Abstract
Background:
Antiphospholipid syndrome (APS) is an autoimmune prothrombotic condition with significant morbidity. The objective of this study was to identify additional clinical and epidemiological risks of arterial thrombosis, venous thrombosis, and pregnancy morbidities in a large cohort of persistent antiphospholipid antibodies (aPLs)-positive carriers.
Methods:
This was a cross-sectional cohort study of 453 consecutive patients with a documented positive aPL who attended Peking University People's Hospital. Among 453 patients screened, 297 patients had persistent positive aPL. We compared asymptomatic aPL carriers with thrombotic and obstetric APS patients. And the univariate analysis and multivariable logistic regression were used to evaluate the association between different risk factors and APS clinical manifestations. The levels of circulating markers of neutrophil extracellular traps (NETs) (cell-free DNA and citrullinated histone H3 [Cit-H3]) were assessed and compared among aPL-positive carriers with or without autoimmune disease and APS patients.
Results:
Additional risk factors associated with arterial thrombosis among aPL-positive carriers included: smoking (odds ratio [OR] = 6.137, 95% confidence interval [CI] = 2.408–15.637, P = 0.0001), hypertension (OR = 2.368, 95% CI = 1.249–4.491, P = 0.008), and the presence of underlying autoimmune disease (OR = 4.401, 95% CI = 2.387–8.113, P < 0.001). Additional risks associated with venous thrombosis among aPL carriers included: smoking (OR = 4.594, 95% CI = 1.681–12.553, P = 0.029) and the presence of underlying autoimmune disease (OR = 6.330, 95% CI = 3.355–11.940, P < 0.001). The presence of underlying autoimmune disease (OR = 3.301, 95% CI = 1.407–7.744, P = 0.006) is the additional risk, which demonstrated a significant association with APS pregnancy morbidity. Higher circulating levels of cell-free DNA and Cit-H3 were observed among APS patients and aPL patients with autoimmune diseases compared with those aPL carriers without underlying autoimmune diseases. Furthermore, control neutrophils that are conditioned with APS patients’ sera have more pronounced NET release compared with those treated with aPL carriers’ sera without underlying autoimmune diseases.
Conclusion:
We identified several potential additional risk factors for APS clinical manifestations among a large cohort of Chinese aPL carriers. Our data may help physicians to risk stratify aPL-positive Asian patients.
Keywords: Antiphospholipid antibodies, Antiphospholipid syndrome, Arterial thrombosis, Pregnancy risk, Venous thrombosis
Introduction
Antiphospholipid syndrome (APS) is an autoimmune prothrombotic condition with significant morbidity. APS can manifest as arterial thrombosis, venous thrombosis, and/or pregnancy morbidities, including premature births as a result of eclampsia, preeclampsia, or placental insufficiency, and recurrent consecutive spontaneous abortions.[1–3] The current classification criteria for APS require a positive test of one or more “criteria”antiphospholipid antibodies (aPL) (anticardiolipin [aCL] IgG or IgM, anti-β2glycoprotein-I [anti-β2GPI] IgG or IgM, and lupus anticoagulant [LA]) in the presence of a thromboembolic event or pregnancy morbidity.[2] However, one of the biggest challenges in daily clinical practice is the management of a patient with positive aPL in absence of thrombotic or obstetric manifestations.
The pathogenesis of thrombotic APS follows a “two hit”model. Autoantibodies trigger initial endothelial damage but a “second hit”insult that disrupts vascular integrity is needed to potentiate thrombus formation.[1] Recent studies have recognized that the presence of aPL does not always lead to adverse clinical outcomes.[1,4,5] Additional risk factors are required to accelerate clinical manifestations in patients with persistent aPL. There are few studies that address the “second hit”risk factors. Data from currently published studies are all acquired from American and European cohorts and are limited by study design, suboptimal statistical models for data analysis, and limited inclusion of specific populations, including aPL-positive Chinese.[6–8] One such study is the global anti-phospholipid syndrome score which was conducted in a crosssectional cohort of European systemic lupus erythematosus (SLE) patients.[9] It is innovative as it included cardiovascular risk factors. However, it has not been widely accepted and further prospective population-based validation studies are needed. Furthermore, a recent study has proven that there are ethnic differences in the thrombotic risk of lupus patients, suggesting that ethnicity may play a role in the risk of aPL-positive patients without lupus.[10] Currently, there is no study assessing additional risk factors in aPL-positive Chinese patients.
Recent studies have demonstrated that neutrophil extracellular traps (NETs) play an important role in APS pathogenesis.[11] Circulating NET levels were increased in APS patients, and that neutrophils isolated from APS patients released more NETs compared with control neutrophils. Control neutrophils that are conditioned with APS patients’ sera have more pronounced NET release compared with those treated with healthy serum.[11] There is a strong association between circulation levels of NETs and traditional aPL, such as anti-β2GPI IgG and lupus anticoagulant (LAC).[11]
Our objective was to investigate the clinical and epidemiological characteristics of a large cohort of Chinese aPL carriers and to identify additional risk factors and the underlying mechanism for APS-related clinical events.
