ABSTRACT
T helper 17 (Th17) cells regulate inflammatory processes and are implicated in pathogenesis of proliferative diabetic retinopathy (PDR) through modulation of interleukin-17 (IL-17). IL-35, anti-inflammatory factor, negatively mediates IL-17 expression and Th17 differentiation. In this study, the role of IL-35 in PDR was assessed. The results showed that IL-35 was down-regulated, while IL-17 was up-regulated, in peripheral blood mononuclear cells (PBMCs) of PDR patients. In addition, immunofluorescence analysis indicated that frequency of Th17 cells was enhanced in the PBMCs of PDR patients. However, incubation with IL-35 reduced the Th17 cell frequency and decreased the level of IL-17 in CD4+ T lymphocytes. Moreover, the levels of transcription factors essential for Th17 differentiation, ROR α (retinoid-related orphan receptor alpha) and ROR γt, were reduced by IL-35 treatment. In conclusion, IL-35 reduced level of IL-17 and inhibited Th17 differentiation to protect against PDR.
KEYWORDS: IL-35, IL-17, Th17 cell, ROR α, ROR γt, proliferative diabetic retinopathy
GRAPHICAL ABSTRACT
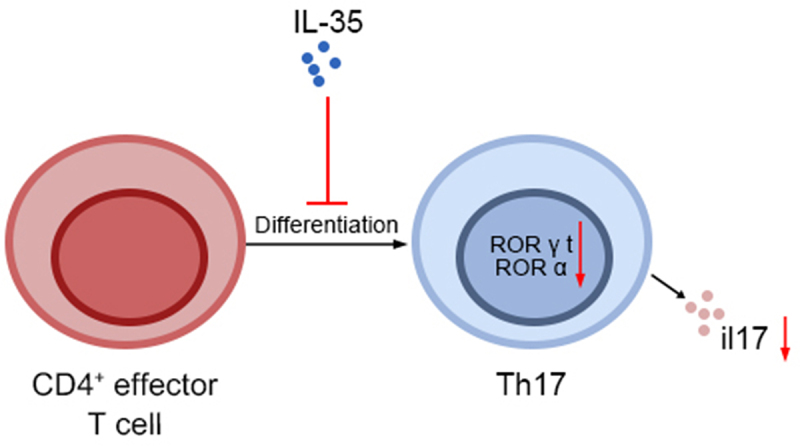
Highlights
IL-35 reduced Th17 frequency and decreased IL-17 in CD4+ T lymphocytes.
IL-35 reduced levels of ROR α and ROR γt to inhibit Th17 differentiation.
IL-35 protected against proliferative diabetic retinopathy.
Introduction
Proliferative diabetic retinopathy (PDR) is a severe stage of DR, characterized by fibrovascular changes that lead to vitreous hemorrhage or traction retinal detachment [1]. Panretinal photocoagulation and anti-vascular endothelial growth factor agents have been widely used in the treatment of PDR [2]. However, intraoperative and postoperative complications from vitrectomy surgery might constrain the benefits of surgical treatment [2]. Therefore, other strategies are urgently needed for the treatment of PDR. Increasing evidence has shown that inflammatory factors were elevated in serum of patients with PDR, local inflammation was implicated in the pathogenesis of PDR [3]. Therefore, regulation of the inflammatory factors might be promising strategies for prevention of PDR.
T helper 17 (Th17) cells belong to CD4+ T lineage, and regulate secretion of IL-17A and the development of autoimmune or inflammatory diseases [4]. IL-17 secreted by Th17 cells initiates secretion of pro-inflammatory factors and further amplifies the inflammatory response in inflammatory and autoimmune diseases [5]. Therefore, Th17 and IL-17 might be involved in pathogenesis of DR. For example, in type 2 diabetes, circulating Th17 cells were up-regulated, promoted production of IL-17 and contributed to the chronic inflammation [6]. Similarly, Th17 cell frequency and concentrations of IL-17A were enhanced in PBMCs of patients with DR [7]. Suppression of Th17 cell differentiation and down-regulation of IL-17 expression ameliorated retinal capillary degeneration to attenuate streptozotocin-induced DR [8]. Th17 cells and IL-17A were also up-regulated in vitreous fluid [9,10] or PBMCs [11] of patients with PDR. However, the effect of Th17-IL-17A pathway on PDR has not been reported yet.
