Abstract
Background:
The role of allogeneic hematopoietic stem cell transplantation (allo-HSCT) in children with high-risk (HR) T-cell acute lymphoblastic leukemia (T-ALL) in first complete remission (CR1) is still under evaluation. Moreover, relapse is the main factor affecting survival. This study aimed to explore the effect of allo-HSCT (especially haploidentical HSCT [haplo-HSCT]) on improving survival and reducing relapse for HR childhood T-ALL in CR1 and the prognostic factors of childhood T-ALL in order to identify who could benefit from HSCT.
Methods:
A total of 74 newly diagnosed pediatric T-ALL patients between January 1, 2012 and June 30, 2018 were enrolled in this retrospective study. Patients were stratified into the low-risk chemotherapy cohort (n = 16), HR chemotherapy cohort (n = 31), and HR transplant cohort (n = 27). Characteristics, survival outcomes, and prognostic factors of all patients were then analyzed.
Results:
Patient prognosis in the HR chemotherapy cohort was significantly worse than that in the low-risk chemotherapy cohort (5year overall survival [OS]: 58.5% vs. 100%, P = 0.003; 5-year event-free survival [EFS]: 54.1% vs. 83.4%, P = 0.010; 5-year cumulative incidence of relapse [CIR]: 45.2% vs. 6.3%, P = 0.011). In HR patients, allo-HSCT improved the 5-year EFS and CIR compared to that of chemotherapy (5-year EFS: 80.1% vs. 54.1%, P = 0.041; 5-year CIR: 11.6% vs. 45.2%, P = 0.006). The 5-year OS was higher in the HR transplant cohort than that in the HR chemotherapy cohort (81.0% vs. 58.5%, P = 0.084). Minimal residual disease re-emergence was an independent risk factor for 5-year OS, EFS, and CIR; age ≥10 years was an independent risk factor for OS and EFS; and high white blood cell count was an independent risk factor for EFS and CIR.
Conclusion:
Allo-HSCT, especially haplo-HSCT, could effectively reduce relapse of children with HR T-ALL in CR1.
Keywords: T-cell acute lymphoblastic leukemia, Allogeneic hematopoietic stem cell transplantation, Haploidentical, Minimal residual disease, Children
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive malignancy with a poor prognosis that accounts for 10% to 15% of all pediatric acute lymphoblastic leukemia (ALL) cases.[1–4] With the advent of various intensive combination chemotherapy regimens in recent years, the 5-year overall survival (OS) and event-free survival (EFS) rates of children with T-ALL have significantly increased to 71.9% to 91.4% and 64% to 87.8%, respectively.[2,3,5–9] However, patients with high-risk (HR) childhood T-ALL have shown unsatisfactory long-term OS and EFS rates of <50%.[10–13] Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is sometimes recommended for children showing HR characteristics at first complete remission (CR1) and is often recommended for patients at second complete remission (CR2) or later complete remission (CR).[14] However, children with T-ALL at CR2 or later CR have significantly worse prognosis even if they were treated with allo-HSCT. In a previous study from our institution, the 3-year leukemia-free survival (LFS) rate of children with TALL at CR2 or later CR was only 26.0%, and the 3-year cumulative incidence of relapse (CIR) was 56.7%.[15] Few studies have demonstrated that the dismal prognosis of HR childhood T-ALL could be improved with allo-HSCT.[10,16,17] In the German ALL-Berlin-Frankfurt-Muenster (BFM) 90 and 95 studies, the 5-year OS and 5-year disease-free survival (DFS) rates of 36 children with HR T-ALL who received allo-HSCT in CR1 were both 67%, while those of 120 children with HR T-ALL who were treated with chemotherapy alone were 47% and 42%, respectively.[10] Therefore, a risk-stratified approach to treat childhood T-ALL is warranted. In recent years, haploidentical HSCT (haplo-HSCT) has become an important alternative for many T-ALL patients undergoing transplantation who could not find a matched related or unrelated donor. Moreover, it has been proven to be a safe and effective treatment option at our institution.[15,18–21] To our knowledge, there have been fewer reports on the effects of chemotherapy compared to allo-HSCT, especially haplo-HSCT for HR childhood T-ALL, and the hierarchical criteria for HR groups remain unclear. Thus, this study aimed to explore the hierarchical criteria, prognostic factors of childhood T-ALL, and the role of allo-HSCT, especially haplo-HSCT, in children with HR T-ALL in CR1.
Methods
Ethical approval
All methods used in this study were carried out in accordance with the Helsinki Declaration of 1975. The Ethics Committee of Peking University People's Hospital approved the collection, analysis, and publication of the data (No. 2021PHB382-001). Informed consent was waived due to the retrospective nature of the study.
Patients and study design
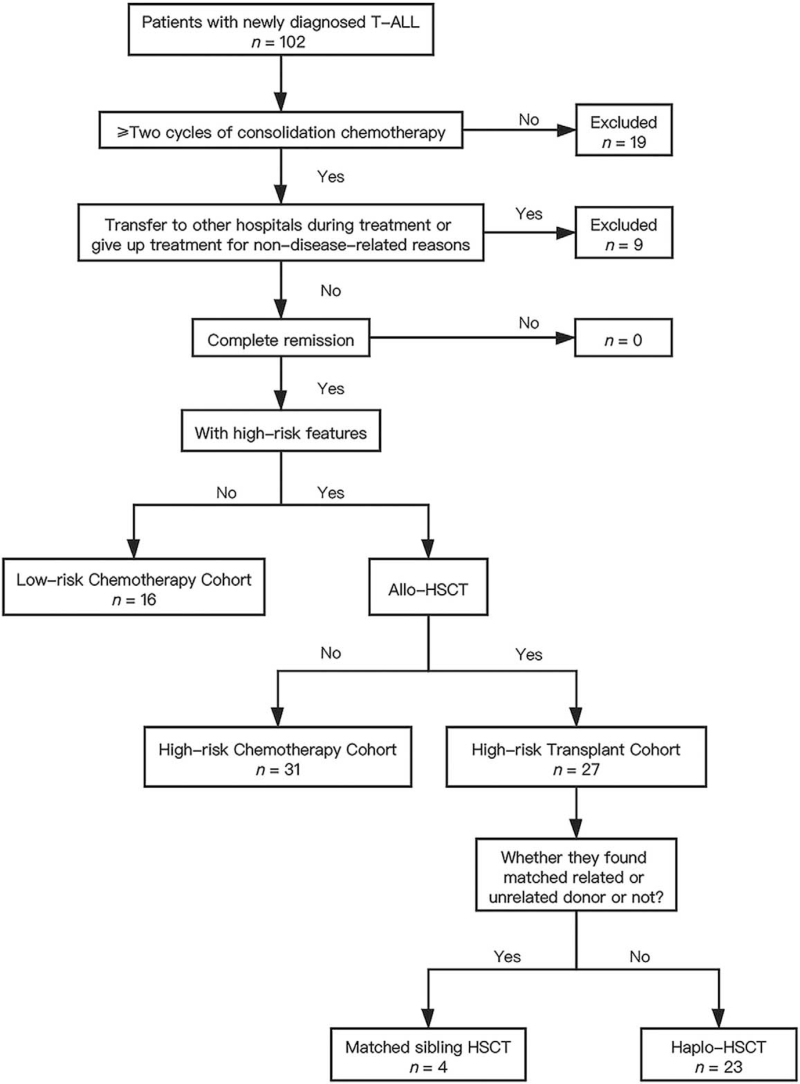
A total of 74 pediatric patients (aged 1–18 years), who were newly diagnosed with T-ALL between January 1, 2012 and June 30, 2018, were enrolled in this retrospective study. Patients were divided into different groups as shown in Figure 1. Patients were assigned to two different chemotherapy regimens: the modified ALL-BFM protocol[22] or the Chinese Children Leukemia Group (CCLG)-ALL 2008 protocol.[23] We recommended that patients in the low-risk group choose chemotherapy and those in the HR group choose bone marrow (BM) transplantation. Decisions for choosing chemotherapy or allo-HSCT were based on patient preferences. Patients who were lost to follow-up were excluded from the final analysis.
