Abstract
Acute kidney injury (AKI), characterized by acute renal dysfunction, is an increasingly common clinical problem and an important risk factor in the subsequent development of chronic kidney disease (CKD). Regardless of the initial insults, the progression of CKD after AKI involves multiple types of cells, including renal resident cells and immune cells such as macrophages. Recently, the involvements of macrophages in AKI-to-CKD transition have garnered significant attention. Furthermore, substantial progress has also been made in elucidating the pathophysiological functions of macrophages from the acute kidney to repair or fibrosis. In this review, we highlight current knowledge regarding the roles and mechanisms of macrophage activation and phenotypic polarization, and transdifferentiation in the development of AKI-to-CKD transition. In addition, the potential of macrophage-based therapy for preventing AKI-to-CKD transition is also discussed.
Keywords: Acute kidney injury, Chronic kidney disease, Macrophage, Inflammation, Repair, Macrophage-based therapy
Introduction
Increasing evidence shows that acute kidney injury (AKI) is a major cause of chronic kidney disease (CKD) and the reoccurrence of AKI in CKD patients may also accelerate CKD progression to end-stage renal disease.[1] Based on the findings of previous studies, the pooled adjusted hazard ratio of AKI-to-CKD is 4.3 compared with CKD without a history of AKI.[2] The development of AKI-to-CKD is heterogeneous as this transition occurs in different patients and can be caused by multiple conditions, such as nephrotoxin, sepsis, ischemia-reperfusion, surgical operation, and cardiovascular diseases.[3]
Currently, the mechanism of this multifactorial process remains unclear. However, such information is crucial for developing treatment strategies to prevent the harmful progression of kidney injury. Multiple cells, including immune cells, tubular epithelial cells (TECs), myofibroblasts, and fibrocytes, are proven to participate in the development of CKD and contribute to progressive fibrosis in the kidney.[4] Of note, immune cells, especially macrophages, are known to play a vital role in the AKI-to-CKD transition. Multiple renal resident mononuclear phagocytic cells and infiltrating immune cells can influence the fate of the injured kidney.[5] Furthermore, distinct macrophage subtypes are involved in the different stages of AKI.[6] Macrophages can evolve into various phenotypes to play multiple roles in causing AKI or promoting kidney repair or fibrosis. There are two major macrophage phenotypes including M1 (classically activated) and M2 (alternatively activated). The activation and transformation of macrophage phenotypes is primarily dependent on the surrounding environment.[7] Macrophages with different phenotypes play diverse roles in mediating renal injury, inflammation, repair, and fibrosis. Of note, several recent studies revealed that macrophages could directly trans-differentiate into myofibroblasts via macrophage-myofibroblast transition (MMT), thereby contributing to renal fibrosis.[8,9] In this review, we highlighted the current advances in cell biology and the impact of different macrophage subtypes on the process of AKI-to-CKD transition. In addition, we also discuss the potential of macrophage-targeted therapy in the prevention of AKI-to-CKD transition.
Macrophages in the Kidney
Origin of macrophages in the kidney
According to recent findings, local macrophages derived from the embryo are proliferating while macrophages derived from bone marrow are recruited into the damaged site during the process of renal inflammation.[10] The characteristics of macrophages are regulated by multiple factors, including external and internal signals, especially cytokines and transcription factors, which determine the tissue specificity.[10] It is important to distinguish macrophages derived from the two different sources as this affects the phenotype and function of macrophages. Based on the transcriptional analysis, resident macrophages have specific functions and characteristics as they possess transcriptional programs specific to tissues.[11] Resident macrophages play anti-inflammatory roles in kidney repair after injury, while circulating macrophages play pro-inflammatory roles after they migrate into tissues in response to injury.[12] Resident embryo-derived macrophages are also gradually replaced by bone marrow-derived macrophages with age during normal postnatal development and dynamic changes in surface marker expression under inflammatory or challenging conditions.[13]
Phenotype and polarization of macrophages in the kidney
Macrophages, the major immune population in normal kidney, are regarded as key sentinels that play a vital role in the establishment and pathogenesis of AKI.[14] Due to different polarization states, macrophages infiltrating the kidney exert a profound impact on renal injury, repair, and fibrosis.[15] Macrophages can change their phenotype in response to their surrounding microenvironment.[16] Generally, macrophages are categorized as M1 and M2. The M1 phenotype is characterized by CD38, CD80, inducible nitric oxide synthase, G-protein-coupled receptor 18, and formyl peptide receptor 2, while the M2 phenotype generally expresses CD163, CD206, early growth response protein 2, c-Myc, arginase, and RELMa.[17,18] Macrophages commonly accumulate in the kidney after injury and undergo a transition from a pro-inflammatory M1 phenotype to an alternatively activated M2 phenotype.
The M1 macrophages participate in the early stage of AKI models, such as contrast-induced and sepsis-induced nephropathy.[19,20] For example, the numbers of neutrophils and M1 macrophages increase in contrast-induced AKI and sepsis-induced AKI in response to heparinbinding protein.[21,22] The activation of the tumor necrosis factor-alpha (TNF-α)/high mobility group box 1 signaling pathway in the M1 macrophage plays an important role in pyroptosis during AKI.[23] Accordingly, interleukin (IL)-1 receptor-associated kinase-M (IRAK-M) induction during the recovery phase of AKI contributes to the resolution of M1 macrophage- and TNF-α-dependent renal inflammation, allowing the repairing and functional recovery of the injured kidney.[24]
M2 macrophages are critical for the inhibition of inflammation, remodeling, and recovery from AKI.[25] Of note, M2 macrophages can initiate pro-inflammatory responses by secreting chemokines that drive the rapid influx of neutrophils and inflammatory leukocytes during tissue injury or infection.[26] In addition, the M2 macrophage phenotype also contributes to long-term AKI outcomes, such as kidney recovery from injury or atrophy and fibrosis.[24] Thus, macrophage phenotype determines the fate of AKI.
Macrophages in Renal Inflammation
AKI may progress to long-term renal damage and cause renal fibrosis and chronic inflammation,[27,28] resulting in CKD.[29] Macrophages are a key inflammatory cell in kidneys with AKI. By using different macrophage depletion and transfer techniques, the pathogenic roles of these pro-inflammatory macrophages in kidney injury and progressive CKD have been revealed. Liposomal clodronate-mediated macrophage depletion in the early stage of ischemia-reperfusion injury and rhabdomyolysis-induced AKI has been found to significantly reduce renal injury and long-term renal fibrosis, indicating the pathogenic role of pro-inflammatory macrophages in the initiation of kidney injury.[30,31] Pro-inflammatory macrophages enhance renal injury possibly by accelerating renal inflammation via the release of several pro-inflammatory cytokines and chemokines or expression of receptors to trigger abnormal wound healing process, eventually leading to renal fibrosis.
