Abstract
Background:
Gestational weight gain (GWG) is associated with the risk of gestational diabetes mellitus (GDM). However, the effect of weight gain in different trimesters on the risk of GDM is unclear. This study aimed to evaluate the effect of GWG on GDM during different trimesters.
Methods:
A birth cohort study was conducted from 2017 to 2020 in Shenzhen, China. In total, 51,205 participants were included comprising two models (early pregnancy model and middle pregnancy model). Gestational weight (kg) was measured at each prenatal clinical visit using a standardized weight scale. Logistic regression analysis was used to assess the risk of GDM. Interaction analysis and mediation effect analysis were performed in the middle pregnancy model.
Results:
In the early pregnancy model, the risk of GDM was 0.858 times lower (95% confidence interval [CI]: 0.786, 0.937) with insufficient GWG (iGWG) and 1.201 times higher (95% CI: 1.097, 1.316) with excessive GWG after adjustment. In the middle pregnancy model, the risk of GDM associated with iGWG increased 1.595 times (95% CI: 1.418, 1.794) after adjustment; for excessive GWG, no significant difference was found (P = 0.223). Interaction analysis showed no interaction between GWG in early pregnancy (GWG-E) and GWG in middle pregnancy (GWG-M) (F = 1.268; P = 0.280). The mediation effect analysis indicated that GWG-M plays a partial mediating role, with an effect proportion of 14.9%.
Conclusions:
eGWG-E and iGWG-M are associated with an increased risk of GDM. Strict control of weight gain in early pregnancy is needed, and sufficient nutrition should be provided in middle pregnancy.
Keywords: Gestational diabetes mellitus, Gestational weight gain, Early pregnancy, Middle pregnancy
Introduction
Gestational diabetes mellitus (GDM) is a common complication during pregnancy and is related to adverse maternal and child outcomes.[1] In recent years, the morbidity of GDM has increased according to multicenter studies.[2] The prevalence of GDM in Korean women showed a significant increasing trend from 2012 to 2016.[3] The prevalence of GDM also increased significantly among the Chinese female population from 2016 to 2018.[4]
Studies have suggested that the risk of adverse maternal and child outcomes varies with gestational weight gain (GWG) and prepregnancy body mass index (BMI).5,6 Our team found that excessive GWG in accordance with Institute of Medicine (IOM) recommendations influences the rate of gestational hypertension[7] and revealed the timing and extent of gestational weight control are relevant to the optimized blood pressure level during pregnancy.[8] Some scholars have proposed that GWG should be acknowledged as an independent factor for screening GDM in clinical guidelines.[9] Studies on the relationship between GWG and GDM are increasing. Brunner et al [10] provided evidence that compared with nonexcessive GWG, excessive GWG before a GDM screening test is associated with an increased risk of GDM. Specific to different trimesters, some studies have found that excessive GWG in the first trimester, rather than the second trimester, is associated with an increased risk of GDM regardless of the prepregnancy BMI.11,12 Another study indicated that excessive GWG in the first and second trimesters may be a risk factor for GDM, highlighting the importance of appropriate weight gain during pregnan-cy.11,12 The implications of GWG in the second trimester are debatable. Some scholars have found that excessive GWG in the second trimester further increased the risk of GDM.[9] A second trimester weight gain >7 kg and an abnormal oral glucose tolerance test (OGTT) value at first screening increased the risk of GDM in at-risk women,[13] but others have found that insufficient GWG (iGWG) in the second trimester increased the risk of GDM.[14] Views on the effect of GWG during different trimesters on the risk of GDM are inconsistent.
Based on our study, a birth cohort study in Shenzhen (BiCoS), we focused on pregnant women from 2017 to 2020 to comprehensively identify the effect of GWG in different trimesters on GDM.
Methods
Ethical approval
The data were collected from the BiCoS conducted at the Shenzhen Maternity and Child Healthcare Hospital, and the study was approved by the Institutional Review Board at Shenzhen Maternity and Child Healthcare Hospital (Shenzhen Maternal and Child Ethics Review No.23).
Study population
Pregnant women were invited to join the BiCoS at their first prenatal clinic visit from 2017 to 2020. In total, 54,435 pregnant women were recruited after providing full informed consent to participate in the study. Finally, 51,205 participants were enrolled according to the following exclusion criteria: multiple pregnancy, heart disease, kidney disease, epilepsy, antiphospholipid antibody syndrome, diagnosis of diabetes and/or hypertension before the current pregnancy, and missing data on the main exposure (ie, GWG) [Figure 1]. In our study, we used two models for analysis. The early model included participants with a gestational weight at 12 ± 1 weeks in the cohort data. The middle model included participants with a gestational weight at 23 ± 1 weeks in the early model. A total of 21,121 participants were enrolled in the early model to verify the effect of GWG in early pregnancy (GWG-E) on the risk of GDM. A total of 13,848 participants enrolled in the middle model were screened from the early model.