Methods
Ethics approval
The study was approved by the Institutional Review Board of Peking University People's Hospital (No. 2019PHB253–01) and was conducted in accordance with the Declaration of Helsinki guidelines for the inclusion of humans in research. Informed consent was obtained from all patients.
aPL patient cohort
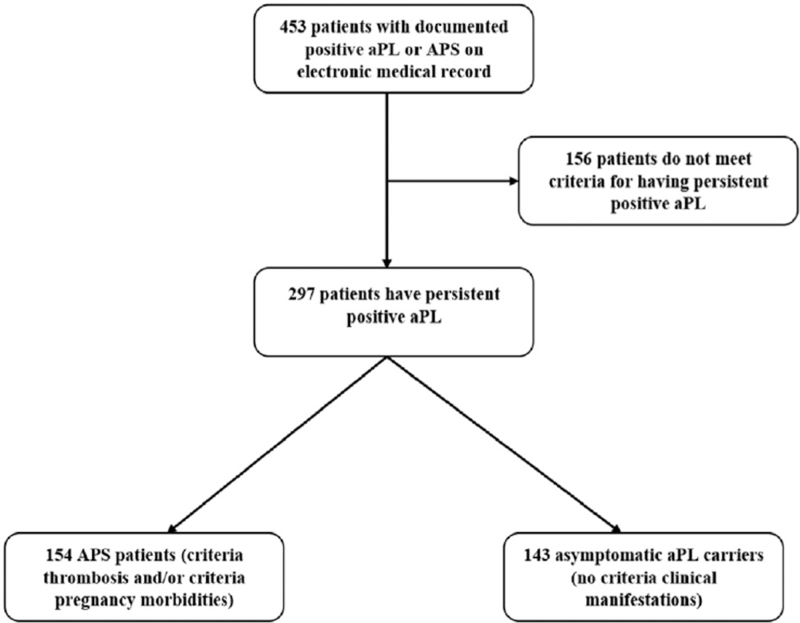
We identified 453 consecutive patients with a documented positive aPL who attended Peking University People's Hospital. Chart review was independently performed by the authors (Yu Zuo and Chun Li). Patients who did not have persistent positivity of one or more “criteria”aPL which include the IgG and IgM isotypes of the aCL or anti-β2GPI immunoassays for at least 12 weeks at People's Hospital established cut-off, as well as the LA were excluded from the study [Figure 1]. Patients who met the revised Sydney classification criteria were defined as APS patients and those who did not meet the criteria were defined as asymptomatic aPL-positive control patients. Asymptomatic aPL-positive patients included both patients with and without underlying connective tissue disease.
Figure 1.
Flow diagram of study design. aPL: Antiphospholipid antibodies; APS: Antiphospholipid syndrome.
Laboratory testing used to confirm aPL positivity
IgG and IgM of aCL and aβ2GPI were determined by enzyme-linked immunosorbent assay kit from EURO-IMMUN (Luebeck, Germany). Values for aCL >12 IU/mL and aβ2GPI >27 RU/mL were considered positive based on local cut-off. The LA assay was performed using Stago STA Compact Hemostasis System, of which the simplified Dilute Russell's Viper Venom Test (dRVVT) was performed using LA1 Screening reagent and LA2 Confirmatory reagent (STA, USA) following the manufacturer's instructions and the dRVVT ratios (LA1 screen/LA2 confirmation) >1.2 were considered positive for LA activity.
APS clinical manifestations
We classified APS-related clinical manifestations into three categories: (1) arterial thrombosis (cerebral, renal, retinal, gastrointestinal, coronary, pulmonary, or peripheral arteries); (2) venous thrombosis (deep venous thrombosis of upper or lower extremities, pulmonary emboli, renal, retinal, and gastrointestinal venous thrombosis); and (3) APS-related pregnancy morbidity.
Data collection
The medical records were reviewed to obtain patients’ demographic information and clinical information. Demographic data included age, sex, and smoking status. Clinical data included APS clinical manifestations defined above, the presence of underlying autoimmune disease, traditional risks of endothelial injury including hypertension (HTN), hyperlipidemia (HLD), diabetes, and traditional signs of endothelial injury, including Raynaud's phenomenon and livedo reticularis. Laboratory data, such as platelet count and complement levels were obtained at the time of APS diagnosis or upon establishment of care for asymptomatic carriers. Smoking status was assessed based on medical record documentation (self-reported). HTN was classified based on the 8th Joint National Committee guideline.[12] HLD was defined as fasting total cholesterol >200 mg/dL. Diabetes was defined as hemoglobin A1c ≥6.5%, or fasting glucose level ≥ 126 mg/dL, or a 2-h 75 g oral glucose tolerance test ≥200 mg/dL. Thrombocytopenia was defined as platelet count <100 × 109/L and hypocomplementemia was defined as either C3 <0.79 g/L or C4 <0.16 g/L.
