Abstract
Background:
Accumulating evidence have revealed that pretreatment albumin to globulin ratio (AGR) may be a predictor of prognosis among patients with colorectal cancer (CRC). However, these findings are inconsistent. The aim of the present study was to investigate the prognostic value of pretreatment AGR in CRC.
Methods:
A systematic meta-analysis was conducted by searching MEDLINE, EMBASE, and Cochrane Library databases.
Results:
A total of 9 studies with 7939 patients were finally included. Low pretreatment AGR was associated with worse overall survival (pooled hazard ratio [HR]: 2.07, 95% CI: 1.60–2.67, P < .001) and disease-free survival/progress-free survival (pooled hazard ratio [HR]: 2.10, 95% confidence interval [CI]: 1.34–3.31, P = .001). Subgroup analyses revealed that the pooled correlation did not alter these results. Moreover, low pretreatment AGR were associated with elderly patients, tumor diameter (≥50 mm), tumor node metastasis stage (III–IV), depth of tumor (T3–4), and CA19-9 (>37 U/mL).
Conclusion:
The present meta-analysis suggests that low pretreatment AGR was associated with advanced clinicopathological features and worse prognosis, suggesting AGR is a useful prognostic biomarker for CRC patients.
Keywords: albumin to globulin ratio, colorectal cancer, meta-analysis, prognosis
1. Introduction
Colorectal cancer (CRC) ranks third and fourth among malignancies in terms of prevalence and lethality, with steadily rising morbidity and mortality in underdeveloped nations, and stable or declining trend in industrialized nations that currently show highest CRC burden around the globe.[1] Despite progress in early detection and treatment strategies, the overall prognosis of CRC remains poor and a major cause of mortality due to the high recurrence rates and distant metastases.[2–4] Therefore, it is critically urgent to identify new clinical biomarkers to predict the poor prognosis of high-risk CRC patients.
Tumor-associated inflammation has been reported to be a key determinant of disease progression and survival in cancer patients.[5] Tumor-associated inflammatory response consists of inflammatory cells and a series of inflammatory mediators.[6] Some inflammation-based factors have been reported to predict prognosis in many cancer types: Glasgow Prognostic Score,[7] C-reactive protein to albumin ratio (CRP/Alb),[8] the neutrophil to lymphocyte ratio,[9] the platelet to lymphocyte ratio,[10] and the lymphocyte to monocyte.[11] These factors, combined with findings of complete blood count and serum chemistry, which are routine tests at the initial visit, help to easily and accurately evaluate the severity of the systemic inflammation in patients with cancer. As a novel inflammation-based score, a combination of albumin and globulin has been reported to be correlated with the prognosis of CRC patients.[12–14] However, these results are inconclusive or even contradictory and there is no meta-analysis performed to evaluate the prognostic value of albumin to globulin ratio (AGR) in CRC.[15,16] Therefore, the present meta-analysis aimed to evaluated the correlation between pretreatment AGR, the clinicopathological feature, and prognosis of CRC patients.
2. Materials and methods
2.1. Search strategy
A comprehensive literature search was carried out on the databses of Embase, MEDLINE, and the Cochrane Central Register of Controlled Trials (CENTRAL) from the inception dates to July 2019. The key words used included: (“albumin/globulin ratio” or “albumin to globulin ratio” or “albumin-to-globulin ratio” or “albumin globulin ratio”) and (“colorectal cancer” or “colon cancer” or “rectal cancer” or “colorectal tumor” or “colorectal carcinoma” or “colorectal neoplasms” or “CRC”). Moreover, the reference lists of the identified studies were manually searched to avoid missing potential studies. Detailed search strategies can be found in Supporting Information. This study was approved by The Institutional Review Board of the Huangshi Central Hospital of Edong Healthcare Group, Hubei Polytechnic University.
2.2. Inclusion and exclusion criteria
The included studies should meet the following criteria: studies reporting the correlation between pretreatment AGR, clinical traits, and prognosis of CRC patients; hazard ratio (HR) and 95% CIs can be extracted directly from the study or sufficient data are provided to calculate these values; and study reported the cut-off value of AGR as a categorical variable. Studies were excluded under the following criteria: duplicate data, with insufficient data such that the statistics of clinical traits or survival could not be calculated, and conference abstracts, case series, reviews, and letters to editors.
2.3. Data extraction and quality assessment
The data of interest were extracted independently by 2 authors, including the following information: study characteristics: basic characteristics (first author, publication year, ethnicity, sample size, cut-off value, analysis method, and Newcastle-Ottawa quality assessment scale [NOS] score; clinicopathological outcomes, including patient age and gender, ethnicity, performance status, tumor location, size, differentiation, tumor, node, metastasis [TNM] stage, depth of tumor, and lymph node metastasis, and venous invasion), and clinicopthological traits (treatment, AGR cut-off values, carbohydrate antigen 19-9 [CA19-9], and carcinoembryonic antigen [CEA] level, patient's survival outcome, and duration of the follow-up period). Performance status is an important factor in determining the quality of life, the choice of treatment, and prognostic tool in patients.[17]
The NOS was used to assess the methodological quality of each included study.[18] There are 3 quality parameters in the NOS tool, which are selection, comparability, and outcome assessment. Studies with NOS values >6 are considered high-quality studies.