Figure 1.
Diagram of all patients enrolled in the study. ALL: Acute lymphoblastic leukemia; Allo-HSCT: Allogeneic hematopoietic stem cell transplantation; Haplo-HSCT: Haploidentical hematopoietic stem cell transplantation; HSCT: Hematopoietic stem cell transplantation; T-ALL: T-cell acute lymphoblastic leukemia.
Definition of HR group
According to the CCLG-ALL 2008 protocol and existing literature, a patient is considered HR if at least one of the following is present: (1) failure to achieve CR after induction chemotherapy; (2) minimal residual disease (MRD) level ≥1 × 10−4 in BM aspirate 3 months after initial diagnosis; (3) age ≥10 years; and (4) re-emergent MRD.[12,23]
Definitions and evaluations
CR was defined as BM leukemic blasts <5% with regenerating hematopoiesis (platelet count >100 × 109/L, neutrophils >1 × 109/L) and no localized leukemic infiltrates. In all patients, MRD of the BM was determined using flow cytometry (FCM) and quantitative polymerase chain reaction (PCR).[24–26] MRD positivity was defined as both FCM ≥1 × 10−4 and presence of other mutated genes (if positive fusion genes were detected in the initial BM specimen) in a single sample or two consecutive positive results with an interval of >2 weeks using FCM or quantitative PCR. Relapse was defined as recurrence of ≥5% BM leukemic blasts and/or localized leukemic infiltrates at any site.
OS was defined as the time from diagnosis to death from any cause or the date of final follow-up. EFS was defined as the time from diagnosis to relapse, second tumor, death, or the date of the final follow-up. CIR was calculated from CR1 to the first relapse.
Transplantation
After induction therapy (IT) and at least two rounds of consolidation therapy, patients who achieved CR1 underwent myeloablative transplant without total body irradiation (TBI) in accordance with their guardians’ wishes. The preconditioning regimen for matched transplants was a modified busulfan-cyclophosphamide (Bu-Cy) conditioning regimen that included hydroxyurea (80 mg · kg−1 · day−1, per os [p.o.], on day -10), cytarabine (2 g · m−2 · day−1, intravenous [i.v.], on day -9), Bu (3.2 mg · kg−1 · day−1, i.v., on days -8 to - 6), Cy (1.8 g · m−2 · day−1, i.v., on days -5 to -4), and methyl-N-(2-chloroethyl)-N-cyclohexyl-N-nitro-sourea (Me-CCNU, 250 mg·kg−1·
day−1, p.o., on day −3). The preconditioning regimen for haplo-transplants consisted of cytarabine (4 g · m−2 · day−1, i.v., on days −10 to −9), Bu, Cy, and Me-CCNU regimens similar to those above and anti-thymocyte immunoglobulin (ATG, 2.5 mg · kg−1 · day−1, i.v., on days −5 to −2). Granulocyte colonystimulating factor was administered to all transplant recipients to mobilize BM and peripheral blood stem cells. Short-term methotrexate, mycophenolate mofetil, and cyclosporine A were administered to all transplant recipients for preventing graft vs. host disease. Supportive care was provided as previously detailed.[15,18–21]
Statistical analyses
For comparison of clinical characteristics of the different groups, quantitative indicators were compared by analysis of variance or the Mann-Whitney U test and Kruskal-Wallis H test according to the data distribution; classification indicators were compared using the χ2 test or exact probability method (if the chi-squared test is not applicable). The Kaplan-Meier method and log-rank test were used for survival analysis. CIR was calculated by competing risk analysis. Factors with P < 0.1 in the univariate analysis were adjusted in the multivariate analysis by Cox regression model, and P < 0.05 indicated statistical significance. All statistical analyses were primarily conducted using R software packages (Bell Labs, New Providence, NJ, USA) and SPSS 26.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
The clinical characteristics of the 74 patients are summarized in Table 1. The median age of the patients was 11 years (range, 2–15 years). The last follow-up was on May 1, 2020, and the median follow-up time was 51.2 months (range, 22.1–99.3 months).
Table 1.
Characteristics of patients with T-ALL (n = 74).
