Abstract
Biological Cr(VI) reduction was studied in anaerobic sediments from an aquifer in Norman, Okla. Microcosms containing sediment and mineral medium were amended with various electron donors to determine those most important for biological Cr(VI) reduction. Cr(VI) (about 340 μM) was reduced with endogenous substrates (no donor), or acetate was added. The addition of formate, hydrogen, and glucose stimulated Cr(VI) reduction compared with reduction in unamended controls. From these sediments, an anaerobic Cr(VI)-utilizing enrichment was obtained that was dependent upon hydrogen for both growth and Cr(VI) reduction. No methane was produced by the enrichment, which reduced about 750 μM Cr(VI) in less than six days. The dissolved hydrogen concentration was used as an indicator of the terminal electron accepting process occurring in the sediments. Microcosms with sediments, groundwater, and chromate metabolized hydrogen to a concentration below the detection limits of the mercury vapor gas chromatograph. In microcosms without chromate, the hydrogen concentration was about 8 nM, a concentration comparable to that under methanogenic conditions. When these microcosms were amended with 500 μM Cr(VI), the dissolved hydrogen concentration quickly fell below the detection limits. These results showed that the hydrogen concentration under chromate-reducing conditions became very low, as low as that reported under nitrate- and manganese-reducing conditions, a result consistent with the free energy changes for these reactions. The utilization of formate, lactate, hydrogen, and glucose as electron donors for Cr(VI) reduction indicates that increasing the availability of hydrogen results in a greater capacity for Cr(VI) reduction. This conclusion is supported by the existence of an enrichment dependent upon hydrogen for growth and Cr(VI) reduction.
More than 170,000 tonnes of chromium wastes are released annually, mainly due to industrial practices, including electroplating, leather tanning, pigment manufacture, corrosion inhibition, and fungicide production (11). These industries generate large quantities of waste, which must be treated before discharge. The widespread use of chromium as well as the improper disposal of by-products and wastes has led to areas of serious environmental contamination, with chromium presently being listed as one of the contaminants in 635 Superfund sites (U. S. Environmental Protection Agency, Office of Health and Environmental Assessment [http://www.epa.gov/ngispgm3/iris]).
Chromium can exist in six valence states, from 0 to +6, but is generally encountered as the trivalent [Cr(III)] or hexavalent [Cr(VI)] form (24). Trivalent chromium, an essential trace element in the human diet, has relatively low toxicity and is nearly insoluble at neutral pH. Thus, it is nearly immobile in the environment (3). Conversely, hexavalent chromium is acutely toxic, mutagenic, teratogenic, and carcinogenic. In addition, Cr(VI) is soluble and, thus, highly mobile in the environment (5, 8). Focusing on its toxicity and exposure potential, the U.S. Environmental Protection Agency recently designated chromium, as well as its compounds, as one of seventeen chemicals posing the greatest threat to human health (U. S. Environmental Protection Agency, Office of Health and Environmental Assessment).
The valence state and relative solubility of chromium are dependent on a variety of environmental conditions (redox potential, pH, and temperature) and the presence of other organic and inorganic molecules (14, 23). Oxidation-reduction (redox) reactions can greatly influence the fate and mobility of these organic and inorganic compounds in both pristine and contaminated aquifers. Many of the significant redox reactions taking place in aquifers, such as nitrate reduction, Fe(III) reduction, sulfate reduction, and methane production, are microbially catalyzed. Lovley and Goodwin (18) have proposed that H2 concentrations in groundwater may indicate which terminal electron accepting process (TEAP) is dominant at a given site, with each TEAP having a characteristic range for its dissolved hydrogen concentration. The dissolved hydrogen concentrations reported for specific terminal electron accepting processes are as follows: methanogenesis, 7 to 10 nM; sulfate reduction, 1 to 1.5 nM; Fe(III) reduction, 0.2 nM; Mn(IV) or nitrate reduction, <0.05 nM (detection limit) (18).
