Abstract
The high-dose glucocorticosteroid (GC) treatment is the first choice for dermatomyositis complicated with interstitial lung disease (DM-ILD) but patients are resistant to the high-dose GC monotherapy. Besides, the high dose of GC, the secondary immunosuppressive agent(s) is necessary but there is controversy for the selection of immunosuppressive agent(s). The objectives of the study were to analyze the efficacy of different therapeutic options for DM-ILD to identify the optimal therapy. A total of 60 patients had received intravenous 1.0–2.0 mg/ kg/day prednisolone for DM-ILD. In severe conditions, patients had received oral 1 to 3 mg/day tacrolimus (TAC), 500 mg/ m2/month cyclophosphamide (CY), and/or 1 g/ day methylprednisolone pulse (TI cohort, n = 24). In severe conditions, patients had received 1 g/day methylprednisolone pulse and 2–3 mg/ kg/day cyclosporine A (CsA) and/or 500 mg/ m2/month CY (existing historical treatment; CT cohort, n = 36). Patients of the TI cohort did not receive CsA. Patients in the CT cohort were received CY in significantly fewer numbers than those of the TI cohort during treatment (P = .0112). A total of 11 (46%) patients from the TI cohort and 14 (39%) patients from the CT cohort were developed relapsed. At the end of the 30-months, higher numbers of patients of the TI cohort had an event(s) free survival than those of the CT cohort (7 (29%) vs 2 (6%), P = .0229). Also, higher numbers of patients of the TI cohort had survived irrespective of an event(s) than those of the CT cohort (21 (87%) vs 22 (61%), P = .0399). Patients of the TI cohort had developed herpes zoster (2 (8%)) and cytomegalovirus (4 (17%)) infections. Patients of the CT cohort developed renal dysfunction (10 (28%)). Hyperglycemia, hyperlipidemia, and fracture (GC-related toxicities) were also reported in both cohorts and these toxicities were fever in the TI cohort. The addition of TAC to high doses GC with CY is an ideal treatment for severe conditions of DM-ILD (Level of Evidence: III; Technical Efficacy Stage: 4).
Keywords: calcineurin inhibitor, dermatomyositis, glucocorticosteroid, immunosuppressive agent, interstitial lung diseases, methylprednisolone, prednisolone, tacrolimus
1. Introduction
Dermatomyositis (DM) is a systemic autoimmune disease in which mainly muscles and skin are affected but also DM pulmonary involvement in the form of interstitial lung disease (ILD) may occur in the course of the disease. The autoimmune-autoinflammatory process may also be involved in other systems and organs such as joints, heart, or vessels.[1] DM belongs to the rheumatic diseases - group connective tissue diseases (CTD). More than 50% of patients with DM have ILD.[2] Progressive, acute, and severe forms of interstitial lung diseases complicated with dermatomyositis (DM-ILD) have diffuse alveolar damage and are restricted to treatment(s).[1] The high-dose glucocorticosteroid (GC) treatment(s) is the first choice for DM-ILD but more than 50% of patients are resistant to the high-dose GC monotherapy and ILD may deteriorate, which requires more aggressive treatment(s).[2] Besides, the high dose of GC, the secondary immunosuppressive agent(s) is necessary but there is controversy for the selection of immunosuppressive agent(s).[1] Cyclophosphamide (CY) is generally used for DM-ILD[3] but patients with severe conditions have often developed resistance to CY.[1] As well as there may be contraindications to such treatment resulting, for example, from the degree of involvement of internal organs, such as the heart. Tacrolimus (TAC) is reported effective for severe forms of DM-ILD.[1,4,5] TAC is a calcineurin inhibitor[6] and has immunosuppressive action by direct reducing activity and growth of T cells and interleukin-2 by calcineurin.[7] Cyclosporine A (CsA) is the first choice of a calcineurin inhibitor for DM-ILD because it induces a faster treatment response.[8] CY is used for severe conditions and the acute or subacute onset of DM-ILD.[3] CY works by inhibiting DNA replication, suppresses B cells more sensitively than T cells.[8]
The objectives of the retrospective study were to compare the effectiveness and safety of TAC plus high doses of GC, and/or CY against existing historical treatment (high doses of GC, CY, and/or CsA) for Chinese patients with DM-ILD.
