Abstract
Neurocritical care (NCC) is not only generally guided by principles of general intensive care, but also directed by specific goals and methods. This review summarizes the common pulmonary diseases and pathophysiology affecting NCC patients and the progress made in strategies of respiratory support in NCC. This review highlights the possible interactions and pathways that have been revealed between neurological injuries and respiratory diseases, including the catecholamine pathway, systemic inflammatory reactions, adrenergic hypersensitivity, and dopaminergic signaling. Pulmonary complications of neurocritical patients include pneumonia, neurological pulmonary edema, and respiratory distress. Specific aspects of respiratory management include prioritizing the protection of the brain, and the goal of respiratory management is to avoid inappropriate blood gas composition levels and intracranial hypertension. Compared with the traditional mode of protective mechanical ventilation with low tidal volume (Vt), high positive end-expiratory pressure (PEEP), and recruitment maneuvers, low PEEP might yield a potential benefit in closing and protecting the lung tissue. Multimodal neuromonitoring can ensure the safety of respiratory maneuvers in clinical and scientific practice. Future studies are required to develop guidelines for respiratory management in NCC.
Keywords: Neurocritical care, Pneumonia, Respiratory management, Multimodel neuromonitoring, Tracheostomy, Mechanical ventilation, Positive end-expiratory pressure
Introduction
Patients in neurocritical care (NCC) compose one of the groups of patients in need of the most intensive care. NCC is not only generally guided by principles used in the general intensive care unit (ICU), but also directed by specific goals and methods for three reasons. First, neurocritical illnesses tend to be severe or emergent. These conditions necessitate the early phase of decision-making regarding the methods implemented for respiratory support. In addition, the incidence of respiratory disorders in the NCC unit is significantly higher than that in the general ICU.[1] Respiratory disturbance, however, has been shown to worsen the outcomes in NCC patients by causing conditions such as delirium and ICU-acquired weakness.[2] Respiratory support, including intubation, ventilation, and sedative choices, directly affects brain perfusion.[3] The goal of respiratory support is different in NCC patients from that in other patients, as the brain or lungs are prioritized.[4] Strategies for respiratory support and management, including artificial airways, the prone position, protective mechanical ventilation (MV), and drugs for airway management, have been summarized in previous studies.[5] Controversies still exist concerning the proper timing of a tracheostomy and levels of positive end-expiratory pressure (PEEP) in neurocritical patients. Although these topics have been widely studied in the general ICU, detailed guidelines on respiratory management in the NCC unit are not available.
We searched the electronic database PubMed and analyzed all the relevant literature. Based on the previous knowledge, this review will describe the research progress made in brain and lung interactions, pulmonary complications, and respiratory strategies in neurocritical patients and emphasize the importance and specifics of respiratory management in NCC.
Pathophysiology
Several theories regarding lung vulnerability after brain damage, including the “blast” theory, secondary inflammatory reaction, “double-hit” model, and pulmonary venule adrenergic hypersensitivity, have been studied in recent years. Inappropriate ventilation and respiratory diseases, however, can lead to secondary brain damage due to vagal signaling and high sensitivity of the brain to CO2 and O2 levels.
Brain to lung pathway
Blast theory is characterized by a transient increase in catecholamine after an acute increase in intracranial pressure (ICP).[6,7] Catecholamine release has been reported to be linked to neurogenic pulmonary edema (NPE) in patients with traumatic brain injury (TBI).[8] It was first defined by Theodore and Robin[7] in 1976 as a catecholamine storm that can cause vasoconstriction of pulmonary venules, followed by a transient increase in intravascular pressure and change in the permeability of the capillary alveolar membrane, which eventually leads to protein leaking into the mesenchyme of the lung.
The emergence of the concept called pulmonary venule adrenergic hypersensitivity has challenged the blast theory. The latter includes the coexistence of high hydrostatic pressure and pulmonary endothelium injury. However, in some cases, after brain injury, despite the occurrence of NPE with direct pulmonary endothelial damage, no changes in systemic pressure have been found.[9,10] In pulmonary venule adrenergic hypersensitivity theory, NPE may result in pulmonary vasoconstriction and endothelial integrity changes following massive sympathetic discharge.[10]
After severe TBI, cerebral and systemic inflammatory reactions are triggered, and systemic inflammation is thought to play a major role in the development of pulmonary edema and alveolar damage.[6,11] The double-hit model was introduced in 2009. The first hit summarizes the effects of the catecholamine storm and systemic inflammatory reactions.[12] After the first hit, the lung is vulnerable to the second hit, which will eventually cause damage to the lung, such as a high tidal volume (Vt), inadequate PEEP during ventilation, and other further injurious events.[12] According to the double-hit model, pulmonary edema is the result of both the first hit and the second hit after brain injury.[6] Another theory is related to the endocrine system.[13,14] When the hypothalamic-pituitary-adrenal (HPA) axis is activated by TBI or surgical stressors, it releases corticotrophin-releasing hormones and arginine vasopressin.[13] These two hormones eventually stimulate the release of corticosteroids through the HPA axis. Corticosteroids mediate the anti-inflammatory response after trauma and are responsible for the hemodynamic response, which maintains blood pressure. In neurocritical patients, the persistence of an anti-inflammatory response can lead to secondary adrenal insufficiency, followed by systemic inflammatory response syndrome. This secondary adrenal insufficiency has been detected in 25% of neurocritical patients (∼50% of patients after TBI or subarachnoid hemorrhage [SAH]).[1,15] Tan et al[16] demonstrated that intracranial hypertension and surgical stress can increase the apoptosis rate of the hypothalamus and pituitary gland in rats and rabbits.
Despite the humoral regulation pathways, TBI can also increase the risk for nosocomial pneumonia following neural circuit deficits, especially in the brain stem, including altered mental status, dysphagia, impaired gag and cough reflexes, and inability to clear secretions.[17]
Lung to brain pathway
Lung injuries and inappropriate ventilation can result in secondary brain damage in neurocritical patients, aggravating the sensitivity of the brain to acute injuries. The mechanisms of secondary brain injuries are closely related to neuroinflammation, hypoxemia, the vagal pathway, and the reactivity of cerebral blood vessels to oxygen and carbon dioxide concentrations.
In a previous study, lung injuries aggravated the sensitivity of the brain to acute injuries.[10] Indeed, lung injuries have been shown to promote the release of proinflammatory cytokines, which can spread into the systemic circulation, cause neuronal apoptosis and disrupt neural circuits.[18,19] Moreover, because hypoxemia and inflammation cause endothelial dysfunction, breakdown of the blood-brain barrier, and subsequent extravasation of erythrocytes, cerebral microbleeds will occur after lung injury, predominantly involving the brainstem, cerebellum, and juxta-cortical white matter.[18,20]
González-López et al[21] reported that MV stimulates type 2 dopamine receptor and inactivates the prosurvival Akt/glycogen synthase kinase 3 beta pathway, which may lead to neural cell apoptosis. These authors also found that pulmonary transient receptor potential vanilloid type-4 mechanoreceptors and purinergic receptors participate in the mechanisms of ventilator-associated brain damage.[22]
Arterial carbon dioxide and oxygen levels are both related to cerebral blood flow (CBF) and ICP.[23–25] Howarth[26] demonstrated a novel role for astrocytes in mediating vasodilation in CBF responses to hypercapnia in vivo. They also demonstrated that ICP increases following the elevation of CBF. On the other hand, hyperventilation, which sometimes results in secondary hypocapnia, can result in cranial vasoconstriction and a decrease in CBF, and it can eventually lead to cerebral ischemia.[27] Oxygen levels in the blood and cerebral tissue are also crucial in neurocritical patients. McBryde et al[28] found that both carotid chemoreceptors and astrocytes can sense hypoxia and ischemia and then determine the level of sympathetic activity and arterial pressure to optimize CBF. Thus, a decrease in arterial oxygen pressure (PaO2) during MV is related to an increase in CBF and ICP.[29,30]
Pulmonary Complications in NCC
Systemic changes secondary to neurocritical injuries can induce impairments in pulmonary function. The conditions and disorders that often occur in these patients include not only pneumonia, adult respiratory distress syndrome (ARDS), and NPE, but also several abnormal respiratory patterns and sleep-disordered breathing.[31] Lung injuries triggered by neurocritical damage can have significant effects on outcomes, such as the selection of treatment plans, disease prognosis, and mortality.
Pneumonia
Pneumonia is commonly seen in stroke patients and is associated with poor outcomes. The most frequently cultured pathogens in NCC patients are gram-negative bacilli and gram-positive cocci, with sputum being the most commonly used sample for cultivation and detec-tion.[32] Risk factors for pneumonia in stroke patients who have been identified in systematic reviews include the following: older age, male, MV, nasogastric tube, dysphagia, diabetes, pre-existing respiratory conditions, atrial fibrillation, and smoking.[33,34] Among the risk factors, ventilation and dysphagia have been studied more widely in recent years regarding the feasibility of an intervention. For example, the timing of tracheostomy in TBI patients[35] and the optimal ventilation strategies of NCC patients have been discussed.[25]
Ventilator-associated pneumonia (VAP) is commonly seen in NCC patients with MV. The incidence of VAP has been shown to be 21% to 60% in patients with severe TBI,[36] 20% to 48% in those with SAH, and approximately 28% in those with stroke.[1] The pathogen that is detected in most cases is S. aureus, followed by H. influenzae, S. pneumoniae, E. coli, and other types of pathogens that are found in some patients.[36–38] Apart from the risk factors mentioned above, other factors associated with the treatment process are worth noting. Esnault et al[36] confirmed that early-onset VAP is associated with therapeutic hypothermia, serious thoracic trauma, and gastric aspiration before intubation. Early enteral feeding, oral care, and prophylactic antibiotics have been reported to protect NCC patients from VAP.[38,39]
Another type of pneumonia associated with a neurocritical state is aspiration pneumonia. Dysphagia, which has been reported in 37% to 45% of stroke patients,[40] is associated with pneumonia and poor outcomes in these patients.[41] Feng et al[42] investigated the mortality rate associated with aspiration pneumonia in stroke patients, and the authors found that dysphagia is a critical factor in the development of aspiration pneumonia. Ding et al[43] studied dysphagia, and it has been considered a common factor in different models for predicting poststroke pneumonia.
