Abstract
Management of candidemia in developing countries like India encounters laxity in appropriate clinical management and challenges in terms of healthcare capacity, despite its association with high morbidity and mortality. Our study aims to evaluate the impact of a comprehensive candidemia care bundle implementation on appropriateness of therapy and major clinical outcomes.
The single-center, quasi-experimental study conducted at a south Indian tertiary care center included adult patients diagnosed with candidemia. Following a retrospective review of candidemia patients of the pre-implementation period (January 2013–December 2015), the hospital antifungal stewardship team instituted a clinical pharmacist driven comprehensive candidemia care bundle for candidemia patients during the post-implementation period (October 2017–2019) and its impact on appropriateness of antifungal prescriptions and inpatient mortality was evaluated.
The study included 175 patients with candidemia, comprising of 103 patients in the pre-implementation period and 72 patients in the post-implementation period. Appropriateness of antifungal prescriptions rose to 65% during post-implementation period from 30% observed in pre-implementation phase (P = .0005). The inhospital mortality rate reduced from 40% in the pre-implementation phase to 36% in the post-implementation phase, recording a 10% reduction over 2 years post-implementation (P = .26). No significant difference was observed in terms length of stay (P = .17).
Our study demonstrates the successful implementation of an antifungal stewardship led comprehensive care bundle in a low middle income countries setting. The results of our study will have profound implications in improving the appropriateness of management of candidemia and feasibility of scaling up to wider settings could be explored.
Keywords: antifungal stewardship, appropriateness of antifungal therapy, candidemia, candidemia care bundle
1. Introduction
With the increasing use of broad spectrum antibiotics, there is a rising trend of nosocomial candida infections. This trend is extremely common in critically ill patients and those with prolonged hospital stay. An overall incidence of candidemia was found to be 6.51 cases/1000 intensive care unit admissions in India. Epidemiological data on candidemia from developing countries are sparse, albeit high mortality, and morbidity. Review of randomized controlled trials (RCTs) report the successful treatment rate of candidemia to be 64.7%.[1] The management of candidemia in low middle income countries (LMICs) is complicated by delayed diagnosis and subsequent lag in antifungal therapy initiation, along with inappropriate antifungal prescriptions such as incorrect dosing and durations.[2] Although international guidelines for the management of candidemia, such as those published by Infectious Diseases Society of America and European Society of Clinical Microbiology & Infectious Diseases, are updated regularly, they have not been able to bring about the desired behavioral changes in the management of candidemia.[3] Coupled with this, there is lack of regional guidelines regarding the use of antifungals in developing countries. Developing countries also face inherent infrastructural issues that undermine proper infection control practices and unavailability of diagnostic techniques compounding proper medical care. On the other hand, inappropriate use of antifungals, has led to the widespread emergence of antifungal resistance and associated high cost of therapy.[4]
This calls in for the implementation of methods to improve the use of antifungals and management of invasive fungal infections and thereby improving the appropriateness of antifungal therapy. Antifungal stewardship (AFS) is an effective program recommended to be incorporated into hospital policies that focuses on established roles such as promoting appropriateness of antifungal therapy, cost containment and reducing the emergence of resistance. However AFS as a standalone programme alone would fall short of optimizing candidemia management in a comprehensive manner. The adoption of care bundles focusing on improving infection related clinical management have been proven successful in improving appropriateness of management of candidemia in developed countries.[5–7] Compliance to candidemia specific management bundles addressing appropriate antifungal prescriptions and follow up of microbiological cultures along with proper source control was reported to be independently associated with clinical success and mortality benefits.[1] To the best of our knowledge, no published reports from India have evaluated the impact of an AFS driven candidemia care bundle on the management of patients with candidemia.
Optimization of antifungal therapy for candidemia represents opportunity for exploring management strategies implementable using existing resources in LMIC setting. We hypothesized that bundling of evidence based strategies for management of candidemia and AFS driven process for implementing the comprehensive care bundle would improve overall patient outcomes. Hence, our objective was to evaluate the impact of a comprehensive candidemia care bundle using a quasi-experimental design.