During AKI, locally-produced cytokines and chemokines can recruit a cascade of inflammatory effector cells into the injured kidney tissue. For example, monocyte chemoattractant protein-1 (MCP-1) (encoded by the Ccl2 gene) functions as an inflammatory cytokine that recruits monocytes to the injured sites and contributes to the AKI.[32] High levels of MCP-1 are commonly observed after renal injury and elevated levels are found in the urine and kidney.[33,34] Granulocyte-macrophage colony-stimulating factor (GM-CSF) promotes macrophage differentiation.[35] According to recent findings, GM-CSF-induced MCP-1/C-C chemokine receptor type 2 (CCR2) signaling plays an important role in the crosstalk between injured tubular cells and immune cells such as infiltrating macrophages, dendritic cells, and T cells to promote renal injury and progressive interstitial fibrosis, resulting in AKI-to-CKD transition.[36]
Macrophage migration inhibitory factor (MIF) is a pro-inflammatory cytokine expressed in multiple cell types, including macrophages. Macrophages secrete MIF in response to a range of stimuli, including lipopolysaccharide (LPS), TNF, and interferon-γ (IFN-γ).[37] MIF has also been shown to regulate the production and secretion of pro-inflammatory cytokines, including TNF, IFN-γ, IL-1β, and IL-6.[38] A recent study revealed that MIF functions as a regulator of the nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome complex in macrophages. Inhibition of MIF in macrophages suppresses NLRP3-dependent secretion of IL-1β and IL-18 in vitro and in vivo.[39]
Macrophages highly express pathogen recognition receptors, such as toll-like receptors (TLRs), which are sensors of pathogen-associated molecules that trigger inflammatory signaling in macrophages.[40]In vitro, activation of macrophages is magnified in the presence of TLR4 ligands, such as LPS.[41] Chemokine receptors, such as CCR2 and CX3CR1, and their cognate ligands, MCP-1 and fractalkine (CX3CL1) can recruit bone marrow-derived monocytes to infiltrate the injured kidney and differentiate into pro-inflammatory macrophages in response to injury signals in several renal injury diseases.[42,43] During the transition from pro-inflammatory to profibrotic macrophages, the predominant role of CCR2 is to induce cell homing/persistence; however, CCR2 is not directly involved in profibrotic macrophage polarization.[36] Macrophage-inducible C-type lectin (Mincle) is a pattern recognition receptor.[44] Mincle is predominantly expressed and persists in macrophages, which exhibit mixed phenotypes with high expression of pro-inflammatory and anti-inflammatory markers in the late phase of AKI.[44] Mincle also contributes to tubular cell death and subsequent renal atrophy in the late phase after AKI. Collectively, the above findings suggest that Mincle is involved in the AKI-to-CKD transition.
Macrophages in Kidney Repair
The anti-inflammatory and reparative roles of macrophages have been well studied.[15] The pro-inflammatory macrophages are a predominant feature of the early injury phase, whereas reparative macrophages are exhibiting properties ensuing epithelial repair and renal function recovery phase.[45] Polarized macrophage populations can orchestrate renal repair during CKD. Ly6Cintermediate macrophages may contribute to renal repair by exerting anti-inflammatory and wound healing functions.[31] Depletion of macrophages in the late stage of the IRI model reduces TEC proliferation and delays renal repair; however, the transferal of IL-4-polarized M2 macrophages induces the repair process.[46] IL-4/IL-13-polarized M2a macrophages are essential for the recovery from ischemic AKI.[47] M2 macrophages exhibit an anti-inflammatory effect mainly by inducing anti-inflammatory factors and high endocytic capacities. M2 macrophages can produce anti-inflammatory cytokines and trophic factors, such as IL-10, transforming growth factor (TGF)-β, insulin-like growth factor, and hepatocyte growth factor. M2 macrophages can also deactivate T cells and macrophages to alleviate renal inflammation.[31]
Macrophages in Renal Fibrosis
Fibrosis is a well-established hallmark of CKD and macrophage infiltration is a common feature of active fibrotic lesions.[48] Infiltrating macrophages from blood monocytes/macrophages and renal resident macrophages proliferations accumulate in the damaged kidney and promote the progression of kidney fibrosis.[49] Generally, macrophage accumulation is significantly linked with the degree of glomerulosclerosis and the extent of interstitial fibrosis and tubular atrophy.[50]
Based on accumulating evidence, the M2 macrophage phenotype is present during kidney fibrosis, although M1 macrophages remain a feature of chronic inflammation. Multiple studies indicate that the accumulation of M2 macrophages expressing CD206 and/or CD163 correlates with renal fibrosis in both human and animal models of kidney disease. The number of glomerular CD163 + macrophages localized in areas of active fibrosis has been confirmed to be associated with glomerulosclerosis, tubular atrophy, interstitial fibrosis, and degree of mesangial matrix expansion.[51] These macrophages are frequently present in areas of interstitial fibrosis, characterized by the deposition of type I collagen and accumulation of myofibroblasts that express smooth muscle α-actin (α-SMA).[52] CD206+ M2 macrophages are indicated to be highly correlated with subclinical inflammation, tubular injury, and the progression of fibrosis.[53]
Depletion of the M2 macrophages is proven to protect against progressive interstitial collagen deposition. For example, the adoptive transfer of M2 macrophages, but not M1 macrophages, reversed the beneficial effects of macrophage depletion on renal fibrosis.[54] Similarly, macrophage depletion from day 4 in a model of unilateral ureteral obstruction (UUO) significantly reduced renal fibrosis, whereas the adoptive transfer of M2 macrophages promoted the accumulation of α-SMA+ cells.[55] These findings suggest a profibrotic role of M2 macrophages and their subsets in renal fibrosis.
Several transcription factors are involved in mediating the functions of macrophages in kidney fibrosis. KLF4 (a zinc-finger transcription factor), an anti-inflammatory signal, is an essential regulator of macrophage polarization. Deletion of KLF4 in macrophages stimulates their production of TNF-α, with consequent TEC necroptosis and exaggerated renal interstitial fibrosis.[56]
Several receptors expressed by macrophages have been shown to play an important role in profibrotic responses after AKI. The small secreted protein, breast regression protein-39 (BRP-39 or Chi3l1), and its receptor, PTGDR2, are highly expressed by macrophages during the early stages of kidney repair and promote tubular cell survival via IL-13 receptor α2-mediated signaling. In addition, BRP-39 can promote macrophage profibrotic response and interstitial fibrosis in the IRI model.[57]
Macrophages in AKI-to-CKD Transition
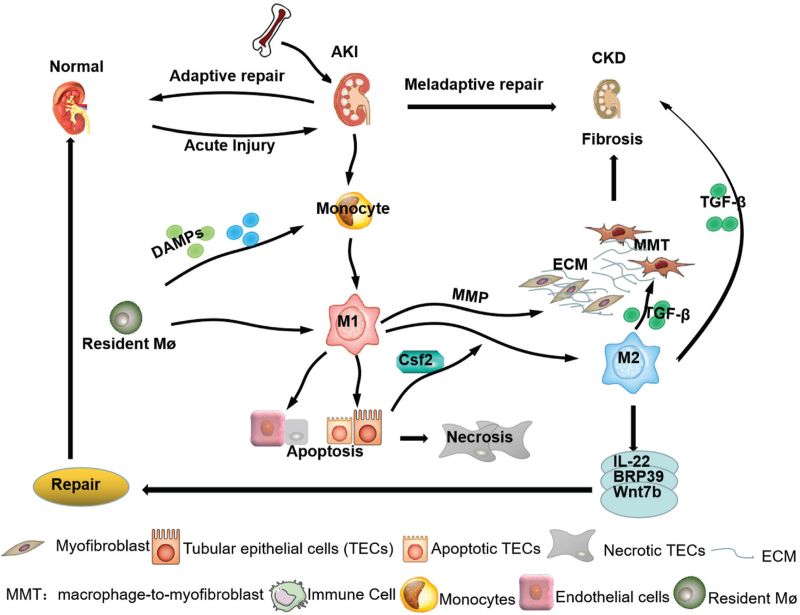
Infiltrating macrophages and other immune cells, damaged TECs, disruption of the renal vascular system, activation of interstitial fibroblasts, hypoxia, inflammatory response, and oxidative stress are involved in AKI-to-CKD transition.[58,59] Among them, macrophages play an important role in AKI-to-CKD through interacting with multiple factors [Figure 1].
Figure 1.
