Abstract
The catabolic IncP1β plasmid pTSA from Comamonas testosteroni T-2 was mapped by subtractive analysis of restriction digests, by sequencing outwards from the tsa operon (toluenesulfonate degradation), and by generating overlapping, long-distance-PCR amplification products. The plasmid was estimated to comprise 72 ± 4 kb. The tsa region was found to be a composite transposon flanked by two IS1071 elements. A cryptic tsa operon was also present in the tsa transposon. Those backbone genes and regions which we sequenced were in the same order as the corresponding genes in resistance plasmid R751, and identities of about 99% were observed. Enrichment cultures with samples from four continents were done to obtain organisms able to utilize p-toluenesulfonate as the sole source of carbon and energy for aerobic growth. Most (15) of the 16 cultures (13 of them isolates) were obtained from contaminated sites and were attributed to three metabolic groups, depending on their metabolism of p-toluenesulfonate. The largest group contained the tsa transposon, usually (six of seven isolates) with negligible differences in sequence from strain T-2.
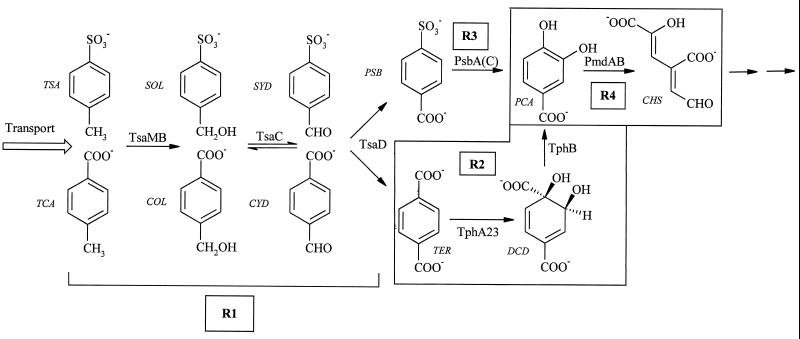
Defined sulfonated aromatic compounds are synthesized only rarely in nature (24) but are produced in large amounts as surfactants (e.g., 2.5 × 106 metric tons of linear alkylbenzenesulfonates per annum [38]), dyestuffs (e.g., 3 × 105 metric tons per annum [2]), and dyestuff precursors and additives in oils and inks (13). The major individual compound is probably p-toluenesulfonate (TSA) (about 2.7 × 104 metric tons per annum in Europe), which is used, for example, in household detergent formulations, preparation of foundry molds, and syntheses of pharmaceuticals (7). Degradation of TSA was established in 1957 (37), and three dissimilatory pathways have been detected in aerobic microorganisms: 2,3-dioxygenation (14); 1,2-dioxygenation, which is frequently plasmid encoded (5, 21); and the best-characterized system, methyl monooxygenation, in Comamonas testosteroni T-2 (Fig. 1), where it is encoded on plasmid pTSA (11).
FIG. 1.
Degradation of TSA and TCA by C. testosteroni T-2. The four regulons are shown as R1, R2, R3, and R4. Reactions that are catalyzed by chromosomal gene products are framed. Regulatory units R1 and R3 are plasmid encoded (pTSA, IncP1β). Abbreviations: TSA, p-toluenesulfonate; SOL, p-sulfobenzylalcohol; SYD, p-sulfobenzaldehyde; PSB, p-sulfobenzoate; PCA, protocatechuate; CHS, 4-carboxy-2-hydroxymuconate semialdehyde; TCA, p-toluenecarboxylate; COL, carboxybenzylalcohol; CYD, carboxybenzaldehyde; TER, terephthalate; DCD, 1,2-dihydroxy-3,5-cyclohexadien-1,4-dicarboxylic acid. Enzymes: TsaMB, toluenesulfonate methylmonooxygenase; TsaC, p-sulfobenzylalcohol dehydrogenase; TsaD, p-sulfobenzaldehyde dehydrogenase; PsbA(C), p-sulfobenzoate-3,4-dioxygenase; PmdAB, protocatechuate-4,5-oxygenase; TphA231, terephthalate dioxygenase (49); TphB, 1,2-dihydroxy-3,5-cyclohexadiene-1,4-dicarboxylic acid dehydrogenase (49).
Junker and Cook (19) concluded that there are two plasmids in C. testosteroni T-2, pTSA and pT2T. The function of plasmid pT2T is unknown. A deletion mutant, strain TER-1, lacks plasmid pTSA, on which regulatory groups R1 (at least tsaMBCD and tsaR) and R3 (at least some genes for the p-sulfobenzoate [PSB]-dioxygenase system) and, presumably, genes encoding TSA transport are believed to be located (11, 19; J. Mampel, unpublished results). Plasmid pTSA belongs to the IncP1β group and is conjugative (19). Regulatory group R4 (encoding ring cleavage and the meta pathway enzymes) is chromosomally located (19).
IncP1 plasmids can be highly promiscuous as either resistance or degradative plasmids (6, 16, 17, 46), and the degradative genes are often found between the oriV and trfA genes (9, 44), although few IncP1 plasmids have been mapped to show this.
Junker and Cook (19) found two copies of the insertion element IS1071 (29, 50) associated with plasmid pTSA and postulated that this could be associated with a transposon involved with regulatory group R3. A similar element was postulated in C. testosteroni PSB-4 associated with plasmid pPSB, where active transposition was observed (19).
We have now mapped the known tsa genes as well as the two known IS1071 elements on pTSA, the first degradative IncP1β plasmid to be mapped. The data indicated a tsa transposon with one functional version of the tsa operon and one cryptic version. The latter contained mutations. In addition, a complete mercury resistance (mer) operon was found. The backbone of the plasmid, where sequenced, showed >99% identity to the sequenced (44) IncP1 resistance plasmid, suggesting a common ancestor. Comparison of pTSA to plasmid R751 showed significant differences concerning the genetic load (12) at the two integration sites. The established tsa genes were found to be widespread and highly conserved in nature.
MATERIALS AND METHODS
Bacteria and growth conditions.
C. testosteroni T-2 (DSM 6577) (10, 45) and its pTSA-negative (pTSA−) mutant strain TER-1 (22) were grown in minimal medium as described previously (22, 45). Enrichment cultures (5 ml) were done with 6 mM TSA as the sole source of carbon and energy for aerobic growth (45). Purity of substrates used as sources of carbon and energy was >99%.
Growth was estimated as turbidity (optical density at 580 nm) and quantified as Lowry-type protein (23). Substrate utilization was determined after separation on reversed-phase high-performance liquid chromatography columns (25, 45), and sulfate was quantified as a suspension of barium sulfate (42). Bacteria were identified by the German Collection of Microorganisms (DSMZ) after analysis of the fatty acid composition of cell membranes. Cleavage of 1 mM 3- or 4-methylcatechol to form the muconate semialdehyde in exponentially growing cells was monitored colorimetrically at 400 nm by adding 200 μl of exponentially growing culture to minimal medium containing 3 mM 3- or 4-methylcatechol.
Preparation and restriction analysis of plasmid DNA.
