Abstract
Background
Whether obesity and being overweight, defined by body mass index (BMI), increase hepatocellular carcinoma (HCC) has been less apparent in Asian populations.
Methods
Overall, 14,265,822 Korean adults who underwent routine health examinations during 2003–2006 were followed up for HCC. Multivariable-adjusted hazard ratios (HRs) associated with BMI were calculated.
Results
During 13.7 years (mean) of follow-up, 47,308 individuals developed HCC. HRs of HCC associated with BMIs of 25.0–26.4, 26.5–27.9, 28.0–29.4, 29.5–30.9 and ≥31 kg/m² compared to those for 23.5–24.9 kg/m² were 1.05, 1.20, 1.39, 1.59 and 2.13, respectively. For BMI < 25 kg/m², linear associations were not apparent. For BMI ≥ 25 kg/m2, the HR per 5 kg/m2 increase in BMI was 1.60 (total), 1.60 (men), and 1.59 (women). The corresponding HRs were 1.56, 1.61 and 1.60 for individuals aged <45, 45–64 and ≥65 years, respectively. Further adjustment for alanine transaminase (ALT) levels substantially reduced the HRs for high BMI, especially in men and younger adults.
Conclusions
Overweight and obesity clearly increase HCC risk in Koreans. ALT levels are a mediator of the impact of obesity, but it may not accurately predict high BMI-induced liver damage that can potentially progress to HCC, especially in women and older adults.
Subject terms: Liver cancer, Liver cancer
Background
Hepatocellular carcinoma (HCC) is the dominant histologic type and accounts for ~80% of primary liver cancer (PLC) [1], which constituted 4.7% of cancer incidence and 8.3% of cancer deaths globally in 2020 [2]. Obesity has been associated with higher HCC/PLC incidence and mortality, as reported in meta-analyses and several, but not all [3, 4], large-scale prospective cohort studies [5–8]. Obesity has been well-accepted as a risk factor for HCC. However, some uncertainties remain in this regard. It is unclear whether lower adiposity, defined by body mass index (BMI), lowers the risk of HCC. Moreover, many large-scale prospective studies have not shown any clear evidence on the association between overweight and the risk of HCC in the general population [3–6, 9, 10].
Among Asian populations, the association of obesity with HCC incidence per se has been less apparent [3–5, 11]. In a recent prospective study of 2744 HCC cases among more than 0.5 million Korean adults, obesity was not associated with an increased risk of HCC [4]. In populations with a substantially slim body shape, wherein the prevalence of obesity is relatively low and generally <10%, as seen among Koreans [12], the associations might be different from those seen in populations with a higher obesity prevalence [13]. The fact that hepatitis B virus (HBV) infection is the primary cause of liver cirrhosis and HCC in Asians [14] was suggested to explain potentially weaker associations [3, 5, 11]. However, there has been little conclusive evidence on this topic.
Through a population-based prospective cohort study of more than 14 million Korean participants, we aimed to investigate the association between BMI, specifically obesity and overweight, and the risk of HCC. We further examined whether the associations differ by sex and age [5, 8, 9]. In addition, we explored the effects of adjustment for mediators of the potential causal pathway linking high BMIs to HCC, such as diabetes, cholesterol, and liver enzymes, because many published studies have included these variables [15–17].
Methods
Study population and follow-up
We conducted a population-based prospective cohort study. Ninety-seven percent of Koreans were insured through the National Health Insurance Service (NHIS) [18]. Korean adults aged 18–99 years who underwent a health examination between 2003 and 2006 were enrolled in the study cohort (n = 15,163,545). From this sample, 122,451 patients with pre-existing cancer were excluded, as were patients (n = 13,476) with missing information on alanine transaminase (ALT), aspartate transaminase (AST), and cardiometabolic factors including fasting glucose levels and BMI; those (n = 1067) with a missing or incorrect health examination date; and those (n = 760,729) with prevalent liver diseases (Supplementary Fig. S1). We excluded individuals with liver diseases, as liver disease status may be an effect modifier [4]. We conducted a follow-up of the remaining 14,265,822 individuals until December 31, 2018, through record linkage to hospital discharge records from the NHIS, in which certified health information managers reviewed the medical records and assigned standardised diagnosis codes. The completeness of the cancer incidence data from the NHIS is comparable to that of the Korea National Cancer Incidence Database [19, 20]. All patients discharged from the hospital due to HCC (the International Classification of Diseases 10th revision: C220) for the first time were considered incident cases.