Detection of NETs
Quantification of cell-free DNA and citrullinated histone H3 (Cit-H3): Cell-free DNA (Invitrogen, Thermo Fisher Scientific, P11496, USA) and Cit-H3 (Cayman, 501620) were quantified in sera according to the manufacturer's instructions. Serum samples were obtained from 30 APS patients, 30 aPL-positive patients with autoimmune disease, 30 aPL patients without autoimmune disease, and 30 healthy controls.
NETosis assay (SYTOX Green): Human neutrophil was purified from healthy volunteers as previously described.[13] For the SYTOX Green assay as previously described,[13] neutrophils were incubated with sera from aPL carriers with or without autoimmune disease and APS patients. All experiments were performed three times.
Statistical analysis
The baseline characteristics of the APS and aPL-positive control patients were summarized using means and standard deviations for continuous variables, and frequencies and proportions for categorical variables. The levels of cell-free DNA and Cit-H3 were expressed using means and standard deviations. The association between clinical risk factors and APS clinical manifestations was examined using Chi-squared or Fisher's exact test, univariate analysis, and multivariable logistic regression analyses. The significance level was set to P < 0.05. Since we were assessing risk factors associated with pregnancy morbidities, only reproductive age (age <45 years) female controls were used (N = 70). All analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Patient demographic and clinical characteristics
Among 453 patients screened, we identified 297 patients with a persistent positive aPL. One hundred and fifty-five patients are primary aPL carriers (aPL-positive individuals without classifiable systemic autoimmune diseases). Two hundred and thirty-five (79.1%) were women and 62 (20.9%) were men. The mean age for the aPL-positive patients in this cohort was 43.1 ± 15.8 years. Of the 297 patients, 154 had APS clinical manifestations and 143 were asymptomatic carriers. Of the 154 APS patients, 85 experienced arterial thrombosis, 76 experienced venous thrombosis, 26 had both arterial and venous thrombosis, 34 patients had criteria pregnancy morbidities, and 15 patients experienced both thrombosis and pregnancy morbidities. Out of the whole cohort (n = 297), 142 patients had an underlying autoimmune disease which included SLE, rheumatoid arthritis (RA), systemic sclerosis, mixed connective tissue disease, undifferentiated connective tissue disease, systemic vasculitis, and adultonset of Still's disease. The most prevalent aPL was aCL antibodies among all groups. Higher frequency of smoking and clinical diagnosis of HTN was observed among patients with APS-related thrombosis. Overall, we found >60% of patients had hypocomplementemia and about 40% had thrombocytopenia [Table 1].
Table 1.
Clinical and demographic characteristics of aPL-positive carriers.
| Characteristics | Arterial thrombosis (n = 85) | Venous thrombosis (n = 76) | Criteria pregnancy morbidities (n = 34) | Asymptomatic aPL carriers (n = 143) |
| Age (year), mean ± SD | 47.0 ± 15.6 | 44.8 ± 16.3 | 36.8 ± 11.6 | 41.8 ± 15.8 |
| Female, n (%) | 55 (64.7) | 55 (72.4) | 34 (100) | 122 (85.3) |
| Primary aPL carriers, n (%) | 32 (37.6) | 22 (28.9) | 13 (38.2) | 102 (71.3) |
| Underlying autoimmune disease, n (%) | 53 (62.4) | 54 (71.1) | 21 (61.8) | 41 (28.7) |
| SLE, n (%) | 32 (37.6) | 38 (50.0) | 12 (35.3) | 26 (18.2) |
| Smoking, n (%) | 22 (25.9) | 15 (19.7) | 0 | 8 (5.6) |
| HTN, n (%) | 37 (43.5) | 26 (34.2) | 6 (17.6) | 35 (24.5) |
| HLD, n (%) | 3 (3.5) | 3 (3.9) | 4 (11.8) | 10 (7.0) |
| Diabetes, n (%) | 14 (16.5) | 8 (10.5) | 2 (5.9) | 13 (9.1) |
| Raynaud's phenomena, n (%) | 11 (12.9) | 9 (11.8) | 4 (11.8) | 9 (6.3) |
| Livedo reticularis, n (%) | 1 (1.2) | 2 (2.6) | 1 (2.9) | 0 |
| Hypocomplementemia, n (%) | 52 (61.2) | 48 (63.2) | 26 (76.5) | 88 (61.5) |
| Thrombocytopenia, n (%) | 36 (42.4) | 31 (40.8) | 14 (41.2) | 56 (39.2) |
| Recurrent 1st trimester pregnancy loss, n (%) | N/A | N/A | 14 (41.2) | N/A |
| 2nd, 3rd trimester pregnancy loss, n (%) | N/A | N/A | 21 (61.8) | N/A |
| Pre-eclampsia, n (%) | N/A | N/A | 6 (17.6) | N/A |
| Intra-uterine growth retardation, n (%) | N/A | N/A | 3 (8.8) | N/A |
| Placenta insufficiency, n (%) | N/A | N/A | 2 (5.9) | N/A |
| aCL IgG/IgM, n (%) | 67 (78.8) | 58 (76.3) | 28 (82.4) | 100 (69.9) |
| A2GPI IgG/IgM, n (%) | 49 (57.6) | 36 (47.4) | 24 (70.6) | 54 (37.8) |
| LA, n (%) | 18 (21.2) | 17 (22.4) | 5 (14.7) | 40 (28.0) |
| Triple positive, n (%) | 11 (12.9) | 8 (10.5) | 3 (8.8) | 13 (9.1) |
aCL: Anticardiolipin; Anti-β2GPI: Anti-β2glycoprotein-I; aPL: Antiphospholipid antibodies; HLD: Hyperlipidemia; HTN: Hypertension; LA: Lupus anticoagulant; N/A: Not applicable; SLE: Systemic lupus erythematosus; Triple positive: Positive aCL, aβ2GPI, and LA.