Large plasmids were prepared with the Plasmid Midi Kit (Qiagen). The manufacturer's protocol for low-copy-number plasmids was optimized for high yields of plasmids larger than 50 kb from C. testosteroni T-2 and found to be effective in other organisms. Lysis time was reduced to the time needed for gentle tube inversion (three times), when it was stopped by addition of the supplemented neutralization buffer. The additional isopropanol precipitation step prior to application to the column was eliminated. Our preparations contained plasmids of up to 80 kb.
Restriction (double) digests of plasmid DNA with BamHI, SmaI, or XhoI (New England BioLabs) were prepared according to the manufacturer's instructions. Restriction fragments were separated on 0.75% agarose gels with 4.4 V/cm as described elsewhere (36). Documentation and analysis were done with the gel documentation system from Bio-Rad and the software Multi Analyst for Macintosh version 1.02. The size of bands was estimated mathematically with the program Gel, version 1.1β, of J. M. Lacroix (http://iubio.bio.indiana.edu/soft/molbio/ibmpc/gel-jml.zip). The physical map of pTSA was generated with the Double Digester software, version 1.1β2, of L. Wright (49).
A technique we term subtractive restriction analysis was used to map pTSA. Samples of plasmid DNA from C. testosteroni T-2 (containing, effectively, pTSA and pT2T) and from strain TER-1 (containing, effectively, only pT2T) were digested separately with the same enzyme or enzyme combination and separated electrophoretically. Fragments that were visible in both digests were not from pTSA.
PCR, quantification of DNA, cycle sequencing, and Southern hybridization.
PCR was conducted in reaction mixtures with final volumes of 20 to 50 μl. All reactions contained 10% (vol/vol) dimethyl sulfoxide. DNA (180 to 200 ng) prepared by cetyltrimethylammonium bromide precipitation (4), or a bacterial culture (3 μl), was used as a template. Templates of ≤5 kb were amplified with Taq polymerase (MWG) and buffer system 2 of Roche's Expand system; templates of >5 kb and <15 kb were amplified with the Expand Long PCR-System (Roche); and templates of >15 kb were amplified with the Expand 20 kb Plus System (Roche). Reactions were conducted according to the manufacturers' recommendations. DNA was quantified fluorimetrically (DyNA Quant 200; Hoefer) according to the manufacturer's instructions.
The following primers were used for PCR: TsaOp 11 (5′-CCG GCG CAG CAC GTA AAT GGT-3′), TsaOp 12 (5′-TGG GCA GGG CGA GGT CAA TGT-3′), TsaOp 21 (5′-CCC CCA AAA CCC CAA CAA GGA GAC-3′), TsaOp 22 (5′-GGG ATC ACG CGG CCA TAG ACG-3′), Tsa M1 (5′-AAA AAT CTT GAG CCA GGT-3′) (22), Tsa B1 (5′-TTG AGC TTT TGG TGA ATC-3′) (22), TsaM Prim 1 (5′-CGT GGT GGC GCT GGA AAA C-3′), TsaM Prim 2 (5′-TCG GGC ACC TGG CTC AAG AT-3′), trf A2-1 (5′-CGA AAT TCR TGG GAG AAG TA-3′) (15), and trf A2-2 (5′-CGY TTG CAA TGC ACC AGG TC-3′) (15).
PCR fragments amplified with the following primer pairs were sequenced by cycle sequencing and primer walking: TrfTsa-a (5′-GGC TGA TGT GCG GCT CGG ATT T-3′), TrfTsa-b (5′-GCC TAC CTG CTC GCG CCA CTT CT-3′), PsTs-0 (5′-ACG GTT TGA CGC CAC GAA TCG CAG ATT T-3′), TsaPsbl (5′-CCG GCG GTT TCC TGG GTG CTG TTC A-3′), PsTs-0a (5′-GTC AAT GAA ATC AAC GAT CTA CCA-3′), TsPs-0b (5′-GAG ATC CCC GGG CAA GAG-3′), PsTsTe (5′-GGC CTA CGG CCC GCA AAC CTA C-3′), IS3062u (5′-CGA CCA CGG TTT GAC GCC ACGA-3′), oriT-2-IncPβ (5′-TAA GGC GGG CAG GAT GTG TGA AG-3′), oriT-1-IncPβ (5′-GCT GCC TCG CAG AGC AGG ATG-3′), oriVb1l (5′-GCA CCT TGG GCC GGT TTG CCG C-3′), merTpTSA1l (5′-CCC CTG GTT TGC AGG CTT GC-3′), trbE1u (5′-GTA CCT CGA TGC GGT AAT CG-3′), trbE4l (5′-CAT GAG CGC CGG CGA CAG CG-3′), and TsPs-11 (5′-GTG CTC AAG GCG GTC GAG GAA ACC-3′).
Amplified template was purified with the Qiaquick PCR Purification Kit (Qiagen), and the fragment (85 ng of DNA/kb) was added to the sequencing reaction in the ABI Prism Big Dye Terminator Kit with the following PCR program: 70 s at 95°C, then 26 cycles of 20 s at 95°C, 30 s at 50°C, and 4 min at 60°C. Reaction products were precipitated with chilled ethanol and sodium acetate (36). Dried pellets were sent to GATC (Konstanz, Germany) for electrophoresis. Sequence data were analyzed using standard software (Edit View from Perkin-Elmer, Genetics Computer Group program package, and DNA-Star package from Lasergene). The National Center for Biotechnology Information (NCBI) BLAST programs were used to search for similarities of the sequences to those in the databases (1).
Southern blot hybridization was performed as described elsewhere (28). Gene probes were generated by PCR with primer pairs described above: TsaOp 11, TsaOp 12, TsaOp 21, TsaOp 22, Tsa M1, Tsa B, trf A2-1, and trf A2-2.
DNA size markers.
The DNA size markers used were λ DNA cut with EcoRI and HindIII from New England BioLabs and the 1-kb ladder of MBI-Fermentas with a range from 0.25 kb to 10 kb.
PFGE.
For pulsed-field gel electrophoresis (PFGE), DNA was prepared from whole cells embedded in low-melting-point agarose (SeaPlaque GTG, FMC, Biozym) as described elsewhere (41). Electrophoresis at 14°C for 66 to 70 h was done with a Rotaphor V (Biometra). For the gel, 1% agarose (UltraPure, electrophoresis grade; Gibco) and 0.025 M Tris-borate buffer (36) were used. A linear gradient from 24 s to 147 s was used with a switching angle of 120° and a field strength of 10 V/cm.
Nucleotide sequence accession numbers.
Nucleotide sequences are available in the NCBI GenBank library under accession numbers AF311820 (tsaMBCD2), AF305549 (oriTpTSA), AF303942 (oriVpTSA), and AF311437 (trfAupf).
RESULTS
Physical map of pTSA.
