Abstract
Introduction:
The objective of this study was to explore the clinical, laboratory, and imaging features of severe Chlamydia psittaci pneumonia in order to improve early diagnosis and treatment success rates.
Methods:
We conducted a retrospective record review of 14 cases of severe Chlamydia psittaci pneumonia diagnosed by metagenomic next-generation sequencing technology in our hospital. We extracted and analyzed data on the clinical symptoms and signs, contact history, laboratory investigations, chest computed tomography, treatment, and clinical outcomes.
Results:
Of the 14 patients, 12 (86%) were male and two (14%) were female, with a mean age of 57 years (SD: 7 years). Eleven patients (79%) had a history of poultry contact. The main clinical manifestations were fever (n = 14, 100%), flu-like symptoms (n = 10, 71%), cough, sputum (n = 9, 64%), and dyspnea (n = 5, 36%). Blood tests revealed marked elevation of neutrophil percentage, C-reactive protein, procalcitonin, brain natriuretic peptide, and creatine kinase levels; slight elevation of aspartate aminotransferase, creatinine, urea, fibrinogen, and D-dimer levels; and decreased albumin, sodium, and calcium levels. Chest computed tomography showed bilateral lesions (n = 7, 50%), middle-lower lobe lesions (n = 10, 71%), lesions in multiple lobes (n = 9, 64%), consolidation shadows (n = 11, 79%), and pleural effusions (n = 11, 79%). The median time from disease onset to hospital admission was 4.5 days (interquartile range: 1–17 days); the mean length of hospital stay was 20.9 ± 8.5 days, and the mean time from admission to diagnosis was 5.1 ± 2.6 days. After diagnosis, patients were either treated with doxycycline alone or doxycycline combined with quinolones. All 14 patients developed respiratory failure and received invasive mechanical ventilation; two (14%) received veno-venous extracorporeal membrane oxygenation, four (29%) received continuous renal replacement therapy, and three (21%) died.
Discussion and conclusion:
A poultry contact history and typical flu-like symptoms are early indicators of Chlamydia psittaci pneumonia. Substantial elevations in procalcitonin, creatine kinase, and brain natriuretic peptide indicate severe disease. Metagenomic next-generation sequencing is useful for diagnosis. Early empirical antibiotic therapy with quinolones can reduce the mortality in critically ill patients.
Keywords: Chlamydia psittaci, doxycycline, metagenomic next-generation sequencing, quinolone, severe pneumonia
1. Introduction
Chlamydia psittaci is an obligate, intracellular gram-negative bacterium that mainly infects poultry but can also cause zoonotic infections. The inhalation of aerosols contaminated by the excreta of infected birds can cause Chlamydia psittaci pneumonia, which can sometimes lead to severe pneumonia and even death.[1] The level of awareness of C. psittaci pneumonia among clinicians is generally low, and its clinical presentation is atypical. Consequently, the disease is easily missed and misdiagnosed. If the diagnosis is delayed, patients may develop severe illness. The traditional culture of C. psittaci is associated with strict requirements, the sensitivity is low, and most laboratories do not routinely culture for Chlamydia. Serological test and polymerase chain reaction testing is available at some medical institutions, but their sensitivity and specificity are generally low.[2] Metagenomic next-generation sequencing (mNGS) has gradually been adopted for the diagnosis of difficult to diagnose, severe pneumonia in recent years.
We retrospectively analyzed the clinical data of 14 patients with severe C. psittaci pneumonia diagnosed by mNGS. The purpose of this report is to help clinicians with the early identification of patients with this disease, and to describe our experience with early administration of empirical antibiotic treatment, in order to improve treatment success rates.
2. Subjects and methods
2.1. Research subjects
Fourteen patients with severe C. psittaci pneumonia who were admitted to our hospital from January 2019 to November 2021 and met the inclusion criteria were selected for the review. The inclusion criteria for were as follows:
-
(1)
complied with the 2019 Infectious Diseases Society of America/American Thoracic Society Guidelines for the diagnosis and treatment of adult community-acquired pneumonia (CAP);[3]
-
(2)
fulfilled the diagnostic criteria for severe pneumonia;[3]
-
(3)
identification of the C. psittaci gene fragments through mNGS analysis of the bronchoalveolar lavage fluid (BALF), blood, or sputum, and met the criteria for a positive mNGS result;[4]
-
(4)
routine microbiological tests, including blood, sputum, and alveolar lavage fluid culture were all negative; and
-
(5)
respiratory failure, defined as an oxygenation index <400 mm Hg (1 mm Hg = 0.133 kPa).
This study was approved by the Ethics Committee of the Zhongshan People's Hospital (approval no.: K2021-127) and proceeded in accordance with the Declaration of Helsinki. All patients or their family members provided signed informed consent. All data in this study were anonymized before analysis.
