Abstract
Purpose
People living with cancer and haematological malignancies are at an increased risk of hospitalisation and death following infection with acute respiratory syndrome coronavirus 2. Coronavirus third dose vaccine boosters are proposed to boost waning immune responses in immunocompromised individuals and increase coronavirus protection; however, their effectiveness has not yet been systematically evaluated.
Methods
This study is a population-scale real-world evaluation of the United Kingdom’s third dose vaccine booster programme for cancer patients from 8th December 2020 to 7th December 2021. The cancer cohort comprises individuals from Public Health England’s national cancer dataset, excluding individuals less than 18 years. A test-negative case-control design was used to assess the third dose booster vaccine effectiveness. Multivariable logistic regression models were fitted to compare risk in the cancer cohort relative to the general population.
Results
The cancer cohort comprised of 2,258,553 tests from 361,098 individuals. Third dose boosters were evaluated by reference to 87,039,743 polymerase chain reaction coronavirus tests. Vaccine effectiveness against breakthrough infections, symptomatic infections, coronavirus hospitalisation and death in cancer patients were 59.1%, 62.8%, 80.5% and 94.5%, respectively. Lower vaccine effectiveness was associated with a cancer diagnosis within 12 months, lymphoma, recent systemic anti-cancer therapy (SACT) or radiotherapy. Patients with lymphoma had low levels of protection from symptomatic disease. In spite of third dose boosters, following multivariable adjustment, individuals with cancer remain at an increased risk of coronavirus hospitalisation and death compared to the population control (OR 3.38, 3.01, respectively. p < 0.001 for both).
Conclusions
Third dose boosters are effective for most individuals with cancer, increasing protection from coronavirus. However, their effectiveness is heterogenous and lower than the general population. Many patients with cancer will remain at the increased risk of coronavirus infections even after 3 doses. In the case of patients with lymphoma, there is a particularly strong disparity of vaccine effectiveness against breakthrough infection and severe disease. Breakthrough infections will disrupt cancer care and treatment with potentially adverse consequences on survival outcomes. The data support the role of vaccine boosters in preventing severe disease, and further pharmacological intervention to prevent transmission and aid viral clearance to limit the disruption of cancer care as the delivery of care continues to evolve during the coronavirus pandemic.
Keywords: COVID-19, Cancer, Vaccination, Effectiveness, SARS-CoV-2, Third dose, Booster
1. Introduction
Patients living with cancer are disproportionally affected by the coronavirus pandemic with the higher levels of morbidity and mortality from SARS-CoV-2 disease (COVID-19) [[1], [2], [3]]. The United Kingdom has a third dose vaccine booster programme for immunocompromised individuals who had completed their primary schedule of COVID-19 vaccination and was concurrently implemented across the general population [4,5]. The programme was initiated in response to work identifying poor immunological responses following the primary (2 dose) vaccination schedule in patients with cancer [[6], [7], [8], [9], [10], [11], [12], [13], [14]], waning or lower vaccine effectiveness in immunocompromised individuals [[15], [16], [17]] and an overrepresentation of immunocompromised individuals amongst coronavirus intensive care unit admissions and deaths in the United Kingdom. [18,19].
The evidence for coronavirus third dose vaccine boosters for patients with cancer is limited to immunological studies. These have identified that third dose vaccine boosters may improve or re-establish immunological or antibody responses [[20], [21], [22], [23], [24]]. However, to date, no studies have demonstrated that third dose boosters provide protection against breakthrough or symptomatic coronavirus infections, coronavirus hospitalisation or death. Furthermore, no studies have performed population-scale evaluations into the risk of patients to haematological malignancies relative to solid malignancies.
Predicted ongoing surges due to new coronavirus variants will increase the risk to patients with cancer, many of whom may have waning efficacy from their primary coronavirus vaccination course [[25], [26], [27]]. The UK Coronavirus Cancer programme is delivering population-scale assessment to evaluate patients with cancer during the COVID-19 pandemic. This study is the largest global coronavirus third dose vaccine booster evaluation, building on data from the UK primary vaccine course effectiveness analyses [28]. We describe how cancer subtype and treatment interact to affect the third dose booster vaccine effectiveness and risk of breakthrough/symptomatic infection, coronavirus hospitalisation and death.
