Abstract
Introduction and Aim:
The prognostic role of neutrophil to lymphocyte ratio (NLR) has been explored extensively in the literature. The aim of this meta-analysis was to evaluate the link between NLR and lymph node metastasis in gastric cancer. A method for increasing specificity and sensitivity of pre-treatment staging has implications on treatment algorithms and survival.
Search Strategy:
The relevant databases were searched as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart. After selection, 12 full text articles that met the inclusion criteria were included for quantitative analysis. 2 × 2 squares were generated using lymph node positive/negative, and NLR high/low data. The effect size for each study was calculated using the DerSimonian–Laird random effects model. P values were calculated using the chi-square method. Finally publication bias was evaluated. All statistics were calculated using R Studio.
Results:
Meta-analysis showed a 1.90 times (odds ratio, with 95% CI 1.52–2.38) increase in risk of positive lymph node status with high neutrophil to lymphocyte ratio. This has significant implications for cancer screening and staging, as NLR is a highly reproducible, cost-effective, and widely available prognostic factor for gastric cancer patients. Additionally, high or low NLR values may have implications for management pathways. Patients with lymph node metastasis can be offered neoadjuvant chemotherapy, avoiding salvage therapy in the form of adjuvant chemoradiotherapy, which is poorly tolerated.
Conclusion:
This meta-analysis shows an association between NLR and positive lymph node status in gastric cancer patients with implications for staging, as well as preoperative personalisation of therapy.
Keywords: cancer diagnosis and workup, gastric cancer, surgical oncology
1. Introduction
Gastric cancer (GC) is one of the most common neoplasms worldwide and is associated with poor prognosis with treatment pathway dependent on tumor staging.[1] Patients with early gastric cancer with no or limited nodal involvement, may be suitable for upfront surgical resection possibly with further adjuvant therapies. More advanced tumors including those with more significant lymph node disease are treated with surgery combined with neoadjuvant and/or adjuvant chemotherapy or chemoradiotherapy, whereas patients with distant metastases (M1) are typically managed non-surgically. TNM staging and categorization reflects the biological behavior and phenotype of a tumor, which is mediated by the systemic inflammatory response. Neutrophils, derived from the common myeloid progenitor, form part of the innate immune system. Lymphocytes (B and T cells), derived from the common lymphoid progenitor, form part of the adaptive immune system. Lymphocytopenia is an impaired cell mediated immune response, whereas neutrophilia is representative of a systematic inflammatory response. Thus the serum neutrophil-lymphocyte ratio (NLR) reflects a balance between activation of antitumor immune function and pro-tumor inflammatory pathways. The aim of this meta-analysis is to show that pre-treatment NLR is associated with increase in risk of lymph node (LN) metastasis in GC. As a simple, inexpensive preoperative investigation, this would be a valuable addition to the existing diagnostic pathway, as it can improve accuracy of staging and prognosis. Patients who have LN spread may benefit from neoadjuvant therapies according to current guidelines, as adjuvant therapy is not well tolerated in western populations. Patients with a higher risk of metastatic disease should receive individualized treatment, as patients with the same TNM stage can have different clinical outcomes.[2,3] Finally, patients with high NLR should be recognized as a high risk group in terms of recurrence, thereby altering the pattern of post-treatment follow-up.
2. Methods
2.1. Literature search
This systematic review with meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.[4] The PubMed, Embase, and Cochrane library were searched for all articles without restriction using the following search criteria: (“neutrophil-lymphocyte ratio” OR “neutrophil-to-lymphocyte ratio” OR “NLR” OR “neutrophils” OR “lymphocytes”) AND (“gastric cancer” OR “gastric adenocarcinoma”) and (“lymph node” OR “lymphadenopathy” OR “metastasis” OR “metastatic spread”). This search strategy was performed until no relevant article was found. Grey literature searches were also performed. Overlapping or duplicate data was excluded. The 3 databases were searched from inception to January 12, 2021. All searches were performed independently by 2 authors. As a meta-analysis of published data, no ethics board approval was required.
2.2. Article selection
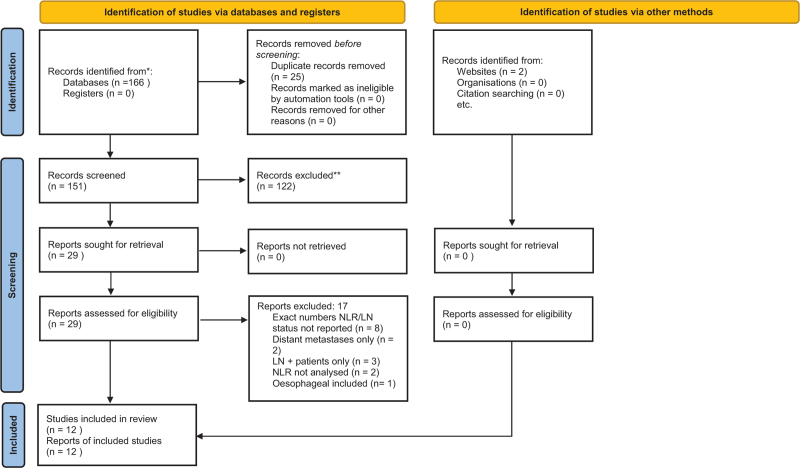
The article selection process is summarized in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart (Fig. 1). The population of interest was patients with a diagnosis of gastric cancer who had pre-treatment neutrophil and lymphocyte count recorded, and the comparison of interest was patients with lymph node metastases.
Figure 1.
PRISMA flowchart. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Studies were included if they provided the following data about gastric cancer patients:
-
(1)
Pre-treatment NLR.
-
(2)
Number of patients in low-NLR and high-NLR groups, with the NLR cutoff defining these groups.
-
(3)
Number of patients in lymph node positive and negative groups.
Studies were excluded if;
-
(1)
Insufficient data was provided, for example, NLR cut off or number of cases with lymph node involvement not provided.
-
(2)
Blood counts were not derived from the patients’ pre-treatment investigations.
-
(3)
They were abstracts, letters, editorials, expert opinion reviews, case reports.
-
(4)
They were not in English.
2.3. Data extraction
Data were extracted independently and manually by 2 investigators. For each study, the following data were extracted; year of publication, author name, country of origin, study design and setting, total number of cases, demographic features, clinicopathological characteristics (sex, age, tumor location, Lauren classification, TNM stage), NLR cut off, number of cases with elevated and reduced NLR, number of cases with positive and negative lymph node status, ages of subjects. No other variables were sought. Missing data from the above fields were marked as such.