Clinical risk factors for thrombosis and pregnancy morbidities
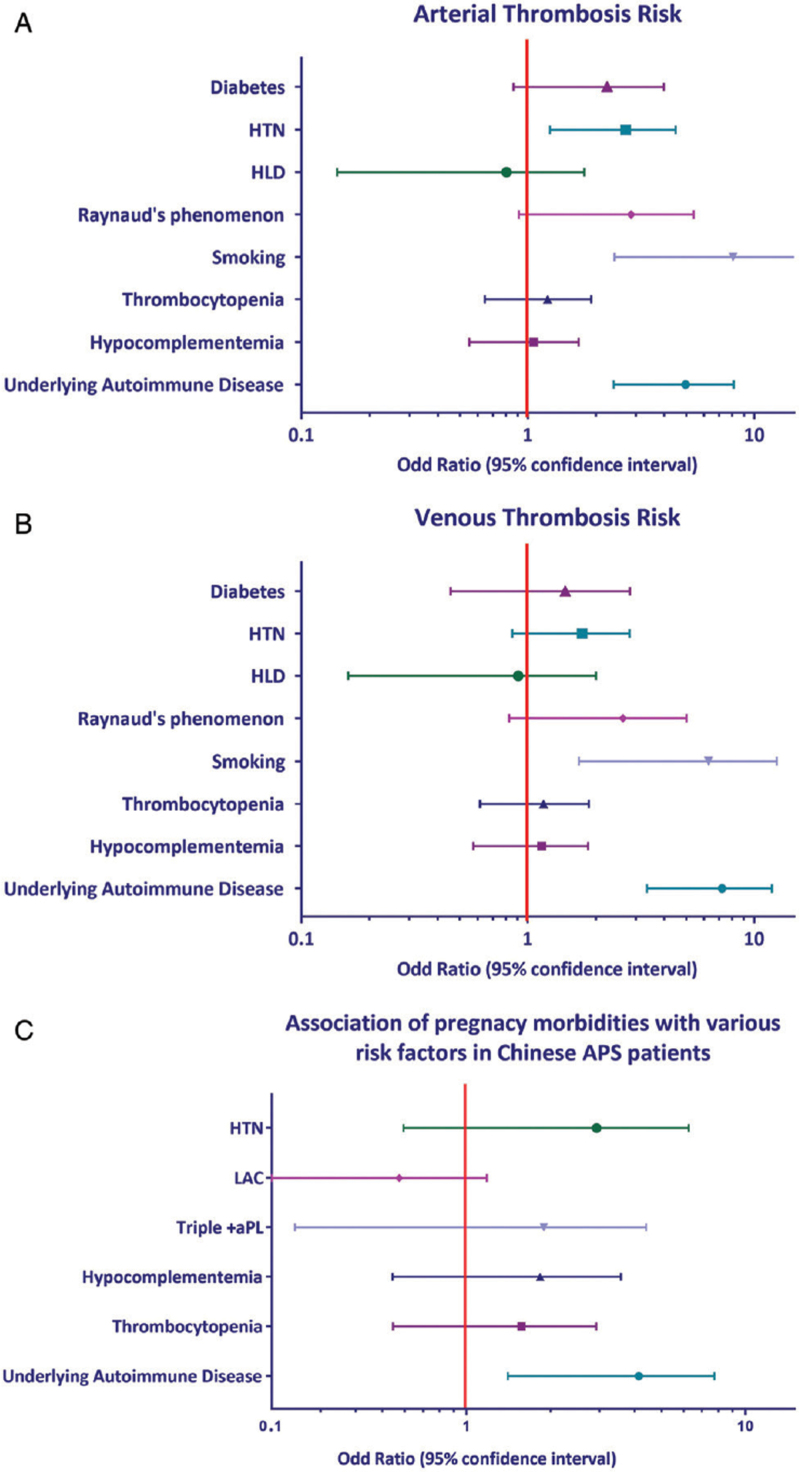
Among traditional risk factors and signs of endothelial injury, the following factors demonstrated independent association with arterial thrombosis: HTN (odds ratio [OR] = 2.368, 95% confidence interval [CI] = 1.249–4.491, P = 0.0083), smoking (OR = 6.137, 95% CI = 2.408–15.637, P = 0.0001), and the presence of underlying autoimmune disease (OR = 4.401, 95% CI = 2.387–8.113, P < 0.001). Among aPL-positive carriers, two risk factors were significantly associated with venous thrombosis: smoking (OR = 4.594, 95% CI = 1.681–12.553, P = 0.029) and the presence of underlying autoimmune disease (OR = 6.330, 95% CI = 3.355–11.940, P < 0.001). Age, diabetes, hypercholesterolemia, Raynaud's phenomena, and livedo reticularis were not significantly associated with any thromboembolic events. Similarly, thrombocytopenia and hypocomplementemia were not associated with thrombosis [Figure 2A and B]. Although evaluating pregnancy morbidities, only the presence of underlying autoimmune disease demonstrated significant association (OR = 3.301, 95% CI = 1.407–7.744, P = 0.006) [Figure 2C]. None of the laboratory characteristics showed statistical associations for pregnancy morbidities.