2. Methodology
2.1. Study setting and population
This was a single-center, pre-post quasi-experimental study done at a 1300 bedded academic tertiary care referral center in the state of Kerala, catering to a high proportion of morbid and critical care patients. The ethical approval for the study has been obtained from the Ethics Committee of Amrita School of Medicine at our hospital. The hospital has a robust antimicrobial stewardship team (antimicrobial stewardship programme [ASP]) reviewing the appropriateness of reserve drug prescriptions.[8] Our study included all adult inpatients (age ≥ 18 years) with provisional blood culture positivity for budding yeast, which was further identified as candida species. Pediatric patients were excluded as well as patients who expired prior to initiating candidemia care bundle since their compliance to the bundle checklist cannot be assessed. As this was a quality improvement initiative, sample size was not estimated prior to initiation of the study.
2.2. Comprehensive candidemia care bundle as an intervention
The pre-implementation phase was from January 2013 to December 2015. A retrospective review of medical charts of adult patients diagnosed with candidemia during this period of time was conducted (Fig. 1). Candidemia refers to the isolation of pathogenic species of Candida from a blood culture specimen of the patient. Specimen positive for Candida species was identified by the updated VITEK 2 system (Biomerieux, Marcy l’Etoile, France).
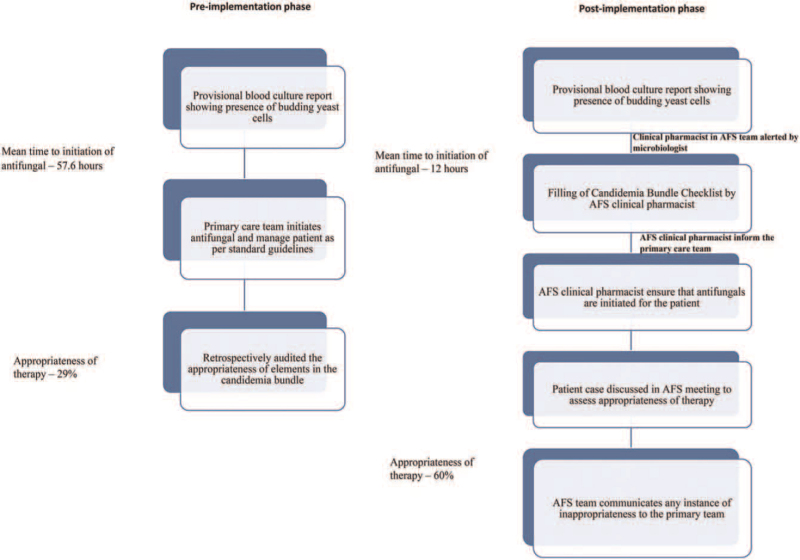
Figure 1.
Process flow of candidemia management in both the pre and post-implementation phase.
The gaps in the management of candidemia including the inappropriateness of antifungal prescriptions and time delay in initiating antifungal therapy were identified as major drawbacks. This was followed by a department wise training to improve the appropriateness of antifungal therapy which was imparted through multiple sessions led by the multidisciplinary antibiotic stewardship team. PDSA cycles were created by the team and an implementation of a bundled approach for Candidemia management was decided upon. A dedicated clinical pharmacist was chosen as the key driver of the process.
