Abstract
Background and Objective
Trastuzumab deruxtecan (T-DXd) is a novel anti-ERBB2 antibody drug conjugate that appears to be associated with an increased risk of lung toxicity. We performed a systematic review to describe the incidence, severity, and management of T-DXd-induced interstitial lung disease (ILD) or pneumonitis.
Methods
We searched PubMed/MEDLINE, Embase, Cochrane, and Web of Sciences through to 1 January, 2022, for human clinical trials that assessed T-DXd in adults with ERBB2-positive advanced solid tumors and described the rate of ILD/pneumonitis. Study screening was performed by two researchers. Data were extracted from the full-text articles.
Results
Fourteen studies with a total of 1193 patients with different types of advanced solid malignancies were included in our systematic review. The overall incidence of all-grade ILD/pneumonitis cases that were adjudicated by an independent committee was 11.40% (ILD/pneumonitis cases, n = 136 out of total n = 1193). Grading of the adjudicated T-DXd-induced ILD/pneumonitis was reported in 122 patients with the majority of the cases (78.69%, n = 96) occurring as grade 1 or 2. Death was reported in 13 out of 122 (10.66%) patients. The highest incidence of ILD/pneumonitis was seen in patients with uterine carcinomatosis (26.47%) and non-small cell lung cancer (24.77%). Interstitial lung disease/pneumonitis events were treated with a dose interruption or reduction, treatment discontinuation, corticosteroids, and supportive care.
Conclusions
Interstitial lung disease/pneumonitis is a well-described, serious, and potentially life-threatening adverse event that is associated with T-DXd. Further studies are needed to identify the risk factors and the underlying pathophysiology of T-DXd-induced ILD/pneumonitis to prevent occurrence and to develop effective management strategies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40265-022-01736-w.
Key Points
| Trastuzumab deruxtecan antibody drug conjugate can lead to a life-threatening interstitial lung disease/pneumonitis. |
| Incidence and severity of trastuzumab deruxtecan-induced interstitial lung disease/pneumonitis are variable among different malignancies. |
| Clinicians should immediately recognize and intervene on any suspected trastuzumab deruxtecan-induced interstitial lung disease/pneumonitis case. |
Introduction
Human epidermal growth factor receptor 2 (ERBB2) is highly expressed in different types of malignancies, making it an attractive target in cancer treatment. Trastuzumab deruxtecan (T-DXd or DS8201a) is a novel antibody drug conjugate (ADC) that consists of an anti-ERBB2 (HER2) monoclonal antibody linked to a topoisomerase I inhibitor, deruxtecan, via a protease cleavable linker [1]. In December 2019, T-DXd was approved by the US Food and Drug Administration for the treatment of unresectable or metastatic ERBB2-positive breast cancer [2]. Later, in January 2021, T-DXd was approved by the Food and Drug Administration for the treatment of ERBB2-positive locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma [3]. Although T-DXd showed encouraging anti-tumor effects, clinical observations showed pulmonary toxicity of drug-induced interstitial lung disease (ILD) or pneumonitis in some patients.
Drug-induced ILD/pneumonitis is a potentially life-threatening lung condition characterized by the inflammation and fibrosis of the lung interstitium [4]. Drug-induced ILD/pneumonitis is a diagnosis of exclusion, and a comprehensive history, physical examination, laboratory investigations, and radiographic imaging are required to establish the diagnosis [5].
There are limited data on the incidence and management of T-DXd-induced ILD/pneumonitis. This could be because of the variability in the terminology used to describe ILD/pneumonitis and the limited experience in routinely diagnosing and managing this disease entity [6]. We conducted a systematic review to describe the incidence, severity, and management of T-DXd-related ILD/pneumonitis across clinical trials in patients with advanced solid malignancies.
Methods
We systematically searched the following databases: PubMed/MEDLINE, Embase, Cochrane, and Web of Sciences through to 1 January, 2022, using Medical Subject Headings as search phrases. All databases were searched for English-language articles only. Human clinical studies that assessed T-DXd in adults with ERBB2-positive advanced solid tumors and described the rate of ILD/pneumonitis were included. Studies other than clinical trials or studies that did not describe ILD/pneumonitis were excluded.
