Abstract
Background:
The increasing number of chronic obstructive pulmonary disease (COPD) incidence has led to a great negative impact on older people's lives. This chronic disease was a critical and independent risk factor for cognitive function impairment in the elderly with mild cognitive impairment as a frequent feature. This systematic review aimed to examine the risk of developing cognitive impairment in COPD.
Methods:
A structured search of the literature was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement guideline, with a pre-determined search strategy starting from study identification, title and abstract screening, eligibility assessment, and inclusion of relevant study. The search was conducted in PubMed and MEDLINE via EBSCOhost, with restriction to human studies. The studies from inception until January 12, 2021.
Results:
Five original articles were included. Most studies found that patients with COPD had a higher chance of developing cognitive impairment, especially when patients were followed up for more than 5 years. We discovered that the risk of cognitive impairment seemed to be correlated with the length of time spent following the participants, with the highest risk of cognitive impairment being identified in those who had the longest observation period. It is critical to conduct cognitive screening from the time a diagnosis of COPD is obtained and on a continuing basis in order to recognize and treat these individuals appropriately.
Conclusion:
There is a potential association between COPD and mild cognitive impairment. We encourage more studies to be done with higher sensitivity and specificity cognitive screening tools in the future to build better evidence and qualify to be analyzed quantitatively with meta-analysis.
Keywords: chronic obstructive pulmonary disease, cognitive dysfunction, COPD, mild cognitive impairment, mild cognitive impairment, pulmonary disease
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a major public health problem due to its high prevalence, devastating systemic effects and associated morbidity and mortality. An estimated 300 million people (as of 2013) are thought to have COPD.[1] The incidence of COPD in the general population is still increasing, and with an ageing population, this number is expected to increase further. COPD is a multisystem disease, with effects beyond the lung and is associated with symptom burden and prognosis that causes chronic airflow limitation, breathlessness, exercise intolerance, cough, difficulty with daily activities, infections, and (re)hospitalization.[2] COPD has great negative impact on the lives of older people, particularly in relation to quality of life, physical functioning, and increased utilization of health care resources.[3]
Attention and executive dysfunctions are frequent features of mild cognitive impairment (MCI) in COPD.[4] Cross-sectional studies have estimated the prevalence of cognitive impairment in general COPD at between 16% and 57%.[3] MCI is defined as a clinical condition characterized by decline of cognitive function greater than expected for a certain age and educational level of the individual but not severe enough to interfere with their daily activities.[5,6] A mechanism proposed for the cognitive impairment in COPD patients is the neuronal damage mediated by hypoxia as a result of the pulmonary disease or the comorbidities that adversely affect the brain, such as vascular disease and smoking.[5] Other study suggested that mechanisms for the association of COPD with higher rates of cognitive impairment, including oxidative stress, inactive state, systemic inflammatory state, and loss of hippocampal volume.[1,7] Patients with COPD report high levels of anxiety and depression, which are associated with disease severity and are related to psychosocial constructs, such as poor quality of life, living alone, female sex, smoking and low socioeconomic status.[4] Identifying pending cognitive impairment at an early stage has become an increasingly important challenge to physicians.[8]
COPD was an important and independent risk factor for cognitive function impairment in the elderly, and the association was more pronounced among those who were current smokers.[9] Identifying cognitive impairment at an early stage has become an increasingly important challenge to physicians.[8] This suggested that clinicians should pay attention to the effect of COPD on cognitive function to avoid more serious cognitive impairment and reduce disease burden, especially among smokers.[9] If the cognitive impairment is confirmed, this would be clinically relevant for COPD management (e.g., it might alter the ability to comply with particular treatment types or benefit from more complex therapies) and for care of their comorbid disease, as specific types of cognitive impairment may have different management strategies.[7]
This article presents a systematic review that examines the risk of cognitive impairment in COPD patients. All of these data bring to light the importance of COPD as a risk factor for MCI, highlighting the need for early detection and intervention, to prevent or delay MCI onset and/or progression for these patients that are likely to require more support and have need of an individualized respiratory care plan which can be beneficial for their cognitive deficits.
2. Methods
A structured search of the literature was conducted to identify research on the effect of COPD on cognitive impairment, using the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement guideline, with a pre-determined search strategy starting from study identification, title and abstract screening, eligibility assessment, and inclusion of relevant study. The search was conducted in PubMed and MEDLINE via EBSCOhost. Search were restricted to human studies, and studies from inception until January 12, 2021 using the MeSH terms and complemented the search strategy using the [All Field], to include terms not found using MeSH term. Combination of search terms we used for search strategy including: chronic obstructive pulmonary disease, cognitve dysfunction, cognitive impairment, neurocognitive disorder, cognitive decline and dementia (Table 1)
Table 1.
