Abstract
As human life expectancy continues to increase and the birth rate continues to decline, the phenomenon of aging is becoming more prominent worldwide. Therefore, addressing the problems associated with global aging has become a current research focus. The main manifestations of human aging are structural degeneration and functional decline of aging tissues and organs, quality of life decline, decreased ability to resist diseases, and high incidence rates of a variety of senile degenerative diseases. Thus far, no ideal treatments have been found. Stem cell (SC) therapies have broad application prospects in the field of regenerative medicine due to the inherent biological characteristics of SCs, such as their plasticity, self-renewal, and multidirectional differentiation potential. Thus, SCs could delay or even reverse aging. This manuscript reviews the causes of human aging, the biological characteristics of SCs, and research progress on age reversal.
Keywords: Aging, anti-aging, Stem cells, Exosome
Introduction
With the anticipation of an aging society, aging research has become an important research direction in the life sciences. The World Health Organization states that population aging is accelerating around the world, and the number of people over the age of 60 worldwide is expected to reach 2 billion by 2050.[1] The latest data from the National Bureau of Statistics show that China's total population is increasing; however, the growth rate is slowing, and the birth rate has been low in recent years. In 2020, the birth rate was 8.52‰, a decrease of 1.89‰ compared with the previous year, and the natural population growth rate was 1.45‰, a decrease of 1.87‰ compared with 2019. People over 65 years old currently account for 13.5% of the total population.[2] It is estimated that China's aging population will reach 480 million by 2050, accounting for approximately 35% of the total population. China's birth rates in the next 5 years are predicted to remain low.[3] This trend will eventually lead to the problem of an aging population. Aging is an unavoidable phenomenon characterized by the appearance of flabby skin, sparse and gray hair, memory decline, and various degenerative diseases. Furthermore, aging results in susceptibility to various chronic diseases, which make the healthy life span expectancy less than the actual life span. This phenomenon will increasingly threaten socioeconomic growth and sustainable development. Therefore, addressing global aging has become a research focus.
With the continuous development of biotechnology, stem cells (SCs) have altered the traditional concept of anti-ging and attracted increasing attention as a new treatment strategy. The Standardization Guide of Stem Cell Antiaging Technology refers to sources of human autograft or allograft SCs that are cultured in vitro and then locally injected into specific parts of the human body or administered via intravenous infusion into the human body. These cells are capable of proliferation and multidirectional differentiation, regulating tissue repair and immune processes to improve skin texture, maintaining skin rejuvenation, improving the mental state, improving the repairability of autologous tissues and organs, and slowing down the aging process, all of which are anti-aging effects.[4] Therefore, fully elucidating how SCs delay or reverse aging is crucial for their future clinical application. In addition, it is important to fully understand current research progress on the ability of SCs to reverse aging and, more importantly, to facilitate the development of increasingly effective anti-aging treatment strategies. This paper summarizes the SC types that have been used in anti-aging research [Table 1].
Table 1.
Research on SCs in aging-related diseases
| Cell type | Involvement | Molecular targets | Mode | Cell dose | Curative effect | References |
| ESCs | POF | PI3K/AKT | Mice with POF | 2 × 107 cells/injection | Improvement in ovarian function | [5] |
| HF | Unknown | Rats with ischemia-reperfusion injury | Unknown | Improvement in ventricular function postinfarction | [6] | |
| iPSCs | Skin defects | RA, BMP-4, EGF | Nude mice with full-thickness skin defects | 1 × 105 cells/cm2 | Reconstruction of skin and skin appendages | [7] |
| Diabetes | Unknown | NOD/SCID mice with diabetes | 4 × 106 cells/mouse | Lower blood glucose levels in diabetic mice and detection of islet-like structures in vivo | [8] | |
| IBD | Unknown | Mice with IBD | 1 × 106 iPSCs/injection (3 injections) | IPSCs stimulated the proliferation of intestinal epithelial cells and increased intestinal angiogenesis | [9] | |
| NSCs | AD | Wnt/β-catenin; RA | Mice with AD | 2.5 × 106 cells/mouse | NEP-NSCs; MOF-NSCs (improve Aβ clearance, cell survival, and neural regeneration) | [10,11] |
| PD | Stromal cell-derived factor-1/CXCR4 | Rats with PD | 7.5 × 105 cells/rat; 3 × 105 cells/site (2 sites) | Increase in the number of dopaminergic neurons | [12] | |
| Stroke | Wnt | Mice with stroke | 3 × 105 cells/rat | Function improvement | [13] | |
| SCI | Notch | Patients with SCI | 2 × 105 cells/injection (6 injections); 1 × 105 cells/site (4 sites) | Safety (phase I clinical trial) | [14,15] | |
| MS | BDNF, FGF | Mice with EAE | 106 cells | Reversal of EAE clinical symptoms and repair of CNS damage | [16] | |
| ALS | Unknown | Patients with ALS | 7.5 × 105 cells/injection | Safety (phase I clinical trial) | [17] | |
| BMSCs | SMI | Unknown | Mice with muscle contusions | various doses (1.25 × 105 cells, 2.5 × 105 cells, 1.25 × 105 cells plus F-127) | Locally injected BMSCs directly participate in myofiber regeneration in the injured muscle tissue | [18] |
| Fractures | miR-140-5p | Mice with fractures | Unknown | miR-140-5p increased the expression of osteocalcin, and miR-140-5p transgenic mice exhibited increased bone mineral density, bone mass, and bone healing | [19] | |
| UCMSCs | POF | JNK/Bcl-2 | Mice with POF | 1 × 106 cells | HO-1 expressed in UCMSCs help recover ovarian function in mice with POF | [20] |
| MS | Unknown | Patients with MS | 2 × 106 cells/day (7 days) | Positive health changes and improved quality of life | [21] | |
| COVID-19 | Unknown | Patients with COVID-19 | 15 mL ExoFloTM | Achieved a survival rate of 83%, 71% of the patients recovered; 13% remained critically ill although stable; 16% died for reasons unrelated to the treatment | [22] | |
| IBD | ERK | Mice with IBD | 3 × 106 cells/day (3 days) | UCMSCs effectively reduce colon shortening and intestinal mucosal injury | [23] | |
| ADMSCs | Ovarian autografting | Unknown | Mice with ovarian autografting | 5 × 104 cells | ADMSC transplantation improved endocrine function in the autografted ovary | [24] |
| DN | miR-486/Smad1/mTOR | Mice with spontaneous diabetes | Unknown | The delivery of miR-486 by ADSC-Exos could promote podocyte functioning by activating autophagy and ameliorating cell damage in high glucose-induced MPC5 cells and DN mice | [25] | |
| DPSCs | CA | Unknown | Rats with CA | 3 × 105 cells | Amelioration of motor coordination, increased cerebellar volumes of molecular and granular layers as well as white matter, and reduced levels of inflammatory cytokines | [26] |
| TMJ | STAT1 | Mice with TMJ | 2 × 105 cells/joint | DPSCs attenuate the pathological changes in experimental subjects with progressive TMJ arthritis | [27] |
AD: Alzheimer's disease; ADMSCs: Adipose-derived mesenchymal stem cells; ALS: Amyotrophic lateral sclerosis; BMP: Bone morphogenetic protein; BMSCs: Bone marrow mesenchymal SCs; CA: Cerebellar ataxias; DN: Diabetic nephropathy; DPSCs: Dental pulp stem cells; EAE mice: Experimental autoimmune encephalomyelitis mouse model; ESCs: Embryonic SCs; ExoFloTM: BMSC-derived exosomes; HF: Heart failure; IBD: Inflammatory bowel disease; iPSCs: Induced pluripotent SCs; MS: Multiple sclerosis; NOD/SCID: Non-obese diabetic-severe combined immunodeficiency; NSCs: Neural SCs; PD: Parkinson's disease; PI3K: Phosphatidylinositol 3-kinase; POF: Premature ovarian failure; SCI: Spinal cord injury; SCs: Stem cells; SMI: Skeletal muscle injuries; TMJ: Temporomandibular joint injuries; UCMSCs: Umbilical cord mesenchymal SCs.