Macrophages in AKI-to-CKD progression. Macrophages derived from bone marrow are recruited into the injured kidney and promote AKI-to-CKD by directly and indirectly Interacting with intrinsic kidney cells and via the process of MMT. AKI: Acute kidney injury; BRP: Breast regression protein; CKD: Chronic kidney disease; DAMPs: Damage-associated molecular patterns; MMT: Macrophage-myofibroblast transition; ECM: Extracellular matrix; MMP: Matrix metalloproteinase; M1: Macrophage 1 phenotype; M2: Macrophage 2 phenotype; Mø: Resident macrophage; TGF-β: Transforming growth factor-β.
Phenotype of macrophages in AKI-to-CKD transition
Macrophages can change their phenotypes from M1 and M2 in response to kidney injury. Both M1 and M2 macrophages may coexist in AKI and CKD. Thus, it is important to understand the roles of macrophages in AKI-to-CKD transition.[60] For example, bone marrow-derived Ly6C− macrophages can induce AKI and subsequently mediate CKD.[61] Ly6Chigh macrophages are essential for renal damage, which is accelerated by the production of pro-inflammatory factors, such as TNF-α and interleukins.[31] IRAK-M is a macrophage-specific inhibitor of TLR and IL-1 receptor signaling that prevents polarization toward a pro-inflammatory phenotype. IRAK-M has been confirmed to be involved in the regulation of macrophage phenotype, wound healing, and tissue regeneration during progressive CKD after AKI.[24] Moreover, macrophage subtypes have been proven to play crucial roles in AKI-to-CKD progression.
Macrophages and renal microenvironment in AKI-to-CKD transition
Pathogen-associated molecular patterns and damage-associated molecular patterns released by infectious organisms and/or cell necrosis may induce and promote the infiltration of bone marrow-derived macrophage via TLRs and other innate pattern recognition receptors toward a pro-inflammatory phenotype in the microenvironments of the kidney.[62] In turn, macrophages can alter the microenvironment of the diseased kidney by interacting with neighboring cells, including TECs, endothelial cells, immune cells, fibroblasts, and myofibroblasts.[63]
Macrophages and microenvironments
Macrophages alter their phenotypes in response to the renal microenvironment; however, macrophages can also modify the microenvironment via a paracrine effect.[64] M1 macrophages are pro-inflammatory and produce cytokines, such as IL-1, IL-6, and TNF-α, whereas M2 are mainly anti-inflammatory and express arginase, mannose receptor, IL-10, and IL-4 receptor-α. Thus, macrophages display considerable diversity and plasticity and can promote or inhibit inflammatory (or other) processes in different contexts. Based on a recent study, pro-inflammatory macrophages from circulating monocytes play an important role in initial IRI and may promote fibrosis.[36] This is confirmed by blocking MCP-1/CCR2 signaling to inhibit macrophage-mediated renal injury.[36] GM-CSF expressed by injured TECs can also trigger macrophages expressing MCP-1 to recruit additional macrophages, dendritic cells, and T cells into the injured kidney. Profibrotic macrophages can also highly express platelet-derived growth factor (PDGF)-β and TGF-β and stimulate PDGFRβ-positive myofibroblasts to produce extracellular matrix (ECM) components, such as collagen 1, COL3a1, and fibronectin.[36] In addition, T cells and dendritic cells are involved in the cytokine microenvironment to sustain kidney injury together with macrophages.[62,63]
The microenvironment status, such as hypoxia, plays a regulatory role in the activation of macrophages. Previous studies revealed that hypoxia can activate TECs, inflammatory cells, and fibroblasts and contributes to the progression of fibrosis.[65] Activation of the hypoxia-inducible factor (HIF) is the central cellular response to hypoxia.[66] Multiple studies have revealed that myeloid HIFs are required for inflammatory macrophage-specific functions in acute ischemic, hypoxic injury, and various types of renal diseases.[67,68] For example, macrophage-specific HIF-1a activation and lowered autophagic flux stimulate an inflammatory response by activating the nuclear factor-kappa B signaling pathway.[69] HIF-1α inducing the exosomal expression of microRNA-23a mediates the crosstalk between TECs and macrophages in tubulointerstitial inflammation.[70] Thus, targeting HIFs can effectively inhibit macrophage activation and the AKI-to-CKD transition. This is confirmed by a study that FG4592 (Roxadustat), which induces HIF stabilization, has been approved to decrease macrophage infiltration and the release of inflammatory cytokines, such as TNF-α and IL-1β, and to reduce collagen deposition as well as the expression of fibrosis biomarkers.[71]
Macrophages and renal TECs
Renal TECs are the major cell type in maintaining the stability of renal homeostasis; however, they are also the main victim of AKI as they are susceptible to various types of cellular stress.[72] Tubular injury is not only a primary trigger of AKI but also a potential determinant of later CKD progression by promoting inflammation and fibrosis.[73,74] These observations indicate that tubules play an essential role in AKI-to-CKD progression. Generally, after AKI, TECs dedifferentiate, proliferate, and re-differentiate, contributing to the repair of nephrons. Macrophages infiltrating the kidney in response to injury can influence these pathophysiological processes by directly or indirectly interesting with TECs and inducing or promoting TEC injury and death.[15,24] Intriguingly, TECs can also regulate the phenotype of macrophages. In sepsis-induced AKI, Csf2 exclusively secreted by injured TECs can promote the transition of M1 macrophages to the M2 phenotype in dose- and time-dependent manners. In addition, Csf2 can also regulate macrophage transition by activating p-Signal transducers and activators of transcription 5.[75]
Macrophages and endothelial cells
The endothelium is a dynamic organ that plays an important role in renal physiopathology. The glomerular endothelial cells contribute to the glomerular filtration barrier and support podocyte structure, whereas endothelial cells of the microvasculature in the kidney contribute to tubular reabsorption. Endothelial cell injury after AKI has long-term outcomes of CKD.[76] Epithelial-endothelial cell interactions exhibit an important effect on capillary rarefaction. Previous studies suggested that injured TECs can cause capillary rarefaction, which further leads to hypoxic TEC injury, thereby resulting in a vicious circle effect.[77] Endothelial cells can also interact with macrophages under kidney disease conditions. Endothelial progenitor cells, which can differentiate into endothelial cells, dwell in stem cell niches and can be derived from the monocyte/macrophage lineage.[78] Pro-inflammatory macrophages release a large amount of TNF-α, which induces the apoptosis of endothelial cells by binding to TNF receptors.[62] Inflammation is closely linked with the endothelial stress response. Factor Xa and the activation of protease-activated receptors (PARs) on the surface of endothelial cells induce glomerular macrophages to release inflammatory mediators, such as MCP-1 and IL-1β.[79]
Macrophages and myofibroblasts
Macrophages can produce fibrotic factors, including fibronectin and collagen, in response to a fibrotic microenvironment under various disease conditions.[80] Moreover, macrophages produce several profibrotic factors (e.g., TGF-β1, galectin-3), pro-inflammatory cytokines, and other factors (e.g., IL-1, TNF-α) to enhance the activation, proliferation, survival, and transdifferentiation of myofibroblasts during CKD progression.[31] Activated α-SMA +, PDGFRβ+ myofibroblasts, the main collagen-producing cells, are believed to be directly responsible for most ECM expression, including ECM proteins and collagen, leading to progressive renal fibrosis.[81] The crosstalk between macrophages and myofibroblasts has been demonstrated by several groups. Macrophage-derived PDGFβ and/or TGF-β have been demonstrated to be potentially important for myofibroblast activation and play a driving role in pericyte-myofibroblast transformation.[82] Based on the above observation, the crosstalk between macrophages and the surrounding cells may also promote the polarization, fate of macrophages, and may be partially responsible for the regenerating kidney.