2.2. mNGS of alveolar lavage fluid
The mNGS utilized for this study was completed in collaboration with the Shenzhen Huada Gene Technology Co. Ltd. (Shenzhen, China). DNA extraction was performed by transferring 3 to 5 mL of alveolar lavage fluid into a centrifuge test tube, placing the test tube at 4°C and centrifuging at a high speed of 4000 rpm for 10 minutes, in accordance with the instructions of the TIANamp Micro DNA Kit (DP316, Tiangen Biotech, Beijing, China). The extracted DNA samples were broken into fragments of approximately 150 bp ultrasonically. An Agilent 2100 Bioanalyzer QC (quality control) library with an insert size of 200 to 300 bp was used; the Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific Inc.) was used to control the DNA library concentration, and cyclization was performed to form a single-chain ring structure. The cyclized library underwent rolling circle replication to generate DNA nanoballs. The prepared DNA nanoballs were loaded into the sequencing chip for sequencing, using a BGISEQ-50/MGISEQ-2000. Low-quality short fragments (length <35 bp) were removed after the sequencing data were derived from the machine to obtain high-quality sequencing data. Burrows-Wheeler Aligner (BWA) (BWA: http://bio-bwa.sourceforge.net/) sequencing analysis software was used to remove data that were matched with the reference human genomic sequence after a comparison with high-quality data. The remaining data were compared with the four microbial databases of bacteria, fungi, viruses, and parasites. After removing low-complexity sequences, the number of sequences that matched certain pathogens was obtained, and the possible pathogens, based on the number of sequences and other clinical tests, were determined.
2.3. Data collection
The demographics of patients, such as sex, age, body mass index, smoking history, and poultry contact history, among others, were collected. The clinical manifestations, physical signs, laboratory examinations, and imaging examination results, as well as the patients’ pneumonia severity scores, acute physiology, and chronic health scores, were recorded.
2.4. Laboratory and imaging tests
On the day of admission, we recorded the patient white blood cell count, erythrocyte sedimentation rate, and blood levels of C-reactive protein, procalcitonin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, creatine kinase (CK), CK-MB isoform, brain natriuretic peptide, albumin, potassium, sodium, calcium, creatinine, blood urea nitrogen, fibrinogen (FIB), and D-dimer. We also performed a blood gas analysis and recorded the oxygenation index when the patients were admitted to the intensive care unit (ICU). All patients underwent chest computed tomography examination prior to admission to determine the distribution, number, and types of lung lesions.
2.5. Statistical methods
All data were analyzed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA). Categorical variables were reported as frequencies and percentages. The one-sample Kolmogorov-Smirnov test was used to assess whether continuous variables followed a normal distribution. Continuous variables were reported as means and standard deviations or medians and interquartile ranges, depending on whether they had a normal or skewed distribution.
3. Results
3.1. Patient characteristics
Of the 14 patients, 12 were male (85.71%) and two were female (14.29%), with an average age of 57.21 ± 7.04 years and body mass index of 23.90 ± 1.19 kg/m2. Eleven patients had a clear history of poultry contact (92.58%), seven had underlying chronic diseases (50%), and eight had a history of smoking (57.14%) (Table 1).
Table 1.
Demographic and clinical characteristics of the 14 patients.
| Case# | Age (years) and sex | History of smoking | History of avian or poultry contact | Underlying diseases | Symptoms |
| 1 | 55 M | Yes | Vegetable market vendor | None | Fever, chest tightness, weakness, abdominal distention, vertigo, nausea, vomiting |
| 2 | 64 M | Yes | Long-term poultry raising | None | Fever, vertigo, weakness, nasal congestion, rhinitis, cough, expectoration, nausea, vomiting |
| 3 | 58 M | No | Farm worker | Renal transplantation, renal insufficiency, hypertension | Fever, dyspnea, diarrhea, vomiting |
| 4 | 50 M | No | Unknown | Gouty arthritis | Fever, cough, expectoration, dyspnea |
| 5 | 59 M | Yes | Poultry butcher | Coronary heart disease, hypertension | Fever, weakness, cough, drowsiness, incoherent speech, mental deterioration |
| 6 | 46 M | Yes | Kept parrots at home | Hemorrhoids, kidney stones, chronic gastritis | Fever, weakness, dyspnea, cough, expectoration, diarrhea |
| 7 | 56 M | Yes | Long-term poultry raising | None | Fever, weakness, cough, expectoration |
| 8 | 62 M | Yes | Frequent visits to the market to buy vegetables | COPD, hypertension, ankylosing spondylitis | Fever, cough, expectoration, dyspnea |
| 9 | 55 M | Yes | Poultry butcher | None | Fever, headache |
| 10 | 70 M | Yes | Unknown | Hypertension, cervical spondylosis, lumbar disc herniation | Fever, myalgia, headache, cough |
| 11 | 60 F | No | Frequent visits to the market to buy vegetables | Chronic hepatitis B, cirrhosis with hepatic decompensation, chronic gastric ulcer | Fever, weakness, headache, myalgia, chest pain, cough |
| 12 | 45 M | No | Raising chickens at home | None | Fever, vertigo, headache, nausea |
| 13 | 56 F | No | Unknown | None | Fever, headache, vertigo, cough, expectoration, chest pain |
| 14 | 65 M | No | Vegetable market vendor | None | Fever, abdominal distention, diarrhea |
COPD = chronic obstructive pulmonary disease.
