Abstract
Background
: Tourette syndrome (TS) is a neuropsychiatric disorder with onset in childhood that warrants effective therapies. Gut microbiota can affect central physiology and function via the microbiota–gut-brain axis. Therefore, the gut microbiota plays an important role in some mental illnesses. A small clinical trial showed that fecal microbiota transplantation (FMT) may alleviate TS symptoms in children. Herein, FMT effects and mechanisms were explored in a TS mouse model.
Methods
: TS mice model (TSMO) (n = 80) were established with 3,3′-iminodipropionitrile, and 80 mice were used as controls. Mice were grouped into eight groups and were subjected to FMT with feces from children or mice with or without TS, or were given probiotics. Fecal specimens were collected 3 weeks after FMT. 16S rRNA sequencing, behavioral observation, and serum serotonin (5-HT) assay were performed. Differences between groups were analyzed using Mann-Whitney U test and Kolmogorov-Smirnov (KS) tests.
Results
: A total of 18 discriminative microbial signatures (linear discriminant analysis score > 3) that varied significantly between TS and healthy mice (CONH) were identified. A significant increase in Turicibacteraceae and Ruminococcaceae in TSMO after FMT was observed (P < 0.05). Compared with non-transplanted TSMO, the symptoms of those transplanted with feces from CONH were alleviated (W = 336, P = 0.046). In the probiotic and FMT experiments, the serum 5-HT levels significantly increased in TSMO that received probiotics (KS = 1.423, P = 0.035) and in those transplanted with feces from CONH (W = 336.5, P = 0.046) compared with TSMO without transplantation.
Conclusions
: This study suggests that FMT may ameliorate TS by promoting 5-HT secretion, and it provides new insights into the underlying mechanisms of FMT as a treatment for TS.
Keywords: Tourette syndrome, Fecal transplantation, Microbiota, Serotonin
Introduction
Tourette syndrome (TS) is a chronic neurological disorder of unknown cause characterized by recurrent motor and vocal tics.[1–4] Although current therapies may partly improve these manifestations, inadequate control of tics and adverse side effects remain as challenges in the treatment of TS.[5–9] The causes of TS are unknown, but some evidence suggests that dysfunction of the dopaminergic pathways within the cortico-striato-cortico-frontal circuitry, failure of cortical inhibition of inappropriate motor programs generated in the basal ganglia, deficits in cerebral maturation, especially for striatal interneuron migration,[2,3] and abnormalities in the cortico-basal ganglia-thalamo-cortical loops may be involved in TS.[10]
The gut microbiota plays an important role in some mental illnesses, such as depression, autism spectrum disorder, and Parkinson disease via the microbiota-gut-brain axis.[11–14] Therefore, fecal microbiota transplantation (FMT) has been considered as a potential method to rebalance the gut microbiota. Indeed, its efficacy has been demonstrated in autism spectrum disorder and epilepsy.[15,16] Herein, we aimed to explore whether the gut microbiota could contribute to TS and if FMT could ameliorate TS symptoms in a mouse model.
Methods
Ethical approval
This study was approved by the biomedical research ethic committee of Cheeloo Children's Hospital of Shandong University (approval No. ETYY-2020230). Every effort was made to minimize the number of animals and reduce their suffering. All procedures used in this study were in accordance with our institutional guidelines and complied with the international ethics and humane standards for animal use.
Animal model
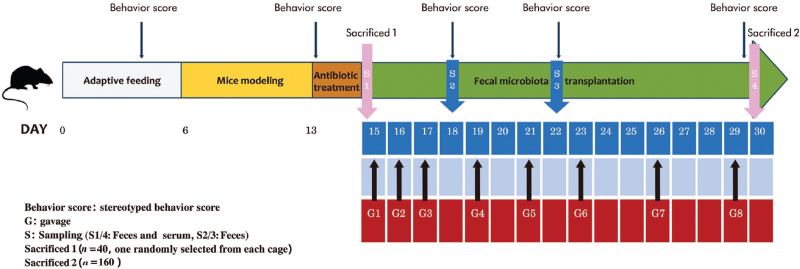
A total of 200 Kunming-specific pathogen-free (SPF) healthy mice (CONH) were provided by the laboratory animal center of Shandong Province. The mice were housed in an SPF animal room in cages. The feeding temperature was maintained at 18 to 29°C, and the humidity was maintained at 40% to 70%. The mice were given SPF maintenance feed and free ultrapure water for drinking. They were used for the experiments after 1 week of acclimation following the task schedule outlined in Figure 1.