IL-35, secreted by Treg cells, is an immunosuppressive factor in distinct inflammatory responses [12]. IL-35 exerted anti-inflammatory effect to alleviate streptozotocin-induced diabetic neuropathic pain [13]. IL-35 were reduced in PDR patients [14]. Moreover, IL-35 has been shown to inhibited the level of IL-17, and decrease infiltrations of CD4+ T cells to prevent inflammatory diseases [15]. IL-35 also inhibited Th17 cell differentiation and down-regulated IL-17 protein expression in PBMCs of patients with periodontitis through down-regulation of regulators for differentiation of Th17 cells, ROR γt and ROR α [16]. Therefore, IL-35 might regulate PDR through Th17-IL-17A pathway. Effects of IL-35 on Th17 cells differentiation and IL-17 protein expression in patients with PDR were evaluated in this study.
Materials and methods
Sample collection
Patients with PDR (N = 21) and diabetic patients with idiopathic macular epiretinal membranes (N = 29) were recruited at the First People’s Hospital of Zunyi. This study was approved by the Ethics Committee of the First People’s Hospital of Zunyi (Approval No. 2021-1-003) and in accordance with World Medical Association Declaration of Helsinki, and the Ethical principles for medical research involving human subjects. Participants signed informed consents. Demographics of participants are shown in Table 1. Blood samples were acquired from the participants to collect the PBMCs according to previous study (Levels of Interleukin 27 and Interleukin 35 in the Serum and Vitreous of Patients with Proliferative Diabetic Retinopathy).
Table 1.
Clinical characteristics of diabetic patients
| Characteristic | Control | PDR | P value |
|---|---|---|---|
| Number of patients | 29 | 21 | |
| Gender (Male/Female) | 15/14 | 11/10 | 0.822 |
| Age(years) | 56.03 ± 7.32 | 58.05 ± 7.27 | 0.330 |
| Duration of diabetes (years) | - | 12.29 ± 3.89 | |
| BMI (kg/m2) | 20.42 ± 6.4 | 24.72 ± 3.49 | 0.01 |
| FPG (mmol/L) | 5.28 ± 0.51 | 8.47 ± 0.76 | <0.001 |
| HbA1c (%) | 4.88 ± 0.56 | 9.2 ± 1.59 | <0.001 |
Cell culture
PBMCs were cultured in RPMI-1640 medium containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) in a 37°C incubator with 5% CO2 according to a previous study (Protective effects of SIRT1 in patients with proliferative diabetic retinopathy via the inhibition of IL-17 expression).
Enzyme linked immunosorbent assay (ELISA)
The blood samples isolated from the participants were centrifuged at 2,000 g to collect supernatants. Serum level of IL-35 was detected by ELISA kit (R&D Systems, Minneapolis, MN, USA). The isolated PBMCs were incubated with ionomycin (250 ng/mL) (Sigma-Aldrich, St Louis, MO, USA) and phytohemagglutinin (5 μg/mL; Sigma-Aldrich) for 2 days. IL-17A was also detected by ELISA kit (R&D Systems) according to previous study (Th17 cell frequency and IL-17A concentrations in peripheral blood mononuclear cells and vitreous fluid from patients with diabetic retinopathy).
Isolation and treatment of CD4 +T lymphocytes
The isolated PBMCs were incubated with CD4 Biotin-Antibody cocktail from CD4+ T cell isolation kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), and treated with Anti-Biotin micro beads. MACS separator was then used to collect CD4+ T lymphocytes. The lymphocytes were incubated with 1 µg/mL anti-CD3 Mabs (Abcam, Cambridge, MA, USA), 10 µg/mL anti-CD28 Mabs (Abcam), 20 ng/mL TGF-β (Sigma-Aldrich), and 50 ng/mL IL-6 (Sigma-Aldrich) for the differentiation to Th17 cells. Differentiated Th17 cells were incubated with IL-35 (50 ng/mL; Sigma-Aldrich) for 3 days according to previous study (Th17 cell frequency and IL-17A concentrations in peripheral blood mononuclear cells and vitreous fluid from patients with diabetic retinopathy).