2. Materials and methods
2.1. Study population
From 15 January 2014 to 14 December 2018, a total of 65 patients were diagnosed with DM-ILD according to the European League Against Rheumatism/ the American College of Rheumatology (EULAR/ACR) criteria[9] and had received treatment for the same at the First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China, and the referring hospitals. Among them, three patients had inclusion body myositis and two patients had malignancy-associated disease. Therefore, these patients (n = 5) were excluded from the study. Data regarding demographical and clinical conditions, outcomes for treatment(s), and treatment-emergent adverse effects of a total of 60 patients who had been treated with a high dose of GC plus calcineurin inhibitor, and/or CY were collected from the medical records of the institutes. The flow diagram of the retrospective analysis is presented in Figure 1.
Figure 1.
The flow diagram of the retrospective analysis.
2.2. Treatments
A total of 24 patients had been treated with intravenous 1.0 to 2.0 mg/ kg/day prednisolone by physicians. In severe conditions, over and above prednisolone, patients had received oral 1 to 3 mg/ ay TAC within one month from the start of prednisolone and/ or methylprednisolone and followed by adjustment to trough levels of 5 to 20 ng/ mL and 500 mg/ m2/month CY within one month from the start of prednisolone and/or methylprednisolone and/ or 1 g/day intravenous methylprednisolone pulse therapy for 3 days (no CsA). These patients were included in the TI cohort. A total of 36 patients had been treated with intravenous 1.0 to 2.0 mg/ kg/day prednisolone by physicians. In severe conditions, over and above prednisolone, patients had received 1 g/day intravenous methylprednisolone pulse therapy for 3 days plus intravenous 2 to 3 mg/ kg/day CsA and/ or 500 mg/ m2/month CY within one month from the start of prednisolone and/or methylprednisolone (no TAC; existing historical treatment). These patients were included in the CT cohort. Therapies for patients were preferred based on the attending physician's discretion.
2.3. Physical examinations
Muscle testing was performed manually for a total of 18 muscles, for example, the bilateral side of the deltoid, flexor, and extensor muscles of the neck, brachioradialis, biceps brachii, iliopsoas, triceps brachii, quadriceps femoris, gluteus maximus, and hamstring. Each muscle was scored on a 0 to 5 scale.[1] 0: no contraction, 1: only visible contraction, 2: moveable with gravity, 3: moveable with and without gravity, 4: slight decrease mobility than normal, and 5: normal. The total score was 90.[10] Neurologists (minimum 3-years of experience) of institutes had evaluated physical examinations.
2.4. Laboratory tests
Serum lactate dehydrogenase (LDH), Krebs von den Lungen-6 (KL-6), and creatine kinase (CK) level were evaluated by pathologists (minimum 3-years of experience) of institutes.
2.5. Radiological examinations
The high-resolution computed tomography (HRCT) was performed and assessed by radiologists (minimum 3-years of experience) of institutes. Radiological parameters were excessed for nodules, irregular linear opacities, ground-glass opacities, honeycombing, and traction bronchiectasis. If predominant ground-glass opacity and/or reticular pattern was homogeneous then that was considered as nonspecific interstitial pneumonia (NSIP) and if that was heterogeneous, patchy, and irregular in size then that was considered as interstitial pneumonia (UIP). Radiologists (minimum 3-years of experience) of institutes had evaluated radiological examinations.
2.6. Pulmonary function
Spirometry parameters, forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), lung capacity that patient can expel in one second (FEV1/ FVC ratio), carbon monoxide diffusion capacity (DLCO), and total lung capacity (the volume in the lungs at maximal inflation; TLC) were evaluated.
2.7. Serious adverse event
Hospitalization due to any cause was considered a serious adverse event (SAE).
2.8. Relapse
Worsening of symptoms, hypoxia, progression of diseases in radiological examinations, and/ or the need for treatment with an increased dose of GC and/ or immunosuppressive agent(s) was considered a relapse.
2.9. Statistical analysis
InStat 3.01, GraphPad Software, San Diego, CA, USA was used for statistical analysis purposes. Data were normally distributed. The Fisher exact test (when the size of compared classes was 2) or Chi-square test for independence (when the size of compared classes was more than 2) was performed for categorical variables. t test or one-way analysis of variance (ANOVA) was performed for continuous variables. Dunnett, multiple comparisons test (considering critical value (q) > 2.261 for within cohort and >2.268 for between cohorts as significant) was used for post hoc analysis to overcome the type-I error. All results were considered significant if P < .05. The numbers of patients who survived with any kind of event(s) or irrespective of an event(s) were taken on the y-axis and time in months were taken on the x-axis, which lead to giving survival curves.