2.4. Quality assessment and risk of bias
All eligible articles were evaluated independently by 2 reviewers for risk of bias according to the Newcastle Ottawa Scale (NOS) (see Supplemental Digital Content Appendix 1; Table containing NOS scoring system of study quality).
2.5. Statistical analysis
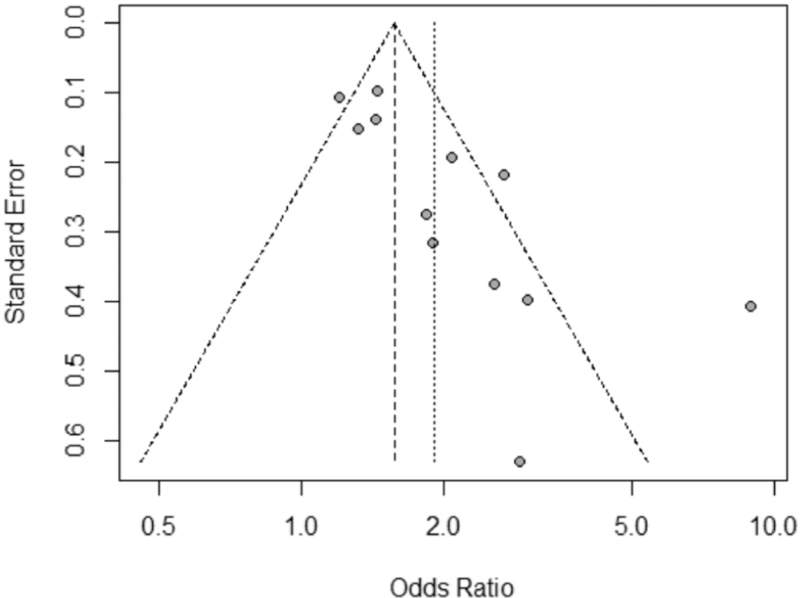
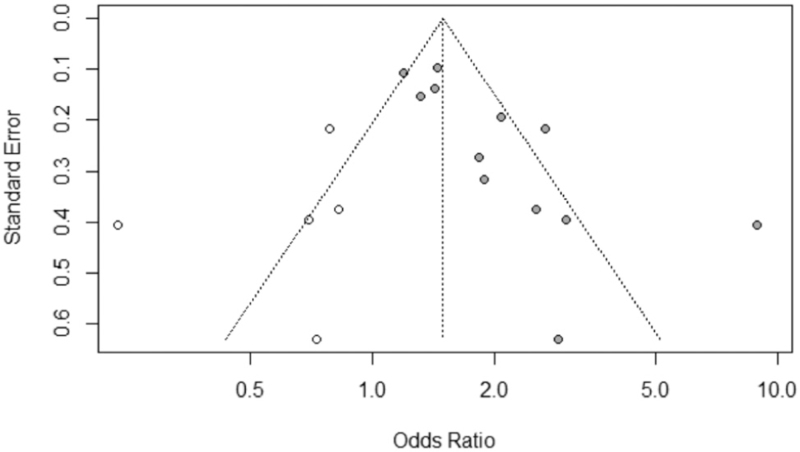
For this meta-analysis, I2 was used to evaluate heterogeneity, with value >50% representing possibility of substantial heterogeneity. If the I2 value exceeded 50%, the effect size (odds ratios and 95% confidence interval) for each study was calculated using the DerSimonian–Laird random effects model. 2 × 2 squares were generated using lymph node positive/negative, and NLR high/low data. This data is available in Appendix 2 (Supplemental Digital Content Appendix 2: 2 × 2 squares containing extracted data from studies). P values were calculated using the chi-square method. Finally publication bias was evaluated by visual inspection of the funnel plot for symmetry, and the Harbord[5] test (see Fig. 2), with P < .05 indicative of possible publication bias. All statistics were calculated using R Studio.[6]
Figure 2.
Funnel plot in assessment of publication bias.
3. Results
3.1. Search results
A total of 168 references were generated: PubMed (n = 96), Embase (n = 54), Cochrane Library (n = 16), grey literature, and references (N = 2). Following the eligibility criteria, only 97 were eligible studies. After screening, 29 studies were reviewed and analyzed. Eight articles[7–14] were excluded as they did not provide the exact number of patients who were NLR positive or negative, and lymph node positive or negative. One article combined platelet-lymphocyte ratio and neutrophil-lymphocyte ratio[15]; 2 articles included patients with distant metastases only[16,17]; 3 articles included lymph node positive patients only[18–20]; 2 articles did not analyze neutrophil-lymphocyte ratio as predictive factors for gastric cancer,[21,22] and 1 article included esophago-gastric cancers.[23] Finally, we identified 12 full text articles[10,24–34] that met the inclusion criteria for quantitative analysis.
3.2. Characteristics of studies
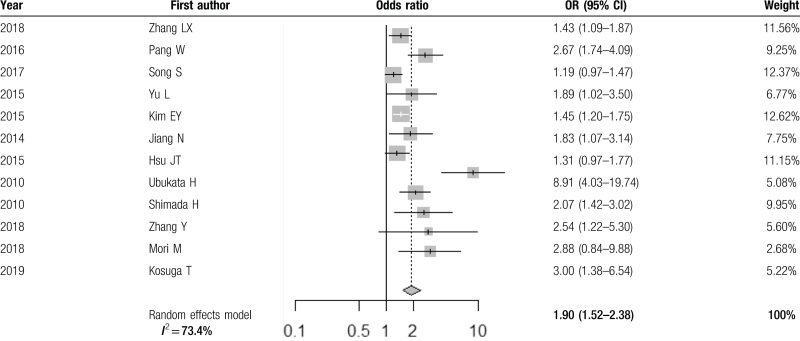
The meta-analysis included 9401 patients, summarized in Table 1. Five studies were identified as high quality (NOS >6).
Table 1.
Summary of results.
| Year | First author | Country | Study design | Patients (n) | Age median | NOS | Cut off value NLR |
| 2018 | Zhang LX | China | R | 904 | N/A | 7 | 2 |
| 2016 | Pang W | China | R | 927 | 63 | 7 | 1.59 |
| 2017 | Song S | China | P | 1990 | 62 | 5 | 2.10 |
| 2015 | Yu L | China | R | 291 | N/A | 8 | 3.5 |
| 2015 | Kim EY | S. Korea | P | 1986 | 58.2 | 6 | 3 |
| 2014 | Jiang N | China | R | 377 | 64 | 6 | 1.44 |
| 2015 | Hsu JT | Taiwan | R | 1030 | N/A | 6 | 3.44 |
| 2010 | Ubukata H | Japan | R | 157 | 65 | 6 | 5 |
| 2010 | Shimada H | Japan | R | 1028 | 65 | 5 | 4 |
| 2018 | Zhang Y | China | P | 182 | 65 | 7 | 2.88 |
| 2018 | Mori M | Japan | R | 100 | 66 | 6 | 1 |
| 2019 | Kosuga T | Japan | R | 429 | 67 | 7 | 1.6 |
| 9401 |
NLR = neutrophil to lymphocyte ratio, NOS = Newcastle Ottawa Scale, P = prospective, R = retrospective.