A process of early notification of blood cultures positive for budding yeast in the form of critical alert from microbiology lab to all stakeholders was generated. Upon receipt of the critical alert, the clinical pharmacist would review the patient medical chart at bedside and file the candidemia care bundle check list in the patient file. The bundle checklist was formulated by aggregating 5 important recommendations for ideal management of candidemia. The components of the checklist were adopted from the Infectious Diseases Society of America's 2016 guidelines for the management of invasive candidiasis. The key elements included drug choice and parameters for appropriateness including early initiation of intravenous antifungals in right loading dose and maintenance dose, optimum duration of treatment (14 days after last negative blood culture), timely repetition of blood cultures in the setting of positive repeat culture, evaluation for infective endocarditis (Echocardiography), and Fundus examination to rule out endophthalmitis (see Table, Supplemental Digital Content 1 listing the comprehensive candidemia bundle checklist components). The references for the components of the checklists including information about loading and maintenance doses, duration of antifungal therapy, ideal time for intravenous to oral conversion were provided in the checklist itself for enhancing the knowledge of the primary clinical care team. It was mandated that the checklist should be filled by the primary physician in stepwise compliance and audited by the clinical pharmacist at regular intervals during the patient's hospital stay and at the time of patient's discharge. The checklist and institutional dosing protocols for antifungals in candidemia were then disseminated through hospital intranet prior to implementation.
In addition to the filing of the checklist, primary team would also be notified through a phone call from the clinical pharmacist regarding the culture positivity, filing of the check list, and choice of antifungals. After the antifungals are prescribed by the primary team, post prescriptive audit for appropriateness would conducted by the Antifungal Stewardship Team. These cases were discussed in the twice weekly AFS meetings and the appropriateness of antifungal therapy was assessed as per 5 R's (Right indication, Right drug, Right dose, Right frequency, and Right duration).[8] Of note, an antifungal prescription will be marked as appropriate only if all 5 R's are fulfilled. Performance of central venous catheter (CVC) removal in non neutropenic cases and repeat blood cultures in appropriate cases were assessed. The clinical pharmacist followed the patient till discharge for subsequent blood cultures, microbiological cure, and outcomes. The stewardship team also reinforced and audited the use of Echocardiogram to rule out endocarditis and ophthalmoscopy to rule out endophthalmitis. The compliance to each element of the candidemia care bundle checklist was audited and the team intervened whenever non-compliance to the checklist was identified. Figure 1 represents the whole process flow regarding management of Candidemia in both the pre and post-implementation phase. An AFS team as part of the ongoing antimicrobial stewardship activities reviewed appropriateness of antifungal prescriptions. The comprehensive 5R system was followed to evaluate the appropriateness of prescriptions. A prescription was marked appropriate only if all R's are fulfilled (Right indication, right drug, right dose, right frequency, and right duration). After completion of training and piloting the process went live from October 1, 2017. The post-implementation phase was from October 2017 to December 2019. Appropriateness of the treatment and mortality are the primary and secondary outcomes. Compliance to bundle elements was evaluated to assess its correlation with mortality.
2.3. Statistical analysis
Descriptive statistics was used summarize the major clinical characteristics and key outcomes. Categorical variables were analyzed by chi-square test or Fisher exact tests and continuous variables by student t test or Mann–Whitney U test based on distribution, to compare key outcomes between pre and post-implementation phase. P < .05 was considered to be statistically significant. All analysis was performed using SPSS version 17 (IBM, Chicago).
3. Results
A total of 175 adult patients diagnosed with candidemia were included into the study, 103 patients in the pre-implementation period (January 2013–December 2015) and 72 patients in the post-implementation period (October 2017–2019). Baseline characteristics including demographics and fungal infection features of the cohort are depicted in Table 1. Incidence of candidemia per 1000 patients was observed to be lower at 0.73 in the post-implementation period compared with 1.38 in the pre-implementation period. Medical specialties dominated both pre-implementation and post-implementation cohorts at 83% and 63% respectively. Frequently isolated Candida species were C tropicalis (30%) and C parapsilosis (32%) over the pre and post-implementation phases respectively. Use of CVC (62% vs 64%) and intensive care unit stay (67% vs 65%) were the most prevalent among the known risk factors for candidemia in pre and post-implementation phases. Mean days to candidemia was observed to be higher in post-implementation phase (11.57 ± 17.5) compared with pre-implementation phase (10.02 ± 12.2).