2.10. Ethics approval and consent to participate
The designed protocol (AMUCL1924 dated 4 June 2021) was approved by the Anhui Medical University review board. The study adheres to the law of China and the V2008 Declarations of Helsinki. Being a retrospective study patients’ consent was not required.
3. Results
3.1. Demographical and clinical conditions
Patients were observed for 30 months regardless of the effects of the outcome. Patients who were put on TAC (the TI cohort) did not receive CsA. However, patients on conventional therapy (without TAC; the CT cohort) were received CY in significantly fewer numbers than those of the TI cohort during treatment (P = .0112, Fisher test). There were no significant differences for laboratory tests, age, sex, manual muscle testing score, radiological examinations, and pulmonary function before treatment, and duration for treatments between patients of both cohorts (P > .0500 for all, Fisher test or χ2 test, or t test). Patients of the CT cohort had higher values of creatinine before treatment than those of the TI cohort, but the difference was not statistically significant between both cohorts (P = .1426, t test). The details of the demographical and clinical conditions, radiological examinations before treatment, and treatment(s) pattern of patients are reported in Table 1.
Table 1.
Demographical and clinical conditions and radiological examinations before treatment and treatment pattern of patients.
| Parameters | Cohorts | |||
| TI | CT | |||
| Therapy | Prednisolone + tacrolimus + methylprednisolone and/or cyclophosphamide | Prednisolone + methylprednisolone and/or cyclophosphamide/ ciclosporin | Comparisons | |
| Numbers of patients | 24 | 36 | P value | df |
| Sex | ||||
| Male | 10 (42) | 13 (36) | .7877 (Fisher test) | N/A |
| Female | 14 (58) | 23 (64) | ||
| Age at time of treatment (years) | ||||
| Minimum | 35 | 35 | .7780 (t test) | N/A |
| Maximum | 63 | 64 | ||
| Mean ± SD | 50.12 ± 15.16 | 51.21 ± 14.23 | ||
| Manual muscle testing score | 79.58 ± 3.61 | 81.00 ± 4.20 | .1813 (t-test) | N/A |
| Serum lactate dehydrogenase (IU/ L) | 552.39 ± 17.71 | 558.07 ± 23.12 | .3122 (t-test) | N/A |
| Laboratory tests | ||||
| Krebs von den Lungen-6 (U/ mL) | 985 ± 121 | 998 ± 136 | .7063 (t-test) | N/A |
| Creatine kinase (IU/ L) | 726 ± 402 | 867 ± 336 | .1426 (t-test) | N/A |
| Nodules | 2 (9) | 5 (14) | ||
| Irregular linear opacities | 13 (54) | 18 (49) | ||
| Radiological examinations | ||||
| Ground glass opacities | 6 (25) | 7 (19) | .9769 (χ2 test) | 5 |
| Honeycombing | 1 (4) | 2 (6) | ||
| Traction bronchiectasis | 1 (4) | 2 (6) | ||
| More than 50% lesion of total lung | 1 (4) | 2 (6) | ||
| Forced vital capacity (% FVC) | 79.63 ± 9.24 | 81.50 ± 6.25 | .3515 (t test) | N/A |
| FEV1/ FVC ratio | 83.51 ± 14.52 | 85.22 ± 15.23 | .6659 (t test) | N/A |
| Pulmonary function | ||||
| % Carbon monoxide diffusing capacity | 48.21 ± 8.43 | 47.83 ± 6.54 | .8472 (t test) | N/A |
| Total lung capacity (L) | 4.13 ± 0.25 | 4.18 ± 0.25 | .4411 (t test) | N/A |
| 1.0–2.0 mg/kg/day prednisolone | 24 (100) | 36 (100) | N/A | N/A |
| Methylprednisolone pulse | 6 (25) | 10 (28) | .9999 (Fisher test) | N/A |
| Treatments | ||||
| Cyclophosphamide | 10 (42) | 4 (11) | .0112 (Fisher test) | N/A |
| Ciclosporin | 0 (0) | 9 (25) | 0.0082 (Fisher test) | N/A |
| Duration of treatment (months) | ||||
| Minimum | 2 | 2 | .3102 (t test) | N/A |
| Maximum | 4 | 4 | ||
| Mean ± SD | 2.5 ± 0.8 | 2.3 ± 0.7 | ||
Categorical variables are demonstrated as frequency (percentages) and continuous variables are demonstrated as mean ± standard deviation (SD).