The screening and selection of studies took place in two stages. In the first step, two investigators (Z.A. and A.A.) assessed the titles and abstracts of papers found in the electronic databases for eligibility using the inclusion and exclusion criteria. In the second step, the same two investigators retrieved the full text of the papers chosen in the first phase and examined them for eligibility using the same inclusion and exclusion criteria. Uncertainty concerning article inclusion was resolved through an agreement between the two investigators and approved by the third investigator (M.N.). A data extraction template with predefined fields was used to extract information from full-text publications.
Results
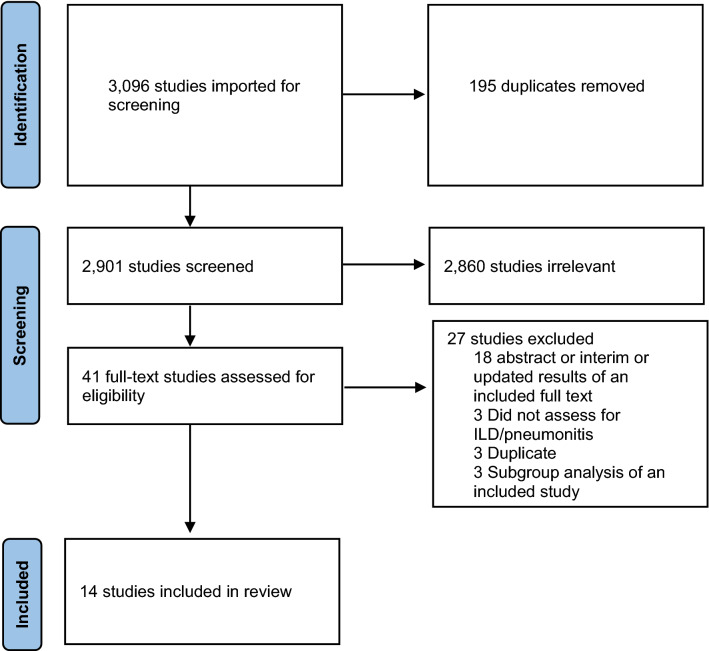
Out of 3096 studies identified through database searching, 2901 titles and abstracts were identified after removing duplicates (Fig. 1). Of them, 41 were eligible for a full-text review based on the inclusion and exclusion criteria. During the full-text review, a total of 27 titles and abstracts were excluded, with the majority (66.67%) excluded because they were abstracts, or interim or updated results of an already included full study. Fourteen articles from the electronic database search were eligible for inclusion in this review.
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram of studies included in the review. ILD interstitial lung disease
A total of 1193 patients with different types of advanced, and previously treated solid malignancies were included. The reported median age range was 48.5–68 years. In the majority of the patients, the Eastern Cooperative Oncology Group performance scale was either 0 or 1. Most of the patients received intravenous T-DXd at either a 5.4-mg/kg or 6.4-mg/kg dose every 3 weeks. Only two studies did not report the dose of T-DXd [7, 8]. Fifty percent of the included studies were phase II clinical trials, 43% were phase I, and only one was a phase III clinical trial. Of the 14 studies, five were conducted globally, four in the USA and Japan, two in Japan only, one in Japan and South Korea, and one each in Taiwan and Austria. The studies and patient characteristics for the included 14 studies are summarized in Table 1.
Table 1.