Overview of aging
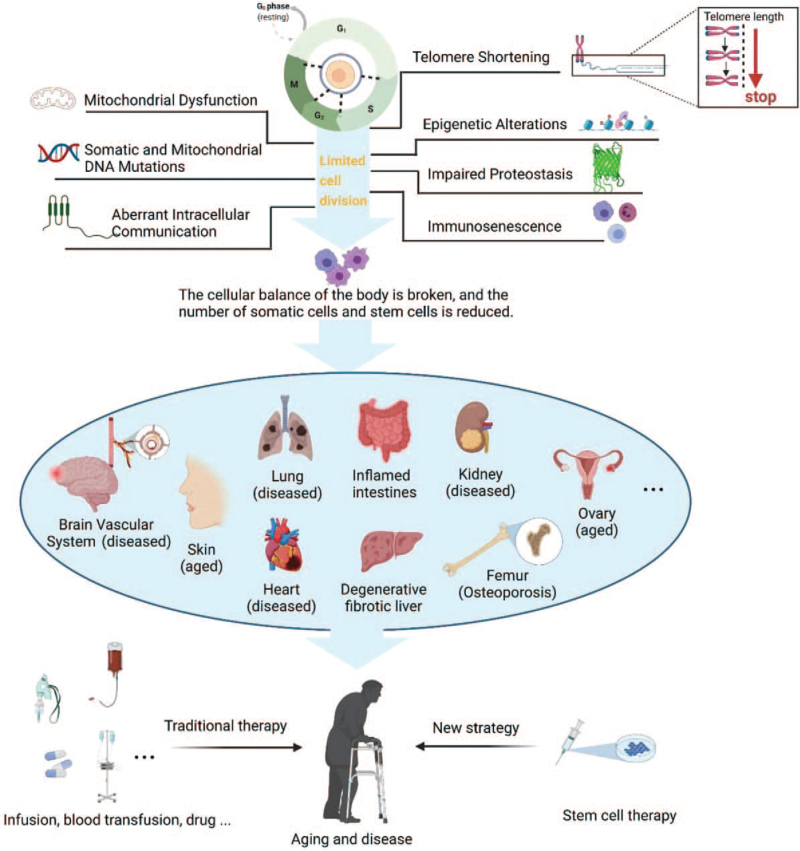
Aging is a biological phenomenon in which the structures and functions of organisms decline over time. Senescent cells were once considered to be potential contributors to the age-associated loss of regenerative potential, but there is increasing evidence of the detrimental role of senescent cells in aging.[28] The main characteristics of senescent cell damage include mitochondrial dysfunction; impaired immune function or immunosenescence; accumulation of damaged proteins (impaired proteostasis) and somatic and mitochondrial DNA mutations; aberrant intracellular communication; telomere shortening; and alteration of autophagy, epigenetics, and nutrient sensing.[29]
Together, these alterations compromise cell and tissue functions and drive aging and diseases, including cancer, diabetes, osteoporosis, sarcopenia, cardiovascular diseases, and neurodegenerative diseases. Aging includes both physiological and pathological aging. The former refers to the development of rough skin, deepening wrinkles, gray hair, memory decline, and lack of energy, whereas pathological aging constitutes excessive aging caused by certain abnormal factors. Aging has always been a fascinating topic for scientists, and despite the performance of numerous studies, no complete or authoritative explanation of the causes and mechanisms of aging has been elucidated.
Aging is a complex change that occurs in organisms at multiple levels, and many theories about the underlying causes have been developed. Of these theories, cellular senescence is currently regarded as the most feasible[30] [Figure 1] because the cell is the basic unit of an organism, and life span is maintained by cell division.[31] However, the division and passage of cells are limited, which is mainly attributed to telomere shortening, which occurs slightly every time a cell divides. When telomeres are shortened such that they are incapable of maintaining DNA replication and chromosome stability, cell senescence and aging-related diseases are induced.[32] Undifferentiated SCs can differentiate into various cells and have been shown to be capable of treating aging-related diseases. Therefore, physiological and pathological aging can be slowed or even reversed to some extent.
Figure 1.
Telomere shortening, DNA mutations, mitochondrial dysfunction, somatic and mitochondrial DNA mutations, epigenetic alterations, impairment of proteostasis, aberrant intracellular communication, immunosenescence, etc., reduce the numbers of somatic cells and SCs under the conditions of limited cell division, disruption of cell homeostasis, and senescence and disease induction. SC therapies represent a new strategy for the treatment of aging-related diseases. SCs: Stem cells.