2.4. Statistical analysis
To evaluate the correlations between AGR and the survival of CRC patients, we combined HRs with their corresponding 95% CIs from each eligible study to estimate the pooled impact of AGR on overall survival (OS) and disease-free survival (DFS)/progress-free survival (PFS). ORs and the corresponding 95% CIs were calculated to assess the correlation between AGR and clinicopathological outcomes. An OR >1 indicated a positive correlation between AGR and clinicopathological outcomes. The HRs and 95% CIs were directly extracted, otherwise, they were calculated according to Tierney method.[19,20] Statistical heterogeneity was tested with I2. In case with significant heterogeneity (I2 > 50%) random-effect model would be employed, while fixed-effect model would be selected when presenting with excellent homogeneity. A subgroup meta-analysis was conducted to identify or confirm the underlying heterogeneity of a given variate. Moreover, sensitivity analysis was conducted to assess the underlying heterogeneity of a single study included in the meta-analysis.[21] If the number of included studies is >11, the publication bias was estimated by visual inspection of Begg funnel plot and Egger linear regression test and defined significantly at a P value <.05.[22,23]
3. Results
3.1. Study retrieving and selection
A total of 38 studies were identified initially in a comprehensive search in Cochrane Library, EMBASE, and Medline databases. The titles and abstracts of 27 articles were reviewed after duplicates removed. After title and abstract screening, 12 studies were omitted due to irrelevance. For further eligibility evaluation, full-text reading was conducted, and 6 studies were removed because of conference abstract (n = 1) and AGR as a continuous variable (n = 1). Nine articles involving 7939 patients were finally included.[12–16,24–27] The flow diagram of the study selection process is shown in Fig. 1.
Figure 1.
Flow diagram of the selection process.
3.2. Study characteristics
The patients were from China (n = 4), Japan (n = 4), and the USA (n = 1). The sample size ranged from 66 to 5336, with a median of 882. Five studies for CRC, 3 for rectum, and 1 for the colon. Most studies were reported at mixed disease, and 2 studies were reported in metastatic disease. Eight studies assessed the association of AGR with OS, and 6 studies assessed the association of AGR with DFS/PFS. The quality of each included study included was assessed based on the NOS tool, and all of the studies were of high quality with scores ranging from 6 to 9 stars. The summarized study characteristics and quality assessment are shown in Table 1.
Table 1.
Characteristics of the studies included in the meta-analysis.
| Author | Year | Country | Ethnicity | Sample size | Location | Treatment | Stage | Cut-off value | Outcome | Analysis | NOS score |
| Azab | 2013 | USA | Caucasian | 651 | CRC | Mixed | Mixed | 1.03 | OS | MV | 8 |
| Fujikawa | 2017 | Japan | Asian | 248 | Colon | Surgery | Mixed | 1.32 | OS/DFS | MV | 6 |
| Hachiya | 2018 | Japan | Asian | 941 | CRC | Mixed | Mixed | 1.20 | OS | MV | 7 |
| Li | 2015 | China | Asian | 293 | Rectum | Mixed | Mixed | 1.20 | OS | MV | 6 |
| Li | 2016 | China | Asian | 5336 | CRC | Mixed | Mixed | 1.50 | OS/DFS | MV | 8 |
| Zhang | 2019 | China | Asian | 71 | CRC | Chemotherapy | Metastatic | 1.40 | PFS | UV | 7 |
| Shibutani | 2015 | Japan | Asian | 66 | CRC | Chemotherapy | Metastatic | 1.25 | OS/PFS | MV | 6 |
| Toiyama | 2018 | Japan | Asian | 114 | Rectum | Mixed | Mixed | 1.18 | OS/DFS | MV | 6 |
| Xu | 2019 | China | Asian | 219 | Rectum | Surgery | Mixed | 1.43 | OS/DFS | MV | 8 |
CRC = colorectal cancer, DFS = disease-free survival, MV = multivariate, NA = not available, OS = overall survival, PFS = progression-free survival, UV = univariate.