Figure 1.
Flowchart of the birth cohort study (BiCoS) conducted at Shenzhen Maternity and Child Healthcare Hospital to verify the effect of GWG in middle pregnancy on the risk of GDM. GDM: Gestational diabetes mellitus; GWG: Gestational weight gain; GWG-E: Gestational weight gain in early pregnancy; GWG-M: Gestational weight gain in middle pregnancy.
Data collection
The baseline data covered a wide range of information concerning the pregnant mother, including general demographic characteristics and socioeconomic characteristics.
Research assistants collected data regarding ethnicity (Han, others), education (schooling years, ≤12 years, 13–15 years, ≥16 years), employment (yes or no), assisted reproduction (yes or no), maternal age (average, ≥35 years, <35 years), gravidity (1,2, ≥3 times), primipara (yes or no) and prepregnancy weight (kg). Height (m) was measured using a standardized height-measuring station at the first clinical visit. Gestational weight (kg) was measured at every prenatal clinical visit using a standardized weight scale. A 75 g OGTT was performed at 24 to 28 weeks of gestation. Fasting glucose levels before 12 weeks and OGTT results were extracted from the electronic medical record system maintained by the hospital.
GWG
The prepregnancy weight was self-reported by the participants. GWG-E was calculated by subtracting the prepregnancy weight from the gestational weight at 12 ± 1 weeks. GWG in middle pregnancy (GWG-M) was calculated by subtracting the gestational weight at 12 ± 1 weeks from that at 23 ± 1 weeks. The BMI (calculation formula: weight/height2) was calculated according to the self-reported prepregnancy weight and height at the first prenatal visit. Based on the World Health Organization adult weight standard,[15] pregnant women were divided into four groups according to the prepregnancy BMI levels. Women were classified as follows: underweight (<18.5 kg/ m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30.0 kg/m2) According to the advice provided by the IOM in 2009,[16] the recommended range of weight gain in early pregnancy is 0.5 to 2.0 kg. In middle pregnancy, the recommended weight gain rates (kg/week) of women with underweight, normal weight, overweight, and obesity before pregnancy were 0.44 to 0.58, 0.35 to 0.50, 0.23 to 0.33, and 0.17 to 0.27, respectively. The recommended range of GWG-M equals the number of gestational weeks multiplied by the recommended weight gain rates. According to the IOM recommendation, GWG was stratified into three categories according to the prepregnancy BMI. Weight gain within the recommended range is considered sufficient GWG, weight gain below the recommended range is considered iGWG, and weight gain above the recommended range is considered excessive GWG (eGWG).
Diagnosis of GDM
GDM can be diagnosed by a 75 g OGTT at 24 to 28 weeks of gestation for all pregnant women without a previous history of diabetes according to the standards issued by the Ministry of Health of China in 2011,[17] based on the International Association of Diabetic Pregnancy Study Group guidelines. Any of the following abnormalities of fasting blood glucose can indicate a GDM diagnosis: ≥5.1 mmol/L (92 mg/dL), 1 h after glucose load ≥10.0 mmol/L (180 mg/dL), and 2 h after glucose load ≥8.5 mmol/L (153 mg/dL).
Statistical analysis
Continuous variables with a normal distribution were presented as means ± standard deviations, and categorical variables were expressed as counts and percentages. Chi-squared test and t-test were used to analyze differences in baseline variables between women with missing data and those without missing data and between GDM and normal pregnant women. Logistic regression was used to evaluate the effects of GWG on GDM. All the variables were entered into the equation at once. Relative risk (RR) was further used to evaluate the risk of GDM after stratifying GWG. The randomized method was used to select participants in the excluded data as the control to match the included data in a 1:1 ratio using the propensity score matching method for comparison. Sensitivity analysis was performed in the middle model to verify the result of the early model. Interaction effect analysis and mediation analysis between GWG-E and GWG-M were also calculated in the middle model. The confounding variables were as follows: maternal age, ethnicity, education years, employment, assisted reproduction, and gravidity. All the RRs were presented with 95% confidence intervals (CIs), with an entry criterion of P < 0.05. All the data were analyzed using SPSS 24.0 (SPSS Inc., Chicago, IL, USA).