Search strategy complemented using MeSH Terms and [All Field].
| Keyword | Results |
| ((“pulmonary disease, chronic obstructive”[MeSH Terms] OR “Chronic Obstructive Pulmonary Disease”[All Fields]) OR (“pulmonary disease, chronic obstructive”[MeSH Terms] OR (“pulmonary”[All Fields] AND “disease”[All Fields] AND “chronic”[All Fields] AND “obstructive”[All Fields]) OR “chronic obstructive pulmonary disease”[All Fields] OR “copd”[All Fields])) AND (((((((“cognitive dysfunction”[MeSH Terms] OR “Cognitive Dysfunction”[All Fields]) OR “Mild Cognitive Impairments”[All Fields]) OR “Cognitive Impairment”[All Fields]) OR “Cognitive Impairments”[All Fields]) OR “Mild Neurocognitive Disorder”[All Fields]) OR “Cognitive Declines”[All Fields]) OR “Cognitive Decline”[All Fields]) AND “humans”[MeSH Terms] | 310 |
| ((“pulmonary disease, chronic obstructive”[MeSH Terms] OR “Chronic Obstructive Pulmonary Disease”[All Fields]) OR (“pulmonary disease, chronic obstructive”[MeSH Terms] OR (“pulmonary”[All Fields] AND “disease”[All Fields] AND “chronic”[All Fields] AND “obstructive”[All Fields]) OR “chronic obstructive pulmonary disease”[All Fields] OR “copd”[All Fields])) AND ((“dementia”[MeSH Terms] OR (“dementia”[MeSH Terms] OR “dementia”[All Fields])) OR (“dementia”[MeSH Terms] OR “dementia”[All Fields] OR “dementias”[All Fields])) AND “humans”[MeSH Terms] | 529 |
| (TI “chronic obstructive pulmonary disease” OR TI copd) AND (TI dementia OR TI “cognitive decline” OR TI “cognitive impairment”) | 77 |
| Total | 916 |
Search strategy were conducted and results were imported into Endnote X9. Duplicates were removed and remaining articles were reviewed for relevance based on the following criteria: studies’ participants were exposed with COPD and compared with control group that is without COPD, outcome assessment were related to risk of developing cognitive impairment evaluated using hazard ratio for a minimum follow up of 1 year, sample size at least 30 participants, and having relevant study design, participant characteristics, and results.
In order to analyse the quality of reporting from cohort studies, we performed assessment according to Strengthening the Reporting of Observational studies in Epidemiology checklist for cohort studies.[10] When quantitative analysis is possible, we extract the reported hazard ratio estimates and their confidence intervals from each study for meta-analysis. We qualify studies to be quantitatively analyzed when having a similar length of follow up and having the similar population characteristic defined by age, and comorbidities. We evaluated methodological quality of cohort studies using Newcastle Ottawa Scale (NOS).[11] This tool allowed assessment for selection, comparability and outcome, with eight items. Each item received a maximum of 1 point for selection and outcome domain, while a maximum of 2 points for comparability domain.
3. Ethics approval
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated in each case.
3.1. Patient and publication consent statement
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the patient consent statement indicated in each case.
4. Results
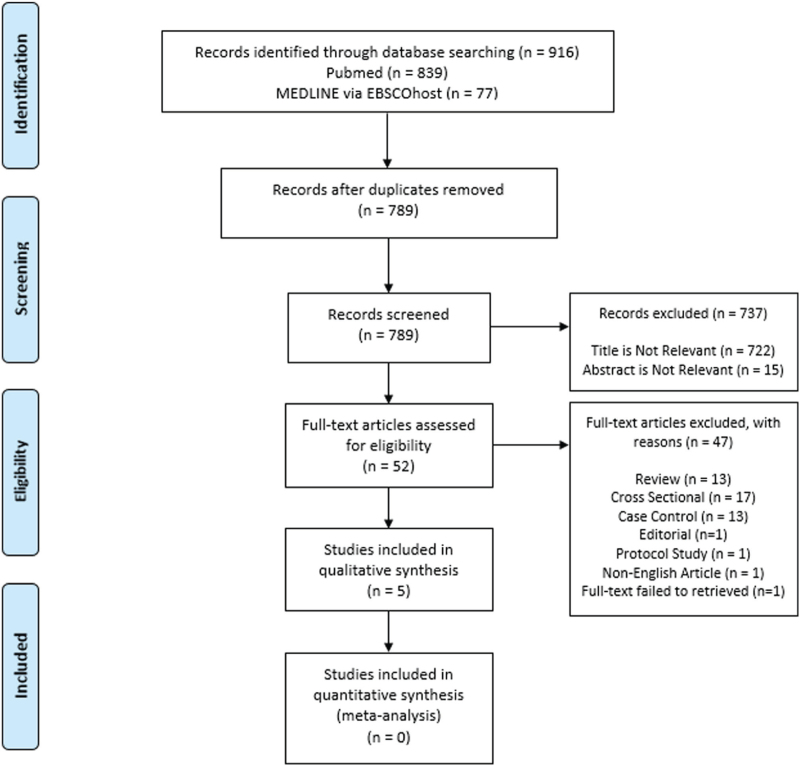
The search strategy identified 916 studies. Results were imported into Endnote X9, and duplicates removed, leaving 789 articles to review. The abstracts of these 789 articles were reviewed for relevance. After screening, 52 articles were retained for full review. Of these, 47 articles were excluded because study design are cross sectional, case control, editorial, review article, protocol study and full-text failed to retrieved. Finally, 5 studies are included for qualitative analysis (Fig. 1).