Respiratory distress and pulmonary edema
ARDS is an alveolar condition characterized by the formation of the hyaline membrane and dysfunction of gas exchange. ARDS has a high mortality rate, and the incidence of ARDS that has been reported in different journals ranges from 19% to 35%.[1,44] Mrozek et al[1] summarized the incidence of ARDS to be 20% to 25% in patients with severe TBI, 20% to 38% in those with SAH,[45] and approximately 4% in those with stroke.[46] The risk factors for developing ARDS in patients with brain injury include severe primary neurological disease, hemodynamic instability, a history of chronic diseases, and other general risk factors.[1,46] ARDS patients in the NCC unit are generally treated with protective MV (pMV) and require restrictive body fluid management.[24] This year, a study in a neurological ICU stated that assisted orthostatism can be a safe auxiliary treatment for severe ARDS, as it improved the PaO2/FiO2 in 95.6% of the patients without causing significant hemodynamic repercus-sions.[47] NPE is a type of protein-rich edema of the lung.
It is diagnosed with bilateral infiltrates and PaO2/FiO2 of < 200 mmHg in patients with severe injury of the central nervous system (CNS) and increased ICP when left atrial hypertension and other common causes of ARDS are not present. With prior participation of the neuropathway, it differs from other types of pulmonary edema and has a higher incidence in neurocritical patients. NPE secondary to neurocritical injuries has an estimated incidence ranging between 2% and 50%, and it occurs more often in patients with severe TBI than in those with SAH.[1,48] Based on the mechanism of NPE related to sympathetic nervous activity, Chen et al[49] examined heart function in 204 patients with SAH and found that heart rate variability can predict the occurrence of NPE.
Respiratory Support in NCC
Respiratory management in NCC follows the general rules of intensive care, but specific aspects are different from general ICU because of the higher incidence of respiratory disorders in the NCC unit.[27,50,51] Patients being treated in the NCC unit are in various states of unconsciousness and have different types of respiratory drive disorders. Thus, patients in the NCC unit are often ventilated because of unconsciousness and potential respiratory disorders or airway obstructions, not primary respiratory failure.
In addition, because various NCC patients have special needs and ventilator targets, the implementation of neuroprotective strategies, including a tailored ventilatory approach on patients with ABI, might effectively improve survival and functional outcome in these patients.[27,52] For example, choking or esophageal reflux should be avoided for patients with cerebral hemorrhage or unruptured aneurysms.[51] Protective ventilation with low tidal volumes (6–8 mL/kg of ideal body weight) could be safely applied to TBI patients.[53]
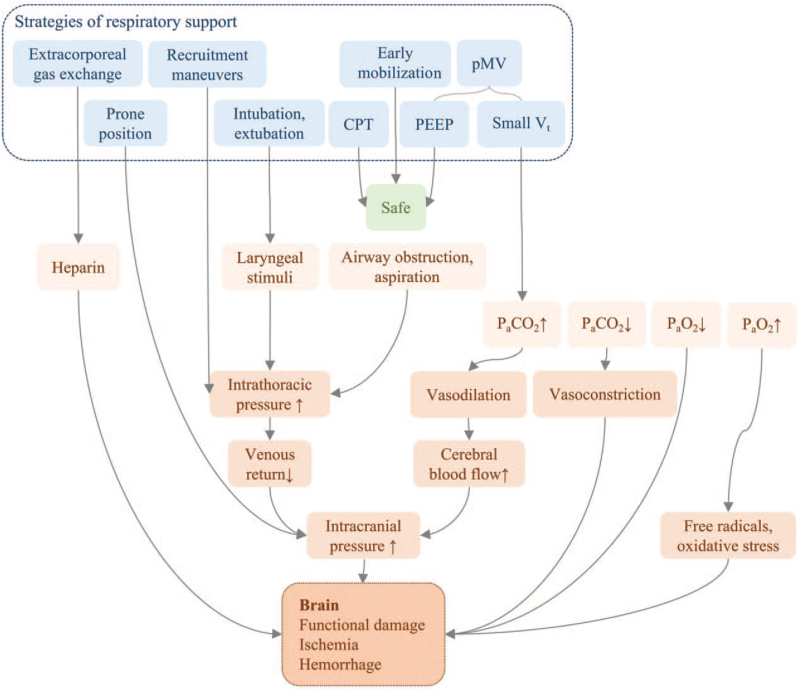
The goal of respiratory support in NCC patients is to avoid fatal secondary damage, including cerebral ischemia, hemorrhage, functional impairment, and even death,[54–56] due to factors such as inappropriate oxygen or carbon dioxide levels, aspiration, and airway obstruction, which differs from the goal in patients in the general ICU, which is to alleviate primary respiratory diseases.[57,58] The reasonable ranges of oxygen and carbon dioxide levels may differ from general ICU patients. We collected the results of different studies concerning respiratory protection maneuvers in NCC patients, and Figure 1 is a summary of the possible potential risks.
Figure 1.
Potential risks of respiratory protective strategies. CPT: Chest physiotherapy; PaCO2: Arterial carbon dioxide pressure; PaO2: Arterial oxygen pressure; PEEP: Positive end-expiratory pressure; pMV: Protective mechanical ventilation; Vt: Tidal volume.
Both hypoxemia and hyperoxia should be avoided because the neurological outcomes can worsen. Hypoxemia is related to cerebral ischemia and functional impairment, while hyperoxia is related to excessive free radicals and oxidative stress, which impair cerebral autoregulation and cause damage to the lung and brain tissue.[56,59] An association between hypoxia and an increase in mortality in NCC patients has been reported,[56,60] while Fallenius et al[29] reported no correlation with long-term mortality in patients with spontaneous intracranial hemorrhage. Both hypercapnia and hypocapnia have a conspicuous effect on ICP. Although hypercapnia may be permissive in the general ICU, it is often prohibitive in NCC patients because of the ability to elevate ICP. However, some researchers have stated that controlled hypercapnia benefits patients with SAH and vasospasm by increasing CBF and tissue oxygenation while monitoring ICP.[58,61] Hypocapnia occurs in approximately 92% of aneurysmal SAH patients, mostly when they breathe spontaneously with minimal ventilation support.[27,58] In addition, hypocapnia is associated with a poor functional outcome in these patients.[58] Low arterial carbon dioxide levels result in a reduction in CBF and vasoconstriction, followed by brain tissue hypoxia.[23] The effects of hypercapnia and hypocapnia necessitate a moderate level of arterial carbon dioxide. Expert consensus for neurosurgical critical care in China suggests maintaining arterial carbon dioxide pressure (PaCO2) at 35 to 45 mmHg, SpO2 > 95%, and PaO2 > 80 mmHg.[51]
Intubation and tracheostomy
Intubation and tracheostomy are ways of creating artificial airways. In the NCC unit, intubation is indicated in patients with unconsciousness or Glasgow coma score (GCS) < 8, an abnormal blood gas composition including hypoxemia and hypercapnia, or respiratory and airway disorders, and it is indicated for treatments such as MV and the control of the ICP or seizures.[3] Tracheostomy is indicated in NCC patients who need long-term (>2 weeks) artificial airway and respiratory support, GCS < 8, and dysphagia. To reduce discomfort and sedation during intubation, better oral and tracheobronchial care that does not hinder the ability to communicate should be provided.[5,62]
The maneuvers of the intubation process and prehospital intubation have been discussed in different studies because of the potential risks of intubation in NCC patients, including an increase in the ICP resulting from laryngoscopy stimuli, hypoxemia during the operation, and aspiration, especially in difficult airways.[63,64] The LEMON approach and amendatory rating scales have been recommended in several studies for the identification of difficult airways.[3,65] These tools may be beneficial in selecting the appropriate technique (awake fiberoptic or rapid sequence induction), tools (video or direct laryngoscopy), and operator (anesthesiologist, attending, or trainee) during the intubation process.[65] In recent years, researchers have recommended rapid sequence intubation (RSI) with sedation during the operation process to reduce the stimuli in laryngoscopy.[3,63] Although a retrospective study reported that trauma severity influenced mortality more than intubation in prehospital admissions, a large cohort-matched study noted that prehospital intubation in patients with TBI was associated with higher in-hospital mortality.[54,66]
Recent studies on tracheostomy in NCC patients mainly focus on the optimal timing and ways of performing a tracheostomy. The optimal timing is still being debated, considering the advantages and disadvantages of performing a tracheostomy earlier or later in time. Early-stage tracheostomy, defined as one with a cutoff of 7 to 8 days,[67] is associated with a reduction in the need for sedation, the incidence of nosocomial infection, and the time of MV and ICU stay compared with prolonged intubation, and it is cost effective.[67–71] Recently, some studies also found that early tracheostomy after severe brain injury is associated with a better neurological outcome, reduced in-hospital time, and reduced risk for VAP.[35,72] However, whether early tracheostomy affects the long-term mortality rate is controversial.[67,68,71] Jibaja et al[57] expressed the view that conducting one primary tracheostomy is advisable in patients at risk (with severe cervical spine injuries, infratentorial severe injuries, repeated failed extubation, prolonged MV, and poor neurological states). The results of tracheostomy performed later in time may exclude a certain percentage of those patients who would have undergone an early-stage tracheostomy, but postponed tracheostomy may be accompanied by a higher incidence of complications.[68] Progress has been made in improving the way tracheostomy is performed to prevent intracranial hypertension by avoiding hyperextension of the neck and shortening the duration of the procedure.[5,73] Different types of tracheos-tomy equipment may have similar outcomes in general patients, but percutaneous/puncture-dilated tracheostomy has been recommended in NCC patients as a safe and quick treatment, as it has the advantages of reducing the incidence of bleeding, tracheal stenosis, and infections.[73,74]
Airway management
Airway management before and after intubation or tracheostomy includes sputum drainage and drugs for RSI and spasmolysis. Sputum is a common cause of airway obstruction, and it produces an artificial airway airflow sound or whistle sound. Sputum drainage is crucial in patients with acute neurological impairment. Thus, optimal methods of achieving airway humidification and clearing in NCC patients are essential and worth discussing. Drugs for airway spasmolysis include sedatives, analgesics, muscle relaxants, and nitric oxide (NO).