Table 1.
Comparison of baseline characteristics of patients with candidemia during the pre-implementation and post-implementation period .
| Characteristics | Pre-implementation (Jan 2014–Dec 2015) | Post-implementation (Oct 2017–Dec 2019) |
|---|---|---|
| N | 103 | 72 |
| Incidence per 1000 patients | 1.4 | 0.73 |
| Age (mean ± SD) | 54.85 ± 16.7 | 57.09 ± 16.39 |
| Advanced age (≥80 yrs) | 7 (7%) | 4 (5%) |
| Gender | ||
| Male | 66 (64%) | 43 (60%) |
| Major Departments | ||
| Medical | 82 (80%) | 47 (65%) |
| Surgical | 21 (20%) | 25 (35%) |
| Fungal species | ||
| Candida tropicalis | 31 (30%) | 16 (22%) |
| Candida parapsilosis | 26 (25%) | 23 (32%) |
| Candida albicans | 16 (16%) | 16 (22%) |
| Candida auris | 0 (%) | 10 (14%) |
| Candida glabrata | 2 (2%) | 2 (3%) |
| Community acquired | 9 (9%) | 14 (19%) |
| Inhospital acquired | 65 (63%) | 58 (81%) |
| Source of infection | ||
| CLABSI | 22 (21%) | 30 (42%) |
| Urinary tract infection | 18 (17%) | 17 (24%) |
| Pneumonia | 2 (2%) | 5 (7%) |
| Skin and soft tissue infection | 1 (1%) | 2 (3%) |
| Osteomyelitis | 0 | 1 (1%) |
| Days to Fungemia (mean) | 10.56 ± 9.3 | 11.57 ± 17.5 |
| Risk factors | ||
| ICU stay | 69 (67%) | 48 (65%) |
| Use of central venous catheter | 64 (62%) | 46 (64%) |
| Use of ventilator | 47 (46%) | 30 (42%) |
| Malignancy | 24 (23%) | 14 (19%) |
| Neutropenia | 15 (15%) | 12 (17%) |
ICU = intensive care unit.
3.1. Outcomes
Comparison of antifungal management and major outcomes of the study between the pre and post-implementation phases are outlined in Table 2.
Table 2.
Comparison of outcome and treatment modalities during the pre-implementation and post-implementation period.
| Characteristics | Pre-implementation (Jan 2014–Dec 2015) | Post-implementation (Oct 2017–Dec 2019) | P |
|---|---|---|---|
| Empirical therapy | 32 (31%) | 68 (94%) | .0001 |
| Definitive therapy | 39 (38%) | 4 (6%) | |
| Antifungal resistance | |||
| Amphotericin B | 18 (17%) | 1/57 (2%) | .0007 |
| Fluconazole | 28 (27%) | 5/57 (9%) | .0007 |
| Appropriateness of antifungal treatment (n = 46)∗ | |||
| Appropriate | 31 (30%) | 30 (65%) | .0005 |
| Inappropriate | 72 (70%) | 16 (35%) | |
| Outcomes | |||
| Alive | 62 (60%) | 46 (64%) | .6 |
| Expired | 41 (40%) | 26 (36%) | |
| Delay in treatment (mean in hours) | 59.07 ± 98.4 | 8.26 ± 16.35 | .0001 |
| Length of stay | |||
| ≤14 days | 43 (42%) | 25 (35%) | .26 |
| 15–29 days | 37 (36%) | 23 (32%) | |
| ≥30 days | 23 (22%) | 24 (33%) | |
∗Twenty six patients in the post-implementation period died before possible completion of all bundle elements; thus, their data were excluded from analysis for appropriateness.