Figure 2.
Logistic regression analyses for arterial thrombosis, venous thrombosis, and pregnancy morbidity risk. Forest plots shows the results of multivariate analysis for additional arterial thrombosis risk (A), venous thrombosis risk (B), and pregnancy morbidities risk (C). aPL: Antiphospholipid antibodies; APS: Antiphospholipid syndrome; HLD: Hyperlipidemia; HTN: Hypertension.
Circulating NETs among APS and aPL carriers’ sera and their abilities to potentiate NETosis
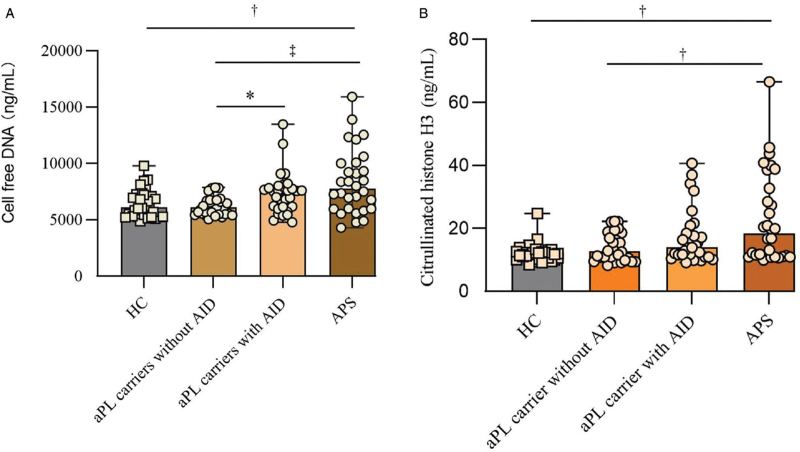
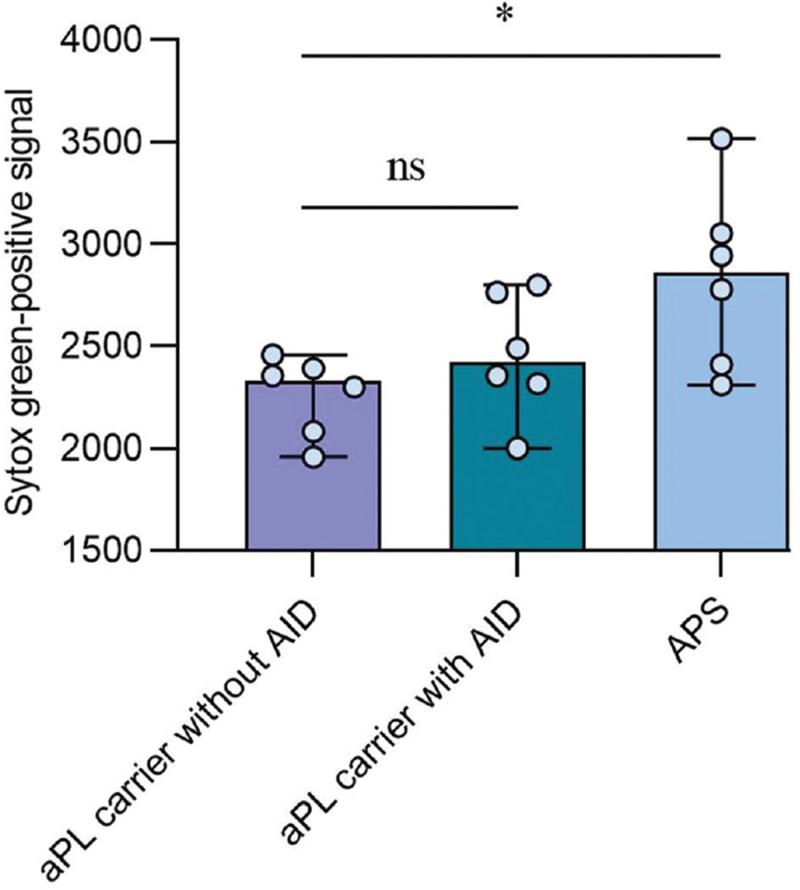
It is well known that NETs can act as a prothrombotic scaffold that promotes platelet activation and tissue factors adhesions among APS patients.[14] We next ask whether some identified additional risk factors such as underlying autoimmune disease promoted thrombosis among aPL-positive carrier dues to exaggerated NETs formation. Indeed, the levels of cell-free DNA were significantly higher among aPL patients with autoimmune disease and APS patients than aPL carriers without the autoimmune disease [Figure 3]. Similarly, levels of Cit-H3 were also higher among APS patients compared with aPL-positive carriers without underlying autoimmune disease. To confirm our observation, we assess the ability of patients’ sera from APS patients and aPL carriers at triggering NETosis in donor neutrophils. Neutrophils conditioned with APS sera have heightened NETosis potential compared with aPL carriers without the underlying autoimmune disease [Figure 4].