3.3. Meta-analysis
The results are displayed with forest plot in Table 2. This showed a 1.90 times pooled OR (95% CI 1.52–2.38) of lymph node metastasis in patients with high neutrophil to lymphocyte ratio. High neutrophil to lymphocyte ratio was defined as a value above the cut-off set for each individual study.
Table 2.
Random effects model showing pooled odds ratio.
3.4. Heterogeneity
Significant heterogeneity among studies was found I2 = 73.4%, Q = 41.33 (P < .01), and therefore the random effects model was used. After performing sensitivity analysis, we found that the study by Ubukata et al[31] contributed more to the heterogeneity—on exclusion analysis, heterogeneity fell to 55.6%, and yet the overall pooled OR was 1.68 (95% CI 1.41–2.00). (See Table in Supplemental Digital Content, Appendix 3: exclusion analysis of studies). To investigate the heterogeneity, metaregression was also performed, including covariates (publication country, study size, publication year, NLR cut-off value).
3.5. Biases
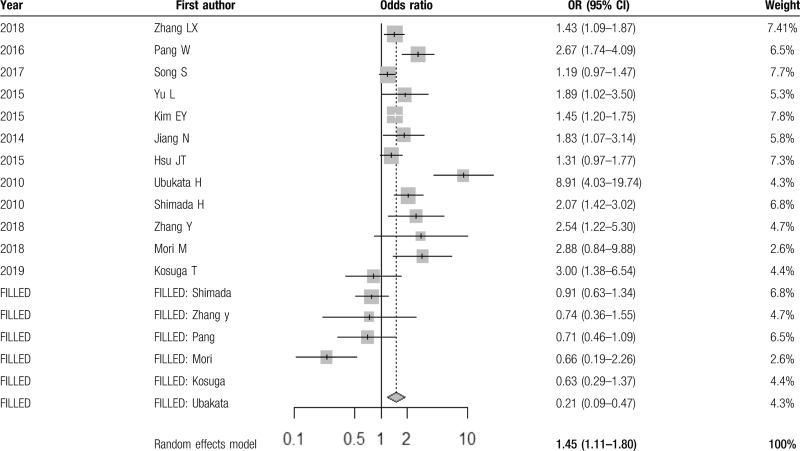
A funnel plot (Fig. 2) demonstrated asymmetrical dispersion of LN metastases. The Harbord test revealed a possibility of publication bias (P < .05). This result must be interpreted with caution, given the low number of studies (10) included in this meta-analysis. Additionally, type II error may exist due to the limited number of publications. The trim-and-fill estimator method[35] to correct for funnel plot asymmetry. These results are available in Table 3 (with filled results in Fig. 3), and show a pooled OR of 1.45 (95% CI 1.11–1.80), a positive association between NLR and LN positive status in patients with GC.
Table 3.
Trim-and-fill method to adjust for funnel plot asymmetry.
Figure 3.
Funnel plot following trim-and-fill estimator correction for asymmetry.
Given the majority of studies originated in Asia, there is the possibility of location bias. There were very few missing results reported in the studies.
4. Discussion
4.1. The role of inflammation in cancer growth and spread is well established
Markers such as C-reactive protein, neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR), represent a non-specific inflammatory response to tumor hypoxia, tissue injury, and necrosis.[36] The ability of gastric cancers to act aggressively and metastasize is dependent on the intrinsic characteristics of the tumor cells themselves, as well as the cancer microenvironment.[37]
Several models of cancer formation have been described. Reactive oxidative species and cytokines formed as part of an inflammatory disease state can initiate oncogenesis, and conversely,[38] existing cancer can provoke an inflammatory response that allows the genetic transformation of a low grade malignancy into a high grade one.[39] These genetic influences are upregulated through transcription factors such as nuclear factor kB, signal transducer, and activator of transcription 3 and hypoxia inducible factor 1a.[40] As part of this process, downregulation of the adaptive immune system creates a self-perpetuating tumor stimulating environment.
Several cell mediated processes are involved in tumor pathogenesis;
-
(1)
Neutrophils play a key role, as they are involved with the initial and subsequent inflammatory response. Neutrophil associated inflammatory mediators include vascular endothelial growth factor and matrix metalloproteinases, which inhibit the antitumor effects of helper T-cells (CD4), cytotoxic T-cells (CD8), and natural killer (NK) cells.[36] They also stimulate tumor angiogenesis via remodeling of the extracellular matrix.[41]
-
(2)
In addition to neutrophils, platelets have several functions in tumor pathogenesis. Although the prognostic role of platelet count, and platelet-to-lymphocyte ratio have been extensively discussed in the literature, they are not the focus of this study. In the tumor microenvironment, platelets facilitate tumor adhesion to vascular endothelium through the formation of tumor thrombi, thus protecting them against immune clearance.[42] Several secretory chemokines (interleukins 1 and 6, tumor necrosis factor α, thrombospondin, leukemia inhibitory factor, and endostatin) are directly released by platelets, potentiating metastatic spread.[9] In particular, the aforementioned vascular endothelial growth factor, along with platelet derived growth factor aid in tumor angiogenesis and metastasis.[43] Additionally, a high platelet count causes a lymphocytopenia, and thus a hypoimmune response linked to lymphocyte-mediated anti-tumor activity at the cellular level, interlinking high platelet count to raised NLR. A robust lymphocyte response is a major factor in the suppression of cancer progression, and therefore, a relative lymphocytopenia leads to a blunted lymphocytic (T4H and T8) anti-tumor response, raising NLR further.