Only 31% (n = 32) of the candidemia patients in the pre-implementation period received antifungal therapy on provisional blood culture report of budding yeast while awaiting species identification and susceptibility data. Initiation of antifungal therapy for candidemia post provisional culture report was significantly higher in post-implementation phase (94%) compared with pre-implementation phase (31%) (P < .001) among patients who received antifungal therapy (P = .0001). Appropriateness of antifungal prescriptions arose to 65% post the implementation of the comprehensive candidemia care bundle from 30% observed during the pre-implementation phase (P = .0005). The all-cause inhospital mortality rate of the candidemia patients was found to relatively reduce from 40% in the pre-implementation phase to 36% in the post-implementation phase, recording a 10% reduction over 2 years post-implementation. However, a statistical significance was not observed. The mean delay in initiating antifungal therapy significantly reduced to 8.26 ± 16.35 hours post candidemia care bundle implementation from 59.07 ± 98.4 hours in pre-implementation phase (P = .0001). However, a shift towards higher hospital length of stay was observed during the post-implementation phase as demonstrated by an increase of candidemia patients with LOS ≥30 days (22% vs 33%) (P = .26). The mean time to clearance of candidemia was found to be 6.2 days.
Among 52 patients for whom microbiological cure could be assessed in the post-implementation phase, 40% and 19% of patients achieved microbiological cure within 48 and 72 hours of initial positive blood culture report. Microbiological cure could not be assessed for 28% of patients in the post-implementation phase who expired before sending repeat cultures.
3.2. Compliance to candidemia care bundle
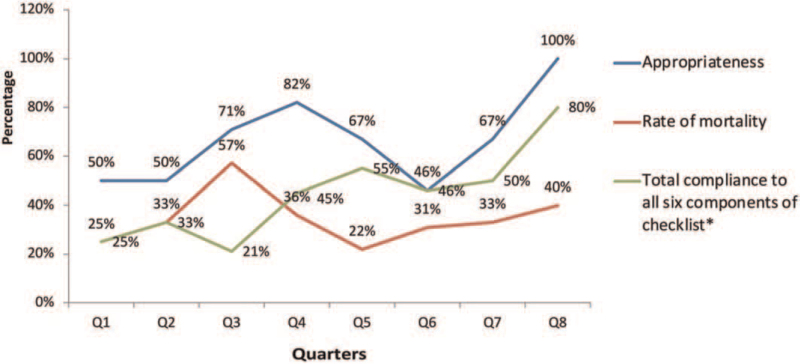
The overall compliance to the comprehensive candidemia care bundle post its implementation was reported to be 40%. No significant association was observed between overall bundle compliance and mortality, though compliance rate was higher among patients who survived (43%) in comparison to expired patients (38%). Similarly, a higher compliance rate was observed among survived patients relative to patients who expired in the case of 4 bundle elements of the candidemia care bundle checklist including removal of CVC (96% vs 84%), sending repeat cultures at 48 hours (69% vs 64%), 14 days of antifungal therapy from first negative blood culture (80% vs 60%), and transthoracic echocardiogram/transesophageal echocardiogram to rule out fungal endocarditis (55% vs 33%). A significant association was however not observed between any of the candidemia care bundle element compliance to mortality (Table 3). Among the 52 patients in the post-implementation phase for which repeat blood culture data were available, higher rates of microbiological cure of 52% and 60% at 48 hours (P = .17) and 72 hours (P = .7) respectively was observed among patients who had bundle compliance in comparison to patients who were non-compliant to the candidemia care bundle (48% in 48 hours and 40% in 72 hours) despite without a significant association. Appropriate antifungal selection rose over the initial quarter to 100% and was sustained throughout the post-implementation period. The total compliance rate exhibited gradual and sustained improvement over the quartiles (Fig. 2).
Table 3.