Figure 1.
Schematic diagram of the in vivo experiment.
A total of 160 mice were randomly divided into CONH and TS mice (TSMO) groups, which comprised healthy and TSMO, respectively. The CONH group was subdivided into four groups (20 mice in each group) that underwent FMT with feces from TS children (HTSC group), feces from healthy children (CHHC group), feces from TS mice (HTSM group), and feces from healthy mice (CHHM group). The TSMO group was similarly divided into four groups (20 mice in each group), which received feces from healthy children transplanted (MFHC group) or from healthy mice (MFHM group), or were administrated probiotics (MPro group) or were left untreated (MCon group). The MPro and MCon groups were used to determine whether probiotic intervention or fecal transplantation was more effective. Pro Chang was obtained from Shandong Tanke Biotechnology Co., Ltd (Shandong, China) which is a complex of seven probiotics and two prebiotics, including Lactobacillus acidophilus, Bifidobacterium longum, Lactobacillus paracasei, Lactobacillus rhamnosus, Lactobacillus fermentum, Lactobacillus belveticus, Streptococcus thermophilus, fructooligosacchar-ides, and isomaltose oligosaccharides. The Pro Chang was diluted 2:50 (m:V) using a gavage (0.3 mL each time).
The TS model was established according to the method described by Diamond et al[17] by administering 150 mg·kg−1·d−1 3,3′-iminodipropionitrile (IDPN; Merck KGaA, Germany). In accordance with the original report,[17] the mice showed no significant change in activity after modeling according to the ethological score; hence the dose of IDPN was increased to 350 mg·kg−1·d−1. All mice showed different degrees of abnormal behavior and activities.[18] The ethological scores were evaluated as outlined in Table 1.[17,18]
Table 1.
Mice stereotyped behavior score.
| Score Stereotype | |
| 0 | Normal activity |
| 1 | Circling behavior (clockwise or counterclockwise rotation) |
| 2 | Excessive vertical movement of the head and neck |
| 3 | Excessive movement of the neck and circling behavior |
| 4 | Swinging the head to the side combined with excessive vertical movement of the head and neck |
Preparation of fecal liquid and FMT
Fecal specimens were collected from TS and healthy children, as well as CONH and TSMO. Fresh feces from each group were collected and immediately weighed. Based on the method of Zhang et al[19] for the preparation of fecal bacteria liquid, fresh feces were mixed immediately with sterile saline (1:5 [w/v]). After homogenization in a biosafety cabinet, the large particles were serially filtered through layers of gauze (10, 30, and 100 mesh), and the suspension was collected in 2-mL sterile centrifuge tubes. The suspension was centrifuged at 1858 × g at 4°C for 3 min. The supernatant was discarded, and the pellet was resuspended in a normal saline. The mixture was vortexed, centrifuged again, and the pellet was resuspended in normal saline solution. All the above operations were performed in a low-temperature environment (4°C), and the processing time was under 1 h.
An antibiotic mixture (500 mg of ampicillin, 250 mg of vancomycin, 500 mg of neomycin, and 250 mg of metronidazole) (Merck KGaA) was administered to the mice by gavage daily for 3 days before FMT. For FMT, the mice were transferred to separate cages (n = 1/cage).
The fresh bacterial solution (0.3 mL) was injected into the stomach of the mouse by gavage for a total of eight times over the subsequent 3 weeks. Afterward, the mice were observed for 30 min.