Overview of SCs
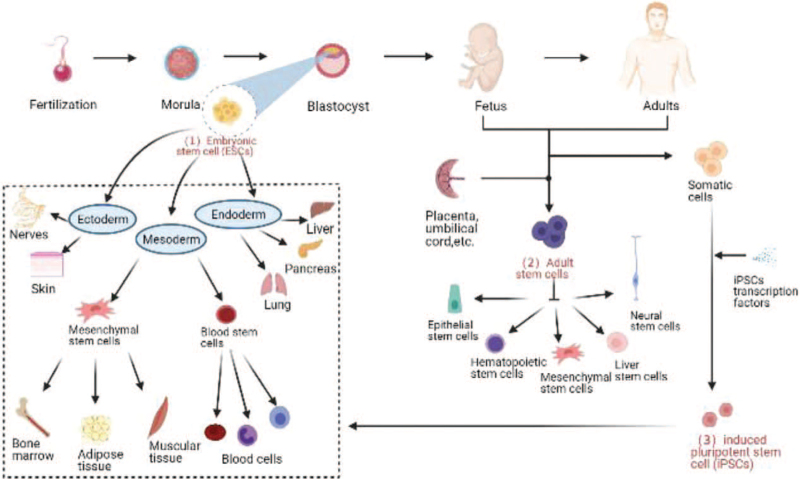
SCs are undifferentiated cells with self-renewal ability and multidirectional differentiation potential. They have the potential to regenerate various cells, tissues, or organs in the body and are the origin of all kinds of cells, tissues, and organs.[33] In recent years, SC therapy has become a promising subject of advanced scientific research. Currently, embryonic SCs (ESCs), induced pluripotent SCs (iPSCs), and adult SCs (ASCs) are being researched in the laboratory [Figure 2].
Figure 2.
Three types of SCs (ESCs, ASCs, and iPSCs). SCs commonly used in the laboratory and their differentiation characteristics. ASCs: Adult SCs; ESCs: Embryonic SCs; iPSCs: Induced pluripotent SCs; SCs: Stem cells.
ESCs and ASCs are classified according to the developmental stage and have different sources and different proliferation and differentiation potentials. ESCs, undifferentiated cells from the inner cell mass in the blastocyst with high differentiation potential, are totipotent SCs that can differentiate into any type of cell in the body, form tissues and organs, and eventually develop into complete individuals. ASCs can be isolated from various human tissues and organs, such as bone marrow, adipose tissue, umbilical cord blood, and placenta. Compared with ESCs, they have a lower differentiation potential and can differentiate into only several or even one type of cell in the body, which are called pluripotent and unipotent SCs, respectively.[34] The discovery of iPSCs in 2006 provided a new idea for cell therapy. These cells play a key role in tissue regeneration and repair. ASCs differentiate into adipocytes, osteoblasts, chondrocytes, hepatocytes, nerve cells, and other downstream SCs to replace or repair aging damaged cells and thereby achieve a balance between cell regeneration and aging.[35] iPSCs are more powerful than ASCs and can differentiate into almost any cell type in the body.
SC research in the field of reversing aging
Global aging is accelerating, and developing a reasonable solution to the social pressure brought about by aging is a difficult problem for not only China but also the world. At the same time, people's quality of life is improving daily, and the desire to be healthy and young has become a common goal. The emergence of SC therapy brings hope for solving these problems. Aureus Paracelsus, a Filipino scholar, proposed cell therapy in the 16th century. In 1930, a Swiss scholar rejuvenated skin by injecting living cells and became known as the “father of cell therapy.”[36] SC therapy has attracted substantial attention in the field of cell therapy and regeneration in recent years. After years of exploration, scientists have made remarkable achievements in anti-aging research with SCs. In 1999, Science placed SC research at the top of the 10 most important research fields in the 21st century. In the current research, neural SCs (NSCs), bone marrow mesenchymal SCs (BMSCs), umbilical cord mesenchymal SCs (UCMSCs), adipose SCs, ESCs, and human iPSCs are the most closely related anti-aging agents and exert direct (cell replacement) and indirect (paracrine) effects. To date, these SCs have been reported to be used in research on autoimmune diseases, nervous system diseases, blood system diseases, cardiovascular system diseases, and other diseases.[37]
ESCs
ESCs have the ability to differentiate into any cell type in the body. Since mouse ESCs were first reported in 1981, ESCs have been studied in different animal models. ESCs, undifferentiated cells in the early stage of embryonic development, are derived from the inner cell mass of blastocysts and obtained from redundant fertilized embryos in vitro. ESCs have pluripotency and the ability to differentiate into mesodermal, endodermal, and ectodermal cells.[38]
Mesodermal cells such as endothelial cells, cardiomyocytes, and hematopoietic cells have been obtained from ESCs by coculture with growth factors. In 2018, a clinical trial on the transplantation of human ESC (hESC)-derived cardiovascular progenitors for severe ischemia was reported, and patients were followed up for 18 months. Among the six patients, one patient died due to treatment- unrelated comorbidities, and all others had uneventful recoveries.[6] In addition, the cardiac differentiation of ESCs can give rise to de novo chamber cardiomyocytes and nodal pacemaker cells. More importantly, researchers have recently discovered a key signaling pathway that exists in the differentiation of ESCs into cardiomyocyte subpopulations.[39] The discovery of the Wnt signaling pathway in the cardiac pacemaker cell population provides a new strategy for cell replacement therapy in patients with heart diseases. ESCs are currently being extensively researched in the context of cardiovascular diseases, and numerous clinical trials have been completed. In addition to cardiovascular diseases, premature ovarian failure (POF) has a great impact on female reproductive function. Liu et al[5] studied the therapeutic effect of ESC-derived small extracellular vesicles on damaged ovaries and explored the underlying molecular mechanism. In this section, the latest research on the roles of ESCs in disease over the past 3 years is summarized. Studies on nervous system diseases, eye diseases, and other diseases were reported as early as a few years ago. Theoretically, ESCs are the most promising strategy for the treatment of clinical diseases. However, SC transplantation requires a highly purified cell population of a specific cell lineage, and ESC technology remains immature and clinically limited by ethical concerns. These problems make the feasibility of hESC therapy uncertain.[40]
IPSCs
iPSCs were established in 2006 when Takahashi and Yamanaka[41] reprogrammed mouse fibroblasts into a pluripotent state with four specific SC transcription factors (Oct4, Sox2, Klf4, and c-Myc). These cells, called iPSCs, are a turning point in SC therapy. A year later, the technology was successfully applied in human cell experiments. In 2007, research teams in the United States and Japan reported the successful induction of human cells into iPSCs, which was ranked as one of the top 10 scientific and technological advances in the world by Science.[42]
Since the discovery of iPSCs that have the potential to differentiate into a broad spectrum of cell types, many studies have reported that iPSCs can be used as a good disease model for research. The emergence of iPSCs has provided a new strategy for SC research, as they can be used for research on embryogenesis, disease modeling, drug experiments, and regenerative medicine. Skin wounds affect the quality of life, and new strategies for wound treatment are being developed. SC-based therapeutic strategies have been proposed to treat these wounds. Human urinary cells isolated from a healthy donor were reprogrammed into iPSCs by Zhou et al.[7] and then induced into epithelial SCs (EpSCs). Human acellular amniotic membranes (HAAMs) were used as a biological scaffold, and HAAMs combined with iPSC-derived EpSCs were transplanted onto defective skin on mice. The iPSC- derived EpSCs were identified as potential candidates for the reparation of skin defects and the recovery of skin functions. Therefore, iPSCs have considerable potential for improving the rate and quality of wound healing and regenerating the skin. Further research on the combination of iPSCs with other biomaterials will promote further technological advances. iPSCs have also been used in the treatment of diabetes, enteritis, and nervous system diseases, among others, and have achieved good results [Table 1].