The Fisher exact test (when the size of compared classes was 2) or Chi-square test for independence (when the size of compared classes was more than 2) was performed for categorical variables.
t-test was performed for continuous variables.
All results were considered significant if P < .05.
χ2 test = the Chi-square test for independence, df = Degree of freedom, N/A = Not applicable.
3.2. Outcome measures
A total of 30 months after the start of treatment, manual muscle testing score, creatine kinase value, and pulmonary function tests (% FVC, % DLCO, and TLC) were improved in both cohorts (P < .05, one-way ANOVA, and q > 2.261, Dunnett multiple comparisons test, for all). However, these results were not significant between both cohorts (P > .05 one-way ANOVA and/ or q < 2.268, Dunnett multiple comparisons test, for all) at 30 months after the start of treatment. The details of outcome measures are reported in Table 2.
Table 2.
Outcome measures.
| Parameters | Cohorts | |||||||||
| TI | CT | Comparisons at EL | ||||||||
| Therapy | Prednisolone + tacrolimus + methylprednisolone and/or cyclophosphamide | Prednisolone + methylprednisolone and/or cyclophosphamide/ ciclosporin | ||||||||
| Level | BL | EL | Comparisons | BL | EL | Comparisons | ||||
| Patients survived | 24 | 21 | P value | q-value | 36 | 22 | P value | q-value | P value | q-value |
| Manual muscle testing score | 79.58 ± 3.61 | 83.95 ± 2.16 | .0039 | 4.0660 | 81.00 ± 4.20 | 83.95 ± 1.76 | .0011 | 3.0990 | .8741 | N/A |
| Creatine kinase (IU/ L) | 726 ± 402 | 81 ± 20 | <.0001 | 6.8790 | 867 ± 336 | 177 ± 10 | <.0001 | 8.1990 | <.0001 | 1.3019 |
| Forced vital capacity (% FVC) | 79.63 ± 9.24 | 85.43 ± 5.82 | .0259 | 2.7090 | 81.50 ± 6.25 | 85.95 ± 3.32 | .0059 | 2.4520 | .7989 | N/A |
| % Carbon monoxide diffusing capacity | 48.21 ± 8.43 | 53.95 ± 5.64 | .0258 | 2.6510 | 52.19 ± 7.23 | 57.68 ± 4.49 | <.0001 | 2.8890 | <.0001 | 1.8839 |
| Total lung capacity (L) | 4.18 ± 0.25 | 4.31 ± 0.20 | .0154 | 2.6369 | 4.18 ± 0.25 | 4.34 ± 0.19 | .0233 | 2.6389 | .6354 | N/A |
Variables are demonstrated as mean ± standard deviation (SD).
One-way ANOVA was performed for statistical analysis.
Dunnett multiple comparisons test was used for post hoc analysis.
BL = Before treatment, EL = 30 months after the start of treatment.
All results were considered significant if P < .05 and q > 2.261 (for within cohort) or >2.268 (for between cohorts).
N/A = Not applicable.
3.3. Safety
A total of 11 (46%) patients from the TI cohort and 14 (39%) patients from the CT cohort were developed relapsed during 30 months after the start of treatment. There were 3 (13%) patients from the TI cohort and 14 (39%) patients from the CT cohort who died during 30 months after the start of treatment, 2 (8%) patients from the TI cohort, and 6 (17%) patients from the CT cohort developed SAE in follow-up. A total of 7 (29%) patients from the TI cohort had developed non-SAE, 30 months after the start of treatment. Among 7 patients (from the TI cohort), 2 (8%) patients had developed cytomegalovirus (CMV) infection and 4 (17%) patients had developed herpes zoster (VZV) infections. All of them were treated with antiviral therapies. A total of 11 (31%) patients from the CT cohort were developed non-SAE (i.e., 10 (28%) patients with renal dysfunction), 30 months after the start of treatment. There were no significant differences for numbers of patients with non-SAE between both cohorts (7 (29%) vs 11 (31%), P = .9999, Fischer exact test). Nausea and vomiting have been experienced by all patients after the start of chemotherapies and for a few days after chemotherapies. The details of adverse events other than GC-related toxicities during the 30-months of observation period are reported in Table 3. Over and above these, GC-related toxicities (e.g., hyperglycemia, hyperlipidemia, fracture) were also reported in both cohorts and these toxicities were fever in the TI cohort (Table 4).