Compliance to candidemia care bundle checklist.
| Steps in candidemia care bundle checklist | N (%) | Alive | Death | P value |
|---|---|---|---|---|
| Appropriate antifungal selection (n = 72) | 68/72 (87%) | 43/46 (93%) | 25/26 (96%) | .63 |
| Appropriate dose of antifungal (n = 72) | 62/72 (86%) | 38/46 (83%) | 24/26 (92%) | .25 |
| Removal of central venous catheter (n = 47) | 43/47 (89%) | 27/28 (96%) | 16/19 (84%) | .14 |
| Sending repeat blood culture at 48 hours (n = 67) | 45/67 (67%) | 31/45 (69%) | 14/22 (64%) | .667 |
| 14 days of antifungal therapy from first negative blood culture (n = 51) | 40/51 (78%) | 37/46 (80%) | 3/5 (60%) | .62 |
| TTE/TEE to rule out fungal endocarditis (n = 15) | 7/15 (47%) | 5/9 (55%) | 2/6 (33%) | .39 |
| Total compliance to all bundle components (n = 72) | 30/72 (42%) | 20/46 (43%) | 10/26 (38%) | .67 |
TEE = transesophageal echocardiogram, TTE = transthoracic echocardiogram.
Figure 2.
Quarterly rates of appropriateness, mortality and compliance to candidemia bundle checklist.
4. Discussion
Our study demonstrated an increase in appropriateness in the management of candidemia with the implementation of an ASP led comprehensive candidemia care bundle checklist with a clinical pharmacist driven process to follow up the patients with candidemia ensuring timely implementation of the bundle elements.
Our bundle checklist included both antifungal drug related and non-drug related aspects of care for patients with candidemia. Compliance to all the elements of the bundle was 35% in our cohort, higher than the previously reported compliance rate at 6.9% by Antworth et al.[5] But the study reported by Takesue et al had a nationwide implementation and the bundle included additional components. Compliance to care elements for managing candidemia ranged from 17% to 83% in literature and was observed to be generally higher in ASP led interventions.[5–11] Survival rates were observed to be higher in subcohorts who complied with individual components and all components in aggregate, although this failed to attain statistical significance in our study. This could be attributed to the relatively small sample size of the subcohorts in our study. The striking contrast of absence of significant mortality benefit as compared with certain published literature may be due to the fact that our bundle was implemented in a tertiary care setting on an institutional basis and hence the cohort could have been sicker than in a nationwide implementation.[1] Moreover, some studies did not observe a beneficial impact on clinical outcomes such as mortality and length of stay.[9,12]
Despite the use of CVC identified as a major risk factor for candidemia in both pre-implementation (62%) and post-implementation periods (64%) and the major focus of infection in expired patients (19/26,73%) in post-implementation phase, CVC removal failed to confer benefit in terms of survival. This is in contrast with the published literature demonstrating favourable effects of CVC removal.[1,13] However overall mortality in candidemia had been known to correlate with the underlying disease.[14] Time window of CVC removal is another factor worth noting which could have tilted the balance. In our study cohort, 33% (17/52) of patients expired before the results arrived/filing of the checklist and thus were not true beneficiaries of the bundle implementation. However, conclusive evidence supporting the removal of CVC is lacking in literature due to heterogeneity of the observational studies and lack of RCTs.
The process of critical alert generation to the stewardship team in addition to the results published in the hospital information system provided the crux of a multipronged approach to improve the time to initiation of antifungal and proved effective in reduction of the same. Evidently, an empiric antifungal was initiated in majority of the patients in the post-implementation period according to the institutional protocol without delaying the therapy until the final culture report was available, thereby reducing the mean delay in treatment initiation. In addition to this, the availability of the checklist and concise treatment guidelines in the patient file guided the clinician in appropriately managing the candidemia patients. Furthermore, the AFS team provided feedback to the clinician whenever additional clarification regarding any of the bundle elements were required.