As a type of artificial pluripotent SC, iPSCs have a utilization value similar to that of ESCs. The emergence of iPSC technology has good prospects in many medical fields, as the derived organoids have substantially advanced the development of disease models, and the possibilities in the field of organ replacement therapy are unlimited. Unlike ESCs, the obtainment of iPSCs does not require the destruction of embryos, thereby eliminating a substantial ethical barrier. iPSCs can utilize a patient's own cells, which provides a rich source and high immune compatibility for cell therapy and avoids the risk of immune rejection.[43] Although iPSCs have numerous advantages, as a new technology in the research stage, many safety concerns remain. It is believed that further research will result in the wide use of iPSCs in the treatment of clinical diseases.
ASCs
Compared with those of ESCs and iPSCs, the differentiation potential of ASCs is limited, but their relatively easy- to-obtain source makes the use of ASCs in medical research and clinical treatment more feasible.
NSCs
Aging has a profound and devastating effect on the brain, and the prevalence of neurodegenerative disorders is increasing as the human lifespan continues to be expanded.[44] However, most inherited neurodegenerative disorders are incurable, and palliative treatment is often the only option available, which undoubtedly has heavy societal and familial burdens. NSCs play a unique role in neural regeneration and can differentiate into neurons, astrocytes, or oligodendrocytes.[45] Increasing evidence suggests that NSC transplantation is a promising tool for the treatment of nervous system diseases. Due to the high complexity of Alzheimer's disease (AD) pathogenesis, AD remains incurable, and existing treatments can only moderately relieve the symptoms.[46] An increasing number of researchers are using NSCs and bioengineering in combination to further develop new strategies for NSC treatment. Recently, Huang et al.[10] reported for the first time that nanoformulation-mediated NSC therapy simultaneously improved the Aß clearance, cell survival, and neural regeneration in a mouse model of AD. Parkinson's disease (PD) is the second most common neurodegenerative disease that is characterized by the progressive death of dopamine neurons,[47] and there are currently numerous treatment methods for PD. Like those for AD, treatments typically lead to the relief of symptoms but lose their potency as the disease progresses. In a recent study,[48] researchers yielded organoids resembling the developing midbrain by inducing the differentiation of human pluripotent stem cells (hPSCs) in 3D and isolated and cultured NSCs for the treatment of rats with PD. They successfully demonstrated the potent therapeutic potential of organoid-based methods for not only central nervous system (CNS) disorders but also extra-CNS disorders.
In addition, NSCs are widely used in the study of other nervous system diseases, such as stroke, spinal cord injury, multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS) [Table 1]. Although no NSC-based treatment strategies have been approved for routine clinical treatment, clinical studies have been performed on all the abovementioned diseases, and the results of the completed clinical studies suggest that NSC treatment is safe and well- tolerated. Given the highly complex pathobiologies of neurodegenerative disorders, they remain incurable. However, therapies involving the combination of NSCs and bioscaffolds/biomaterials continue to be developed and will eventually lead to a new breakthrough in NSC research.
BMSCs
BMSCs are ASCs present in bone marrow tissue that was discovered early. Similar to other SCs, BMSCs have the potential for self-renewal, proliferation, and multidirectional differentiation. In addition to the characteristics of SCs, BMSCs also have the characteristics of stromal cells, which can secrete various cytokines, play roles in immune regulation and chemotaxis, and support hematopoiesis in damaged areas.[49]
Numerous animal experiments have suggested that BMSCs can delay or reverse aging. Zhou et al.[50] injected BMSCs from young mice into old mice, and the aging of the spleen and the transcription of some genes related to SCs in old mice were partially or completely reversed. According to the database of the U.S. Library of Medicine, most of the 759 registered studies on BMSCs have completed phase I and phase II clinical trials. Studies investigating the treatment of cardiovascular diseases, acute leukemia, rheumatoid arthritis, and diabetes have completed phase III/IV clinical trials.[49] A phase I clinical trial on the use of autologous BMSCs indicated that BMSCs were safe and feasible for the treatment of ALS, as no adverse reactions were observed, and symptoms were improved after treatment.[51] BMSCs have also shown potential in the treatment of other diseases [Table 1], but most studies utilized mouse models; because differences exist between mice and humans, more studies utilizing primate models are needed.
Although BMSCs have always been a popular type of ASC and are considered to be a good source of mesenchymal SCs (MSCs) for the treatment of various diseases, there are limitations related to the acquisition and clinical application of BMSCs, which are easily affected by health status and aging. Most studies have shown that the number and differentiation ability of BMSCs decrease with age, that the process of bone marrow puncture is very painful, and that the content of SCs in bone marrow is very low.[35] To some extent, their clinical application is limited. BMSC transplantation has also been reported to be associated with the risk of tumor formation, but this phenomenon remains controversial.
UCMSCs
UCMSCs can be obtained from different structural components of the umbilical cord, such as umbilical cord blood, umbilical cord Wharton's jelly (UCWJ), and perivascular tissue of the umbilical cord. In current clinical research, UCMSCs derived from cord blood and UCWJ are the most widely used.[52] Compared with BMSCs, UCMSCs have stronger self-renewal and proliferation capabilities and rich sources and can differentiate into different cell lineages.
UCMSCs exert anti-aging effects by regenerating and repairing aging cells, tissues, and organs. An experiment on the use of UCMSCs as a treatment for POF showed that UCMSCs express the HO-1 gene, which can activate autophagy regulated by the JNK/Bcl-2 signaling pathway and upregulate the CD8+CD28–T cell cycle, thereby restoring ovarian function in mice with POF.[53] The autophagy system generates new building blocks and energy for cellular renovation and bodily homeostasis, and the study of molecular mechanisms has substantially contributed to the development of targeted therapies for human diseases. Lanzoni et al.[22] conducted an early safety assessment of UCMSCs for the treatment of COVID-19. Twenty-four eligible COVID-19 patients were treated in a double-blind controlled trial; the survival rate of patients treated with UCMSCs was significantly higher than that of patients in the conventional treatment group, and UCMSCs were deemed to be safe for the treatment of this disease. Cumulative studies have also investigated the use of UCMSCs in some systemic diseases and local pathological injuries, such as nervous system, immune system, cardiovascular system, kidney, liver, lung, and skin injuries.[54] In many studies, UCMSCs have been combined with biomaterials and scaffolds for treatment, and biomaterials and stents can feasibly enhance the efficacy of SC treatments.