Some doctors have compared different liquids for airway humidification in the lungs in patients with TBI, and they found that 0.9% sodium chloride (NaCl) with ambroxol is an ideal airway humidification liquid.[75] It was found to have anti-inflammatory and antioxidant properties, as it promoted the synthesis and secretion of pulmonary surfactants and inhibited the release of inflammatory factors and cytokines.
The inflated cuff of the endotracheal tube is designed to prevent microaspiration; thus, the cuff pressure should be at a moderate level at 20 to 30 cm of water, as air leakage or aspiration occurs when it is too low, and tracheal or subglottic stenosis occurs when it is too high.[5,76,77] In NCC patients, the cuff pressure may decline with extubation and the transition to a prone position.[78] In addition, the high incidence of pneumonia further emphasized the necessity of determining and monitoring cuff pressure. However, the palpation method performed with the operator's fingers was suggested to be inadequate to determine the cuff pressure;[77] thus, guidelines and equipment are required.
Chest physiotherapy (CPT) can mobilize respiratory secretions and increase the amount of tracheobronchial mucus that is cleared from the respiratory tree. Although CPT can increase ICP,[79] it has been indicated to be safe in NCC patients.[80–82] Tomar et al[81] evaluated the safety of different CPT techniques in patients with TBI, and they found that an automated or mechanical method of performing CPT can be executed without a transient rise in ICP, while the manual method might jeopardize cerebral circulatory pathophysiology. CPT is safe in patients with ICP monitoring in situ.[83]
Adequate sedation in neurocritical patients is paramount. Sedation can ease fear and anxiety, reduce ICP and cerebral oxygen consumption, facilitate tolerance of the endotracheal tube and MV, and reduce sympathetic nervous activity.[3] Rajajee et al[3] summarized the variety of sedatives available and the common sedatives used in NCC patients. Alpha-2 agonists such as dexmedetomidine have been shown to have no effect on the ICP and hemodynamic variability when they are included in standard sedation.[84] However, in several studies, they have been reported to reduce the duration of MV and ICU stays more than traditional sedatives such as propofol and remifentanil.[85,86]
Although analgesics such as fentanyl were found to be ineffective in treating episodic intracranial hypertension, analgesia is recommended before sedation in some studies.[87] Because the analgesic effect of most sedatives is unsatisfactory and sedation without pain control is a risk factor for delirium,[3] analgesia with the use of short-acting opioids is recommended. Additionally, patients in a coma with adequate pain control and airway construction do not need sedatives.
Muscle relaxants and NO also help restore respiratory function in neurocritical patients. Muscle relaxants can correct hypoxemia and prevent MV-associated lung injury.[5] Sugammadex has been used in some cases to accelerate weaning from the ventilator after prolonged MV.[88] NO inhalation is immunomodulatory and pathogen static, and it assists in the reversal of pulmonary hypertension.[4] Terpolilli et al[89] reported that in mice, NO inhalation can reduce secondary brain damage after TBI. Guo et al[90] discussed the influence of NO on cerebral autoregulation and noted that NO may assist in the regulation of CBF, which can be considered a new therapeutic target.
Parameters of MV
Regarding MV, compared with patients without neurological conditions, patients with neurological conditions have shown a longer ventilation duration, higher rates of tracheostomy, and less extracerebral organ dysfunc-tion.[91] Considering the effect of standard MV on lung tissue damage, clinicians and researchers promote pMV in patients with respiratory disorders and intensive care. To open the lung and keep it open in the NCC unit, patients are recommended to undergo pMV with a small Vt, elevated PEEP, and recruitment maneuvers (RMs).[1] However, in recent years, controversies have been discussed in several studies on the effect of high PEEP and low PEEP.[92,93] Little progress has been made in clarifying the role and safety of high-frequency ventilation (respiratory rate > 150 breaths per minute, Vt 1–5 mL/kg[94]) in patients with neurological diseases.
PEEP is a method of keeping the alveoli open at the end of expiration. Although inducing alveolar hyperinflation has been shown to increase PaCO2 and ICP in a previous study,[95] moderate PEEP (5–15 cm H2O) is beneficial for mechanically ventilated patients with acceptable hemody-namic changes; it has been shown to be beneficial for improving oxygenation, preventing and recruiting alveolar collapse, and reducing the risk of atelectasis in patients with low Vt.[27,96] In clinical practice, in the management of neurocritical patients with ARDS, PEEP has few negative effects on the intracranial condition and can even benefit brain tissue oxygenation.[50,97,98] A study conducted by Boone et al[97] demonstrated that the fluctuations of the ICP and cerebral perfusion pressure (CPP) in patients with severe lung injury are more sensitive to PEEP than those in the other patients, but the application of PEEP does not appear to have a clinical effect overall. However, recent studies have suggested that instead of keeping the lungs open, the goal should be to close down the lungs and keep them closed to protect the lung tissue when low PEEP (≤3 cm H2O) is applied in ARDS patients, but data on this topic are not available in neurocritical patients.[92,93]
pMV with low Vt and moderate PEEP is safe for patients after brain injury, but its positive effects on the outcome must be better delineated.[50,69] A small Vt is related to hypercapnia in patients with pMV. It may be permissive in the general ICU, but it deserves vigilance in the NCC unit.[12] Vt is positively associated with the incidence of ARDS and negatively associated with PaCO2 and ICP in a dose-response relationship.[99] Thus, a low Vt decreases the incidence of lung injuries but is accompanied by PaCO2 and ICP increases, which may affect most NCC patients. However, a randomized clinical trial involving 961 patients without ARDS in the ICU found that a low Vt was not more effective than an intermediate Vt.[92] Avoiding hypercapnia necessitates the monitoring of the cerebral hemodynamic index and carbon dioxide level, which is part of the multimodal neuro-monitoring process.[4,27] Intra-operative pMV with low tidal volumes (6–8 mL/kg) has been shown to reduce the incidence of post-operative pulmonary complications, while intraoperative high PEEP might negatively affect hemodynamics in nonobese patients.[100] Intra-operative pMV will be studied in a single-center, parallel-group randomized controlled trial to determine its efficiency and safety in neurosurgical patients undergoing a craniotomy.[101]
Recruitment maneuvers (RMs) for collapsed pulmonary alveoli can open the lung and improve oxygenation and respiratory system compliance in ARDS patients.[102,103] However, the management of ICP hinders reaeration by lung units in neurocritical patients undergoing RMs. RMs may interfere with venous blood return and increase intrathoracic pressure, which increases ICP and decreases cerebral arterial blood pressure.[104,105] Although continuous positive airway pressure (CPAP) is currently the most common RM, clinical experiments comparing different RMs in NCC patients have been conducted by several researchers, who reported that maneuvers with a lower airway pressure and longer duration are better than traditional CPAP.[27,106] RMs are safe with the strict monitoring of systemic and cerebral parameters.
Time of weaning and extubation
The extubation failure rate of NCC patients has been reported to be 17.2% to 38.0% in different studies, and ventilation discontinuation accounted for 50% of the deaths in neurovascular patients.[50,57,107] Strategies of weaning from MV and extubation have been developed from studies and protocols in patients without neuro-critical conditions.[57] Patients with brain injuries were rarely described in the latest guidelines for weaning or extubation strategies.[50] Waiting for full neurological recovery is not mandatory. Prolonged MV in patients with subdural hematoma (>4 days) is associated with pulmonary complications and a longer hospital stay.[108]
Factors associated with the success of MV and extubation withdrawals have been identified and summarized in several studies.[50,57,107] Anderson et al[109] found that following four commands, closing the eyes, showing two fingers, wiggling the toes, and coughing, were protective factors for extubation success. A multicentric cohort study of patients with severe brain injury identified four features associated with extubation success: an age of < 40 years, visual pursuit, swallowing attempts, and a GCS of > 10.[107] Jibaja et al[57] expressed the view that not answering verbal commands or a low GCS does not indicate a delay or contraindication for MV or extubation withdrawal.