3.2. Clinical manifestations of Chlamydia psittaci pneumonia
All patients had fever (100%), with headaches, dizziness, muscle aches, fatigue, sneezing, nasal congestion, runny nose, and other flu-like symptoms occurring in 10 patients (71%), cough and sputum in nine patients (64%), nausea, vomiting, abdominal distension, abdominal pain, diarrhea, and other abdominal symptoms in four patients (29%), dyspnea in five patients (36%), altered mental state in one patient (7%), and chest pain in one patient (7%) (Table 1).
3.3. Laboratory findings
Blood tests revealed markedly elevated neutrophil percentages, and blood levels of C-reactive protein (CRP), procalcitonin (PCT), brain natriuretic peptide (BNP), lactate dehydrogenase, and CK. Blood level of aspartate aminotransferase, creatinine, urea, FIB, D-dimer, and troponin were slightly elevated; and the albumin, sodium, and calcium levels were slightly decreased. All patients had a significant decrease in oxygen partial pressure and oxygenation index on admission to the ICU (Table 2).
Table 2.
Laboratory parameters of the fourteen patients.
| Parameter (units) | Normal range | Median (range) or (mean ± SD) |
| Blood tests | ||
| WBC (×109/L) | 4–10 | 7.48 ± 3.29 |
| Neutrophil (%) | 50–70 | 87 (7–95) |
| HGB (g/L) | 120–160 | 123.2 ± 22.9 |
| PLT (×109/L) | 100–300 | 178 (24–590) |
| CRP (mg/L) | 0–8 | 150 ± 48 |
| PCT (ng/mL) | 0–5 | 1.6 (0.5–48.8) |
| AST (U/L) | 13–40 | 78 (13–382) |
| ALT (U/L) | 7–50 | 47 (9–1621) |
| TBIL (μmol/L) | 1.7–21 | 15.8 (4.5–61.3) |
| ALB (g/L) | 35–50 | 31.7 ± 4.5 |
| LDH (U/L) | 0–247 | 387 (180–2348) |
| CK (U/L) | 26–174 | 567 (25–16620) |
| CK-MB (U/L) | 0–24 | 19 (3–170) |
| BNP (pg/mL) | 0–100 | 430 (10–9000) |
| K (mmol/L) | 3.5–5.5 | 3.7 ± 0.35 |
| Na (mmol/L) | 135–145 | 133.8 ± 4.7 |
| Ca (mmol/L) | 2.25–2.75 | 2.08 ± 0.17 |
| Cr (μmol/L) | 59–104 | 120.3 ± 55.6 |
| BUN (mmol/L) | 3.2–7.1 | 8.53 ± 3.08 |
| FIB (g/L) | 2–4 | 6.2 ± 1.7 |
| D-dimer (mg/L) | 0–0.5 | 2.64 ± 1.51 |
| Cardiac function | ||
| LVEF (%) | 50–70 | 57 ± 13 |
| Respiratory function | ||
| PO2 (mmHg) | 80–100 | 74.9 ± 26.4 |
| PaO2/FiO2 (mmHg) | 400–500 | 233 (205–422) |
ALB = albumin, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BNP = brain natriuretic peptide, BUN = blood urea nitrogen, Ca = calcium, CK = creatine kinase, CK-MB = creatine kinase MB isoenzyme, Cr = creatinine, CRP = C-reactive protein, FIB = fibrinogen, HGB = hemoglobin, K = potassium, LDH = lactate dehydrogenase, LVEF = left ventricular ejection fraction, Na = sodium, PaO2/FiO2 = oxygenation index, PCT = procalcitonin, PLT = platelets, PO2 = partial pressure of oxygen, TBIL = total bilirubin, WBC = white blood cells.