| Parameters | All patients cohort (n = 74) | Low-risk chemotherapy cohort (n = 16) | High-risk chemotherapy cohort (n = 31) | High-risk transplant cohort (n = 27) | χ2 | P value |
| Age (years) | 11 (2–15) | – | 11 (3–15) | 12 (4–15) | NA | 0.261∗ |
| Gender | 1.351 | 0.520 | ||||
| Male | 52 (70.3) | 10 (62.5) | 21 (67.7) | 21 (77.8) | ||
| Female | 22 (29.7) | 6 (37.5) | 10 (32.3) | 6 (22.2) | ||
| WBC count (×109/L) | 73.7 (1.1–691.9) | 72.5 (1.8–691.9) | 87.6 (1.1–614.8) | 60.5 (1.4–629.3) | NA | 0.857† |
| Hemoglobin (g/L) | 108 (40–157) | 97 (59–124) | 104 (50–146) | 114 (40–157) | NA | 0.308‡ |
| Platelet count (×109/L) | 57 (8–461) | 51 (10–399) | 75 (12–461) | 57 (8–390) | NA | 0.463† |
| Immunophenotype | 2.203 | 0.361 | ||||
| ETP | 13 (17.6) | 2 (12.5) | 4 (12.9) | 7 (25.9) | ||
| Non-ETP | 58 (78.4) | 14 (87.5) | 26 (83.9) | 18 (66.7) | ||
| SIL/TAL1 | 5.500 | 0.162 | ||||
| Positive | 13 (17.6) | 2 (12.5) | 3 (9.7) | 8 (29.6) | ||
| Negative | 59 (79.7) | 14 (87.5) | 26 (83.9) | 19 (70.4) | ||
| TCR gene rearrangement | 3.975 | 0.364 | ||||
| Positive | 14 (18.9) | 4 (25.0) | 7 (22.6) | 3 (11.1) | ||
| Negative | 58 (78.4) | 12 (75.0) | 22 (71.0) | 24 (88.9) | ||
| WT1 | 4.463 | 0.288 | ||||
| Positive | 40 (54.1) | 11 (68.8) | 17 (54.8) | 12 (44.4) | ||
| Negative | 32 (43.2) | 5 (31.2) | 12 (38.7) | 15 (55.6) | ||
| CNSL at initial diagnosis | 2.618 | 0.261 | ||||
| Yes | 4 (5.4) | 1 (6.2) | 3 (9.7) | 0 (0) | ||
| No | 70 (94.6) | 15 (93.8) | 28 (90.3) | 27 (100) | ||
| Chemotherapy regimen | 1.466 | 0.482 | ||||
| CCLG-ALL-2008 protocol | 37 (50) | 7 (43.8) | 14 (45.2) | 16 (59.3) | ||
| Modified BFM protocol | 37 (50) | 9 (56.2) | 17 (54.8) | 11 (40.7) | ||
| CR after IT | 66 (89.2) | 16 (100) | 28 (90.3) | 22 (81.5) | 3.257 | 0.211 |
| Follow-up time (months) | 51.2 (22.1–99.3) | 48.1 (34.0–88.7) | 51.8 (22.1–92.3) | 54.6 (22.2–99.3) | NA | 0.592† |
Data are presented as median (range) or n (%).
P value was calculated by Mann-Whitney U test.
P value was calculated by Kruskal-Wallis H test.
P value was calculated by analysis of variance. ALL: Acute lymphoblastic leukemia; BFM: Berlin-Frankfurt-Muenster; CNSL: Central nervous system leukemia; CCLG-ALL-2008: Chinese Children Leukemia Group-Acute Lymphoblastic Leukemia-2008; CR: Complete remission; ETP: Early T-cell precursor; IT: Induction therapy; NA: Not available; TCR: T-cell receptor; T-ALL: T-cell acute lymphoblastic leukemia; WBC: White blood cell; WT1: Wilms tumor 1 gene.
Early treatment response
Sixty-six patients (89.2%) achieved CR at the end of induction chemotherapy and 74 (100%) eventually achieved CR. Early T-cell precursor (ETP) was a factor related to CR after induction chemotherapy (P < 0.001). Other clinical characteristics such as sex, age, white blood cell (WBC) count, SIL/TAL1 transcript, T-cell receptor gene rearrangement, Wilms tumor 1 gene positivity, and central nervous system leukemia (CNSL) at initial diagnosis had no influence on the early treatment response [Table 2].
Table 2.
Factors affecting early treatment response.
| Factors | CR | χ 2 | P value |
| Age (years) | 0.011 | 1.000 | |
| <10 | 26 (89.7) | ||
| ≥10 | 40 (88.9) | ||
| Gender | 1.764 | 0.227 | |
| Male | 48 (92.3) | ||
| Female | 18 (81.8) | ||
| WBC count (×109/L) | 2.926 | 0.132 | |
| <100 | 37 (84.1) | ||
| ≥100 | 29 (96.7) | ||
| ETP | 28.856 | <0.001 | |
| Yes | 6 (46.2) | ||
| No | 57 (98.3) | ||
| SIL/TAL1 | 1.804 | 0.473 | |
| Negative | 51 (86.4) | ||
| Positive | 13 (100) | ||
| TCR gene rearrangement | 2.038 | 0.476 | |
| Negative | 50 (86.2) | ||
| Positive | 14 (100) | ||
| WT1 | 1.596 | 0.433 | |
| Negative | 30 (93.8) | ||
| Positive | 34 (85.0) | ||
| CNSL at initial diagnosis | 0.513 | 1.000 | |
| Yes | 4 (100) | ||
| No | 62 (88.6) | ||
| Chemotherapy regimen | 0.561 | 0.711 | |
| CCLG-ALL-2008 protocol | 32 (86.5) | ||
| Modified BFM protocol | 34 (91.9) |
Data are presented as n (%). ALL: Acute lymphoblastic leukemia; BFM: Berlin-Frankfurt-Muenster; CNSL: Central nervous system leukemia; CCLG-ALL-2008: Chinese Children Leukemia Group-Acute Lymphoblastic Leukemia-2008; CR: Complete remission; ETP: Early T-cell precursor; TCR: T cell receptor; WBC: White blood cell; WT1: Wilms tumor 1 gene.
Outcomes
The study process is detailed in Figure 1. Low-risk chemotherapy, HR chemotherapy, and HR transplant cohorts included 16, 31, and 27 patients, respectively. There were no statistically significant differences in the baseline characteristics among the three cohorts [Table 1].
Of the 27 patients in the HR transplant cohort, 23 received haplo-HSCT and four had a matched sibling donor. Three of the 27 transplant recipients were MRD positive before allo-HSCT, and the remaining 24 patients were MRD negative before allo-HSCT. The median time between diagnosis and transplant was 6.4 months (range, 3.4–15.6 months).