Characteristics of the included clinical trials in the systematic review
| Author, year | Study type | Trial No. | Country | Intervention | Subjects | Sample size, n | Age, years median (range) | Female, n | ECOG status | ILD/pneumonitis, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Modi (2020) [9] | Open-label, single-arm, multicenter, phase II | NCT03248492 | International | T-DXd 5.4 mg/kg q3w | Previously treated, ERBB2(+) breast CA | 184 | 55 (28–96) | 184 |
0: 102 1: 81 2: 1 |
25 (13.59) |
| Cortes (2021) [8] | Open-label, randomized, multicenter, phase III | NCT03529110 | International | T-DXd, NR dose | Previously treated, ERBB2(+) breast CA | 257 | 54 (20–83) | NR | NR | 27 (10.51) |
| Modi (2020) [10] | Open-label, single-arm, multicenter, phase Ib | NCT03248492 | USA and Japan | T-DXd 5.4 or 6.4 mg/kg q3w | Previously treated, ERBB2(+) breast CA | 54 | 56.6 (33–75) | NR |
0: 36 1: 18 2: 0 |
8 (14.81) |
| Chang (2019) [11] | Open-label, single-arm, multicenter, phase I | NCT03368196 | Taiwan | T-DXd 6.4 mg/kg q3w | Breast CA | 12 | 55 (36–69) | 12 | NR | 0 |
| Tamura (2019) [12] | Open-label, single-arm, multicenter, phase I | NCT02564900 | USA and Japan | T-DXd 5.4 or 6.4 mg/kg q3w | Previously treated, ERBB2(+) breast CA | 115 | 55 (47–66) | 114 |
0: 72 1: 43 2: 0 |
13 (11.30) |
| Yamashita (2020) [13] | Open-label, single-arm, multicenter, phase I | NCT03366428 | Japan | T-DXd 6.4 mg/kg q3w | Advanced ERBB2(+) or low breast CA | 51 | 56 (31–79) | 51 | NR | 1 (1.96) |
| Bartsch (2021) [7] | Open-label, single-arm, single center, phase II | NCT04752059 | Austria | T-DXd, NR dose | Previously treated, ERBB2(+), breast CA with brain mets | 10 | 48.5 (NR) | NR | NR | 0 |
| Shitara (2020) [14] | Open-label, randomized, multicenter, phase II | NCT03329690 | Japan and South Korea | T-DXd 6.4 mg/kg q3w | Previously treated, ERBB2(+) gastric/GEJ CA | 125 | 65 (34–82) | 30 |
0: 62 1: 63 2: 0 |
12 (9.60) |
| Van Cutsem (2021) [15] | Open-label, single arm, multicenter, phase II | NCT04014075 | International | T-DXd 6.4 mg/kg q3w | Previously treated, ERBB2(+) gastric/GEJ CA | 79 | 61 (20–78) | NR | NR | 6 (7.59) |
| Shitara (2019) [16] | Open-label, single-arm, multicenter, phase I | NCT02564900 | USA and Japan | T-DXd 5.4 or 6.4 mg/kg q3w | Previously treated, ERBB2(+) gastric/GEJ CA | 44 | 68 (62.5–72) | 12 |
0: 32 1: 12 2: 0 |
1 (2.27) |
| Siena (2021) [17] | Open-label, single arm, multicenter, phase II | NCT03384940 | International | T-DXd 6.4 mg/kg q3w | Previously treated, ERBB2(+) colorectal CA | 78 | 58.5 (50–60)a | 37 |
0: 49 1: 28 2: 1 |
5 (6.41) |
| Hasegawa (2021) [19] | Open-label, single-arm, multicenter, phase II | STATICE trial, NCCH1615 | Japan | T-DXd 6.4 mg/kg q3w | Previously treated, ERBB2(+) uterine carcinosarcoma | 34 | NR | 34 | NR | 9 (26.47) |
| Li (2022) [20] | Open-label, single-arm, multicenter, phase II | NCT03505710 | International | T-DXd 6.4 mg/kg q3w | Previously treated, ERBB2(+) NSCLC | 91 | 60 (29–88) | 60 |
0: 23 1: 68 2: 0 |
24 (26.37) |
| Tsurutani (2020) [18] | Open-label, single-arm, multicenter, phase I | NCT02564900 | USA and Japan | T-DXd 6.4 mg/kg q3w | Previously treated, ERBB2(+) non-breast or gastric solid tumors | 59 | 58 (23–83) | 31 |
0: 29 1: 31 2: NR |
5 (8.47) |
CA cancer, ECOG Eastern Cooperative Oncology Group, ERBB2 human epidermal growth factor receptor 2, GEJ gastroesophageal junction, ILD interstitial lung disease, mets metastasis, NR not reported, NSCLC non-small cell lung cancer, q3w every 3 weeks, T-DXd trastuzumab deruxtecan
aAge is the median (interquartile range)
Incidence and Severity of Trastuzumab-Deruxtecan-Induced Interstitial Lung Disease (ILD)/Pneumonitis
In the included studies, out of a total of 1193 patients, a total of 136 (11.40%) patients were reported to have adjudicated T-DXd-induced ILD/pneumonitis after evaluation by an independent adjudication committee (Table 1). Twelve out of the 14 included studies reported the grading of the adjudicated ILD/pneumonitis cases (n = 122). Most of the adjudicated T-DXd-induced ILD/pneumonitis adverse events were grades 1 or 2, seen in 96 patients (78.69%), while grades 3 and 4 were noted in 13 patients (10.66%). Death related to T-DXd-induced ILD/pneumonitis was reported in 13 out of 122 (10.66%) patients. Differences in the incidence and severity of ILD/pneumonitis were seen among different types of solid tumors.