In our system, the standard turnaround times of susceptibility data, especially in relation to fungal cultures, has been a major hurdle in timely initiation of antifungal therapy. Post-implementation period witnessed a decrease in the delay in initiating therapy (12 hours compared with 58 hours) and initiation of systemic antifungals before susceptibility results (96% compared with 19%) but these could not be translated to independent determinants of survival in our cohort. Similar reduction in time to effective therapy was also reported by a Pharm D driven ASP intervention study, akin to our clinical pharmacist led process.[12]
Majority of the patients in the post-implementation phase complied to the drug related parameters in the bundle checklist including appropriate drug selection, appropriate dose of antifungal, and 14-day antifungal therapy from the first negative blood culture. There was an apprehension from the clinicians to comply to some of the non-drug therapy elements in the bundle checklist such as sending repeat blood culture at 48 hours and transthoracic echocardiogram/transesophageal echocardiogram to rule out fungal endocarditis due to lack of strong evidence supporting these recommendations. These were also considered as moderate recommendation according the Equal Candida score developed by Mellinghoff et al.[15] One of the major reasons behind the non-compliance to the bundle elements was the resistance from a few clinicians despite our initial sensitization. Evidently, this could represent one of the major barriers for incorporation of care bundles in clinical practice in low middle income group countries like ours. Quarterly evaluation of compliance of individual components of the bundle revealed a sustained positive trend for selection of antifungals, appropriate dose of antifungals, and sending of repeat cultures. This also highlights the need and importance of continued sensitization and feedback. The involvement of dedicated clinical pharmacist as the key driver of the process was unique in our setting and this could have translated in the improvement in the drug related appropriateness as reported by Reed et al.[12]
4.1. Limitations of the study
Due to pre post quasi experimental design of study, it was not possible to determine causality of outcomes. The major limitation was the small sample size because of which we were not able to show statistically significant results in many aspects of the bundle checklist. Another limitation was that we did not receive real time notifications during off hours on evenings and weekends.
5. Conclusion
The current study demonstrates the successful implementation of an ASP led comprehensive care bundle approach in improving the appropriateness of management of candidemia. This is especially beneficial in LMIC like India where there is a limited availability of resources.
Acknowledgments
The authors would like to acknowledge the senior administrators and microbiology staff at Amrita Institute of Medical Sciences for facilitating the study.
Author contributions
Conceptualization: Merlin Moni, Sanjeev Singh, Dipu Sathyapalan.
Formal analysis: Merlin Moni, Jini James, Fabia Edathadathil, Dipu Sathyapalan.
Investigation: Merlin Moni, Neeraj Sidharthan, Sangita Sudhir, Binny Prabhu, Vrinda Nampoothiri, Jeslyn Mary Philip, Jisha Thomas, Remya Antony, Zubair Umer Mohamed, Preetha Prasanna, Dipu Sathyapalan.
Writing – original draft: Merlin Moni, Vrinda Nampoothiri, Jini James, Dipu Sathyapalan.
Writing – review & editing: Merlin Moni, Anil Kumar, Fabia Edathadathil, Dipu Sathyapalan.
Supplementary Material
Footnotes
Abbreviations: AFS = antifungal stewardship, ASP = antimicrobial stewardship programme, CVC = central venous catheter, LMIC = low middle income countries.
How to cite this article: Moni M, Sidharthan N, Sudhir S, Prabhu B, Nampoothiri V, James J, Philip JM, Thomas J, Antony R, Mohamed ZU, Kumar A, Prasanna P, Edathadathil F, Singh S, Sathyapalan D. A quality improvement initiative to improve the appropriateness of candidemia management by the implementation of a comprehensive candidemia care bundle at a tertiary care hospital in South India: results of a quasi-experimental study. Medicine. 2022;101:13(e28906).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.;
Supplemental digital content is available for this article.
Contributor Information
Merlin Moni, Email: drmerlin.blessan@gmail.com.
Neeraj Sidharthan, Email: neerajsidharthan@aims.amrita.edu.
Sangita Sudhir, Email: sangitas@aims.amrita.edu.
Binny Prabhu, Email: binnypp@yahoo.co.uk.