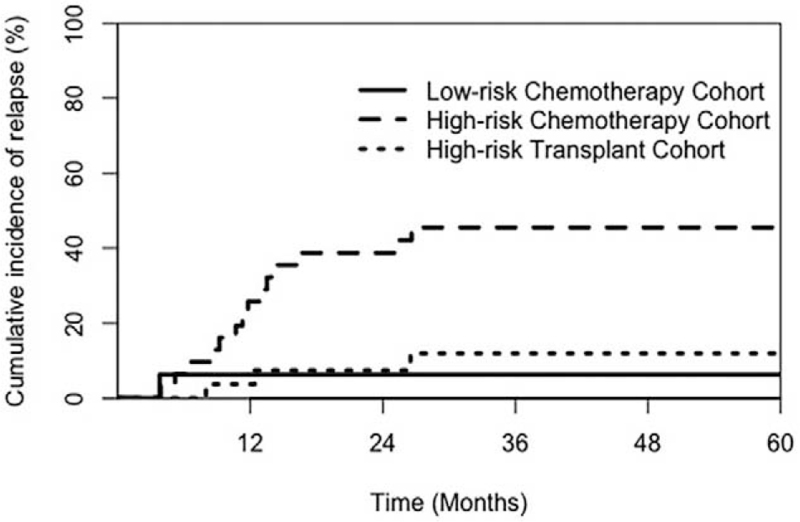
Relapse occurred in 18 patients (24.3%): 1 (6.3%), 14 (45.2%), and 3 (11.1%) in the low-risk, HR chemotherapy cohorts, and HR transplant cohort, respectively. The median time of continuous CR was 11.55 months (range, 3.9–26.6 months) in the HR chemotherapy cohort and 12.4 months (range, 8–26.5 months) in the HR transplant cohort. Fifteen patients had hematologic relapse, and three had extramedullary leukemia relapse. Twenty patients died at the median follow-up of 15.8 months (range, 8.2–59.9 months). Of these, 16 patients died due to relapse (13 in the HR chemotherapy cohort and three [haplo-HSCT] in the HR transplant cohort), three (haploidentical) died due to transplant-related complications, and one died due to severe pneumonia. Thirteen patients (17.6%) had the ETP immunophenotype; six were in the chemotherapy cohort and seven were in the transplant cohort. Of the 13 patients, two in the chemotherapy cohort (one due to relapse and one due to severe pneumonia) and two in the transplant cohort (one due to relapse and one due to transplant-related complications) died. Ten patients (13.5%) were MRD positive at 3 months; four patients were in the HR chemotherapy cohort and six were in the HR transplant cohort. Of the ten patients, three died due to relapse in the HR chemotherapy cohort and one died due to multiple organ dysfunction failure in the HR transplant cohort. All 74 patients achieved MRD-negative status before transplantation but 19 (25.7%) had re-emergent MRD. Of the 19 patients, 13 were in the HR chemotherapy cohort and six were in the HR transplant cohort. In addition, ten of the 19 patients died due to relapse (eight in the HR chemotherapy cohort and two in the HR transplant cohort). The median time for MRD reemergence was 7.9 months (range, 2.2–25.5 months).
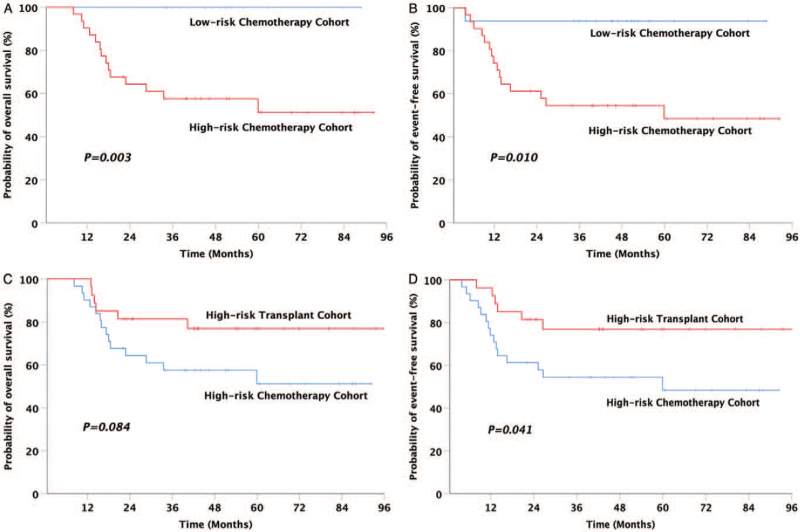
In all 74 patients, the 5-year OS, EFS, and CIR rates were 76.6%, 73.2%, and 24.6%, respectively. The 5-year OS, EFS, and CIR rates of the HR chemotherapy cohort were significantly inferior to those of the low-risk chemotherapy cohort (5-year OS: 58.5% vs. 100%, P = 0.003; 5-year EFS: 54.1% vs. 83.4%, P = 0.010; 5-year CIR: 45.2% vs. 6.3%, P = 0.011) [Figures 2A, 2B, and 3]. The prognosis of the HR transplant cohort was significantly better than that of the HR chemotherapy cohort (5-year OS: 81.0% vs. 58.5%, P = 0.084; 5-year EFS: 80.1% vs. 54.1%, P = 0.041; 5-year CIR: 11.6% vs. 45.2%, P = 0.006) [Figures 2C, 2D, and 3].
Figure 2.
Comparison of the outcomes in patients among the three cohorts. (A) OS for low-risk chemotherapy cohort (100%) vs. high-risk chemotherapy cohort (58.5%, 95% CI: 45.4–71.7%), (B) EFS for low-risk chemotherapy cohort (83.4%, 95% CI: 73.3–93.5%) vs. high-risk chemotherapy cohort (54.1%, 95% CI: 40.1–68.0%), (C) OS for high-risk chemotherapy cohort (58.5%, 95% CI: 45.4–71.7%) vs. high-risk transplant cohort (81.0%, 95% CI: 68.1–93.9%), and (D) EFS for high-risk chemotherapy cohort (54.1%, 95% CI: 40.1–68.0%) vs. high-risk transplant cohort (80.1%, 95% CI: 66.6–93.7%). CI: Confidence interval; EFS: Event-free survival; OS: Overall survival.
Figure 3.
Comparison of the 5-year CIR in patients among the three cohorts. CIR: Cumulative incidence of relapse; CI: Confidence interval.
Factors related to OS, EFS, and CIR
The univariate analysis of factors for OS, EFS, and CIR is shown in Table 3. MRD re-emergence and high WBC count were poor risk factors for OS, EFS, and CIR. Modified BFM protocol and age ≥10 years were poor risk factors for OS and EFS, while non-transplantation was a poor risk factor for CIR. The multivariate analysis revealed that MRD re-emergence was an independent risk factor for OS, EFS, and CIR, age ≥10 years was an independent poor risk factor for OS and EFS, and high WBC count was an independent poor risk factor for EFS and CIR, and nontransplantation was an independent poor risk factor for CIR [Table 4].
Table 3.
Univariate analysis of factors associated with long-term prognosis in all T-ALL patients (n = 74).