3.3. Impact of AGR on OS
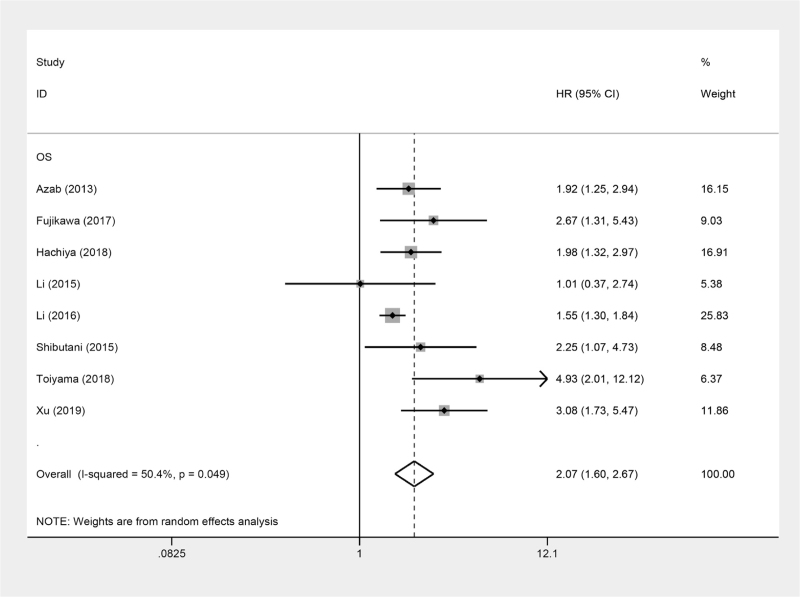
Eight studies involving 7868 patients were enrolled in the analysis of the relationship between AGR and OS of CRC patients. A random-effects model was used to conduct the meta-analysis because of the heterogeneity (I2 = 50.4%), the OS was indicated to be obviously different between the high AGR and low AGR group (pooled HR: 2.07, 95% CI: 1.60–2.67, P < 0.001; Fig. 2). Furthermore, to further investigate the underlying heterogeneity, we performed subgroup analyses according to ethnicity, sample size, treatment, tumor stage, and cut-off value for AGR (Table 2). The results revealed that neither the pooled heterogeneity nor the correlation between AGR and OS of CRC patients in each subgroup analysis was altered. As tumor stage was of great importance in the prognosis of CRC patients, a subgroup meta-analysis was conducted based on stage (mixed/metastatic). Patients with metastatic stage (pooled HR: 2.25; 95% CI = 1.07–4.72; P = .032) and mixed stage (pooled HR: 2.07; 95% CI = 1.56–2.74; P < .001) were all obviously correlated with poor OS. For the analysis stratified by cut-off for AGR, significant worse OS was observed in patients with patients with CAR <1.20 (pooled HR: 2.82; 95% CI = 1.14–7.00; P = .025) and CAR ≥1.20 (pooled HR: 1.94; 95% CI = 1.48–2.54; P < .001). Furthermore, the ethnicity, sample size, and treatment also did not affect the significant prognostic role of AGR in patients with CRC.
Figure 2.
Forest plots of the correlation between AGR and OS in CRC patients. AGR = albumin to globulin ratio, CRC = colorectal cancer, OS = overall survival.
Table 2.
Pooled hazard ratios (HRs) for OS according to subgroup analyses.
| Subgroup | No. of studies | No. of patients | HR (95% CI) | P value | Heterogeneity | |
| I2 (%) | Ph | |||||
| Overall | 8 | 7868 | 2.07 (1.60–2.67) | <.001 | 50.4 | 0.049 |
| Ethnicity | ||||||
| Asian | 7 | 7217 | 2.14 (1.57- 2.92) | <.001 | 57.1 | 0.030 |
| Caucasian | 1 | 658 | 1.92 (1.25–2.94) | .003 | – | – |
| Sample size | ||||||
| ≥300 | 2 | 6928 | 1.63 (1.34–1.98) | <.001 | 15.5 | 0.277 |
| <300 | 6 | 940 | 2.39 (1.71–3.32) | <.001 | 30.5 | 0.206 |
| Treatment | ||||||
| Surgery | 2 | 467 | 2.91 (1.86–4.55) | <.001 | 0 | 0.759 |
| Mixed | 5 | 7335 | 1.85 (1.38–2.47) | <.001 | 52.0 | 0.080 |
| Chemotherapy | 1 | 66 | 2.25 (1.07–4.72) | .032 | – | – |
| Stage | ||||||
| Mixed | 7 | 7802 | 2.07 (1.56–2.74) | <.001 | 56.3 | 0.033 |
| Metastatic | 1 | 66 | 2.25 (1.07–4.72) | .032 | – | – |
| Cut-off for AGR | ||||||
| <1.20 | 2 | 870 | 2.82 (1.14–7.00) | .025 | 71.0 | 0.063 |
| ≥1.20 | 6 | 6998 | 1.94 (1.48–2.54) | <.001 | 43.6 | 0.115 |
AGR = albumin to globulin ratio, OS = overall survival.