MMT in AKI-to-CKD transition
Inflammation is regarded as one of the earliest processes after renal injury. Acute inflammation is followed by tissue repair or scarring. During successful repair, macrophages regress or undergo apoptosis. However, if severe or unresolved injury exists, macrophages can become a profibrotic phenotype, thereby promoting kidney fibrosis through myofibroblast activation.[83] Unexpectedly, our recent studies uncovered that monocyte/macrophages from bone marrow can acquire myofibroblast phenotype via the process of MMT in a mouse model of unilateral ureteric obstruction.[84] This novel finding is consistent with the profibrotic effect of macrophages as reported by other studies.[85] Macrophages infiltrating the kidney in response to injury can transition directly or via paracrine effects into myofibroblasts.[31,48] Indeed, MMT cells are prominently accumulated in sites of fibrosis by co-expressing macrophage (CD68) and myofibroblast (α-SMA) markers and are an active form of fibroblasts with both fibrogenic and pro-inflammatory properties.[86] Thus, MMT cells correlate better with the CKD progression including chronic renal allograft rejection.[87] Thus, MMT may be a newly identified source of myofibroblast origin during renal fibrosis and may play an important role in progressive renal fibrosis.
MMT contributes to fibrosis in diverse diseases and is mainly driven by the TGF-β1/Smad3 signaling pathway.[88,89] To identify alternative therapeutic targets downstream of TGF-β/Smad3 signaling, single-cell RNA-seq has been employed in an in vitro model of MMT. Accordingly, Src is identified as a key regulator of the MMT-dependent gene network. Furthermore, Pou4f1 is the only transcription factor involved in TGF-β1/Smad3-mediated MMT based on unbiased gene network analysis.[90] Pou4f1 is highly expressed by macrophages undergoing MMT in fibrotic lesions in human and experimental kidney disease and is considered to stimulate renal fibrosis directly through the MMT.[86] Fatty acid-binding protein 4 (FABP4) has also been revealed to be responsible for the MMT process. According to Feng et al[89], FABP4 is co-expressed in MMT cells and serves as an important factor that contributes to renal interstitial fibrosis by mediating the MMT process.
The role of the MMT process is still debatable, as MMT has not been identified in all myeloid lineage tracing studies in models of renal fibrosis.[91] Furthermore, another study revealed that bone marrow-derived macrophages account for only a small fraction that directly contributes to myofibroblasts.[92] This discrepancy may partially be associated with the nature, the stage of the disease, and the severity of renal fibrosis. In addition, the MMT process is dynamic and can only be observed in active inflammatory lesions with progressive renal fibrosis, but is rare in diseases with acute inflammation or non-progressive renal fibrosis.[84] These conflicting results warrant further investigation.
MMT may account for a substantial component of the myofibroblast population, arguing for MMT as a common mechanism of renal fibrosis. However, whether MMT plays key roles in the AKI-to-CKD model remains undetermined and needs to be further explored.
Macrophage-related signal pathways in AKI-to-CKD transition
Notch signaling pathway
The macrophage Notch signaling pathway is known for its indispensable role in various types of AKI (e.g., ischemic AKI) and CKD by increasing inflammation and apoptosis, as well as promoting renal fibrosis.[93,94] Recently, a study revealed that myeloid-specific Notch activation aggravates renal fibrosis, which is mediated by CCR2 + macrophages infiltration.[95] The Notch signaling pathway is also responsible for determining the balance between the M1/M2 phenotype in CKD.[96]
TGF-β/Smad signaling pathway
The TGF-β/Smad signaling pathway plays a significant role in the fibrogenesis of the kidney and is a central mediator in renal fibrosis.[97] Tubular cells, the primary target of AKI, could proliferate and regenerate after injury.[29] However, the TEC injury is persistent and becomes unrepaired, TECs can contribute to the transition from AKI to CKD. TGF-β/Smad signaling has been considered as an important factor during this progression.[98] This pathway not only perpetuates TECs injury but also promotes macrophage chemotaxis after AKI. TGF-β/Smad signaling acts as a potent chemoattractant for macrophages and may promote injury by augmenting macrophage infiltration and activation,[98] although macrophage-derived TGF-β1 may not be essential for UUO-induced renal interstitial fibrosis.[99] TGF-β/Smad signaling also promotes progressive renal fibrosis by mediating ECM synthesis, inhibiting its degradation, and stimulating TECs and endothelial cells to undergo epithelial-to-mesenchymal transition or endothelial-to-mesenchymal transition.[100] Most significantly, our recent studies identified that TGF-β can induce MMT via the Smad3-dependent mechanism as deletion of Smad3 from macrophages and targeting the Smad3-Src-POU4f1 pathway can block MMT and renal fibrosis.[86,87,90]
Wnt/β-catenin signaling pathway
Under normal conditions, the Wnt/β-catenin pathway is silent in the kidney; however, Wnt/β-catenin signaling becomes activated once an injury occurs.[101] Sustained activation of the Wnt/β-catenin signaling pathway plays a decisive role in driving the AKI-to-CKD continuum and accelerates renal fibrosis.[102] This is confirmed by treating macrophages with Wnt3a to exacerbate IL-4- or TGF-β1-induced macrophage alternative (M2) polarization during kidney fibrosis.[59]
Macrophage-targeted Therapy for AKI-to-CKD Transition
To date, therapeutic strategies that interfere with monocyte/macrophage recruitment, activation, and polarization, or macrophage-cell-based therapy have been widely studied. Liposomal clodronate-mediated clearance of macrophage alleviates renal fibrosis.[103] Modification of macrophage activation and infiltration may also prevent renal fibrosis. For example, macrophage TGF-βRII deletion results in inhibition of macrophage infiltration and renal fibrosis after AKI.[104] Blocking NF-kB signaling using antisense oligonucleotides or its natural inhibitor, IκB, suppresses the classical activation of macrophages, but increases anti-inflammatory macrophages, thereby alleviating kidney injury.[105] Additionally, treatment with Quercetin or inhibition of Wnt5a has been shown to block macrophage infiltration and M2 polarization, thereby preventing ECM production and interstitial fibrosis in a TGF-β1/Smad-dependent mechanism.[106,107]
Multiple factors, including CSF-1 receptor (CSF-1R), can influence the proliferation, differentiation, and survival of macrophages.[108] Dysfunction in the Csf1r gene selectively inhibits the development of tissue macrophage populations and leads to an almost complete lack of F4/80+ cells in tissues.[109] Deletion of the super-enhancer of the Csf1r gene or blocking CSF-1R depletes macrophages in the kidney.[109] Treatment with the CSF-1R inhibitor, GW2580, in an I/R-induced AKI model can inhibit Ly6C+ M2-like macrophage infiltration.[110] The protease-activated receptor (PAR)-1 antagonist, vorapaxar, is reported to suppress macrophage infiltration by inhibiting MAPK ERK1/2 and TGF-β/Smad signaling during AKI-to-CKD transition.[111] Intriguingly, macrophage-derived microvesicles for kidney targeted delivery of dexamethasone produce a superior suppressive effect on renal inflammation and fibrosis.[112]
Multiple cell-based hematopoietic products are another therapeutic approach.[113] For example, macrophages could be adoptively transferred after ex vivo genetic modification.[114] Recently, exogenous macrophage-based therapies for inflammatory and degenerative diseases, including renal disease, have been developed.[115,116] For example, genetically modified bone-marrow-derived macrophages to produce IL-4 or IL-10 (anti-inflammatory cytokines associated with M2 polarization) have been shown to effectively inhibit experimental glomerulonephritis.[116]
Conclusion
AKI is a major cause of CKD and AKI-to-CKD transition is a key process leading to progressive renal injury. Macrophages are a key immune cell that plays a driving role in AKI-to-CKD. M1 macrophages are pro-inflammatory and cause AKI by directly interacting with intrinsic kidney cells such as TECs and endothelial cells or indirectly by producing abundant pro-inflammatory cytokines. M2 macrophages can be renal reparative involving the repair process by secreting anti-inflammatory cytokines. However, they can become pro-fibrogenic and again the myofibroblast phenotype via the MMT process when a renal injury is unresolved and progressive. MMT cells are of pro-inflammatory and profibrotic phenotype and may play a driving role from AKI-to-CKD, which is tightly regulated by TGF-β/Smad3 signaling. Thus, modified macrophage phenotypes or targeting the pathways that regulate M1 macrophage activation and MMT may be a promising therapeutic approach for the treatment of kidney disease by preventing AKI-to-CKD progression.
