the EZH2-related genes suggested that many of these genes were also enriched in the MAPK signaling pathway (Figure 4(h)). The WB analysis suggested the phosphorylation of p38, the MAPK pathway marker protein, was significantly suppressed after GADD45A inhibition (Figure 4(i)). These results indicated that silencing of GADD45A triggers proliferation, migration, and invasiveness of trophoblast cells possibly by blocking the MAPK signaling pathway.
Figure 4.
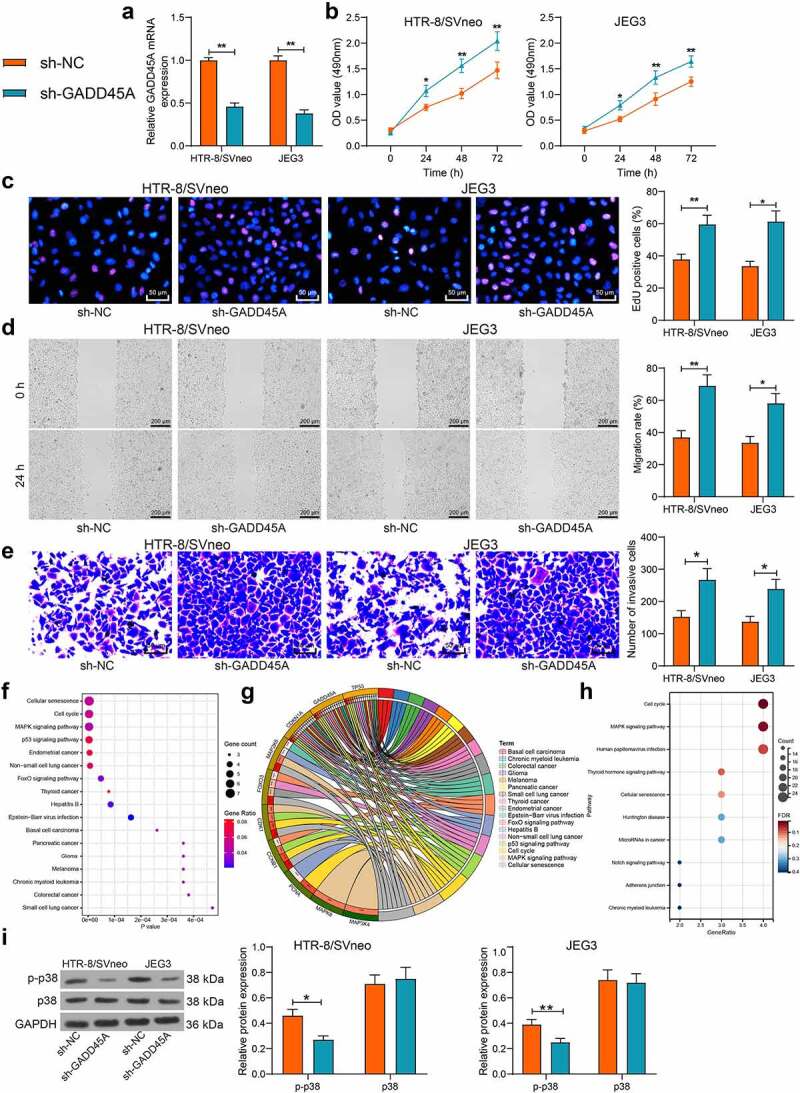
GADD45A silencing promotes proliferation and invasiveness of trophoblast cell lines possibly through suppressing the MAPK signaling pathway. A, GADD45A mRNA in HTR-8/SVneo and JEG3 cells after transfection of sh-NC or sh-GADD45A determined by qPCR analysis; B, viability of cells examined by the MTT assay; C, proliferation activity cells examined by the EdU labeling assay; D, migration ability of HTR-8/SVneo and JEG3 cells examined by the scratch test; E, invasiveness of HTR-8/SVneo and JEG3 cells examined by the transwell assay; F-H, GADD45A enriched signaling pathways analyzed by the KEGG enrichment analysis; I, protein level and phosphorylation of p38 in cells examined by WB analysis. Repetition = 3. *p < 0.05; **p < 0.01.