3.4. Impact of AGR on DFS/PFS
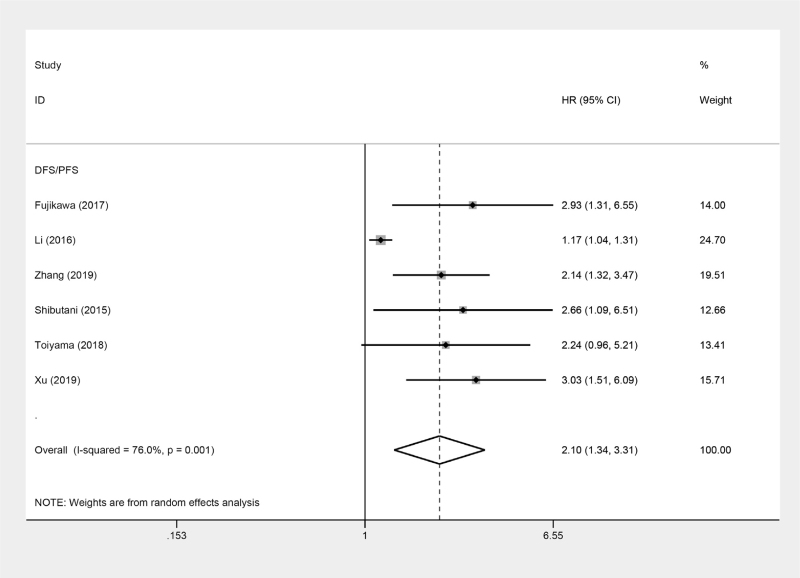
For studies evaluating DFS/PFS, a random-effects model was used to conduct the meta-analysis because of an obvious heterogeneity (I2 = 76.0%, P = .001). According to the final pooled HR of 2.10 (95% CI = 1.34–3.31, P = .001), it revealed that low AGR was correlated with decreased DFS/PFS (Fig. 3).
Figure 3.
Forest plots of the correlation between AGR and DFS/PFS in CRC patients. AGR = albumin to globulin ratio, CRC = colorectal cancer, DFS = disease-free survival, PFS = progress-free survival.
3.5. AGR and clinicopathological features
A comprehensive meta-analysis of 12 studies was conducted to evaluate the correlation between AGR and patient age, gender, performance status, tumor site, tumor size, depth of tumor, differentiation, TNM stage, CA19-9 level, CEA level, lymph node metastasis, and venous invasion. The results (Table 3) indicated that low AGR was positively correlated with age (>median vs <median; OR = 1.72, 95% CI: 1.56–1.90, P < .001), tumor size (≥50 mm vs <50 mm; OR = 2.33, 95% CI: 1.59–3.42, P < .001), TNM stage (III–IV vs I–II; OR = 1.48, 95% CI: 1.21–1.80, P < .001), depth of tumor (T3–4 vs T1–2; OR = 1.81, 95% CI: 1.38–2.38, P < .001), CA19-9 (>37 U/mL vs <37 U/mL; OR = 2.95, 95% CI: 1.46–5.94, P = .002). Neverthless, AGR was not correlated with gender (male vs female; OR = 0.9, 95% CI: 0.51–1.58, P = .72), performance status (≥1 vs 0; OR = 5.59, 95% CI: 0.73–42.80, P = .10), tumor site (colon vs rectum; OR = 1.38, 95% CI: 0.98–1.94, P = .07), tumor differentiation (low vs moderate/high; OR = 1.29, 95% CI: 0.87–1.90, P = .20), CEA (>5 ng/mL vs <5 ng/mL; OR = 1.09, 95% CI: 0.68–1.74, P = .73), lymph node metastasis (yes vs no; OR = 1.09, 95% CI: 0.99–1.20, P = .88), and venous invasion (yes vs no; OR = 1.22, 95% CI: 0.87–1.72, P = .24).
Table 3.
Meta-analysis of the association between AGR and clinicopathological features of CRC.
| Characteristics | No. of studies | No. of patients | OR (95% CI) | P | Heterogeneity | |
| I2 (%) | Ph | |||||
| Age (>median vs <median) | 4 | 6568 | 1.72 (1.56–1.90) | <.001 | 0 | 0.82 |
| Gender (male vs female) | 3 | 6496 | 0.9 (0.51–1.58) | .72 | 92 | <0.001 |
| Performance status (≥1 vs 0) | 3 | 1076 | 5.59 (0.73–42.80) | .10 | 92 | <0.001 |
| Tumor site (colon vs rectum) | 3 | 6343 | 1.38 (0.98–1.94) | .07 | 74 | 0.02 |
| Tumor diameter (≥50 mm vs <50) | 3 | 6413 | 2.33 (1.59–3.42) | <.001 | 80 | 0.007 |
| Differentiation (low vs moderate/high) | 6 | 7161 | 1.29 (0.87–1.90) | .20 | 70 | 0.005 |
| TNM Stage (III–IV vs I–II) | 3 | 1634 | 1.48 (1.21–1.80) | <.001 | 0 | 0.56 |
| Depth of tumor (T3–4 vs T1–2) | 3 | 6134 | 1.81 (1.38–2.38) | <.001 | 55 | 0.11 |
| CEA (>5 ng/mL vs <5 ng/mL) | 3 | 356 | 1.09 (0.68–1.74) | .73 | 32 | 0.23 |
| CA19-9 (>37 U/mL vs <37 U/mL) | 2 | 137 | 2.95 (1.46–5.94) | .002 | 40 | 0.20 |
| Lymph node metastasis (yes vs no) | 3 | 6417 | 1.09 (0.99–1.20) | .08 | 0 | 0.86 |
| Venous invasion (yes vs no) | 2 | 5848 | 1.22 (0.87–1.72) | .24 | 76 | 0.04 |
CA19-9 = carbohydrate antigen 19-9, CEA = carcinoembryonic antigen.