Many bacterial strains have been shown to mediate reduction of Cr(VI) to Cr(III) both aerobically (7, 13, 15, 16) and anaerobically (17, 19, 20 25, 28, 30, 32); however, few studies have examined the in situ potential of microbial reduction in aquifers (12). The stimulation of existing microbial populations with bioavailable electron donors may result in microbial chromium reduction, potentially preventing the migration, and reducing the toxicity, of Cr(VI) in aquifers. However, it is not clear whether highly reducing conditions (e.g., sulfate reducing, iron reducing, or methanogenic) are needed, or whether microbial Cr(VI) reduction will occur under more oxidized conditions (aerobic or nitrate reducing). Electron donors used as stimulants for microbially mediated Cr(VI) reduction in aquifers, as well as the prevailing H2 concentration during Cr(VI) reduction, were assessed in this study.
MATERIALS AND METHODS
Sample collection and microcosm construction.
Subsurface sediments from an aquifer underlying the municipal landfill in Norman, Okla., were collected by digging to the top of the water table (5 to 6 ft below the surface) with a post-hole digger and collecting aquifer material in sterile glass jars. The jars were filled to capacity, sealed, and transported to the laboratory, where they were stored in an anaerobic chamber at room temperature until used.
Microcosms were prepared in an anaerobic glove box (Coy Laboratory Products, Inc., Ann Arbor, Mich.) by placing 5 g (wet weight) of sediment into sterile serum bottles (160-ml capacity) and adding 50 ml of sterile, anaerobic mineral medium. The composition of the mineral medium has been previously described by Tanner (27) and was prepared according to the procedure described by Balch and Wolfe (4). No reductant was added to the microcosms. All serum bottles were sealed with butyl rubber stoppers, and the gas headspace was exchanged with 80% N2–20% CO2 (≈125 kPa); the final pH of the microcosms was 7.2. Each microcosm typically had an initial Cr(VI) concentration of approximately 500 μM, added from a sterile stock of K2CrO4, which was replenished after complete reduction. Triplicate microcosms were used for each treatment, and heat-killed controls were included for each experiment. The heat-killed controls were autoclaved twice at 121°C for 20 min and then amended with 20 mg of HgCl2 per liter. All incubations were carried out at room temperature in the dark. Samples were collected regularly from each microcosm.
Effect of electron donor.
To determine the effect of electron donor additions, microcosms were incubated without a donor until the rate of Cr(VI) reduction slowed in order to exhaust any endogenous electron donor present in the sediment. After 161 days, the microcosms were amended with donor and reamended with Cr(VI) before an additional 360-day incubation period began. During this second incubation period, microcosms were reamended with Cr(VI) on one occasion, 217 days after donor addition. Statistical differences between the mean Cr(VI) reductions for each donor, compared with the no donor control, were determined with the Student t test (P = 0.05).
Enrichment for Cr(VI)-reducing, H2-utilizing consortium.
A Cr(VI)-reducing, H2-utilizing enrichment was obtained from aquifer sediment and serially transferred in a mineral salts medium with an 80% H2–20% CO2 (≈125 kPa) headspace and an initial Cr(VI) concentration of approximately 750 μM. The mineral medium contained the following components (per liter of deionized water): Tanner's metal solution (27), 5 ml; Tanner's vitamin solution (27), 10 ml; Pfennig's mineral solution (22), 10 ml; yeast extract, 0.1 g; and NaHCO3, 10 g. The enrichment was maintained by transferring a 1 to 5% inoculum to fresh media bimonthly.
Determination of H2 concentration under Cr(VI)-reducing conditions.
Microcosms were constructed as described above, with 5 g (wet weight) of sediment, 50 ml of groundwater and 500 μM Cr(VI). Hydrogen was added with a Hamilton syringe to give an initial soluble H2 concentration of approximately 300 nM (≈0.8%). Microcosms were stored in a stationary position at room temperature in the dark.
Analytical methods.
The Cr(VI) concentration was determined colorimetrically by reaction with diphenylcarbazide in acid solution, having a detection limit of approximately 5 μM Cr(VI) (9, 31). The coefficient of variation was 4.7%. Hydrogen was quantitated with a mercury vapor reduction gas analyzer (26). Fatty acids were analyzed with a high-performance liquid chromatograph (HPLC) equipped with a Bio-Rad Aminex HPX-87H column (300 by 7.8 mm) and an isocratic mobile phase of 0.016 N H2SO4 at a flow rate of 0.9 ml/min (2). Benzoate was analyzed with an HPLC equipped with an Alltech Econosphere C18 column (250 by 4.6 mm; reversed phase) and a UV detector set at 254 nm (Alltech Inc., Deerfield, Ill.). The HPLC was operated at a flow rate of 1.0 ml/min with a mobile phase of 80% (vol/vol) sodium acetate (50 mM, pH 4.5) and 20% (vol/vol) acetonitrile. At the conclusion of each experiment, methane production was measured by gas chromatography (6).