Breast Cancer
Seven clinical trials that assessed the efficacy and safety of T-DXd in previously treated, advanced ERBB2-positive breast cancer were included in our systematic review [7–13]. The T-DXd safety profile was assessed in a total of 683 patients with breast cancer with a median age range of 48.5–56.6 years. Adjudicated T-DXd-induced ILD/pneumonitis was reported in 74 patients (10.83%). All the included studies except one [12] provided the grading of the adjudicated ILD/pneumonitis adverse events (n = 61). Most of the adverse events were grades 1 or 2 (51 of 61, 83.60%), while grade 3 or 4 events were seen in only three patients (4.92%). Death due to T-DXd-induced ILD/pneumonitis was reported in only seven patients (11.48%). Only one study [9] reported the median time until the onset of the lung disease in investigator-reported cases of ILD/pneumonitis of any grade, and it was 193 (range: 42–535) days. Furthermore, the same study reported a median time from the onset of lung disease to recovery of 34 (range: 3–179) days (Table 2).
Table 2.
Incidence of the adjudicated cases of T-DXd-induced ILD/pneumonitis according to the type of malignancy
| Malignancy | Author, year | Sample size, n | ILD/pneumonitis, n (%) | G1 or 2, n (%) | G3, n (%) | G4, n (%) | G5, n (%) | Median time in days until onset of lung disease (range) | Median time in days from the onset to recovery (range) |
|---|---|---|---|---|---|---|---|---|---|
| Breast cancer | Modi (2020) [9] | 184 | 25 (13.59) | 20 (80) | 1 (4) | 0 | 4 (16) | 193 (42–535) | 34 (3–179) |
| Cortes (2021) [8] | 257 | 27 (10.51) | 25 (92.59) | 2 (7.40) | 0 | 0 | NR | NR | |
| Modi (2020) [10] | 54 | 8 (14.81) | 5 (62.5) | 0 | 0 | 3 (37.5) | NR | NR | |
| Chang (2019) [11] | 12 | 0 | 0 | 0 | 0 | 0 | NR | NR | |
| Tamura (2019) [12] | 115 | 13 (11.30)a | NR | NR | NR | NR | NR | NR | |
| Yamashita (2020) [13] | 51 | 1 (1.96) | 1 (100) | 0 | 0 | 0 | NR | NR | |
| Bartsch (2021) [7] | 10 | 0 | 0 | 0 | 0 | 0 | NR | NR | |
| Gastric and GEJ cancer | Shitara (2020) [14] | 125 | 12 (9.60) | 9 (75) | 2 (16.67) | 1 (8.33) | 0 | 84.5 (36–638) | 57 (NR) |
| Van Cutsem (2021) [15] | 79 | 6 (7.59) | 5 (83.33) | 0 | 0 | 1 (16.67) | NR | NR | |
| Shitara (2019) [16] | 44 | 1 (2.27)b | NR | NR | NR | NR | NR | NR | |
| Colorectal carcinoma | Siena (2021) [17] | 78 | 5 (6.41) | 2 (40) | 1 (20) | 0 | 2 (40) | 77 (42–84) | NR |
| Tsurutani (2020) [18] | 20 | 2 (10)c | NR | NR | NR | NR | NR | NR | |
| Uterine carcinosarcoma | Hasegawa (2021) [19] | 34 | 9 (26.47) | 8 (88.89) | 1 (11.11) | 0 | 0 | NR | NR |
| NSCLC | Li (2022) [20] | 91 | 24 (26.37) | 18 (75) | 4 (16.67) | 0 | 2 (8.33) | 141 (14–462) | 43 (24–94)c |
| Tsurutani (2020) [18] | 18 | 3 (16.67) | NR | NR | NR | 1 (33.33) | NR | NR |
G1 or 2 grade 1 or 2, G3 grade 3, G4 grade 4, G5 grade 5, GEJ gastroesophageal junction, ILD interstitial lung disease, NR not reported, NSCLC non-small cell lung cancer, T-DXd trastuzumab deruxtecan
a20 cases of ILD, pneumonitis, or organizing pneumonia were reported by investigators (G1 or 2: 17, G3: 1, G5: 2). Of the 20 cases, 13 were adjudicated as T-DXd-induced ILD, 1 confirmed ILD but not due to T-DXd, 2 were not ILD, and 4 were pending adjudication. Data regarding grading of the 13 confirmed cases were not reported