2. Methods
2.1. Study description
The UK Coronavirus Cancer Programme is part of the United Kingdom’s COVID-19 cancer pandemic response to safeguard, evaluate and protect patients with cancer (www.ukcovidcancerprogramme.org). The study period was from the start of the COVID-19 vaccination in England from 8th December 2020 to 7th December 2021. The United Kingdom’s third dose booster vaccination programme for patients with immunocompromised cancer was launched on 1st September 2021 and the booster vaccination programme for the general population was launched on 14th September 2021.
2.2. Study design, data and sampling
The dataset contains all SARS-CoV-2 polymerase chain reaction (PCR) test results from England, obtained from the second-generation surveillance system. National Health Service (NHS) England and NHS Test and Trace use PCR testing for those with symptoms and antigen-detecting lateral flow testing (also known as rapid diagnostic testing) for asymptomatic case identification. During the study period, confirmatory PCR testing was mandatory for individuals testing positive by lateral flow tests. In the NHS, infection and prevention control measures in secondary care requires coronavirus testing of asymptomatic patients prior to many procedures or treatments. PCR records were linked to vaccination records from the National Immunisation Management Service. Data linkage required exact matching of NHS identifier number. The cancer cohort comprises adults (18 years or older) identified from Public Health England’s rapid registration national cancer dataset between 1st January 2018 and 30th April 2021. The population control cohort consists of tests from adults who were not contained within this national cancer dataset. Breakthrough infection was defined as a PCR positive test in a symptomatic or asymptomatic individual following vaccination. Hospital records were obtained from the secondary use statistics datasets. Coronavirus hospitalisation was defined as a hospitalisation episode from 1 prior to 14 days following a positive PCR test. Coronavirus death was a death within 28 days of a PCR positive test, in keeping with how COVID-19 deaths are reported by our UK Office for National Statistics [19]. SACT is an umbrella term of cancer treatments including cytotoxics (chemotherapy), targeted, immune or hormonal treatments. The study was designed as a public health surveillance analysis to support rapid clinical decision making in accordance with the UK Policy Framework for Health and Social Care Research. This study was supported by the Department of Health and Social Care, UK Health Security Agency, University of Oxford, University of Southampton, University of Birmingham and Blood Cancer UK with ethical approval from the Health Research Authority (20/WA/0181). The corresponding authors and senior author had final responsibility for the decision to submit for publication. The funders had no formal role in data analysis, interpretation or decision to submit.
2.3. Statistical analyses
The primary outcomes of the study were vaccine effectiveness against coronavirus breakthrough infections, symptomatic infections, hospitalisation and death. This was calculated following receipt of the third dose and the receipt of the second dose. Post-dose 2 vaccine effectiveness was calculated for individuals at least 20 weeks (140 days) after the administration of second dose, but who had not yet received their third dose. This is in keeping with existing UK population vaccination study methodology [29]. A test-negative case-control analysis was used to estimate vaccine effectiveness in cancer and general populations. This methodology was used as it gives high concordance with findings from randomised clinical trials and is a standardised measure of vaccine effectiveness for phase 4 surveillance studies. [30,31] Vaccine effectiveness was calculated with the test-negative case–control methodology formula: 1 minus, the ratio of third dose vaccinated to unvaccinated individuals who met a coronavirus end-point (either breakthrough infection, hospitalisation, death), divided by the ratio of third dose vaccinated to unvaccinated individuals with negative PCR tests. The negative tests act as an internal control, comprising individuals who might have symptoms from non-COVID-19 causes. Higher vaccine effectiveness is demonstrated if there are lower levels of vaccinated individuals amongst those who have a coronavirus end-point, compared to those who have a negative test. Predefined subgroup analyses for vaccine effectiveness against coronavirus breakthrough or symptomatic infections included analyses of the effect of cancer type and subtype, date of diagnosis, receipt of cancer treatment and vaccination manufacturer/combination. These subgroups have an established clinical rationale as individuals with a cancer diagnosis are a heterogenous group. An additional post-hoc analysis of vaccine effectiveness against coronavirus hospitalisation and death was performed in the lymphoma subgroup following clinical evaluation review. A multivariable logistic regression model was fitted to identify risk to patients with cancer of coronavirus hospitalisation/death amongst those had received a third dose. This was adjusted for the following clinically important variables: age, sex, cancer diagnosis, ethnicity, level of deprivation (based on English Index of Multiple Deprivation) [32], primary vaccination manufacturer and booster manufacturer. As the cancer cohort differed from the population control by having an older age distribution, sensitivity analyses were performed within age bands.