The authors were granted access to anonymized data by the NHIS. This study was approved by the Institutional Review Board of Catholic Kwandong University, Republic of Korea. The need for informed consent was waived because the data used were anonymized, constructed, and provided by the NHIS according to a strict confidentiality protocol.
Data collection
Data were collected during baseline health examinations conducted from 2003 to 2006 through measurements and a questionnaire. ALT and AST levels were measured using the NADH-UV or Reitman–Frankel methods. Fasting serum glucose and total cholesterol (TC) levels were assayed using enzymatic methods [21]. Blood pressure was measured using a standard mercury sphygmomanometer. BMI was calculated as the measured weight (kg) divided by the square of the measured height (m2) [18]. Smoking status, alcohol use and history of cancer and cardiovascular diseases were assessed using a questionnaire. Patients with a self-reported history of cancer or those who were admitted to a medical institution for cancer before the baseline health examination were considered to have pre-existing cancer. The health examinations and data collection followed a standard protocol documented by the government. The data collection methods for smoking and alcohol use were similar to those used in our previous study [4]. We considered individuals to have prevalent diabetes (E10-E14) or liver diseases (B15-B19, K70-K77) at baseline if they had visited a medical institution for the diseases at least once within 12 months before, or two months after the baseline health examination date.
Statistical analysis
BMI was categorised into 11 groups (kg/m2; <17.5, 17.5–18.9 to 29.5–30.9 by 1.5, ≥31.0, with 23.5–24.9 as reference) and seven groups (<18.5, 18.5–20.9, 21–22.9, 23–24.9 [reference], 25–27.4, 27.5–29.9 and ≥30 kg/m2) [18]. BMI was also analysed as a continuous variable (per 5 kg/m2 increase), assuming a linear association in the full, lower (<25 kg/m2), and upper (≥25 kg/m2) ranges.
Hazard ratios (HRs) for HCC incidence were obtained using Cox proportional hazards models stratified by age (years) at baseline (18–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84 and 85–99 years, using the STRATA statement). Multivariable analysis was adjusted for age at baseline (continuous variable), sex, smoking status (never, former or current smoking [<10, 10–19 or ≥20 cigarettes/day]), alcohol use (none, <10, 10–19, 20–39, ≥40 g ethanol use/day, and missing information), physical activity (exercise with light sweating, none, 1–2 times/week and 3–7 times/week), and income status (quartiles; 1 [low income], 2, 3, 4 [high income]). Mediators of the effect of BMI, such as diabetes status (normoglycemia [fasting glucose <100 mg/dL], impaired fasting glucose [100–125 mg/dL], and diabetes [≥126 mg/dL or prevalent diabetes]), TC (continuous variable) [22], and ALT (natural log-transformed levels), were further adjusted for in sensitivity analyses. In addition, an analysis stratified by sex (male and female) and age (<45, 45–64 and ≥65 years old) was performed. Differences in effect size between sexes and age groups were evaluated using Cochrane Q statistics as the interaction test. The proportional hazards assumption was tested using Schoenfeld residuals. All P values were two-sided.
Results
Baseline characteristics
During 13.7 years (mean) of follow-up, a total of 47,308 individuals were diagnosed with HCC. At baseline, mean (±SD) BMI was 23.5 ± 3.2 kg/m2 and mean patient age was 45.0 ± 14.5 years. The BMI range with the highest proportion of participants was 22–22.9 kg/m2 in women and 23–23.9 kg/m2 in men (Supplementary Fig. S2). The group with the lowest BMI comprised the youngest individuals and had the highest proportion of women and never smokers. Higher BMI groups were associated with heavy alcohol use and higher levels of fasting glucose, TC and ALT (Table 1).
Table 1.
Participants’ characteristics at baseline according to seven BMI categories.