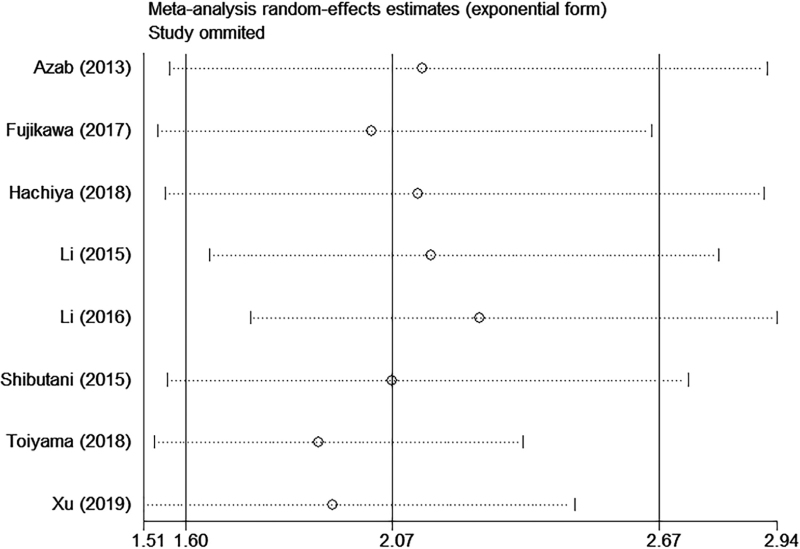
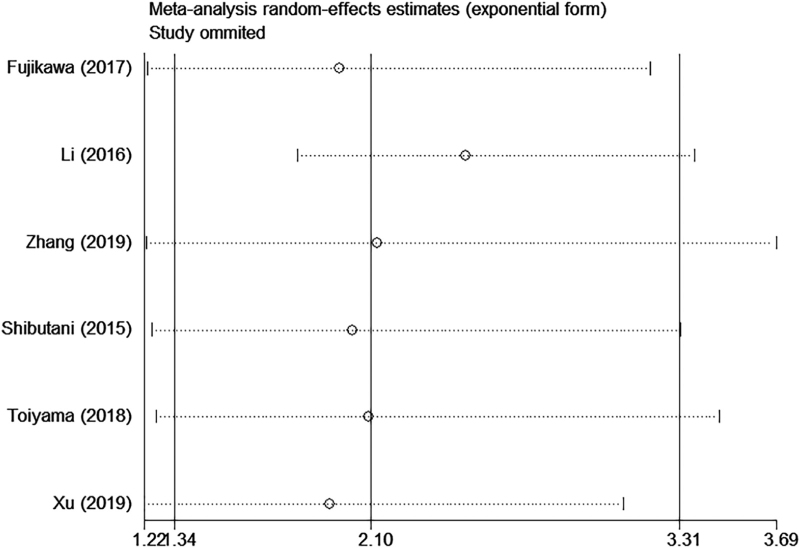
3.6. Sensitivity analysis
We conducted sequential leave-one-out sensitivity analysis to assess the stability and reliability of the results. As shown in (Figs. 4 and 5), no substantial changes were observed in the pooled results after removing each study, which indicated the robustness of the results.
Figure 4.
Sensitivity analysis of AGR on OS in CRC patients. AGR = albumin to globulin ratio, CRC = colorectal cancer, OS = overall survival.
Figure 5.
Sensitivity analysis of AGR on DFS/RFS in CRC patients. AGR = albumin to globulin ratio, CRC = colorectal cancer, DFS = disease-free survival, PFS = progress-free survival.
4. Discussion
Strength of this study is that a large cohort of CRC was used for the first time to systematically to explore the prognostic role of pretreatment AGR in CRC. Our study gives robust evidence of an association between low pretreatment AGR and worse prognosis based upon 9 studies including 7939 CRC patients. The prognostic value of AGR remained regardless of ethnicity, sample size, treatment, tumor stage, and cut-off value for AGR. Furthermore, low pretreatment AGR was linked to advanced clinical traits, such as elderly patients, large tumor size, advanced tumor stage and depth of tumor, and high CA19-9 level. Therefore, AGR might be a potential and valuable prognosticator for CRC patients.