GADD45A silencing restores the proliferation and invasiveness of trophoblast cell lines suppressed by sh-EZH2
To examine the interaction between GADD45A and EZH2 in trophoblast cells, the HTR-8/SVneo cells with higher activity were administrated with sh-EZH2 alone or sh-EZH2 + sh-GADD45A. The qPCR analysis indicated that sh-EZH2 suppressed the EZH2 expression but increased the expression of GADD45A, whereas sh-GADD45A reduced the expression of GADD45A but had no effect on the expression of EZH2 (Figure 5(a)). It was found that the viability, proliferation activity, and migration ability and invasiveness of cells were significantly suppressed by EZH2 silencing alone but then rescued following GADD45A suppression (Figure 5(b-e)). Moreover, the WB assay suggested that the phosphorylation of p38 in cells was promoted after EZH2 suppression but inhibited after GADD45A knockdown (figure 5(f)). This body of evidence supports that EZH2 possibly suppresses GADD45A and inactivates the MAPK signaling to enhance proliferation and invasiveness of cells.
Figure 5.
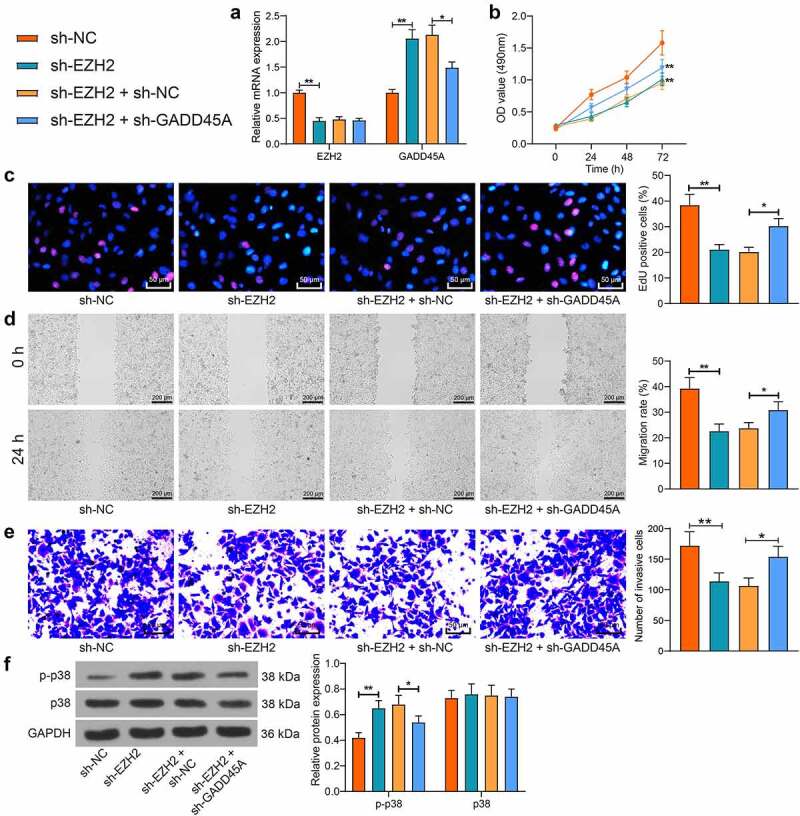
Silencing of GADD45A restores proliferation and invasiveness of trophoblast cell lines suppressed by sh-EZH2. A, EZH2 and GADD45A mRNA in HTR-8/SVneo cells after transfection of sh-EZH2 alone or sh-EZH2 + sh-GADD45A determined by qPCR analysis; B, viability of cells determined by the MTT assay; C, proliferation activity of cells measured by the EdU labeling assay; D, migration of HTR-8/SVneo and JEG3 cells examined by the scratch test; E, invasiveness of HTR-8/SVneo and JEG3 cells examined by the transwell assay; F, protein level and phosphorylation of p38 in cells determined by WB analysis. Repetition = 3. *p < 0.05; **p < 0.01.
Inhibition of p38 blocks the suppressive role of sh-EZH2 in the proliferation and invasiveness of trophoblast cell lines
To confirm whether the p38/MAPK pathway is implicated in the events above, a p38-specific antagonist SB203580 was administrated into HTR-8/SVneo cells following sh-EZH2 transfection. After that, the phosphorylation of p38 in cells was suppressed (Figure 6(a)). Thereafter, it was observed that the proliferation, migration, and invasiveness of cells suppressed by sh-EZH2 was rescued by p38 inhibition (Figure 6(b-e)). Collectively, it can be inferred that EZH2 enhances the proliferation and migration of trophoblast cells by blocking the p38/MAPK signaling pathway.