Flow cytometry
The isolated PBMCs, CD4+ T lymphocytes or differentiated Th17 cells were incubated with 25 ng/mL phorbol myristate acetate, Brefeldin A (1 µg/mL) and Ionomycin (1 µg/mL) (Sigma-Aldrich) for 4 hours. Cells were then incubated with fluorescein-isothiocyanate-labeled anti-CD4 (BioLegend, San Diego, CA, USA) antibody, stained with phycoerythrin-Cy7-labeled anti-IL-17 (Abcam). Th17, CD4+ IL-17 +, were analyzed by FACSCalibur flow cytometer (BD Biosciences, San Diego, CA, USA) according to previous study (Th17 cell frequency and IL-17A concentrations in peripheral blood mononuclear cells and vitreous fluid from patients with diabetic retinopathy).
qRT-PCR (Quantitative Reverse Transcription PCR)
Isolated PBMCs, CD4+ T lymphocytes or differentiated Th17 cells were lyzed in TRIzol kit (Invitrogen), and the isolated RNAs were synthesized into cDNAs. PreTaq II kit (Takara, Dalian, Liaoning, China) was used to determine mRNA expression of IL-35, ROR γt, ROR α with following primers: IL-35 (F: 5’-CGGTGCCCTACATGCTAAAT-3’ and R: 5’-GCGGAGTCGGTACTTGAGAG-3’); ROR γt (F: 5’-CTCCATCTTTGACTTCTCCCACTCCCTA-3’ and R: 5’-CACATGCTGGCTACACAGGCTC-3’); ROR α (F: 5’-GCTTCGGCAGCACATATACTAAAAT-3’ and R: 5’-CGCTTCACGAATTTGCGTGTCAT-3’). The relative expression of IL-35, ROR γt, and ROR α were calculated using 2 −∆∆Cq method through normalization to GAPDH according to previous study (Lower level of IL‑35 and its reduced inhibition in Th17 cells in patients with bone marrow mononuclear cell Coombs test‑positive hemocytopenia).
Western blot
CD4+ T lymphocytes or differentiated Th17 cells were lyzed in RIPA buffer (Beyotime, Beijing, China). Protein samples were then separated by 10% SDS-PAGE. Following transferred onto nitrocellulose membranes and blocked in 5% bovine serum albumin, the membranes were probed with specific antibodies: anti-ROR γt (1:2000), anti-ROR α (1:2500), and anti-GAPDH (1:3000). The membranes were incubated with horseradish peroxidase-conjugated secondary antibody (1:4000). Immunoreactivities were visualized using enhanced chemiluminescence (Sigma-Aldrich) according to previous study (Protective effects of SIRT1 in patients with proliferative diabetic retinopathy via the inhibition of IL-17 expression).
Statistical analysis
All the data with at least triple replicates were expressed as mean ± SEM, and analyzed by Student’s t test or one-way analysis of variance under SPSS software. A p value of <0.05 was considered statistically significant.
Results
IL-35 was downregulated in PBMCs of patients with PDR
To investigate the role of IL-35 in PDR, PBMCs were isolated from PDR patients. The mRNA expression of IL-35 was decreased in PBMCs of PDR patients (Figure 1(a)). Moreover, patients with PDR also demonstrated lower IL-35 than in patients with idiopathic macular epiretinal membranes (Figure 1(b)), suggesting the possible relation between IL-35 and PDR.
Figure 1.
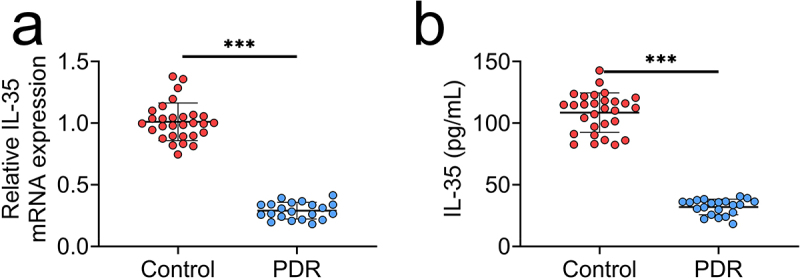
IL-35 was down-regulated in PBMCs of patients with PDR.