Funding
This work was supported by the Research Grants Council of Hong Kong (Nos. 14117418, 14104019, 14101121, and C7018-16G), the Lui Che Woo Institute of Innovative Medicine (CARE program), and the Guangdong-Hong Kong-Macao-Joint Labs Program from Guangdong Science and Technology (No. 2019B121205005). This work was also supported by the National Natural Science Foundation of China (Nos. 81970584 and 82100727).
Conflicts of interest
None.
Footnotes
How to cite this article: Meng X, Jin J, Lan HY. Driving role of macrophages in transition from acute kidney injury to chronic kidney disease. Chin Med J 2022;135:757–766. doi: 10.1097/CM9.0000000000002100
Xiaoming Meng and Juan Jin contributed equally to this work.
References
- 1.Charlton JR, Xu Y, Wu T, deRonde KA, Hughes JL, Dutta S, et al. Magnetic resonance imaging accurately tracks kidney pathology and heterogeneity in the transition from acute kidney injury to chronic kidney disease. Kidney Int 2021; 99:173–185. doi: 10.1016/j.kint.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdala PM, Swanson EA, Hutchens MP. Meta-analysis of AKI to CKD transition in perioperative patients. Perioper Med (Lond) 2021; 10:24.doi: 10.1186/s13741-021-00192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuang Q, Wu S, Xue N, Wang X, Ding X, Fang Y. Selective Wnt/beta-catenin pathway activation concomitant with sustained overexpression of miR-21 is responsible for aristolochic acid-induced AKI-to-CKD transition. Front Pharmacol 2021; 12:667282.doi: 10.3389/fphar.2021.667282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 2006; 69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 5.Nelson PJ, Rees AJ, Griffin MD, Hughes J, Kurts C, Duffield J. The renal mononuclear phagocytic system. J Am Soc Nephrol 2012; 23:194–203. doi: 10.1681/ASN.2011070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen T, Cao Q, Wang Y, Harris DCH. M2 macrophages in kidney disease: biology, therapies, and perspectives. Kidney Int 2019; 95:760–773. doi: 10.1016/j.kint.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 7.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng XM, Wang S, Huang XR, Yang C, Xiao J, Zhang Y, et al. Inflammatory macrophages can transdifferentiate into myofibroblasts during renal fibrosis. Cell Death Dis 2016; 7:e2495.doi: 10.1038/cddis 2016.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuppe C, Ibrahim MM, Kranz J, Zhang X, Ziegler S, Perales-Patón J, et al. Decoding myofibroblast origins in human kidney fibrosis. Nature 2021; 589:281–286. doi: 10.1038/s41586-020-2941-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gentek R, Molawi K, Sieweke MH. Tissue macrophage identity and self-renewal. Immunol Rev 2014; 262:56–73. doi: 10.1111/imr.12224. [DOI] [PubMed] [Google Scholar]
- 11.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 2014; 159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol 2016; 17:2–8. doi: 10.1038/ni.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molawi K, Wolf Y, Kandalla PK, Favret J, Hagemeyer N, Frenzel K, et al. Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med 2014; 211:2151–2158. doi: 10.1084/jem.20140639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nash WT, Okusa MD. Chess not checkers: complexities within the myeloid response to the acute kidney injury syndrome. Front Med (Lausanne) 2021; 8:676688.doi: 10.3389/fmed.2021.676688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 2011; 22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engel JE, Chade AR. Macrophage polarization in chronic kidney disease: a balancing act between renal recovery and decline? Am J Physiol Renal Physiol 2019; 317:F1409–F1413. doi: 10.1152/ajprenal 00380.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Kubes P. A reservoir of mature cavity macrophages that can rapidly invade visceral organs to affect tissue repair. Cell 2016; 165:668–678. doi: 10.1016/j.cell.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Le K, Sun J, Khawaja H, Shibata M, Maggirwar SB, Smith MR, et al. Mantle cell lymphoma polarizes tumor-associated macrophages into M2-like macrophages, which in turn promote tumorigenesis. Blood Adv 2021; 5:2863–2878. doi: 10.1182/bloodadvances.2020003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubio-Navarro A, Carril M, Padro D, Guerrero-Hue M, Tarín C, Samaniego R, et al. CD163-macrophages are involved in rhabdomyolysis-induced kidney injury and may be detected by MRI with targeted gold-coated iron oxide nanoparticles. Theranostics 2016; 6:896–914. doi: 10.7150/thno.14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan RZ, Liu J, Zhang YY, Wang HL, Li JC, Liu YH, et al. Curcumin relieved cisplatin-induced kidney inflammation through inhibiting Mincle-maintained M1 macrophage phenotype. Phytomedicine 2019; 52:284–294. doi: 10.1016/j.phymed.2018.09.210. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Shi D, Zhang H, Yao X, Wang S, Wang R, et al. The application of functional magnetic resonance imaging in type 2 diabetes rats with contrast-induced acute kidney injury and the associated innate immune response. Front Physiol 2021; 12:669581.doi: 10.3389/fphys 2021.669581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xing L, Zhongqian L, Chunmei S, Pingfa C, Lei H, Qin J, et al. Activation of M1 macrophages in sepsis-induced acute kidney injury in response to heparin-binding protein. PLoS One 2018; 13:e0196423.doi: 10 1371/journal pone.0196423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Zhang H, Chen Q, Jiao F, Shi C, Pei M, et al. TNF-alpha/HMGB1 inflammation signalling pathway regulates pyroptosis during liver failure and acute kidney injury. Cell Prolif 2020; 53:e12829.doi: 10.1111/cpr.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lech M, Gröbmayr R, Ryu M, Lorenz G, Hartter I, Mulay SR, et al. Macrophage phenotype controls long-term AKI outcomes — kidney regeneration versus atrophy. J Am Soc Nephrol 2014; 25:292–304. doi: 10.1681/ASN.2013020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singbartl K, Formeck CL, Kellum JA. Kidney-immune system crosstalk in AKI. Semin Nephrol 2019; 39:96–106. doi: 10.1016/j.semnephrol.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Hu Q, Lyon CJ, Fletcher JK, Tang W, Wan M, Hu TY. Extracellular vesicle activities regulating macrophage- and tissue-mediated injury and repair responses. Acta Pharm Sin B 2021; 11:1493–1512. doi: 10.1016/j.apsb.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 2012; 81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 2012; 82:516–524. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 29.Sato Y, Yanagita M. Immune cells and inflammation in AKI to CKD progression. Am J Physiol Renal Physiol 2018; 315:F1501–F1512. doi: 10.1152/ajprenal.00195.2018. [DOI] [PubMed] [Google Scholar]
- 30.Belliere J, Casemayou A, Ducasse L, Zakaroff-Girard A, Martins F, Iacovoni JS, et al. Specific macrophage subtypes influence the progression of rhabdomyolysis-induced kidney injury. J Am Soc Nephrol 2015; 26:1363–1377. doi: 10.1681/ASN.2014040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng XM, Mak TS, Lan HY. Macrophages in renal fibrosis. Adv Exp Med Biol 2019; 1165:285–303. doi: 10.1681/ASN.2014040320. [DOI] [PubMed] [Google Scholar]