UCMSCs are characterized by faster self-renewal and proliferation, having a wider range of sources and easier access, and having better advantages than other types of SCs. After delivery, the umbilical cord is usually discarded as medical waste. The isolation and extraction of MSCs from the umbilical cord not only turn waste into treasure but also cause no pain for the donor. The cells obtained by this process are characterized by stable proliferation and long-term storage, low immunogenicity, low incidence of graft-versus-host disease, low risk of viral transmission, lack of tumorigenicity, and no ethical controversy.[55] As a star cell type of the SC family, UCMSCs have been investigated in clinical research studies and have applications in many fields.
Adipose-derived mesenchymal stem cells
Adipose tissue is an endocrine organ derived from the mesoderm during embryonic development. Adipose- derived mesenchymal stem cells (ADMSCs) are ASCs abundant in adipose tissue that can differentiate into other mesodermal tissue types, including bone, cartilage, muscle, and adipose tissue.[52] The paracrine effect of ADMSCs is particularly significant, as they can secrete various cell growth factors and chemokines, thus promoting angiogenesis, stimulating the activation of endogenous SCs, regulating inflammation, and promoting wound healing.[56]
In terms of anti-aging effects, the efficacy of ADMSCs in aging skin has been proven. ADMSCs can differentiate into keratinocytes, dermal fibroblasts, and other skin cells to repair damaged and apoptotic cells or stimulate cell regeneration through paracrine action.[57] These processes can improve various skin defects, such as wrinkles, skin thickness, skin whitening, and ultraviolet-induced skin injury. An animal study examined the effect of ADMSC transplantation at the site of autologous ovarian transplantation in mice, revealing that the transplantation of ADMSCs enhanced the structure and function of transplanted ovaries.[24] ADMSCs secrete various cytokines, which can stimulate hair follicles and promote hair regeneration. Some researchers have treated patients with alopecia by injecting proteins secreted by adipose SCs (ADSCs-CM), thereby improving the hairlines of most patients and increasing the amount of hair.[58] However, the clinical use of ADMSCs to stimulate hair follicles in the treatment of alopecia is ineffective, and the specific molecular mechanism is not clear. The field of ADMSC research is application-driven. Although many studies have investigated ADMSCs, basic research lags behind their clinical application, which seriously hinders their safe and efficient application.
In 2001,[59] SCs in adipose tissue were reported for the first time. Adipose tissue is one of the largest tissue types in the human body, and inguinal subcutaneous adipose tissue is usually an important source of the material. An increasing number of obese people are undergoing liposuction for weight loss, and excess adipose tissue is often discarded. Research materials containing ADMSCs for regenerative medicine treatment are easier to obtain than BMSCs and are not associated with the same ethical problems as ESCs. Numerous related studies have confirmed that ADMSCs have weak immunogenicity and express histocompatibility complex molecules at low levels; moreover, their proliferation in vitro is difficult, which limits their clinical application.
Dental pulp stem cells
The dental pulp is a type of oral tissue located in the central pulp cavity of each tooth. Dental pulp stem cells (DPSCs) are SCs isolated from dental pulp tissue that can be obtained from deciduous or extracted teeth. At present, the most commonly used teeth are extracted from caries-free teeth, mainly the third molars. In 2000, Gronthos et al.[60] reported for the first time the isolation of DPSCs from the pulp tissue of the third molar. DPSCs are ectoderm-derived SCs that have the characteristics of MSCs. Under suitable culture conditions, DPSCs have the potential to differentiate into odontoblasts, chondrocytes, adipocytes, and nerve- like cells.[61] Based on studies on the functional characterization and mechanism of DPSCs, attempts to regenerate dental pulp tissue by DPSCs have been widely performed in preclinical studies. Xuan et al.[62] showed that dental pulp with an odontoblast layer, blood vessels, and nerves could be regenerated in animal models by the implantation of autologous DPSCs, and they further verified this phenomenon in a clinical trial. Research has shown that human DPSCs can be used to regenerate complete dental pulp tissue and may be used to treat tooth injuries caused by trauma. All of the results from their animal experiments and clinical trial support the hypothesis that DPSCs can regenerate dental pulp and have led to breakthroughs in the study of dental pulp regeneration.
DPSCs also have potential in the field of regenerative medicine and have been used in the study of skin, pancreas, and heart tissues, among others. Li et al.[63] studied the effects of DPSCs on skin senescence and cultured fibroblasts with a DPSC-conditioned medium, concluding that DPSCs had an anti-aging effect on fibroblasts. DPSCs represent a promising new therapeutic method, as they have numerous sources and are easy to obtain; however, research remains in the exploratory stage, and many challenges remain.
Role of SC exosomes in reversing aging
Definition and characteristics of exosomes
Exosomes are double-layered model vesicles ranging in diameter from 30 to 1150 nm that are released by nearly all types of living cells and carry cargo, such as proteins, nucleic acids, cytokines, and lipids based on the cell origin.[64] Exosomes play roles in intercellular communication (signal transmission), intracellular metabolite clearance, and the immune response.
Research on the application of stem cells exosomes
SCs are commonly used as a cellular therapy source due to their strong immunosuppressive and regenerative effects. This article has discussed several types of SCs that are most closely related to the reversal of aging. SCs were previously proposed to exert their therapeutic effect by migrating to sites of damage, engrafting, and interacting with other cells after infusion. Recent investigations have demonstrated that the treatment mechanism of SCs is not associated with their differentiation ability but rather principally orchestrated by the secretion of paracrine factors.[65]
Since the discovery of exosomes, an increasing number of researchers have turned their attention to the development of SC-derived exosomes. Tatullo[66] reviewed the application of exosomes from human periapical cyst-MSCs in the treatment of PD, which provided a new idea for the treatment of PD. Intravenously injected SCs have frequently exhibited therapeutic activity in numerous animal models, but the majority of SCs do not significantly home to sites of injury; thus, more research is needed on the mechanism of SC therapy. Recently, researchers assessed the ability of exosomes secreted from MSCs (MSC-exos) to treat MS using an experimental autoimmune encephalomyelitis (EAE) mouse model, and the results suggested that MSC-exos ameliorated EAE.[67] Therefore, we believe that stem cells exosomes (SC-exos) are a mechanism by which SCs contribute to tissue regeneration at distant sites.