Chromatographic assay of serum serotonin (5-HT)
Mice in each group were fasted without water for 24 h after the last administration, and were randomly selected from each group. 2 to 3 mL of blood was collected from the orbit and stored in an eppendorf tube. After standing at 4°C for 2 h, the blood was centrifuged at 2500 ×g for 10 min in a refrigerated centrifuge. The upper serum was collected and stored at −80°C for later use. The same amount of 5% perchloric acid solution was added into serum, and then mixed evenly in a vortex mixer for 30 s. Then the mixture was placed at room temperature for 10–15 min, and centrifuged at a rate of 11,100 ×g for 5 min. Finally 25 μL of supernatant was taken for chromatographic analysis.
Sample collection, DNA extraction, and sequencing
Fecal specimens were collected 2 weeks after FMT in sterile 2-mL tubes containing pure chilled ethanol, frozen within 30 min, and stored at − 80°C until analysis. Genomic DNA was extracted using the cetyltrimethylammonium bromide method. An equivalent of 1 μL of each sample was used for DNA quantification using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The amplification of the V3-V4 region of the 16S rRNA was performed to analyze the bacterial population and execute amplification of the variable region. Polymerase chain reaction (PCR) was conducted using the bacterial universal forward primers 319F (5′-ACT CCT ACG GGA GGC AGC AG-3′) and the reverse 806R (5′-GGA CTA CHV GGG TWT CTA AT-3′). The PCR products were verified by electrophoresis on 1% (w/v) agarose gels in Tris-borate-EDTA (TBE) buffer stained with Genecolour I (GeneBio Systems, Oakville, ON, Canada) and visualized under ultraviolet (UV) light. Amplicons were first purified using the QIAquick PCR Purification Kit (Qiagen, Hilden, Germany), quantified using NanoDrop 2000, and then pooled in equal concentrations. Pooled amplicons (2 nmol/ L) were subjected to sequencing using an Illumina HiSeq 2500 (Illumina, San Diego, CA, USA), following the standard Illumina platform protocols.
Analysis of 16S rRNA sequence
The 16S rRNA sequence paired-end data set was joined and quality-filtered using the FastLength Adjustment Shortreads software (FLASH, http://ccb.jhu.edu/software/FLASH/index.shtml). All sequence analyses were conducted using the Quantitative Insights Into Microbial Ecology (QIIME, version 1.9.1, http://qiime.org/) software suite,[20] as per the QIIME tutorial (http://qiime.org/). Chimeric sequences were removed using usearch61 (http://www.drive5.com/usearch/) with de novo models. The sequences were clustered against the 2013 Green (13_8 release) ribosomal database and 97% reference data set. Sequences that did not match with any entries in this reference were subsequently clustered into de novo operational taxonomic units (OTUs) at 97% similarity with USEARCH clustering. Taxonomy was assigned to all OTUs using the ribosomal database project classifier within QIIME and the Greengenes reference data set.[21]
To account for any bias caused by uneven sequencing depth, the least number of sequences present in any given sample was selected randomly from a sample category before calculating community-wide dissimilarity measures (α and β diversities). The OTU table was rarified to a sequencing depth of 22,000 per sample for both diversity analyses. All principal coordinate analyses (PCoAs) were based on unweighted and weighted UniFrac distances using evenly sampled OTU abundances. Linear discriminant effect size (LEfSe) analysis was performed to identify features (taxa) that were differentially represented between the two groups. LEfSe combines the Kruskal-Wallis test or pairwise Wilcoxon rank-sum test with linear discriminant analysis (LDA). It ranks features by an effective size, which explains most of the biological differences at the top. The LEfSe analysis was performed based on the threshold of the logarithmic LDA score for discriminative features, which was equal to 2.0. Prediction of the functional composition of a metagenome, using marker gene data and a database of reference genomes, was performed using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt).[22] The graphical representation of the results was performed using R[23] and Statistical Analysis of Metagenomic Profiles (STAMP).