RESULTS
Effect of electron donor.
The addition of formate, hydrogen, and glucose to aquifer sediments stimulated Cr(VI) reduction compared to that in unamended controls (Table 1). Cr(VI) reduction in microcosms amended with lactate, benzoate, and acetate was not significantly different from that observed in microcosms with no exogenous donor. Little loss of Cr(VI) occurred in heat-killed controls, indicating that reduction of Cr(VI) in viable microcosms was microbiologically mediated.
TABLE 1.
Effect of electron donor on chromate reduction by aquifer material
| Donor | Initial [Cr(VI)] (μM) | Final [Cr(VI)]a (μM) | Total [Cr(VI)] added (μM) | Total [Cr(VI)] reducedb (μM) | % of electrons available from donord |
|---|---|---|---|---|---|
| Heat-killed control | 509 ± 42e | 404 ± 38 | 510 ± 42 | 106 ± 11 | |
| No donor | 458 ± 9 | 115 ± 159 | 820 ± 128 | 344 ± 166 | |
| Formate | 699 ± 190 | 83 ± 134 | 1,174 ± 179 | 1,050 ± 73c | 17 |
| Lactate | 615 ± 143 | 5 ± 1 | 932 ± 322 | 899 ± 370 | 16 |
| Hydrogen | 561 ± 37 | 5 ± 1 | 1,018 ± 259 | 895 ± 250c | 2.3 |
| Glucose | 568 ± 63 | 245 ± 48 | 1,068 ± 165 | 823 ± 166c | 0.25 |
| Benzoate | 740 ± 246 | 230 ± 218 | 892 ± 180 | 510 ± 63 | 80 |
| Acetate | 549 ± 67 | 255 ± 31 | 549 ± 67 | 294 ± 48 | 0 |
The concentration of Cr(VI) was measured after a 360-day incubation period.
The value shown is additive on the basis of Cr(VI) amendments. Data have been corrected for Cr(VI) loss prior to donor addition.
Significantly different from the value for the unamended control.
The amount of electrons available for Cr(VI) reduction for each donor was calculated by multiplying the amount of electrons produced if the donor was completely oxidized to CO2 by the moles of electron donor used and then by subtracting this value from the amount of electrons present in each product (e.g., amount of electrons released if the product was oxidized to CO2) multiplied by the amount of product detected. The percent of electrons available from the donor was then calculated by dividing the amount of electrons used for Cr(VI) reduction [three times the total Cr(VI) reduced after subtracting the amount of Cr(VI) reduced in no-donor controls] by the amount of electrons available for Cr(IV) reduction (see above) multiplied by 100.
Values shown are mean ± standard deviation.
Measurement of the donors revealed that formate (2.1 mM) was completely utilized and that the 1.6 mM lactate added was converted to about 1.2 mM acetate. Hydrogen, initially 80% of the gas headspace, was below detection limits as measured by mercury vapor gas chromatography at the conclusion of the experiment. Glucose (approximately 58 mM) was converted to about 80 mM acetate, 4 mM propionate, 9 mM isobutyrate, and 3 mM butyrate. Taking into account methane production (≈1.8 mmol) and an assumed production of CO2 (one CO2 molecule produced for every two carbon compounds produced) resulted in a glucose carbon recovery of about 91%. Approximately 62 μM of the 639 μM benzoate added was used and no depletion of acetate (2.1 mM) occurred during the 360-day incubation period. At the conclusion of the experiment, methane was measured. Microcosms supplied with H2 produced 0.84 mmol of methane, and glucose-amended microcosms produced 1.8 mmol of methane. Lactate- and benzoate-amended microcosms produced 0.04 mmol of methane; 0.05 mmol of methane was formed when formate was the donor. Little methane (0.001 mmol) was detected in acetate-amended and non-acetate-amended microcosms. Cr(VI) reduction accounted for about 80% of the electrons available from benzoate and about 16 to 17% of the electrons available from lactate and formate. Glucose- and hydrogen-amended microcosms were primarily fermentative and methanogenic, respectively, with these processes accounting for the majority of the electrons available.