Figure 6.
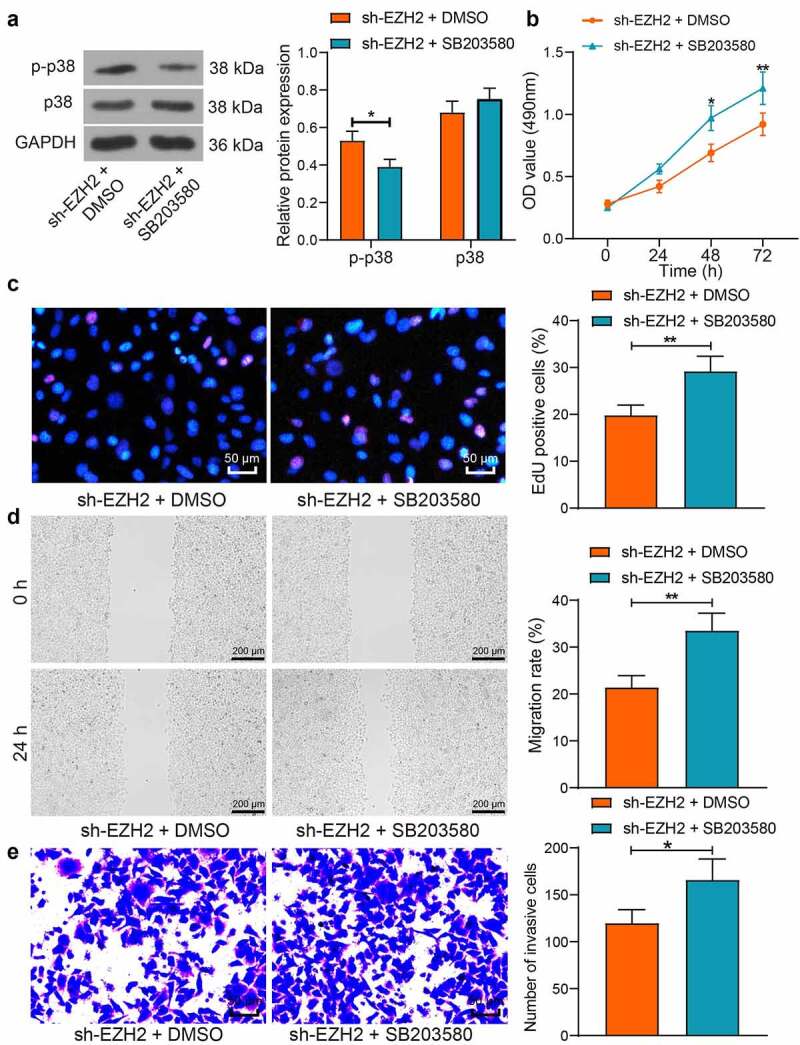
Inhibition of p38 blocks the suppressive role of sh-EZH2 in the proliferation and invasiveness of trophoblast cell lines. A, protein level and phosphorylation of p38 in HTR-8/SVneo cells after SB203580 treatment determined by WB analysis; B, viability of cells examined by the MTT assay; C, proliferation activity cells examined by the EdU labeling assay; D, migration ability of HTR-8/SVneo and JEG3 cells examined by the scratch test; E, invasiveness of HTR-8/SVneo and JEG3 cells examined by the transwell assay. Repetition = 3. *p < 0.05; **p < 0.01.
Discussion
The etiology of PE remains elusive despite decades of investigation, and immediate termination of pregnancy has been the most effective treatment [31]. Except this, there is limiting strategy in the prevention or treatment of PE, with approximately 300,000 mothers and 500,000 fetuses died during the disease progression each year [32]. In the present study, the authors report that the histone methyltransferase EZH2 can strengthen trophoblast proliferation and migration by inhibiting GADD45A-mediated p38/MAPK pathway.