Results
Participant characteristics
Based on the BiCoS, 51,205 participants were included and the rate of GDM was 17.0% (8691/51,205) [Supplementary Table 1]. The mean maternal age was 31.34 years, and the proportion of women >35 years was 24.3% (12,426/51,205). The mean prepregnancy BMI was 21.05 kg/m2, and most of the women had a normal BMI (73.0% [37,387/51,205]). A total of 2095 (4.1% [2095/51,205]) participants accepted assisted reproduction, and most of the participants were of Han ethnicity (97.1% [49,698/51,205]). A total of 67.8% (34,723/51,205) of the participants had a high education, while 75.6% (38,734/51,205) were working. Participants with more than three pregnancies (34.0% [17,424/51,205]) and a parity of two or more (51.4% [26,333/51,205]) accounted for the largest proportion.
Relationship of GWG-E with GDM
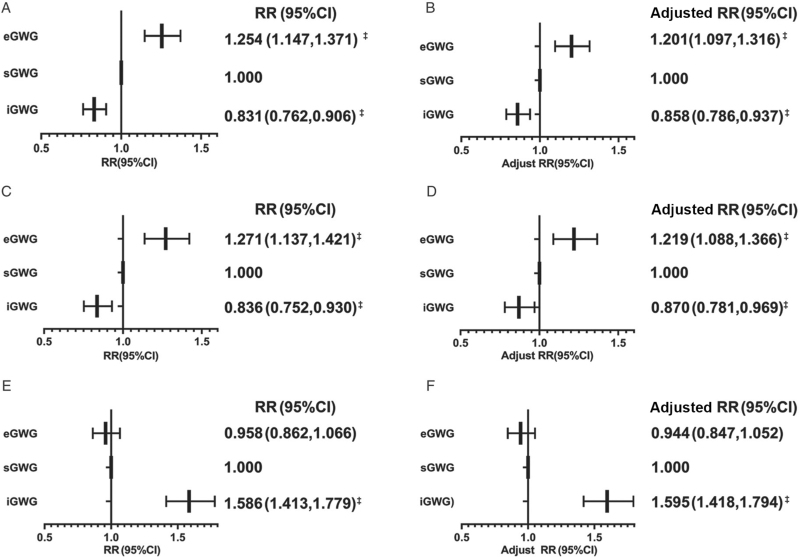
Comparison of the characteristics between normal and GDM women in early pregnancy revealed that GDM women were older (33.00 ± 4.42 years vs. 30.95 ± 4.45 years; P < 0.001) and had a higher prepregnancy BMI (22.00 ± 3.21 kg/m2 vs. 20.78 ± 2.75 kg/m2; P < 0.001) than normal women [Table 1]. Overweight plus obesity was dominant in the GDM group (16.1% vs. 7.4%). The fasting blood glucose levels (FPGs) before 12 weeks were higher in the GDM group (4.57 ± 0.47 mmol/L vs. 5.15 ± 0.57 mmol/L; P < 0.001). The average GWG-E was 1.10 ± 2.77 kg overall, 1.02 ± 2.77 kg in normal women, and 1.45 ± 2.74 kg in GDM women. Additionally, women with GDM had more gravidities (P < 0.001) in the early model. Most of the women with GDM were not primipara (P < 0.001). Women with GDM had a higher rate of excessive GWG (33.3% [1237/3710] vs. 26.5% [4608/17,411]) in early pregnancy. Logistic regression analyses showed that the risk of GDM increased from iGWG to eGWG in GWG-E [Figure 2]. After adjustment and accession, the risk of GDM associated with iGWG was 0.858 times lower (95% CI: 0.786–0.937) in early pregnancy. For eGWG in GWG-E, the risk of GDM increased 1.201 times (95% CI: 1.097, 1.316).
Table 1.