| Variables | Characteristics | BMI <18.5 kg/m2 | 18.5–20.9 kg/m2 | 21–22.9 kg/m2 | 23–24.9 kg/m2 | 25–27.4 kg/m2 | 27.5–29.9 kg/m2 | ≥30 kg/m2 | |
|---|---|---|---|---|---|---|---|---|---|
| n = 14,265,822 | n = 616,199 | n = 2,548,372 | n = 3,318,420 | n = 3,498,272 | n = 2,830,851 | n = 1,047,337 | n = 406,371 | ||
| Age | years | 45.0 ± 14.5 | 38.5 ± 17.2 | 40.5 ± 15.2 | 44.6 ± 14.3 | 46.8 ± 13.6 | 47.7 ± 13.3 | 47.8 ± 13.5 | 46.0 ± 14.2 |
| BMI | kg/m2 | 23.5 ± 3.2 | 17.6 ± 0.8 | 19.9 ± 0.7 | 22.0 ± 0.6 | 24.0 ± 0.6 | 26.1 ± 0.7 | 28.5 ± 0.7 | 31.9 ± 2.0 |
| Total cholesterola | mg/dL | 193.0 ± 37.3 | 174.2 ± 32.0 | 180.3 ± 33.7 | 189.2 ± 35.8 | 196.5 ± 36.9 | 201.9 ± 37.6 | 205.8 ± 38.2 | 208.2 ± 39.0 |
| Fasting glucoseb | mg/dL | 94.8 ± 26.0 | 89.3 ± 22.6 | 90.4 ± 22.1 | 93.1 ± 24.5 | 95.6 ± 26.3 | 97.9 ± 27.8 | 100.0 ± 29.6 | 102.2 ± 32.3 |
| SBP | mmHg | 123.8 ± 17.0 | 114.8 ± 15.5 | 117.5 ± 15.5 | 121.5 ± 16.2 | 125.1 ± 16.5 | 128.3 ± 16.6 | 131.3 ± 17.0 | 134.2 ± 17.4 |
| AST | IU/L | 24.9 ± 17.0 | 22.5 ± 17.5 | 22.7 ± 16.2 | 23.7 ± 15.7 | 24.9 ± 16.5 | 26.6 ± 17.5 | 28.4 ± 19.5 | 31.0 ± 21.0 |
| ALT | IU/L | 24.4 ± 21.0 | 16.8 ± 15.8 | 18.3 ± 16.7 | 21.2 ± 17.5 | 24.7 ± 19.4 | 29.1 ± 22.0 | 33.6 ± 27.4 | 39.6 ± 35.9 |
| Loge(ALT) | IU/L | 3.0 ± 0.5 | 2.7 ± 0.5 | 2.8 ± 0.5 | 2.9 ± 0.5 | 3.1 ± 0.5 | 3.2 ± 0.5 | 3.3 ± 0.6 | 3.5 ± 0.6 |
| Age, years | 18–44 | 7,429,014 (52.1) | 445,503 (72.3) | 1,697,452 (66.6) | 1,793,804 (54.1) | 1,612,704 (46.1) | 1,229,274 (43.4) | 451,985 (43.2) | 198,292 (48.8) |
| 45–64 | 5,277,607 (37.0) | 92,625 (15.0) | 606,049 (23.8) | 1,177,442 (35.5) | 1,497,735 (42.8) | 1,273,833 (45.0) | 467,623 (44.6) | 162,300 (39.9) | |
| ≥65 | 1,559,201 (10.9) | 78,071 (12.7) | 244,871 (9.6) | 347,174 (10.5) | 387,833 (11.1) | 327,744 (11.6) | 127,729 (12.2) | 45,779 (11.3) | |
| Sex | Women | 6,570,282 (46.1) | 410,865 (66.7) | 1,460,118 (57.3) | 1,583,448 (47.7) | 1,436,420 (41.1) | 1,057,268 (37.3) | 430,082 (41.1) | 192,081 (47.3) |
| Diabetes status | Normoglycemia | 10,374,717 (72.7) | 517,579 (84.0) | 2,081,439 (81.7) | 2,533,093 (76.3) | 2,484,643 (71.0) | 1,872,803 (66.2) | 647,811 (61.9) | 237,349 (58.4) |
| IFG | 2,755,852 (19.3) | 75,951 (12.3) | 359,808 (14.1) | 575,514 (17.3) | 716,212 (20.5) | 657,729 (23.2) | 264,351 (25.2) | 106,287 (26.2) | |
| Diabetes | 1,135,253 (8.0) | 22,669 (3.7) | 107,125 (4.2) | 209,813 (6.3) | 297,417 (8.5) | 300,319 (10.6) | 135,175 (12.9) | 62,735 (15.4) | |
| Smoking status | Never smoker | 8,992,962 (63.0) | 436,503 (70.8) | 1,708,289 (67.0) | 2,118,868 (63.9) | 2,152,234 (61.5) | 1,685,461 (59.5) | 638,260 (60.9) | 253,347 (62.3) |
| Past smoker | 1,181,594 (8.3) | 28,537 (4.6) | 146,194 (5.7) | 253,463 (7.6) | 329,529 (9.4) | 293,265 (10.4) | 98,987 (9.5) | 31,619 (7.8) | |