(a) mRNA expression of IL-35 was decreased in the PBMCs of patients with PDR compared to the control (patients with idiopathic macular epiretinal membranes).(b) Serum level of IL-35 was decreased in the PBMCs of patients with PDR compared to the control. *** p < 0.001.
Th17 frequency and IL-17 were upregulated in PDR
Th17-IL17 were then assessed in patients with PDR. Level of IL-17 (Figure 2(a)) and frequency of Th17 cells (Figure 2(b)) were enhanced in PBMCs of PDR patients compared to patients with idiopathic macular epiretinal membranes. IL-17 expression showed negative correlation with IL-35 in patients with PDR (Figure 2(c)).
Figure 2.
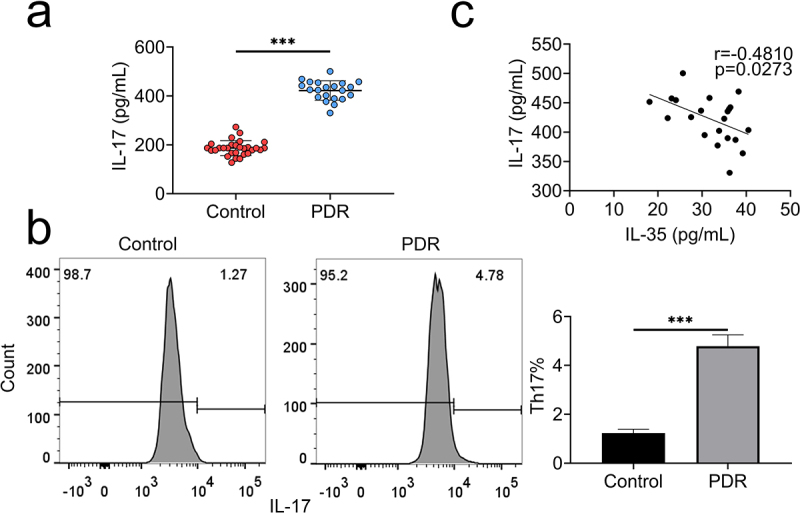
Th17 frequency and IL-17 were up-regulated in PDR.
(a) IL-17 was increased in PBMCs of patients with PDR compared to the control.(b) The frequency of Th17 cells was enhanced in the PBMCs of patients with PDR compared to the control.(c) IL-17 showed negative correlation with IL-35 expression in patients with PDR. *** p < 0.001.
IL-35 suppressed Th17 differentiation
CD4+ T lymphocytes of PBMCs were incubated with IL-35 to investigate the role of IL-35 in Th17 differentiation. Th17 cell frequency was increased in CD4+ T lymphocytes post differentiation (Figure 3(a)), while incubation with IL-35 reduced the frequency (Figure 3(a,b)). Moreover, IL-35 also reduced the level of IL-17 in differentiated Th17 cells (Figure 3(c)), indicating that IL-35 suppressed Th17 differentiation in PDR.
Figure 3.
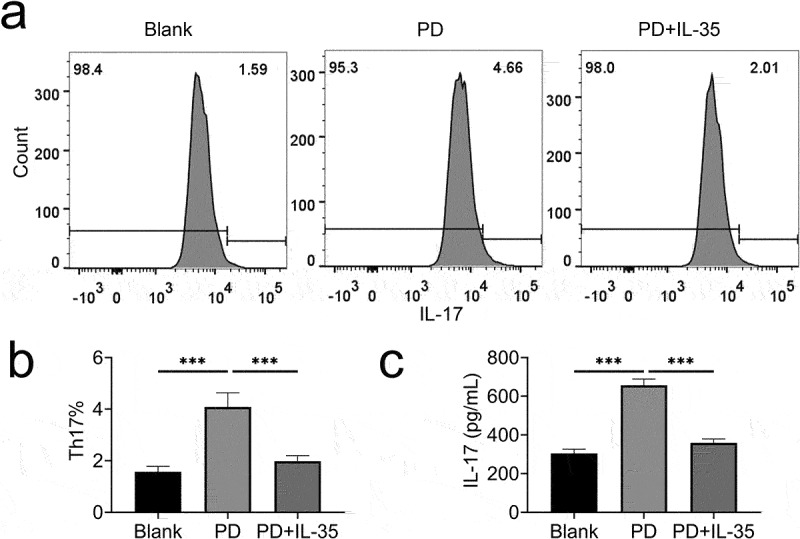
IL-35 suppressed Th17 differentiation.