Differences between normal and GDM women in early and middle pregnancy (N = 21,121).
| Early pregnancy | Middle pregnancy | |||||||
| Characteristics∗ | Total (n = 21,121) | Normal (n = 17,411) | GDM (n = 3710) | P values† | Total (n = 13,848) | Normal (n = 11,432) | GDM (n = 2416) | P values† |
| Maternal age (years) | 31.31 ± 4.45 | 30.95 ± 4.45 | 33.00 ± 4.42 | <0.001 | 31.40 ± 4.41 | 31.03 ± 4.32 | 33.13 ± 4.43 | <0.001 |
| Maternal age (years) | ||||||||
| ≥35 | 5157 (24.4) | 3775 (21.7) | 1382 (37.3) | <0.001 | 3433 (24.8) | 2510 (22.0) | 923 (38.2) | |
| <35 | 15,964 (75.6) | 13,636 (78.3) | 2328 (62.7) | 10,415 (75.2) | 8922 (78.0) | 1493 (61.8) | ||
| Prepregnancy BMI (kg/m2) | 21.00 ± 2.88 | 20.78 ± 2.75 | 22.00 ± 3.21 | <0.001 | 20.97 ± 2.86 | 20.76 ± 2.74 | 21.97 ± 3.20 | <0.001 |
| Prepregnancy BMI (kg/m2) | ||||||||
| <18.5 | 3859 (18.3) | 3414 (19.6) | 445 (12.0) | <0.001 | 2539 (18.3) | 2245 (19.6) | 294 (12.2) | <0.001 |
| 18.5–24.9 | 15,291 (72.4) | 12,649 (72.6) | 2642 (71.2) | 10,054 (72.6) | 8330 (72.9) | 1724 (71.4) | ||
| 25.0–29.9 | 1698 (8.0) | 1178 (6.8) | 520 (14.0) | 1078 (7.8) | 750 (6.6) | 328 (13.6) | ||
| ≥30.0 | 181 (0.9) | 103 (0.6) | 78 (2.1) | 115 (0.8) | 65 (0.6) | 50 (2.1) | ||
| Assisted reproduction | ||||||||
| Yes | 1012 (4.8) | 774 (4.4) | 238 (6.4) | <0.001 | 760 (5.5) | 584 (5.1) | 176 (7.3) | <0.001 |
| No | 20,069 (95.0) | 16,606 (95.4) | 3463 (93.3) | 13,070 (94.4) | 10,834 (94.8) | 2236 (92.5) | ||
| Missing | 40 (0.2) | 31 (0.2) | 9 (0.2) | 18 (0.1) | 14 (0.1) | 4 (0.2) | ||
| Gravidity (times) | ||||||||
| 1 | 7031 (33.3) | 6070 (34.9) | 961 (25.9) | <0.001 | 4687 (33.9) | 4072 (35.6) | 615 (25.5) | <0.001 |
| 2 | 6987 (33.1) | 5795 (33.3) | 1192 (32.1) | 4588 (33.9) | 3797 (33.2) | 791 (32.8) | ||
| ≥3 | 7100 (33.6) | 5544 (31.8) | 1556 (25.9) | 4571 (33.0) | 3562 (31.2) | 1009 (41.8) | ||
| Missing | 3 (<0.1) | 2 (<0.1) | 1 (<0.1) | 2 (<0.1) | 1 (<0.1) | 1 (<0.1) | ||
| Primipara | ||||||||
| Yes | 10,160 (48.1) | 8629 (49.6) | 1531 (41.3) | <0.001 | 6915 (50.0) | 5901 (51.6) | 1014 (42.0) | <0.001 |
| No | 10,802 (51.1) | 8652 (49.7) | 2150 (58.0) | 6833 (49.3) | 5450 (47.7) | 1383 (57.2) | ||
| Missing | 159 (0.8) | 130 (0.7) | 29 (0.8) | 100 (0.7) | 81 (0.7) | 19 (0.8) | ||
| Ethnicity | ||||||||
| Han | 20,518 (97.1) | 16,919 (97.2) | 3599 (97.0) | 0.580 | 13,451 (97.1) | 11,114 (97.2) | 2337 (96.7) | 0.190 |
| Others | 603 (2.9) | 492 (2.8) | 111 (3.0) | 397 (2.9) | 318 (2.8) | 79 (3.3) | ||
| Employment | ||||||||
| Yes | 15,788 (74.8) | 12,971 (74.5) | 2817 (75.9) | 0.070 | 10,519 (76.0) | 8664 (75.8) | 1855 (76.8) | 0.300 |
| No | 5333 (25.2) | 4440 (25.5) | 893 (24.1) | 3329 (24.0) | 2768 (24.2) | 561 (23.2) | ||
| Education (schooling years) | ||||||||
| <12 | 97 (0.5) | 81 (0.5) | 16 (0.4) | 0.680 | 53 (0.4) | 41 (0.4) | 12 (0.5) | 0.200 |
| 13–15 | 7155 (33.9) | 5876 (33.7) | 1279 (34.5) | 4426 (32.0) | 3624 (31.7) | 802 (33.2) | ||
| ≥16 | 13,869 (65.7) | 11,454 (65.8) | 2415 (65.1) | 9369 (67.7) | 7767 (67.9) | 1602 (66.3) | ||
| FPG (mmol/L) | 4.59 ± 0.48 | 4.57 ± 0.47 | 5.15 ± 0.57 | <0.001 | 4.61 ± 0.50 | 4.59 ± 0.49 | 5.43 ± 0.60 | <0.001 |
| GWG-E/M (kg) | 1.10 ± 2.77 | 1.02 ± 2.77 | 1.45 ± 2.74 | <0.001 | 5.86 ± 2.52 | 5.97 ± 2.47 | 5.31 ± 2.63 | <0.001 |
| GWG-E/M | ||||||||
| iGWG | 8709 (41.2) | 7394 (42.5) | 1315 (35.4) | <0.001 | 3253 (23.5) | 2498 (21.9) | 755 (31.3) | <0.001 |
| sGWG | 6567 (31.1) | 5409 (31.1) | 1158 (31.2) | 4316 (31.2) | 3625 (31.7) | 691 (28.6) | ||
| eGWG | 5845 (27.7) | 4608 (26.5) | 1237 (33.3) | 6279 (45.3) | 5309 (46.4) | 970 (40.1) | ||
The data are presented as means ± SD for continuous variables or n (%) for categorical variables.