Real-time PCR analysis
To confirm the relative abundance of Turicibacteraceae and Ruminococcaceae observed in TSMO by 16S sequencing, total DNA of the mouse feces was extracted using the DNeasy mini kit (Qiagen). The Te oligonucleotide primers for target genes were as follows: rpsD of Turicibacteraceae (5′-AGCGTCAATTCCGTCGTACA-3′ and 5′-GACGACGAGTCGCAGCTAAT-3′), 16S of Turicibacteraceae (5′-CCGTGGAGGGTCATTGGAAA-3′ and 5′-GTGTCAGTTGCAGACCAGGA-3′), FECF of Ruminococcaceae (5′-CTGGAAGATACGCTGCCGAT-3′ and 5′-CGCTTTCCGCTGTGAAACAA-3′), and 16S of Ruminococcaceae (5′-GGGCTGCATCCAAAACTGTG-3′ and 5′-CAGCGTCAGAAAATGCCCAG-3′). The 2—ΔΔCt method was used to calculate the relative DNA expression.
Statistical analysis
The diversities were analyzed using the Mann-Whitney U and Kolmogorov-Smirnov (KS) tests. Data in the behavioral and 5-HT experiments are expressed as the mean ± standard error (SE). All analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). The P values for PICRUSt and STAMP were calculated using the Kruskal-Wallis H-test and Welch t-test. The effects were considered significant if the P values were < 0.05.
Results
Mice with TS harbor a different gut microbiome compared with CONH
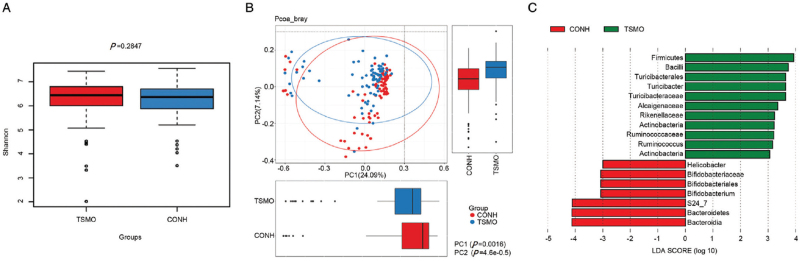
To evaluate the differences between TS and CONH, we compared a and β diversities between the TSMO and CONH groups. Analysis of a diversity revealed that the Shannon index did not differ significantly between the groups (Figure 2A; Wilcoxon rank-sum test, P > 0.05). However, analysis of β diversity based on the unweighted UniFrac distances showed that the gut microbiome of the TSMO group was significantly different from that of the CONH group (analysis of similarities [ANOSIM], r = 0.143, P < 0.05, unweighted UniFrac; Figure 2B). These significant differences were further confirmed by LEfSe analysis, which identified 18 discriminative microbial signatures (LDA score > 3) that varied significantly between the TSMO and CONH groups [Figure 2B]. At the genus level, a significant increase in the relative abundance of Turicibacteraceae and Ruminococcaceae was observed in TS compared with CONH (LDA > 3) [Figure 2C], an observation that was further confirmed by quantitative real-time PCR (Supplementary Figure 1).
Figure 2.
The microbiota compartment in TS and CONH before FMT. (A) α diversity in the TSMO and CONH groups. (B) PCoA of bacterial β diversity between TSMO and CONH groups. (C) LEfSe indicating the differences in the bacterial taxa between the CONH and TSMO groups. CONH: Healthy mice; FMT: Fecal microbiota transplantation; LDA: Linear discriminant analysis; LEfSe: Linear discriminant effect size; PCoA: Principal coordinate analyses; TS: Tourette syndrome; TSMO: TS mice.
Fecal transplantation is effective for alleviating symptoms of Tourette syndrome
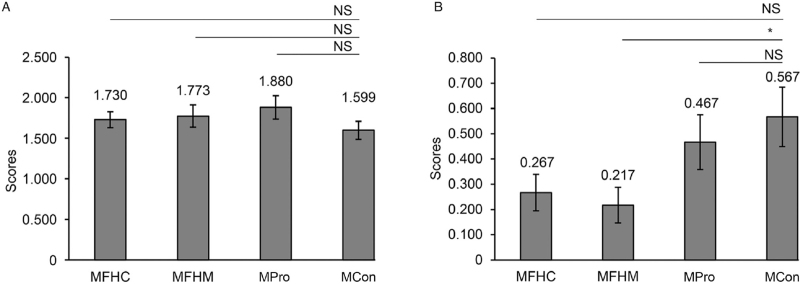
Furthermore, fecal transplantation showed a therapeutic effect on IDPN-induced TS. Compared with non-transplanted TS mice (MCon group), the symptoms of TS mice transplanted with feces of healthy mice (MFHM group) were ameliorated (W = 336, P = 0.046; Figure 3B). Thus, these findings suggest that fecal transplantation may be effective for the treatment of TS.