(a) Incubation with IL-35 reduced the frequency of Th17.(b) Relative frequency of Th17 cells.(c) Incubation with IL-35 reduced IL-17 in differentiated Th17. *** p < 0.001.
IL-35 reduced expression of ROR γt and ROR α
Underlying mechanism of IL-35-mediated Th17 differentiation was then evaluated. The mRNA (Figure 4(a)) and protein (Figure 4(b)) expression of ROR γt and ROR α were enhanced in differentiated Th17 cells. However, incubation with IL-35 reduced ROR γt and ROR α (Figure 4(a,b)), revealing that IL-35 suppressed Th17 cell differentiation through decreasing of ROR γt and ROR α.
Figure 4.
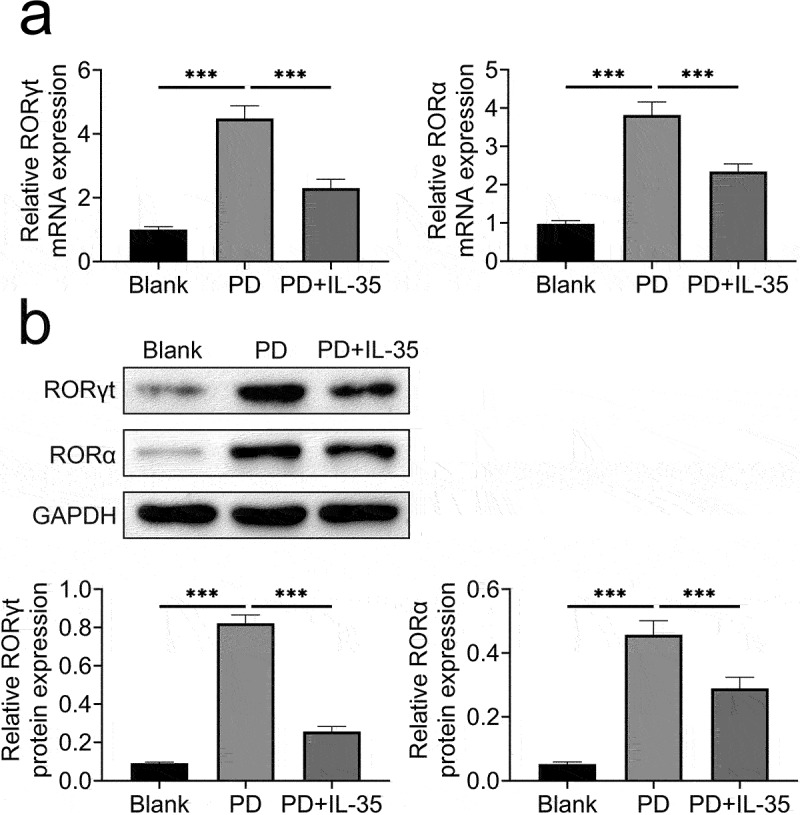
IL-35 reduced ROR γt and ROR α expression.
(a) Incubation with IL-35 reduced ROR γt and ROR α mRNAs in differentiated Th17 cells.(b) Incubation with IL-35 reduced ROR γt and ROR α proteins in differentiated Th17 cells. *** p < 0.001.
Discussion
Emerging evidence has shown that inflammation functions as a key player in DR, especially PDR [17]. Th2 and Th17 cells, and the associated cytokines were enhanced in vitreous fluid of patients with PDR [10]. Inhibition of Th17 cell frequency and the related cytokine, IL-17A, attenuated retinal capillary degeneration in streptozotocin-induced diabetic mice [8]. This study for the first time showed that IL-35 repressed differentiation of Th17 cells and the production of IL-17, providing a potential strategy for the prevention of PDR.