Increasing evidence supports that SC-exos are the main mechanism of SC therapy. In addition, SC-exos with low immunogenicity are incapable of directly forming tumors and have diminished safety risks regarding the administration of live cells.[68] Therefore, exosome transplantation overcomes the limitations of SCs and has a more obvious application advantage. As of September 19, 2021, 110 registered clinical studies on the use of exosome treatment for diseases were found on the clinicaltrials.gov website.
Exosomes are currently the biggest hot spot in precision and transformation medicinal research, and strategies involving exosomes are being developed at an astonishing speed.
Conclusion
At present, SCs have become the top priority for cell biology and even whole-life science researchers. As a natural law of human life, aging occurs slowly over time, and the risk of age-related diseases gradually increases. The possibilities provided by the continuous enhancement of SC research are unlimited, and SC therapy appears to be an ideal medical strategy. Thus far, a substantial amount of research has been performed on SCs. Based on the biological characteristics of SCs, they are theoretically expected to be used in the clinical treatment of various diseases and to delay human aging.
Although studies on SCs have shown broad application prospects in the field of cell therapy and regenerative medicine, many challenges remain. First, research on the mechanism of SCs is insufficient; for example, basic research on ADSCs lags behind their clinical application, and apoptosis may occur after SC transplantation.[69] Second, the source and application of ESCs are still subject to legal and ethical restrictions. Third, tumorigenicity (eg, iPSCs) may increase the expression of oncogenes upon the reprogramming of cells. Fourth, immune rejection remains a major obstacle to SC transplantation.[70] Therefore, extensive research still needs to be performed before SC therapies can be widely used in the clinic as a mature technology.
Even so, based on the understanding of the biological characteristics of several types of SCs presented herein, iPSC technology will be the focus of SC developmental strategies in the future. UCMSCs have low immunogenicity, a strong proliferation ability, and a wide range of donors; moreover, they are easy to harvest, are not damaging to donors, are limited by no ethical restrictions, and have broad prospects in the field of cell therapy and regeneration. SC therapies are thought to be useful for counteracting the traditional concept of aging, and although many challenges remain, SC therapies are projected to move from the experimental research stage to practical clinical applications upon the development of biotechnologies and more extensive research.
Funding
This project was supported by grants from Yunnan Talent Project (No. 2018HB002) and Army Animal Special Project (No. SYDW[2020]19).
Conflicts of interest
None.
Footnotes
How to cite this article: Chang L, Fan W, Pan X, Zhu X. Stem cells to reverse aging. Chin Med J 2022;135:901–910. doi: 10.1097/CM9.0000000000001984
References
- 1.Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel JP, et al. The world report on ageing and health: a policy framework for healthy ageing. Lancet 2016; 387:2145–2154. doi: 10.1016/s0140-6736(15)00516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annual population data of the National Bureau of Statistics. China: National Bureau of Statistics; 2021. Available from: https://data.stats.gov.cn/easyquery.htm?cn=C01. [Google Scholar]
- 3.Sun XX, Gao Y, Yuan X, Wang ZX. Analysis and prediction of birth rate in China (in Chinese). J Shandong Normal Univ (Nat Sci) 2020; 35:256–264. doi: 10.3969/j.issn.1001-4748.2020.03.002. [Google Scholar]
- 4.Cong XL, Wang XJ, Cui L. Standardized guide for anti-aging technology of stem cells (in Chinese). Chin J Health Care Med 2017; 19:185–186. doi: CNKI:SUN:JFJB.0.2017-02-033. [Google Scholar]
- 5.Liu M, Qiu Y, Xue Z, Wu R, Li J, Niu X, et al. Small extracellular vesicles derived from embryonic stem cells restore ovarian function of premature ovarian failure through PI3K/AKT signaling pathway. Stem Cell Res Ther 2020; 11:3.doi: 10.1186/s13287-019-1508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Parouchev A, et al. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J Am Coll Cardiol 2018; 71:429–438. doi: 10.1016/j. jacc.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 7.Zhou H, Wang L, Zhang C, Hu J, Chen J, Du W, et al. Feasibility of repairing full-thickness skin defects by iPSC-derived epithelial stem cells seeded on a human acellular amniotic membrane. Stem Cell Res Ther 2019; 10:155.doi: 10.1186/s13287-019-1234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yabe SG, Fukuda S, Takeda F, Nashiro K, Shimoda M, Okochi H. Efficient generation of functional pancreatic beta-cells from human induced pluripotent stem cells. J Diabetes 2017; 9:168–179. doi: 10.1111/1753-0407.12400. [DOI] [PubMed] [Google Scholar]
- 9.Soontararak S, Chow L, Johnson V, Coy J, Wheat W, Regan D, et al. Mesenchymal stem cells (MSC) derived from induced pluripotent stem cells (iPSC) equivalent to adipose-derived MSC in promoting intestinal healing and microbiome normalization in mouse inflammatory bowel disease model. Stem Cells Transl Med 2018; 7:456–467. doi: 10.1002/sctm.17-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang D, Cao Y, Yang X, Liu Y, Zhang Y, Li C, et al. A Nanoformulation-mediated multifunctional stem cell therapy with improved beta-amyloid clearance and neural regeneration for Alzheimer's disease. Adv Mater 2021; 33:e2006357.doi: 10.1002/adma.202006357. [DOI] [PubMed] [Google Scholar]
- 11.Yu D, Ma M, Liu Z, Pi Z, Du X, Ren J, et al. MOF-encapsulated nanozyme enhanced siRNA combo: control neural stem cell differentiation and ameliorate cognitive impairments in Alzheimer's disease model. Biomaterials 2020; 255:120160.doi: 10.1016/j. biomaterials.2020.120160. [DOI] [PubMed] [Google Scholar]