Studies about extubation failure in NCC patients have mentioned predictors, such as airway dysfunctions (pneumonia, atelectasis, thick secretion, no gag reflex, weak cough, and deglutition), neurological statuses (a GCS of < 7–9, inability to follow commands), and the duration of MV.[110–113] Several studies have emphasized the predictive role of fundamental state (age, fluid balance) and upper-airway functions irrespective of neurological status.[112,114] However, Mayer et al[113] stated that neurological status is more important than pulmonary status in deciding whether to perform extubation. Cohn et al[111] retrospectively reviewed the data in pediatric NCC patients and found that a weak cough reflex might be a risk factor for failed extubation.
Algorithms and criteria before extubation have been reported by researchers.[115,116] The spontaneous breathing trial (SBT) is a consensus approach used to predict extubation success. Mullaguri et al[115] studied an algorithm in 108 NCC patients using zero pressure support and a zero-PEEP SBT, followed by 5-cm H2O pressure support and a 5-cm H2O PEEP SBT, in patients who failed the zero-PEEP SBT, and the researchers found that most NCC patients who were otherwise ready to be extubated could safely be extubated after passing a zero-PEEP SBT. Tanwar et al[116] suggested that the airway care score can be used as a criterion for early extubation success.
Extracorporeal gas exchange
Extracorporeal decarboxylation is the normalization of the serum carbon dioxide level in vitro through the canalization of arteries and veins. Blood flow occurs in a pumpless arteriovenous system to create pressure gradients for the two blood vessels. As the blood flows across the device, it normalizes the carbon dioxide level, resulting in an improvement in the blood pH and a decrease in the occurrence of ventilator-induced lung injuries.[117] This approach is applied in patients with normal oxygenation and severe hypercapnia.[118] The use of this approach is restricted because of the potential intracranial hemorrhagic risk with the application of a large dose of heparin and the lack of evidence regarding improved outcomes in patients.[118,119]
Extracorporeal membrane oxygenation (ECMO) is more effective than extracorporeal decarboxylation in patients with both hypercapnia and hypoxemia. It can reduce the aggressiveness of MV and reduce the mortality rate in patients with indications for ECMO.[5] Two types of ECMO exist: venovenous ECMO (vv-ECMO), where blood is taken from the inferior vena cava and returned to the superior vena cava, and veno-arterial ECMO, where blood is returned to the aorta. Starting ECMO > 7 days after the initiation of MV yields no benefits.[120] The need for relatively high doses of heparin increases the risk of these complications in NCC patients.[121] To date, only a few cases of the use of ECMO in NCC patients have been reported; because intracranial hemorrhage complications can occur after prolonged ECMO or vv-ECMO, ECMO is considered a rescue for severe hypoxemia respiratory failure in trauma patients.[120–124] The use of special biomaterials in modern ECMO without initial anticoagulation has been considered a valid option for patients with a high risk of bleeding.[5,122]
Positioning and mobilization
Different positions and postures are suggested for NCC patients because of their pulmonary disorders and altered ICP. The proclive or reverse Trendelenburg position is a position where the level of the patient's head and chest is higher than the feet, which decreases the ICP and end-tidal carbon dioxide partial pressure.[125] It is commonly used in patients with intracranial hypertension. The supine position is the position where a patient lies down horizontally and faces upward, and this position is commonly used in patients without intracranial hypertension or difficulty in gas exchange. The prone position is the position where a patient lies down and faces downward; it facilitates venous return, as the heart and lungs are at the lowest level of the body. Because it improves pulmonary drainage and oxygenation, the prone position is commonly used in patients with severe ARDS, but to date, it has been used only in a few cases in the NCC unit. In patients without head injury or a risk of intracranial hypertension, the prone position is associated with a moderate elevation of the ICP and an increase in oxygenation.[126,127] A retrospective descriptive study suggested that the prone position is safe in patients with severe ARDS, even in patients at risk of intracranial hypertension.[128] ICP monitoring in the prone position is required in patients who are at risk of intracranial hypertension or have a history of neurosurgery.[126–128]
Early mobilization is not widely deployed in NCC patients, but recently, it has been reported to be safe, feasible, and potentially beneficial.[129,130] A multicenter study conducted in 10 patients with acute brain injury found that early mobilization appears to favor clinical and functional recovery.[130] Bahouth et al[129] proposed a formalized Neurocritical Care Unit mobility algorithm for adult patients with primary intracerebral hemorrhage. The study suggested that within the first week after a hemorrhagic stroke, a large percentage of patients can be mobilized without additional adverse events, and the implementation of a standardized algorithm is feasible and reduces the incidence of pulmonary embolism.[131]
Gas exchange in neuro-monitoring
An appropriate blood gas composition is crucial for neurocritical patients to avoid secondary brain damage. Pandin et al[132] systemically introduced the concept of multimodel neuromonitoring. Compared with other means, multimodel neuromonitoring can detect early neurological deterioration, consider individual pathophysiological variations, and allow clinicians to make individualized management decisions.[133] The process of monitoring brain and spinal cord metabolism and function can be summarized by five aspects: ICP and CPP, which represent the driving pressure of brain perfusion; trans-cranial Doppler, which shows the local and regional CBF; brain tissue oxygen pressure (PbtO2), which reflects the CBF and oxygen diffusion; the result of microdialysis, which is associated with CNS metabolism; and electroencephalogram monitoring, which reflects CNS function. Multimodel neuromonitoring is useful for monitoring gas exchange in brain tissues.[27,134]
Assessing pulmonary function and testing the blood gas composition are important parts of a gas exchange evaluation. Ventilator parameters, such as PEEP and Vt, and gas composition parameters, such as PaO2 and PaCO2, are indices that directly represent the gas exchange level in the lung. Corradi et al[104] combined the use of lung ultrasound and brain ultrasound in NCC patients with demanding MV needs in ventilation management, with the aim of tailoring the balance in intracranial hypertension-directed and lung-protective therapy.[104]
Limitations
This article has several limitations. The first is that we primarily searched a single English database. Consequently, our search terms possibly did not capture all aspects of the topic. However, we minimized the likelihood of missed articles by applying a broad search strategy. Second, some of the included articles were case reports, method introductions, or small sample studies. Thus, the interpretation of some results may be limited.
Conclusion
Systemic changes secondary to neurocritical injuries can induce impairments in pulmonary function. Although it has been discussed for many years, the pathomechanisms remain poorly defined. With the development of brain science, brain-lung crosstalk is becoming a research hotspot. Respiratory management in NCC follows the general rules of intensive care, but in specific aspects, it is different due to the higher incidence of respiratory disorders and the prioritization of protecting the brain in NCC. Thus, strategies to protect the lungs and the brain are recommended for NCC patients. However, the optimal strategies for the management of NCC patients remain controversial, and further guidelines and criteria are urgently needed.
Funding
This work was supported by the National Key Research & Development Program of China (No. 2018YFA0108603), the Beijing Tianjin Hebei basic research cooperation project (No. 19JCZDJC64600(Z)), the CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 020-I2M-C&T-B-028), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (No. 2020-JKCS-026), and the National Natural Science Foundation of China (Nos. 81601033, 81974183).
Conflicts of interest
None.