Table 3.
Adverse events other than glucocorticosteroid related toxicities during 30-months of the observation period.
| Parameters | Cohorts | ||
| TI | CT | Comparisons | |
| Therapy | Prednisolone + tacrolimus + methylprednisolone and/or cyclophosphamide, n (%) | Prednisolone + methylprednisolone and/or cyclophosphamide/ ciclosporin, n (%) | |
| Numbers of patients | 24 | 36 | P value |
| Relapse | 11 (46) | 14 (39) | .6056 |
| Death | 3 (13) | 14 (39)∗ | .0399 |
| Serious adverse effects | |||
| Hepatic cirrhosis | 2 (8) | 0 (0) | .1559 |
| Malignancy | 0 (0) | 6 (17) | .0722 |
| Total | 2 (8) | 6 (17) | .4571 |
| Non- serious adverse effects | |||
| Cytomegalovirus infection | 2 (8) | 0 (0) | .1559 |
| Herpes zoster infections | 4 (17)∗ | 0 (0) | .0218 |
| Renal dysfunction | 0 (0) | 10 (28)∗ | .0039 |
| Cataract | 1 (4) | 1 (3) | .9999 |
| Total | 7 (29) | 11 (31) | .9999 |
Variables are demonstrated as frequency (percentages).
The Fisher exact test was used for statistical analysis.
All results were considered significant if P < .05.
Significant higher value than that of the other cohort.
The enrolled patients had reported one or more event (s).
Relapse: Worsening of symptoms, hypoxia, progression of diseases in radiological examinations, and/ or the need for treatment for increased dose of corticosteroid (s) and/ or immunosuppressive agent.
Table 4.
Glucocorticosteroid related toxicities during 30-months of the observation period.
| Parameters | Cohorts | ||
| TI | CT | ||
| Therapy | Prednisolone + tacrolimus + methylprednisolone and/or cyclophosphamide, n (%) | Prednisolone + methylprednisolone and/or cyclophosphamide/ ciclosporin, n (%) | Comparisons |
| Numbers of patients | 24 | 36 | P value |
| Hyperglycemia | 1 (4) | 4 (11) | .6392 |
| Hyperlipidemia | 2 (8) | 7 (19) | .2930 |
| Fracture | 0 (0) | 3 (8) | .2678 |
| Cataract | 3 (13) | 7 (19) | .7255 |
| Hemorrhagic gastric ulcer | 0 (0) | 3 (8) | .2678 |
| Hypertension | 0 (0) | 2 (6) | .5209 |
Variables are demonstrated as frequency (percentages).
The Fisher exact test was used for statistical analysis.
All results were considered significant if P < .05.