-
(3)
NLR can therefore be considered as the balance between pro tumor inflammatory status and immune mediated tumor suppression. Patients with elevated NLR have a relative lymphocytopenia and neutrophilic leucocytosis, shifting the balance in favor of protumor inflammation. This systemic inflammation is associated with functional and nutritional decline,[44] a poor oncological outcome,[45] and poorer survival in gastric cancer.[46]
4.2. Elevated NLR is a proven negative prognostic factor in many cancers, including gastric cancer
NLR has been shown to be a negative prognostic factor in other cancers, including breast, colorectal, esophageal, liver, melanoma, ovarian, pancreatic, and prostate.[47] As a result, there have been numerous novel studies incorporating NLR into preoperative staging. Huang et al[48] recommended a preoperative COCT-NLR (a combination of NLR and contrast enhanced computed tomography) to detect LN metastasis in non-small cell lung cancer patients, with a high sensitivity (70.59%) and specificity (74.89%). Similarly, Ertas et al[49] showed an independent association between both preoperative NLR and PLR and lymph node metastasis in vulvar squamous cell sarcoma patients. Similarly, elevated NLR, along with other inflammation based scores such as PLR, is associated with poorer outcomes in gastric cancer. A meta-analysis by Kim et al[50] found that the combined hazard ratio of mortality was markedly higher in GC patients with elevated NLR than in patients with normal NLR, and patients with higher C-reactive protein, NLR, and Glasgow prognostic score (GPS/mGPS) had lower overall survival. The meta-analysis showed that collectively, these inflammatory markers are prognostic for GC outcomes regardless of country, quality of study, cancer stage, study design, or the inclusion of patients with no-or adjuvant chemotherapy.
Additionally, Miyamoto et al[8] found that their high NLR group had worse preoperative symptoms, postoperative complications (greater than Clavein-Dindo III), intraoperative blood loss, intraoperative transfusion requirement, and median disease free survival. It is evident that preoperative NLR is not only a predictor of short term outcomes (including perioperative complications), but of cancer recurrence, impacting follow up. Li et al[51] describe this as the product of the extended inflammatory postoperative state, which then leads to an extended period of immunosuppression, facilitating micrometastatic spread.
Interestingly, Ishiziuka et al[52] found no relationship between Combination Of PLR and NLR and the levels of tumor markers, for example, CEA,[53] and Ca199.[54] It is not unusual for patients with advanced GC to have tumor marker levels within normal ranges, and such patients would benefit from postoperative surveillance using an inflammation based score such as NLR. The same study found that age, tumor type, lymph node metastasis, and the serum level of albumin are closely associated with the postoperative survival of patients with gastric cancer. Therefore, these other inflammation based factors, along with NLR, may also be useful in the prognostication of gastric cancer.
4.3. Elevated preoperative NLR is associated with lymph node metastasis, which could be a valuable adjunct to the current staging pathway
Patients with gastric cancer are staged via a variety of invasive and non-invasive modalities according to the American Joint Committee for Cancer (AJCC) 8th edition guidelines, summarized in Appendix 4 and 5 (Supplemental Digital Content Appendix 4: TNM staging of gastric cancer as per the AJCC Guidelines, 8th edition; and Supplemental Digital Content Appendix 5: Anatomic stage/prognostic groups as per AJCC, 8th edition). There is significant overall variability in these techniques in terms of sensitivity, specificity, cost, and consistency.
Computed tomography remains the gold standard in evaluating nodal status, but can be costly, invasive and inconsistent in accuracy.[55,56] In our experience, finally, nodal metastases are often found that are not enlarged as per CT criteria, that is, nodes <10 mm in diameter. CT is often combined with fluoro-deoxyglucose positron emission tomography (FDG-PET). FDG PET-CT is utilized in esophageal cancer due to its high diagnostic sensitivity for the primary tumor,[57] nodal status,[58] and detection of distant metastases.[59,60] This is crucial for identifying patients with occult metastatic or advanced locoregional disease that would benefit from neoadjuvant chemotherapy. The published sensitivity of FDG PET-CT in staging gastric adenocarcinoma ranges from 60% to 94%.[61] For example, Bosch et al[62] in a retrospective series an additional 16% of their patients to have occult metastases (distant lymph nodes or solid organ disease) detected solely via FDG PET-CT.
In primary GC tumor detection, the sensitivity of FDG-PET is greater for the intestinal histological subtype, and lowest for the sig histology. Kodou et al[60] found that FDG uptake in the primary tumor and lymph nodes was significantly lower with the Sig histology, and sensitivities for lymph node metastasis in Stage III and IV disease were also lower in GC with the Sig histology. By comparison, greater avidity of both primary and metastatic intestinal subtype gastric tumors results in a FDG-PET sensitivity of <50%[63–65] as the spatial resolution of FDG-PET-CT cannot differentiate the primary tumor from positive perigastric nodes.[64] The same study also found that FDG uptake in the primary tumor or lymph nodes was independently associated with Stage III or IV GC, suggesting a more aggressive phenotype. This is explained by Bosch et al,[62] who also found that a significant portion of tumors have no FDG uptake, so no staging information is added in this subset of patients, despite the average size of 18F-FDG negative tumors in their study being 40 mm, with the smallest tumor measuring 10 mm. Additionally, physiological or inflammatory uptake in non-malignant gastric mucosa can obscure a gastric cancer and provide difficulty with primary tumor identification, limiting the specificity of PET-CT.[66]
Other more invasive staging methods, such as endoscopic ultrasound, are also utilized. Meta-analysis by Chen et al[56] showed a relatively high sensitivity of EUS for gastric cancer N staging (82%), but lower specificity (68%). However, this method is invasive, and operator dependent. NLR as an adjunct nodal detection method would reflect the inflammatory balance of malignancy.
Preoperative NLR allows accurate staging of nodal status and guides the therapeutic strategy and prognosis,[46] with randomized trials proving the benefit of neoadjuvant chemotherapy (NAC) in patients presenting with advanced disease.[67]
The addition of NLR to the staging process has the potential to expand personalized medicine in this field. Stratification of high-risk patients means they can be targeted for intensive staging via the above imaging methods. By pre-treatment detection of nodal disease, patients may be offered neoadjuvant therapy, thus potentially avoiding salvage therapy in the form of adjuvant chemoradiotherapy for intraoperatively detected metastatic disease. Patients with nodal disease can be counseled more fully as to their prognosis, allowing them to make more informed decisions, and undergo tailored surveillance.