3. Results
Over the course of the study period, 87,039,743, PCR tests were performed from 29,929,073 individuals. A total of 2,258,553 PCR tests were identified from 361,098 individuals within the national cancer registry, forming the cancer cohort. 8,371,139 individuals had a third dose booster, of whom 230,666 were in the cancer cohort.
The majority of the cancer cohort (97.8%) had a BNT162b2 (Pfizer-BioNTech) third dose with a minority of individuals (1.5%) having a mRNA-1273 (Moderna) third dose. In terms of the baseline characteristics of the PCR positive cases, we observed that severe coronavirus outcomes (hospitalisation or death) were associated with tests from individuals of advancing age (Table 1 ).
Table 1.
Table demonstrating baseline characteristics between the cancer cohort and the population control.
| Cancer cohort |
Population control |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | SARS-CoV-2 PCR Negative | Breakthrough infections | Symptomatic infections | Hospital admission | Death | Cohort | SARS-CoV-2 PCR negative | Breakthrough infections | Symptomatic infections | Hospital admission | Death | |
| Total | 2258553 | 1906816 | 351737 | 328084 | 5467 | 6946 | 84781190 | 77135957 | 7645233 | 6402708 | 221964 | 81,271 |
| Age group | ||||||||||||
| 18–19 | 6704 | 3275 | 3429 | 3227 | 1 | 1 | 1542445 | 1312966 | 229479 | 183459 | 1659 | 13 |
| 20–29 | 32592 | 22606 | 9986 | 8628 | 33 | 7 | 12268104 | 10915482 | 1352622 | 1100890 | 16007 | 193 |
| 30–39 | 79174 | 59537 | 19637 | 18327 | 85 | 45 | 15032642 | 13640231 | 1392411 | 1142049 | 26715 | 768 |
| 40–49 | 157804 | 125757 | 32047 | 28688 | 201 | 104 | 14212766 | 12899883 | 1312883 | 1056818 | 28973 | 2078 |
| 50–59 | 371635 | 305755 | 65880 | 61299 | 579 | 459 | 15638289 | 14397690 | 1240599 | 1043152 | 39339 | 5767 |
| 60–69 | 531067 | 449631 | 81436 | 76142 | 1145 | 1187 | 10557704 | 9710645 | 847059 | 737430 | 35608 | 11917 |
| 70–79 | 638202 | 545798 | 92,404 | 87646 | 1745 | 2392 | 7058259 | 6396119 | 662140 | 601,039 | 33360 | 19553 |
| 80–89 | 359827 | 319012 | 40,815 | 38745 | 1272 | 2063 | 5729098 | 5277821 | 451277 | 408,365 | 28585 | 26793 |
| 90+ | 81548 | 75445 | 6103 | 5382 | 406 | 688 | 2741883 | 2585120 | 156763 | 129,506 | 11718 | 14189 |
| Sex | ||||||||||||
| Male | 1128166 | 944990 | 183176 | 172585 | 3157 | 4253 | 32588547 | 28961653 | 3626894 | 3091561 | 116504 | 46532 |
| Female | 1130385 | 961824 | 168561 | 155499 | 2310 | 2693 | 52155575 | 48137490 | 4018085 | 3310919 | 105446 | 34736 |
| Ethnicity | ||||||||||||
| White/White British | 2008740 | 1716193 | 292,547 | 272362 | 4647 | 5934 | 64909441 | 60035859 | 4873582 | 4038278 | 120279 | 24786 |