Cr(VI)-reducing, H2-utilizing consortium.
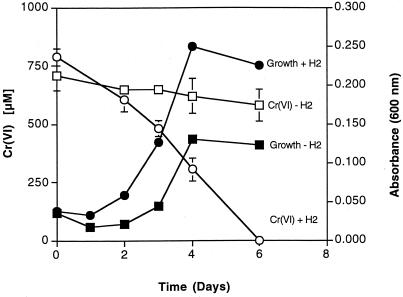
From these sediments a Cr(VI)-reducing, H2-utilizing enrichment was obtained, which was maintained sediment-free for over two years. This enrichment was able to reduce approximately 750 μM Cr(VI) in 6 days under growing conditions when hydrogen was supplied as the donor (Fig. 1). Some growth, but no chromium reduction, occurred in the absence of hydrogen.
FIG. 1.
Growth and chromate reduction by H2-utilizing, Cr(VI)-reducing enrichment. (Error bars represent the standard deviation of triplicate samples; if there are no error bars, then standard deviation is less than the width of the data point.)
Growth and Cr(VI) reduction by the enrichment were completely inhibited in the presence of 10 mM formaldehyde. The enrichment was able to grow and reduce Cr(VI) when either 10 mM bromoethanesulfonic acid (BESA), an inhibitor of methanogenic bacteria, or 10 mM molybdate, an inhibitor of sulfate-reducing bacteria, was present. However, molybdate appeared to partially inhibit growth of the enrichment, since the optical densities were not equivalent to those of cultures with no inhibitor present. In addition, cultures grown with molybdate required an additional day to completely reduce the Cr(VI). No methane was produced by the enrichment at any time. Based on the lack of methane production and tolerance to BESA it was concluded that methanogenic bacteria were not important members of this consortium. The fact that molybdate had little effect on growth or Cr(VI) reduction argues against the possibility that Cr(VI) reduction was the result of the cycling of low levels of sulfate to S2−, which could then react with Cr(VI). Neither growth nor Cr(VI) reduction was affected by the addition of 40 mM NaCl, which was included as an ionic strength control (data not shown).
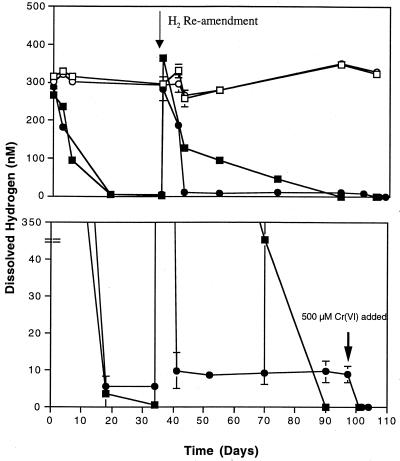
H2 concentration under Cr(VI)-reducing conditions.
The dissolved hydrogen concentration in the presence of Cr(VI) was below detection limits (Fig. 2). This has also been observed when either nitrate or manganese serves as the terminal electron acceptor (18). After 34 days, bottles were reamended with hydrogen and the concentrations again fell to below detection limits. No loss of Cr(VI) could be measured due to the small amount of added hydrogen, and no methane was detected when Cr(VI) was present. Microcosms to which no chromate was added had a dissolved hydrogen concentration of 5.6 nM, which falls in the range reported for methanogenesis as the terminal electron accepting process (5 to 10 nM) (18). Upon reamendment with hydrogen, the concentration of dissolved hydrogen fell to 9.7 nM and was stable for over 50 days. The addition of 500 μM Cr(VI) to microcosms that previously had not received Cr(VI) caused the hydrogen concentration to drop from 9.7 nM to below detection levels, as was found for the other Cr(VI)-amended microcosms (Fig. 2). Stoichiometric balances between hydrogen consumption and Cr(VI) reduction were not possible since the amount of hydrogen added would result in very little Cr(VI) reduction. However, previous experiments showed a decrease in hydrogen concentration concomitant with Cr(VI) reduction.
FIG. 2.
Hydrogen concentrations in aquifer microcosms in the presence and absence of Cr(VI). (The lower graph has an expanded axis of the upper graph.) Symbols: □, heat-killed control with Cr(VI); ○, heat-killed control without Cr(VI); ▪, live incubation with Cr(VI); ●, live incubation without Cr(VI).