- 12.Xu JT, Qian Y, Wang W, Chen XX, Li Y, Li Y, et al. Effect of stromal cell-derived factor-1/CXCR4 axis in neural stem cell transplantation for Parkinson's disease. Neural Regen Res 2020; 15:112–119. doi: 10.4103/1673-5374.264470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu D, Li F, Xue G, Hou K, Fang W, Li Y. Effect of Wnt signaling pathway on neurogenesis after cerebral ischemia and its therapeutic potential. Brain Res Bull 2020; 164:1–13. doi: 10.1016/j.brainres-bull.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Curtis E, Martin JR, Gabel B, Sidhu N, Rzesiewicz TK, Mandeville R, et al. A first-in-human, phase I study of neural stem cell transplantation for chronic spinal cord injury. Cell Stem Cell 2018; 22: 941-950.e6. doi: 10.1016/j.stem.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Khazaei M, Ahuja CS, Rodgers CE, Chan P, Fehlings MG. Generation of definitive neural progenitor cells from human pluripotent stem cells for transplantation into spinal cord injury. Methods Mol Biol 2019; 1919:25–41. doi: 10.1007/978-1-4939-9007-8_3. [DOI] [PubMed] [Google Scholar]
- 16.Brown C, McKee C, Halassy S, Kojan S, Feinstein DL, Chaudhry GR. Neural stem cells derived from primitive mesenchymal stem cells reversed disease symptoms and promoted neurogenesis in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Stem Cell Res Ther 2021; 12:499.doi: 10.1186/s13287-021-02563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzini L, Gelati M, Profico DC, Soraru G, Ferrari D, Copetti M, et al. Results from phase I clinical trial with intraspinal injection of neural stem cells in amyotrophic lateral sclerosis: a long-term outcome. Stem Cells Transl Med 2019; 8:887–897. doi: 10.1002/sctm.18-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu CH, Chang TH, Chang SS, Chang GJ, Chen AC, Cheng CY, et al. Application of bone marrow-derived mesenchymal stem cells for muscle healing after contusion injury in mice. Am J Sports Med 2020; 48:1226–1235. doi: 10.1177/0363546520905853. [DOI] [PubMed] [Google Scholar]
- 19.Jiao J, Feng G, Wu M, Wang Y, Li R. Liu J. miR-140-5p promotes osteogenic differentiation of mouse embryonic bone marrow mesenchymal stem cells and post-fracture healing of mice. Cell Biochem Funct 2020; 38:1152–1160. doi: 10.1002/cbf.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin N, Wu C, Qiu J, Zhang Y, Bo L, Xu Y, et al. Protective properties of heme oxygenase-1 expressed in umbilical cord mesenchymal stem cells help restore the ovarian function of premature ovarian failure mice through activating the JNK/Bcl-2 signal pathway regulated autophagy and upregulating the circulating of CD8+ CD28− T cells. Stem Cell Res Ther 2020; 11:49.doi: 10.1186/s13287-019-1537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riordan NH, Morales I, Fernandez G, Allen N, Fearnot NE, Leckrone ME, et al. Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. J Transl Med 2018; 16:57.doi: 10.1186/s12967-018-1433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med 2021; 10:660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang G, Joel MDM, Yuan J, Wang J, Cai X, Ocansey DKW, et al. Human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease by inhibiting ERK phosphorylation in neutrophils. Inflammopharmacology 2020; 28:603–616. doi: 10.1007/s10787-019-00683-5. [DOI] [PubMed] [Google Scholar]
- 24.Shojafar E, Soleimani Mehranjani M, Shariatzadeh SMA. Adipose-derived mesenchymal stromal cell transplantation at the graft site improves the structure and function of autografted mice ovaries: a stereological and biochemical analysis. Cytotherapy 2018; 20:1324–1336. doi: 10.1016/j.jcyt.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Jin J, Shi Y, Gong J, Zhao L, Li Y, He Q, et al. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res Ther 2019; 10:95.doi: 10.1186/s13287-019-1177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aliaghaei A, Boroujeni ME, Ahmadi H, Bayat AH, Tavirani MR, Abdollahifar MA, et al. Dental pulp stem cell transplantation ameliorates motor function and prevents cerebellar atrophy in rat model of cerebellar ataxia. Cell Tissue Res 2019; 376:179–187. doi: 10.1007/s00441-018-02980-x. [DOI] [PubMed] [Google Scholar]
- 27.Cui SJ, Zhang T, Fu Y, Liu Y, Gan YH, Zhou YH, et al. DPSCs attenuate experimental progressive TMJ arthritis by inhibiting the STAT1 pathway. J Dent Res 2020; 99:446–455. doi: 10.1177/0022034520901710. [DOI] [PubMed] [Google Scholar]
- 28.Rivero-Segura NA, Bello-Chavolla OY, Barrera-Vazquez OS, Gutierrez-Robledo LM, Gomez-Verjan JC. Promising biomarkers of human aging: in search of a multi-omics panel to understand the aging process from a multidimensional perspective. Ageing Res Rev 2020; 64:101164.doi: 10.1016/j.arr.2020.101164. [DOI] [PubMed] [Google Scholar]
- 29.Johnson AA, Stolzing A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 2019; 18:e13048.doi: 10.1111/acel.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borghesan M, Hoogaars WMH, Varela-Eirin M, Talma N, Demaria M. A senescence-centric view of aging: implications for longevity and disease. Trends Cell Biol 2020; 30:777–791. doi: 10.1016/j. tcb.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Polymenis M, Kennedy BK. Unbalanced growth, senescence and aging. Adv Exp Med Biol 2017; 1002:189–208. doi: 10.1007/978-3-319-57127-0_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu Y, Liu X, Ding X, Wang F, Geng X. Telomere and its role in the aging pathways: telomere shortening, cell senescence and mitochondria dysfunction. Biogerontology 2019; 20:1–16. doi: 10.1007/s10522-018-9769-1. [DOI] [PubMed] [Google Scholar]
- 33.Jin J. Stem cell treatments. JAMA 2017; 317:330.doi: 10.1001/jama.2016.17822. [DOI] [PubMed] [Google Scholar]
- 34.Cable J, Fuchs E, Weissman I, Jasper H, Glass D, Rando TA, et al. Adult stem cells and regenerative medicine—a symposium report. Ann N Y Acad Sci 2020; 1462:27–36. doi: 10.1111/nyas.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, et al. Mesenchymal stem cells: cell therapy and regeneration potential. J Tissue Eng Regen Med 2019; 13:1738–1755. doi: 10.1002/term.2914. [DOI] [PubMed] [Google Scholar]
- 36.Shan SR, Huang GZ. Research and progress in stem cell anti-aging theory (in Chinese). Chinese J Tissue Eng Res 2013; 17:4347–4354. doi: 10.3969/j.issn.2095-4344.2013.23.024. [Google Scholar]
- 37.Liu SY, Liu LN, Yang YQ. Progress in application of mesenchymal stem cells (in Chinese). J Hebei North Univ (Nat Sci) 2020; 36:61–65. doi: CNKI:SUN:ZJKN.0.2020-10-020. [Google Scholar]