Figure 1.
Flow diagram of the identification and selection of studies included in the analysis.
This systematic review used 5 observational study including 3 cohort retrospective and 2 prospective studies from 11 countries (Table 2). We identified from all 5 studies that they have evaluation of risk from patients having COPD to develop cognitive impairment as analyzed by hazard ratio.[9,12–15] Every study have a different length of follow up. Various length of follow up including around 3 years for Xie and Xie[9] and Cherbuin et al[15]; 5 years for Singh et al[14]; 12 years for Liao et al[12]; and 25 years for Rusanen et al.[13] We analyzed 80,026 participants with baseline age of older than 44 years old. Cognitive impairment was defined by various methods that is according to Diagnostic and Statistical Manual (DSM) – IV and Mayo Clinic AD Research Center criteria; neuropsychological testing; International Classification of Diseases, Ninth Revision, Clinical Modification and Mini Mental State Examination (MMSE), cognitive test battery, clinical interviews, and informant reports using various diagnostic tools.
Table 2.
Characteristics of included studies.
| Study | Country | Design | Subjects | Baseline age | Definition | Inclusion criteria | Exclusion criteria | Follow up | Assessment | Outcome |
| Rusanen M, et al (2013) | Finland | Cohort Retrospective | Cognitive impairment = 289Control = 1222 | Cognitive impairment = 51.5 ± 5.9Control = 50.0 ± 6.0 | Cognitive impairment group is the combination of patient with MCI and patient with dementiaThe diagnoses of MCI and dementia were made in the meetings of a review board. The diagnosis of dementia was based on DSM-IV criteria and MCI onMayo Clinic AD Research Center (MCADRC) criteria | Population from four independent, randomly selected, population-based samples originally studied within the framework of the North Karelia Project and the FINMONICA study in 1972, 1977, 1982 or 1987 (midlife visit). | N/A | 25 years | DSM IV and Mayo Clinic AD Research Center (MCADRC) criteria | HR for Cognitive Impairment∗: 1.85 (1.05 – 3.28) |
| Singh B, et al (2014) | United States | Cohort Prospective | MCI = 370Normal cognition = 1055 | MCI = 82.8 (77.42 – 85.16)Normal cognition = 78.33 (74.37 – 82.73) | A domain-specific (memory, language, executive function and visuospatial skills)score less than 1.0 standard deviation (SD) below the age-specific mean among the generalpopulation was considered as possible cognitive impairment.A diagnosis of normalcognition, MCI, or dementia was made according to published criteria and was based on aconsensus agreement between the interviewing nurse, examining physician, and theneuropsychologist taking into account all the information collected | Residency in Olmsted County, absence of dementia (determined through medical record review by a behavioral neurologist), and not terminally ill or in hospice. Cognitively normal subjects at baseline. | Prevalent cases of MCI, and individuals who died or dropped out prior to any follow-up | 5.1 years | Nurse interview, neurological examination, and neuropsychological testing. | HR for MCI, > 5 years†: 1.58 (1.04-2.40)HR for MCI, ≤ 5 years†: 1.11 (0.70–1.74) |
| Liao WC, et al (2015) | Taiwan | Cohort Retrospective | COPD + = 20492COPD - = 40765 | COPD + = 68.2 ± 12.4COPD - = 67.0 ± 12.5 | Not reported | COPD and non-COPD patients newly diagnosed between 1998 and 2008 from Taiwan National Health Insurance Research Database (NHIRD) | Patients with a history of dementiaor who were younger than 20 years, loss to follow-up, death or withdrawal from the database, orthe end of 2010 | 12 years | International Classification of Diseases, Ninth Revision, Clinical Modification | HR for COPD vs non-COPD group for dementia‡:1.27 (1.20–1.36)P < .001 |
| Cherbuin N, et al (2018) | Cuba, Dominican Republic, Peru, Venezuela, Mexico, China, Puerto Rico | Cohort Retrospective | COPD + = 11098 | COPD + = 74 (65–80 + ) | Not reported | COPD patients age 65 years and over | Not reported | 3 years | Cognitive tests battery, clinical interviews, and informant reports (including Community Screening Instrument for Dementia; the CERAD wordlist learning and animal naming tests; the GeriatricMental State Examination, and the History and Aetiology Schedule – Dementia Diagnosis and Subtype), and was validated against local clinicians DSM-IVDiagnoses | HR for COPD for dementia§:0.74 (0.43–1.04)P > .05 |
| Xie and Xie (2019) | China | Cohort Prospective | COPD + = 515COPD - = 4220 | Male = 82.21 ± 9.33Female = 83.74 ± 10.13 | MCI was defined as positive if the MMSE score was below 17for illiterate participants, below 20 for those with 1–6 years of education, or below 24 for those who had over 6 years of education among Chinese people | Population from Chinese LongitudinalHealth Longevity Survey (CLHLS) 2011/2012 wave | lacked follow-up information,were missing important variable data, or had been diagnosedwith mild cognitive impairment (MCI) or dementia at baseline | 3 years | Mini Mental State (MMS) was used to evaluate cognitive status. | HR for MCI, 3 years¶: 1.486 (1.207 – 1.855) |
Adjusted for age, sex, education, midlife smoking, APOE, midlife physical activity, systolic blood pressure, body mass index, and total serum cholesterol and late-life vascular diseases.