Footnotes
How to cite this article: Wen J, Chen J, Chang J, Wei J. Pulmonary complications and respiratory management in neurocritical care: a narrative review. Chin Med J 2022;135:779–789. doi: 10.1097/CM9.0000000000001930
References
- 1.Mrozek S, Constantin JM, Geeraerts T. Brain-lung crosstalk: implications for neurocritical care patients. World J Crit Care Med 2015; 4:163–178. doi: 10.5492/wjccm.v4.i3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oddo M, Bracard S, Cariou A, Chanques G, Citerio G, Clerckx B, et al. Update in neurocritical care: a summary of the 2018 Paris International Conference of the French Society of Intensive Care. Ann Intensive Care 2019; 9:47.doi: 10.1186/s13613-019-0523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajajee V, Riggs B, Seder DB. Emergency neurological life support: airway, ventilation, and sedation. Neurocrit Care 2017; 27: (Suppl 1): 4–28. doi: 10.1007/s12028-017-0451-2. [DOI] [PubMed] [Google Scholar]
- 4.Della Torre V, Badenes R, Corradi F, Racca F, Lavinio A, Matta B, et al. Acute respiratory distress syndrome in traumatic brain injury: how do we manage it? J Thorac Dis 2017; 9:5368–5381. doi: 10.21037/jtd.2017.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nazarenko MB, Kruglyakov NM, Semenov MS, Zabelin MV, Udalov YD, Samoylov AS, et al. Topical respiratory strategies in neurocritical care. Burdenko's J Neurosurg 2017; 81:104–116. doi: 10.17116/engneiro201781590-98. [DOI] [PubMed] [Google Scholar]
- 6.Mascia L. Acute lung injury in patients with severe brain injury: a double hit model. Neurocrit Care 2009; 11:417–426. doi: 10.1007/s12028-009-9242-8. [DOI] [PubMed] [Google Scholar]
- 7.Theodore J, Robin ED. Speculations on neurogenic pulmonary edema (NPE). Am Rev Respir Dis 1976; 113:405–411. doi: 10.1164/arrd.1976.113.4.405. [DOI] [PubMed] [Google Scholar]
- 8.Thalanayar PM, James LO, Infeld M. Impact of sympathetic storm (from brain trauma) on ventilator dependence. Am J Respir Crit Care Med 2017; 195:A2021.doi: 10.1164/ajrccm-confer-ence.2017.A58. [Google Scholar]
- 9.Keegan MT, Lanier WL. Pulmonary edema after resection of a fourth ventricle tumor: possible evidence for a medulla-mediated mechanism. Mayo Clin Proc 1999; 74:264–268. doi: 10.4065/74.3.264. [DOI] [PubMed] [Google Scholar]
- 10.Mrozek S, Gobin J, Constantin JM, Fourcade O, Geeraerts T. Crosstalk between brain, lung and heart in critical care. Anaesth Crit Care Pain Med 2020; 39:519–530. doi: 10.1016/j.accpm.2020.06.016. [DOI] [PubMed] [Google Scholar]
- 11.McKeating EG, Andrews PJ, Signorini DF, Mascia L. Transcranial cytokine gradients in patients requiring intensive care after acute brain injury. Br J Anaesth 1997; 78:3.doi: 10.1093/bja/78.5.520. [DOI] [PubMed] [Google Scholar]
- 12.Han DW. Brain and lung: dangerous crosstalk. Korean J Anesthesiol 2017; 70:116–117. doi: 10.4097/kjae.2017.70.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol 2002; 20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- 14.Choi KE, Sim T, Berger K, Lahiri S, Lee T, Park B, et al. Sympathetic excess drives systemic response syndrome after brain injury. Neurocrit Care 2014; 21:S220.doi: 10.1007/s12028-014-0034-4. [Google Scholar]
- 15.Cárdenas J, Cuadrado A, Medina L, Cánovas J, Rosado L. Incidence of systemic immune response syndrome (SIRS) in patients with subarachnoid aneurysmal haemorrhage (SAH) and its association with morbidity and mortality. Intensive Care Med 2014; 40:S120–S121. doi: 10.1007/s00134-013-3451-5. [Google Scholar]
- 16.Tan H, Yang W, Wu C, Liu B, Lu H, Wang H, et al. Assessment of the role of intracranial hypertension and stress on hippocampal cell apoptosis and hypothalamic-pituitary dysfunction after TBI. Sci Rep 2017; 7:3805.doi: 10.1038/s41598-017-04008-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu PJ, Pittet JF, Kerby JD, Bosarge PL, Wagener BM. Acute brain trauma, lung injury, and pneumonia: more than just altered mental status and decreased airway protection. Am J Physiol Lung Cell Mol Physiol 2017; 313:L1–L15. doi: 10.1152/ajplung.00485.2016. [DOI] [PubMed] [Google Scholar]
- 18.Huang M, Gedansky A, Hassett CE, Price C, Fan TH, Stephens RS, et al. Pathophysiology of brain injury and neurological outcome in acute respiratory distress syndrome: a scoping review of preclinical to clinical studies. Neurocrit Care 2021; 23:1–10. doi: 10.1007/s12028-021-01309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bilotta F, Giordano G, Sergi PG, Pugliese F. Harmful effects of mechanical ventilation on neurocognitive functions. Crit Care 2019; 23:273.doi: 10.1186/s13054-019-2546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breit H, Jhaveri M, John S. Concomitant delayed posthypoxic leukoencephalopathy and critical illness microbleeds. Neurol Clin Pract 2018; 8:e31–e33. doi: 10.1212/cpj.0000000000000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González-López A, López-Alonso I, Aguirre A, Amado-Rodríguez L, Batalla-Solís E, Astudillo A, et al. Mechanical ventilation triggers hippocampal apoptosis by vagal and dopaminergic pathways. Am J Respir Crit Care Med 2013; 188:693–702. doi:10.1164/rccm.201304-0691OC. [DOI] [PubMed] [Google Scholar]
- 22.González-López A, López-Alonso I, Pickerodt PA, von Haefen C, Amado-Rodríguez L, Reimann H, et al. Lung purinoceptor activation triggers ventilator-induced brain injury. Crit Care Med 2019; 47:e911–e918. doi: 10.1097/CCM.0000000000003977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grüne F, Kazmaier S, Stolker RJ, Visser GH, Weyland A. Carbon dioxide induced changes in cerebral blood flow and flow velocity: role of cerebrovascular resistance and effective cerebral perfusion pressure. J Cereb Blood Flow Metab 2015; 35:1470–1477. doi: 10.1038/jcbfm.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Roux PD, Levine JM, Andrew Kofke W. Monitoring in Neurocritical Care. Singapore: Elsevier; 2013. [Google Scholar]
- 25.Robba C, Poole D, McNett M, Asehnoune K, Bösel J, Bruder N, et al. Mechanical ventilation in patients with acute brain injury: recommendations of the European Society of Intensive Care Medicine consensus. Intensive Care Med 2020; 46:2397–2410. doi: 10.1007/s00134-020-06283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howarth C. The contribution of astrocytes to the regulation of cerebral blood flow. Front Neurosci 2014; 8:103.doi: 10.3389/fnins.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borsellino B, Schultz MJ, Gama de Abreu M, Robba C, Bilotta F. Mechanical ventilation in neurocritical care patients: a systematic literature review. Expert Rev Respir Med 2016; 10:1123–1132. doi: 10.1080/17476348.2017.1235976. [DOI] [PubMed] [Google Scholar]
- 28.McBryde FD, Malpas SC, Paton JF. Intracranial mechanisms for preserving brain blood flow in health and disease. Acta Physiol (Oxf) 2017; 219:274–287. doi: 10.1111/apha.12706. [DOI] [PubMed] [Google Scholar]
- 29.Fallenius M, Raj R, Reinikainen M, Bendel S, Skrifvars MB. Association between high arterial oxygen tension and long-term survival after spontaneous intracerebral hemorrhage. Crit Care Med 2016; 44:180–187. doi: 10.1097/CCM.0000000000001281. [DOI] [PubMed] [Google Scholar]
- 30.Ghosh A, Highton D, Kolyva C, Tachtsidis I, Elwell CE, Smith M. Hyperoxia results in increased aerobic metabolism following acute brain injury. J Cereb Blood Flow Metab 2017; 37:2910–2920. doi: 10.1177/0271678X16679171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balofsky A, George J, Papadakos P. Wijdicks EFM, Kramer AH. Chapter 3 - Neuro-pulmonology. Handbook of Clinical Neurology. Cambridge, MA: Elsevier; 2017. 33–48. [DOI] [PubMed] [Google Scholar]
- 32.Kishore AK, Vail A, Jeans AR, Chamorro A, Di Napoli M, Kalra L, et al. Microbiological etiologies of pneumonia complicating stroke: a systematic review. Stroke 2018; 49:1602–1609. doi: 10.1161/STROKEAHA.117.020250. [DOI] [PubMed] [Google Scholar]
- 33.Wastfelt M, Cao Y, Strom JO. Predictors of post-stroke fever and infections: a systematic review and meta-analysis. BMC Neurol 2018; 18:49.doi: 10.1186/s12883-018-1046-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapman C, Morgan P, Cadilhac DA, Purvis T, Andrew NE. Risk factors for the development of chest infections in acute stroke: a systematic review. Top Stroke Rehabil 2018; 25:445–458. doi: 10.1080/10749357.2018.1481567. [DOI] [PubMed] [Google Scholar]
- 35.Robba C, Galimberti S, Graziano F, Wiegers EJA, Lingsma HF, Iaquaniello C, et al. Tracheostomy practice and timing in traumatic brain-injured patients: a CENTER-TBI study. Intensive Care Med 2020; 46:983–994. doi: 10.1007/s00134-020-05935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esnault P, Nguyen C, Bordes J, D’Aranda E, Montcriol A, Contargyris C, et al. Early-onset ventilator-associated pneumonia in patients with severe traumatic brain injury: incidence, risk factors, and consequences in cerebral oxygenation and outcome. Neurocrit Care 2017; 27:187–198. doi: 10.1007/s12028-017-0397-4. [DOI] [PubMed] [Google Scholar]