Figure 3.
FMT is effective for managing TS. TS scores in MFHC, MFHM, MPro, and MCon (A) before and (B) after FMT. ∗P < 0.05. FMT: Fecal microbiota transplantation; MFHC: Feces from healthy children transplanted into TS mice; MFHM: Feces from healthy mice transplanted to TS mice; MCon: TS mice without transplantation; MPro: Probiotics given to TS mice; TS: Tourette syndrome.
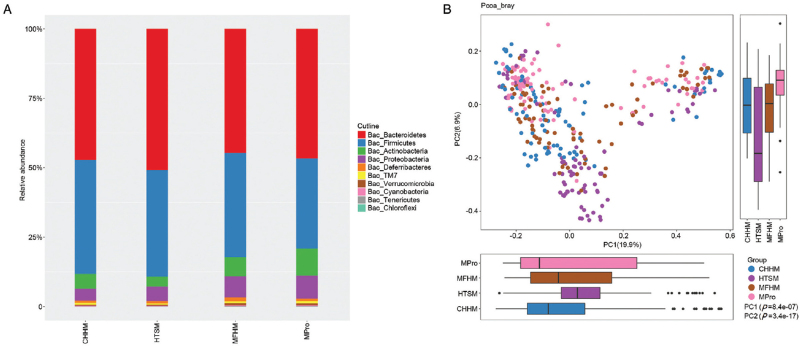
Fecal transplantation changes the gut microbiota of the mice
To reveal the effect of fecal transplantation on the microbiota community, we analyzed the microbiota from CHHM, HTSM, MFHM, and MPro groups. The bacterial 16S rRNA was sequenced after 2 weeks, revealing that the microbiota of CONH changed after transplanting the feces of mice with TS, and the microbiota of TSMO changed after transplanting feces from CONH or MPro treatment. Clustering was observed in the PCoA between CHHM, HTSM, MFHM, and MPro groups (unweighted UniFrac Distance, ANOSIM, P < 0.05; Figure 4). Next, we explored the gut microbial community features (relative taxon abundance of the microbiome) of mice that received FMT. In HTSM, Firmicutes and Actinobacteria were decreased, whereas Bacteroidetes and Proteobacteria were increased compared with CHHM [Figure 4].
Figure 4.
The microbiota compartment after FMT. (A) Comparison of relative taxa abundance between the CHHM, HTSM, MFHM, and MPro groups. (B) PCoA of bacterial β diversity based on the unweighted UniFrac between the CHHM, HTSM, MFHM, and MPro groups. CHHM: Feces from healthy mice transplanted into healthy mice; FMT; Fecal microbiota transplantation; HTSM: Feces from TS mice transplanted into healthy mice; MFHM: Feces from healthy mice transplanted to TS mice; MPro: Probiotics given to TS mice; PCoA: Principal coordinate analyses.
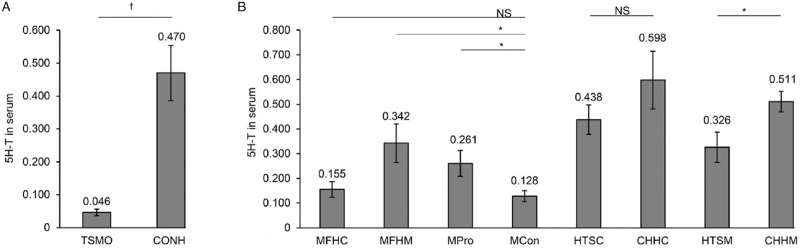
FMT affects 5-HT levels
5-HT is critical for the development of TS. To explore the effect of fecal transplantation on 5-HT, we analyzed 5-HT in the serum of TSMO and CONH mice. TSMO showed significantly decreased 5-HT levels compared with CONH (W = 212, P < 0.001; Figure 5A). Moreover, the serum 5-HT levels significantly increased in the Mpro and MFHM groups compared with those in the MCon group (Mpro vs. MCon: KS = 1.423, P = 0.035; MFHM vs. MCon: W = 336.5, P = 0.046; Figure 5B). Furthermore, transplantation of fecal samples from TSMO significantly decreased serum 5-HT levels as compared with fecal samples from CONH (W = 299.5, P = 0.002; Figure 5B). These results suggest that FMT ameliorates TS and promotes 5-HT secretion.