Adjusted for education as a continuous variable, sex where applicable, age at baseline as time variable, Beck Depression Inventory-II, history of stroke, APOEe4 genotype, smoking, diabetes, hypertension, coronary artery disease, z-scores and BMI.
Adjusted for age, sex, urbanization and comorbidities (diabetes, hypertension, stroke, coronary artery disease, depression, head injury).
Adjusted for age, sex, education level, smoking, physical activity, hypertension, depression and hazardous alcohol consumption.
Adjusted for baseline prevalence of hypertension, diabetes, stroke, alcohol drinking, current exercise, baseline body mass index, age, gender, marital status, education level.
All studies present results with risk using hazard ratio adjusted by confounders mainly age, sex and comorbidities. Three studies,[9,12,13] showed a significant risk for patients with COPD to develop cognitive impairment. One study[14] only found a significant risk for a period of follow up around more than 5 years but the risk was become insignificant when follow up was less than 5 years. One study[15] did not find a significant risk for dementia among COPD patients during a follow up of around 3 years.
4.1. Quality of reporting cohort studies
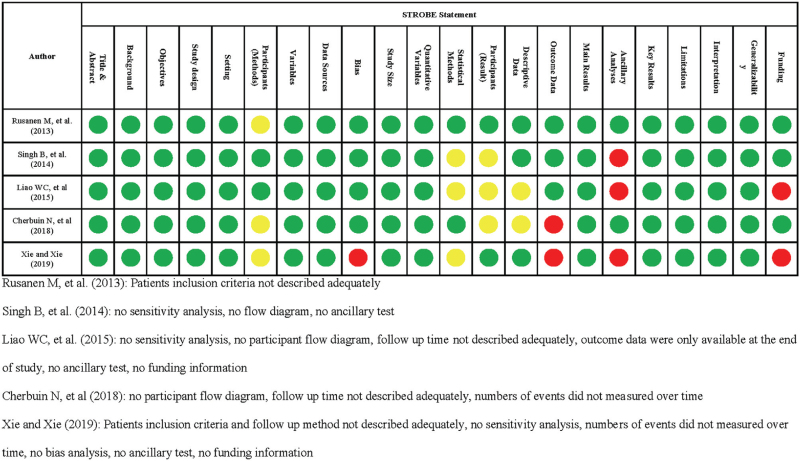
We conducted quality of reporting cohort studies in accordance with Strengthening the Reporting of Observational studies in Epidemiology guideline (Fig. 2).[10] Study designs were described in title or abstract and explaination related to what was done and found had been explained adequately in all studies.[9,12–14] Scientific background and rational for investigation, and objectives were described completely in all studies.[9,12–14] For the reported methods, we found that patient inclusion or eligibility criteria are not described adequately in 3 studies,[9,13,15] while there was not enough information related to methods on patients follow up in one study.[9] All studies[9,12–14] had clearly defined all variables used in their studies. Data sources and measurements had been described in all studies.[9,12–14] One study had not described any efforts to address potential bias.[9] All studies provided explaination regarding on how to handle quantitative variables. Of 5 studies, 2 provided sensitivity analysis detect outcome of interest.[13,15] Two studies considered use of flow diagram.[12,14] Follow up time not describe adequately in 2 study.[12,15] Number of events of cognitive impairment was not shown over time in 2 study.[9,15] Main results was informed completely in all studies provided by unadjusted and adjusted estimation. There was no additional test done in 3 studies[9,12,14] All studies[9,12–14] provide complete explanation related to key results, limitation, interpretation, generalizability. Two studies[9,12] did not provide any funding information.
Figure 2.
STROBE Statement for Evaluating Quality of Reporting in Cohort Studies. Clear information provided; , Some information but insufficient; , No information provided or unclear description.
4.2. Methodological quality for cohort study
We used NOS to evaluate methodological quality across studies (Table 3).[11] We evaluate each study and have score ranged between 7/9 and 9/9. A study by Xie and Xie[9] have a score 7/9 due to self reported COPD and no information regarding on how the assessment of outcome performed. A study by Rusanen et al[13] and Cherbuin et al[15] also showed that COPD is self reported and thus revealing a total NOS score of 8/9. Other 2 studies[12,14] has enough information across items and thus showing a 9/9 NOS score.
Table 3.
Methodological quality as evaluated by Newcastle Ottawa Scale.
| Selection | Comparability | Outcome | |||||||
| Author | Representativeness of the exposed cohort | Selection of the non exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at the start of study | Comparability of cohort on the basis of the design and analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up cohorts | Newcastle Ottawa Scale Grade |
| Rusanen M, et al (2013) | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 8 |
| Singh B, et al (2014) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Liao WC, et al (2015) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Cherbuin N, et al (2018) | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 8 |
| Xie and Xie (2019) | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 1 | 7 |
5. Discussion
Our systematic review included 5 cohort studies with different follow up times. Results from over 5 years follow up showed risk of cognitive impairment in COPD patients, meanwhile a follow up of less than 5 years are currently showing a mixed result.