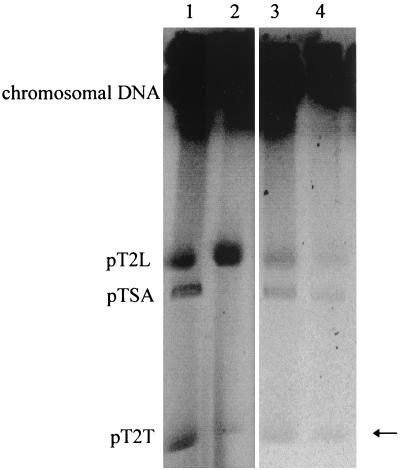
We planned to purify pTSA from C. testosteroni T-2 and generate a restriction map directly. Conjugation experiments failed to yield strains with pTSA as the sole episomal element (19). Electroporation of single plasmids, separated by PFGE, into the plasmid-free C. testosteroni type strain (DSM 50244T) also failed. Preparative PFGE yielded insufficient material to elute it from the gel, and the quality of the DNA was too low for in-gel restriction. The new experiments did, however, establish the presence of a third, larger, plasmid in strain T-2, and we termed it pT2L (Fig. 2). The pTSA− mutant, strain TER-1, contained two of the three plasmids (Fig. 2), pT2T and pT2L, the latter of which was recovered in low yield in plasmid preparations.
FIG. 2.
Electropherogram of the PFGE of C. testosteroni T-2 (lane 1), its pTSA− derivative TER-1 (lane 2) (Table 2), and of the isolates D. acidovorans Mu4 (lane 3) and S. maltophilia MuF (lane 4). The arrow points to the weak band representing pT2T.
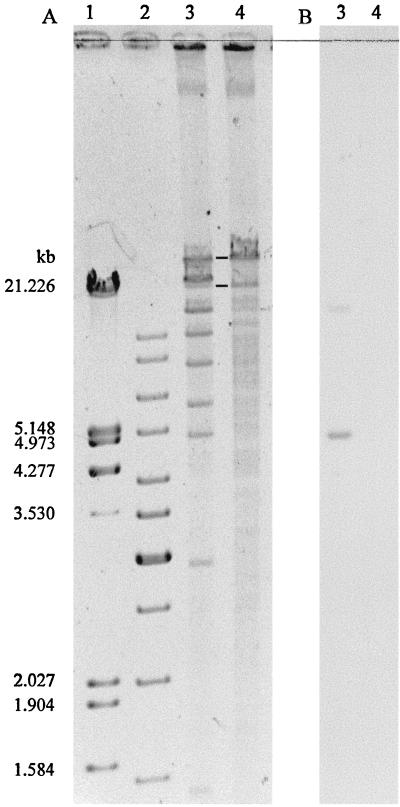
The first physical characterization of pTSA was generated by subtractive restriction mapping of preparations containing plasmids from strain T-2 or from strain TER-1. BamHI, SmaI (Fig. 3), and XhoI were suitable. Double digests were prepared with BamHI-SmaI, BamHI-XhoI, and SmaI-XhoI. The mean plasmid size calculated from 13 different restriction digestions was 72 ± 4 kb.
FIG. 3.
Electropherogram and Southern hybridization of the double digestion of the plasmids in C. testosteroni T-2 and its derivative TER-1. Restriction enzymes used were BamHI and SmaI. (A) Agarose gel electrophoresis. Lanes: 1 and 2, λ marker and 1-kb ladder; 3 and 4, digested plasmid DNA from strains T-2 (plasmids pT2L, pTSA, and pT2T) and TER-1 (plasmids pT2L and pT2T), respectively. Fragments present in lane 3 but not in lane 4 belong to pTSA. Fragments present in both lanes are marked with bold lines and belong to pT2T. (B) Southern hybridization with a tsaM1 probe; signals occur only in lane 3 (T-2).
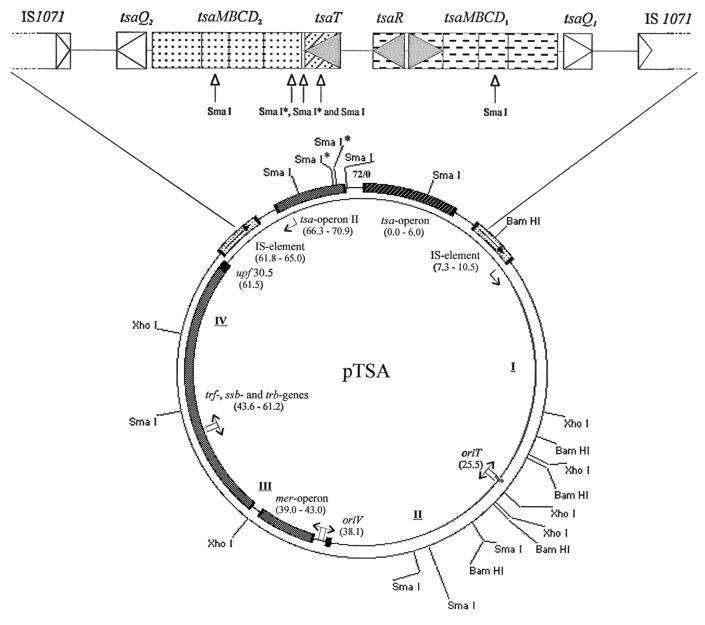
Southern hybridizations with probes for the tsa operon (tsaRM) and for trfA (data not shown) showed that these sequences are encoded on a 4.9-kb SmaI fragment (Fig. 3) and on a 7.3-kb SmaI-XhoI fragment, respectively; the only possible synthesis of the bands we generated led to the map shown in Fig. 4. We sequenced several regions of the plasmid and derived several sets of PCR primers from either our own results or published data (44), which gave us access to four overlapping, long-distance-PCR fragments (I to IV in Fig. 4). These fragments, with the tsa region, represented the whole plasmid, which was not sequenced in its entirety.
FIG. 4.
Map of plasmid pTSA. The resolution is about 1%; fragments of <0.8 kb are not resolved. Sites with an asterisk (SmaI) are sequenced but not cut by SmaI in these mapping assays. The tsa region is shown in enlarged form at the top of the diagram. I to IV refer to PCR fragments; the map positions and genes are indicated in kilobases. Triangles indicate the direction of transcription.
General structure of pTSA.
The long-distance-PCR products (Fig. 4) confirmed the estimate of the size of the plasmid (72 ± 4 kb) and enabled us to refine the genetic map. The known tsa region (tsaR and tsaMBCD [20]) was located, and the 3′ end of the tsaR gene was selected as the starting position for mapping.
The oriT region was located about 26 kb from the start (Fig. 4), and the oriV region was located at about 38 kb. The trf and trb genes were found at 44 to 62 kb with the upf region at 62 kb (cf. reference 44 and Table 1). A complete mer operon, which will be described elsewhere (J. Ruff, unpublished data), was found between the oriV and trf regions (Fig. 4), and the complex of tsa genes, as well as the IS1071 elements (19), was located between 62 kb and 11 kb.
TABLE 1.