3.4. Microbiology results
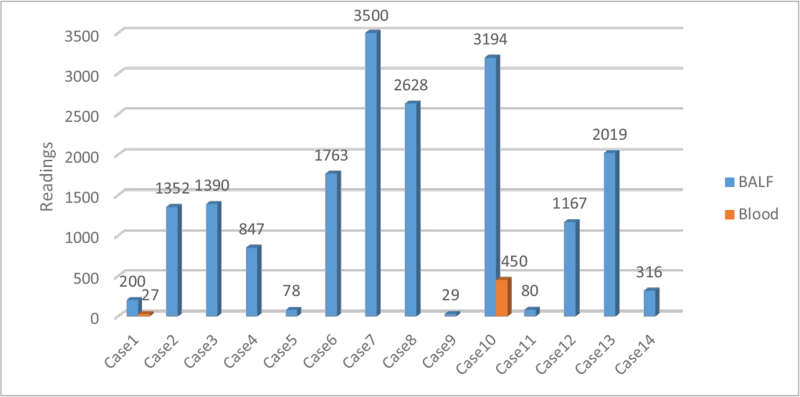
The traditional microbiological examination of the 14 patients, including sputum, blood, and BALF culture, did not reveal any other pathogens. All patients received mNGS testing of the BALF, and two patients had simultaneous BALF and blood testing using mNGS. The median sequence number for mNGS analysis of alveolar lavage fluid samples was 1325.93 ± 1174.14 (Fig. 1). The mNGS sequence numbers in the two blood samples tested were 27 and 450. The sequence numbers for the corresponding BALF samples were 200 and 3194, respectively (Fig. 1). In six patients (42.86%), the mNGS results of alveolar lavage fluid samples also revealed that the numbers of sequences corresponding to Chlamydia abortus were 12, 31, 97, 115, 173, and 194.
Figure 1.
Metagenomic next-generation sequencing results and number of Chlamydia psittaci readings obtained for each patient. BALF = bronchoalveolar lavage fluid.
3.5. Chest computed tomography
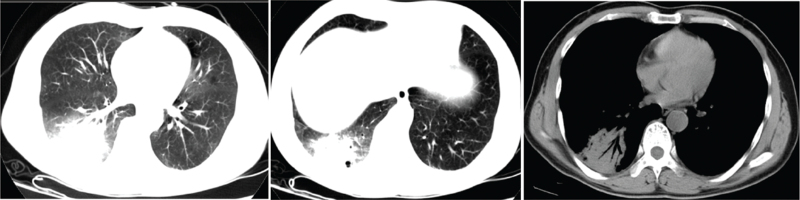
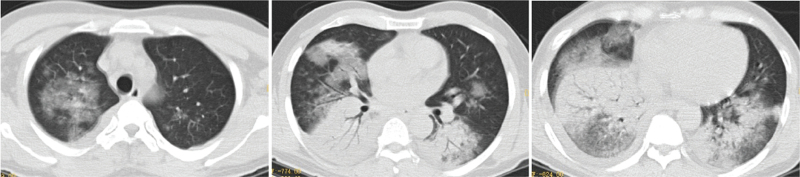
All patients underwent preadmission chest computed tomography examination, of which two patients (14%) had left-sided lesions, five patients (36%) had right-sided lesions, seven patients (50%) had bilateral lesions, four patients (29%) had upper lobe lesions, 10 patients (71%) had middle-lower lobe lesions, five patients (36%) had single lobe lesions, and nine patients (64%) had multilobar lesions; reflected as consolidation occurred in six patients (43%), consolidation shadows mixed with ground-glass opacities were present in five patients (36%), two patients (14%) had patchy shadows, and one patient (7%) had consolidation shadows and mixed ground-glass opacities with patchy shadows. Eleven patients (79%) had pleural effusions (Figs. 2–6).
Figure 2.
Chest computed tomography of Case 3, a 58-year-old male, showing patchy consolidation of both lungs with blurred edges, and a small right pleural effusion.
Figure 6.
Chest computed tomography of Case 12, a 45-year-old male, showing patchy increased density shadows and consolidation shadows in the right lower lung, with air bronchograms.
Figure 3.
Chest computed tomography of Case 4, a 50-year-old male, showing multiple patchy, patchy and nodular consolidation shadows, ground-glass opacity, air-bronchogram, blurred edges, slightly thickened interlobular septa in both lungs, and a small right pleural effusion.
Figure 4.
Chest computed tomography of Case 6, a 46-year-old male, showing large consolidation shadows and ground-glass opacity in both lungs, air bronchograms, and a small bilateral pleural effusion.
Figure 5.
Chest computed tomography of Case 10, a 70-year-old male, showing consolidation shadows in the right lung, scattered cavities, and a small right pleural effusion.