Tests for differences between GDM and non-GDM were performed using Student's t-test for continuous variables or Chi-squared test for categorical variables; P < 0.05 indicates significance. BMI: Body mass index; eGWG: Excessive gestational weight gain; FPG: Fasting blood glucose level; GDM: Gestational diabetes mellitus; GWG: Gestational weight gain; GWG-E: Gestational weight gain in early pregnancy; GWG-M: Gestational weight gain in middle pregnancy; iGWG: Insufficient gestational weight gain; SD: Standard deviation; sGWG: Sufficient gestational weight gain.
Figure 2.
Effect of GWG on GDM. (A) Effect of GWG-E on the risk of GDM. (B) Effect of GWG-E on the risk of GDM after adjustment. (C) Sensitivity analysis of the effect of GWG-E on the risk of GDM in the middle model. (D) Sensitivity analysis of the effect of GWG-E on the risk of GDM in the middle model after adjustment. (E) Effect of GWG-M on the risk of GDM. (F) Effect of GWG-M on the risk of GDM after adjustment. CI: Confidence intervals; eGWG: Excessive gestational weight gain; GDM: Gestational diabetes mellitus; GWG: Gestational weight gain; GWG-E: Gestational weight gain in early pregnancy; GWG-M: Gestational weight gain in middle pregnancy; iGWG: Insufficient gestational weight gain; RR: Relative risks; sGWG: Sufficient gestational weight gain. All models were adjusted for maternal age, ethnicity, assisted reproduction, gravidity, education, and employment. Logistic regression method: enter. ∗ P < 0.05, † P < 0.01, ‡ P < 0.001.
Before analyzing the effects on GDM according to different trimesters of GWG, we compared the basic information of the included and excluded population. No significant difference was found in the maternal age, the prepregnancy BMI, gravidity, parity, or ethnicity (P > 0.05) [Supplementary Table 1]. The GDM rate was 17.6% in the included data and 16.6% in the excluded data. Additionally, a significant difference was found in the conception mode, employment and education between the groups (P < 0.001) [Supplementary Table 1]. Considering that there may be selection bias, randomized method was used to select the excluded population as the control and propensity score matching method was used to match the included population. The characteristics of included population after propensity score matching were shown in Supplementary Table 2. Sensitivity analysis of the included population after propensity score matching showed the same result that the risk of GDM increased from iGWG to eGWG in GWG-E [Supplementary Table 3].