Figure 5.
Comparison of plasma 5-HT levels among the different groups. (A) TSMO vs. CONH. (B) Among MFHC, MFHM, MPro, MCon, HTSC, CHHC, HTSM, and CHHM groups. ∗P < 0.05, +P < 0.01. 5-HT: Serotonin; CONH: Healthy mice; CHHC: Feces from healthy children transplanted to healthy mice; CHHM: Feces from healthy mice transplanted into healthy mice; HTSC: Feces from TS children transplanted into healthy mice; HTSM: Feces from TS mice transplanted into healthy mice; MFHC: Feces from healthy children transplanted into TS mice; MFHM: Feces from healthy mice transplanted to TS mice; MPro: Probiotics given to TS mice; MCon: TS mice without transplantation; TSMO: TS mice.
Discussion
The results of this study showed that IDPN-induced TSMO harbor different gut microbiomes compared with CONH, especially regarding the relative abundance of Turicibacteraceae and Ruminococcaceae. This finding is consistent with another study showing increased Ruminococcaceae in adults and adolescents with TS and the positive correlation with inattentive symptoms.[24] Studies on the intestinal flora of patients with neurological diseases, such as Alzheimer disease and autism, also revealed significant increases in Firmicutes and/or decreases in Bacteroidetes,[25–27] as well as lower ratios of Firmicutes to Bacteroidetes.[27] As a mucin-degrading bacterium, Ruminococcus can lead to compromised intestinal permeability,[28–30] which may increase the translocation of intestinal bacterial products to the brain. Proteobacteria are major Gram-negative bacteria and include various opportunistic pathogens.[31] In this study, similarly, FMT from healthy to TSMO decreased Firmicutes and increased Bacteroidetes. Moreover, FMT from an ill donor altered the microbiota of CONH, and conversely FMT from a healthy donor changed the intestinal environment of TSMO. Hence, these results suggest that the gut-brain axis is involved in the development of TS.
FMT is effective in treating autism and epilepsy.[15,16] Acase was recently reported of a child with TS whose symptoms were successfully managed with FMT.[32] Another case reported that four patients (4/5) responded positively to FMT (Yale Global Tic Severity Scale-total tic score reduction rate > 25%) at week 8 with high safety.[33] A clinical trial of mini-FMT is currently underway to determine the effects of mini-FMT in patients with TS (ClinicalTrials.gov: NCT03764748). In line with these results, we observed that the symptoms of TSMO transplanted with feces of CONH had declined, thereby suggesting that FMT may represent an alternative treatment, either alone or in combination with a classical treatment, for TS.
Abnormal dopaminergic and serotonergic transmission may be involved in the pathogenesis of TS.[34,35] Studies have suggested that reduced 5-HT bioavailability in the brain is associated with the severity of TS and obsessive-compulsive disorder.[36–38] The gut microbiota can promote the metabolism of tryptophan into precursors of 5-HT, which can pass the blood-brain barrier.[39] In this study, similarly, the microbiota of TSMO was associated with the lowest 5-HT levels, whereas the microbiota from CONH was associated with the highest 5-HT levels. Therefore, tryptophan and 5-HT metabolism may be involved in the effects of FMT on TS. Nevertheless, additional studies are necessary to examine these relationships.
There are still many limitations to this study. Blood markers of the microbiota-gut-brain axis (including lipopolysaccharides and inflammation markers) were not assessed nor were the levels of neurotransmitters.