3.6. Treatment and outcome
The median time from disease onset to hospital admission for the 14 patients was 4.5 days (interquartile range [IQR]: 1–17 days); the median time from admission to diagnosis was 4.5 days (IQR: 2–12 days); and the mean length of hospital stay was 20.93 ± 8.46 days. Eight patients (57%) were admitted directly to the ICU and six patients (43%) were initially admitted to the general ward and then transferred to the ICU. The median time from hospital admission until transfer to the ICU was 3 days (IQR: 0.5–11 days). The mean Pneumonia Severity Index score of the 14 patients on admission to the ICU was 146.71 ± 21.23, and the median Acute Physiology and Chronic Health Evaluation I score was 17 (IQR: 15–39). The mean time from admission to diagnosis for the 14 patients was 5.1 ± 2.6 days. All 14 patients presented with respiratory failure, and all patients (100%) received invasive mechanical ventilation therapy, of which two patients (14%) received venous-venous extracorporeal membrane oxygenation therapy and four patients received continuous renal replacement therapy. Three of 14 patients (21%) died, and 11 patients (79%) recovered fully and were discharged (Table 3). The three patients who died were all treated with β-amides or β-amides in combination with glycopeptide antibiotics before diagnosis. One patient died of multiple organ failure before the mNGS report was ready, and the other two patients who died were treated with doxycycline in combination with quinolones after being diagnosed. The 11 patients who recovered fully were all treated with β-amides in combination with quinolone antibiotics before the diagnosis and were subsequently treated with either doxycycline alone or doxycycline in combination with a quinolone after diagnosis (Table 3).
Table 3.
Treatment and outcome of 14 patients with severe Chlamydia psittaci pneumonia diagnosed by metagenomic next-generation sequencing.
| Case # | Length of hospital stay (days) | Time from admission to diagnosis (days) | PSI score | APACHE II score | Supportive treatment | mNGS C. psittaci reads∗ (n) | Antibiotics before diagnosis | Antibiotics after diagnosis | Outcome |
| 1 | 22 | 5 | 130 | 15 | Mechanical ventilation, V-V ECMO, CRRT | 200 (blood 27) | MEM | DOX + MXF | Death |
| 2 | 4 | 3 | 179 | 16 | Mechanical ventilation | 1352 | MEM + VAN | NA† | Death |
| 3 | 18 | 3 | 146 | 39 | Mechanical ventilation, CRRT | 1390 | MEM | DOX + MXF | Death |
| 4 | 34 | 3 | 152 | 17 | Mechanical ventilation | 847 | MEM + MXF | DOX + MXF | Survived |
| 5 | 15 | 5 | 192 | 24 | Mechanical ventilation | 78 | MEM + MXF | DOX + MXF | Survived |
| 6 | 18 | 2 | 147 | 16 | Mechanical ventilation | 1763 | MEM + LVX | DOX + LVX | Survived |
| 7 | 17 | 4 | 153 | 17 | Mechanical ventilation | 3500 | TZP + MXF | DOX + MXF | Survived |
| 8 | 20 | 4 | 139 | 23 | Mechanical ventilation | 2628 | TZP + LVX | DOX + LVX | Survived |
| 9 | 18 | 7 | 135 | 15 | Mechanical ventilation | 29 | TZP + LVX | DOX + MXF | Survived |
| 10 | 40 | 12 | 150 | 30 | Mechanical ventilation, V-V ECMO | 3194 (blood 450) | TZP + MXF | DOX | Survived |
| 11 | 29 | 8 | 125 | 18 | Mechanical ventilation | 80 | TZP + LVX | DOX | Survived |
| 12 | 21 | 7 | 165 | 19 | Mechanical ventilation, CRRT | 1167 | IPM/cilastin + LVX | DOX + LVX | Survived |
| 13 | 22 | 3 | 136 | 17 | Mechanical ventilation, CRRT | 2019 | TZP + LVX | DOX | Survived |
| 14 | 15 | 6 | 105 | 17 | Mechanical ventilation | 316 | CFP/SUL +LVX | DOX + LVX | Survived |
APACHE = The Acute Physiology and Chronic Health Evaluation, CFP = cefoperazone, CRRT = continuous renal replacement therapy, DOX = doxycycline, IPM = imipenem, LVX = levofloxacin, MEM = meropenem, MXF = moxifloxacin, NA = not applicable, PSI = Pneumonia Severity Index, SUL = sulbactam, TZP = piperacillin-tazobactam, VAN = vancomycin, V-V ECMO = venous-venous extracorporeal membrane oxygenation.
The metagenomic next-generation sequencing was performed on bronchoalveolar lavage fluid unless stated otherwise.
The patient died before the metagenomic next-generation sequencing results were reported.