A follow up study for about 12 years in newly diagnosed COPD patients between 1998 and 2008 by Liao et al,[12] revealed that there will be 27% higher chance of getting dementia in COPD group than non-COPD group. For a follow up at about 25 years, Rusanen et al[13] showed 85% higher chance of COPD patients getting cognitive impairment. A study by Singh et al[14] revealed that patients with COPD had 58% higher chance of getting MCI after more than 5 years of follow up but no significant risk of MCI when patients were followed up for less than 5 years of follow up. Another study by Cherbuin et al,[15] also showed a similar result favoring a non-significant risk of dementia when patient followed up for only 3 years. On the contrary, Xie and Xie[9] show 49% higher chance for MCI during a 3 years follow up.
During follow up of less than 5 years, different results from Singh et al,[14] Xie and Xie,[9] and Cherbuin et al[15] may be due to different adjustment conducted during analysis and different diagnostic tools that were being used. Study by Xie and Xie[9] conducted adjustment for baseline prevalence of hypertension, diabetes, stroke, alcohol drinking, current exercise, baseline body mass index, age, gender, marital status, and education level. This study also used MMSE to evaluate cognitive status while Singh et al[14] refered to nurse interview, neurological examination, and neuropsychological testing to diagnose MCI. Meanwhile Singh et al[14] made adjusment for education as a continuous variable, sex where applicable, age as time variable, Beck Depression Inventory-II, history of stroke, apolipoprotein-E4 genotype, smoking, diabetes, hypertension, coronary artery disease, z-scores and body mass index. Furthermore, when looking at study by Cherbuin et al,[15] the author adjusted confounding factors such as age, sex, education level, smoking, physical activity, hypertension, depression and hazardous alcohol consumption. Study by Singh et al[14] also reviewed the medical records to confirm a diagnosis of COPD and while Xie and Xie[9] and Cherbuin et al[15] had a self reported COPD and thus different effects may be introduced due to uncertain COPD diagnosis from the latter study.
Lower hazard ratio in study by Liao et al[12] than study conducted by Rusanen et al[13] probably due to different type of cognitive impairment that were being measured and the length of follow up. Cohort retrospective study by Liao et al[12] measured the chance of COPD patients getting dementia in 12 years follow up while cohort prospective study by Rusanen et al[13] measured in 25 years follow up, the chance of developing cognitive impairment in which analysis of mild cognitive impairment and dementia group were combined. The overall result difference between the 4 studies probably caused by different diagnostic tools that being used. All the 4 studies[9,12–14] used different diagnostic tools: International Classification of Diseases, Ninth Revision, Clinical Modification in study by Liao et al[12]; DSM IV and Mayo Clinic AD Research Center in study by Rusanen et al[13]; nurse interview, neurological examination, and neuropsychological testing in study by Singh et al[14]; and MMSE in study by Xie and Xie.[9] ICD-9 that was being used by Liao et al[12] has 100% sensitivity and 90.4% specificity in diagnosing dementia with positive predictive value at 11.2%.[16] A study by Solomon et al[17] evaluated standard clinical instruments to measure cognitive impairment, included MMSE, Montreal Cognitive Assessment (MOCA), and Alzheimer's Disease Assessment Scale – Cognitive Subscale. The findings, showed that the MOCA is superior to the MMSE as screening tool, particularly in discerning the earliest symptoms of cognitive decline. Similarly, Alzheimer's Disease Assessment Scale –Cognitive Subscale also demonstrated good diagnostic and classification accuracy in differentiating between diagnoses. The addition of a subjective measure of functional impairment can improve overall diagnostic accuracy.[17] MMSE that being used by Xie and Xie[9] has 66.23% sensitivity and 72.94% specificity to differentiate MCI group and control group. Meanwhile, MoCA has greater sensitivity at 80.48% and specificity at 81.19%. These results suggested that diagnostic tools with higher sensitivity and specificity with the addition of subjective measure could be used in future study as a tool to diagnose MCI in COPD patients.[16]
Previous systematic review in 2017 had highlighted that at least 1 out of 4 people with COPD had MCI and that the prevalence of any cognitive impairment for COPD was 32% among COPD patients.[3] However, this systematic review used prevalence for effect measurement.[3] Here, we present a systematic review that is able to represent a more appropriate estimate to identify the risk of cognitive impairment in COPD by evaluating risk estimation instead of prevalence which is used more appropriately in reflecting disease burden.[18] Therefore, despite the burden of MCI in COPD patients had been reported by previous review,[3] it needs to be emphasized that there were still limited evidence concerning the risk of cognitive impairment in COPD patients and future larger trials with longer follow-up duration will be important in order to give a more robust evidence. Follow-up duration is 1 important factor that should be considered due to the fact that the length of follow-up used by each study showed that there was a higher risk of cognitive impairment in patients with COPD with longer interval of follow-up observation. This was shown by Rusanen, et al,[13] Singh et al,[14] Xie and Xie[9] with 85%, 58%, 49% higher risk of cognitive impairment after 25 years, 5 years, and 3 years follow up respectively.