| <0.5 pack/day | 874,831 (6.1) | 42,360 (6.9) | 165,727 (6.5) | 212,107 (6.4) | 214,894 (6.1) | 165,193 (5.8) | 54,512 (5.2) | 20,038 (4.9) | |
| 0.5–0.9 pack/day | 2,068,329 (14.5) | 71,293 (11.6) | 354,033 (13.9) | 487,114 (14.7) | 519,354 (14.8) | 428,272 (15.1) | 150,728 (14.4) | 57,535 (14.2) | |
| 1–1.9 pack/day | 784,352 (5.5) | 18,804 (3.1) | 104,339 (4.1) | 162,962 (4.9) | 196,247 (5.6) | 189,542 (6.7) | 78,787 (7.5) | 33,671 (8.3) | |
| ≥2 pack/day | 338,681 (2.4) | 17,671 (2.9) | 65,341 (2.6) | 78,069 (2.4) | 79,947 (2.3) | 63,951 (2.3) | 24,280 (2.3) | 9,422 (2.3) | |
| Missing | 25,073 (0.2) | 1,031 (0.2) | 4,449 (0.2) | 5,837 (0.2) | 6,067 (0.2) | 5,167 (0.2) | 1,783 (0.2) | 739 (0.2) | |
| Alcohol use, g ethanol/day | None | 7,237,398 (50.7) | 345,454 (56.1) | 1,338,897 (52.5) | 1,703,360 (51.3) | 1,740,510 (49.8) | 1,370,380 (48.4) | 525,950 (50.2) | 212,847 (52.4) |
| <10 | 3,521,061 (24.7) | 172,903 (28.1) | 707,339 (27.8) | 851,111 (25.6) | 848,960 (24.3) | 643,245 (22.7) | 217,215 (20.7) | 80,288 (19.8) | |
| 10–19 | 2,000,611 (14.0) | 56,868 (9.2) | 294,808 (11.6) | 444,003 (13.4) | 522,015 (14.9) | 458,036 (16.2) | 164,480 (15.7) | 60,401 (14.9) | |
| 20–39 | 600,340 (4.2) | 12,885 (2.1) | 74,587 (2.9) | 124,652 (3.8) | 158,213 (4.5) | 150,457 (5.3) | 57,793 (5.5) | 21,753 (5.4) | |
| ≥40 | 594,739 (4.2) | 14,006 (2.3) | 76,842 (3.0) | 123,192 (3.7) | 152,665 (4.4) | 147,020 (5.2) | 58,824 (5.6) | 22,190 (5.5) | |
| Missing | 311,673 (2.2) | 14,083 (2.3) | 55,899 (2.2) | 72,102 (2.2) | 75,909 (2.2) | 61,713 (2.2) | 23,075 (2.2) | 8,892 (2.2) | |
| Physical activity, time/week | None | 8,151,093 (57.1) | 458,019 (74.3) | 1,638,989 (64.3) | 1,893,757 (57.1) | 1,876,721 (53.6) | 1,489,656 (52.6) | 566,222 (54.1) | 227,729 (56.0) |
| 1–2 | 3,616,156 (25.3) | 108,760 (17.7) | 577,655 (22.7) | 841,786 (25.4) | 929,838 (26.6) | 770,950 (27.2) | 280,217 (26.8) | 106,950 (26.3) | |
| ≥3 | 2,498,573 (17.5) | 49,420 (8.0) | 331,728 (13.0) | 582,877 (17.6) | 691,713 (19.8) | 570,245 (20.1) | 200,898 (19.2) | 71,692 (17.6) | |
| Income status, quartile | Q1 (low) | 3,660,816 (25.7) | 205,152 (33.3) | 767,330 (30.1) | 874,558 (26.4) | 825,402 (23.6) | 635,462 (22.4) | 245,104 (23.4) | 107,808 (26.5) |
| Q2 | 3,227,728 (22.6) | 182,415 (29.6) | 673,407 (26.4) | 753,353 (22.7) | 725,852 (20.7) | 576,308 (20.4) | 221,384 (21.1) | 95,009 (23.4) | |
| Q3 | 3,564,114 (25.0) | 135,245 (21.9) | 594,671 (23.3) | 812,962 (24.5) | 891,993 (25.5) | 745,568 (26.3) | 278,641 (26.6) | 105,034 (25.8) | |
| Q4 | 3,813,164 (26.7) | 93,387 (15.2) | 512,964 (20.1) | 877,547 (26.4) | 1,055,025 (30.2) | 873,513 (30.9) | 302,208 (28.9) | 98,520 (24.2) |
Data are expressed as mean ± SD or n (%).