Neoadjuvant therapies are effective in controlling LN metastasis in gastric cancer, thereby reducing N stage and increasing complete resection.[68–70] The benefits of neoadjuvant therapy have been analyzed in previous studies,[71,72] with increasingly more studies recommending preoperative therapies for GC patients with nodal involvement.[73,74] In contrast, adjuvant only therapies are regarded as salvage treatments for advanced disease following resection in Western practice, though are often used routinely in Asia. Current ESMO[75] and NCCN[76] guidelines recommend patients with stage II disease or greater undergo neoadjuvant chemotherapy For stage IB GC, the recommendation is for patients to have neoadjuvant chemotherapy if lymph node positive, or chemoradiation if lymph node positive after resection. By comparison, Japanese guidelines[77] recommend patients with Stage II disease and above with lymph node involvement undergo adjuvant chemotherapy, and for stage 2 and above with “bulky” lymph node disease to undergo neoadjuvant therapy. Adjuvant therapy is not easily tolerated by a more debilitated postoperative cohort, particularly in the case of chemoradiotherapy.[78,79] In fact, only almost 50% of patients randomized in the MAGIC and FNCLCC-ACCORD[80] trials completed adjuvant therapy, due to early death after surgery, disease progression, postoperative complications, or toxicity.[81] Certainly, this may explain the findings of a meta-analysis by Hu et al[82] showing neoadjuvant chemotherapy as superior in terms of overall survival at 1, 3, and 5 years compared with surgery only, or surgery with adjuvant chemotherapy. Certainly, adjuvant treatment has an important role in the general management of a case, especially in patients with positive nodal disease (ypN) or >50% vital tumor cells after neoadjuvant chemotherapy.[83] There potential benefit in this cohort should be balanced against the risk of chemotherapy related effects. Additionally, regarding chemoradiotherapy, a meta-analysis of 13 randomized clinical trials including nearly 3000 patients did not find any difference in outcomes between neoadjuvant and adjuvant chemoradiotherapy.[84]
Additionally, minimally invasive procedures such as endoscopic submucosal dissection (ESD), and endoscopic mucosal dissection (EMD) are becoming increasingly frequent in early gastric cancer without lymph node metastasis,[85] as they are physiologically less stressful, resulting in a shorter postoperative hospital stay and has a lower postoperative morbidity rate. There was no difference in survival between the 2 methods in a study comparing curative ESD/EMD and gastrectomy.[85] The major risk of ESD is that pathological N status is not determined, because lymph node resection is not performed.[86] Early gastric cancer is resected specifically for lymph node resection, as the risk of lymph node metastases is 2% and 5%.[87] Determining N stage is also valuable in selecting an appropriate surgical method, given that the degree of lymph node metastasis will influence the course of management.
The approach to lymph node dissection varies, with debate over the extent of lymphadenectomy by comparison of D1, D1+, and D2 resections. Currently, the 8th edition of the UICC/AJCC staging system for gastric cancer recommends the removal of at least 16 lymph nodes (D1+ resection) for correct lymph node assessment.[88] Previous randomized trials have shown that D2 lymphadenectomy was related to a higher possibility of reoperation, morbidity, and mortality, and no prolonged survival was observed compared with D1+ lymphadenectomy.[89,90] However these are older studies where splenectomy and often distal pancreatectomy was included in the resection. Current Japanese guidelines now avoid dissection of station 10 (perisplenic) lymph nodes, unless the greater curve is involved.[91] Another RCT suggested that D2 resection may be more suitable than D1 resection for advanced gastric cancer patients with LN metastasis.[92] High likelihood of node negative disease through conventional staging and the addition of NLR means that more extensive lymphadenectomy could be avoided, with ESD, EMD, or D1 lymphadenectomy used to achieve curative resection. Combined with neoadjuvant therapy, the addition of NLR to preoperative staging will allow accurate staging of LN status prior to surgery, and therefore allocation of patients to an appropriate personalized therapeutic pathway.
Finally, survival analysis by Miyamoto et al[8] based on cancer related prognostic factors, for example, serum CEA, differentiation, and stage, found that elevated NLR is also associated with a reduced survival in gastric cancer. Detection of distant metastatic disease through NLR and appropriate diagnostic imaging might allow the patients to commence palliative therapies sooner, and reduce the risks, complications, trauma, and costs (financial, physical, and psychological) of well-intentioned, but ultimately futile treatment
4.4. Several score models have been developed to detect LN metastasis in gastric cancer
For example, Li et al[93] described a nodal status predictive score system in pT2 stage gastric cancer and similarly, Shida et al[94] also recommended a preoperative score system to predict LN metastasis in early gastric cancer. Only tumor-related factors were analyzed and the accuracy, specificity, and sensitivity rates of the model were only 70%, 61.6%, and 63.2% respectively. Comparatively, Pang et al[28] included variables such as tumor size, macroscopic type, depth of invasion, PLR, and NLR predicted LN metastasis with a sensitivity of 82.7% and a specificity of 72.4%. The positive and negative predictive values of the model were 88.7% and 61.5% respectively, and unlike EUS, which is operator dependent, models such as these can be combined with traditional imaging protocols to decrease false positive results, especially for understaged patients who could benefit from adjuvant or neoadjuvant treatment. Further studies are needed in this area, including the development of a stratification score validated in a large cohort of patients.
4.5. Limitations
This metanalysis focused on retrospective cohort studies that are prone to faults, including that of publication bias:
Studies did not specify how they eliminated or reduced the rate of false positive, some tumors may have been misclassified by histology or location.
Only included English articles, and small studies with cumulative results were not published, leading to potential bias.
The authors of each manuscript mathematically derived their cut-off value for NLR based on their population data, with high variability noted in the optimal NLR cut-off, between 1[10] to 3.5.[27] The translation of the NLR into the clinical setting remains challenging, as the strength of association between NLR and all outcomes, including overall survival, varies between studies. A study by Howard et al[47] found that average values of NLR varies between subgroups of the population, and the magnitude of association between high NLR and survival is greater for certain patients—significantly higher as a baseline in white patients, male patients, over 60s, patients with stage IV disease, and patients with ovarian or pancreatic cancer. Some of these have been addressed in the literature, for example, existing observations of benign ethnic neutropenia, and decreasing immune function with age. However, the same study notes that the assumption that the NLR has equal prognostic value for all patients regardless of demographic factors or clinical characteristics of the disease is highly unlikely to be correct. Similarly, Vano et al[34] found that their optimal NLR cut-off value was highly variable based on the time at which survival was assessed and the indices used for optimality assessment, bringing into question the application of such a cut-off across different populations.
Further prospective, randomized controlled studies are needed to further investigate the role of NLR in N staging and also in the post-treatment phase, such as in the monitoring of response to chemotherapy and prognostic assessment.
Finally, it is important to remember that inflammatory markers are influenced by a number of other factors, for example, age, nutritional status, adjuvant therapy, concurrent infective process, and thus can be falsely elevated.
5. Conclusions
This meta-analysis shows an association between NLR and positive lymph node status in gastric cancer patients. As a simple, inexpensive preoperative investigation, this would be a valuable addition to the existing diagnostic pathway, as it can stage patients with LN positive disease, and further identify patients who may benefit from neoadjuvant therapies according to current guidelines. Patients with high NLR should be recognized as a high risk group in terms of recurrence, and should be monitored closely in follow-up.