b4 cases of ILD/pneumonitis were reported by investigators (G2: 3, G3: 1). Only 2 cases were assessed by an independent adjudication committee, one was ILD related to T-DXd, and the other was ILD not related to T-DXd. Data regarding grading the confirmed case were not reported
c2 cases of ILD/pneumonitis reported by the investigators. No reported data on the grading or number of adjudicated ILD/pneumonitis cases among the colorectal carcinoma cohort
dMedian (95% confidence interval)
Gastric and Gastroesophageal Junction Cancers
Three studies assessed T-DXd in 248 patients with previously treated, advanced, and ERBB2-positive gastric or gastroesophageal junction cancer [14–16]. The median age range in the included gastric or gastroesophageal junction cancer studies was 61–68 years. Adjudicated T-DXd induced ILD/pneumonitis cases were reported in 19 patients (7.66%). The grading of the adjudicated T-DXd-induced ILD/pneumonitis adverse events were reported in two studies (n = 18) [14, 16]. Most of the cases were grade 1 or 2 (n = 14, 77.78%), while three or four adverse events were seen in three (16.67%) patients. Only one (5.56%) patient had a grade 5 fatal outcome. The median time until the onset of lung disease was reported in one study [14], and it was 84.5 (range: 36–638) days. The same study reported a median of 57 days from the onset of the disease until recovery (Table 2).
Colorectal Carcinoma
Two studies that assessed T-DXd in patients with advanced colorectal adenocarcinoma (n = 98) were included in our systematic review [17, 18]. Adjudicated T-DXd-induced ILD/pneumonitis was reported in only one study (n = 78) [17]. In that study, five out of 78 (6.41%) had T-DXd-induced ILD/pneumonitis. Two (40%) were grade 2, one (20%) was grade 3, and two (40%) were associated with fatal outcomes. The same study reported the median time until the onset of lung disease and it was 77 (range 42–84) days. The other study assessed the efficacy and safety of T-DXd in 59 patients with advanced solid malignancies; of them, 20 patients had colorectal carcinoma [18]. The investigators reported two cases (10%) of ILD/pneumonitis among the patients. The study did not specifically report the number of adjudicated ILD/pneumonitis cases among the colorectal carcinoma cohort (Table 2).
Uterine Carcinosarcoma
One study assessed T-DXd in 34 patients with advanced ERBB2-positive uterine carcinosarcoma [19]. Nine (26.47%) cases of adjudicated T-DXd-induced ILD/pneumonitis were reported. Eight (88.89%) were grade 1 or 2, and one (11.11%) was grade 3. There were no reported deaths among the patients due to ILD/pneumonitis (Table 2).
Non-Small Cell Lung Cancer
Two studies assessed the use of T-DXd in a total of 109 patients with ERBB2-positive advanced non-small cell lung cancer [18, 20]. The mean median age was 59 years. Twenty-seven (24.77%) patients had T-DXd-induced ILD/pneumonitis that a committee adjudicated. Among them, three (11.11%) patients had a fatal outcome. Adjudicated T-DXd-induced ILD/pneumonitis grading was reported in one study [20] (n = 24), and most of the cases were grade 1 or 2, seen in 18 patients (75%), while grade 3 or 4 adverse events were seen in four (14.67%) patients. The same study reported a median time until the onset of the lung disease of 141 (range: 14–462) days, and a median time from the onset to the recovery of 43 (95% confidence interval 24–94) days (Table 2).