- 32.Kitagawa K, Wada T, Furuichi K, Hashimoto H, Ishiwata Y, Asano M, et al. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol 2004; 165:237–246. doi: 10.1016/S0002-9440(10)63292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng D, Wolfe M, Cowley BD, Jr, Wallace DP, Yamaguchi T, Grantham JJ. Urinary excretion of monocyte chemoattractant protein-1 in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2003; 14:2588–2595. doi: 10.1097/01.asn.0000088720.61783.19. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Zhou X, Fan LX, Yao Y, Swenson-Fields KI, Gadjeva M, et al. Macrophage migration inhibitory factor promotes cyst growth in polycystic kidney disease. J Clin Invest 2015; 125:2399–2412. doi: 10.1172/JCI80467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamilton JA. GM-CSF in inflammation. J Exp Med 2020; 217:e20190945.doi: 10.1084/jem.20190945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu L, Sharkey D, Cantley LG. Tubular GM-CSF promotes late MCP-1/CCR2-mediated fibrosis and inflammation after ischemia/reperfusion injury. J Am Soc Nephrol 2019; 30:1825–1840. doi: 10.1681/ASN.2019010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol 2003; 3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bozza FA, Gomes RN, Japiassú AM, Soares M, Castro-Faria-Neto HC, Bozza PT, et al. Macrophage migration inhibitory factor levels correlate with fatal outcome in sepsis. Shock 2004; 22:309–313. doi: 10.1097/01.shk.0000140305.01641.c8. [DOI] [PubMed] [Google Scholar]
- 39.Lang T, Lee JPW, Elgass K, Pinar AA, Tate MD, Aitken EH, et al. Macrophage migration inhibitory factor is required for NLRP3 inflammasome activation. Nat Commun 2018; 9:2223.doi: 10.1038/s41467-018-04581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fitzpatrick JM, Minogue E, Curham L, Tyrrell H, Gavigan P, Hind W, et al. MyD88-dependent and -independent signalling via TLR3 and TLR4 are differentially modulated by Delta(9)-tetrahydrocannabinol and cannabidiol in human macrophages. J Neuroimmunol 2020; 343:577217.doi: 10.1016/j.jneuroim.2020.577217. [DOI] [PubMed] [Google Scholar]
- 41.Wu H, Wang Y, Zhang Y, Xu F, Chen J, Duan L, et al. Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox Biol 2020; 32:101500.doi: 10.1016/j.redox.2020.101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chousterman BG, Boissonnas A, Poupel L, Baudesson de Chanville C, Adam J, Tabibzadeh N, et al. Ly6Chigh monocytes protect against kidney damage during sepsis via a CX3CR1-dependent adhesion mechanism. J Am Soc Nephrol 2016; 27:792–803. doi: 10.1681/ASN.2015010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huen SC, Cantley LG. Macrophages in renal injury and repair. Annu Rev Physiol 2017; 79:449–169. doi: 10.1146/annurev-physiol-022516-034219. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka M, Saka-Tanaka M, Ochi K, Fujieda K, Sugiura Y, Miyamoto T, et al. C-type lectin Mincle mediates cell death-triggered inflammation in acute kidney injury. J Exp Med 2020; 217:e20192230.doi: 10.1084/jem.20192230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar S. Cellular and molecular pathways of renal repair after acute kidney injury. Kidney Int 2018; 93:27–40. doi: 10.1016/j.kint.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 46.Vinuesa E, Hotter G, Jung M, Herrero-Fresneda I, Torras J, Sola A. Macrophage involvement in the kidney repair phase after ischaemia/reperfusion injury. J Pathol 2008; 214:104–113. doi: 10.1002/path.2259. [DOI] [PubMed] [Google Scholar]
- 47.Zhang MZ, Wang X, Wang Y, Niu A, Wang S, Zou C, et al. IL-4/IL-13-mediated polarization of renal macrophages/dendritic cells to an M2a phenotype is essential for recovery from acute kidney injury. Kidney Int 2017; 91:375–386. doi: 10.1016/j.kint.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang PM, Nikolic-Paterson DJ, Lan HY. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol 2019; 15:144–158. doi: 10.1038/s41581-019-0110-2. [DOI] [PubMed] [Google Scholar]
- 49.Day YJ, Huang L, Ye H, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: Role of macrophages. Am J Physiol Renal Physiol 2005; 288:F722–F731. doi: 10.1152/ajprenal 00378.2004. [DOI] [PubMed] [Google Scholar]
- 50.Klessens CQF, Zandbergen M, Wolterbeek R, Bruijn JA, Rabelink TJ, Bajema IM, et al. Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol Dial Transplant 2017; 32:1322–1329. doi: 10.1093/ndt/gfw260. [DOI] [PubMed] [Google Scholar]
- 51.Ikezumi Y, Suzuki T, Karasawa T, Hasegawa H, Yamada T, Imai N, et al. Identification of alternatively activated macrophages in new-onset paediatric and adult immunoglobulin A nephropathy: potential role in mesangial matrix expansion. Histopathology 2011; 58:198–210. doi: 10.1111/j.1365-2559.2011.03742.x. [DOI] [PubMed] [Google Scholar]
- 52.Ikezumi Y, Suzuki T, Yamada T, Hasegawa H, Kaneko U, Hara M, et al. Alternatively activated macrophages in the pathogenesis ofchronic kidney allograft injury. Pediatr Nephrol 2015; 30:1007–1017. doi: 10.1007/s00467-014-3023-0. [DOI] [PubMed] [Google Scholar]
- 53.Toki D, Zhang W, Hor KL, Liuwantara D, Alexander SI, Yi Z, et al. The role of macrophages in the development of human renal allograft fibrosis in the first year after transplantation. Am J Transplant 2014; 14:2126–2136. doi: 10.1111/ajt.12803. [DOI] [PubMed] [Google Scholar]
- 54.Kim MG, Kim SC, Ko YS, Lee HY, Jo SK, Cho W. The role of M2 macrophages in the progression of chronic kidney disease following acute kidney injury. PLoS One 2015; 10:e0143961.doi: 10 1371/journal pone.0143961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen B, Liu X, Fan Y, Qiu J. Macrophages regulate renal fibrosis through modulating TGFbeta superfamily signaling. Inflammation 2014; 37:2076–2084. doi: 10.1007/s10753-014-9941-y. [DOI] [PubMed] [Google Scholar]
- 56.Wen Y, Lu X, Ren J, Privratsky JR, Yang B, Rudemiller NP, et al. KLF4 in macrophages attenuates TNFalpha-mediated kidney injury and fibrosis. J Am Soc Nephrol 2019; 30:1925–1938. doi:10 1681/ASN.2019020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Montgomery TA, Xu L, Mason S, Chinnadurai A, Lee CG, Elias JA, et al. Breast regression protein-39/chitinase 3-like 1 promotes renal fibrosis after kidney injury via activation of myofibroblasts. J Am Soc Nephrol 2017; 28:3218–3226. doi: 10.1681/ASN.2017010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shu S, Wang Y, Zheng M, Liu Z, Cai J, Tang C, et al. Hypoxia and hypoxia-inducible factors in kidney injury and repair. Cells 2019; 8:207.doi: 10.3390/cells8030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feng Y, Ren J, Gui Y, Wei W, Shu B, Lu Q, et al. Wnt/beta-cateninpromoted macrophage alternative activation contributes to kidney fibrosis. J Am Soc Nephrol 2018; 29:182–193. doi: 10.1681/ASN.2017040391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Landis RC, Quimby KR, Greenidge AR. M1/M2 macrophages in diabetic nephropathy: Nrf2/HO-1 as therapeutic targets. Curr Pharm Des 2018; 24:2241–2249. doi: 10.2174/1381612824666180716163845. [DOI] [PubMed] [Google Scholar]