4. Discussion
Chlamydia psittaci can be divided into 10 genotypes, of which genotypes A and E can infect humans.[5] The lungs are the most common site of C. psittaci infection. According to Hogerwerf et al,[6]C. psittaci pneumonia account for approximately 1% of CAP, of which approximately 30% have severe pneumonia.[6,7] One study reported that the carrier rate of C. psittaci amongst poultry that were commercially sold in the markets was 13% in chickens, 39% in ducks, and 31% in pigeons.[8] Contact with birds or poultry is regarded as the main risk factor of C. psittaci pneumonia,[9] and 11 patients (92.58%) in this study had a history of poultry or bird contact. Hence, newly admitted patients with severe pneumonia should be asked about their poultry contact history. The patients’ average age in this study was 57 years, which was similar to that reported by Rybarczyk et al;[10] male patients accounted for 86%, which was is similar to the male predominance reported by Raeven et al.[11]
Chlamydia psittaci pneumonia mostly manifests as flu-like symptoms, such as fever, fatigue, myalgia, headache, and cough.[12] In this study, most patients had flu-like symptoms at the early stage. C. psittaci infection needs to be ruled out in patients with severe pneumonia and typical flu-like symptoms. In this study, 36% of the patients experienced dyspnea before hospital admission, whereas another study focusing on severe pneumonia caused by Chlamydia psittaci reported that 100% of patients had dyspnea. The difference in the prevalence of dyspnea in different studies may be related to differences in the diagnostic classification and treatment approaches between areas. In the study by Chen et al[13] patients were first treated in primary medical institutions and then transferred to higher-level hospitals when their conditions worsened. However, another study focusing on severe pneumonia caused by Chlamydia psittaci reported that 100% of patients had dyspnea
In this study, the neutrophil percentage, erythrocyte sedimentation rate, CRP, PCT, lactate dehydrogenase, CK, and BNP were significantly increased. Su et al[14] reported that CK and BNP were independent risk factors for severe C. psittaci pneumonia, and PCT was also increased in patients with severe pneumonia. CK is distributed in many tissues and is mainly found in muscle tissues, such as skeletal muscles and cardiac muscle; high levels of CK occur when C. psittaci pneumonia is coupled with rhabdomyolysis, of which the mechanism is currently unclear. CK levels greater than 174 U/L comprise a predictive factor for severe C. psittaci pneumonia. In addition to being a biomarker for heart failure, BNP is also one of the biomarkers for the severity and prognosis of CAP.[15] The significant elevation of the BNP levels in patients with severe C. psittaci pneumonia was probably due to hypoxia and increased pro-inflammatory cytokine secretion.[16] The high levels of inflammatory factors (CRP, PCT) and low oxygenation index observed in this study supports this hypothesis. In this study, the patients’ FIB and D-dimer levels were slightly elevated, as reported by Zhou et al,[17] the severity of CAP is positively correlated with abnormal blood coagulation, which further indicates that C. psittaci pneumonia is more likely to develop into severe illness.
It is not common for psittacosis to affect the heart. The CK-MB levels of the patients in this study were not significantly elevated, and the left ventricular ejection fraction did not decrease significantly. The patients’ aspartate aminotransferase, creatinine, and urea levels were elevated, and this occurred in combination with varying degrees of liver and kidney damage, which were complicated by severe renal failure, with four patients receiving continuous renal replacement therapy. Severely ill patients are prone to electrolyte disturbances, such as decreased blood sodium and calcium.
In this study, patients mainly had lesions in the bilateral, middle-lower lobe, and multilobar disease, generally presented as consolidation. In patients with C. psittaci pneumonia, the extent of the lesion is closely related to the severity of the disease, and the risk of respiratory failure with multilobar lesions is higher than in other types of CAP. All 14 patients had respiratory failure and received invasive mechanical ventilation, and two of these patients received venous-venous extracorporeal membrane oxygenation therapy Further, 79% of the patients had pleural effusions, which was higher than the incidence rates of 15% reported in Australia[12] and 44.4% reported in another Chinese study.[13] Concomitant pleural effusion can be considered related to the involvement of the pleural membrane and secondary hypoproteinemia.
Metagenomic NGS can be used to rapidly diagnose infectious pathogens, including bacteria, fungi, viruses, mycoplasma, chlamydia, and parasites.[18] For the diagnosis of C. psittaci pneumonia, mNGS is not only highly sensitive, but it can also provide pathogen load information (based on the number of reads). In this study, mNGS analysis of the alveolar lavage fluid of six patients concurrently discovered sequences of C. abortus. This may be because C. abortus and C. psittaci have similar genetic backgrounds.[19] In this study, the patients’ time from admission to the diagnosis of C. psittaci pneumonia was approximately 5 days. Considering the mNGS costs and timely opportunities for use, it is not accepted as a routine investigation for severe pneumonia. Instead, mNGS testing is recommended only when empirical antibiotic therapy fails.