DISCUSSION
Cr(VI) is a strong oxidant that can be reduced abiotically in the presence of electron donors commonly found in soils, such as aqueous Fe(II), ferrous iron minerals, reduced sulfur, and soil organic matter (10, 24). In addition to abiotic reduction, many microorganisms have been shown to mediate reduction of Cr(VI) to the trivalent form (17, 33). However, under the conditions used in this study, namely a mixed culture with sediment, it would be difficult to determine whether Cr(VI) reduction occurred by direct enzymatic reduction of the chromate anion or by the continual production of small amounts of Fe(II) or S2−, which can then abiotically react with Cr(VI). However, the establishment of a sediment-free enrichment in medium with very low levels of sulfate and Fe(II) is consistent with the conclusion that direct microbial reduction of Cr(VI) occurred. Also, the addition of molybdate to the enrichment resulted in slight inhibition of Cr(VI) reduction, suggesting that sulfur cycling was not an important mechanism for Cr(VI) reduction in the enrichment.
Regardless of the mechanism of Cr(VI) reduction, our studies show the potential for stimulating Cr(VI) reduction by naturally occurring microorganisms. Addition of electron donors that increase the bioavailable hydrogen for microbial use, such as formate, hydrogen, and glucose, resulted in a greater extent of Cr(VI) reduction. Benzoate, which is thermodynamically more difficult to degrade with hydrogen production, and acetate, which may be degraded without any hydrogen production, did not stimulate Cr(VI) reduction to the extent of the other electron donors. Thus, the addition of suitable electron donors may be an effective method for treating chromium contamination in aquifers owing to stimulation of organisms indigenous to the aquifer. The existence of a Cr(VI)-reducing enrichment that is dependent upon H2 for both growth and Cr(VI) reduction supports the conclusion that increasing the availability of hydrogen promotes greater reduction of Cr(VI).
This study documents the dissolved hydrogen concentration during Cr(VI)-reducing conditions. Our results indicate that very low hydrogen concentrations occur under Cr(VI)-reducing conditions, as has been reported for nitrate- and manganese-reducing conditions (18). Furthermore, the dissolved hydrogen concentration in methanogenic microcosms fell below the detection limits within 2 days of Cr(VI) addition. The fact that very low hydrogen concentrations were observed in Cr(VI)-amended microcosms, similar to that reported for nitrate- and manganese-reducing conditions, is logical given the Gibbs free energy changes for these reduction reactions when coupled to hydrogen oxidation (Table 2) (1, 29). The Gibbs free energy change for Cr(VI) reduction is less favorable than for nitrate or manganese reduction but much more favorable than for sulfate reduction or methanogenesis. This is also consistent with our previous experiments, in which Cr(VI) was added in combination with NO3−, Fe(III), and SO42− (21). Cr(VI) reduction was found to occur simultaneously with nitrate reduction but prior to iron or sulfate reduction. When Cr(VI) was present, no Fe(II) or S2− production was observed. However, in the absence of Cr(VI), nitrate utilization was followed by Fe(II) production and, later, sulfate reduction.
TABLE 2.
Gibbs free energies for terminal electron accepting processes coupled to H2 oxidation
| Reaction | ΔG′ (kJ/mol of e−)a |
|---|---|
| 1/2 MnO2 (s) + H+ + 1/2 H2 → 1/2 Mn2+ + H2O | −94.06 |
| 1/5 NO3− + 1/5 H+ + 1/2 H2 → 1/10 N2 (g) + 3/5 H2O | −86.44 |
| 1/3 CrO42− + 5/3 H+ + 1/2 H2 → 1/3 Cr3+ + 4/3 H2O | −45.02 |
| Fe(OH)3(am) + 2 H+ + 1/2 H2 → Fe2+ + 3 H2O | −43.89 |
| 1/8 SO42− + 1/8 H+ + 1/2 H2 → 1/8 HS− + 1/2 H2O | −0.42 |
| 1/8 HCO3− + 1/8 H+ + 1/2 H2 → 1/8 CH4 + 3/8 H2O | +0.67 |
All values are taken from the data of Ahmann (1), except for methane production, which was calculated on the basis of data from Thauer et al. (29). Partial pressure of hydrogen (PH2) = 10−6.6 atm; PN2 = 0.78 atm; PO2 = 0.21 atm; PCH4 = 2.45 × 10−4 atm; pH = 7.0; [Mn2+] = [NO3−] = [CrO42] = [Cr3+] = [Fe2+] = [SO42−] = [HS−] = [HCO3−] = 10−6 M.