- 61.Yang Q, Wang Y, Pei G, Deng X, Jiang H, Wu J, et al. Bone marrow-derived Ly6C(-) macrophages promote ischemia-induced chronic kidney disease. Cell Death Dis 2019; 10:291.doi: 10.1038/s41419-019-1531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int 2011; 80:915–925. doi: 10.1038/ki.2011.217. [DOI] [PubMed] [Google Scholar]
- 63.Meng XM, Tang PM, Li J, Lan HY. Macrophage phenotype in kidney injury and repair. Kidney Dis (Basel) 2015; 1:138–146. doi: 10.1159/000431214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meng XM. Inflammatory mediators and renal fibrosis. Adv Exp Med Biol 2019; 1165:381–406. doi: 10.1007/978-981-13-8871-2_18. [DOI] [PubMed] [Google Scholar]
- 65.Anders HJ. Immune system modulation of kidney regeneration — mechanisms and implications. Nat Rev Nephrol 2014; 10:347–358. doi: 10.1038/nrneph.2014.68. [DOI] [PubMed] [Google Scholar]
- 66.Gonzalez FJ, Xie C, Jiang C. The role of hypoxia-inducible factors in metabolic diseases. Nat Rev Endocrinol 2018; 15:21–32. doi: 10.1038/s41574-018-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cowman SJ, Fuja DG, Liu XD, Tidwell RSS, Kandula N, Sirohi D, et al. Macrophage HIF-1alpha is an independent prognostic indicator in kidney cancer. Clin Cancer Res 2020; 26:4970–4982. doi: 10.1158/1078-0432.CCR-19-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tateishi Y, Osada-Oka M, Tanaka M, Shiota M, Izumi Y, Ishimura E, et al. Myeloid HIF-1 attenuates the progression of renal fibrosis in murine obstructive nephropathy. J Pharmacol Sci 2015; 127:181–189. doi: 10.1016/j.jphs.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 69.Wang X, de Carvalho Ribeiro M, Iracheta-Vellve A, Lowe P, Ambade A, Satishchandran A, et al. Macrophage-specific hypoxia-inducible factor-1alpha contributes to impaired autophagic flux in nonalcoholic steatohepatitis. Hepatology 2019; 69:545–563. doi: 10.1002/hep.30215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li ZL, Lv LL, Tang TT, Wang B, Feng Y, Zhou LT, et al. HIF-1alpha inducing exosomal microRNA-23a expression mediates the cross-talk between tubular epithelial cells and macrophages in tubulointerstitial inflammation. Kidney Int 2019; 95:388–404. doi: 10.1016/j.kint.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 71.Li X, Zou Y, Xing J, Fu YY, Wang KY, Wan PZ, et al. Pretreatment with roxadustat (FG-4592) attenuates folic acid-induced kidney injury through antiferroptosis via Akt/GSK-3beta/Nrf2 pathway. Oxid Med Cell Longev 2020; 2020:6286984.doi: 10.1155/2020/6286984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chevalier RL. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am J Physiol Renal Physiol 2016; 311:F145–F161. doi: 10.1152/ajprenal 00164.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu BC, Tang TT, Lv LL, Lan HY. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int 2018; 93:568–579. doi: 10.1016/j.kint.2017.09.033. [DOI] [PubMed] [Google Scholar]
- 74.Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 2015; 11:264–276. doi: 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Y, Zhai P, Zheng Y, Zhang J, Kellum JA, Peng Z. Csf2 attenuated sepsis-induced acute kidney injury by promoting alternative macrophage transition. Front Immunol 2020; 11:1415.doi: 10.3389/fimmu 2020.01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang L. How acute kidney injury contributes to renal fibrosis. Adv Exp Med Biol 2019; 1165:117–142. doi: 10.1007/978-981-13-8871-2_7. [DOI] [PubMed] [Google Scholar]
- 77.Bonventre JV. Can we target tubular damage to prevent renal function decline in diabetes? Semin Nephrol 2012; 32:452–462. doi: 10.1016/j.semnephrol.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kiewisz J, Kaczmarek MM, Pawlowska A, Kmiec Z, Stompor T. Endothelial progenitor cells participation in cardiovascular and kidney diseases: a systematic review. Acta Biochim Pol 2016; 63:475–482. doi: 10.18388/abp.2016_1284. [DOI] [PubMed] [Google Scholar]
- 79.Oe Y, Hayashi S, Fushima T, Sato E, Kisu K, Sato H, et al. Coagulation factor Xa and protease-activated receptor 2 as novel therapeutic targets for diabetic nephropathy. Arterioscler Thromb Vasc Biol 2016; 36:1525–1533. doi: 10.1161/ATVBAHA.116.307883. [DOI] [PubMed] [Google Scholar]
- 80.Schnoor M, Cullen P, Lorkowski J, Stolle K, Robenek H, Troyer D, et al. Production of type VI collagen by human macrophages: a new dimension in macrophage functional heterogeneity. J Immunol 2008; 180:5707–5719. doi: 10.4049/jimmunol.180.8.5707. [DOI] [PubMed] [Google Scholar]
- 81.Djudjaj S, Boor P. Cellular and molecular mechanisms of kidney fibrosis. Mol Aspects Med 2019; 65:16–36. doi: 10.1016/j.mam.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 82.Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL, Chiang WC, et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int 2011; 80:1170–1181. doi: 10.1038/ki.2011.208. [DOI] [PubMed] [Google Scholar]
- 83.Huen SC, Cantley LG. Macrophage-mediated injury and repair after ischemic kidney injury. Pediatr Nephrol 2015; 30:199–209. doi: 10.1007/s00467-013-2726-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang S, Meng XM, Ng YY, Ma FY, Zhou S, Zhang Y, et al. TGF-beta/Smad3 signalling regulates the transition of bone marrow-derived macrophages into myofibroblasts during tissue fibrosis. Oncotarget 2016; 7:8809–8822. doi: 10.18632/oncotarget.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen J, Xia Y, Lin X, Feng XH, Wang Y. Smad3 signaling activates bone marrow-derived fibroblasts in renal fibrosis. Lab Invest 2014; 94:545–556. doi: 10.1038/labinvest.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang PM, Zhang YY, Xiao J, Tang PC, Chung JY, Li J, et al. Neural transcription factor Pou4f1 promotes renal fibrosis via macrophage-myofibroblast transition. Proc Natl Acad Sci USA 2020; 117:20741–20752. doi: 10.1073/pnas.1917663117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang YY, Jiang H, Pan J, Huang XR, Wang YC, Huang HF, et al. Macrophage-to-myofibroblast transition contributes to interstitial fibrosis in chronic renal allograft injury. J Am Soc Nephrol 2017; 28:2053–2067. doi: 10.1681/ASN.2016050573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Little K, Llorián-Salvador M, Tang M, Du X, Marry S, Chen M, et al. Macrophage to myofibroblast transition contributes to subretinal fibrosis secondary to neovascular age-related macular degeneration. J Neuroinflammation 2020; 17:355.doi: 10.1186/s12974-020-02033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feng Y, Guo F, Xia Z, Liu J, Mai H, Liang Y, et al. Inhibition of fatty acid-binding protein 4 attenuated kidney fibrosis by mediating macrophage-to-myofibroblast transition. Front Immunol 2020; 11:566535.doi: 10.3389/fimmu.2020.566535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tang PM, Zhou S, Li CJ, Liao J, Xiao J, Wang QM, et al. The proto-oncogene tyrosine protein kinase SRC is essential for macrophage-myofibroblast transition during renal scarring. Kidney Int 2018; 93:173–187. doi: 10.1016/j.kint.