| Asian/Asian British | 99,253 | 76218 | 23,035 | 21722 | 310 | 281 | 5783735 | 5194104 | 589631 | 505,886 | 19604 | 2008 |
| Black/Black British | 72380 | 55433 | 16,947 | 16254 | 253 | 187 | 3051051 | 2842800 | 208251 | 177,571 | 6792 | 819 |
| Mixed/Other Ethnic Group | 22143 | 16853 | 5290 | 4967 | 38 | 45 | 1235403 | 1146947 | 88456 | 74,036 | 2283 | 163 |
| Social deprivation index (IMD) | ||||||||||||
| IMD = Low (1–3) | 631193 | 517,019 | 114,174 | 107,506 | 1906 | 2088 | 25984682 | 23377311 | 2607371 | 2225455 | 85767 | 28956 |
| IMD = Medium (4–7) | 932921 | 787,675 | 145,246 | 135,226 | 2165 | 2888 | 34658310 | 31625313 | 3032997 | 2530216 | 86104 | 32318 |
| IMD=High (8–10) | 694385 | 602071 | 92314 | 85349 | 1396 | 1970 | 24120895 | 22118571 | 2002324 | 1644819 | 50062 | 19993 |
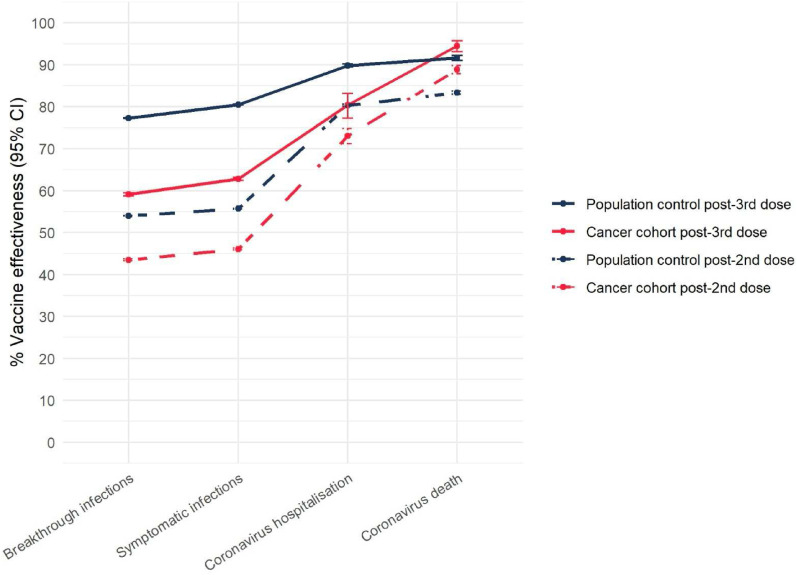
Following receipt of a third dose booster, we evaluated vaccine effectiveness (VE) against breakthrough infection, symptomatic infection, coronavirus hospitalisation and death in the population and cancer cohort. In both cohorts, third dose boosters increased vaccine effectiveness against all primary outcome measures (Supplementary Table 1) (Fig. 1 ).
Fig. 1.
Vaccine effectiveness following third dose coronavirus vaccine booster in the cancer cohort and population control. Error bars represent 95% confidence intervals for vaccine effectiveness.
Within the cancer dataset, we observed that vaccine effectiveness was similar among individuals who had a BNT162b2 third dose booster following a primary vaccination schedule with ChAdOx1 nCov-19 (AZD1222, AstraZeneca), compared to those who had a primary vaccination schedule with BNT162b2 (Supplementary Table 2). The number of individuals who had a third dose booster with mRNA-1273 (Moderna) was not sufficient to confidently report vaccination effectiveness.
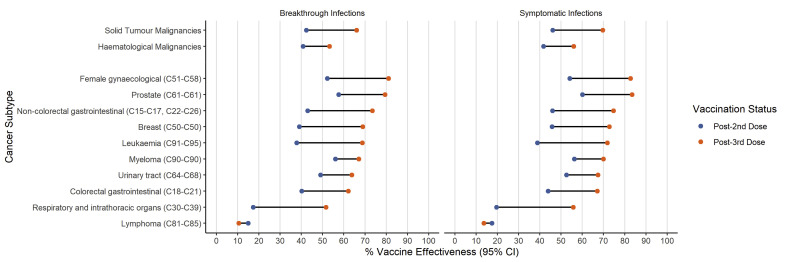
To identify groups that may show differences in vaccine effectiveness, analyses were performed with predefined subgroups measuring breakthrough and symptomatic infections. The number of events following subgrouping was not sufficient to perform analyses for coronavirus hospitalisation or death.
There was evidence of an increase in vaccine effectiveness against breakthrough and symptomatic infections for most cancer subtypes following the third dose booster (Fig. 2 , Supplementary Table 3). Vaccine effectiveness was higher following third dose boosters in solid organ malignancies for breakthrough and symptomatic infections (66.0%, 95% CI: 65.5–66.4 and 69.6%, 95% CI 69.2–70.1 respectively), compared to individuals with haematological malignancies (53.2%, 95% CI: 52.8–53.6 and 56.0%, 95% CI: 55.5–56.4).
Fig. 2.