Understanding the processes that stimulate naturally occurring microorganisms to reduce Cr(VI) is essential to improving present remediation strategies for contaminated sites by optimizing Cr(VI)-reducing conditions. For example, addition of electron donors that make more hydrogen available for microbial use, thereby resulting in a greater extent of Cr(VI) reduction, may offer a straightforward approach to the treatment of Cr(VI)-contaminated aquifers. Second, the very low H2 concentrations observed during Cr(VI) reduction (Fig. 2) and the observation that Cr(VI) reduction occurs before iron or sulfate reduction (21) suggest that highly reducing conditions (e.g., iron-reducing, sulfate-reducing, or methanogenic conditions) will not likely be required for Cr(VI) reduction.
ACKNOWLEDGMENTS
Support for this research was provided by the U.S. Department of Energy (grants DE-FG03–96ER202/14 and DE-FG02–97ER62478).
REFERENCES
- 1.Ahmann D. Bioremediation of metal-contaminated soil. Soc Ind Microbiol News. 1997;47:218–233. [Google Scholar]
- 2.Amos D A, McInerney M J. Growth of Syntrophomonas wolfei on short chain unsaturated fatty acids. Arch Microbiol. 1990;154:31–36. [Google Scholar]
- 3.Anderson R A, Kozlovsky A S. Chromium uptake, absorption, and excretion of subjects consuming self-selected diets. Am J Clin Nutr. 1985;41:1177–1183. doi: 10.1093/ajcn/41.6.1177. [DOI] [PubMed] [Google Scholar]
- 4.Balch W E, Wolfe R S. New approach to the cultivation of bacteria: 2-mercaptoethanesulfonic acid (Hs-CoM)-dependent Methanobacterium ruminantium in a pressurized atmosphere. Appl Environ Microbiol. 1976;32:781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett R J. Chromium cycling in soils and water: links, gaps, and methods. Environ Health Perspect. 1991;92:17–24. doi: 10.1289/ehp.919217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beeman R E, Suflita J M. Microbial ecology of a shallow unconfined ground water aquifer polluted by municipal landfill leachate. Microb Ecol. 1987;14:39–54. doi: 10.1007/BF02011569. [DOI] [PubMed] [Google Scholar]
- 7.Bopp L H, Ehrlich H L. Chromate resistance and reduction in Pseudomonas fluorescens strain LB300. Arch Microbiol. 1988;150:426–431. [Google Scholar]
- 8.Cervantes C. Bacterial interactions with chromate. Antonie Leeuwenhoek. 1991;59:229–233. doi: 10.1007/BF00583675. [DOI] [PubMed] [Google Scholar]
- 9.Clesceri L S, Greenberg A E, Trussel R R, editors. Standard methods for the examination of water and wastewater, 17th ed. Washington, D.C.: American Public Health Association; 1989. [Google Scholar]
- 10.Eary L E, Rai D. Chromate removal from aqueous wastes by reduction with ferrous iron. Environ Sci Technol. 1988;22:972–977. doi: 10.1021/es00173a018. [DOI] [PubMed] [Google Scholar]
- 11.Gadd G M, White C. Microbial treatment of metal pollution—a working biotechnology? Trends Biotechnol. 1993;11:353–359. doi: 10.1016/0167-7799(93)90158-6. [DOI] [PubMed] [Google Scholar]
- 12.Gvozdyak P I, Mogilevich N F, Ryl'skii A F, Grishchenko N I. Reduction of hexavalent chromium by collection strains of bacteria. Microbiology. 1987;55:770–773. [Google Scholar]
- 13.Horitsu H, Futo S, Miyazawa Y, Ogai S, Kawai K. Enzymatic reduction of hexavalent chromium by hexavalent chromium tolerant Pseudomonas ambigua G-1. Agric Biol Chem. 1987;51:2417–2420. [Google Scholar]
- 14.Katz S. The analytical biochemistry of chromium. Environ Health Perspect. 1991;92:13–16. doi: 10.1289/ehp.919213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kvasnikov E I, Stepanyuk V V, Klyushnikova T M, Serpokrylov N S, Simonova G A, Kasatkina T P, Pachenko L P. A new chromium reducing, gram-variable bacterium with mixed type of flagellation. Microbiology. 1985;54:69–75. [Google Scholar]