Author contributions
Conceptualization: Animesh Singla, Neil Merrett, Philip Townend
Data curation: Animesh Singla, Krishna Kotecha, Neil Merrett, Philip Townend
Formal analysis: Animesh Singla, Krishna Kotecha, Philip Townend
Investigation: Animesh Singla, Krishna Kotecha
Methodology: Animesh Singla, Philip Townend
Project administration: Krishna Kotecha, Philip Townend
Resources: Krishna Kotecha
Software: Krishna Kotecha
Supervision: Neil Merrett
Validation: Neil Merrett
Visualization: Neil Merrett, Philip Townend
Writing – original draft: Krishna Kotecha
Writing – review & editing: Animesh Singla, Neil Merrett, Philip Townend
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AJCC = American Joint Committee for Cancer, EMD = endoscopic mucosal dissection, ESD = endoscopic submucosal dissection, FDG-PET = fluoro-deoxyglucose positron emission tomography, GC = gastric cancer, GPS = Glasgow prognostic score, LN = lymph node, NLR = neutrophil to lymphocyte ratio, NOS = Newcastle Ottawa Scale, PDGF = platelet derived growth factor, PLR = platelet-lymphocyte, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
How to cite this article: Kotecha K, Singla A, Townend P, Merrett N. Association between neutrophil-lymphocyte ratio and lymph node metastasis in gastric cancer: a meta-analysis. Medicine. 2022;101:25(e29300).
Institution: Royal North Shore Hospital, Reserve Road, St Leonards 2065 NSW, Australia.
Disclosure: The authors did not receive any grant support and declare no conflicts of interest. The first author is not a surgeon in training. All authors are in agreement with the content of the manuscript. The manuscript has not been published previously, and is not under consideration elsewhere.
All data generated or analyzed during this study are included in this published article [and itssupplementary information files]
Supplemental digital content is available for this article.
References
- [1].Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet 2020;396:635–48. [DOI] [PubMed] [Google Scholar]
- [2].Peng CW, Wang LW, Zeng WJ, Yang XJ, Li Y. Evaluation of the staging systems for gastric cancer. J Surg Oncol 2013;108:93–105. [DOI] [PubMed] [Google Scholar]
- [3].Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol 2010;17:2077–9. [DOI] [PubMed] [Google Scholar]
- [4].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cochrane; 2021;Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). [Google Scholar]
- [6].R Core Team. R: A Language and Environment for Statistical Computing. 2017;Vienna, Austria: R Foundation for Statistical Computing, https://www.R-project.org/ [Google Scholar]
- [7].Dutta S, Crumley AB, Fullarton GM, Horgan PG, McMillan DC. Comparison of the prognostic value of tumour and patient related factors in patients undergoing potentially curative resection of gastric cancer. Am J Surg 2012;204:294–9. [DOI] [PubMed] [Google Scholar]
- [8].Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol 2018;44:607–12. [DOI] [PubMed] [Google Scholar]
- [9].Murakami Y, Saito H, Shimizu S, et al. Neutrophil-to-lymphocyte ratio as a prognostic indicator in patients with unresectable gastric cancer. Anticancer Res 2019;39:2583–9. [DOI] [PubMed] [Google Scholar]
- [10].Mori M, Shuto K, Kosugi C, et al. An increase in the neutrophil-to-lymphocyte ratio during adjuvant chemotherapy indicates a poor prognosis in patients with stage II or III gastric cancer. BMC Cancer 2018;18:1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xu AM, Huang L, Zhu L, Wei ZJ. Significance of peripheral neutrophil-lymphocyte ratio among gastric cancer patients and construction of a treatment-predictive model: a study based on 1131 cases. Am J Cancer Res 2014;4:189–95. [PMC free article] [PubMed] [Google Scholar]
- [12].Aliustaoglu M, Bilici A, Ustaalioglu BB, et al. The effect of peripheral blood values on prognosis of patients with locally advanced gastric cancer before treatment. Med Oncol 2010;27:1060–5. [DOI] [PubMed] [Google Scholar]
- [13].Aldemir MN, Turkeli M, Simsek M, et al. Prognostic value of baseline neutrophil-lymphocyte and platelet-lymphocyte ratios in local and advanced gastric cancer patients. Asian Pac J Cancer Prev 2015;16:5933–7. [DOI] [PubMed] [Google Scholar]
- [14].Gunaldi M, Goksu S, Erdem D, et al. Prognostic impact of platelet/lymphocyte and neutrophil/lymphocyte ratios in patients with gastric cancer: a multicenter study. Int J Clin Exp Med 2015;8:5937–42. [PMC free article] [PubMed] [Google Scholar]
- [15].Ishizuka M, Oyama Y, Abe A, Kubota K. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients undergoing surgery for gastric cancer. J Surg Oncol 2014;110:935–41. [DOI] [PubMed] [Google Scholar]
- [16].Abu-Shawer O, Abu-Shawer M, Haimour A, et al. Hematologic markers of distant metastases in gastric cancer. J Gastrointest Oncol 2019;10:529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lou N, Zhang L, Chen XD, et al. A novel scoring system associating with preoperative platelet/lymphocyte and clinicopathologic features to predict lymph node metastasis in early gastric cancer. J Surg Res 2017;209:153–61. [DOI] [PubMed] [Google Scholar]
- [18].Cho IR, Park JC, Park CH, et al. Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer 2014;17:703–10. [DOI] [PubMed] [Google Scholar]
- [19].Lee S, Oh SY, Kim SH, et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer 2013;13:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kim H, Ro SM, Yang JH, et al. The neutrophil-to-lymphocyte ratio prechemotherapy and postchemotherapy as a prognostic marker in metastatic gastric cancer. Korean J Intern Med 2018;33:990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gür EÖ, Karaisli S, Kamer E, et al. Predictive factors for lymph node metastasis and the effect on survival in early gastric cancer patients with radical gastric resection. Sisli Etfal Hastan Tip Bul 2019;53:371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wu Y, Jiang M, Qin Y, Lin F, Lai M. Single and combined use of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and carcinoembryonic antigen in diagnosing gastric cancer. Clin Chim Acta 2018;481:20–4. [DOI] [PubMed] [Google Scholar]
- [23].Grenader T, Waddell T, Peckitt C, et al. Prognostic value of neutrophil-to-lymphocyte ratio in advanced oesophago-gastric cancer: exploratory analysis of the REAL-2 trial. Ann Oncol 2016;27:687–92. [DOI] [PubMed] [Google Scholar]
- [24].Zhang LX, Wei ZJ, Xu AM, Zang JH. Can the neutrophil-lymphocyte ratio and platelet-lymphocyte ratio be beneficial in predicting lymph node metastasis and promising prognostic markers of gastric cancer patients? Tumor maker retrospective study. Int J Surg 2018;56:320–7. [DOI] [PubMed] [Google Scholar]