Subgroup analyses of vaccine effectiveness in cancer subtypes following third dose booster and following primary vaccination course (Post-2nd dose).
For patients with lymphoma, 21,594 PCR results were available following primary dose vaccination, and 9,582 PCR results were available following third dose booster. Low levels of vaccine effectiveness against breakthrough and symptomatic infections were observed in this group (10.5%, 95% CI: 9.9–11.1 and 13.6% 95% CI: 12.9–14.3), which did not improve following a third vaccine dose. Nevertheless, in this lymphoma subgroup, similar to the wider cancer cohort, third dose boosters conferred higher levels of protection against more severe coronavirus outcomes (coronavirus hospitalisation 23.2% 95% CI: −2.4-43.5 and coronavirus death 80.1%, 95% CI: 69.7–87.0).
We also observed that a recent cancer diagnosis or receipt of SACT or radiotherapy within 12 months were associated with lower vaccine effectiveness against breakthrough and symptomatic coronavirus infections (Supplementary Table 3).
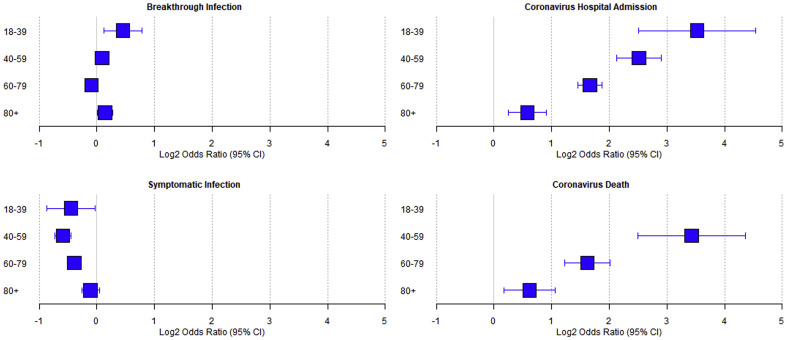
The age distribution differed between the cancer cohort and the population control. To explore the differences in risk for coronavirus outcomes between the general population and patients with cancer, we fitted a multivariable logistic regression model to tests from individuals who had received a third dose booster (Supplementary Table 4). The model was fitted to adjust for the effects of age, sex, levels of deprivation, ethnicity, primary dose manufacturer and booster dose manufacturer. This analysis did not find that the cancer cohort were at increased risk of breakthrough infections (OR 0.99, 95% CI 0.96–1.03, p = 0.77) and were at reduced risk of symptomatic infections (OR 0.73, 95% CI 0.70–0.76, p < 0.00001) compared to the population control. However, following a positive coronavirus test, cancer patients were at an increased risk of hospitalisation (OR 3.38, 95% CI: 3.03–3.77, p < 0.0001) and death (OR 3.01, 95% CI 2.48–3.65, p < 0.0001) (Fig. 3 , Table 2 ) compared to the population control.
Fig. 3.
Forest plot showing the multivariable fitted odds ratio in patients with cancer compared to the population control for coronavirus breakthrough infections, symptomatic infections, coronavirus hospitalisation and death. Data are displayed as a log2 odds ratio.
Table 2.
Multivariable logistic regression models demonstrating the odds ratio in patients with cancer compared to the general population who had received a third dose booster.
| Age, | Breakthrough infections | p | Symptomatic infections | p | Hospitalisation | p | Death | p |
|---|---|---|---|---|---|---|---|---|
| All ages | 0.99 (0.96–1.03) | 0.77 | 0.73 (0.70–0.76) | <0.00001 | 3.38 (3.03–3.77) | <0.00001 | 3.01 (2.48–3.65) | <0.00001 |
| 18–39 | 1.37 (1.09–1.71) | 0.0064 | 0.73 (0.55–0.98) | 0.033 | 11.50 (5.67–23.34) | <0.00001 | 67.15 (5.05–893.14) | 0.0014 |
| 40–59 | 1.06 (0.98–1.15) | 0.15 | 0.66 (0.60–0.73) | <0.00001 | 5.71 (4.36–7.48) | <0.00001 | 10.77 (5.66–20.51) | <0.00001 |
| 60–79 | 0.94 (0.89–0.99) | 0.013 | 0.76 (0.72–0.81) | <0.00001 | 3.17 (2.75–3.66) | <0.00001 | 3.07 (2.33–4.05) | <0.00001 |
| 80+ | 1.10 (1.00–1.21) | 0.043 | 0.93 (0.83–1.03) | 0.16 | 1.49 (1.19–1.87) | 0.00057 | 1.53 (1.12–2.09) | 0.0073 |
4. Discussions
This work has confirmed that the third dose booster programme has increased vaccine effectiveness in most of the cancer population. Overall, this is a reassuring finding that should add impetus for global efforts to maintain vaccine effectiveness in patients with cancer over time. However, our study also demonstrates the benefits of the third dose in patients with cancer are less substantial than the general population, and in some, the benefits are not apparent. While the absolute risk is low, when adjusted for salient parameters including age, following infection, patients with cancer remain at a higher risk of the consequences of infection, specifically hospitalisation and death, despite receipt of a third dose booster. Therefore, while behaviour modification may attenuate the loss of protection from infection, it cannot compensate for the increased risk of complications once infection has occurred.