3.4. Survival
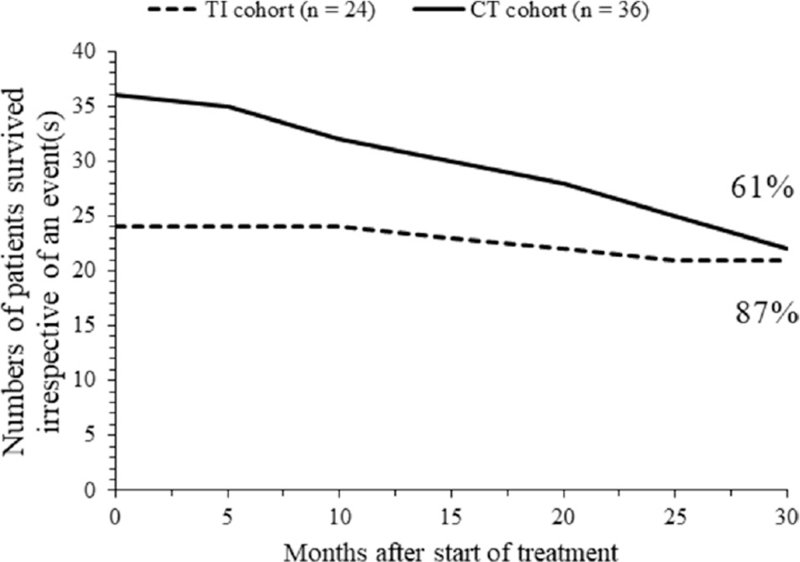
At the end of the 30-months of the followed-up period, higher numbers of patients of the TI cohort had an event(s) free survival than those of the CT cohort (7 (29%) vs 2 (6%), P = .0229, Fischer exact test). The event(s) free survival curves crossed during the follow-up period of the 20 to 25-months. Any kind of event(s) free survival of patients is reported in Figure 2. Also, higher numbers of patients of the TI cohort had survived irrespective of an event(s) than those of the CT cohort (21 (87%) vs 22 (61%), P = .0399, Fischer exact test). The survival curves irrespective of an event(s) crossed above 30-months of the follow-up period. Patients’ survival curve irrespective of an event(s) is reported in Figure 3.
Figure 2.
Numbers of the event(s)-free surviving patients in TI and CT cohorts vs treatment time.
Figure 3.
Total numbers of surviving patients in TI and CT cohorts irrespective of the event(s) vs treatment time.
4. Discussion
Both cohorts have reported improvement in manual muscle testing scores, creatine kinase value, and pulmonary function tests among survivals. The results of outcome measures of the current study are consistent with those of retrospective studies[1,11] and a randomized trial.[12] It was possible to get targeted outcome measures without the addition of TAC in a high dose of GC with an immunosuppressive agent(s) during the treatment of patients with DM-ILD.
The current study reported that the addition of TAC with GC plus CY increased the survival of patients, irrespective of an event(s) and an event(s) free survival of patients with DM-ILD. The results of survival of the current study are consistent with those of retrospective analyses[1,4,7,11] and a randomized trial.[12] Bronchoalveolar lavage fluid has increased lymphocytes in patients with DM-ILD.[1] TAC acts on the growth of T cells.[7] The current study recommended the addition of TAC with high doses of GC and CY as an ideal treatment for DM-ILD.
Patients in the CT cohort had reported renal dysfunction. Patients of the CT cohort had received CsA. A CsA is induced by renal dysfunction through renal cell apoptosis.[13] Patients of the TI cohort had reported VZV infections. Patients of the TI cohort had received TAC, which is responsible for the development of VZV infections.[14] Also, higher numbers of patients in the TI cohort had received CY, which is responsible for VZV infections.[15] The results of incidences of events and risk factors of the current study are consistent with those of retrospective studies[1,11] and a randomized trial.[12] The risk of renal complications would be higher if patients of DM-ILD were treated without TAC. The very high levels of immunosuppression utilized were responsible for SAE and non-SAE. Therefore, discontinuation of an immunosuppressive agent(s), TAC, or CsA is a possible way to overcome adverse effects. TAC has fewer side effects compared to CsA.
Patients of the TI cohort had reported fewer SAE, non-SAE, and GC-related toxicities than those of the CT cohort. Patients of the TI cohort had not received CsA and received oral 1 to 3 mg/day TAC. TAC is given optimal effect with the least adverse effects if less than 3 mg/day TAC was given initially and followed by adjustment to a trough level of more than 5 ng/ mL.[16] This led to less toxicity due to TAC among the patients of the TI cohort. A total of 1 to 3 mg/day TAC with a trough level of >5 ng/ mL is the optimum dose of TAC. Also, prednisolone with CsA administered in the CT cohort had higher adverse effects than those of only prednisolone in the TI cohort.
Survival curves crossed during the follow-up period. The presented work combines several clinical situations by entering into one comparative model-TAC as the main distractor, but in two separate groups there is also variability in the form of taking or not taking CY (and/or), which may change the assessed parameters-treatment effectiveness, and side effects. The clinical features before treatments are also the possible confounders for these results. Some additional clinical features are needed to be involved: anti-MDA5 antibody, pathotyping of clinically amyopathic dermatomyositis (CADM), oxygen administration, recent exacerbation history, disease duration, and the previous history of medication.