- 37.Kalanuria AA, Fellerman D, Nyquist P, Geocadin R, Kowalski RG, Nussenblatt V, et al. Variability in diagnosis and treatment of ventilator-associated pneumonia in neurocritical care patients. Neurocrit Care 2015; 23:44–53. doi: 10.1007/s12028-015-0109-x. [DOI] [PubMed] [Google Scholar]
- 38.Cinotti R, Dordonnat-Moynard A, Feuillet F, Roquilly A, Rondeau N, Lepelletier D, et al. Risk factors and pathogens involved in early ventilator-acquired pneumonia in patients with severe subarachnoid hemorrhage. Eur J Clin Microbiol Infect Dis 2014; 33:823–830. doi: 10.1007/s10096-013-2020-8. [DOI] [PubMed] [Google Scholar]
- 39.Lord AS, Nicholson J, Lewis A. Infection prevention in the neurointensive care unit: a systematic review. Neurocrit Care 2019; 31:196–210. doi: 10.1007/s12028-018-0568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 2005; 36:2756–2763. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- 41.Al-Khaled M, Matthis C, Binder A, Mudter J, Schattschneider J, Pulkowski U, et al. Dysphagia in patients with acute ischemic stroke: early dysphagia screening may reduce stroke-related pneumonia and improve stroke outcomes. Cerebrovasc Dis 2016; 42:81–89. doi: 10.1159/000445299. [DOI] [PubMed] [Google Scholar]
- 42.Feng MC, Lin YC, Chang YH, Chen CH, Chiang HC, Huang LC, et al. The mortality and the risk of aspiration pneumonia related with dysphagia in stroke patients. J Stroke Cerebrovasc Dis 2019; 28:1381–1387. doi: 10.1016/j.jstrokecerebrovas-dis.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 43.Ding Y, Yan Y, Niu J, Zhang Y, Gu Z, Tang P, et al. Braden scale for assessing pneumonia after acute ischaemic stroke. BMC Geriatr 2019; 19:259.doi: 10.1186/s12877-019-1269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muehlschlegel S, Flahive J, Osgood M, Carandang R, Hall W, Goldberg R. Incidence and impact of intensive care unit complications on short-and long-term outcomes after moderate-severe traumatic brain injury-results from a 7-year prospective cohort study. Neurocrit Care 2017; 27:S79–S80. doi: 10.1007/s12028-017-0465-9. [Google Scholar]
- 45.Veeravagu A, Chen YR, Ludwig C, Rincon F, Maltenfort M, Jallo J, et al. Acute lung injury in patients with subarachnoid hemorrhage: a nationwide inpatient sample study. World Neuro-surg 2014; 82:e235–241. doi: 10.1016/j.wneu.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 46.Rincon F, Maltenfort M, Dey S, Ghosh S, Vibbert M, Urtecho J, et al. The prevalence and impact of mortality of the acute respiratory distress syndrome on admissions of patients with ischemic stroke in the United States. J Intensive Care Med 2014; 29:357–364. doi: 10.1177/0885066613491919. [DOI] [PubMed] [Google Scholar]
- 47.Travassos P, Vale R, Geres W, Teixeira É, Coscrato L, Veiga V, et al. Use of the orthostatic board as an additional resource for the treatment of acute respiratory distress syndrome. Crit Care 2019; 23: (Suppl 2): 72.doi: 10.1186/s13054-019-2358-0.30890150 [Google Scholar]
- 48.Davison DL, Terek M, Chawla LS. Neurogenic pulmonary edema. Crit Care 2012; 16:7.doi: 10.1007/978-3-642-25716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen WL, Chang SH, Chen JH, Tai HC, Chan CM, Wang YC. Heart rate variability predicts neurogenic pulmonary edema in patients with subarachnoid hemorrhage. Neurocrit Care 2016; 25:71–78. doi: 10.1007/s12028-015-0237-3. [DOI] [PubMed] [Google Scholar]
- 50.Asehnoune K, Roquilly A, Cinotti R. Respiratory management in patients with severe brain injury. Crit Care 2018; 22:76.doi: 10.1186/s13054-018-1994-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao JZ, Zhou DB, Zhou LF, Wang RZ, Zhang JN, et al. China Neurosurgical Critical Care Specialist Council (CNCCSC). The experts consensus for patient management of neurosurgical critical care unit in China (2015). Chin Med J 2015; 128:1252–1267. doi: 10.4103/0366-6999.156146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chesnut R, Aguilera S, Buki A, Bulger E, Citerio G, Cooper DJ, et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med 2020; 46:919–929. doi: 10.1007/s00134-019-05900-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cinotti R, Bouras M, Roquilly A, Asehnoune K. Management and weaning from mechanical ventilation in neurologic patients. Ann Transl Med 2018; 6:381.doi: 10.21037/atm.2018.08.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haltmeier T, Benjamin ER, Siboni S, Dilektasli E, Inaba K, Demetriades D. Prehospital intubation for isolated severe blunt traumatic brain injury: worse outcomes and higher mortality. J Am Coll Surg 2015; 221:S97.doi: 10.1016/j.jamcollsurg.2015.07.224. [DOI] [PubMed] [Google Scholar]
- 55.Gravesteijn BY, Sewalt CA, Nieboer D, Menon DK, Maas A, Lecky F, et al. Tracheal intubation in traumatic brain injury: a multicentre prospective observational study. Br J Anaesth 2020; 125:505–517. doi: 10.1016/j.bja.2020.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stocchetti N, Taccone FS, Citerio G, Pepe PE, Le Roux PD, Oddo M, et al. Neuroprotection in acute brain injury: an up-to-date review. Crit Care 2015; 19:186.doi: 10.1186/s13054-015-0887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jibaja M, Sufan JL, Godoy DA. Controversies in weaning from mechanical ventilation and extubation in the neurocritical patient. Med Intensiva (Engl Ed) 2018; 42:551–555. doi: 10.1016/j.medine.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 58.Badenes R, Bilotta F. Neurocritical care for intracranial haemorrhage: a systematic review ofrecent studies. Br J Anaesth 2015; 115: (Suppl 2): ii68–ii74. doi: 10.1093/bja/aev379. [DOI] [PubMed] [Google Scholar]
- 59.Hanafy KA, Selim MH. Antioxidant strategies in neurocritical care. Neurotherapeutics 2012; 9:44–55. doi: 10.1007/s13311-011-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rincon F, Kang J, Maltenfort M, Vibbert M, Urtecho J, Athar MK, et al. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med 2014; 42:387–396. doi: 10.1097/CCM.0b013e3182a27732. [DOI] [PubMed] [Google Scholar]
- 61.Westermaier T, Stetter C, Kunze E, Willner N, Holzmeier J, Weiland J, et al. Controlled hypercapnia enhances cerebral blood flow and brain tissue oxygenation after aneurysmal subarachnoid hemorrhage: results of a phase 1 study. Neurocrit Care 2016; 25:205–214. doi: 10.1007/s12028-016-0246-x. [DOI] [PubMed] [Google Scholar]
- 62.Yaghi S, Moore P, Ray B, Keyrouz SG. Predictors of tracheostomy in patients with spontaneous intracerebral hemorrhage. Clin Neurol Neurosurg 2013; 115:695–698. doi: 10.1016/j.clineuro.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 63.Bucher J, Koyfman A. Intubation of the neurologically injured patient. J Emerg Med 2015; 49:920–927. doi: 10.1016/j.jemermed.2015.06.078. [DOI] [PubMed] [Google Scholar]
- 64.Perkins ZB, Wittenberg MD, Nevin D, Lockey DJ, O’Brien B. The relationship between head injury severity and hemodynamic response to tracheal intubation. J Trauma Acute Care Surg 2013; 74:1074–1080. doi: 10.1097/TA.0b013e3182827305. [DOI] [PubMed] [Google Scholar]
- 65.Reed MJ, Dunn MJ, McKeown DW. Can an airway assessment score predict difficulty at intubation in the emergency department? Emerg Med J 2005; 22:99–102. doi: 10.1136/emj.2003.008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gutierrez Rodriguez R, Arias Verdu MD, De Miguel Aparicio FJ. Effects of prehospital intubation and sedation on mortality after traumatic brain injury. Eur J Anaesthesiol 2014; 31:117–118. doi: 10.1097/00003643-201406001-00325.24047728 [Google Scholar]
- 67.Raimondi N, Vial MR, Calleja J, Quintero A, Cortés Alban A, Celis E, et al. Evidence-based guides in tracheostomy use in critical patients. Med Intensiva 2017; 41:94–115. doi: 10.1016/j.medin.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 68.McCredie VA, Alali AS, Scales DC, Adhikari NK, Rubenfeld GD, Cuthbertson BH, et al. Effect of early versus late tracheostomy or prolonged intubation in critically ill patients with acute brain injury: a systematic review and meta-analysis. Neurocrit Care 2017; 26:14–25. doi: 10.1007/s12028-016-0297-z. [DOI] [PubMed] [Google Scholar]
- 69.Asehnoune K, Mrozek S, Perrigault PF, Seguin P, Dahyot-Fizelier C, Lasocki S, et al. A multi-faceted strategy to reduce ventilation-associated mortality in brain-injured patients. The BI-VILI project: a nationwide quality improvement project. Intensive Care Med 2017; 43:957–970. doi: 10.1007/s00134-017-4764-6. [DOI] [PubMed] [Google Scholar]