IL-35 was highly upregulated in PDR patients [14]. IL-17A was also elevated in vitreous [3] and aqueous humor [18] of patients with PDR. This study also confirmed that IL-35 was upregulated in PDR patients, and PBMCs isolated from patients with PDR expressed higher IL-35 and IL-17 than the patients with idiopathic macular epiretinal membranes. Similarly, in line with previous studies that Th17 cells were elevated in patients with PDR [9–11], this study also confirmed the enhanced frequency of Th17 cells in patients with PDR. Moreover, IL-17 expression showed a negative correlation with IL-35 in patients with PDR. The role of IL-35/IL-17 in PDR was then evaluated.
Previous study has shown that treatment with IL-35 reduced IL-17 expression and the production of Th17 cells in CD4 + T lymphocytes [19]. IL-35 exerted anti-inflammatory effect against psoriasis and inflammatory bowel disease through promoting of Treg infiltration [15]. Results indicated that incubation with IL-35 recombinant protein reduced frequency of Th17 cells, and down-regulated IL-17 in the differentiated Th17 cells. Therefore, IL-35 suppressed Th17 differentiation and reduced IL-17 production, thus contributing to attenuation of PDR. Moreover, angiogenesis, with the degradation of basement membrane and formation of capillary tubes, also contribute to inflammatory responses, and promote the development of PDR [17]. IL-35 has been shown to regulate pro-angiogenic molecules through Th17/IL-17 signaling [20]. The role of IL-35/Th17/IL-17 in PDR-associated angiogenesis should be investigated in further research.
ROR γt and ROR α are critical regulators for Th17 differentiation, and are regarded as potential targets for Th17 cells-mediated diseases [21]. Deficiency of ROR γt and ROR α resulted in abrogation of Th17 differentiation [22]. IL-35 has been reported to down-regulate the expression of ROR γt and ROR α, and repressed frequency of Th 17 cells [16]. Here, IL-35 also attenuated Th17 differentiation-induced increase in ROR γt and ROR α, thus suppressing differentiation of Th17 in PDR. Therefore, IL-35 suppressed differentiation of Th17 through down-regulation of ROR γt and ROR α to ameliorate PDR.
Conclusion
In summary, IL-35 functioned as an anti-inflammatory cytokine in PDR through inhibition of Th17 differentiation and down-regulation of IL-17. This study suggested the potential clinical significance of IL-35 therapy in the treatment of PDR patients, and confirmed the correlation between IL-35 and Th17/IL-17 in PDR patients. However, the role of IL-35 in in vivo animal model of PDR remains elusive and will be investigated in the future.
Funding Statement
This work was supported by Zunyi Science and Technology Bureau Joint Research and Development Fund Project (Grant No. (2017)35), National Nature Science Foundation of China (Grant No. 82160200), R&D Program of The First People’s Hospital of Zunyi (Grant No. (2020)4), Science and Technology Fund Project of Guizhou Provincial Health Commission in 2020 (Grant No. gzwjk2020-1-155).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Ethics approval
Ethical approval was obtained from the Ethics Committee of the First People’s Hospital of Zunyi (Approval No. 2021-1-003).
Contribution of authors
Ai Yan and Ying Zhang designed the study and carried them out, Xiaocong Wang supervised the data collection, analyzed the data, interpreted the data, Yueling Cui and Wei Tan prepare the manuscript for publication and reviewed the draft of the manuscript. All authors have read and approved the manuscript.
Statement of human and animal rights
All procedures in this study were conducted in accordance with the Ethics Committee of the First People’s Hospital of Zunyi approved protocols.