Among the limitations of the presented study should be listed: small sample size and retrospective design of this study. The prospective study would be a source of wider data. Such an incomplete structure may be burdened with a large error of inference, for example, about the risk of renal complications – by definition, has a better chance of being higher in the CT cohort (a known complication of CsA is nephrotoxicity). However, it is difficult to prepare in the case of a rare disease and a larger group of patients at the same time. As DM-ILD is a life-threatening disease, a prospective trial is difficult to design. ILD affects only some patients with DM, and it is difficult to gather more patients representing this group. Numbers of patients with fibrotic phenotype and no response to the high degree of immunosuppression were not reported and thus were not identified in this study.
5. Conclusions
The addition of TAC in a high dose of GC with CY could improve muscle testing score, creatine kinase value, pulmonary function, and survival for DM-ILD compared to existing historical treatment (high dose of GC with an immunosuppressive agent(s)). Also, decreased adverse effects and death. In the future, a prospective study is necessary to state the hypothesis clearly.
Acknowledgments
The authors are thankful for the medical and non-medical staff of the First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China.
Author contributions
Conceptualization: Mu Li.
Data curation: Kang Wang.
Formal analysis: Kang Wang.
Investigation: Yingchun Li.
Methodology: Kang Wang, Mu Li, Shengqian Xu, Yingchun Li.
Project administration: Lian Li.
Resources: Kang Wang, Lian Li, Shengqian Xu, Yingchun Li.
Software: Lian Li, Shengqian Xu.
Supervision: Lian Li, Mu Li.
Validation: Yingchun Li.
Visualization: Mu Li.
Writing – original draft: Shengqian Xu.
Writing – review & editing: Shengqian Xu.
Footnotes
Abbreviations: ANOVA = analysis of variance, CADM = clinically amyopathic dermatomyositis, CK = creatine kinase, CMV = cytomegalovirus, CsA = cyclosporin, Ciclosporin A, Cyclosporine A, Cyclosporin A, or, Cyclosporine, CT cohort = patients had received intravenous 1.0–2.0 mg/kg/day prednisolone and/ or 1 g/day intravenous methylprednisolone pulse therapy for 3 days and/ or intravenous 2–3 mg/kg/day ciclosporin or 500 mg/m2/month cyclophosphamide, CTD = connective tissue diseases, DLCO = Carbon monoxide diffusion capacity, DM = dermatomyositis, DM-ILD = interstitial lung diseases complicated with dermatomyositis, EULAR/ACR criteria = the European League Against Rheumatism/ the American College of Rheumatology criteria, FEV1 = forced expiratory volume in 1 second, FEV1/ FVC ratio = lung capacity that patient can expel in one second, FVC = forced vital capacity, GC = glucocorticosteroid, HRCT = the high-resolution computed tomography, ILD = interstitial lung disease, KL-6 = Krebs von den Lungen-6, LDH = serum lactate dehydrogenase, NSIP = nonspecific interstitial pneumonia, q = critical value for Dunnett, multiple comparisons test, χ2 test: the Chi-square test for independence, SAE = serious adverse event, SD = standard deviation, TAC = Tacrolimus, TI cohort = patients had received intravenous 1.0–2.0 mg/kg/day prednisolone and/ or 1 g/day intravenous methylprednisolone pulse therapy for 3 days + oral 1–3 mg/day tacrolimus and 500 mg/m2/month cyclophosphamide, TLC = total lung capacity, UIP = interstitial pneumonia, VZV = Herpes zoster virus.
How to cite this article: Lian L, Li M, Li Y, Wang K, Xu S. Combination therapy of tacrolimus, high doses of glucocorticosteroids, and cyclophosphamide against existing historical treatment for patients in severe conditions of interstitial lung diseases complicated with dermatomyositis: A retrospective analysis. Medicine. 2022;101:24(e29108).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Contributor Information
Lian Li, Email: vdfgad589@163.com.
Mu Li, Email: n267298@163.com.
Yingchun Li, Email: v506575@163.com.
Kang Wang, Email: j794162@163.com.
Shengqian Xu, Email: shengqian.xu1@gmail.com.