Relationship of GWG-M with GDM
Further study comparing the characteristics between normal and GDM women in the middle pregnancy model [Table 1] revealed the same trend as that observed in early pregnancy: GDM women were more likely to be older (33.13 ± 4.43 years vs. 31.03 ± 4.32 years; P < 0.001) and have a higher prepregnancy BMI (21.97 ± 3.20 kg/m2 vs. 20.76 ± 2.74 kg/m2; P < 0.001). Additionally, overweight plus obesity was dominant in the GDM group (15.7% [378/2416] vs. 7.2% [815/11,432]), and most of the women with GDM were not primipara (P < 0.001). However, gravidity was lower in GDM women (P < 0.001). FPGs before 12 weeks were higher in the GDM group (4.59 ± 0.49 mmol/L vs. 5.43 ± 0.60 mmol/L; P < 0.001). The average GWG-M was 6.01 ± 2.91 kg in total, 5.97 ± 2.47 kg in normal women, and 5.31 ± 2.63 kg in GDM women. In contrast to early pregnancy, the eGWG rate in the normal group was higher than that in the GDM group (46.4% [5309/11,432] vs. 40.1% [970/2416]) at middle pregnancy. The risk of GDM decreased from iGWG to eGWG-M [Figure 2]. Regarding GWG-M, the risk of GDM associated with iGWG increased 1.595 times (95% CI: 1.418, 1.794); for eGWG, it showed no significant difference (P = 0.22). Moreover, the finding that eGWG-E increases the risk of GDM was also found in middle model [Figure 2], which was consistent with the early model.
In the middle model, we also compared the included and excluded data. Similarly, no significant difference was found (P > 0.05) [Supplementary Table 1] in the maternal age, the prepregnancy BMI, ethnicity, or education between the included and excluded data (P > 0.05). Conception, gravidity, and parity showed significant differences (P < 0.01). The randomized method and propensity score matching method were also used to select the included population with the excluded population as control in the middle pregnancy model as described aboved and the characteristics of included population after propensity score matching were shown in Supplementary Table 4. Sensitivity analysis of the included population after propensity score matching showed that the risk of GDM decreased from iGWG to eGWG-M [Supplementary Table 3].
Interaction and mediation analyses in the middle model
To determine whether GWG-E influences GWG-M, we performed an interaction analysis in the middle model and found no interaction between GWG-E and GWG-M (F = 1.268; P = 0.280). For further consideration, we performed mediation effect analysis to determine whether GWG-M was a mediating factor for the increased risk of GDM caused by GWG-E. The regression coefficient of the total effect was 0.009 (95% CI: 0.006, 0.011) and that of the direct effect was 0.008 (95% CI: 0.005, 0.010). The regression coefficient of the mediation effect was 0.001 (95% CI: 0.006, 0.015), indicating that GWG-M plays a partial mediating role in the effect of GWG-E on GDM, with an effect proportion of 14.9% [Figure 3].
Figure 3.
Analysis of the mediation effect of GWG-M on the relationship between GWG-E and GDM (n = 13,848). a, Regression coefficient of GWG-E vs. GWG-M adjusted for prepregnancy BMI, maternal age, ethnicity, assisted reproduction, gravidity, education, and employment; b, regression coefficient of GWG-M vs. GDM adjusted for prepregnancy BMI, maternal age, ethnicity, assisted reproduction, gravidity, education, and employment; c, total effect, regression coefficient when GWG-E vs. GDM (no mediator variable GWG-M in the model); c’, direct effect, regression coefficient when GWG-E vs. GDM (mediator variable GWG-M in the model). BMI: Body mass index; GDM: Gestational diabetes mellitus; GWG: Gestational weight gain; GWG-E: Gestational weight gain in early pregnancy; GWG-M: Gestational weight gain in middle pregnancy. The continuous variable gestational weight gain in early and middle pregnancy was analyzed as an independent variable in these causal mediation analyses. ∗ P < 0.05, † P < 0.01, ‡ P < 0.001.
Discussion
GDM can lead to adverse pregnancy outcomes.[18] To distinguish the risk factors for GDM, precautions should be taken. In our study, we focused on GWG in different trimesters to clarify the impact of different periods on diabetes risk. We examined the association of GWG with GDM based on BiCoS to provide more evidence.
Some studies have focused on the risk of total GWG during pregnancy with GDM. However, GDM was diagnosed at 24 to 28 weeks by OGTT. Thus, we believe that analyzing the risks of GDM with GWG before 24 weeks is more reasonable. Our study found that iGWG-E decreased the risk of GDM and that excessive eGWG-E increased the risk of GDM, findings that were consistent with other studies.11,19 During pregnancy, the mother develops physiological insulin resistance to ensure fetal growth and development. Additionally, islet β cells compensate for increased insulin secretion to maintain normal blood glucose.[20] Excessive weight gain in early pregnancy may increase insulin resistance and weaken the secretion of islet β cells, leading to GDM.