The benefits of third dose boosters for people with cancer are also heterogeneous, and individuals at higher risk of breakthrough and symptomatic infections include those with haematological malignancies (particularly lymphoma), those with a recent diagnosis and those who received anti-cancer treatments in the last year. For patients with a history of lymphoma, similar to the rest of the cohort, vaccination provided good protection against severe disease and death. This population-scale study replicates immunological studies, such as the UK PROSECO study, that analysed 457 patients with lymphoma who had received two or three vaccine doses. This showed undetectable humoural responses following vaccination in over half of patients on active treatment and 6 in 10 patients on anti-CD20 therapy [33].
Breakthrough infection has the potential to disrupt cancer therapy and adversely affect cancer outcomes. Patients with lymphoma and other haematological malignancies who have mild or asymptomatic COVID-19 infection are likely to have treatment delayed due to concerns about the spread of COVID disease within chemotherapy centres. Additionally, we have previously shown that disease control in itself is important for reducing the risks of COVID-19 mortality in patients with haematological malignancy [34].
The data presented in this study would support supplementary measures in addition to third dose booster to mitigate the risks posed by breakthrough infection to healthcare delivery. Additional pharmaceutical interventions should be recommended to prevent transmission to patients with cancer and additionally help patients to clear viral load more quickly in order to limit treatment disruption to often highly effective cancer therapy [35]. Such evaluations need not be restricted to patients with haematological malignancies, but across all cancer subtypes, as we develop strategies to deliver healthcare within a population with an ongoing risk of COVID-19 infection.
Whilst, this is the largest third dose booster cohort to date, there are limitations to this study. We have included patients recorded as having cancer before 30th April 2021, excluding those diagnosed more recently. This is likely to have resulted in an underestimate of the reduction in vaccine effectiveness seen in patients with cancer, as those recently diagnosed are more likely to have been receiving active treatment but will not have been counted among the positive infection results of the cancer cohort. Second, the underlying variant was not known. However, we have reported on third dose boosters during a period that was driven in the majority of cases with the Delta coronavirus variant (B.1.617) and pre-dates the most recent wave of infection driven by the Omicron variants (B.1.1.529). Finally, the cancer cohort may display differences in behaviour compared to the general population. Patients with cancer and haematological malignancies may be monitored more closely through access to pre-chemotherapy or pre-radiotherapy testing and this may confound our estimates of breakthrough and symptomatic infections.
Placing our findings in the wider context, our analysis has found that third dose boosters induce demonstrable, albeit attenuated, vaccine effectiveness for patients with cancer. Priority should be given to measure and sustain vaccine effectiveness in this group. A pragmatic approach for patients with cancer is to ensure good education about risk in order to maximise their quality of life. Cancer patients, and particularly those with lymphoma, should be encouraged to take additional measures to reduce their risk of infection when community prevalence and transmission are high. This study also supports the prioritisation of further interventions for patients with cancer to maintain the risk-benefit ratio for those on immunosuppressive treatments. Targeted interventions might include immunological studies assessing coronavirus antibody testing or T cell assays as a risk-management test, coronavirus pre-exposure prophylaxis programmes by neutralising antibodies, such as Evusheld, or early post-infection anti-viral treatments. In combination, these measures will help mitigate the inequality of outcomes from SARS-CoV-2 infection, maintain the ability to deliver effective timely anti-cancer treatment and maximise their prognosis and quality of life during the ongoing pandemic.