- [25].Pang W, Lou N, Jin C, et al. Combination of preoperative platelet/lymphocyte and neutrophil/lymphocyte rates and tumor-related factors to predict lymph node metastasis in patients with gastric cancer. Eur J Gastroenterol Hepatol 2016;28:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Song S, Li C, Li S, Gao H, Lan X, Xue Y. Derived neutrophil to lymphocyte ratio and monocyte to lymphocyte ratio may be better biomarkers for predicting overall survival of patients with advanced gastric cancer. Onco Targets Ther 2017;10:3145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yu L, Lv CY, Yuan AH, Chen W, Wu AW. Significance of the preoperative neutrophil-to-lymphocyte ratio in the prognosis of patients with gastric cancer. World J Gastroenterol 2015;21:6280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim EY, Lee JW, Yoo HM, Park CH, Song KY. The platelet-to-lymphocyte ratio versus neutrophil-to-lymphocyte ratio: which is better as a prognostic factor in gastric cancer? Ann Surg Oncol 2015;22:4363–70. [DOI] [PubMed] [Google Scholar]
- [29].Jiang N, Deng JY, Liu Y, Ke B, Liu HG, Liang H. The role of preoperative neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after radical resection for gastric cancer. Biomarkers 2014;19:444–51. [DOI] [PubMed] [Google Scholar]
- [30].Hsu JT, Liao CK, Le PH, et al. Prognostic value of the preoperative neutrophil to lymphocyte ratio in resectable gastric cancer. Medicine (Baltimore) 2015;94:e1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ubukata H, Motohashi G, Tabuchi T, Nagata H, Konishi S, Tabuchi T. Evaluations of interferon-γ/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol 2010;102:742–7. [DOI] [PubMed] [Google Scholar]
- [32].Shimada H, Takiguchi N, Kainuma O, et al. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer 2010;13:170–6. [DOI] [PubMed] [Google Scholar]
- [33].Zhang Y, Lu JJ, Du YP, Feng CX, Wang LQ, Chen MB. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in gastric cancer. Medicine (Baltimore) 2018;97:e0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kosuga T, Konishi T, Kubota T, et al. Clinical significance of neutrophil-to-lymphocyte ratio as a predictor of lymph node metastasis in gastric cancer. BMC Cancer 2019;19:1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shi L, Lin L. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Medicine (Baltimore) 2019;98:e15987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin Cancer Biol 2013;23:141–8. [DOI] [PubMed] [Google Scholar]
- [37].Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- [39].Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010;141:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shau HY, Kim A. Suppression of lymphokine-activated killer induction by neutrophils. J Immunol 1988;141:4395–402. [PubMed] [Google Scholar]
- [41].De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res 2004;10:4895–900. [DOI] [PubMed] [Google Scholar]
- [42].Nieswandt B, Hafner M, Echtenacher B, Männel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 1999;59:1295–300. [PubMed] [Google Scholar]
- [43].Giralt J, Navalpotro B, Hermosilla E, et al. Prognostic significance of vascular endothelial growth factor and cyclooxygenase-2 in patients with rectal cancer treated with preoperative radiotherapy. Oncology 2006;71:312–9. [DOI] [PubMed] [Google Scholar]
- [44].Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology 2007;73:215–20. [DOI] [PubMed] [Google Scholar]
- [45].McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009;12:223–6. [DOI] [PubMed] [Google Scholar]
- [46].Xin-Ji Z, Yong-Gang L, Xiao-Jun S, Xiao-Wu C, Dong Z, Da-Jian Z. The prognostic role of neutrophils to lymphocytes ratio and platelet count in gastric cancer: a meta-analysis. Int J Surg 2015;21:84–91. [DOI] [PubMed] [Google Scholar]
- [47].Howard R, Kanetsky PA, Egan KM. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep 2019;9:19673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Huang C, Yue J, Li Z, Li N, Zhao J, Qi D. Usefulness of the neutrophil-to-lymphocyte ratio in predicting lymph node metastasis in patients with non-small cell lung cancer. Tumour Biol 2015;36:7581–9. [DOI] [PubMed] [Google Scholar]
- [49].Ertas IE, Gungorduk K, Akman L, et al. Can preoperative neutrophil:lymphocyte and platelet:lymphocyte ratios be used as predictive markers for lymph node metastasis in squamous cell carcinoma of the vulva? Eur J Obstet Gynecol Reprod Biol 2013;171:138–42. [DOI] [PubMed] [Google Scholar]
- [50].Kim MR, Kim AS, Choi HI, Jung JH, Park JY, Ko HJ. Inflammatory markers for predicting overall survival in gastric cancer patients: a systematic review and meta-analysis. PLoS One 2020;15:e0236445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Li QG, Li P, Tang D, Chen J, Wang DR. Impact of postoperative complications on long-term survival after radical resection for gastric cancer. World J Gastroenterol 2013;19:4060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br J Cancer 2013;109:401–7. Kochi M, Fujii M, Kanamori N, et al. Evaluation of serum CEA and CA19-9 levels as prognostic factors in patients with gastric cancer. Gastric Cancer. 2000;3(4):177-186.doi:10.1007/pl00011715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Reiter W, Stieber P, Reuter C, Nagel D, Lau-Werner U, Lamerz R. Multivariate analysis of the prognostic value of CEA and CA 19-9 serum levels in colorectal cancer. Anticancer Res 2000;20:5195–8. [PubMed] [Google Scholar]
- [54].Seevaratnam R, Cardoso R, McGregor C, et al. How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? A meta-analysis. Gastric Cancer 2012;15: (suppl): S3–18. [DOI] [PubMed] [Google Scholar]
- [55].Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer 2009;12:06–22. [DOI] [PubMed] [Google Scholar]
- [56].Chen J, Zhou C, He M, Zhen Z, Wang J, Hu X. A meta-analysis and systematic review of accuracy of endoscopic ultrasound for N staging of gastric cancers. Cancer Manag Res 2019;11:8755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Flamen P, Lerut A, Van Cutsem E, et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol 2000;18:3202–10. [DOI] [PubMed] [Google Scholar]
- [58].Block MI, Patterson GA, Sundaresan RS, et al. Improvement in staging of esophageal cancer with the addition of positron emission tomography. Ann Thorac Surg 1997;64:770–7. [DOI] [PubMed] [Google Scholar]
- [59].Findlay JM, Antonowicz S, Segaran A, et al. Routinely staging gastric cancer with 18F-FDG PET-CT detects additional metastases and predicts early recurrence and death after surgery. Eur Radiol 2019;29:2490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kudou M, Kosuga T, Kubota T, et al. Value of preoperative PET-CT in the prediction of pathological stage of gastric cancer. Ann Surg Oncol 2018;25:1633–9. [DOI] [PubMed] [Google Scholar]