Genes identified on pTSA and comparison with sequences from plasmids R751 (IncP1β) and RK2 (IncP1α)a
| Gene | Size (nt)b
|
% Identity (Id) or similarity (Sim) tob:
|
Putative function in pTSA | ||||
|---|---|---|---|---|---|---|---|
| pTSA | R751 | R751
|
RK2
|
||||
| aa (Id) | nt (Id) | aa (Id) | aa (Sim) | ||||
| trfA1/A2 | 1,221 | 1,224 | 96 | 99 | 65 | 76 | Replication, DNA binding |
| ssb | 342 | 342 | 100 | 100 | 72 | 93 | Single-stranded DNA binding |
| trbA | 309 | 309 | 100 | 99 | 76 | 85 | Regulation of transfer |
| trbB | 963 | 963 | 99 | 99 | 83 | 89 | ATPase, protein kinase, Mpf |
| trbC | 465 | 465 | 99 | 99 | Export signal sequence, Mpf | ||
| trbD | 312 | 312 | 98 | 98 | 85 | 92 | Bacterial zipper, Mpf |
| trbE | 2,559 | 2,559 | 99 | 99 | 87 | 94 | Mating pair formation, Mpf |
| trbF | 783 | 783 | 99 | 99 | 67 | 78 | Mating pair formation, Mpf |
| trbG | 921 | 921 | 99 | 99 | 78 | 91 | Mating pair formation, Mpf |
| trbH | 489 | 489 | 99 | 99 | 60 | 71 | Mating pair formation, Mpf |
| trbI | 1,422 | 1,422 | 99 | 99 | 65 | 74 | Mating pair formation, Mpf |
| trbJ | 765 | 765 | 100 | 99 | 71 | 88 | Mating pair formation, Mpf |
| trbK | 228 | 228 | 99 | 99 | Entry exclusion | ||
| trbL | 1,719 | 1,719 | 99 | 99 | Mpf, topoisomerase? | ||
| trbM | 588 | 591 | 91 | 98 | Signal sequence motif | ||
| trbN | 636 | 636 | 99 | 99 | Muramidase | ||
| trbO | 267 | 267 | 100 | 100 | |||
| trbP | 699 | 699 | 99 | 99 | |||
| upf30.5 | 432 | 429 | 99 | 99 | Outer membrane protein? | ||
| oriV | 500 | 500 | 100 | 500 bp upstream of the mer operon | |||
| Spacer | 24 | Downstream of the mer operon | |||||
| Spacer | 481 | 477 | 98 | Spacer between mer and trf | |||
| 5′ trfA1 | 46 | 46 | 100 | Spacer between trfA1 and ssb | |||
| 5′ trbA | 167 | 167 | 99 | Spacer with trbA, ssb, trfA promoter | |||
| 5′ trbB | 309 | 304 | 97 | Spacer including trbB promoter | |||
| 3′ upf30.5 | 97 | 97 | 100 | Spacer between upf30.5 and upf31.0 | |||
| oriT | 300 | 300 | 100 | Including the 99-bp relaxation region | |||
Alignments were performed with the NCBI-BLAST service (1). Putative functions of gene products and plasmid regions were ascribed as described elsewhere (44). The complete sequence of R751 (GenBank accession number U67194) was used for alignments, except for the 5′ trbA region (GenBank accession number U07618). Amino acid sequence alignments with the corresponding sequences of RK2 could reasonably be done with pirS08595 (TrfA), pirS07258 (Ssb), pirS26290 (TrbA), pirC44020 (TrbB), priD4985a2 (TrbD), pirF44020 (TrbE), pirG44020 (TrbF), pirH44020 (TrbG), pirI44020 (TrbH), pirA46449 (TrbI), and pirB46449 (TrbJ).
Numbers in boldface type indicate where gaps in the DNA sequence cause frameshifts.
The overall structure of the degradative plasmid pTSA thus has many similarities to that of the sequenced IncP1β resistance plasmid, R751. The gene order is the same (see also below). The mer operon is in the same relative position as the remnant in R751. The degradative genes may be considered to take the place of the Tn402/5090 region in plasmid R751.
A second tsa operon as part of a composite transposon.
We sequenced downstream of the tsaMBCD1 operon defined by Junker et al. (20) and found an open reading frame termed tsaQ1 (which will be described elsewhere [T. Tralau, unpublished data]) and then a region with no significant homologies in BLAST searches and an IS1071 element (Fig. 4).
When we sequenced downstream of the tsaR gene (Fig. 4), we located another open reading frame, termed tsaT (which is described elsewhere [27]), and a second version of the tsaMBCD genes, which we termed tsaMBCD2 (GenBank accession number AF311820). A second, identical, copy of tsaQ, tsaQ2, was found, as well as a second putative noncoding region and the second IS1071 element.
There was no promoter-like sequence upstream of tsaM2 (GenBank accession numberAF311820). The 5′ region of tsaM2, representing about 135 amino acids, gave a derived sequence, e.g., GMATDCSRKP (positions 11 to 20), which differed from the protein microsequence data of the enzyme isolated from strain T-2 (AWDTEIPAEG [20]), and the latter was confirmed in the DNA sequence (20). Therefore, we concluded that tsaMBCD2 was not expressed, and we confirmed this by obtaining no reverse transcription-PCR signal for tsaM2, whereas inducible transcription of tsaM1 was detected with the same method.
The sequence of tsaMBCD2 after the initial 410 bp was essentially identical to that of tsaMBCD1. The three differences altered about seven amino acids in putative TsaB2. tsaQ2 and tsaQ1 were identical. The sequences between tsaQ and the appropriate insertion element were almost identical (5 different nucleotides in 1,500). The first 500 bp of the IS1071 element at about 8 kb were identical with the left-hand end of the published sequence (30), whereas the first 700 bp of the IS1071 element at about 65 kb were identical with the right-hand end of the published sequence (30). The junctions to these elements were also identical.
Backbone of pTSA.
IncP plasmids are routinely classified by amplifying and sequencing a 241-bp segment of the trfA2 gene (12, 15). Junker and Cook (19) reported 97% identity with plasmid R751 on the DNA level for pTSA. We have now sequenced this and another 18 backbone genes (Table 1) and found only two genes altered in comparison to the backbone of R751. The trfA and trfA2 genes were 99% identical to R751 on the DNA sequence level and 96% identical on the amino acid level. The lower value is due to frameshifts and a deletion at four positions. Three different frameshifts result in three altered amino acids; a gap is offset after 16 bp by an additional base in pTSA, resulting in five altered amino acids. The second altered backbone gene in pTSA was trbM, which showed a deletion; three gaps resulted in seven altered amino acids and one amino acid fewer in the gene product from pTSA. The other identified genes are 99 or 100% identical to R751 on the DNA and the amino acid sequence levels (Table 1), which shows that this part of the plasmid backbone is even more closely related to the corresponding R751 region than the sequence comparison of trfA2 (12, 19) indicated. On the other hand, we found much lower identity (65 to 87%) for those genes which are comparable on the IncP1α plasmids (32) on the amino acid level (76 to 94% similarity) (Table 1). In contrast to R751, there is no upf31.0 gene at 61.5 kb, upstream of the putative tsa transposon.
The 300-bp oriT region (25 kb on map) between the plasmid backbone genes traJ and traK (44) is 100% identical to the corresponding R751 region and showed only 60% identity to the appropriate region on the IncP1α plasmid RK2. The 99-bp relaxation region in the first half of the 300-bp segment adjacent to traJ, however, is 80% identical to the corresponding region on RK2. These differences, observed in restriction maps, are the original basis of the subdivision of the IncP1 group (52).