| Factors | n | 5-year OS (%) | P value | 5-year EFS (%) | P value | 5-year CIR (%) | P value |
| Age (years) | |||||||
| <10 | 29 | 84.6 (76.4–92.9) | 0.013 | 78.3 (67.2–89.5) | 0.080 | 17.2 (6.1–33.1) | 0.250 |
| ≥10 | 45 | 68.0 (56.3–79.7) | 66.6 (54.3–78.9) | 29.5 (16.8–43.4) | |||
| Gender | |||||||
| Male | 52 | 77.9 (67.8–88.0) | 0.655 | 75.4 (64.7–86.1) | 0.451 | 21.4 (11.4–33.5) | 0.350 |
| Female | 22 | 72.3 (57.1–87.4) | 66.4 (49.5–83.4) | 32.4 (14.0–52.5) | |||
| WBC (×109/L) | |||||||
| <100 | 44 | 82.3 (72.4–92.2) | 0.090 | 81.8 (71.6–92.0) | 0.015 | 14.0 (5.6–26.2) | 0.006 |
| ≥100 | 30 | 67.2 (53.1–81.2) | 59.5 (44.1–74.9) | 40.2 (22.5–57.3) | |||
| Hemoglobin (g/L) | |||||||
| <100 | 25 | 82.3 (69.0–95.6) | 0.445 | 77.4 (62.1–92.7) | 0.609 | 20.0 (7.1–37.6) | 0.740 |
| ≥100 | 43 | 70.6 (60.3–80.9) | 67.8 (56.8–78.8) | 26.2 (13.9–40.2) | |||
| Platelet count (×109/L) | |||||||
| <50 | 29 | 71.9 (58.3–85.6) | 0.345 | 67.6 (52.9–82.4) | 0.327 | 24.8 (10.7–41.9) | 0.760 |
| ≥50 | 40 | 80.8 (70.3–91.4) | 77.9 (66.4–89.4) | 22.5 (11.0–36.4) | |||
| ETP | |||||||
| Yes | 13 | 65.7 (48.4–83.1) | 0.846 | 64.4 (46.3–82.6) | 0.924 | 16.7 (2.3–42.6) | 0.380 |
| No | 58 | 77.7 (68.2–87.1) | 73.5 (63.1–83.8) | 26.0 (15.5–37.9) | |||
| SIL/TAL1 | |||||||
| Negative | 59 | 76.3 (66.6–85.9) | 0.829 | 72.4 (62.0–82.7) | 0.703 | 24.2 (14.0–35.9) | 0.950 |
| Positive | 13 | 75.3 (57.4–93.3) | 74.4 (55.5–93.3) | 23.1 (5.1–48.5) | |||
| TCR gene rearrangement | |||||||
| Negative | 58 | 78.6 (69.1–88.1) | 0.587 | 74.7 (64.5–85.0) | 0.686 | 22.6 (12.8–34.2) | 0.640 |
| Positive | 14 | 66.0 (46.6–85.3) | 64.5 (44.2–84.8) | 29.3 (8.3–54.6) | |||
| WT1 | |||||||
| Negative | 32 | 73.0 (59.5–86.5) | 0.538 | 66.5 (51.8–81.2) | 0.275 | 28.7 (14.0–45.2) | 0.360 |
| Positive | 40 | 75.5 (65.8–85.1) | 74.3 (64.0–84.6) | 20.0 (9.3–33.7) | |||
| CNSL at initial diagnosis | |||||||
| Yes | 4 | 48.0 (13.3–82.7) | 0.183 | 46.6 (10.5–82.7) | 0.294 | 50.0 (2.3–88.1) | 0.210 |
| No | 70 | 77.8 (69.2–86.4) | 74.2 (64.9–83.5) | 23.2 (14.0–33.7) | |||
| CR after IT | |||||||
| Yes | 66 | 78.9 (70.2–87.6) | 0.105 | 75.4 (65.9–84.8) | 0.164 | 22.9 (13.5–33.6) | 0.360 |
| No | 8 | 40.1 (26.1–54.2) | 36.4 (20.4–52.5) | 41.7 (6.9–75.1) | |||
| MRD after IT | |||||||
| Negative | 55 | 76.4 (67.0–85.7) | 0.816 | 72.2 (61.9–82.4) | 0.978 | 25.5 (14.8–33.7) | 0.620 |
| Positive | 16 | 64.5 (47.9–81.2) | 63.8 (46.7–81.0) | 19.8 (4.4–43.1) | |||
| MRD at 3 months | |||||||
| Negative | 63 | 79.4 (70.6–88.2) | 0.163 | 75.6 (66.0–85.2) | 0.299 | 22.4 (13.0–33.5) | 0.530 |
| Positive | 10 | 63.4 (37.8–88.9) | 62.3 (35.8–88.7) | 31.4 (6.3–61.6) | |||
| MRD re-emergence | |||||||
| Yes | 19 | 56.0 (38.3–73.6) | 0.005 | 48.9 (30.3–67.5) | 0.002 | 57.9 (32.0–76.9) | <0.001 |
| No | 55 | 83.5 (74.6–92.4) | 81.3 (71.7–90.8) | 12.9 (5.6–23.3) | |||
| Chemotherapy regimen | |||||||
| CCLG-ALL-2008 protocol | 37 | 81.1 (72.1–90.1) | 0.046 | 77.9 (67.6–88.3) | 0.060 | 16.4 (6.5–30.2) | 0.100 |
| Modified BFM protocol | 37 | 68.1 (55.2–81.0) | 64.6 (50.9–78.2) | 32.8 (18.2–48.2) | |||
| Transplant | |||||||
| Yes | 27 | 81.0 (68.1–93.9) | 0.505 | 80.1 (66.6–93.7) | 0.272 | 11.6 (2.8–27.4) | 0.050 |
| No | 47 | 69.5 (59.5–79.6) | 64.7 (53.8–75.7) | 31.9 (19.1–45.5) |
Data are presented as mean percentage of incidence (95% CI). ALL: Acute lymphoblastic leukemia; BFM: Berlin-Frankfurt-Muenster; CNSL: Central nervous system leukemia; CCLG-ALL-2008: Chinese Children Leukemia Group-Acute Lymphoblastic Leukemia-2008; CR: Complete remission; CI: Confidence interval; CIR: Cumulative incidence of relapse; ETP: Early T-cell precursor; EFS: Event-free survival; IT: Induction therapy; MRD: Minimal residual disease; OS: Overall survival; TCR: T-cell receptor; T-ALL: T-cell acute lymphoblastic leukemia; WBC: White blood cell; WT1: Wilms tumor 1 gene.
Table 4.
Multivariate analysis of factors associated with long-term prognosis in all T-ALL patients (n = 74).
| OS | EFS | CIR | ||||
| Variable | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value |
| Age ≥10 years | 5.798 (1.659–20.256) | 0.006 | 3.555 (1.267–9.975) | 0.016 | 3.008 (0.992–9.123) | 0.052 |
| WBC ≥100 × 109/L | 1.917 (0.782–4.703) | 0.155 | 2.765 (1.166–6.554) | 0.021 | 3.815 (1.582–9.200) | 0.003 |
| MRD re-emergence | 2.942 (1.176–7.359) | 0.021 | 3.636 (1.471–8.986) | 0.005 | 6.061 (1.963–18.713) | 0.002 |
| CCLG-ALL-2008 protocol | 0.454 (0.168–1.231) | 0.121 | 0.595 (0.229–1.545) | 0.287 | 0.756 (0.278–2.056) | 0.580 |
| Transplant | 0.548 (0.206–1.460) | 0.229 | 0.436 (0.165–1.153) | 0.094 | 0.227 (0.068–0.757) | 0.016 |
Data are presented as hazard ratio (95% CI). ALL: Acute lymphoblastic leukemia; CCLG-ALL-2008: Chinese Children Leukemia Group-Acute Lymphoblastic Leukemia-2008; CI: Confidence interval; CIR: Cumulative incidence of relapse; EFS: Event-free survival; MRD: Minimal residual disease; OS: Overall survival; T-ALL: T-cell acute lymphoblastic leukemia; WBC: White blood cell.