- [61].Räsänen JV, Sihvo EI, Knuuti MJ, et al. Prospective analysis of accuracy of positron emission tomography, computed tomography, and endoscopic ultrasonography in staging of adenocarcinoma of the esophagus and the esophagogastric junction. Ann Surg Oncol 2003;10:954–60. [DOI] [PubMed] [Google Scholar]
- [62].Bosch KD, Chicklore S, Cook GJ, et al. Staging FDG PET-CT changes management in patients with gastric adenocarcinoma who are eligible for radical treatment. Eur J Nucl Med Mol Imaging 2020;47:759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kim EY, Lee WJ, Choi D, et al. The value of PET/CT for preoperative staging of advanced gastric cancer: comparison with contrast-enhanced CT. Eur J Radiol 2011;79:183–8. [DOI] [PubMed] [Google Scholar]
- [64].Yun M. Imaging of gastric cancer metabolism using 18 F-FDG PET/CT. J Gastric Cancer 2014;14:01–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yang QM, Kawamura T, Itoh H, et al. Is PET-CT suitable for predicting lymph node status for gastric cancer? Hepatogastroenterology 2008;55:782–5. [PubMed] [Google Scholar]
- [66].Kobayashi S, Ogura M, Suzawa N, et al. 18F-FDG uptake in the stomach on screening PET/CT: value for predicting Helicobacter pylori infection and chronic atrophic gastritis. BMC Med Imaging 2016;16:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Fu T, Bu ZD, Li ZY, et al. Neoadjuvant chemoradiation therapy for resectable esophago-gastric adenocarcinoma: a meta-analysis of randomized clinical trials. BMC Cancer 2015;15:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Dong S, Yu JR, Zhang Q, Liu XS. Neoadjuvant chemotherapy in controlling lymph node metastasis for locally advanced gastric cancer in a Chinese population. J Chemother 2016;28:59–64. [DOI] [PubMed] [Google Scholar]
- [69].Wu ZM, Teng RY, Shen JG, Xie SD, Xu CY, Wang LB. Reduced lymph node harvest after neoadjuvant chemotherapy in gastric cancer. J Int Med Res 2011;39:2086–95. [DOI] [PubMed] [Google Scholar]
- [70].Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol 2010;28:5210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Leong T, Smithers BM, Michael M, et al. TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer 2015;15:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Schirren R, Reim D, Novotny AR. Adjuvant and/or neoadjuvant therapy for gastric cancer? A perspective review. Ther Adv Med Oncol 2015;7:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Schwarz RE. Current status of management of malignant disease: current management of gastric cancer. J Gastrointest Surg 2015;19:782–8. [DOI] [PubMed] [Google Scholar]
- [74].Newton AD, Datta J, Loaiza-Bonilla A, Karakousis GC, Roses RE. Neoadjuvant therapy for gastric cancer: current evidence and future directions. J Gastrointest Oncol 2015;6:534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27: (suppl): v38–49. [DOI] [PubMed] [Google Scholar]
- [76].National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Gastric Cancer. Version 2; 2020. Available at: www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. Accessed July 8, 2021. [Google Scholar]
- [77].Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021;24:01–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kundel Y, Purim O, Idelevich E, et al. Postoperative chemoradiation for resected gastric cancer--is the Macdonald Regimen Tolerable? A retrospective multi-institutional study. Radiat Oncol 2011;6:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Foo M, Leong T. Adjuvant therapy for gastric cancer: current and future directions. World J Gastroenterol 2014;20:13718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715–21. [DOI] [PubMed] [Google Scholar]
- [81].Petrillo A, Pompella L, Tirino G, et al. Perioperative treatment in resectable gastric cancer: current perspectives and future directions. Cancers (Basel) 2019;11:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hu Y, Hu D, Li W, Yu X. Neoadjuvant chemotherapy brings more survival benefits than postoperative chemotherapy for resectable gastric cancer: a meta-analysis of randomized controlled trials. JBUON 2019;24:201–14. [PubMed] [Google Scholar]
- [83].Glatz T, Bronsert P, Schäfer M, et al. Perioperative platin-based chemotherapy for locally advanced esophagogastric adenocarcinoma: postoperative chemotherapy has a substantial impact on outcome. Eur J Surg Oncol 2015;41:1300–7. [DOI] [PubMed] [Google Scholar]
- [84].Qu JL, Qu XJ, Li X, et al. Early initiation of fluorouracil-based adjuvant chemotherapy improves survival in patients with resectable gastric cancer. JBUON 2015;20:800–7. [PubMed] [Google Scholar]
- [85].Bausys R, Bausys A, Stanaitis J, et al. Propensity score-matched comparison of short-term and long-term outcomes between endoscopic submucosal dissection and surgery for treatment of early gastric cancer in a Western setting. Surg Endosc 2019;33:3228–37. [DOI] [PubMed] [Google Scholar]
- [86].Wang S, Zhang Z, Liu M, Li S, Jiang C. Endoscopic resection compared with gastrectomy to treat early gastric cancer: a systematic review and meta-analysis. PLoS One 2015;10:e0144774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Pyo JH, Byeon SJ, Lee H, et al. Measurement of tumor volume is not superior to diameter for prediction of lymph node metastasis in early gastric cancer with minute submucosal invasion. Oncotarget 2017;8:113758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Coburn N, Cosby R, Klein L, et al. Staging and surgical approaches in gastric cancer: a systematic review. Cancer Treat Rev 2018;63:104–15. [DOI] [PubMed] [Google Scholar]
- [89].Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 1999;79:1522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 2004;22:2069–77. [DOI] [PubMed] [Google Scholar]
- [91].Sano T, Sasako M, Mizusawa J, et al. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma. Ann Surg 2017;265:277–83. [DOI] [PubMed] [Google Scholar]
- [92].Degiuli M, Sasako M, Ponti A, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg 2014;101:23–31. [DOI] [PubMed] [Google Scholar]
- [93].Li W, Ye F, Wang D, et al. Protein predictive signatures for lymph node metastasis of gastric cancer. Int J Cancer 2013;132:1851–9. [DOI] [PubMed] [Google Scholar]
- [94].Shida A, Fujioka S, Kawamura M, et al. Prediction of lymph node metastasis in patients with submucosa-invading early gastric cancer. Anticancer Res 2014;34:4471–4. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.