The 500-bp oriV region upstream of the mer operon is 100% identical to the R751 oriV region. Other noncoding sequences in the backbone region (Table 1) also show identity values of 97 to 100%. One short 24-bp sequence (Table 1; 43 kb on map) adjacent to the mer sequences shows no homology to R751 or other prokaryotic sequences. A BLAST search with the subsequent 481 bp shows not only a 98% identity to R751 at the corresponding plasmid location but also 92% identity to a stretch of 462 bp on R906, another resistance IncP1β plasmid (35, 39). This sequence comprises repeat regions and a protein binding region for TrfA, suggesting that it is an essential oriV-related region of IncP1β plasmids (39, 40).
Distribution of the tsa genes.
Samples (n = 98) from 84 sites on four continents (France, Germany, Iceland, Ireland, Norway, Spain, Switzerland; Argentina, United States, Greenland; India, Taiwan; Tahiti) were used as inocula for enrichment cultures to detect utilization of TSA as the sole source of carbon and energy for aerobic growth. The samples were largely (n = 72) from pristine soils, lakes, and rivers; the others were from sewage works, landfills, and contaminated rivers. The 16 positive enrichment samples, all prokaryotic, were largely (n = 15) from wastewater or sites of industrial pollution; only 1 enrichment sample (from Tahiti) was from a pristine site. Most enrichments (n = 13) yielded a pure culture, and all were examined (Table 2).
TABLE 2.
Comparison of 16 isolates and mixed cultures of TSA-utilizing bacteria with C. testosteroni T-2 and its pTSA− mutant, strain TER-1
| Culture | Origin | Phylogenya | Substrate rangeb
|
tsa genes presentc | MC23O activityd | Plasmidse
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BS | TSA | PSB | TCA | TER | PCA | IncP1 | Total | |||||
| TER-1 | Switzerland | C. testosteroni (β) | − | − | − | − | + | + | − | − | − | 2 |
| T-2 | Switzerland | C. testosteroni (β) | − | + | + | + | + | + | + | − | + | 32 |
| Mu4 | Germany | Delftia acidovorans (β) | − | + | + | + | + | + | + | − | + | 32 |
| MuF | Germany | Stenotrophomonas maltophilia (γ) | − | + | + | + | + | + | + | − | + | 43 |
| MoP1 | Germany | Delftia acidovorans (β) | − | + | + | − | − | + | + | − | − | 00 |
| EWL2 | Switzerland | Pseudomonas pseudoalcaligenes (γ) | − | + | + | − | − | + | + | − | − | 4 |
| TKR | Taiwan | Ralstonia pickettii (β) | − | + | + | − | − | + | + | − | − | 20 |
| TA12 | Tahiti | Unidentified isolate | − | + | + | − | + | + | + | − | − | 0 |
| ISP2 | Germany | Pseudomonas sp. (γ) | − | + | + | − | + | − | + | − | − | 00 |
| SCM | Germany | Mixture | + | + | + | − | + | + | − | + | + | 3 |
| KNP1 | Germany | Mixture | + | + | − | − | + | + | − | + | − | 0 |
| EWM1 | Switzerland | Hydrogenophaga taeniospiralis (β) | + | + | − | − | + | + | − | + | + | 2 |
| EW13 | Switzerland | C. testosteroni (β) | + | + | − | − | − | + | − | + | − | 0 |
| RLB | Germany | Stenotrophomonas maltophilia (γ) | + | + | − | − | − | + | − | + | − | 1 |
| VLB1 | Germany | Mixture | + | + | − | − | − | + | − | + | − | 2 |
| VLB | Germany | Stenotrophomonas maltophilia (γ) | − | + | − | − | − | + | − | + | + | 0 |
| TKA | Germany | Acidovorax delafieldii (β) | − | + | − | − | − | + | − | + | − | 2 |
| OrL1 | USA | C. testosteroni (β) | − | + | − | − | − | + | − | + | − | 1 |
Organisms were tentatively identified on the basis of their fatty acid content and found to belong to two subgroups of the Proteobacteria.
We observed quantitative substrate utilization in liquid culture; in the case of sulfonates, the sulfonate moiety was recovered quantitatively as sulfate. For abbreviations, see the legend to Fig. 1. BS, benzenesulfonate.
The presence of the tsa genes was considered positive when three different PCR amplifications were positive: 825 bp of tsaM, 2,119 bp overlapping tsaMB, and 3,186 bp overlapping tsaCD (cf. Fig. 4).
4-Methylcatechol 2,3-dioxygenase (MC23O) activity was tested in cells growing on TSA salts medium. 3-Methylcatechol was also a substrate, often with higher activity.
The number of plasmids was estimated by PFGE, after which the localization of tsa genes was determined by Southern analysis. Plasmid-located tsa genes are indicated by a superscript which gives the corresponding plasmid when counted from the loading slot. 0 indicates chromosomal localization of the tsa genes. In Mu4 and MuF, the plasmid sizes are the same as in C. testosteroni T-2 (Fig. 2). MuF seems to have one additional plasmid bigger than pT2L. The presence of IncP1 plasmids was tested at the whole-cell level with a PCR probe for tftrA (15). VLB seems to have a chromosomally integrated IncP1 plasmid.
We considered the new cultures to fall into three groups on the basis of their metabolism of TSA (Table 2). Group I (seven isolates) resembled C. testosteroni T-2 in degrading TSA and PSB without expressing 4-methylcatechol 2,3-dioxygenase activity. Group II (three isolates and three mixed cultures) resembled Alcaligenes sp. strain O-1, which involves 1,2-dioxygenation of TSA in degrading both BS and TSA via 4-methylcatechol 2,3-dioxygenase. Group III (three isolates) degraded TSA but not BS or PSB and expressed 4-methylcatechol 2,3-dioxygenase (21).
The Group I organisms all excreted 4-sulfobenzylalcohol (Fig. 1) transiently during growth in TSA salts medium, as does strain T-2 (20), and all behaved as strain T-2 and excreted PSB transiently (20, 26) except for EWL2, which was nevertheless able to use PSB. All seven Group I organisms contained the tsa genes (Table 2, footnote c), but only two of them (strains Mu4 and MuF) resembled strain T-2 in containing an IncP1β plasmid. Indeed, three tsa-positive isolates (strains MoP1, TA12, and ISP2) contained no detectable plasmid and presumably have chromosomally encoded tsa genes (Table 2).
The putative tsa transposon (Fig. 4) was found in its entirety in six of the seven Group I organisms; in strain TA12, tsaT was missing. The distribution of this structure on different plasmids and on the chromosome presumably confirms that the structure is a mobile element and capable of transposition.
There was considerable diversity in Group I. Strains Mu4 and MuF (β- and γ-Proteobacteria) most closely resembled strain T-2 metabolically and physiologically, including having the tsa transposon encoded on a 72-kb plasmid which could belong to the IncP1 group (Fig. 2 and Table 2). Three isolates (MoP1, EWL2, and TKR) did not utilize terephthalate and presumably as a consequence could not utilize toluenecarboxylate (Fig. 1). In contrast, strain TA12 could utilize terephthalate but not TCA, and so it may lack a TCA transport gene. We presume that strain ISP2 has an altered R4 operon lacking the PCA transport gene (M. Providenti, unpublished data). There was much less diversity in the tsa genes, where we compared an 830-bp section overlapping tsaM1 and the promoter region (position 1370 to 2200 in U32622 [20]). One mutation (1393 [C to T]) was found in strain EWL2 in the promoter region, and two mutations were found in strain ISP2: 1398 (T to C), in the promoter region, and 2214 (C to A), giving an alternative R codon.