Bioinformatics analysis using gene expression chips has been helpful tools for the identification of key genes implicated in diseases, including PE [33,34]. In this study, the analysis on a GSE147776 gene chip suggested EZH2 as a poorly expressed gene in PE. EZH2 has been reported to be crucial for the early embryogenesis of mice [35]. Likewise, EZH2 deficiency in mouse embryos was associated with impaired embryonic development but increased embryonic lethality [36]. Moreover, EZH2 suppression was implicated in the development of human recurrent miscarriage, in which reduced trophoblast activity plays a major role [37]. This body of evidence suggested that downregulation of EZH2 is potentially correlated with reduced trophoblast activity in PE as well. In the subsequent experiments, reduced expression of EZH2 was detected in the placentas of PE, and upregulation of EZH2 enhanced the activity of HTR-8/SVneo and JEG3 cells, validating that EZH2 can enhance the activity of trophoblast cells in vitro. As aforementioned, EZH2 suppresses transcription activity of genes by catalyzing H3K27me3 modification [15]. Here, the mechanism of action involved in the EZH2-mediated trophoblast activity required further investigation.
Positive H3K27me3 has been found in most of insufficient extravillous trophoblasts in human placental villi [14]. Also, H3K27me3 modification site was found in GADD45A promoter [20]. Moreover, the GADD45A signaling was activated in response to the PE-associated stresses [17]. In line, in this work, high GADD45A expression was detected in the placental tissues of PE, and EZH2 upregulation elevated the level of H3K27me3 and epigenetically suppressed the expression of GADD45A. Importantly, artificial silencing of GADD45A suppressed the activity of trophoblast cell lines. Similar results have been observed in previous reports that restoration of GADD45A inhibited the viability, migration, invasiveness and angiogenesis of HTR-8/SVneo cells but promoted cell apoptosis [38,39]. Thereafter, our pathway enrichment analysis revealed that the GADD45A-related genes were mainly enriched in the p38/MAPK pathway. Of note, this signaling has been implicated in oxidative stress in the pathogenesis of PE mediated by GADD45A [19]. Aberrant activation of the p38/MAPK signaling leads to PE by regulating oxidative stress and apoptosis of endothelial cells and affecting the angiogenesis of extravillous trophoblasts [40]. Studies have demonstrated the important involvement of MAPK cascades, including the p38 MAPK, JNK and ERK, in the regulation of trophoblast function [41,42]. Recent evidence has also validated that decreased phosphorylation of p38 and JNK was correlated with increased proliferation and colony formation abilities of trophoblast cells [43,44]. In the present study, silencing of EZH2 led to increased phosphorylation of p38, and the rescue experiments suggested that either inhibition of GADD45A or p38 restored the proliferation, migration, and invasiveness of the trophoblast cell lines suppressed by EZH2 silencing, indicating that EZH2 enhances the activity of trophoblast cells, at least partially, through suppressing GADD45A and the p38/MAPK axis.
Conclusion
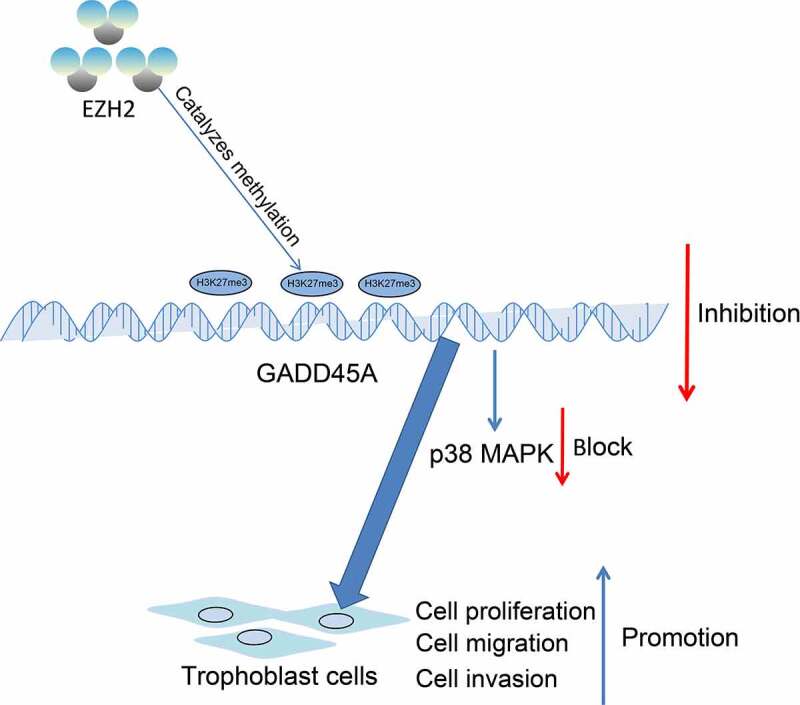
In conclusion, this study demonstrates that EZH2 plays a promoting role in the proliferation and motility of trophoblast cell lines, at least partially, via the epigenetic suppression of the GADD45A transcription and the subsequent inhibition of the p38/MAPK axis (Figure 7). These results validated that reduced EZH2 or increased GADD45A might serve as biological markers for the early prediction of PE or other pregnancy pathologies caused by reduced activity of trophoblast cell lines. However, there might be more signaling pathways and other molecules in the MAPK pathway such as JNK and ERK that are involved in the GADD45A-manipulated events. We would like to probe more molecules responsible for impaired activity of trophoblasts. We also hope more researches will be launched to probe more molecular mechanisms implicated in PE.