Chlamydia psittaci belongs to the Chlamydia genus, and hence, tetracyclines, macrolides, and quinolones can interfere with the synthesis of DNA and protein, which can be used as treatment options.[20] At present, tetracyclines such as doxycycline, tetracycline, oxytetracycline, and minocycline are the first-choice for the treatment of C. psittaci pneumonia.[5] Azithromycin and quinolones also have antibacterial activity against C. psittaci, of which moxifloxacin works best,[21] whereas azithromycin is considered the best drug alternative for patients with contraindications to tetracyclines and quinolones. However, macrolides might not be effective for patients with severe illness and those who are pregnant.[2] For severely ill patients, oxytetracycline is recommended.[3] Because oxytetracycline is not available in our hospital, we used doxycycline in combination with quinolones to achieve better results. The recommended duration of antibiotic treatment for C. psittaci pneumonia is 14 to 21 days to prevent recurrence.[5] The mortality rate of patients with severe C. psittaci pneumonia was 21% in this study, which is similar to the 10% to 20% mortality reported by Rybarczyk et al.[10] None of the three patients who died had been treated with antibiotic regimens that included quinolones before diagnosis, and the 11 patients who survived had received β-amides in combination with quinolones before diagnosis. We recommend the empirical use of β-amides in combination with quinolones as empirical antibiotics in critically ill patients before diagnosis.[3] This enables the treatment to be customized after the patient is diagnosed. This empirical antibiotic strategy needs to be validated by prospective studies with a larger sample size.
5. Conclusions
In conclusion, poultry contact history and typical flu-like symptoms are early indicators of C. psittaci pneumonia, whereas significant increases in PCT, CK, and BNP indicate severe illness. mNGS provides a rapid diagnostic tool. Moreover, anti-infection treatment plans that include quinolones should be used early and empirically for critically ill patients, to gain valuable time for the diagnosis and “targeted” treatment of patients.
Acknowledgments
We thank the patients enrolled in our study.
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors have read and approved the manuscript.
Conceptualization: Anbing Zhang, Xiaoling Yuan, Xiuqiong Xia
Data curation: Anbing Zhang, Dandan Zhang
Formal analysis: Anbing Zhang, Dandan Zhang, Xiuqiong Xia
Investigation: Anbing Zhang, Dandan Zhang, Haiming Niu, Xiuqiong Xia, Yanhua Lv, Yuxia Liu
Methodology: Anbing Zhang, Dandan Zhang, Haiming Niu, Jianping Liang, Xiaoling Yuan, Xiuqiong Xia, Yuxia Liu
Project administration: Dandan Zhang, Jianping Liang, Xiaoling Yuan, Xiuqiong Xia, Yanhua Lv
Resources: Anbing Zhang, Jianping Liang, Xiaoling Yuan, Xiuqiong Xia, Yuxia Liu
Software: Anbing Zhang, Haiming Niu, Yanhua Lv
Supervision: Anbing Zhang, Haiming Niu, Jianping Liang
Validation: Anbing Zhang, Jianping Liang, Xiaoling Yuan, Yanhua Lv
Visualization: Anbing Zhang, Haiming Niu, Jianping Liang, Xiaoling Yuan, Yanhua Lv
Writing – original draft: Anbing Zhang
Writing – review & editing: Anbing Zhang, Jianping Liang
Footnotes
Abbreviations: BNP = brain natriuretic peptide, CAP = community-acquired pneumonia, CK = creatine kinase, CRP = C-reactive protein, FIB = fibrinogen, ICU = intensive care unit, mNGS = metagenomic next-generation sequencing, PCT = procalcitonin.
How to cite this article: Zhang A, Xia X, Yuan X, Lv Y, Liu Y, Niu H, Zhang D, Liang J. Clinical characteristics of 14 cases of severe Chlamydia psittaci pneumonia diagnosed by metagenomic next-generation sequencing: a case series. Medicine. 2022;101:24(e29238).
AZ and XX contributed equally to this work.
This work was supported by Guangdong Medical Science and Technology Research Project Foundation (No. C2019032), and Zhongshan Medical Research Project (No. 2021J096).
The Ethics Committees of Zhongshan People's Hospital (K2021-127) approved this study. Informed consent for publication of images was obtained from the patients or their next-of-kin.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Anbing Zhang, Email: 15876064629@163.com.
Xiuqiong Xia, Email: zabzhongshan@163.com.
Xiaoling Yuan, Email: 15989107884@163.com.
Yanhua Lv, Email: liuyuxia1109@163.com.
Yuxia Liu, Email: 383118404@qq.com.
Haiming Niu, Email: nhmlyzh@qq.com.
Dandan Zhang, Email: medidan@163.com.
Jianping Liang, Email: ljpzhongshan@163.com.