Figure 3.
Detection of cell-free DNA and Cit-H3 in sera of APS and aPL carriers. The level of cell-free DNA in APS patients was compared with aPL carriers with autoimmune disease, aPL carriers without autoimmune disease, and healthy controls by Mann-Whitney U test (A). The level of Cit-H3 in APS patients was compared with aPL carriers with autoimmune disease, aPL carriers without autoimmune disease, and healthy controls by Mann-Whitney U test (B); ∗P < 0.05, +P < 0.01, ‡P < 0.001. AID: Autoimmune diseases; aPL: Antiphospholipid antibodies; APS: Antiphospholipid syndrome; Cit-H3: Citrullinated histone H3.
Figure 4.
The abilities of sera from APS and aPL carriers to potentiate NETosis. The samples from three groups (n = 6) were selected to test their abilities to stimulate donor neutrophils for NET release. The extracellular DNA was measured as relative fluorescence units of SYTOX Green. Each data point represents a patient. ∗P < 0.01. aPL: Antiphospholipid antibodies; APS: Antiphospholipid syndrome; NET: Neutrophil extracellular trap.
Discussion
In this cross-sectional cohort study of aPL-positive Chinese carriers, we assessed multiple potential additional clinical, epidemiological, and laboratory risk factors for arterial thrombosis, venous thrombosis, and pregnancy morbidities. NETs might play a very important role in the pathogenesis of APS in aPL carriers. This is the largest study of Chinese aPL-positive carriers to specifically characterize potential additional risk factors for the APS clinical manifestations.
HTN has been shown in various studies to increase the risk of thrombosis in aPL-positive patients. Hypertensive aPL-positive patients had more frequent cerebral vascular accidents than normotensive aPL-positive patients.[15] Other groups reported that HTN was significantly associated with arterial thrombosis but not venous thrombosis.[7] A cross-sectional study of the Hopkins lupus cohort showed that HTN was associated with a 2.4-fold increase in the risk of arterial thrombosis among aPL-positive lupus patients.[8] A cross-sectional study of 163 Polish APS patients also demonstrated HTN as an independent risk factor for arterial thrombosis.[16] A prospective multicenter follow-up study of 258 aPL carriers showed that HTN is significantly associated with the first thrombotic event.[17] Previously published studies were all based on data from European or North American APS patient cohorts. Our study demonstrates that HTN is a potential independent risk factor for arterial thrombosis but not venous thrombosis among aPL-positive Chinese carriers.
Unlike HTN, there have been conflicting data on the role of smoking in predicting thromboembolic events. A crosssectional study of 77 APS patients and 56 asymptomatic aPL control patients showed that smoking is significantly associated with arterial thrombosis.[7] Three studies on aPL and cerebrovascular events showed that smoking is one of the most important predictors of cerebral vascular events in patients with high titer aPL.[18–20] In a recent study of 107 Chinese primary APS patients, cigarette smoking was observed as an independent risk factor for arterial thrombosis.[21] In contrast, a study of aPL-related arterial thrombosis risks did not observe a significant association between smoking and arterial thrombosis in Polish APS patients.[16] Similarly, several other reports did not detect a significant association between smoking and either arterial or venous thrombosis.[8,9] We observed a strong association of smoking with both arterial and venous thromboembolic events in our study of Chinese aPL-positive patients. We suspect the variation in the prevalence of smoking among patients from different cultural and ethnic backgrounds may contribute to the discrepancies observed in different studies. According to the World Health Organization 2014 report, China has one of the highest per capita rates of consumption of cigarettes.[22]
Inflammation plays a very important mechanistic role in hemostasis. Pro-inflammatory cytokines can cause endothelial damage, activate platelet and tissue factor, suppress anti-coagulant function, and impair fibrinolytic activities. Inflammatory mediators tip the hemostatic balance to a thrombophilia state.[23] Systemic autoimmune disease-related hypercoagulability has gained increasing attention among clinicians. A recent study evaluated the thrombophilia state of RA patients using a thrombin generation assay. Compared with healthy controls, a significantly higher thrombin generation potential was observed among both active RA patients and inactive RA patients.[24] Other studies suggest that vascular events occur more often in aPL-positive patients with lupus compared with aPL-positive patients without lupus.[25] Our study further confirms that the presence of underlying autoimmune disease is an important additional risk factor for both arterial and venous thrombosis among aPL-positive Chinese carriers. The pregnancy risk stratification and association between aPL and adverse obstetric events remains controversial. Based on the report of the 14th International Congress on Obstetric APS, currently available studies on the aPL-related pregnancy morbidities are limited by study design and inconsistent application of stringent classification criteria.[26] A recently completed multicenter, prospective, observational study of risk factors for adverse pregnancy outcomes in patients with aPL concluded that LA, the coexistence of lupus, age, nonwhite race, and prior history of thrombosis were risk factors contributing to adverse pregnancy outcomes among aPL-positive patients.[27] Another study suggested that complement activation measured by Bb and sC5b-9 levels is a strong predictor of adverse pregnancy outcomes among patients with lupus and/or positive aPL.[28] Kim et al[29] recently reported that imbalance of angiogenic factor levels, such as high fms-like tyrosine kinase level, high soluble endoglin level, and low placental growth factors level during pregnancy may serve as predictors of adverse pregnancy outcomes among lupus and/or aPL-positive patients. Among the tested traditional APS risk factors, only the presence of underlying autoimmune disease showed a significant association with pregnancy morbidities among aPL-positive Chinese patients. The coexistence of autoimmune disease remains the most consistent aPL-associated obstetric risk among various cohorts. This is the first attempt to identify additional risks for aPL-related adverse pregnancy outcomes among a large group of Asian aPL-positive carriers. Non-traditional APS risk factors, new biomarkers (i.e., complement breaking products and angiogenic factors), and noncriteria aPL need to be evaluated in future studies for their ability to predict pregnancy risk in this population.