In addition, previous study by Fekri, et al[19] have shown that COPD patients have a higher prevalence of cognitive impairment and that this was related to COPD severity, arterial oxygen saturation, and older age. Another review article by Ranzini, et al[20] have also suggested that cognitive impairment is 1 commorbidity that often can be found in COPD patients and that cognitive functioning should be always evaluated in COPD patients as different because varying levels of cognitive impairment might impede self-management, adherence and personal independence. Our systematic review in turn have found additional important determinant that should be considered that is a longer period of COPD as shown by follow-up duration might provide a better correlation with cognitive impairment.
All studies included in this systematic review have similar methodological properties and only cognitive tests were different. Meanwhile, a study design using observational method stilll suffer from its limitation to determine a robust causality evidence between COPD and cognitive impairment primarily due to shared risk factors with smoking.[21] In recent publications, new approach using Mendelian Randomization (MR) which is a novel epidemiological tool for inferring causation may provide a chance to investigate this significant knowledge gap.[22] MR is a technique that may be used to circumvent the issues associated with unmeasured confounding and reverse causation that afflict standard observational epidemiology. Through the use of genetic variations as proxy for modifiable risk variables and health outcomes, MR enables causal inference. Multiple advantages of MR include the fact that it is unaffected by behavioral or environmental influences and reduces reverse causality. Additionally, the effects are equal to lifetime changes, which alleviates concerns about transitory exposure fluctuations.[21] A study by Higbee et al[21] using MR, had found no evidence the causation between lung function on COPD and cognitive impairment. It was then suggested that the results from previous observational studies were more likely influenced by residual confoundings.[21]
However, it should be noted that only 1 MR study is available within current publication and that this study is still suffering from several limitations as noted by the authors.[21] The authors noted that COPD might still have causal effects on certain domains not detected by analysis that had been performed. The use of MR to elucidate causal relationship between COPD and cognitive impairment were evaluated by using Single Nucleotide Polymorphisms (SNPs) and that there were no SNPs which can guarantee future COPD diagnosis although for a binary exposure, MR remains a viable test of the causal null hypothesis.[21] Furthermore, the outcome of this study was performed to evaluate global cognitive function for general population as a contiuous outcome and have not performed evaluation on the impact of lung function on MCI.[21]
6. Conclusion
Our systematic review found that COPD might have a role in developing cognitive impairment. In addition to previous review that have been published earlier, we found that the risk of cognitive impairment seems to have a correlation with the follow-up duration with the highest risk of cognitive impairment are found in the longest observation time. It is important to do cognitive screening since the first-time diagnosis of COPD is established and do periodically in order to detect and give appropriate treatment for these patients. More studies are necessary to draw a robust conclusion for the causative connection between COPD and cognitive impairment particularly with consideration of longer follow-up duration.
Author contribution
Conceptualization: Yopi Simargi, Muchtaruddin Mansyur, Yuda Turana, Alida R Harahap, Yetty Ramli, Kristiana Siste, Marcel Prasetyo, Cleopas Martin Rumende.
Data curation: Yopi Simargi, Muchtaruddin Mansyur, Yuda Turana, Alida R Harahap, Yetty Ramli, Kristiana Siste, Marcel Prasetyo, Cleopas Martin Rumende.
Formal analysis: Yopi Simargi, Muchtaruddin Mansyur, Yuda Turana, Alida R Harahap, Yetty Ramli, Kristiana Siste, Marcel Prasetyo, Cleopas Martin Rumende.
Funding acquisition: Yopi Simargi, Muchtaruddin Mansyur, Yuda Turana, Alida R Harahap, Yetty Ramli, Kristiana Siste, Marcel Prasetyo, Cleopas Martin Rumende.
Investigation: Yopi Simargi, Muchtaruddin Mansyur, Yuda Turana, Alida R Harahap, Yetty Ramli, Kristiana Siste, Marcel Prasetyo, Cleopas Martin Rumende.
Methodology: Yopi Simargi, Muchtaruddin Mansyur, Yuda Turana, Alida R Harahap, Yetty Ramli, Kristiana Siste, Marcel Prasetyo, Cleopas Martin Rumende.
Project Administration: Yopi Simargi, Muchtaruddin Mansyur, Yuda Turana, Alida R Harahap, Yetty Ramli, Kristiana Siste, Marcel Prasetyo, Cleopas Martin Rumende.
Resources: Yopi Simargi, Muchtaruddin Mansyur, Yuda Turana, Alida R Harahap, Yetty Ramli, Kristiana Siste, Marcel Prasetyo, Cleopas Martin Rumende.
Software: Yopi Simargi, Muchtaruddin Mansyur, Yuda Turana, Alida R Harahap, Yetty Ramli, Kristiana Siste, Marcel Prasetyo, Cleopas Martin Rumende.
Supervision: Yopi Simargi, Muchtaruddin Mansyur, Yuda Turana, Alida R Harahap, Yetty Ramli, Kristiana Siste, Marcel Prasetyo, Cleopas Martin Rumende.