Figure 7.
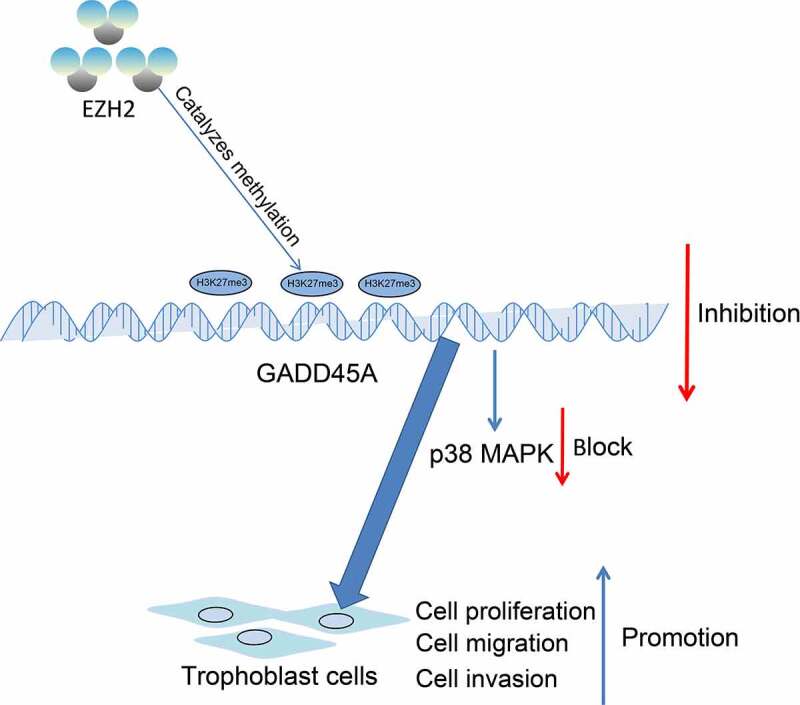
EZH2 epigenetically suppresses GADD45A transcription by catalyzing H3K27me3 modification, which subsequently inhibits the p38/MAPK axis and promotes the proliferation and invasiveness of trophoblast cells.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
References
- [1].Mol BWJ, Roberts CT, Thangaratinam S, et al. Pre-eclampsia. Lancet. 2016;387(10022):999–1011. [DOI] [PubMed] [Google Scholar]
- [2].von Dadelszen P, Magee LA.. Pre-eclampsia: an update. Curr Hypertens Rep. 2014;16(8):454. [DOI] [PubMed] [Google Scholar]
- [3].Zhang S, Zou Y, Tang X, et al. Silencing of AFAP1-AS1 lncRNA impairs cell proliferation and migration by epigenetically promoting DUSP5 expression in pre-eclampsia. J Cell Biochem. 2021;122(10):1506–1516. [DOI] [PubMed] [Google Scholar]
- [4].Staff AC. The two-stage placental model of preeclampsia: an update. J Reprod Immunol. 2019;134-135:1–10. [DOI] [PubMed] [Google Scholar]
- [5].Ferreira MB, Silveira CF, Silva SR, et al. Nursing care for women with pre-eclampsia and/or eclampsia: integrative review. Rev Esc Enferm USP. 2016;50(2):324–334. [DOI] [PubMed] [Google Scholar]
- [6].Anderson CM, Schmella MJ. CE: preeclampsia: current approaches to nursing management. Am J Nurs. 2017;117(11):30–38. [DOI] [PubMed] [Google Scholar]
- [7].Rana S, Lemoine E, Granger JP, et al. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124(7):1094–1112. [DOI] [PubMed] [Google Scholar]
- [8].Zakiyah N, Postma MJ, Baker PN, et al. Pre-eclampsia diagnosis and treatment options: a review of published economic assessments. Pharmacoeconomics. 2015;33(10):1069–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Witcher PM. Preeclampsia: acute complications and management priorities. AACN Adv Crit Care. 2018;29(3):316–326. [DOI] [PubMed] [Google Scholar]
- [10].Kuo CY, Guo T, Cabrera-Luque J, et al. Placental basement membrane proteins are required for effective cytotrophoblast invasion in a three-dimensional bioprinted placenta model. J Biomed Mater Res A. 2018;106(6):1476–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].McNally R, Alqudah A, Obradovic D, et al. Elucidating the pathogenesis of pre-eclampsia using in vitro models of spiral uterine artery remodelling. Curr Hypertens Rep. 2017;19(11):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kohan-Ghadr HR, Kadam L, Jain C, et al. Potential role of epigenetic mechanisms in regulation of trophoblast differentiation, migration, and invasion in the human placenta. Cell Adh Migr. 2016;10(1–2):126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074–1080. [DOI] [PubMed] [Google Scholar]
- [14].Fogarty NM, Burton GJ, Ferguson-Smith AC. Different epigenetic states define syncytiotrophoblast and cytotrophoblast nuclei in the trophoblast of the human placenta. Placenta. 2015;36(8):796–802. [DOI] [PubMed] [Google Scholar]
- [15].Margueron R, Reinberg D. The polycomb complex PRC2 and its mark in life. Nature. 2011;469(7330):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xu Y, Lian Y, Zhang Y, et al. The long non-coding RNA PVT1 represses ANGPTL4 transcription through binding with EZH2 in trophoblast cell. J Cell Mol Med. 2018;22(2):1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xiong Y, Liebermann DA, Holtzman EJ, et al. Preeclampsia-associated stresses activate Gadd45a signaling and sFlt-1 in placental explants. J Cell Physiol. 2013;228(2):362–370. [DOI] [PubMed] [Google Scholar]