Author contributions
The following authors were involved in the study design (LYWL, MI, TS, ML, MT, AT, HM, LB, BM, SR, EK, TR, RK, AP, GM, MM, MF, TF, PJ), data acquisition (LYWL, MI, LB, MB, JC, SR, TM, LP, MP, MPa, NR), interpretation (LYWL, MI, TS, PJ), writing of manuscript (all), decision to submit (LYWL, MI, TS, ML, MT, AT, HM, MF, TF, PJ). The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements and Funding
The authors thank our patients, oncologists, physicians and health-care staff working tirelessly on the frontlines of the COVID-19 pandemic.
The authors would like to thank Department of Health and Social Care (DHSC), UK Health Security Agency (UKHSA), University of Oxford, University of Birmingham, University of Southampton and Blood Cancer UK for providing funding and support for this study. The research was supported by the National Institute of Health Research (NIHR) Oxford Biomedical Research Centre (BRC). Lennard Lee is supported by grants from the Academy of Medical Sciences (AMS) and the Government Department of Business, Energy and Industrial Strategy (BEIS). The authors are grateful for Mark Purver (UKHSA) for supporting this work. The authors would also like to acknowledge the work by the NCRI consumer forum for initiating this project. This work uses data provided by patients and collected by the NHS as part of their care and support.
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2022.06.038.
Contributor Information
UK Coronavirus Cancer Programme:
Emma Kinloch, Emily Lam, Gillian Murphy, Malcolm Rhodes, Kate Robinson, Sanskriti Swarup, Keeley Bernhardt, Jola Bytyci, Yuxin Ying, Sukhmunni Johal, and Remarez Sheehan
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lee L.Y.W., Cazier J-B., Angelis V., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee L.Y.W., Cazier J-B., Starkey T., et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. J: Lancet Oncol. 2020;21(10):1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.JCVI statement regarding a COVID-19 booster vaccine programme for winter 2021 to 2022. GOV.UK https://www.gov.uk/government/publications/jcvi-statement-september-2021-covid-19-booster-vaccine-programme-for-winter-2021-to-2022/jcvi-statement-regarding-a-covid-19-booster-vaccine-programme-for-winter-2021-to-2022.
- 5.Third primary COVID-19 vaccine dose for people who are immunosuppressed: JCVI advice. GOV.UK https://www.gov.uk/government/publications/third-primary-covid-19-vaccine-dose-for-people-who-are-immunosuppressed-jcvi-advice.
- 6.Fendler A., Au L., Shepherd S.T.C., et al. Functional antibody and T cell immunity following SARS-CoV-2 infection, including by variants of concern, in patients with cancer: the CAPTURE study. Nat Can (Que) 2021:1–17. doi: 10.1038/s43018-021-00275-9. [DOI] [PubMed] [Google Scholar]
- 7.Monin L., Laing A.G., Muñoz-Ruiz M., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agha M., Blake M., Chilleo C., Wells A., Haidar G. Suboptimal response to COVID-19 mRNA vaccines in hematologic malignancies patients. medRxiv. 2021 doi: 10.1101/2021.04.06.21254949. 04.06.21254949 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrière J., Chamorey E., Adjtoutah Z., et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021;32:1053–1055. doi: 10.1016/j.annonc.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madelon N., Lauper K., Breville G., et al. Robust T cell responses in anti-CD20 treated patients following COVID-19 vaccination: a prospective cohort study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021:ciab954. doi: 10.1093/cid/ciab954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Oekelen O., Gleason C.R., Agte S., et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39:1028–1030. doi: 10.1016/j.ccell.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer J., Le N-S., Mattes D., et al. Evaluation of antibody responses to COVID-19 vaccines among solid tumor and hematologic patients. Cancers. 2021;13:4312. doi: 10.3390/cancers13174312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavriatopoulou M., Terpos E., Kastritis E., et al. Low neutralizing antibody responses in WM, CLL and NHL patients after the first dose of the BNT162b2 and AZD1222 vaccine. Clin Exp Med. 2021 doi: 10.1007/s10238-021-00746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim S.H., Campbell N., Johnson M., et al. Antibody responses after SARS-CoV-2 vaccination in patients with lymphoma. Lancet Haematol. 2021;8:e542–e544. doi: 10.1016/S2352-3026(21)00199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.PHE: Duration of protection of COVID-19 vaccines against clinical disease, 9 September 2021. GOV.UK https://www.gov.uk/government/publications/phe-duration-of-protection-of-covid-19-vaccines-against-clinical-disease-9-september-2021.