Discussion
In recent years, high-dose and multi-agent chemotherapy regimens have improved the outcomes of childhood TALL. In this study, we explored the hierarchical criteria, prognostic factors of childhood T-ALL, and the role of allo-HSCT in the treatment of children with HR T-ALL in CR1. The 5-year OS and EFS rates in 74 enrolled patients were 76.6% and 73.2%, respectively, which are compa-rable to those reported by other centers.[2,3,5–8]
ETP was a poor risk factor related to CR after induction chemotherapy. The Italian Association of Pediatric Hematology Oncology (AIEOP) centers’ study confirmed that ETP-ALL had poor early treatment response, and ETP-ALL patients obtained favorable outcomes due to administration of cyclophosphamide, 6-mercaptopurine, and cytarabine.[27] In the Children's Oncology Group (COG) AALL0434 study, 1144 patients were divided into three groups (ETP, near-ETP, and non-ETP). There were no statistically significant differences in the 5-year OS and EFS rates among the three groups, which showed a lack of significance of the ETP immunophenotype in pediatric T-ALL.[28] In our study, the number of patients with ETP was relatively small. ETP was not a risk factor affecting prognosis, possibly because our intensive chemotherapy regimen contained cytarabine, cyclophosphamide, and 6-mercaptopurine. Moreover, 7/13 ETP patients underwent transplantation, and it is possible that transplantation has also improved the prognosis of patients with the ETP immunophenotype.
HR T-ALL prognosis remains unsatisfactory[10–13]; hence, a risk-stratified approach to treat childhood T-ALL is warranted. In childhood ALL, age, WBC count, and response to treatment were independent risk factors. However, the prognostic factors are different between B-cell acute lymphoblastic leukemia (B-ALL) and T-ALL.[29] Herein, according to the CCLG-ALL 2008 protocol and existing literature, we considered CR after IT, MRD at 3 months, MRD re-emergence, and age ≥10 years as the hierarchical criteria.[12,23]
Patients with BM leukemic blasts >25% after induction chemotherapy, age >10 years, or those with T-ALL were considered to be at particular risk.[30] Failure of IT is rare in pediatric ALL (<2% of patients), but those who experience IT failure may have a worse outcome.[30,31] In our study, eight patients did not achieve CR at the end of induction chemotherapy, and three patients eventually died due to relapse.
Children with T-ALL have poor tolerance to chemotherapy and an increased risk of extramedullary relapse; as a result, they are generally older than children with B-ALL. This indicates that older age at presentation may lead to a worse prognosis in patients with T-ALL.[2] In this study, age ≥10 years was an independent risk factor affecting the 5-year OS and EFS rates, indicating that children ≥10 years of age have a worse prognosis and are more likely to experience relapse. In the univariate analysis, the P value corresponding to the age ≥10 years for EFS showed a downward trend that was not statistically significant, indicating that patients ≥10 years of age may have poorer tolerance to chemotherapy and are more likely to experience treatment complications.
The initial WBC count is an important factor that affects ALL prognosis. In successive EORTC-CLG 58881 and 58951 trials, HR T-ALL patients were identified based on their WBC count at presentation, central nervous system (CNS) positivity, and treatment response.[6] However, in the UK trial UKALL 2003, EFS was inversely related to WBC count in patients with B-ALL but not in those with T-ALL.[32] The Nordic Society of Pediatric Hematology and Oncology[32] and COG[33] also reported that the initial WBC count was not a risk factor in T-ALL patients. In this study, high initial WBC count was an independent risk factor that affected the 5-year EFS and CIR rates, indicating that children with high initial WBC counts may be at greater risk of relapse. However, the 5-year OS was unaffected by this factor, possibly owing to the application of intensive combination chemotherapy and BM transplantation.
In childhood T-ALL, genetic subtypes such as SIL/TAL1 and t(v; 11q23)/MLL rearranged are not meaningful, but MRD is a significant factor related to long-term outcomes in most cooperative group studies.[1,2] Improved risk stratification eliminated the previous independent prognostic significance of gender and CNSL, whereas the MRD level after IT emerged as a risk-stratifying feature.[34] A large percentage of childhood T-ALL patients have detectable MRD after induction chemotherapy; however, they could have had a favorable outcome if MRD converted to negative at post-consolidation.[12] The AIEOP-BFM 2000 trial showed an excellent 7-year EFS in childhood T-ALL patients with positive MRD after induction and MRD converting to negative at day 78. Conversely, T-ALL patients who were MRD positive at day 78 had a relatively high 7-year CIR and were considered for HSCT at CR1.[1,12,28,29] In this study, MRD positivity at 3 months was not an independent risk factor, possibly due to the small sample size and the application of allo-HSCT. However, MRD re-emergence was an independent risk factor affecting 5-year OS, EFS, and CIR rates. Most of the patients who had re-emergent MRD treated with chemotherapy alone relapsed and eventually died, but those who had re-emergent MRD and chose chemotherapy combined with transplantation rarely relapsed, which indicated that patients with MRD reemergence during treatment had a relatively high relapse risk that seriously affected the prognosis.
Although intensive combination chemotherapy regimens are now widely used, allo-HSCT is still valuable for the treatment of pediatric T-ALL. Allo-HSCT should be strongly recommended for childhood T-ALL patients with positive MRD after consolidation.[1] It is suggested that patients with continuous CR and low-level MRD undergo allo-HSCT.[1,12,35] In one study, childhood T-ALL patients older than 6 years who received allo-HSCT had a favorable survival compared to those who received chemothera-py.[31] The German ALL-BFM 90 and 95 studies reported that the 5-year DFS in the allo-HSCT group was significantly higher than that in the chemotherapy group.[10] In the AIEOP ALL 2000 study, children with T-ALL seemingly benefitted from allo-HSCT, resulting in a relatively high 5-year DFS.[16] For all children presenting with T immunophenotype, irrespective of other very highrisk features, 5-year disease-free survival was 47.9% in children assigned chemotherapy compared with 62.2% in those assigned related- donor transplantation.[17] A previous study conducted at our institution showed a relatively higher 3-year LFS and lower 3-year CIR in HR childhood T-ALL patients who received haplo-HSCT in CR1.[15] In this study, the outcomes in the low-risk chemotherapy cohort showed that the therapeutic effects exceeded those of international studies. Patients in the HR chemotherapy cohort had significantly worse outcomes, and the prognosis in the HR transplant cohort was significantly better than that in the HR chemotherapy cohort, which demonstrated good risk stratification of patients in this cohort. More importantly, the long-term survival of patients in the HR transplant cohort in our study was relatively excellent when compared with that in the treatment of HR childhood T-ALL in other international collaborative groups. Meanwhile, the therapeutic effect in this study exceeded the level shown in our previous institutional study, which may have been due to the improvement of transplantation technology. It is suggested that allo-HSCT was an effective strategy to reduce relapse, and it had the tendency to improve the long-term survival in childhood HR T-ALL in CR1.