- [18].Liu X, Deng Q, Luo X, et al. Oxidative stress-induced Gadd45alpha inhibits trophoblast invasion and increases sFlt1/sEng secretions via p38 MAPK involving in the pathology of pre-eclampsia. J Matern Fetal Neonatal Med. 2016;29(23):3776–3785. [DOI] [PubMed] [Google Scholar]
- [19].Li FH, Han N, Wang Y, et al. Gadd45a knockdown alleviates oxidative stress through suppressing the p38 MAPK signaling pathway in the pathogenesis of preeclampsia. Placenta. 2018;65:20–28. [DOI] [PubMed] [Google Scholar]
- [20].Ma P, Pan Y, Yang F, et al. KLF5-Modulated lncRNA NEAT1 contributes to tumorigenesis by acting as a scaffold for BRG1 to silence GADD45A in gastric cancer. Mol Ther Nucleic Acids. 2020;22:382–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cheng D, Jiang S, Chen J, et al. The increased lncRNA MIR503HG in preeclampsia modulated trophoblast cell proliferation, invasion, and migration via regulating matrix metalloproteinases and NF-kappaB signaling. Dis Markers. 2019;2019:4976845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang Y, Zou Y, Wang W, et al. Down-regulated long non-coding RNA MEG3 and its effect on promoting apoptosis and suppressing migration of trophoblast cells. J Cell Biochem. 2015;116(4):542–550. [DOI] [PubMed] [Google Scholar]
- [23].Zhou X, Liu X, Zhang G, et al. Knockdown THOC2 suppresses the proliferation and invasion of melanoma. Bioengineered. 2019;10(1):635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liu B, Sun X. miR-25 promotes invasion of human non-small cell lung cancer via CDH1. Bioengineered. 2019;10(1):271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang J, Sun M, Hao M, et al. FAM53A affects breast cancer cell proliferation, migration, and invasion in a p53-dependent manner. Front Oncol. 2019;9:1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li Y, Li X, Qu J, et al. Cas9 mediated correction of beta-catenin mutation and restoring the expression of protein phosphorylation in colon cancer HCT-116 cells decrease cell proliferation in vitro and hamper tumor growth in mice in vivo. Onco Targets Ther. 2020;13:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang L, Li H, Li M, et al. LRP6 is involved in the proliferation, migration and invasion of trophoblast cells via miR346. Int J Mol Med. 2020;46(1):211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Suo M, Sun Y, Yang H, et al. miR-183-5p suppressed the invasion and migration of HTR-8/SVneo trophoblast cells partly via targeting MMP-9 in preeclampsia. Biosci Rep. 2020;40:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li N, Zhao Z, Liu P, et al. Upregulation of deubiquitinase USP7 by transcription factor FOXO6 promotes EC progression via targeting the JMJD3/CLU axis. Mol Ther Oncolytics. 2021;20:583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sheng W, Gu Y, Chu X, et al. Upregulation of histone H3K9 methylation in fetal endothelial cells from preeclamptic pregnancies. J Cell Physiol. 2021;236(3):1866–1874. [DOI] [PubMed] [Google Scholar]