Validation: Yopi Simargi, Muchtaruddin Mansyur, Yuda Turana, Alida R Harahap, Yetty Ramli, Kristiana Siste, Marcel Prasetyo, Cleopas Martin Rumende.
Visualization: Yopi Simargi, Muchtaruddin Mansyur, Yuda Turana, Alida R Harahap, Yetty Ramli, Kristiana Siste, Marcel Prasetyo, Cleopas Martin Rumende.
Writing – original draft: Yopi Simargi, Muchtaruddin Mansyur, Yuda Turana, Alida R Harahap, Yetty Ramli, Kristiana Siste, Marcel Prasetyo, Cleopas Martin Rumende.
Writing – review & editing: Yopi Simargi, Muchtaruddin Mansyur, Yuda Turana, Alida R Harahap, Yetty Ramli, Kristiana Siste, Marcel Prasetyo, Cleopas Martin Rumende.
Conceptualization: Alida Harahap, Cleopas Rumende, Kristiana Siste, Marcel Prasetyo, Muchtaruddin Mansyur, Yetty Ramli, Yopi Simargi, Yuda Turana.
Data curation: Alida Harahap, Cleopas Rumende, Kristiana Siste, Marcel Prasetyo, Muchtaruddin Mansyur, Yetty Ramli, Yopi Simargi, Yuda Turana.
Formal analysis: Alida Harahap, Cleopas Rumende, Kristiana Siste, Marcel Prasetyo, Muchtaruddin Mansyur, Yetty Ramli, Yopi Simargi, Yuda Turana.
Funding acquisition: Alida Harahap, Cleopas Rumende, Kristiana Siste, Marcel Prasetyo, Muchtaruddin Mansyur, Yetty Ramli, Yopi Simargi, Yuda Turana.
Investigation: Alida Harahap, Cleopas Rumende, Kristiana Siste, Marcel Prasetyo, Muchtaruddin Mansyur, Yetty Ramli, Yopi Simargi, Yuda Turana.
Methodology: Alida Harahap, Cleopas Rumende, Kristiana Siste, Marcel Prasetyo, Muchtaruddin Mansyur, Yetty Ramli, Yopi Simargi, Yuda Turana.
Project administration: Alida Harahap, Cleopas Rumende, Kristiana Siste, Marcel Prasetyo, Muchtaruddin Mansyur, Yetty Ramli, Yopi Simargi, Yuda Turana.
Resources: Alida Harahap, Cleopas Rumende, Kristiana Siste, Marcel Prasetyo, Muchtaruddin Mansyur, Yetty Ramli, Yopi Simargi, Yuda Turana.
Software: Alida Harahap, Cleopas Rumende, Kristiana Siste, Marcel Prasetyo, Muchtaruddin Mansyur, Yetty Ramli, Yopi Simargi, Yuda Turana.
Supervision: Alida Harahap, Cleopas Rumende, Kristiana Siste, Marcel Prasetyo, Muchtaruddin Mansyur, Yetty Ramli, Yopi Simargi, Yuda Turana.
Validation: Alida Harahap, Cleopas Rumende, Kristiana Siste, Marcel Prasetyo, Muchtaruddin Mansyur, Yetty Ramli, Yopi Simargi, Yuda Turana.
Visualization: Alida Harahap, Cleopas Rumende, Kristiana Siste, Marcel Prasetyo, Muchtaruddin Mansyur, Yetty Ramli, Yopi Simargi, Yuda Turana.
Writing – original draft: Alida Harahap, Cleopas Rumende, Kristiana Siste, Marcel Prasetyo, Muchtaruddin Mansyur, Yetty Ramli, Yopi Simargi, Yuda Turana.
Writing – review & editing: Alida Harahap, Cleopas Rumende, Kristiana Siste, Marcel Prasetyo, Muchtaruddin Mansyur, Yetty Ramli, Yopi Simargi, Yuda Turana.
Footnotes
Abbreviations: COPD = chronic obstructive pulmonary disease, DSM = diagnostic and statistical manual, MCI = mild cognitive impairment, MMSE = mini mental state examination, NOS = Newcastle Ottawa Scale.
How to cite this article: Simargi Y, Mansyur M, Turana Y, Harahap AR, Ramli Y, Siste K, Prasetyo M, Rumende CM. Risk of developing cognitive impairment on patients with chronic obstructive pulmonary disease: a systematic review. Medicine. 2022;101:25(e29235).
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated in each case.
The authors have no funding and conflicts of interests to disclose.
Contributor Information
Muchtaruddin Mansyur, Email: muchtaruddin.mansyur@ui.ac.id.
Yuda Turana, Email: yuda.turana@atmajaya.ac.id.
Alida R. Harahap, Email: alida@eijkman.go.id.
Yetty Ramli, Email: yettyramli@yahoo.com.
Kristiana Siste, Email: ksiste@yahoo.com.
Marcel Prasetyo, Email: marcel.prasetyo@gmail.com.
Cleopas Martin Rumende, Email: rumende_martin@yahoo.com.