It is well recognized that inflammatory cells are known to be powerful tumor promoters.[28,29] However, the precise mechanisms between the inflammatory response and tumor progression are not entirely elucidated. Accumulating evidence indicates that these inflammatory factors are associated with malnutrition, poor immune response, up-regulation of growth factors, and angiogenesis, thereby leading to carcinogenesis.[30,31] Several inflammatory/immune cells have been identified in the tumor microenvironment, such as tumor-associated macrophages and tumor-infiltrating lymphocytes.[32,33] Due to this inflammatory tumor microenvironment, inflammation-based factors are increasingly being investigated for their ability to predict cancer-specific outcomes. AGR, as an inflammatory marker, has been confirmed as a prognostic factor in various cancers, including CRC.[34]
AGR has been confirmed as a prognostic factor in many solid organ malignant tumors.[35–37] A previous meta-analysis revealed that high preoperative AGR was correlated with poor OS, DFS, and PFS across various types of cancer.[34] Moreover, the prognostic value of preoperative AGR remained constant regardless of AGR cut-off values and cancer type, although AGR cut-off values were heterogeneous, ranging from 0.9 to 1.93 for some cancers. In present study, the AGR cut-off values were relatively uniform, ranging from 1.01 to 1.50. The prognostic role of AGR in patients with CRC may be explained by several possible mechanisms involved in patients’ inflammatory and nutritional status. The AGR was calculated from the serum albumin and globulin levels. Serum albumin, accounting for approximately 60% of the total protein,[38] is typically used to reflect nutritional status.[39] It is produced by the liver and is the major protein in the blood that helps maintain intravascular osmotic pressure and acts as a free radical scavenger.[40] It is one of the most commonly used markers for evaluating patients’ nutritional status. More than half of CRC patients are diagnosed with malnutrition at presentation, possibly due to the progression of masticatory dysfunction, dysphagia, and cachexia, which are associated with adverse survival outcomes. Malnutrition may impair a number of human defense mechanisms, such as anatomic barriers, humoral and cellular immunity, and phagocytic function,[41] which can be accurately detected based on serum albumin levels.[42] Therefore, patients may not be sufficiently fit to receive treatment, resulting in poorer survival than patients who have normal serum albumin.
In addition to being a nutritional marker, albumin has also been considered a marker of inflammatory responses.[26] Decreased albumin levels are associated with increased inflammatory responses to tumors, poor nutritional status, and increased cytokine release. Albumin acts as an antioxidant against carcinogens by organizing cell growth and DNA stabilization.[43] Several studies have shown that hypoproteinemia is associated with cancer.[44] Hypoalbuminemia may be caused by the decreased production of ALB by hepatocytes because of cytokines released by the tumor, such as interleukin (IL)-1, IL-6, and tumor necrotic factor α, which blocks hepatocyte albumin production.[45,46] Increased TNF-α and cytokine levels may be a surrogate for more aggressive disease.[47] Hypoproteinemia may also be due to the intense systemic inflammatory response that accompanies the tumor, which may be a surrogate for more aggressive malignancies.[48] Due to possible gastrointestinal obstruction and malabsorption, gastrointestinal tumor patients have a higher risk of hypoproteinemia than other cancer patients.[49]
Globulin is the main protein produced by immune organs, which reflects the body's inflammation and immune status.[50] According to its electropherogram, globulin includes α1, α2, β, and γ globulin. The γ globulin, also known as immunoglobulins or antibodies, play an important role in immunity and inflammation.[51] In chronic inflammation, the level of globulin gradually increases due to the activity of inflammatory cytokines.[52] High levels of globulin resulting from immunoglobulin and acute phase protein aggregation may indicate inflammation and host immune responses in the malignancy microenvironment. As a biomarker of systemic inflammation, an increased preoperative C-reactive protein (CRP) level predicts poor survival in CRC.[53] Furthermore, elevated complement 3 and IgA levels have been considered as a prognostic factors in patients with CRC.[54] Because albumin and globulin are affected by many factors such as stress, liver failure, and dehydration, they also show measurement variability. However, because the AGR is represents albumin/globulin ratio and is therefore less affected by measurement variability, making it a more appropriate indicator than serum albumin or globulin alone. We believe that nutritional status and the systemic inflammatory response have important effects on the oncological outcomes of patients with CRC. The AGR is a combination of these 2 predictors of adverse outcome, which may enhance its predictive value. Taken together, AGR may represent a balance between the nutritional status and inflammation, may serve as a predictor of prognosis in CRC patients.
The present meta-analysis has several limitations. First, most of the studies included were from Asia countries. Hence, our findings need to validate in populations of other ethnicities. Second, there existed a moderate between-study heterogeneity, which might have originated from different definitions of cut-off value. Random-effects model, subgroup analysis, and sensitivity analysis were performed to adjust this shortcoming. Finally, all included studies were retrospective studies. It would give rise to select bias when getting past information. In addition, they are more prone to selection bias due to the retrospective nature of the study design.
5. Conclusions
The present meta-analysis suggests that low pretreatment AGR was associated with advanced clinicopathological features and worse prognosis, suggesting AGR is a useful prognostic biomarker for CRC patients.
Author contributions
Jian-Ying Ma and Zhong-Zhong Zhu designed the study. Gang Liu, Liang-Zhi Pan, and Zhong-Zhong Zhu contributed to the collection of clinical information and data analysis. Jian-Ying Ma, Gang Liu, Liang-Zhi Pan, and Min Hu analyzed and interpreted the data. Jian-Ying Ma and Gang Liu wrote and revised the manuscript. Zhong-Zhong Zhu supervised the study.
Footnotes
Abbreviations: AGR = albumin to globulin ratio, CEA = carcinoembryonic antigen, CRC = colorectal cancer, DFS = disease-free survival, NOS = Newcastle-Ottawa quality assessment scale, OS = overall survival, PFS = progress-free survival, TNM = tumor node metastasis.