Our study firstly showed that iGWG in middle pregnancy increased the risk of GDM. Some scholars have considered that excessive GWG in the second trimester might be a risk factor for GDM.[21] However, others have considered that excessive GWG-M is not associated with an increased risk of GDM.11,12 iGWG may increase the risk of fetal growth restriction (FGR).[5] Mechanistically, when FGR occurs, 11 β d-hydroxysteroid dehydrogenase type II genes are induced, increasing the risk of maternal glucocorticoid exposure,[22] which may eventually increase the risk of GDM. Compared with other studies, our study was different regarding the calculation of GWG-M. Additionally, different standards of the prepregnancy BMI were used in different studies. However, we showed that excessive GWG does not change the risk of GDM. Whether because of more energy required for fetal growth or other reasons, further exploration is needed to pursue the mechanism.
Additionally, mediation analysis has suggested that GWG-M accounted for 16.8% of the effect of GWG-E on GDM. This finding represents another new discovery of our study. GWG-E partly affected the risk of GDM by influencing GWG-M. A small proportion of pregnant women might experience impaired glucose regulation before diagnosis, which must be considered in clinical management. Whether screening for impaired glucose regulation is necessary in the first trimester requires more detailed exploration.
When analyzing the data, we first performed sensitivity analysis in the early and middle models. From the overall sample, the GDM rate was 17.0% among the total participants, a value consistent with that in previous multicenter studies.[22] The GDM rates in the early and middle models were 17.6% and 17.4%, respectively, representing the total population. The prepregnancy BMI, assisted reproduction, employment, education, and the GDM rate were significantly different in the early model. Participants with employment and higher education included in our study may focus more on their pregnancy and have regular prenatal examinations. Thus, collecting their data was easy, similar to that for participants who accepted assisted reproduction. The data included have more participants with lower BMI. These unbalanced characteristics may influence the risks of GWG on GDM. Sensitivity analysis in the middle model revealed the same result as that obtained in the early model, indicating that our data were robust. We performed interaction analysis in the middle model and ensured no interaction between GWG-E and GWG-M. Therefore, we believe our results are highly credible.
Overall, our study was based on a birth cohort study with a large sample size. Hence, we have robust data to show that excessive weight gain in early pregnancy will increase the risk of GDM. What is more meaningful is that our findings have indicated that insufficient weight gain in middle pregnancy will also increase the risk of GDM. However, the present study has some limitations. First, the prepregnancy weight was self-reported by the participants, likely miscalculating the association of GWG with GDM. Second, although our study benefited from the large sample size, it had a single-center design. Despite these limitations, the results of this study have important implications.
Overall, eGWG-E and iGWG-M are related to an increased risk of GDM, highlighting that strengthening the control of pregnant women's weight gain during early pregnancy and appropriately controlling weight gain in middle pregnancy are highly significant to prevent the occurrence of GDM.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81830041 and 81771611) and Shenzhen Science and Technology Innovation Committee Special Funding for Future Industry (No. JCYJ20170412140326739).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Yin A, Tian F, Wu X, Chen Y, Liu K, Tong J, Guan X, Zhang H, Wu L, Niu J. Excessive gestational weight gain in early pregnancy and insufficient gestational weight gain in middle pregnancy increased risk of gestational diabetes mellitus. Chin Med J 2022;135:1057–1063. doi: 10.1097/CM9.0000000000001972
Supplemental digital content is available for this article.
References
- 1.Coustan DR, Lowe LP, Metzger BE, Dyer AR. International Association of Diabetes and Pregnancy Study Groups. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. Am J Obstet Gynecol 2010; 202:654.e1–654.e6. doi: 10.1016/j.ajog.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Summary of revisions: standards of medical care in diabetes-2021. Diabetes Care 2021; 44:S4–S6. doi: 10.2337/dc21-Srev. [DOI] [PubMed] [Google Scholar]
- 3.Jung CH, Jung SH, Choi D, Kim BY, Kim CH, Mok JO. Gestational diabetes in Korea: temporal trends in prevalence, treatment, and short-term consequences from a national health insurance claims database between 2012 and 2016. Diabetes Res Clin Pract 2021; 171:108586.doi: 10.1016/j.diabres.2020.108586. [DOI] [PubMed] [Google Scholar]