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 91.Gomez I.G., Duffield J.S.. The FOXD1 lineage of kidney perivascular cells and myofibroblasts: functions and responses to injury. Kidney Int 2014; 4: (Suppl 2011): 26–33. doi: 10.1038/kisup.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kramann R, Machado F, Wu H, Kusaba T, Hoeft K, Schneider RK, et al. Parabiosis and single-cell RNA sequencing reveal a limited contribution of monocytes to myofibroblasts in kidney fibrosis. JCI Insight 2018; 3:e99561.doi: 10 1172/jci insight99561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang R, Zhou Q, Veeraragoo P, Yu H, Xiao Z. Notch2/Hes-1 pathway plays an important role in renal ischemia and reperfusion injury-associated inflammation and apoptosis and the gamma-secretase inhibitor DAPT has a nephroprotective effect. Ren Fail 2011; 33:207–216. doi: 10.3109/0886022X.2011.553979. [DOI] [PubMed] [Google Scholar]
- 94.Djudjaj S, Chatziantoniou C, Raffetseder U, Guerrot D, Dussaule JC, Boor P, et al. Notch-3 receptor activation drives inflammation and fibrosis following tubulointerstitial kidney injury. J Pathol 2012; 228:286–299. doi: 10.1002/path.4076. [DOI] [PubMed] [Google Scholar]
- 95.Jiang Y, Wang Y, Ma P, An D, Zhao J, Liang S, et al. Myeloid-specific targeting of Notch ameliorates murine renal fibrosis via reduced infiltration and activation of bone marrow-derived macrophage. Protein Cell 2019; 10:196–210. doi: 10.1007/s13238-018-0527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiandong L, Yang Y, Peng J, Xiang M, Wang D, Xiong G, et al. Trichosanthes kirilowii lectin ameliorates streptozocin-induced kidney injury via modulation of the balance between M1/M2 phenotype macrophage. Biomed Pharmacother 2019; 109:93–102. doi: 10.1016/j.biopha.2018.10.060. [DOI] [PubMed] [Google Scholar]
- 97.Meng XM, Tang PM, Li J, Lan HY. TGF-beta/Smad signaling in renal fibrosis. Front Physiol 2015; 6:82.doi: 10.3389/fphys.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gewin LS. Transforming growth factor-beta in the acute kidney injury to chronic kidney disease transition. Nephron 2019; 143:154-157.doi: 10 1159/000500093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huen SC, Moeckel GW, Cantley LG. Macrophage-specific deletion of transforming growth factor-beta1 does not prevent renal fibrosis after severe ischemia-reperfusion or obstructive injury. Am J Physiol Renal Physiol 2013; 305:F477–F484. doi: 10.1152/ajprenal 00624.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li J, Qu X, Bertram JF. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am J Pathol 2009; 175:1380–1388. doi: 10.2353/ajpath.2009.090096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen DQ, Cao G, Chen H, Liu D, Su W, Yu XY, et al. Gene and protein expressions and metabolomics exhibit activated redox signaling and wnt/beta-catenin pathway are associated with metabolite dysfunction in patients with chronic kidney disease. Redox Biol 2017; 12:505–521. doi: 10.1016/j.redox.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou FF, et al. Sustained activation of Wnt/beta-catenin signaling drives AKI to CKD progression. J Am Soc Nephrol 2016; 27:1727–1740. doi: 10.1681/ASN.2015040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kitamoto K, Machida Y, Uchida J, Izumi Y, Shiota M, Nakao T, et al. Effects of liposome clodronate on renal leukocyte populations and renal fibrosis in murine obstructive nephropathy. J Pharmacol Sci 2009; 111:285–292. doi: 10.1254/jphs.09227fp. [DOI] [PubMed] [Google Scholar]
- 104.Chung S, Overstreet JM, Li Y, Wang Y, Niu A, Wang S, et al. TGF-beta promotes fibrosis after severe acute kidney injury by enhancing renal macrophage infiltration. JCI Insight 2018; 3:e123563.doi: 10 1172/jci insight.123563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wilson HM, Chettibi S, Jobin C, Walbaum D, Rees AJ, Kluth DC. Inhibition of macrophage nuclear factor-kappaB leads to a dominant anti-inflammatory phenotype that attenuates glomerular inflammation in vivo. Am J Pathol 2005; 167:27–37. doi: 10.1016/s0002-9440(10)62950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Feng Y, Liang Y, Zhu X, Wang M, Gui Y, Lu Q, et al. The signaling protein Wnt5a promotes TGFbeta1-mediated macrophage polarization and kidney fibrosis by inducing the transcriptional regulators Yap/Taz. J Biol Chem 2018; 293:19290–19302. doi: 10.1074/jbc.RA118.005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lu H, Wu L, Liu L, Ruan Q, Zhang X, Hong W, et al. Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization. Biochem Pharmacol 2018; 154:203–212. doi: 10.1016/j.bcp.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 108.Carrington JM, Hershberger DM. Pulmonary Alveolar Proteinosis. Treasure Island (FL): StatPearls; 2021. [PubMed] [Google Scholar]
- 109.Rojo R, Raper A, Özdemir DD, Lefevre L, Grabert K, Wollscheid-Lengeling E, et al. Deletion of a Csf1r enhancer selectively impacts CSF1R expression and development of tissue macrophage populations. Nat Commun 2019; 10:3215.doi: 10.1038/s41467-019-11053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Deng X, Yang Q, Wang Y, Zhou C, Guo Y, Hu Z, et al. CSF-1R inhibition attenuates ischemia-induced renal injury and fibrosis by reducing Ly6C(+) M2-like macrophage infiltration. Int Immunopharmacol 2020; 88:106854.doi: 10.1016/j.intimp.2020.106854. [DOI] [PubMed] [Google Scholar]
- 111.Lok SWY, Yiu WH, Li H, Xue R, Zou Y, Li B, et al. The PAR-1 antagonist vorapaxar ameliorates kidney injury and tubulointerstitial fibrosis. Clin Sci (Lond) 2020; 134:2873–2891. doi: 10.1042/CS20200923. [DOI] [PubMed] [Google Scholar]
- 112.Tang TT, Lv LL, Wang B, Cao JY, Feng Y, Li ZL, et al. Employing macrophage-derived microvesicle for kidney-targeted delivery of dexamethasone: an efficient therapeutic strategy against renal inflammation and fibrosis. Theranostics 2019; 9:4740–4755. doi: 10.7150/thno.33520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee S, Kivimäe S, Dolor A, Szoka FC. Macrophage-based cell therapies: the long and winding road. J Control Release 2016; 240:527–540. doi: 10.1016/j.jconrel.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anderson NR, Minutolo NG, Gill S, Klichinsky M. Macrophage-based approaches for cancer immunotherapy. Cancer Res 2021; 81:1201–1208. doi: 10.1158/0008-5472.CAN-20-2990. [DOI] [PubMed] [Google Scholar]
- 115.Chan MWY, Viswanathan S. Recent progress on developing exogenous monocyte/macrophage-based therapies for inflammatory and degenerative diseases. Cytotherapy 2019; 21:393–415. doi: 10.1016/j.jcyt.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 116.Wilson HM, Stewart KN, Brown PA, Anegon I, Chettibi S, Rees AJ, et al. Bone-marrow-derived macrophages genetically modified to produce IL-10 reduce injury in experimental glomerulonephritis. Mol Ther 2002; 6:710–717. doi: 10.1006/mthe.2002.0802. [DOI] [PubMed] [Google Scholar]