References
- [1].Kakkera K, Padala KP, Kodali M, Padala PR. Association of chronic obstructive pulmonary disease with mild cognitive impairment and dementia. Curr Opin Pulm Med 2018;24:173–8. [DOI] [PubMed] [Google Scholar]
- [2].Prinzi G, Santoro A, Lamonaca P, Cardaci V, Fini M, Russo P. Cognitive impairment in chronic obstructive pulmonary disease (COPD): possible utility of marine bioactive compounds. Mar Drugs 2018;16:313.doi:10.3390/md16090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yohannes AM, Chen W, Moga AM, Leroi I, Connolly MJ. Cognitive impairment in chronic obstructive pulmonary disease and chronic heart failure: a systematic review and meta-analysis of observational studies. J Am Med Dir Assoc 2017;18:451e1–1. doi:10.1016/j.jamda.2017.01.014. [DOI] [PubMed] [Google Scholar]
- [4].Pierobon A, Sini Bottelli E, Ranzini L, et al. COPD patients’ self-reported adherence;1; psychosocial factors and mild cognitive impairment in pulmonary rehabilitation. Int J Chron Obstruct Pulmon Dis 2017;12:2059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mourad S, Abd Al-Ghaffar M, Abdellah AH, Al-Amir Bassiony M. Cognitive profile in patients with bronchial asthma and chronic obstructive pulmonary disease (COPD). Egypt J Ear Nose Throat Allied Sci 2017;18:61–5. [Google Scholar]
- [6].Amezquita-Sanchez JP, Adeli A, Adeli H. A new methodology for automated diagnosis of mild cognitive impairment (MCI) using magnetoencephalography (MEG). Behav Brain Res 2016;305:174–80. [DOI] [PubMed] [Google Scholar]
- [7].Morris C, Mitchell JW, Moorey H, Younan HC, Tadros G, Turner AM. Memory, attention and fluency deficits in COPD may be a specific form of cognitive impairment. ERJ Open Res 2019;5:00229–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Petersen RC. Mild cognitive impairment. Contin Lifelong Learn Neurol 2016;22:404–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xie F, Xie L. COPD and the risk of mild cognitive impairment and dementia: a cohort study based on the Chinese Longitudinal Health Longevity Survey. Int J Chron Obstruct Pulmon Dis 2019;14:403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Elm E von, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. doi:10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [12].Liao WC, Lin CL, Chang SN, Tu CY, Kao CH. The association between chronic obstructive pulmonary disease and dementia: a population-based retrospective cohort study. Eur J Neurol 2015;22:334–40. [DOI] [PubMed] [Google Scholar]
- [13].Rusanen M, Ngandu T, Laatikainen T, Tuomilehto J, Soininen H, Kivipelto M. Chronic obstructive pulmonary disease and asthma and the risk of mild cognitive impairment and dementia: a population based CAIDE study. Curr Alzheimer Res 2013;10:549–55. [DOI] [PubMed] [Google Scholar]
- [14].Singh B, Mielke MM, Parsaik AK, et al. A prospective study of chronic obstructive pulmonary disease and the risk for mild cognitive impairment. JAMA Neurol 2014;71:581.doi:10.1001/jamaneurol.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cherbuin N, Walsh EI, Prina AM. Chronic obstructive pulmonary disease and risk of dementia and mortality in lower to middle income countries. Anstey K, Peters R, eds. J Alzheimers Dis 2019;70(s1):S63–73. doi:10.3233/JAD-180562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ciesielska N, Sokołowski R, Mazur E, Podhorecka M, Polak-Szabela A, Kędziora-Kornatowska K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis Psychiatr Pol 2016;50:1039–52. [DOI] [PubMed] [Google Scholar]
- [17].Solomon TM, DeBros G, Byrnes A, Murphy C, Solomon P. Comparative and combined accuracies of objective versus subjective screening instruments in distinguishing Alzheimer's disease, mild cognitive impairment, and age-related cognitive decline. Alzheimers Dement 2015;11:439.doi:10.1016/j.jalz.2015.06.427. [Google Scholar]
- [18].Noordzij M, Dekker FW, Zoccali C, Jager KJ. Measures of disease frequency: prevalence and incidence. Nephron Clin Pract 2010;115:c17–20. [DOI] [PubMed] [Google Scholar]
- [19].Fekri MS, Hashemi-Bajgani SM, Naghibzadeh-Tahami A, Arabnejad F. Cognitive impairment among patients with chronic obstructive pulmonary disease compared to normal individuals. Tanaffos 2017;16:34. [PMC free article] [PubMed] [Google Scholar]
- [20].Ranzini L, Schiavi M, Pierobon A, Granata N, Giardini A. From mild cognitive impairment (MCI) to dementia in chronic obstructive pulmonary disease. Implications for clinical practice and disease management: a mini-review. Front Psychol 2020;11:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Higbee DH, Granell R, Hemani G, Smith GD, Dodd JW. Lung function, COPD and cognitive function: a multivariable and two sample Mendelian randomization study. BMC Pulm Med 2021;21:01–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Higbee DH, Dodd JW. Cognitive impairment in COPD: an often over looked co-morbidity. Expert Rev Respir Med 2021;15:09–11. [DOI] [PubMed] [Google Scholar]