Induced ILD/Pneumonitis in 5.4 versus 6.4 mg/kg Subgroups
Most of the patients in the included studies received T-DXd at a dose of 6.4 mg/kg every 3 weeks. Seventy (12.46%) out of 562 patients who received T-DXd at 6.4 mg/kg every 3 weeks were reported to have ILD/pneumonitis that was confirmed by an independent committee (Electronic Supplementary Material). In contrast, among 205 patients who received the 5.4-mg/kg dose, 25 (12.20%) had ILD/pneumonitis. Data are limited on the dosing subgroups as not all studies reported the adverse events according to the dose.
Monitoring and Management of Induced ILD
Seven studies mentioned the guidelines used to manage suspected T-DXd-induced ILD/pneumonitis adverse events [9, 10, 12, 14, 17, 18, 20]. In these studies, suspected cases of lung toxicity were treated with a dose interruption, reduction, or discontinuation, corticosteroids, and supportive care. According to the guidelines used in these studies, if a case of ILD/pneumonitis was suspected, treatment with T-DXd was to be interrupted until a further evaluation, and corticosteroids were started promptly. If the adverse event was confirmed to be grade 1, T-DXd was restarted only if the event had fully resolved. The dose was maintained if resolved in ≤ 28 days from the day of onset, but if resolved in >28 days from the day of onset, the dose was to be reduced by one level. For grade 2, 3, or 4 events, T-DXd was permanently discontinued.
Discussion
Novel anti-ERBB2 ADCs are currently showing promise in studies for different malignancies. Because numerous malignancies express ERBB2, anti-ERBB2 ADCs could become a therapy option for a variety of tumors in addition to the already Food and Drug Administration-approved breast and gastric/gastroesophageal junctional tumor types. Given promising efficacy results in multiple settings, the management of potentially fatal adverse events such as ILD would be critical in improving the overall therapeutic index of T-DXd. The underlying mechanism for anti-ERBB2-induced lung damage is yet to be elucidated; however, it is likely to be associated with the cytotoxic agent (payload) [21]. Induced ILD/pneumonitis-related deaths have been documented in anti-ERBB2 trials with varying occurrence rates.
In our systematic review, among all the patients with different types of malignancies who received T-DXd, the rate of adjudicated cases of T-DXd-induced ILD/pneumonitis was 11.4%. Drug-induced ILD/pneumonitis is a known serious adverse event induced by many drugs such as anti-ERBB2 monoclonal antibodies. Hackshaw et al. reported a rate of 9.9% for trastuzumab-induced ILD in ERBB2-positve metastatic breast cancer [22]. The rate of T-DXd-induced ILD/pneumonitis that has been reported from studies enrolling patients with breast cancer was higher, and this could be because of the different molecular and structural characteristics between trastuzumab and T-DXd. In addition to trastuzumab in the T-DXd structure, a cytotoxic topoisomerase inhibitor “deruxtecan” is present. Although the mechanism of T-DXd-induced ILD remains unknown, it is likely associated, at least in part, with the carried cytotoxic agent, deruxtecan [21]. Additionally, comparing T-DXd-induced ILD/pneumonitis to other anti-ERBB2 ADCs, the incidence of T-DXd-induced ILD/pneumonitis was higher than the reported incidence of trastuzumab emtansine. Among 3290 patients with advanced ERBB2-positive breast cancer treated with trastuzumab emtansine, ILD was seen in only 0.5% [22]. Another study by Emens et al. showed a rate of 4% in 202 patients with advanced ERBB2-positive breast cancer when atezolizumab was added to trastuzumab emtansine [23].
Our systematic review reported the highest incidence of T-DXd-induced ILD/pneumonitis in patients with uterine carcinosarcoma (26.47%) and NSCLC (24.77%). In patients with NSCLC, the higher incidence of ILD/pneumonitis could be attributed to the known underlying diseased lung, and the likely limited baseline pulmonary function because of previous lung resection surgeries or radiation therapy, and or possible emphysematous changes due to smoking that may make the lungs more vulnerable to pulmonary toxicity. However, in the uterine carcinosarcoma cohort, reasons are unclear, and the higher incidence may be because of the small sample size (n = 34). The lowest incidence of T-DXd-related ILD/pneumonitis was seen among patients with colorectal carcinoma (6.41%) followed by patients with gastric and gastroesophageal junction (7.66%).