- 70.Alali AS, Scales DC, Fowler RA, Mainprize TG, Ray JG, Kiss A, et al. Tracheostomy timing in traumatic brain injury: a propensity-matched cohort study. J Trauma Acute Care Surg 2014; 76:70–76. discussion 76-78. doi: 10.1097/TA.0b013e3182a8fd6a. [DOI] [PubMed] [Google Scholar]
- 71.Bosel J, Schiller P, Hook Y, Andes M, Neumann JO, Poli S, et al. Stroke-related early tracheostomy versus prolonged orotracheal intubation in neurocritical care trial (SETPOINT): a randomized pilot trial. Stroke 2013; 44:21–28. doi: 10.1161/STROKEAHA. 112.669895. [DOI] [PubMed] [Google Scholar]
- 72.de Franca SA, Tavares WM, Salinet ASM, Paiva WS, Teixeira MJ. Early tracheostomy in severe traumatic brain injury patients: a meta-analysis and comparison with late tracheostomy. Crit Care Med 2020; 48:e325–e331. doi: 10.1097/ccm.0000000000004239. [DOI] [PubMed] [Google Scholar]
- 73.Cheung NH, Napolitano LM. Tracheostomy: epidemiology, indications, timing, technique, and outcomes. Respir Care 2014; 59:895–915. discussion 916-919. doi: 10.4187/respcare.02971. [DOI] [PubMed] [Google Scholar]
- 74.Bosel J. Tracheostomy in stroke patients. Curr Treat Options Neurol 2014; 16:274.doi: 10.1007/s11940-013-0274-1. [DOI] [PubMed] [Google Scholar]
- 75.Su X, Li Z, Wang M, Li Z, Wang Q, Lu W, et al. The protective effect of different airway humidification liquids to lung after tracheotomy in traumatic brain injury: the role of pulmonary surfactant protein-A (SP-A). Gene 2016; 577:89–95. doi: 10.1016/j.gene.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 76.Khan AB, Omar S, Thandrayen K. Tracheal tube cuff pressure monitoring: assessing current practice in critically ill patients at Chris Hani Baragwanath Academic Hospital. S Afr J Crit Care 2019; 35:8–13. doi: 10.7196/SAJCC.2019.v35i1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giusti GD, Rogari C, Gili A, Nisi F. Cuff pressure monitoring by manual palpation in intubated patients: how accurate is it? A manikin simulation study. Aust Crit Care 2017; 30:234–238. doi: 10.1016/j.aucc.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 78.Athiraman U, Gupta R, Singh G. Endotracheal cuff pressure changes with change in position in neurosurgical patients. Int J Crit Illn Inj Sci 2015; 5:237–241. doi: 10.4103/2229-5151.170841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferreira LL, Valenti VE, Vanderlei LC. Chest physiotherapy on intracranial pressure of critically ill patients admitted to the intensive care unit: a systematic review. Rev Bras Ter Intensiva 2013; 25:327–333. doi: 10.5935/0103-507X.20130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thiesen RA, Dragosavac D, Roquejani AC, Falcão ALE, Araujo S, Dantas Filho VP, et al. Influence of the respiratory physioterapy on intracranial pressure in severe head trauma patients. Arq Neuropsiquiatr 2005; 63:110–113. doi: 10.1590/S0004-282X2005000100020. [DOI] [PubMed] [Google Scholar]
- 81.Tomar GS, Singh GP, Bithal PK. To compare the effects of 2 different techniques of chest physiotherapy on intracranial pressure in traumatic brain injury patients: a randomized cross over study. J Neurosurg Anesthesiol 2016; 28:S18.doi: 10.1097/ANA.0000000000000287. [Google Scholar]
- 82.Harvey J, Hurst B. An evaluation of the effect of respiratory physiotherapy on intracranial pressure and cerebral perfusion pressure in ventilated, adult neuro-trauma patients. Physiotherapy 2016; 102:e88.doi: 10.1016/j.physio.2016.10.088. [Google Scholar]
- 83.Olson DM, Bader MK, Dennis C, Mahanes D, Riemen K. Multicenter pilot study: safety of automated chest percussion in patients at risk for intracranial hypertension. J Neurosci Nurs 2010; 42:119–127. doi: 10.1097/JNN.0b013e3181d4a3aa. [PubMed] [Google Scholar]
- 84.Hendry R, Mace B, Riemen K, James M, Olson DW, Dombrowski K. Cerebral perfusion pressure using precedex and other sedatives (C3PO). Neurocrit Care 2015; 23:S146.doi: 10.1007/s12028-015-0193-y. [Google Scholar]
- 85.Chen K, Lu Z, Xin YC, Cai Y, Chen Y, Pan SM. Alpha-2 agonists for long-term sedation during mechanical ventilation in critically ill patients. Cochrane Database Syst Rev 2015; 1:CD010269.doi: 10.1002/14651858.CD010269.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tran A, Blinder H, Hutton B, English S. Alpha-2 agonists for sedation in mechanically ventilated neurocritical care patients: a systematic review protocol. Syst Rev 2016; 5:154.doi: 10.1186/s13643-016-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Welch TP, Wallendorf MJ, Kharasch ED, Leonard JR, Doctor A, Pineda JA. Fentanyl and midazolam are ineffective in reducing episodic intracranial hypertension in severe pediatric traumatic brain injury. Crit Care Med 2016; 44:18.doi: 10.1097/CCM.0000000000001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oreshnikov E, Oreshnikova S. Successful use of sugammadex for weaning from mechanical ventilation in ARDS with the previous use of aminosteroid muscle relaxants. Crit Care Med 2012; 40:320.doi: 10.1097/01.ccm.0000425605.04623.4b.22179360 [Google Scholar]
- 89.Terpolilli NA, Kim SW, Thal SC, Kuebler WM, Plesnila N. Inhaled nitric oxide reduces secondary brain damage after traumatic brain injury in mice. J Cereb Blood Flow Metab 2013; 33:311–318. doi: 10.1038/jcbfm.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo ZN, Shao A, Tong LS, Sun W, Liu J, Yang Y. The role of nitric oxide and sympathetic control in cerebral autoregulation in the setting of subarachnoid hemorrhage and traumatic brain injury. Mol Neurobiol 2016; 53:3606–3615. doi: 10.1007/s12035-015-9308-x. [DOI] [PubMed] [Google Scholar]
- 91.Pelosi P, Ferguson ND, Frutos-Vivar F, Anzueto A, Putensen C, Raymondos K, et al. Management and outcome of mechanically ventilated neurologic patients. Crit Care Med 2011; 39:11.doi: 10.1097/CCM.0b013e31821209a8. [DOI] [PubMed] [Google Scholar]
- 92.Simonis FD, Neto AS, Binnekade JM, Braber A, Bruin KCM, Determann RM, et al. Effect of a low vs intermediate tidal volume strategy on ventilator-free days in intensive care unit patients without ARDS: a randomized clinical trial. JAMA 2018; 320:1872–1880. doi: 10.1001/jama.2018.14280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pelosi P, Rocco PRM, Gama de Abreu M. Close down the lungs and keep them resting to minimize ventilator-induced lung injury. Crit Care 2018; 22:72.doi: 10.1186/s13054-018-1991-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chang WT, Nyquist PA. Strategies for the use of mechanical ventilation in the neurologic intensive care unit. Neurosurg Clin N Am 2013; 24:407–416. doi: 10.1016/j.nec.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 95.Mascia L, Grasso S, Fiore T, Bruno F, Berardino M, Ducati A. Cerebro-pulmonary interactions during the application of low levels of positive end-expiratory pressure. Intensive Care Med 2005; 31:373–379. doi: 10.1007/s00134-004-2491-2. [DOI] [PubMed] [Google Scholar]
- 96.Lou M, Xue F, Chen L, Xue Y, Wang K. Is high PEEP ventilation strategy safe for acute respiratory distress syndrome after severe traumatic brain injury? Brain Inj 2012; 26:887–890. doi: 10.3109/02699052.2012.660514. [DOI] [PubMed] [Google Scholar]
- 97.Boone MD, Jinadasa SP, Mueller A, Shaefi S, Kasper EM, Hanafy KA, et al. The effect of positive end-expiratory pressure on intracranial pressure and cerebral hemodynamics. Neurocrit Care 2017; 26:174–181. doi: 10.1007/s12028-016-0328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nemer SN, Caldeira JB, Santos RG, Guimaraes BL, Garcia JM, Prado D, et al. Effects of positive end-expiratory pressure on brain tissue oxygen pressure of severe traumatic brain injury patients with acute respiratory distress syndrome: a pilot study. J Crit Care 2015; 30:1263–1266. doi: 10.1016/j.jcrc.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 99.Mascia L, Zavala E, Bosma K, Pasero D, Decaroli D, Andrews P, et al. High tidal volume is associated with the development of acute lung injury after severe brain injury: an international observational study. Crit Care Med 2007; 35:1815–1820. doi: 10.1097/01.CCM.0000275269.77467.DF. [DOI] [PubMed] [Google Scholar]
- 100.Güldner A, Kiss T, Neto AS, Hemmes SNT, Canet J, Spieth PM, et al. Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications: a comprehensive review of the role of tidal volume, positive end-expiratory pressure, and lung recruitment maneuvers. Anesthesiology 2015; 123:22.doi: 10.1097/ALN.0000000000000754. [DOI] [PubMed] [Google Scholar]