References
- [1].Kurita T, Yasuda S, Oba K, et al. The efficacy of tacrolimus in patients with interstitial lung diseases complicated with polymyositis or dermatomyositis. Rheumatology 2015;54:1536. [DOI] [PubMed] [Google Scholar]
- [2].Marie I, Hatron PY, Dominique S, Cherin P, Mouthon L, Menard JF. Short-term and long-term outcomes of interstitial lung disease in polymyositis and dermatomyositis: A series of 107 patients. Arthritis Rheum 2011;63:3439–47. [DOI] [PubMed] [Google Scholar]
- [3].Mira-Avendano IC, Parambil JG, Yadav R, et al. A retrospective review of clinical features and treatment outcomes in steroid-resistant interstitial lung disease from polymyositis/dermatomyositis. Respir Med 2013;107:890–6. [DOI] [PubMed] [Google Scholar]
- [4].Takada K, Katada Y, Ito S, et al. Impact of adding tacrolimus to initial treatment of interstitial pneumonitis in polymyositis/dermatomyositis: a single-arm clinical trial. Rheumatology 2020;59:1084–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yokoyama Y, Furuta S, Ikeda K, Hirose K, Nakajima H. Corticosteroid-sparing effect of tacrolimus in the initial treatment of dermatomyositis and polymyositis. Mod Rheumatol 2015;25:888–92. [DOI] [PubMed] [Google Scholar]
- [6].Witt LJ, Demchuk C, Curran JJ, Strek ME. Benefit of adjunctive tacrolimus in connective tissue disease-interstitial lung disease. Pulm Pharmacol Ther 2016;36:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ueno KI, Shimojima Y, Kishida D, Sekijima Y, Ikeda SI. Advantage of administering tacrolimus for improving prognosis of patients with polymyositis and dermatomyositis. Int J Rheum Dis 2016;19:1322–30. [DOI] [PubMed] [Google Scholar]
- [8].Shimojima Y, Ishii W, Matsuda M, Kishida D, Ikeda SI. Effective use of calcineurin inhibitor in combination therapy for interstitial lung disease in patients with dermatomyositis and polymyositis. J Clin Rheumatol 2017;23:87–93. [DOI] [PubMed] [Google Scholar]
- [9].Barsotti S, Dastmalchi M, Notarnicola A, et al. Performance of the new EULAR/ACR classification criteria for idiopathic inflammatory myopathies (IIM) in a large monocentric IIM cohort. Semin Arthritis Rheum 2020;50:492–7. [DOI] [PubMed] [Google Scholar]
- [10].Shimojima Y, Ishii W, Matsuda M, Tazawa K, Ikeda S. Coadministration of tacrolimus with corticosteroid accelerates recovery in refractory patients with polymyositis/dermatomyositis: a retrospective study. BMC Musculoskelet Disord 2012;13:01–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tsuji H, Nakashima R, Hosono Y, et al. Multicenter Prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheumatol 2020;72:488–98. [DOI] [PubMed] [Google Scholar]
- [12].Fujisawa T, Hozumi H, Kamiya Y, et al. Prednisolone and tacrolimus versus prednisolone and cyclosporin A to treat polymyositis/dermatomyositis-associated ILD: A randomized, open-label trial. Respirology 2021;26:370–7. [DOI] [PubMed] [Google Scholar]
- [13].Xiao Z, Shan J, Li C, et al. Mechanisms of cyclosporine-induced renal cell apoptosis: A systematic review. Am J Nephrol 2013;37:30–40. [DOI] [PubMed] [Google Scholar]
- [14].Takeuchi T, Wakasugi N, Uno S, Makino H. Long-term safety and effectiveness of tacrolimus in patients with lupus nephritis: 5-year interim postmarketing surveillance study in Japan (TRUST). J Rheumatol 2021;48:74–81. [DOI] [PubMed] [Google Scholar]
- [15].Ge Y, Peng Q, Zhang S, Zhou H, Lu X, Wang G. Cyclophosphamide treatment for idiopathic inflammatory myopathies and related interstitial lung disease: a systematic review. Clin Rheumatol 2015;34:99–105. [DOI] [PubMed] [Google Scholar]
- [16].Ge Y, Zhou H, Shi J, et al. The efficacy of tacrolimus in patients with refractory dermatomyositis/polymyositis: A systematic review. Clin Rheumatol 2015;34:01–7. [DOI] [PubMed] [Google Scholar]