References
- [1].Lamoth F, Greub G. Fastidious intracellular bacteria as causal agents of community-acquired pneumonia. Expert Rev Anti Infect Ther 2010;8:775–90. doi: 10.1586/eri.10.52. [DOI] [PubMed] [Google Scholar]
- [2].Nieuwenhuizen AA, Dijkstra F, Notermans DW, et al. Laboratory methods for case finding in human psittacosis outbreaks: a systematic review. BMC Infect Dis 2018;18:442.doi: 10.1186/s12879-018-3317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019;200:e45–67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Miao Q, Ma Y, Wang Q, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis 2018;67: (Suppl 2): S231–40. doi: 10.1093/cid/ciy693. [DOI] [PubMed] [Google Scholar]
- [5].Balsamo G, Maxted AM, Midla JW, et al. Compendium of measures to control Chlamydia psittaci infection among humans (psittacosis) and pet birds (avian chlamydiosis), 2017. J Avian Med Surg 2017;31:262–82. doi: 10.1647/217-265. [DOI] [PubMed] [Google Scholar]
- [6].Hogerwerf L, DE Gier B, Baan B, et al. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect 2017;145:3096–105. doi: 10.1017/S0950268817002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Knittler MR, Berndt A, Bocker S, et al. Chlamydia psittaci: new insights into genomic diversity, clinical pathology, host-pathogen interaction and anti-bacterial immunity. Int J Med Microbiol 2014;304:877–93. doi: 10.1016/j.ijmm.2014.06.010. [DOI] [PubMed] [Google Scholar]
- [8].Chau S, Tso EY, Leung WS, et al. Three cases of atypical pneumonia caused by Chlamydophila psittaci. Hong Kong Med J 2015;21:272–5. doi: 10.12809/hkmj144321. [DOI] [PubMed] [Google Scholar]
- [9].Hahn DL, Azenabor AA, Beatty WL, et al. Chlamydia pneumoniae as a respiratory pathogen. Front Biosci 2002;7:e66–76. doi: 10.2741/hahn. [DOI] [PubMed] [Google Scholar]
- [10].Rybarczyk J, Versteele C, Lernout T, et al. Human psittacosis: a review with emphasis on surveillance in Belgium. Acta Clin Belg 2020;75:42–8. doi: 10.1080/17843286.2019.1590889. [DOI] [PubMed] [Google Scholar]
- [11].Raeven VM, Spoorenberg SM, Boersma WG, et al. Atypical aetiology in patients hospitalised with community-acquired pneumonia is associated with age, gender and season; a data-analysis on four Dutch cohorts. BMC Infect Dis 2016;16:299.doi: 10.1186/s12879-016-1641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Branley JM, Weston KM, England J, et al. Clinical features of endemic community-acquired psittacosis. New Microbes New Infect 2014;2:07–12. doi: 10.1002/2052-2975.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen X, Cao K, Wei Y, et al. Metagenomic next-generation sequencing in the diagnosis of severe pneumonias caused by Chlamydia psittaci. Infection 2020;48:535–42. doi: 10.1007/s15010-020-01429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Su S, Su X, Zhou L, et al. Severe Chlamydia psittaci pneumonia: clinical characteristics and risk factors. Ann Palliat Med 2021;10:8051–60. doi: 10.21037/apm-21-1502. [DOI] [PubMed] [Google Scholar]
- [15].Christ-Crain M, Breidthardt T, Stolz D, et al. Use of B-type natriuretic peptide in the risk stratification of community-acquired pneumonia. J Intern Med 2008;264:166–76. doi: 10.1111/j.1365-2796.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- [16].Post F, Weilemann LS, Messow CM, et al. B-type natriuretic peptide as a marker for sepsis-induced myocardial depression in intensive care patients. Crit Care Med 2008;36:3030–7. doi: 10.1097/CCM.0b013e31818b9153. [DOI] [PubMed] [Google Scholar]
- [17].Zhou Y, Dai YM, Wang YP, et al. Analysis of influencing factors on cellular inflammatory factors, coagulation function and prognosis in elderly patients with severe pneumonia. J Clin Pulmonology 2020;25:70–3. doi:10.3969/j.issn.1009-6663.2020.01.017. [Google Scholar]
- [18].Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol 2019;14:319–38. doi: 10.1146/annurev-pathmechdis-012418-012751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Van Loock M, Vanrompay D, Herrmann B, et al. Missing links in the divergence of Chlamydophila abortus from Chlamydophila psittaci. Int J Syst Evol Microbiol 2003;53(Pt 3):761–70. doi: 10.1099/ijs.0.02329-0. [DOI] [PubMed] [Google Scholar]
- [20].Kohlhoff SA, Hammerschlag MR. Treatment of Chlamydial infections: 2014 update. Expert Opin Pharmacother 2015;16:205–12. doi: 10.1517/14656566.2015.999041. [DOI] [PubMed] [Google Scholar]
- [21].De Boeck C, Dehollogne C, Dumont A, et al. Managing a cluster outbreak of psittacosis in Belgium linked to a pet shop visit in The Netherlands. Epidemiol Infect 2016;144:1710–6. doi: 10.1017/S0950268815003106. [DOI] [PMC free article] [PubMed] [Google Scholar]