In conclusion, FMT can alleviate tic severity in a TS mouse model by modulating the intestinal flora and upregulating the serum 5-HT levels, further supporting the hypothesis of microbe-intestine-brain axis in TS. Thereby, this study provides valuable insights into the mechanisms involved in TS, which may serve as the foundation for the development of treatments. In the future, underlying mechanisms will be explored from the molecular, protein, and ethological perspective along with the brain-intestine-axis direction.
Availability of data and materials
The data set supporting the results of this article are included within the article. The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Li H, Wang Y, Zhao C, Liu J, Zhang L, Li A. Fecal transplantation can alleviate tic severity in a Tourette syndrome mouse model by modulating intestinal flora and promoting serotonin secretion. Chin Med J 2022;135:707–713. doi: 10.1097/CM9.0000000000001885
Supplemental digital content is available for this article.
References
- 1.Rampello L, Alvano A, Battaglia G, Bruno V, Raffaele R, Nicoletti F. Tic disorders: from pathophysiology to treatment. J Neurol 2006; 253:1–15. doi: 10.1007/s00415-005-0008-8. [DOI] [PubMed] [Google Scholar]
- 2.Cavanna AE, Seri S. Tourette's syndrome. BMJ 2013; 347:f4964.doi: 10.1136/bmj.f4964. [DOI] [PubMed] [Google Scholar]
- 3.Jankovic J, Kurlan R. Tourette syndrome: evolving concepts. Mov Disord 2011; 26:1149–1156. doi: 10.1002/mds.23618. [DOI] [PubMed] [Google Scholar]
- 4.Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr Neurol 2012; 47:77–90. doi: 10.1016/j.pediatrneurol.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Murphy TK, Lewin AB, Storch EA, Stock S. American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI). Practice parameter for the assessment and treatment of children and adolescents with tic disorders. J Am Acad Child Adolesc Psychiatry 2013; 52:1341–1359. doi: 10.1016/j.jaac.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Weisman H, Qureshi IA, Leckman JF, Scahill L, Bloch MH. Systematic review: pharmacological treatment of tic disorders - efficacy of antipsychotic and alpha-2 adrenergic agonist agents. Neurosci Biobehav Rev 2013; 37:1162–1171. doi: 10.1016/j.neu-biorev.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roessner V, Plessen KJ, Rothenberger A, Ludolph AG, Rizzo R, Skov L, et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part II: pharmacological treatment. Eur Child Adolesc Psychiatry 2011; 20:173–196. doi: 10.1007/s00787-011-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farag M, Stern JS, Simmons H, Robertson MM. Serial pharmacological prescribing practices for tic management in Tourette syndrome. Hum Psychopharmacol 2015; 30:435–441. doi: 10.1002/hup.2495. [DOI] [PubMed] [Google Scholar]
- 9.Schrock LE, Mink JW, Woods DW, Porta M, Servello D, Visser-Vandewalle V, et al. Tourette syndrome deep brain stimulation: a review and updated recommendations. Mov Disord 2015; 30:448–471. doi: 10.1002/mds.26094. [DOI] [PubMed] [Google Scholar]
- 10.Ganos C, Roessner V, Munchau A. The functional anatomy of Gilles de la Tourette syndrome. Neurosci Biobehav Rev 2013; 37:1050–1062. doi: 10.1016/j.neubiorev.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, et al. Gut microbiome alterations in Alzheimer's disease. Sci Rep 2017; 7:13537.doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 2015; 28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 13.Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev 2019; 99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 14.Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell 2016; 167:915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Micro-biome 2017; 5:10.doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Z, Cui BT, Zhang T, Li P, Long CY, Ji GZ, et al. Fecal microbiota transplantation cured epilepsy in a case with Crohn's disease: the first report. World J Gastroenterol 2017; 23:3565–3568. doi: 10.3748/wjg.v23.i19.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diamond BI, Reyes MG, Borison R. A new animal model for Tourette syndrome. Adv Neurol 1982; 35:221–225. [PubMed] [Google Scholar]