How to cite this article: Ma JY, Liu G, Pan LZ, Hu M, Zhu ZZ. Clinical impact of pretreatment albumin–globulin ratio in patients with colorectal cancer: a meta-analysis. Medicine. 2022;101:20(e29190).
JYM, GL, and LZP contributed equally to this work.
The authors declare that they have no competing interests.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Jian-Ying Ma, Email: majianying2015@sina.com.
Gang Liu, Email: gangliu2017@126.com.
Liang-Zhi Pan, Email: 695375957@qq.com.
Min Hu, Email: 46679555@qq.com.
Zhong-Zhong Zhu, Email: drzhuzz@sina.com.
References
- [1].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Van Cutsem E, Nordlinger B, Adam R, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer 2006;42:2212–21. [DOI] [PubMed] [Google Scholar]
- [3].Roxburgh CS, McMillan DC. The role of the in situ local inflammatory response in predicting recurrence and survival in patients with primary operable colorectal cancer. Cancer Treat Rev 2012;38:451–66. [DOI] [PubMed] [Google Scholar]
- [4].Ludmir EB, Palta M, Willett CG, Czito BG. Total neoadjuvant therapy for rectal cancer: an emerging option. Cancer 2017;123:1497–506. [DOI] [PubMed] [Google Scholar]
- [5].Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- [7].Lu X, Guo W, Xu W, et al. Prognostic value of the Glasgow prognostic score in colorectal cancer: a meta-analysis of 9,839 patients. Cancer Manag Res 2019;11:229–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Konishi S, Hatakeyama S, Tanaka T, et al. C-reactive protein/albumin ratio is a predictive factor for prognosis in patients with metastatic renal cell carcinoma. Int J Urol 2019;26:992–8. [DOI] [PubMed] [Google Scholar]
- [9].Xia WK, Liu ZL, Shen D, Lin QF, Su J, Mao WD. Prognostic performance of pre-treatment NLR and PLR in patients suffering from osteosarcoma. World J Surg Oncol 2016;14:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kitano Y, Yamashita YI, Yamamura K, et al. Effects of preoperative neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios on survival in patients with extrahepatic cholangiocarcinoma. Anticancer Res 2017;37:3229–37. [DOI] [PubMed] [Google Scholar]
- [11].Cong X, Li S, Xue Y. Impact of preoperative lymphocyte to monocyte ratio on the prognosis of the elderly patients with stage II(-III (gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi 2016;19:1144–8. [PubMed] [Google Scholar]
- [12].Hachiya H, Ishizuka M, Takagi K, et al. Clinical significance of the globulin-to-albumin ratio for prediction of postoperative survival in patients with colorectal cancer. Ann Gastroenterol Surg 2018;2:434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang L, Zhang J, Wang Y, et al. Potential prognostic factors for predicting the chemotherapeutic outcomes and prognosis of patients with metastatic colorectal cancer. J Clin Lab Anal 2019;33:e22958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xu Y, Xu X, Xi C, Ye N, Wang Y. Prognostic value of preoperative albumin to globulin ratio in elderly patients with rectal cancer. Medicine (Baltimore) 2019;98:e16066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li Q, Meng X, Liang L, Xu Y, Cai G, Cai S. High preoperative serum globulin in rectal cancer treated with neoadjunctive chemoradiation therapy is a risk factor for poor outcome. Am J Cancer Res 2015;5:2856–64. [PMC free article] [PubMed] [Google Scholar]
- [16].Toiyama Y, Oki S, Okugawa Y, et al. Clinical impact of preoperative albumin-globulin ratio in patients with rectal cancer treated with preoperative chemoradiotherapy. Oncology 2018;95:270–80. [DOI] [PubMed] [Google Scholar]
- [17].Sok M, Zavrl M, Greif B, Srpčič M. Objective assessment of WHO/ECOG performance status. Support Care Cancer 2019;27:3793–8. [DOI] [PubMed] [Google Scholar]
- [18].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [19].Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- [20].Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kriston L. Dealing with clinical heterogeneity in meta-analysis. Assumptions, methods, interpretation. Int J Methods Psychiatr Res 2013;22:01–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [24].Li Y, Jia H, Yu W, et al. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int J Cancer 2016;139:220–31. [DOI] [PubMed] [Google Scholar]
- [25].Shibutani M, Maeda K, Nagahara H, et al. The pretreatment albumin to globulin ratio predicts chemotherapeutic outcomes in patients with unresectable metastatic colorectal cancer. BMC Cancer 2015;15:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fujikawa H, Toiyama Y, Inoue Y, et al. Prognostic impact of preoperative albumin-to-globulin ratio in patients with colon cancer undergoing surgery with curative intent. Anticancer Res 2017;37:1335–42. [DOI] [PubMed] [Google Scholar]