- [31].Du MR, Wang SC, Li DJ. The integrative roles of chemokines at the maternal-fetal interface in early pregnancy. Cell Mol Immunol. 2014;11(5):438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ahmed A, Rezai H, Broadway-Stringer S. Evidence-based revised view of the pathophysiology of preeclampsia. Adv Exp Med Biol. 2017;956:355–374. [DOI] [PubMed] [Google Scholar]
- [33].He J, Liu K, Hou X, et al. Identification and validation of key non-coding RNAs and mRNAs using co-expression network analysis in pre-eclampsia. Medicine (Baltimore). 2021;100(14):e25294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu K, Fu Q, Liu Y, et al. An integrative bioinformatics analysis of microarray data for identifying hub genes as diagnostic biomarkers of preeclampsia. Biosci Rep. 2019;39:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ge SQ, Lin SL, Zhao ZH, et al. Epigenetic dynamics and interplay during spermatogenesis and embryogenesis: implications for male fertility and offspring health. Oncotarget. 2017;8(32):53804–53818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Huang XJ, Wang X, Ma X, et al. EZH2 is essential for development of mouse preimplantation embryos. Reprod Fertil Dev. 2014;26(8):1166–1175. [DOI] [PubMed] [Google Scholar]
- [37].Lv S, Wang N, Lv H, et al. The attenuation of trophoblast invasion caused by the downregulation of EZH2 is involved in the pathogenesis of human recurrent miscarriage. Mol Ther Nucleic Acids. 2019;14:377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tan Y, Xiao D, Xu Y, et al. Long non-coding RNA DLX6-AS1 is upregulated in preeclampsia and modulates migration and invasion of trophoblasts through the miR-376c/GADD45A axis. Exp Cell Res. 2018;370(2):718–724. [DOI] [PubMed] [Google Scholar]
- [39].Yang Y, Shang H. Silencing lncRNA-DGCR5 increased trophoblast cell migration, invasion and tube formation, and inhibited cell apoptosis via targeting miR-454-3p/GADD45A axis. Mol Cell Biochem. 2021;476(9):3407–3421. [DOI] [PubMed] [Google Scholar]
- [40].Lu F, Gong H, Lei H, et al. Downregulation of cathepsin C alleviates endothelial cell dysfunction by suppressing p38 MAPK/NF-kappaB pathway in preeclampsia. Bioengineered. 2022;13(2):3019–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chakraborty C, Gleeson LM, McKinnon T, et al. Regulation of human trophoblast migration and invasiveness. Can J Physiol Pharmacol. 2002;80(2):116–124. [DOI] [PubMed] [Google Scholar]
- [42].Wong CH, Cheng CY. Mitogen-activated protein kinases, adherens junction dynamics, and spermatogenesis: a review of recent data. Dev Biol. 2005;286(1):1–15. [DOI] [PubMed] [Google Scholar]
- [43].Che G, Wang Y, Zhou B, et al. Knockdown of heparanase suppresses invasion of human trophoblasts by activating p38 MAPK signaling pathway. Dis Markers. 2018;2018:7413027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jiang J, Zhao ZM. LncRNA HOXD-AS1 promotes preeclampsia progression via MAPK pathway. Eur Rev Med Pharmacol Sci. 2018;22(24):8561–8568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.