Vrinda Nampoothiri, Email: vrindan@aims.amrita.edu.
Jini James, Email: jinijames@aims.amrita.edu.
Jeslyn Mary Philip, Email: jeslynmp@aims.amrita.edu.
Jisha Thomas, Email: jishathomas@aims.amrita.edu.
Remya Antony, Email: remyaantony@aims.amrita.edu.
Zubair Umer Mohamed, Email: zubairum22623@aims.amrita.edu.
Anil Kumar, Email: vanilkumar@aims.amrita.edu.
Preetha Prasanna, Email: preethap@aims.amrita.edu.
Fabia Edathadathil, Email: fabiaet22441@aims.amrita.edu.
Sanjeev Singh, Email: sanjeevksingh@aims.amrita.edu.
Dipu Sathyapalan, Email: diputsmck@gmail.com.
References
- [1].Takesue Y, Ueda T, Mikamo H, et al. Management bundles for candidaemia: the impact of compliance on clinical outcomes. J Antimicrob Chemother 2015;70:587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kaur H, Chakrabarti A. Strategies to reduce mortality in adult and neonatal candidemia in developing countries. J Fungi (Basel) 2017;3:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Murakami M, Komatsu H, Sugiyama M, et al. Antimicrobial stewardship without infectious disease physician for patients with candidemia: a before and after study. J Gen Fam Med 2018;19:82–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vitale RG, Nucci M. Diagnosis of candidemia. Curr Fungal Infect Rep 2014;8:90–4. [Google Scholar]
- [5].Antworth A, Collins CD, Kunapuli A, et al. Impact of an antimicrobial stewardship program comprehensive care bundle on management of candidemia. Pharmacother J Hum Pharmacol Drug Ther 2013;33:137–43. [DOI] [PubMed] [Google Scholar]
- [6].Lavallée JF, Gray TA, Dumville J, Russell W, Cullum N. The effects of care bundles on patient outcomes: a systematic review and meta-analysis. Implement Sci 2017;12:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Morris AC, Hay AW, Swann DG, et al. Reducing ventilator-associated pneumonia in intensive care: impact of implementing a care bundle. Crit Care Med 2011;39:2218–24. [DOI] [PubMed] [Google Scholar]
- [8].Singh S, Menon VP, Mohamed ZU, et al. Implementation and impact of an antimicrobial stewardship program at a tertiary care center in South India. Open Forum Infect Dis 2019;6:ofy290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pettit NN, Han Z, Nguyen CT, et al. Antimicrobial stewardship review of automated candidemia alerts using the epic stewardship module improves bundle-of-care adherence. Open Forum Infect Dis 2019;6:ofz412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vena A, Bouza E, Corisco R, et al. Efficacy of a “Checklist” intervention bundle on the clinical outcome of patients with candida bloodstream infections: a quasi-experimental pre-post study. Infect Dis Ther 2020;9:119–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rac H, Wagner JL, King ST, Barber KE, Stover KR. Impact of an antifungal stewardship intervention on optimization of candidemia management. Ther Adv Infect Dis 2018;5:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Reed EE, West JE, Keating EA, et al. Improving the management of candidemia through antimicrobial stewardship interventions. Diagn Microbiol Infect Dis 2014;78:157–61. [DOI] [PubMed] [Google Scholar]
- [13].Andes DR, Safdar N, Baddley JW, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis 2012;54:1110–22. [DOI] [PubMed] [Google Scholar]
- [14].Puig-Asensio M, Padilla B, Garnacho-Montero J, et al. Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: a population-based surveillance in Spain. Clin Microbiol Infect 2014;20:O245–54. [DOI] [PubMed] [Google Scholar]
- [15].Mellinghoff SC, Hoenigl M, Koehler P, et al. EQUAL candida score: an ECMM score derived from current guidelines to measure QUAlity of Clinical Candidaemia Management. Mycoses 2018;61:326–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.