The strengths of this study include its size, unique Chinese aPL-positive patient cohort, its rigorous clinical and laboratory definitions, and its consideration of multiple clinical, demographic, and laboratory variables. The crosssectional design is a limitation of our study design as we are unable to make causal inferences between risk factors and clinical outcomes. We are not able to account for medication use, genetic thrombophilia risks which may be confounding variables for thrombosis. Our cohort is also limited by lacking information on peripartum management and underlying autoimmune disease activities, which may be confounding variables for pregnancy morbidities.
To conclude, this is a cross-sectional comparison between Chinese APS patients and asymptomatic aPL-positive patient controls where we can identify possible additional risk factors for thromboembolic events and pregnancy morbidities. Our data support the existing literature on the additional risk conferred by HTN, smoking, and underlying systemic autoimmunity. It highlights the additional need for research in aPL-related pregnancy morbidities risk. Longitudinal assessment to evaluate chronology between risk factors and APS clinical manifestations is needed to confirm our findings. Our data may help physicians to risk stratify aPL-positive Asian patients.
Conflicts of interest
None.
Footnotes
How to cite this article: Li C, Zuo Y, Zhang S, Makris UE, Karp DR, Li Z. Additional risk factors associated with thrombosis and pregnancy morbidity in a unique cohort of antiphospholipid antibody-positive patients. Chin Med J 2022;135:658–664. doi: 10.1097/CM9.0000000000001964
Chun Li and Yu Zuo contributed equally to the work.
References
- 1.Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med 2013; 368:1033–1044. doi: 10.1056/NEJMra1112830. [DOI] [PubMed] [Google Scholar]
- 2.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 3.Zuo Y, Shi H, Li C, Knight JS. Antiphospholipid syndrome: a clinical perspective. Chin Med J 2020; 133:929–940. doi: 10.1097/CM9. 0000000000000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischetti F, Durigutto P, Pellis V, Debeus A, Macor P, Bulla R, et al. Thrombus formation induced by antibodies to beta2-glycoprotein I is complement dependent and requires a priming factor. Blood 2005; 106:2340–2346. doi: 10.1182/blood-2005-03-1319. [DOI] [PubMed] [Google Scholar]
- 5.Meroni PL, Borghi MO, Raschi E, Tedesco F. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat Rev Rheumatol 2011; 7:330–339. doi: 10.1038/nrrheum.2011.52. [DOI] [PubMed] [Google Scholar]
- 6.Bertolaccini ML, Amengual O, Andreoli L, Atsumi T, Chighizola CB, Forastiero R, et al. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmun Rev 2014; 13:917–930. doi: 10.1016/j.autrev.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Erkan D, Yazici Y, Peterson MG, Sammaritano L, Lockshin MD. A cross-sectional study of clinical thrombotic risk factors and preventive treatments in antiphospholipid syndrome. Rheumatology (Oxford) 2002; 41:924–929. doi: 10.1093/rheumatology/41.8.924. [DOI] [PubMed] [Google Scholar]
- 8.Danowski A, de Azevedo MN, de Souza Papi JA, Petri M. Determinants of risk for venous and arterial thrombosis in primary antiphospholipid syndrome and in antiphospholipid syndrome with systemic lupus erythematosus. J Rheumatol 2009; 36:1195–1199. doi: 10.3899/jrheum.081194. [DOI] [PubMed] [Google Scholar]
- 9.Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. GAPSS: the global anti-phospholipid syndrome score. Rheumatology (Oxford) 2013; 52:1397–1403. doi: 10.1093/rheumatology/kes388. [DOI] [PubMed] [Google Scholar]
- 10.Scalzi LV, Hollenbeak CS, Wang L. Racial disparities in age at time of cardiovascular events and cardiovascular-related death in patients with systemic lupus erythematosus. Arthritis Rheum 2010; 62:2767–2775. doi: 10.1002/art.27551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yalavarthi S, Gould TJ, Rao AN, Mazza LF, Morris AE, Núñez-Álvarez C, et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: A newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol 2015; 67:2990–3003. doi: 10.1002/art.39247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 13.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020; 5:e138999.doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tambralli A, Gockman K, Knight JS. NETs in APS: current knowledge and future perspectives. Curr Rheumatol Rep 2020; 22:67.doi: 10.1007/s11926-020-00936-1. [DOI] [PubMed] [Google Scholar]