- 16.Lebedeva E V, Lyalikova N N. Reduction of crocoite by Pseudomonas chromatophilia sp. nov. Mikrobiologiya. 1979;48:517–522. [PubMed] [Google Scholar]
- 17.Lovley D R. Dissimilatory metal reduction. Annu Rev Microbiol. 1993;47:263–290. doi: 10.1146/annurev.mi.47.100193.001403. [DOI] [PubMed] [Google Scholar]
- 18.Lovley D R, Goodwin S. Hydrogen concentrations as an indicator of the predominant terminal electron-accepting reactions in aquatic sediment. Geochim Cosmochim Acta. 1988;52:2993–3003. [Google Scholar]
- 19.Lovley D R, Phillips E J P. Reduction of chromate by Desulfovibrio vulgaris and its c3 cytochrome Appl. Environ Microbiol. 1994;60:726–728. doi: 10.1128/aem.60.2.726-728.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovley D, Widman P, Woodward J, Phillips E. Reduction of uranium by cytochrome c3 of Desulfovibrio vulgaris. Appl Environ Microbiol. 1993;59:3572–3576. doi: 10.1128/aem.59.11.3572-3576.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsh T L, Leon N C, McInerney M J. Physiochemical factors affecting chromate reduction by aquifer materials. Geomicrobiol J. 2000;17:291–303. [Google Scholar]
- 22.McInerney M J, Bryant M P, Pfennig N. Anaerobic bacterium that degrades fatty acids in syntrophic association with methanogens. Arch Microbiol. 1979;122:129–135. doi: 10.1007/s002030050685. [DOI] [PubMed] [Google Scholar]
- 23.Moore J W, Ramamoorthy S. Chromium. In: Moore J W, Ramamoorthy S, editors. Heavy metals in natural waters. New York, N.Y: Springer-Verlag; 1984. pp. 58–76. [Google Scholar]
- 24.Palmer C D, Puls R W. Natural attenuation of hexavalent chromium in ground water and soils. U.S. EPA/540/S-94/505. U.S. Cincinnati, Ohio: Environmental Protection Agency; 1994. [Google Scholar]
- 25.Romanenko V I, Koren'kov V N. A pure culture of bacteria utilizing chromates and bichromates as hydrogen acceptors in growth under anaerobic conditions. Mikrobiologiya. 1977;46:414–417. [PubMed] [Google Scholar]
- 26.Seiler W, Giehl H, Roggendorf P. Detection of carbon monoxide and hydrogen by conversion of mercury oxide to mercury vapor. Atmos Technol. 1980;12:40–45. [Google Scholar]
- 27.Tanner R S. Monitoring sulfate-reducing bacteria: comparison of enumeration media. J Microbiol Methods. 1989;10:83–90. [Google Scholar]
- 28.Tebo B M, Obraztsova A Y. Sulfate-reducing bacterium grows with Cr(VI), U(VI), Mn(IV), and Fe(III) as electron acceptors. FEMS Microbiol Lett. 1998;162:193–198. [Google Scholar]
- 29.Thauer R K, Jungermann K, Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tucker M D, Barton L L, Thomas B M. Reduction of Cr, Mo, Se and U by Desulfovibrio desulfuricans immobilized in polyacrylamide gels. J Ind Microbiol Biotechnol. 1998;20:13–19. doi: 10.1038/sj.jim.2900472. [DOI] [PubMed] [Google Scholar]
- 31.Urone P F. Stability of colorimetric reagent for chromium, s-diphenylcarbazide, in various solvents. Anal Chem. 1955;27:1354–1355. [Google Scholar]
- 32.Wang P, Mori T, Komori K, Sasatsu M, Toda K, Ohtake H. Isolation and characterization of an Enterobacter cloacae strain that reduces hexavalent chromium under anaerobic conditions. Appl Environ Microbiol. 1989;55:1665–1669. doi: 10.1128/aem.55.7.1665-1669.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y T, Shen H. Bacterial reduction of hexavalent chromium. J Ind Microbiol. 1995;14:159–163. doi: 10.1007/BF01569898. [DOI] [PubMed] [Google Scholar]