- 4.Wang M, Hu RY, Gong WW, Pan J, Fei FR, Wang H, et al. Trends in prevalence of gestational diabetes mellitus in Zhejiang Province, China, 2016–2018. Nutr Metab (Lond) 2021; 18:12.doi: 10.1186/s12986-020-00539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voerman E, Santos S, Inskip H, Amiano P, Barros H, et al. LifeCycle Project-Maternal Obesity and Childhood Outcomes Study Group. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA 2019; 321:1702–1715. doi: 10.1001/jama.2019.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pouliot A, Elmahboubi R, Adam C. Incidence and outcomes of gestational diabetes mellitus using the New International Association of Diabetes in Pregnancy Study Group Criteria in Hôpital Maisonneuve-Rosemont. Can J Diabetes 2019; 43:594–599. doi: 10.1016/j.jcjd.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Ren M, Li H, Cai W, Niu X, Ji W, Zhang Z, et al. Excessive gestational weight gain in accordance with the IOM criteria and the risk of hypertensive disorders of pregnancy: a meta-analysis. BMC Pregnancy Childbirth 2018; 18:281.doi: 10.1186/s12884-018-1922-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lei Q, Zhou X, Duan DM, Lv LJ, Lin XH, Ji WJ, et al. Trimester-specific weight gain and midpregnancy diastolic blood pressure rebound during normotensive pregnancy. Hypertension 2017; 70:804–812. doi: 10.1161/HYPERTENSIONAHA.117. 09760. [DOI] [PubMed] [Google Scholar]
- 9.Yong HY, Shariff ZM, Yusof BNM, Rejali Z, Tee YYS, Bindels J, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep 2020; 10:8486.doi: 10.1038/s41598-020-65251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunner S, Stecher L, Ziebarth S, Nehring I, Rifas-Shiman SL, Sommer C, et al. Excessive gestational weight gain prior to glucose screening and the risk of gestational diabetes: a meta-analysis. Diabetologia 2015; 58:2229–2237. doi: 10.1007/s00125-015-3686-5. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald SC, Bodnar LM, Himes KP, Hutcheon JA. Patterns of gestational weight gain in early pregnancy and risk of gestational diabetes mellitus. Epidemiology 2017; 28:419–427. doi: 10.1097/EDE.0000000000000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lan X, Zhang YQ, Dong HL, Zhang J, Zhou FM, Bao YH, et al. Excessive gestational weight gain in the first trimester is associated with risk of gestational diabetes mellitus: a prospective study from Southwest China. Public Health Nutr 2020; 23:394–401. doi:10.1017/S1368980019003513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boriboonhirunsarn D. Second trimester weight gain >7 kg increases the risk of gestational diabetes after normal first trimester screening. J Obstet Gynaecol Res 2017; 43:462–467. doi: 10.1111/jog.13231. [DOI] [PubMed] [Google Scholar]
- 14.Dai ZY, Liu D, Li R, Wang Y, Zhang J, Liu J, et al. Association between gestational weight gain per trimester/total gestational weight gain and gestational diabetes mellitus (in Chinese). Chin J Epidemiol 2016; 37:1336–1340. doi: 10.3760/cma.j.issn.0254-6450. 2016.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Kabiru W, Raynor BD. Obstetric outcomes associated with increase in BMI category during pregnancy. Am J Obstet Gynecol 2004; 191:928–932. doi: 10.1016/j.ajog.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen KM, Yaktine AL. Institute of Medicine (IS) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC): National Academies Press (US); 2009. [PubMed] [Google Scholar]
- 17.Yang HX. Diagnostic criteria for gestational diabetes mellitus (WS 331-2011). Chin Med J 2012; 125:1212–1213. doi: 10.3760/cma.j.issn.0366-6999.2012.07.004. [PubMed] [Google Scholar]
- 18.Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012; 35:780–786. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robitaille J. Excessive gestational weight gain and gestational diabetes: importance of the first weeks of pregnancy. Diabetologia 2015; 58:2203–2205. doi: 10.1007/s00125-015-3725-2. [DOI] [PubMed] [Google Scholar]
- 20.Freemark M. Regulation of maternal metabolism by pituitary and placental hormones: roles in fetal development and metabolic programming. Horm Res 2006; 65: (Suppl 3): 41–49. doi: 10.1159/000091505. [DOI] [PubMed] [Google Scholar]
- 21.Yang Z, Zhu P, Guo C, Zhu X, Sun K. Expression of 11beta-hydroxysteroid dehydrogenase type 1 in human fetal lung and regulation of its expression by interleukin-1beta and cortisol. J Clin Endocrinol Metab 2009; 94:306–313. doi: 10.1210/jc.2008-1534. [DOI] [PubMed] [Google Scholar]
- 22.Qi Y, Sun X, Tan J, Zhang G, Chen M, Xiong Y, et al. Excessive gestational weight gain in the first and second trimester is a risk factor for gestational diabetes mellitus among women pregnant with singletons: a repeated measures analysis. J Diabetes Investig 2020; 11:1651–1660. doi: 10.1111/jdi.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.