- [27].Azab B, Kedia S, Shah N, et al. The value of the pretreatment albumin/globulin ratio in predicting the long-term survival in colorectal cancer. Int J Colorectal Dis 2013;28:1629–36. [DOI] [PubMed] [Google Scholar]
- [28].Wisastra R, Dekker FJ. Inflammation, cancer and oxidative lipoxygenase activity are intimately linked. Cancers (Basel) 2014;6:1500–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer 2006;6:24–37. [DOI] [PubMed] [Google Scholar]
- [30].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- [31].Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565–70. [DOI] [PubMed] [Google Scholar]
- [32].Zhao Y, Ge X, He J, et al. The prognostic value of tumor-infiltrating lymphocytes in colorectal cancer differs by anatomical subsite: a systematic review and meta-analysis. World J Surg Oncol 2019;17:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pathria P, Louis TL, Varner JA. Targeting tumor-associated macrophages in cancer. Trends Immunol 2019;40:310–27. [DOI] [PubMed] [Google Scholar]
- [34].Lv GY, An L, Sun XD, Hu YL, Sun DW. Pretreatment albumin to globulin ratio can serve as a prognostic marker in human cancers: a meta-analysis. Clin Chim Acta 2018;476:81–91. [DOI] [PubMed] [Google Scholar]
- [35].Atsumi Y, Kawahara S, Kakuta S, et al. Low preoperative albumin-to-globulin ratio is a marker of poor prognosis in patients with esophageal cancer. In Vivo 2021;35:3555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shinde R, Bhandare MS, Chaudhari V, Sarodaya V, Agarwal V, Shrikhande S. Preoperative albumin-globulin ratio and its association with perioperative and long-term outcomes in patients undergoing pancreatoduodenectomy. Dig Surg 2021;38:275–82. [DOI] [PubMed] [Google Scholar]
- [37].Utsumi M, Kitada K, Tokunaga N, et al. Preoperative albumin-to-globulin ratio predicts prognosis in hepatocellular carcinoma: a cohort study including non-hepatitis virus-infected patients. Dig Surg 2021;38:307–15. [DOI] [PubMed] [Google Scholar]
- [38].Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Inflammation-based prognostic system predicts postoperative survival of colorectal cancer patients with a normal preoperative serum level of carcinoembryonic antigen. Ann Surg Oncol 2012;19:3422–31. [DOI] [PubMed] [Google Scholar]
- [39].Caraceni P, Tufoni M, Bonavita ME. Clinical use of albumin. Blood Transfus 2013;11:s18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Asher V, Lee J, Bali A. Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Med Oncol 2012;29:2005–9. [DOI] [PubMed] [Google Scholar]
- [41].Liu J, Dai Y, Zhou F, et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomy. Urol Oncol 2016;34:484.e1–8. [DOI] [PubMed] [Google Scholar]
- [42].Laky B, Janda M, Cleghorn G, Obermair A. Comparison of different nutritional assessments and body-composition measurements in detecting malnutrition among gynecologic cancer patients. Am J Clin Nutr 2008;87:1678–85. [DOI] [PubMed] [Google Scholar]
- [43].Seaton K. Albumin concentration controls cancer. J Natl Med Assoc 2001;93:490–3. [PMC free article] [PubMed] [Google Scholar]
- [44].Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr 2019;43:181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol 2005;39:S143–6. [DOI] [PubMed] [Google Scholar]
- [46].Onate-Ocana LF, Aiello-Crocifoglio V, Gallardo-Rincon D, et al. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol 2007;14:381–9. [DOI] [PubMed] [Google Scholar]
- [47].Brenner DA, Buck M, Feitelberg SP, Chojkier M. Tumor necrosis factor-alpha inhibits albumin gene expression in a murine model of cachexia. J Clin Invest 1990;85:248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].McMillan DC, Watson WS, O’Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer 2001;39:210–3. [DOI] [PubMed] [Google Scholar]
- [49].Alkan A, Koksoy EB, Utkan G. Albumin to globulin ratio, a predictor or a misleader? Ann Oncol 2015;26:443–4. [DOI] [PubMed] [Google Scholar]
- [50].Ballow M. Mechanisms of action of intravenous immune serum globulin in autoimmune and inflammatory diseases. J Allergy Clin Immunol 1997;100:151–7. [DOI] [PubMed] [Google Scholar]
- [51].Kubczak M, Rogalinska M. Evolution of monoclonal antibodies in cancer treatment. Postepy Biochem 2016;62:518–25. [PubMed] [Google Scholar]
- [52].Du XJ, Tang LL, Mao YP, et al. The pretreatment albumin to globulin ratio has predictive value for long-term mortality in nasopharyngeal carcinoma. PloS One 2014;9:e94473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Björkman K, Kaprio T, Beilmann-Lehtonen I, Stenman UH, Böckelman C, Haglund C. TATI, TAT-2, and CRP as prognostic factors in colorectal cancer. Oncology 2022;100:22–30. [DOI] [PubMed] [Google Scholar]
- [54].Codina Cazador A, Salvá Lacombe JA, Fernández-Llamazares Rodríguez J, Ruiz Feliu B, Codina Barreras A, Moreno Aguado V. Immunoglobulins and the complement system in colorectal cancer. Rev Esp Enferm Apar Dig 1989;75:143–8. [PubMed] [Google Scholar]