- 18.Lin L, Yu L, Xiang H, Hu X, Yuan X, Zhu H, et al. Effects of acupuncture on behavioral stereotypies and brain dopamine system in mice as a model of Tourette syndrome. Front Behav Neurosci 2019; 13:239.doi: 10.3389/fnbeh.2019.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang F, Cui B, He X, Nie Y, Wu K, Fan D, et al. Microbiota transplantation: concept, methodology and strategy for its modernization. Protein Cell 2018; 9:462–473. doi: 10.1007/s13238-018-0541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010; 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, et al. The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 2009; 37:D141–D145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 2013; 31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMurdie PJ, Holmes S. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 2013; 8:e61217.doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szopinska-Tokov J, Dam S, Naaijen J, Konstanti P, Rommelse N, Belzer C, et al. Investigating the gut microbiota composition of individuals with attention-deficit/hyperactivity disorder and association with symptoms. Microorganisms 2020; 8:406.doi: 10.3390/microorganisms8030406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, Hayek J, et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017; 5:24.doi: 10.1186/s40168-017-0242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams BL, Hornig M, Buie T, Bauman ML, Paik MC, Wick I, et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One 2011; 6:e24585.doi: 10.1371/journal.pone.0024585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariat D, Firmesse O, Levenez F, Guimaraes V, Sokol H, Dore J, et al. The firmicutes/bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol 2009; 9:123.doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One 2013; 8:e68322.doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quevrain E, Maubert MA, Michon C, Chain F, Marquant R, Tailhades J, et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn's disease. Gut 2016; 65:415–425. doi: 10.1136/gutjnl-2014-307649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mancuso C, Santangelo R. Alzheimer's disease and gut microbiota modifications: the long way between preclinical studies and clinical evidence. Pharmacol Res 2018; 129:329–336. doi: 10.1016/j.phrs.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 2015; 33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Zhao H, Shi Y, Luo X, Peng L, Yang Y, Zou L. The effect of fecal microbiota transplantation on a child with Tourette syndrome. Case Rep Med 2017; 2017:6165239.doi: 10.1155/2017/6165239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao HJ, Luo X, Shi YC, Li JF, Pan F, Ren RR, et al. The efficacy of fecal microbiota transplantation for children with Tourette syndrome: a preliminary study. Front Psychiatry 2020; 11:554441.doi: 10.3389/fpsyt.2020.554441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong DF, Brasic JR, Singer HS, Schretlen DJ, Kuwabara H, Zhou Y, et al. Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology 2008; 33:1239–1251. doi: 10.1038/sj.npp.1301528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller-Vahl KR, Szejko N, Wilke F, Jakubovski E, Geworski L, Bengel F, et al. Serotonin transporter binding is increased in Tourette syndrome with obsessive compulsive disorder. Sci Rep 2019; 9:972.doi: 10.1038/s41598-018-37710-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller-Vahl KR, Meyer GJ, Knapp WH, Emrich HM, Gielow P, Brucke T, et al. Serotonin transporter binding in Tourette syndrome. Neurosci Lett 2005; 385:120–125. doi: 10.1016/j.neulet.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 37.Zitterl W, Aigner M, Stompe T, Zitterl-Eglseer K, Gutierrez-Lobos K, Wenzel T, et al. Changes in thalamus-hypothalamus serotonin transporter availability during clomipramine administration in patients with obsessive-compulsive disorder. Neuropsychopharmacology 2008; 33:3126–3134. doi: 10.1038/npp.2008.35. [DOI] [PubMed] [Google Scholar]
- 38.Stengler-Wenzke K, Muller U, Barthel H, Angermeyer MC, Sabri O, Hesse S. Serotonin transporter imaging with [123I]beta-CIT SPECT before and after one year of citalopram treatment of obsessive-compulsive disorder. Neuropsychobiology 2006; 53:40–45. doi: 10.1159/000090702. [DOI] [PubMed] [Google Scholar]
- 39.Kaur H, Bose C, Mande SS. Tryptophan metabolism by gut microbiome and gut-brain-axis: an in silico analysis. Front Neurosci 2019; 13:1365.doi: 10.3389/fnins.2019.01365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data set supporting the results of this article are included within the article. The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.