Strains of C. testosteroni were found in Groups I (T-2), II (EW13), and III (OrL1) (Table 1). This indicates a high mobility of the genetic elements encoding the degradative genes both within the Proteobacteria and across continents.
DISCUSSION
This is the first degradative plasmid to be mapped by subtractive analysis of restriction digests. Large plasmids are usually mapped by restriction analysis (e.g., see reference 51), which requires separation of the plasmid, if several are present in the organism under study. We had to use an alternative strategy, because we could not isolate pTSA. Our data (72 kb [Fig. 4]) show the plasmid to be smaller than our original estimate of 85 kb but within the error range (19); our new data with several enzymes and improved markers are supported by the sequenced tsa region and the lengths of the long-distance-PCR fragments. With hindsight, the mer operon might serve as a selective marker, but mercury resistance is apparently weak in this strain (19).
The use of long-distance PCR was a key method in establishing the structure of pTSA. Much work was done before the map of plasmid R751 was available (44), but the latter data showed the near identity of backbone genes in both plasmids (Table 1) and allowed us to choose primers for suitably sized amplification products (Fig. 4 and Materials and Methods).
IncP1β plasmids are considered to form a structurally more diverse subgroup than IncP1α plasmids (12, 40). Here, however, we present an IncP1β plasmid, whose backbone, where sequenced, is almost identical to that of the IncP1β plasmid R751. The overall structure is also highly homologous to that of plasmid R751, though it confers degradative capabilities rather than resistance factors. Degradative IncP plasmids were shown to have their degradative genes integrated between the oriV and trfA regions (9). In contrast, the degradative genes of pTSA are integrated within a transposon-like structure adjacent to the upf30.5 gene, a region where R751 carries a transposon conferring trimethoprim resistance (Tn402/5090 [18, 44]). No trimethoprim resistance is found in C. testosteroni T-2 (19). The Tn501-related mer operon found between oriV and trfA, a common feature of IncPβ plasmids (40), is complete on pTSA but only a remnant on R751. One characteristic feature of R751 is the inverted repeats found in the oriV/trfA and tra-1/tra-2 gaps (44). These repeats are discussed as hot spots of recombination. On the sequenced backbone of pTSA we found seven of eight possible inverted repeats. Between the mer operon and the trf region all five repeats could be located, two of them with minor mutations—a point mutation changing C to G in one repeat and a 1-bp shift of a second repeat. The other two repeats are positioned at the insertion site of the tsa transposon. Compared to R751, one repeat is missing (44). It seems that these repeats are linked to the insertion sites on the plasmid—on pTSA as well as on R751.
The major difference between the structure of the plasmid and the predictions is that genes encoding regulatory unit R3 (Fig. 1) are not present. Junker and Cook (19) predicted a transposon carrying the R3 genes between two IS1071 elements. There is indeed a transposon of this kind, which has been characterized in C. testosteroni PSB-4 (J. Ruff et al., unpublished data). Furthermore, a copy of this transposon, with a deleted psbC, is present in C. testosteroni T-2 (11), but it is encoded on pT2L (J. Ruff et al., unpublished data). The IS1071 elements, which Junker and Cook (19) detected on pTSA, can now be seen to belong to a tsa transposon-like structure (Fig. 4). The separation of the regulons R1 and R3 on two plasmids, pTSA and pT2L, explains why pTSA was not separable by conjugation to, e.g., C. testosteroniT, which encodes neither R1 nor R3 (19).
The tsa operon discovered by Junker et al. (20) can now be seen to be part of a composite transposon (Fig. 4). The transposon has been found on three continents (Table 2), sometimes plasmid encoded and sometimes chromosomally encoded (Table 2), and so we can assume that the transposon has an active transposase.
We now have a total of eight bacteria that carry this transposon with essentially the same structure (except that strain TA12 lacks tsaT) (Table 2). Each organism from among seven genera and three continents thus contains a second, silent, and almost perfect duplicate set of genes (tsaMBCD2 with tsaQ2 and junction to the insertion element) (Fig. 4). Duplication of degradative genes is not uncommon, as in 3-chlorobenzoate metabolism in Pseudomonas sp. strain B13 (34) or on the TOL plasmid pWW53, where two homologous and functional, but not identical, meta pathway operons were characterized (31), and these duplications are discussed as an important mechanism for the genetic adaptation of microorganisms to xenobiotics (47).
The duplication within the tsa transposon (Fig. 4) may allow the development of novel degradative pathways in the future, but the plasmid location should enhance this (43) as well as the location within a transposon. The evolution of the silent operon is coupled to the spread of the active tsa genes and therefore dependent on the release of TSA. To our knowledge this is the first description of a genetic constellation where the possible evolution of a duplicated catabolic operon may be enhanced by the release of a xenobiotic substance. Furthermore, the nature of pTSA, one of the highly promiscuous IncPβ plasmids (33), coupled to the obviously active transposase of the tsa transposon, has presumably contributed to its widespread occurrence. Götz et al. (15) have linked IncP1β plasmids to contaminated sites, and we have found degradation of TSA almost exclusively in contaminated areas. In corroboration of the results of Götz et al. (15), IncP1 genes occur in 6 of our 19 isolates (Table 2).
In contrast to the Group I isolates (Table 2), with their genetic and physiological similarities to C. testosteroni T-2 (Fig. 1 and Table 4), we had trouble obtaining isolates of Group II, in which our three mixed cultures are found in these six cultures (Table 2). Group II organisms can grow on BS and TSA and show a 4-methylcatechol 2,3-dioxygenase activity, properties which indicate the presence of a desulfonative TSA dioxygenase, as in Alcaligenes sp. strain O-1 (11, 21). This pathway is frequently plasmid encoded (5), but two cultures (mixture KNP1 and isolate EW13 [Table 2]) contain no detectable plasmid. We consider it possible that the Group III organisms encode the TSA 2,3-dioxygenase pathway enzymes (14), but we have no proof as yet. Further analysis may show whether there are pathways for the degradation of TSA that have not been described up to now.
Strain TA12 seemed to adapt to the utilization of TSA during enrichment, because the growth rate increased steadily during the enrichment (T. Tralau, unpublished data). This could have been the result of mutations in regulatory genes as described by Arai et al. (3) for the utilization of phenol by C. testosteroni TA441.
Two of the newly isolated TSA degraders (EW13 and OrL1) are strains of C. testosteroni but do not have tsa genes and belong to Groups II and III, respectively. Similarly, strains of Stenotrophomonas maltophilia are found in each group (Table 2). S. maltophilia is of clinical relevance and is well known for its multiple antibiotic resistance (e.g., see reference 53). Strains of this genus reveal a high intraspecies diversity and do not show any resistance patterns correlating specifically with the source of isolation, clinical or environmental (8). We presume that Stenotrophomonas is a highly adaptive genus that reacts rapidly towards clinical and metabolic selection.