- 16.Wu J.T.-Y., La J., Branch-Elliman W., et al. Association of COVID-19 vaccination with SARS-CoV-2 infection in patients with cancer: a us nationwide veterans affairs study. JAMA Oncol. 2022;8(2):281–286. doi: 10.1001/jamaoncol.2021.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song Q., Bates B., Shao Y.R., et al. Risk and outcome of breakthrough COVID-19 infections in vaccinated patients with cancer: real-world evidence from the national COVID cohort collaborative. J. Clin. Oncol. JCO. 2022;21 doi: 10.1200/JCO.21.02419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.reportICNARC report on COVID-19 in cri cal care: England, Wales and Northern Ireland 4 March 2022.
- 19.Deaths involving COVID-19, England and wales - Office for national Statistics. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsinvolvingcovid19englandandwales/deathsoccurringinmarch2020#characteristics-of-those-dying-from-covid-19.
- 20.Bagacean C., Letestu R., Al-Nawakil C., et al. Humoral response to mRNA anti–COVID-19 vaccines BNT162b2 and mRNA-1273 in patients with chronic lymphocytic leukemia. Blood Adv. 2022;6:207–211. doi: 10.1182/bloodadvances.2021006215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ligumsky H., Dor H., Etan T., et al. Immunogenicity and safety of BNT162b2 mRNA vaccine booster in actively treated patients with cancer. Lancet Oncol. 2022;23(2):193–195. doi: 10.1016/S1470-2045(21)00715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro L.C., Thakkar A., Campbell S.T., et al. Efficacy of booster doses in augmenting waning immune responses to COVID-19 vaccine in patients with cancer. Cancer Cell. 2022;40(1):3–5. doi: 10.1016/j.ccell.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fendler A., Shepherd S.T.C., Au L., et al. Immune responses following third COVID-19 vaccination are reduced in patients with hematological malignancies compared to patients with solid cancer. Cancer Cell. 2022;40:114–116. doi: 10.1016/j.ccell.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prendecki M., Thomson T., Clarke C.L., et al. Immunological responses to SARS-CoV-2 vaccines in kidney transplant recipients. Lancet. 2021;398:1482–1484. doi: 10.1016/S0140-6736(21)02096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinato D.J., Aguilar-Company J., Ferrante D., et al. Outcomes of the SARS-CoV-2 omicron (B.1.1.529) variant outbreak among vaccinated and unvaccinated patients with cancer in Europe: results from the retrospective, multicentre, OnCovid registry study. Lancet Oncol. 2022;23(7):865–875. doi: 10.1016/S1470-2045(22)00273-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Update on Omicron. https://www.who.int/news/item/28-11-2021-update-on-omicron.
- 27.COVID-19: Omicron daily overview. GOV.UK https://www.gov.uk/government/publications/covid-19-omicron-daily-overview.
- 28.Lee L.Y.W., Starkey T., Ionescu M.C., et al. Vaccine effectiveness against COVID-19 breakthrough infections in patients with cancer (UKCCEP): a population-based test-negative case-control study. J Lancet Oncol. 2022;23(6):748–757. doi: 10.1016/S1470-2045(22)00202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews N., Stowe J., Kirsebom F., et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022;28(4th ed.):831–837. doi: 10.1038/s41591-022-01699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez Bernal J., Andrews N., Gower C., et al. Effectiveness of covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez Bernal J., Andrews N., Gower C., et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.English indices of deprivation 2019. GOV.UK https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019.
- 33.Lim S.H., Stuart B., Joseph-Pietras D., et al. Immune responses against SARS-CoV-2 variants after two and three doses of vaccine in B-cell malignancies: UK PROSECO study. Nat Can (Que) 2022;3:552–564. doi: 10.1038/s43018-022-00364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Booth S., Curley H.M., Varnai C., et al. Key findings from the UKCCMP cohort of 877 patients with haematological malignancy and COVID-19: disease control as an important factor relative to recent chemotherapy or anti-CD20 therapy. Br J Haematol. 2022;196:892–901. doi: 10.1111/bjh.17937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rockett R., Basile K., Maddocks S., et al. Resistance mutations in SARS-CoV-2 Delta variant after sotrovimab use. N Engl J Med. 2022;386:1477–1479. doi: 10.1056/NEJMc2120219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.