In previous international studies, the conditioning regimen was usually based on TBI.[10,16,17] However, the associated side effects were significant. Recently, allo-HSCT without TBI has been proven effective for childhood ALL.[36] It has been demonstrated that CNS relapse of childhood ALL could be effectively prevented by risk-adjusted chemotherapy without cranial radiotherapy.[29,37] In this study, the patients who received allo-HSCT with a TBI-free Bu-based conditioning regimen had excellent outcomes.
However, this study had limitations because it was a nonrandomized retrospective study with a small sample size performed in a single center. In addition, two different chemotherapy regimens were applied to patients which may have caused bias; however, there were no statistically significant differences in terms of long-term survival between patients treated with those two regimens.
In conclusion, the results of our study indicate that the effect of chemotherapy for children with HR T-ALL in CR1 is still unsatisfactory. Allo-HSCT, especially haplo-HSCT, could be a feasible option, as it effectively reduced relapse of children with HR T-ALL in CR1. However, the results should be confirmed by further prospective, multicenter, randomized controlled clinical trials.
Funding
The present study was supported by the 2018 Beijing Municipal Key Clinical Specialty Construction ProjectPediatrics (No. 2199000726).
Conflicts of interest
None.
Footnotes
How to cite this article: Zhang Y, Bai L, Cheng Y, Lu A, Wang Y, Wu J, Zhang X, Zuo Y, Xu L, Jia Y, Huang X, Zhang L. Haploidentical hematopoietic stem cell transplantation may improve long-term survival for children with high-risk T-cell acute lymphoblastic leukemia in first complete remission. Chin Med J 2022;135:940–949. doi: 10.1097/CM9.0000000000001999
Yongzhan Zhang and Lu Bai contributed equally to this work.
References
- 1.Teachey DT, O’Connor D. How I treat newly diagnosed T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma in children. Blood 2020; 135:159–166. doi: 10.1182/blood.2019001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teachey DT, Pui CH. Comparative features and outcomes between paediatric T-cell and B-cell acute lymphoblastic leukaemia. Lancet Oncol 2019; 20:e142–e154. doi: 10.1016/s1470-2045(19)30031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raetz EA, Teachey DT. T-cell acute lymphoblastic leukaemia. Hematol Am Soc Hematol Educ Program 2016; 2016:580–588. doi: 10.1182/asheducation-2016.1.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet 2008; 371:1030–1043. doi: 10.1016/s0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 5.Karrman K, Johansson B. Pediatric T-cell acute lymphoblastic leukemia. Genes Chromosomes Cancer 2017; 56:89–116. doi: 10.1002/gcc.22416. [DOI] [PubMed] [Google Scholar]
- 6.Hofmans M, Suciu S, Ferster A, Van Vlierberghe P, Mazingue F, Sirvent N, et al. Results of successive EORTC-CLG 58,881 and 58,951 trials in paediatric T-cell acute lymphoblastic leukaemia (ALL). BrJ Haematol 2019; 186:741–753. doi: 10.1111/bjh.15983. [DOI] [PubMed] [Google Scholar]
- 7.Winter SS, Dunsmore KP, Devidas M, Wood BL, Esiashvili N, Chen Z, et al. Improved survival for children and young adults with T-lineage acute lymphoblastic leukemia: results from the Children's Oncology Group AALL0434 Methotrexate Randomization. J Clin Oncol 2018; 36:2926–2934. doi: 10.1200/JCO.2018.77.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asselin BL, Devidas M, Wang C, Pullen J, Borowitz MJ, Hutchison R, et al. Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: a randomized study by the Children's Oncology Group (POG 9404). Blood 2011; 118:874–883. doi: 10.1182/blood-2010-06-292615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns MA, Place AE, Stevenson KE, Gutierrez A, Forrest S, Pikman Y, et al. Identification of prognostic factors in childhood T-cell acute lymphoblastic leukemia: results from DFCI ALL Consortium Protocols 05-001 and 11-001. Pediatr Blood Cancer 2021; 68:e28719.doi: 10.1002/pbc.28719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrauder A, Reiter A, Gadner H, Niethammer D, Klingebiel T, Kremens B, et al. Superiority of allogeneic hematopoietic stem-cell transplantation compared with chemotherapy alone in high-risk childhood T-cell acute lymphoblastic leukemia: results from ALL-BFM 90 and 95. J Clin Oncol 2006; 24:5742–5749. doi: 10.1200/JCO.2006.06.2679. [DOI] [PubMed] [Google Scholar]
- 11.Dunsmore KP, Devidas M, Linda SB, Borowitz MJ, Winick N, Hunger SP, et al. Pilot study of nelarabine in combination with intensive chemotherapy in high-risk T-cell acute lymphoblastic leukemia: a report from the Children's Oncology Group. J Clin Oncol 2012; 30:2753–2759. doi: 10.1200/jco.2011.40.8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schrappe M, Valsecchi MG, Bartram CR, Schrauder A, Panzer-Grumayer R, Moricke A, et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood 2011; 118:2077–2084. doi: 10.1182/blood-2011-03-338707. [DOI] [PubMed] [Google Scholar]
- 13.Willemse MJ, Seriu T, Hettinger K, d’Aniello E, Hop WCJ, Panzer-Grumayer ER, et al. Detection of minimal residual disease identifies differences in treatment response between T-ALL and precursor BALL. Blood 2002; 99:4386–4393. doi: 10.1182/blood.v99.12.4386. [DOI] [PubMed] [Google Scholar]
- 14.Hochberg J, Khaled S, Forman SJ, Cairo MS. Criteria for and outcomes of allogeneic haematopoietic stem cell transplant in children, adolescents and young adults with acute lymphoblastic leukaemia in first complete remission. Br J Haematol 2013; 161:27–42. doi: 10.1111/bjh.12239. [DOI] [PubMed] [Google Scholar]
- 15.Xu ZL, Huang XJ, Liu KY, Chen H, Zhang XH, Han W, et al. Haploidentical hematopoietic stem cell transplantation for paediatric high-risk T-cell acute lymphoblastic leukaemia. Pediatr Transplant 2016; 20:572–580. doi: 10.1111/petr.12704. [DOI] [PubMed] [Google Scholar]
- 16.Conter V, Valsecchi MG, Parasole R, Putti MC, Locatelli F, Barisone E, et al. Childhood high-risk acute lymphoblastic leukemia in first remission: results after chemotherapy or transplant from the AIEOP ALL 2000 study. Blood 2014; 123:1470–1478. doi: 10.1182/blood-2013-10-532598. [DOI] [PubMed] [Google Scholar]