We conclude that there was independent evolution of several pathways for the degradation of TSA and that there was an intergeneric distribution of the genetic modules necessary for TSA utilization.
The research was funded by the Deutsche Forschungsgemeinschaft (T. Tralau), the University of Konstanz, and the Fonds der Chemischen Industrie.
ACKNOWLEDGMENTS
We are grateful to Frithjof Küpper, who collected most of the environmental samples, and to Kornelia Smalla for helpful discussions.
REFERENCES
- 1.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anliker R. Color chemistry and the environment. Ecotoxicol Environ Safety. 1977;1:211–237. doi: 10.1016/0147-6513(77)90037-9. [DOI] [PubMed] [Google Scholar]
- 3.Arai H, Akahira S, Ohishi T, Maeda M, Kudo T. Adaptation of Comamonas testosteroni TA441 to utilize phenol: organization and regulation of the genes involved in phenol degradation. Microbiology (Reading) 1998;144:2895–2903. doi: 10.1099/00221287-144-10-2895. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1987. [Google Scholar]
- 5.Balashov S V, Boronin A M. Benzenesulphonic and p-toluenesulphonic acid degrading plasmids of Comamonas testosteroni. Russ J Genet. 1997;33:498–503. [Google Scholar]
- 6.Bates S, Cashmore A M, Wilkins B M. IncP plasmids are unusually effective in mediating conjugation of Escherichia coli and Saccharomyces cerevisiae: involvement of the Tra2 mating system. J Bacteriol. 1998;180:6538–6543. doi: 10.1128/jb.180.24.6538-6543.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behret H, Ahlers J, Ettel S, Feicht E, Futterer E, Mangelsdorf I, Pohlenz-Michel C, Roβ H, Sterzl-Eckert H, Vogel D, Weis L, Widmann K. p-Toluolsulfonsaeure. Beratergremium für umweltrelevante Altstoffe (BUA)–Stoffberichte. Vol. 63. Weinheim, Germany: Verlag Chemie; 1991. [Google Scholar]
- 8.Berg G, Roskot N, Smalla K. Genotypic and phenotypic relationships between clinical and environmental isolates of Stenotrophomonas maltophilia. J Clin Microbiol. 1999;37:3594–3600. doi: 10.1128/jcm.37.11.3594-3600.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burlage R S, Bemis L A, Layton A C, Sayler G S, Larimer F. Comparative genetic organization of incompatibility group P degradative plasmids. J Bacteriol. 1990;172:6818–6825. doi: 10.1128/jb.172.12.6818-6825.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busse H-J, El-Banna T, Oyaizu H, Auling G. Identification of xenobiotic-degrading isolates from the beta subclass of the Proteobacteria by a polyphasic approach including 16S rRNA partial sequencing. Int J Syst Bacteriol. 1992;42:19–26. doi: 10.1099/00207713-42-1-19. [DOI] [PubMed] [Google Scholar]
- 11.Cook A M, Laue H, Junker F. Microbial desulfonation. FEMS Microbiol Rev. 1998;22:399–419. doi: 10.1111/j.1574-6976.1998.tb00378.x. [DOI] [PubMed] [Google Scholar]
- 12.Dröge M, Pühler A, Selbitschka W. Phenotypic and molecular characterization of conjugative antibiotic resistance plasmids isolated from bacterial communities of activated sludge. Mol Gen Genet. 2000;263:471–482. doi: 10.1007/s004380051191. [DOI] [PubMed] [Google Scholar]
- 13.Elvers B, Hawkins S, Russey W. Ullmann's encyclopedia of industrial chemistry. Weinheim, Germany: VCH Verlagsgesellschaft mbH; 1994. [Google Scholar]
- 14.Focht D D, Williams F D. The degradation of p-toluenesulphonate by a Pseudomonas. Can J Microbiol. 1970;16:309–316. doi: 10.1139/m70-056. [DOI] [PubMed] [Google Scholar]
- 15.Götz A, Pukall R, Smith E, Tietze E, Prager R, Tschäpe H, van Elsas J D, Smalla K. Detection and characterization of broad-host-range plasmids in environmental bacteria by PCR. Appl Environ Microbiol. 1996;62:2621–2628. doi: 10.1128/aem.62.7.2621-2628.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guiney D G, Lanka E. Conjugative transfer of IncP plasmids. In: Thomas C M, editor. Promiscuous plasmids of gram-negative bacteria. London, United Kingdom: Academic Press; 1989. pp. 27–56. [Google Scholar]
- 17.Heinemann J A, Sprague G F. Bacterial conjugative plasmids mobilize DNA transfer berween bacteria and yeast. Nature (London) 1989;340:205–209. doi: 10.1038/340205a0. [DOI] [PubMed] [Google Scholar]
- 18.Jobanputra R S, Datta N. Trimethoprim R factors in enterobacteria from clinical specimens. J Med Microbiol. 1974;7:169–177. doi: 10.1099/00222615-7-2-169. [DOI] [PubMed] [Google Scholar]
- 19.Junker F, Cook A M. Conjugative plasmids and the degradation of arylsulfonates in Comamonas testosteroni. Appl Environ Microbiol. 1997;63:2403–2410. doi: 10.1128/aem.63.6.2403-2410.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Junker F, Kiewitz R, Cook A M. Characterization of the p-toluenesulfonate operon tsaMBCD and tsaR in Comamonas testosteroni T-2. J Bacteriol. 1997;179:919–927. doi: 10.1128/jb.179.3.919-927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Junker F, Leisinger T, Cook A M. 3-Sulphocatechol 2,3-dioxygenase and other dioxygenases (EC 1.13.11.2 and EC 1.14.12.-) in the degradative pathways of 2-aminobenzenesulphonic, benzenesulphonic and 4-toluenesulphonic acids in Alcaligenes sp. strain O-1. Microbiology (Reading) 1994;140:1713–1722. doi: 10.1099/13500872-140-7-1713. [DOI] [PubMed] [Google Scholar]
- 22.Junker F, Saller E, Schläfli Oppenberg H R, Kroneck P M H, Leisinger T, Cook A M. Degradative pathways for p-toluenecarboxylate and p-toluenesulfonate and their multicomponent oxygenases in Comamonas testosteroni strains PSB-4 and T-2. Microbiology (Reading) 1996;142:2419–2427. doi: 10.1099/00221287-142-9-2419. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy S I T, Fewson C A. Enzymes of the mandelate pathway in bacterium N.C.I.B. 8250. Biochem J. 1968;107:497–506. doi: 10.1042/bj1070497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laskin A I, Lechevalier H A. CRC handbook of microbiology. Vol. 5. Boca Raton, Fla: CRC Press; 1984. [Google Scholar]
- 25.Laue H, Field J A, Cook A M. Bacterial desulfonation of the ethanesulfonate metabolite of the chloroacetanilide herbicide metazachlor. Environ Sci Technol. 1996;30:1129–1132. [Google Scholar]