- 101.Zhang L, Xiong W, Peng Y, Zhang W, Han R. The effect of an intraoperative, lung-protective ventilation strategy in neurosurgi-cal patients undergoing craniotomy: study protocol for a randomized controlled trial. Trials 2018; 19:85.doi: 10.1186/s13063-018-2447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kung SC, Hung YL, Chen WL, Wang CM, Chang HC, Liu WL. Effects of stepwise lung recruitment maneuvers in patients with early acute respiratory distress syndrome: a prospective, randomized, controlled trial. J Clin Med 2019; 8:231.doi: 10.3390/jcm8020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Keenan JC, Formenti P, Marini JJ. Lung recruitment in acute respiratory distress syndrome: what is the best strategy? Curr Opin Crit Care 2014; 20:63–68. doi: 10.1097/MCC.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 104.Corradi F, Robba C, Tavazzi G, Via G. Combined lung and brain ultrasonography for an individualized “brain-protective ventilation strategy” in neurocritical care patients with challenging ventilation needs. Crit Ultrasound J 2018; 10:24.doi: 10.1186/s13089-018-0105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Flexman AM, Gooderham PA, Griesdale DE, Argue R, Toyota B. Effects of an alveolar recruitment maneuver on subdural pressure, brain swelling, and mean arterial pressure in patients undergoing supratentorial tumour resection: a randomized crossover study. Can J Anesth 2017; 64:626–633. doi: 10.1007/s12630-017-0863-7. [DOI] [PubMed] [Google Scholar]
- 106.Nemer SN, Caldeira JB, Azeredo LM, Garcia JM, Silva RT, Prado D, et al. Alveolar recruitment maneuver in patients with subarachnoid hemorrhage and acute respiratory distress syndrome: a comparison of 2 approaches. J Crit Care 2011; 26:22–27. doi: 10.1016/j.jcrc.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 107.Asehnoune K, Seguin P, Lasocki S, Roquilly A, Delater A, Gros A, et al. Extubation success prediction in a multicentric cohort of patients with severe brain injury. Anesthesiology 2017; 127:338–346. doi: 10.1097/ALN.0000000000001725. [DOI] [PubMed] [Google Scholar]
- 108.Busl KM, Ouyang B, Boland TA, Pollandt S, Temes RE. Prolonged mechanical ventilation is associated with pulmonary complications, increased length of stay, and unfavorable discharge destination among patients with subdural hematoma. J Neurosurg Anesthesiol 2015; 27:31–36. doi: 10.1097/ANA.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 109.Anderson CD, Bartscher JF, Scripko PD, Biffi A, Chase D, Guanci M, et al. Neurologic examination and extubation outcome in the neurocritical care unit. Neurocrit Care 2011; 15:490–497. doi:10.1007/s12028-010-9369-7. [DOI] [PubMed] [Google Scholar]
- 110.Wang S, Zhang L, Huang K, Lin Z, Qiao W, Pan S. Predictors of extubation failure in neurocritical patients identified by a systematic review and meta-analysis. PLoS One 2014; 9:e112198.doi: 10.1371/journal.pone.0112198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cohn EC, Robertson TS, Scott SA, Finley AM, Huang R, Miles DK. Extubation failure and tracheostomy placement in children with acute neurocritical illness. Neurocrit Care 2018; 28:83–92. doi: 10.1007/s12028-017-0429-0. [DOI] [PubMed] [Google Scholar]
- 112.Godet T, Chabanne R, Marin J, Kauffmann S, Futier E, Pereira B, et al. Extubation failure in brain-injured patients: risk factors and development of a prediction score in a preliminary prospective cohort study. Anesthesiology 2017; 126:104–114. doi: 10.1097/ALN.0000000000001379. [DOI] [PubMed] [Google Scholar]
- 113.Mayer GF, Ciarrocchi N, Goldenberg F, Hardy V, Venuti M, Tessore N, et al. Predictors of tracheal extubation success in neurocritically ill patients. Crit Care Med 2018; 46:502.doi:10.1097/01.ccm.0000529044.82229.96. [Google Scholar]
- 114.McCredie VA, Ferguson ND, Pinto RL, Adhikari NK, Fowler RA, Chapman MG, et al. Airway management strategies for braininjured patients meeting standard criteria to consider extubation. A prospective cohort study. Ann Am Thorac Soc 2017; 14:85–93. doi: 10.1513/AnnalsATS.201608-620OC. [DOI] [PubMed] [Google Scholar]
- 115.Mullaguri N, Khan Z, Nattanmai P, Newey CR. Extubating the neurocritical care patient: a spontaneous breathing trial algorithmic approach. Neurocrit Care 2018; 28:93–96. doi: 10.1007/s12028-017-0398-3. [DOI] [PubMed] [Google Scholar]
- 116.Tanwar G, Singh U, Kundra S, Chaudhary A, Kaytal S, Grewal A. Evaluation of airway care score as a criterion for extubation in patients admitted in neurosurgery intensive care unit. J Anaesth Clin Pharmacol 2019; 35:85–91. doi: 10.4103/joacp.JOACP_362_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nentwich J, John S. Current techniques for extracorporeal decarboxylation. Med Klin Intensivmed Notfmed 2019; 114:733–740. doi: 10.1007/s00063-019-0567-6. [DOI] [PubMed] [Google Scholar]
- 118.Munoz-Bendix C, Beseoglu K, Kram R. Extracorporeal decarbox-ylation in patients with severe traumatic brain injury and ARDS enables effective control of intracranial pressure. Crit Care 2015; 19:381.doi: 10.1186/s13054-015-1088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Deniau B, Ricard JD, Messika J, Dreyfuss D, Gaudry S. Use of extracorporeal carbon dioxide removal (ECCO2R) in 239 intensive care units: results from a French national survey. Intensive Care Med 2016; 42:624–625. doi: 10.1007/s00134-016-4226-6. [DOI] [PubMed] [Google Scholar]
- 120.Rozencwajg S, Pilcher D, Combes A, Schmidt M. Outcomes and survival prediction models for severe adult acute respiratory distress syndrome treated with extracorporeal membrane oxygenation. Crit Care 2016; 20:392.doi: 10.1186/s13054-016-1568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Luyt CE, Brechot N, Demondion P, Jovanovic T, Hekimian G, Lebreton G, et al. Brain injury during venovenous extracorporeal membrane oxygenation. Intensive Care Med 2016; 42:897–907. doi: 10.1007/s00134-016-4318-3. [DOI] [PubMed] [Google Scholar]
- 122.Robba C, Ortu A, Bilotta F, Lombardo A, Sekhon MS, Gallo F, et al. Extracorporeal membrane oxygenation for adult respiratory distress syndrome in trauma patients. J Trauma Acute Care Surg 2017; 82:165–173. doi: 10.1097/ta.0000000000001276. [DOI] [PubMed] [Google Scholar]
- 123.Darsie M, Cadena RR, Sasaki-Adams DD, Charles AA, Olm-Shipman CC. Veno-venous ECMO as rescue therapy for severe neurogenic pulmonary edema due to aneurysmal subarachnoid hemorrhage. Neurocrit Care 2016; 25:S301.doi: 10.1007/s12028-016-0301-7. [Google Scholar]
- 124.Guerrero B, Kuo G, Kim-Tenser MA. ECMO related intracerebral hemorrhage. Neurocrit Care 2017; 27:S464.doi: 10.1007/s12028-017-0465-9. [Google Scholar]
- 125.Dogru S, Karaman T, Tapar H, Sahin A, Karakiscedil A. The relationship between end-tidal carbon dioxide levels and patient positions. Acta Medica Mediterranea 2016; 32:791–794. doi: 10.19193/0393-6384_2016_3_91. [Google Scholar]
- 126.Roth C, Ferbert A, Deinsberger W, Kleffmann J, Kastner S, Godau J, et al. Does prone positioning increase intracranial pressure? A retrospective analysis of patients with acute brain injury and acute respiratory failure. Neurocrit Care 2014; 21:186–191. doi:10.1007/s12028-014-0004-x. [DOI] [PubMed] [Google Scholar]
- 127.Robba C, Bragazzi NL, Bertuccio A, Cardim D, Donnelly J, Sekhon M, et al. Effects of prone position and positive end-expiratory pressure on noninvasive estimators of ICP: a pilot study. J Neurosurg Anesthesiol 2017; 29:243–250. doi: 10.1097/ANA.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 128.Bourenne J, Guerin V, Perrin G, Benarous L, Bouzana F, Lambert D, et al. Prone position: Impact on intracranial pressure and cerebral perfusion pressure in ARDS patients with brain injury. Ann Intensive Care 2017; 7:175–176. doi: 10.1186/s13613-016-0224-7. [Google Scholar]
- 129.Bahouth MN, Power MC, Zink EK, Kozeniewski K, Kumble S, Deluzio S, et al. Safety and feasibility of a neuroscience critical care program to mobilize patients with primary intracerebral hemorrhage. Arch Phys Med Rehabil 2018; 99:1220–1225. doi: 10.1016/j.apmr.2018.01.034. [DOI] [PubMed] [Google Scholar]
- 130.Bartolo M, Bargellesi S, Castioni CA, Intiso D, Fontana A, Copetti M, et al. Mobilization in early rehabilitation in intensive care unit patients with severe acquired brain injury: an observational study. J Rehabil Med 2017; 49:715–722. doi: 10.2340/16501977-2269. [DOI] [PubMed] [Google Scholar]
- 131.AVERT Trial Collaboration group. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet 2015; 386:46–55. doi: 10.1016/S0140-6736(15)60690-0. [DOI] [PubMed] [Google Scholar]
- 132.Pandin P, Renard M, Bianchini A, Desjardin P, Obbergh LV. Monitoring brain and spinal cord metabolism and function. Open J Anesthesiol 2014; 04:131–152. doi: 10.4236/ojanes.2014.46020. [Google Scholar]
- 133.Yang MT. Multimodal neurocritical monitoring. Biomed J 2020; 43:226–230. doi: 10.1016/j.bj.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ngwenya LB, Burke JF, Manley GT. Brain tissue oxygen monitoring and the intersection of brain and lung: a comprehensive review. Respir Care 2016; 61:1232–1244. doi: 10.4187/respcare.04962. [DOI] [PubMed] [Google Scholar]