- 38.Weatherbee BAT, Cui T, Zernicka-Goetz M. Modeling human embryo development with embryonic and extra-embryonic stem cells. Dev Biol 2021; 474:91–99. doi: 10.1016/j.ydbio.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang W, Han P, Kim EH, Mak J, Zhang R, Torrente AG, et al. Canonical Wnt signaling promotes pacemaker cell specification of cardiac mesodermal cells derived from mouse and human embryonic stem cells. Stem Cells 2020; 38:352–368. doi: 10.1002/stem.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu J, Wang J. From embryonic stem cells to induced pluripotent stem cells—Ready for clinical therapy? Clin Transplant 2019; 33:e13573.doi: 10.1111/ctr.13573. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 43.Karagiannis P, Takahashi K, Saito M, Yoshida Y, Okita K, Watanabe A, et al. Induced pluripotent stem cells and their use in human models of disease and development. Physiol Rev 2019; 99:79–114. doi: 10.1152/physrev.00039.2017. [DOI] [PubMed] [Google Scholar]
- 44.Navarro Negredo P, Yeo RW, Brunet A. Aging and rejuvenation of neural stem cells and their niches. Cell Stem Cell 2020; 27:202–223. doi: 10.1016/j.stem.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahroba H, Ramezani B, Maadi H, Sadeghi MR, Jaberie H, Ramezani F. The role of Nrf2 in neural stem/progenitors cells: from maintaining stemness and self-renewal to promoting differentiation capability and facilitating therapeutic application in neurodegenerative disease. Ageing Res Rev 2021; 65:101211.doi: 10.1016/j. arr.2020.101211. [DOI] [PubMed] [Google Scholar]
- 46.Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell 2019; 179:312–339. doi: 10.1016/j.cell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jankovic J, Tan EK. Parkinson's disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry 2020; 91:795–808. doi: 10.1136/jnnp-2019-322338. [DOI] [PubMed] [Google Scholar]
- 48.Kim SW, Woo HJ, Kim EH, Kim HS, Suh HN, Kim SH, et al. Neural stem cells derived from human midbrain organoids as a stable source for treating Parkinson's disease: midbrain organoid-NSCs (Og-NSC) as a stable source for PD treatment. Prog Neurobiol 2021; 204:102086.doi: 10.1016/j.pneurobio.2021.102086. [DOI] [PubMed] [Google Scholar]
- 49.Chu DT, Phuong TNT, Tien NLB, Tran DK, Thanh VV, Quang TL, et al. An update on the progress of isolation, culture, storage, and clinical application of human bone marrow mesenchymal stem/stromal cells. Int J Mol Sci 2020; 21:708.doi: 10.3390/ijms21030708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou B, Zhao Z, Zhang X, Deng W, Li Y. Effect of allogenic bone marrow mesenchymal stem cell transplantation on T cells of old mice. Cell Reprogram 2020; 22:30–35. doi: 10.1089/cell.2019.0055. [DOI] [PubMed] [Google Scholar]
- 51.Nabavi SM, Arab L, Jarooghi N, Bolurieh T, Abbasi F, Mardpour S, et al. Safety, feasibility of intravenous and intrathecal injection of autologous bone marrow derived mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: an open label phase I clinical trial. Cell J 2019; 20:592–598. doi: 10.22074/cellj.2019.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazini L, Rochette L, Amine M, Malka G. Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). Int J Mol Sci 2019; 20:2523.doi: 10.3390/ijms20102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yin N, Wu C, Qiu J, Zhang Y, Bo L, Xu Y, et al. Protective properties of heme oxygenase-1 expressed in umbilical cord mesenchymal stem cells help restore the ovarian function of premature ovarian failure mice through activating the JNK/Bcl-2 signal pathway-regulated autophagy and upregulating the circulating of CD8+ CD28− T cells. Stem Cell Res Ther 2020; 11:49.doi: 10.1186/s13287-019-1537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lelek J, Zuba-Surma EK. Perspectives for future use of extracellular vesicles from umbilical cord-and adipose tissue-derived mesenchymal stem/stromal cells in regenerative therapies-synthetic review. Int J Mol Sci 2020; 21:799.doi: 10.3390/ijms21030799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anwar I, Ashfaq UA, Shokat Z. Therapeutic potential of umbilical cord stem cells for liver regeneration. Curr Stem Cell Res Ther 2020; 15:219–232. doi: 10.2174/1568026620666200220122536. [DOI] [PubMed] [Google Scholar]
- 56.Hong P, Yang H, Wu Y, Li K, Tang Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: a comprehensive review. Stem Cell Res Ther 2019; 10:242.doi: 10.1186/s13287-019-1358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazini L, Rochette L, Admou B, Amal S, Malka G. Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) inwoundhealing. Int J Mol Sci 2020; 21:1306.doi: 10.3390/ijms21041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fukuoka H, Narita K, Suga H. Hair regeneration therapy: application of adipose-derived stem cells. Curr Stem Cell Res Ther 2017; 12:531–534. doi: 10.2174/1574888x12666170522114307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001; 7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 60.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 2000; 97:13625–13630. doi: 10.1073/pnas. 240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sui B, Wu D, Xiang L, Fu Y, Kou X, Shi S. Dental pulp stem cells: from discovery to clinical application. J Endod 2020; 46:S46–S55. doi: 10.1016/j.joen.2020.06.027. [DOI] [PubMed] [Google Scholar]
- 62.Xuan K, Li B, Guo H, Sun W, Kou X, He X, et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med 2018; 10: eaaf3227. doi: 10.1126/scitranslmed.aaf3227. [DOI] [PubMed] [Google Scholar]
- 63.Li YL, Yang JY, Xu R. Anti-aging effect of dental pulp stem cells on skin fibroblasts. Shanghai Kou Qiang Yi Xue 2020; 29:466–470. [PubMed] [Google Scholar]
- 64.Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem 2019; 88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 65.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020; 367: eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tatullo M, Marrelli B, Zullo MJ, Codispoti B, Paduano F, Benincasa C, et al. Exosomes from human periapical cyst-MSCs: theranostic application in Parkinson's disease. Int J Med Sci 2020; 17:657–663. doi: 10.7150/ijms.41515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riazifar M, Mohammadi MR, Pone EJ, Yeri A, Lasser C, Segaliny AI, et al. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano 2019; 13:6670–6688. doi: 10.1021/acsnano.9b01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant 2019; 54:789–792. doi: 10.1038/s41409-019-0616-z. [DOI] [PubMed] [Google Scholar]
- 69.Seo YS, Ko IO, Park H, Jeong YJ, Park JA, Kim KS, et al. Radiation-induced changes in tumor vessels and microenvironment contribute to therapeutic resistance in glioblastoma. Front Oncol 2019; 9:1259.doi: 10.3389/fonc.2019.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamanaka S. Pluripotent stem cell-based cell therapy—Promise and challenges. Cell Stem Cell 2020; 27:523–531. doi: 10.1016/j.stem. 2020.09.014. [DOI] [PubMed] [Google Scholar]