- 15.Asherson RA, Khamashta MA, Gil A, Vazquez JJ, Chan O, Baguley E, et al. Cerebrovascular disease and antiphospholipid antibodies in systemic lupus erythematosus, lupus-like disease, and the primary antiphospholipid syndrome. Am J Med 1989; 86:391–399. doi: 10.1016/0002-9343(89)90335-5. [DOI] [PubMed] [Google Scholar]
- 16.Matyja-Bednarczyk A, Swadzba J, Iwaniec T, Sanak M, Dziedzina S, Kmiel A, et al. Risk factors for arterial thrombosis in antiphospholipid syndrome. Thromb Res 2014; 133:173–176. doi: 10.1016/j. thromres.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Ruffatti A, Del Ross T, Ciprian M, Bertero MT, Sciascia S, Scarpato S, et al. Risk factors for a first thrombotic event in antiphospholipid antibody carriers: a prospective multicentre follow-up study. Ann Rheum Dis 2011; 70:1083–1086. doi: 10.1136/ard.2010.142042. [DOI] [PubMed] [Google Scholar]
- 18.Verro P, Levine SR, Tietjen GE. Cerebrovascular ischemic events with high positive anticardiolipin antibodies. Stroke 1998; 29:2245–2253. doi: 10.1161/01.str.29.11.2245. [DOI] [PubMed] [Google Scholar]
- 19.Levine SR, Deegan MJ, Futrell N, Welch KM. Cerebrovascular and neurologic disease associated with antiphospholipid antibodies: 48 cases. Neurology 1990; 40:1181–1189. doi: 10.1212/wnl.40.8.1181. [DOI] [PubMed] [Google Scholar]
- 20.Hansen KE, Kong DF, Moore KD, Ortel TL. Risk factors associated with thrombosis in patients with antiphospholipid antibodies. J Rheumatol 2001; 28:2018–2024. [PubMed] [Google Scholar]
- 21.Zhao JL, Sun YD, Zhang Y, Xu D, Wang Q, Li MT, et al. The clinical manifestations and thrombotic risk factors in primary antiphospholipid syndrome (in Chinese). Chin J Intern Med 2016; 55:386–391. doi: 10.3760/cma.j.issn.0578-1426.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 22. Stewart WB, Wild CP. World Cancer Report 2014. International Agency for Research on Cancer/World Health Organization, 2014. [Google Scholar]
- 23.Margetic S. Inflammation and haemostasis. Biochem Med (Zagreb) 2012; 22:49–62. [PMC free article] [PubMed] [Google Scholar]
- 24.Solfietti L, Binello GB, Stella S, Bazzan M, Salierno M, Roccatello D. Thrombin generation assay: interactions between chronic inflammation and haemostasis in patients with autoimmune diseases. Clin Exp Rheumatol 2016; 34:925–928. [PubMed] [Google Scholar]
- 25.Laskin CA, Clark CA, Spitzer KA. Antiphospholipid syndrome in systemic lupus erythematosus: is the whole greater than the sum of its parts. Rheum Dis Clin North Am 2005; 31:255–272. doi: 10.1016/j. rdc.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 26.de Jesus GR, Agmon-Levin N, Andrade CA, Andreoli L, Chighizola CB, Porter TF, et al. 14th International Congress on Antiphospholipid Antibodies Task Force report on obstetric antiphospholipid syndrome. Autoimmun Rev 2014; 13:795–813. doi: 10.1016/j. autrev.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Buyon JP, Kim MY, Guerra MM, Laskin CA, Petri M, Lockshin MD, et al. Predictors of pregnancy outcomes in patients with lupus: a cohort study. Ann Intern Med 2015; 163:153–163. doi: 10.7326/M14-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim MY, Guerra MM, Kaplowitz E, Laskin CA, Petri M, Branch DW, et al. Complement activation predicts adverse pregnancy outcome in patients with systemic lupus erythematosus and/or antiphospholipid antibodies. Ann Rheum Dis 2018; 77:549–555. doi:10.1136/annrheumdis-2017-212224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim MY, Buyon JP, Guerra MM, Rana S, Zhang D, Laskin CA, et al. Angiogenic factor imbalance early in pregnancy predicts adverse outcomes in patients with lupus and antiphospholipid antibodies: results of the PROMISSE study. Am J Obstet Gynecol 2016; 214:108e1–108e14. doi: 10.1016/j.ajog.2015.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]