Statement of informed consent
Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
References
- [1].Kroll P, Büchele Rodrigues E, Hoerle S.. Pathogenesis and classification of proliferative diabetic vitreoretinopathy. Ophthalmologica. 2007;221(2):78–94. [DOI] [PubMed] [Google Scholar]
- [2].Gündüz K, Bakri SJ. Management of proliferative diabetic retinopathy. Compr ophthalmol update. 2007;8(5):245–256. [PubMed] [Google Scholar]
- [3].Chernykh VV, Varvarinsky EV, Smirnov EV, et al. Proliferative and inflammatory factors in the vitreous of patients with proliferative diabetic retinopathy. Indian J Ophthalmol. 2015;63(1):33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Qu N, Xu M, Mizoguchi I, Furusawa J-I, Kaneko K, Watanabe K, Mizuguchi J, et al. Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22, and IL-23, in inflammatory diseases. Clin Dev Immunol. 2013;2013:968549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kuwabara T, Ishikawa F, Kondo M, Kakiuchi T. The role of IL-17 and related cytokines in inflammatory autoimmune diseases. Mediators Inflamm. 2017;2017:3908061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jagannathan-Bogdan M, McDonnell ME, Shin H, et al. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol (Baltimore, Md: 1950). 2011;186(2):1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen H, Ren X, Liao N, et al. Th17 cell frequency and IL-17A concentrations in peripheral blood mononuclear cells and vitreous fluid from patients with diabetic retinopathy. J Int Med Res. 2016;44(6):1403–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zapadka TE, Lindstrom SI, Batoki JC, et al. Aryl hydrocarbon receptor agonist VAF347 impedes retinal pathogenesis in diabetic mice. Int J Mol Sci. 2021;22(9):4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Takeuchi M, Sato T, Sakurai Y, et al. Association between aqueous humor and vitreous fluid levels of Th17 cell-related cytokines in patients with proliferative diabetic retinopathy. PloS one. 2017;12(5):e0178230–e0178230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Takeuchi M, Sato T, Tanaka A, et al. Elevated levels of cytokines associated with Th2 and Th17 cells in vitreous fluid of proliferative diabetic retinopathy patients. PloS one. 2015;10(9):e0137358–e0137358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yu H, Liu B, Wu G, et al. Dysregulation of circulating follicular helper T cells in type 2 diabetic patients with diabetic retinopathy. Immunol Res. 2021;69(2):153–161. [DOI] [PubMed] [Google Scholar]
- [12].Li X, Fang P, Yang WY, et al. IL-35, as a newly proposed homeostasis-associated molecular pattern, plays three major functions including anti-inflammatory initiator, effector, and blocker in cardiovascular diseases. Cytokine. 2019;122:154076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jiang Y, Wang J, Li H, et al. IL-35 alleviates inflammation progression in a rat model of diabetic neuropathic pain via inhibition of JNK signaling. J Inflamm (Lond). 2019;16(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yan A, You H, Zhang X. Levels of Interleukin 27 and Interleukin 35 in the serum and vitreous of patients with proliferative diabetic retinopathy. Ocul Immunol Inflamm. 2018;26(2):273–279. [DOI] [PubMed] [Google Scholar]
- [15].Wang Y, Mao Y, Zhang J, et al. IL-35 recombinant protein reverses inflammatory bowel disease and psoriasis through regulation of inflammatory cytokines and immune cells. J Cell Mol Med. 2018;22(2):1014–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Okada K, Fujimura T, Kikuchi T, et al. Effect of interleukin (IL)-35 on IL-17 expression and production by human CD4+ T cells. PeerJ. 2017;5:e2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rübsam A, Parikh S, Fort PE. Role of inflammation in diabetic retinopathy. Int J Mol Sci. 2018;19(4):942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Feng S, Yu H, Yu Y, et al. Levels of inflammatory cytokines IL-1β, IL-6, IL-8, IL-17A, and TNF-α in aqueous humour of patients with diabetic retinopathy. J Diabetes Res. 2018;2018:8546423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li Y, Wang Y, Liu H, et al. Lower level of IL‑35 and its reduced inhibition in Th17 cells in patients with bone marrow mononuclear cells Coombs test‑positive hemocytopenia. Mol Med Rep. 2018;17(2):2973–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu S, Li Y, Xia L, et al. IL-35 prevent bone loss through promotion of bone formation and angiogenesis in rheumatoid arthritis. Clin Exp Rheumatol. 2019;37(5):820–825. [PubMed] [Google Scholar]
- [21].Castro G, Liu X, Ngo K, et al. RORγt and RORα signature genes in human Th17 cells. PLoS ONE. 2017;12(8):e0181868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yang XO, Pappu BP, Nurieva R, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28(1):29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]