General guidelines are available in the package insert to establish the diagnosis or manage T-DXd-induced ILD/pneumonitis[24]. All patients receiving T-DXd should be monitored for signs and symptoms of ILD/pneumonitis. If a patient develops cough, dyspnea, fever and/or new or worsening respiratory symptoms, evaluation of ILD/pneumonitis should be initiated promptly. Radiographic imaging should be obtained in all suspected cases, and a pulmonologist consultation should be considered. For asymptomatic ILD/pneumonitis (grade 1), T-DXd should be interrupted until resolved and corticosteroids can be considered (e.g. ≥ 0.5 mg/kg/day of prednisolone or equivalent). If the adverse event resolved at ≤ 28 days, the same dose can be maintained, and if resolved at ≥ 28 days, the dose is to be reduced by one level. However, if a patient develops symptomatic ILD/pneumonitis (grade ≥ 2), T-DXd should be discontinued permanently, and corticosteroid treatment should be promptly initiated (e.g., ≥ 1 mg/kg/day of prednisolone or equivalent) for at least 14 days followed by a gradual taper for at least 4 weeks. Similar recommendations on the diagnosis and management of T-DXd-induced ILD/pneumonitis can also be adopted from drug-induced lung injury guidelines [25].
Our systematic review has certain limitations, including the limited sample size of the included studies. The majority of the patients had breast cancer; thus, results may not generalize to other tumor types. Second, some study investigators reported ILD/pneumonitis events rather than the adjudicated cases. Third, our data are limited on the correlation between the T-DXd dose and ILD/pneumonitis events as not all the studies documented the dose and/or the events according to the received dose. Fourth, we could not describe event timelines, such as the time to onset of ILD/pneumonitis and the time to recovery, owing to limited reported data. Last, we could not retrieve patient-level information on prior treatment history; for example, having exposure to immunotherapy within a certain period may increase a patient’s risks for ILD/pneumonitis, although the Destiny-Lung01 protocol mandated ≥4 weeks from previous immunotherapy [20].
Conclusions
Induced ILD/pneumonitis is a well-described adverse drug event associated with anti-ERBB2 ADC T-DXd with the highest incidence in patients with uterine carcinomatosis and NSCLC. Trastuzumab deruxtecan-induced ILD/pneumonitis is typically managed via a dose reduction, interruption, treatment discontinuation, corticosteroids, and supportive care. Additional studies are needed to identify the risk factors and the underlying pathophysiology of T-DXd-induced ILD/pneumonitis to prevent the occurrence and to develop effective management strategies.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
No external funding was received for the preparation of this article.
Conflicts of interest/Competing interests
MN is on the advisory board for AstraZeneca, Daiichi Sankyo, Takeda, Novartis, EMD Serono, Janssen, Pfizer, Eli Lilly and Company, and Genentech; is a consultant for Caris Life Sciences (virtual tumor board); a speaker for Blueprint Medicines and Takeda; and reports travel support from AnHeart Therapeutics. All other authors have no conflicts of interest that are directly relevant to the contents of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
Z. Abuhelwa, A. Allghbi, A. Alqahtani and M. Nagasaka all contributed to the writing and revision of the manuscript.
References
- 1.Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22(20):5097–5108. doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration. FDA approves fam-trastuzumab deruxtecan-nxki for unresectable or metastatic HER2-positive breast cancer. 2019. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2-positive-breast-cancer. Accessed 20 June 2022.
- 3.US Food and Drug Administration. FDA approves fam-trastuzumab deruxtecan-nxki for HER2-positive gastric adenocarcinomas. 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-her2-positive-gastric-adenocarcinomas. Accessed 20 June 2022.