- 17.Balduzzi A, Valsecchi MG, Uderzo C, De Lorenzo P, Klingebiel T, Peters C, et al. Chemotherapy versus allogeneic transplantation for very-high-risk childhood acute lymphoblastic leukaemia in first complete remission: comparison by genetic randomisation in an international prospective study. Lancet 2005; 366:635–642. doi: 10.1016/s0140-6736(05)66998-x. [DOI] [PubMed] [Google Scholar]
- 18.Liu D, Huang X, Liu K, Xu L, Chen H, Han W, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for treatment of hematological malignancies in children. Biol Blood Marrow Transplant 2008; 14:469–477. doi: 10.1016/j. bbmt.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant 2006; 38:291–297. doi: 10.1038/sj.bmt.1705445. [DOI] [PubMed] [Google Scholar]
- 20.Liu DH, Xu LP, Liu KY, Wang Y, Chen H, Han W, et al. Long-term outcomes of unmanipulated haploidentical HSCT for paediatric patients with acute leukaemia. Bone Marrow Transplant 2013; 48:1519–1524. doi: 10.1038/bmt.2013.99. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Chen YH, et al. Haploidentical/mismatched hematopoietic stem cell transplantation without in vitro T cell depletion for T cell acute lymphoblastic leukemia. Biol Blood Marrow Transplant 2012; 18:716–721. doi: 10.1016/j.bbmt.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Xue YJ, Cheng YF, Lu AD, Wang Y, Zuo YX, Yan CH, et al. Allogeneic hematopoietic stem cell transplantation, especially haploidentical, may improve long-term survival for high-risk pediatric patients with philadelphia chromosome-positive acute lymphoblastic leukemia in the tyrosine kinase inhibitor era. Biol Blood Marrow Transplant 2019; 25:1611–1620. doi: 10.1016/j. bbmt.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Cui L, Li ZG, Chai YH, Yu J, Gao J, Zhu XF, et al. Outcome of children with newly diagnosed acute lymphoblastic leukemia treated with CCLG-ALL 2008: the first nation-wide prospective multicenter study in China. Am J Hematol 2018; 93:913–920. doi: 10.1002/ajh.25124. [DOI] [PubMed] [Google Scholar]
- 24.Pui CH, Pei D, Raimondi SC, Coustan-Smith E, Jeha S, Cheng C, et al. Clinical impact of minimal residual disease in children with different subtypes of acute lymphoblastic leukemia treated with response-adapted therapy. Leukemia 2017; 31:333–339. doi: 10.1038/leu.2016.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parekh C, Gaynon PS, Abdel-Azim H. End of induction minimal residual disease alone is not a useful determinant for risk stratified therapy in pediatric T-cell acute lymphoblastic leukemia. Pediatr Blood Cancer 2015; 62:2040–2043. doi: 10.1002/pbc.25582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng SH, Lau KM, Li CK, Chan NPH, Ip RKL, Cheng CK, et al. Minimal residual disease-based risk stratification in Chinese childhood acute lymphoblastic leukemia by flow cytometry and plasma DNA quantitative polymerase chain reaction. PLoS One 2013; 8:e69467.doi: 10.1371/journal.pone.0069467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conter V, Valsecchi MG, Buldini B, Parasole R, Locatelli F, Colombini A, et al. Early T-cell precursor acute lymphoblastic leukaemia in children treated in AIEOP centres with AIEOP-BFM protocols: a retrospective analysis. Lancet Haematol 2016; 3:e80–e86. doi: 10.1016/s2352-3026(15)00254-9. [DOI] [PubMed] [Google Scholar]
- 28.Hefazi M, Litzow MR. Recent advances in the biology and treatment of T cell acute lymphoblastic leukemia. Curr Hematol Malig Rep 2018; 13:265–274. doi: 10.1007/s11899-018-0455-9. [DOI] [PubMed] [Google Scholar]
- 29.Patrick K, Vora A. Update on biology and treatment of T-cell acute lymphoblastic leukaemia. Curr Opin Pediatr 2015; 27:44–49. doi: 10.1097/mop.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 30.Merli P, Algeri M, Del Bufalo F, Locatelli F. Hematopoietic stem cell transplantation in pediatric acute lymphoblastic leukemia. Curr Hematol Malig Rep 2019; 14:94–105. doi: 10.1007/s11899-019-00502-2. [DOI] [PubMed] [Google Scholar]
- 31.Schrappe M, Hunger SP, Pui CH, Saha V, Gaynon PS, Baruchel A, et al. Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med 2012; 366:1371–1381. doi: 10.1056/NEJMoa1110169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaitkeviciene G, Forestier E, Hellebostad M, Heyman M, Jonsson OG, Lähteenmäki PM, et al. High white blood cell count at diagnosis of childhood acute lymphoblastic leukaemia: biological background and prognostic impact. Results from the NOPHO ALL-92 and ALL-2000 studies. Eur J Haematol 2011; 86:38–46. doi: 10.1111/j.1600-0609.2010.01522.x. [DOI] [PubMed] [Google Scholar]
- 33.Hastings C, Gaynon PS, Nachman JB, Sather HN, Lu X, Devidas M, et al. Increased post-induction intensification improves outcome in children and adolescents with a markedly elevated white blood cell count (≥200 × 10(9) /l) with T cell acute lymphoblastic leukaemia but not B cell disease: a report from the Children's Oncology Group. Br J Haematol 2015; 168:533–546. doi: 10.1111/bjh.13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmiegelow K, Forestier E, Hellebostad M, Heyman M, Kristinsson J, Soderhall S, et al. Long-term results of NOPHO ALL-92 and ALL-2000 studies of childhood acute lymphoblastic leukemia. Leukemia 2010; 24:345–354. doi: 10.1038/leu.2009.251. [DOI] [PubMed] [Google Scholar]
- 35.O’Connor D, Moorman AV, Wade R, Hancock J, Tan RMR, Bartram J, et al. Use of minimal residual disease assessment to redefine induction failure in pediatric acute lymphoblastic leukemia. J Clin Oncol 2017; 35:660–667. doi: 10.1200/jco.2016.69.6278. [DOI] [PubMed] [Google Scholar]
- 36.Hamidieh AA, Monzavi SM, Kaboutari M, Behfar M, Esfandbod M. Outcome analysis of pediatric patients with acute lymphoblastic leukemia treated with total body irradiation-free allogeneic hematopoietic stem cell transplantation: comparison of patients with and without central nervous system involvement. Biol Blood Marrow Transplant 2017; 23:2110–2117. doi: 10.1016/j.bbmt.2017.08.036. [DOI] [PubMed] [Google Scholar]
- 37.Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 2009; 360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]