- 26.Locher H H, Leisinger T, Cook A M. Degradation of p-toluenesulphonic acid via sidechain oxidation, desulphonation and meta ring cleavage in Pseudomonas (Comamonas) testosteroni T-2. J Gen Microbiol. 1989;135:1969–1978. doi: 10.1099/00221287-135-7-1969. [DOI] [PubMed] [Google Scholar]
- 27.Mampel J. Ph.D. thesis. Konstanz, Germany: University of Konstanz; 2000. [Google Scholar]
- 28.Mampel J, Ruff J, Junker F, Cook A M. The oxygenase component of the 2-aminobenzenesulfonate dioxygenase system from Alcaligenes sp. strain O-1. Microbiology (Reading) 1999;145:3255–3264. doi: 10.1099/00221287-145-11-3255. [DOI] [PubMed] [Google Scholar]
- 29.Nakatsu C, Ng J, Singh R, Straus N, Wyndham R C. Chlorobenzoate catabolic transposon Tn5271 is a composite class I element with flanking class II insertion sequences. Proc Natl Acad Sci USA. 1991;88:8312–8316. doi: 10.1073/pnas.88.19.8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakatsu C H, Straus N A, Wyndham R C. The nucleotide sequence of the Tn5271 3-chlorobenzoate 3,4-dioxygenase genes (cbaAB) unites the class IA oxygenases in a single lineage. Microbiology (Reading) 1995;141:485–495. doi: 10.1099/13500872-141-2-485. [DOI] [PubMed] [Google Scholar]
- 31.Osborne D J, Pickup R W, Williams P A. The presence of two complete homologous meta pathway operons on TOL plasmid pWW53. J Gen Microbiol. 1988;134:2965–2975. doi: 10.1099/00221287-134-11-2965. [DOI] [PubMed] [Google Scholar]
- 32.Pansegrau W, Lanka E, Barth P T, Figurski D H, Guiney D G, Haas D, Helinski D R, Schwab H, Stanisich V A, Thomas C M. Complete nucleotide sequence of Birmingham IncPα plasmids. J Mol Biol. 1994;239:623–663. doi: 10.1006/jmbi.1994.1404. [DOI] [PubMed] [Google Scholar]
- 33.Pukall R, Tschaepe H, Smalla K. Monitoring the spread of broad host and narrow host range plasmids in soil microcosms. FEMS Microbiol Ecol. 1996;20:53–66. [Google Scholar]
- 34.Rangnekar V M. Variation in the ability of Pseudomonas sp. strain B13 cultures to utilize meta-chlorobenzoate is associated with tandem amplification and deamplification of DNA. J Bacteriol. 1988;170:1907–1912. doi: 10.1128/jb.170.4.1907-1912.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakanian V A, Azarian N G, Krupenko M A. Molecular organization of plasmid R906 (Inc P-1) Mol Biol (Moscow) 1985;19:964–973. [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 37.Sawyer C N, Ryckman D W. Anionic synthetic detergents and water supply problems. J Am Water Works Assoc. 1957;49:480–490. [Google Scholar]
- 38.Schulze K. Der westeuropäische Tensidmarkt 1994/1995. Tenside Surfactants Deterg. 1996;33:94–95. [Google Scholar]
- 39.Smith C A, Pinkney M, Guiney D G, Thomas C M. The ancestral IncP replication system consisted of contiguous oriV and trfA segments as deduced from a comparison of the nucleotide sequences of diverse IncP plasmids. J Gen Microbiol. 1993;139:1761–1766. doi: 10.1099/00221287-139-8-1761. [DOI] [PubMed] [Google Scholar]
- 40.Smith C A, Thomas C M. Comparison of the organisation of the genomes of phenotypically diverse plasmids of incompatibility group P: members of the IncP β sub-group are closely related. Mol Gen Genet. 1987;206:419–427. doi: 10.1007/BF00428881. [DOI] [PubMed] [Google Scholar]
- 41.Smith C L, Klco S R, Cantor C R. Pulsed-field gel electrophoresis and the technology of large DNA molecules. In: Davies K E, editor. Genome analysis: a practical approach. Oxford, United Kingdom: IRL Press; 1988. pp. 40–72. [Google Scholar]
- 42.Tabatabai M A. Determination of sulfate in water sample. Sulphur Inst J. 1974;10:11–13. [Google Scholar]
- 43.Tan H M. Bacterial catabolic transposons. Appl Microbiol Biotechnol. 1999;51:1–12. doi: 10.1007/s002530051356. [DOI] [PubMed] [Google Scholar]
- 44.Thorsted P B, Macartney D P, Akhtar P, Haines A S, Ali N, Davidson P, Stafford T, Pocklington M J, Pansegrau W, Wilkins B M, Lanka E, Thomas C M. Complete sequence of the IncPβ plasmid R751: implications for evolution and organisation of the IncP backbone. J Mol Biol. 1998;282:969–990. doi: 10.1006/jmbi.1998.2060. [DOI] [PubMed] [Google Scholar]
- 45.Thurnheer T, Köhler T, Cook A M, Leisinger T. Orthanilic acid and analogues as carbon sources for bacteria: growth physiology and enzymic desulphonation. J Gen Microbiol. 1986;132:1215–1220. [Google Scholar]
- 46.Trieu-Cuot P, Carlier C, Martin P, Courvalin P. Plasmid transfer by conjugation from Escherichia coli to Gram-positive bacteria. FEMS Microbiol Lett. 1987;48:289–294. [Google Scholar]
- 47.van der Meer J R, de Vos W M, Harayama S, Zehnder A J B. Molecular mechanisms of genetic adaption to xenobiotic compounds. Microbiol Rev. 1992;56:677–694. doi: 10.1128/mr.56.4.677-694.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y Z, Zhou Y, Zylstra G J. Molecular analysis of isophthalate and terephthalate degradation by Comamonas testosteroni YZW-D. Environ Health Perspect. 1995;130:9–12. doi: 10.1289/ehp.95103s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wright L. Double Digester 1.1 beta. 2nd ed. New Haven, Conn: Yale University Center for Medical Informatics; 1993. [Google Scholar]
- 50.Wyndham R C, Cashore A E, Nakatsu C H, Peel M C. Catabolic transposons. Biodegradation. 1994;5:323–342. doi: 10.1007/BF00696468. [DOI] [PubMed] [Google Scholar]
- 51.Xia X S, Aathithan S, Oswiecimska K, Smith A R, Bruce I J. A novel plasmid pIJB1 possessing a putative 2,4-dichlorophenoxyacetate degradative transposon Tn5530 in Burkholderia cepacia strain 2a. Plasmid. 1998;39:154–159. doi: 10.1006/plas.1997.1332. [DOI] [PubMed] [Google Scholar]
- 52.Yakobson E, Guiney D. Homology in the transfer origins of broad host range IncP plasmids: definition of two subgroups of P plasmids. Mol Gen Genet. 1983;192:436–438. doi: 10.1007/BF00392187. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L, Li X Z, Poole K. Multiple antibiotic resistance in Stenotrophomonas maltophilia: involvement of a multidrug efflux system. Antimicrob Agents Chemother. 2000;44:287–293. doi: 10.1128/aac.44.2.287-293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]