- 4.Meyer KC. Diagnosis and management of interstitial lung disease. Transl Respir Med. 2014;2(1):4. doi: 10.1186/2213-0802-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skeoch S, et al. Drug-induced interstitial lung disease: a systematic review. J Clin Med. 2018;7(10):356. doi: 10.3390/jcm7100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells AU, Hirani N. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63(Suppl. 5):v1–58. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]
- 7.Bartsch R, et al. 280P Intracranial activity of trastuzumab-deruxtecan (T-DXd) in HER2-positive breast cancer patients with active brain metastases: results from the first stage of the phase II TUXEDO-1 trial. Ann Oncol. 2021;32:S486. doi: 10.1016/j.annonc.2021.08.563. [DOI] [Google Scholar]
- 8.Cortés J, et al. LBA1 trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): results of the randomized phase III DESTINY-Breast03 study. Ann Oncol. 2021;32:S1287–S1288. doi: 10.1016/j.annonc.2021.08.2087. [DOI] [Google Scholar]
- 9.Modi S, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modi S, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. 2020;38(17):1887. doi: 10.1200/JCO.19.02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang D-Y, Lin C-C, Chen TW, et al. Abstract C041: Safety and pharmacokinetic results from a phase 1, multicenter, open-label study of [fam-] trastuzumab deruxtecan (T-DXd; DS-8201a) in subjects with advanced HER2-positive breast cancer. AACR. March 29-April 3, 2019. Atlanta, GA, USA.
- 12.Tamura K, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20(6):816–826. doi: 10.1016/S1470-2045(19)30097-X. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita T, Shimomura A, Takano T, et al. Abstract P1-18-12: a phase 1, multicenter, open-label study to assess the effect of [fam-] trastuzumab deruxtecan (T-DXd; DS-8201a) on QTc and pharmacokinetics in subjects with HER2-expressing metastatic and/or unresectable breast cancer. AACR. April 24-29, 2020. San Diego, CA, USA.
- 14.Shitara K, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419–2430. doi: 10.1056/NEJMoa2004413. [DOI] [PubMed] [Google Scholar]
- 15.Van Cutsem E, et al. LBA55 primary analysis of a phase II single-arm trial of trastuzumab deruxtecan (T-DXd) in western patients (pts) with HER2-positive (HER2+) unresectable or metastatic gastric or gastroesophageal junction (GEJ) cancer who progressed on or after a trastuzumab-containing regimen. Ann Oncol. 2021;32:S1332. doi: 10.1016/j.annonc.2021.08.2135. [DOI] [Google Scholar]
- 16.Shitara K, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20(6):827–836. doi: 10.1016/S1470-2045(19)30088-9. [DOI] [PubMed] [Google Scholar]
- 17.Siena S, et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2021;22(6):779–789. doi: 10.1016/S1470-2045(21)00086-3. [DOI] [PubMed] [Google Scholar]
- 18.Tsurutani J, et al. Targeting HER2 with trastuzumab deruxtecan: a dose-expansion, phase I study in multiple advanced solid tumors. Cancer Discov. 2020;10(5):688–701. doi: 10.1158/2159-8290.CD-19-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasegawa K, et al. 813P Efficacy and safety of trastuzumab deruxtecan in HER2-expressing uterine carcinosarcoma (STATICE trial, NCCH1615): a multicenter, phase II clinical trial. Ann Oncol. 2021;32:S767. doi: 10.1016/j.annonc.2021.08.1255. [DOI] [Google Scholar]
- 20.Li BT, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. 2022;386(3):241–251. doi: 10.1056/NEJMoa2112431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarantino P, et al. Interstitial lung disease induced by anti-ERBB2 antibody-drug conjugates: a review. JAMA Oncol. 2021;7(12):1873–1881. doi: 10.1001/jamaoncol.2021.3595. [DOI] [PubMed] [Google Scholar]
- 22.Hackshaw MD, et al. Incidence of pneumonitis/interstitial lung disease induced by HER2-targeting therapy for HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2020;183(1):23–39. doi: 10.1007/s10549-020-05754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emens LA, et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020;21(10):1283–1295. doi: 10.1016/S1470-2045(20)30465-4. [DOI] [PubMed] [Google Scholar]
- 24.US Food and Drug Administration. Highlights of prescribing information 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761139s011lbl.pdf. Accessed 21 Mar 2022.
- 25.Kubo K, et al. Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respir Invest. 2013;51(4):260–277. doi: 10.1016/j.resinv.2013.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.