ALT alanine aminotransferase, ANOVA analysis of variance, AST aspartate aminotransferase, BMI body mass index, IFG impaired fasting glucose, SBP systolic blood pressure, SD standard deviation.
The P values, which were calculated using the chi-square test and one-way ANOVA across the BMI groups, were <0.001 for each variable.
aTo convert cholesterol from mg/dL to mmol/L, multiplied by 0.02586.
bTo convert glucose from mg/dL to mmol/L, multiply by 0.0555.
Categorical analyses
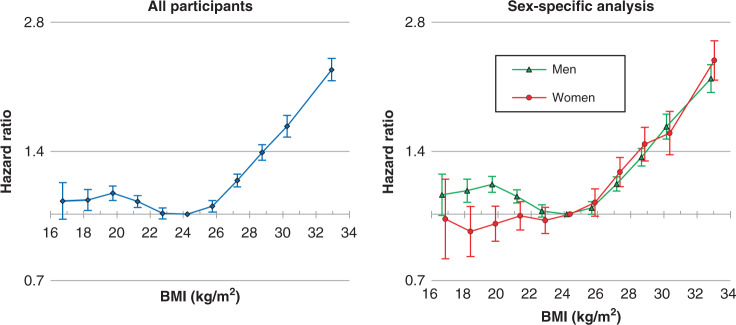
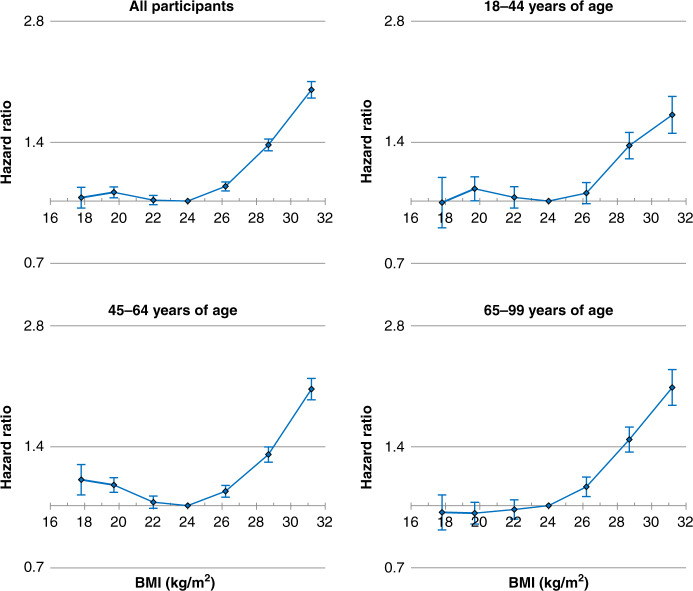
BMI had a non-linear association with HCC: BMI was positively associated with the risk of HCC in the BMI range of ≥25 kg/m² but not in the BMI range of <25 kg/m2 (Figs. 1 and 2, Supplementary Figs. S3 and S4 and Supplementary Tables S1 and S2). Compared to the BMI range 23.5–24.9 kg/m2, multivariable-adjusted HRs of HCC associated with BMI ranges of 25.0–26.4, 26.5–27.9, 28.0–29.4, 29.5–30.9 and ≥31 kg/m² were 1.05, 1.20, 1.39, 1.59 and 2.13, respectively (Supplementary Table S1, P < 0.001, except for the BMI range 25.0–26.4 [P = 0.003]). The corresponding HRs associated with BMI ranges of 31.0–32.9, 33.0–34.9 and ≥35 kg/m² were 1.98 (95% confidence interval: 1.84–2.13), 2.36 (2.08–2.67) and 2.73 (2.33–3.20), respectively (Supplementary Fig. S4). Linear associations between BMI and HCC were not apparent in the BMI range of <25 kg/m². In general, the HRs associated with BMI < 25 kg/m² were not substantially different from unity. In the sex- and age-specific analyses, the association was similar between sexes and age groups in the BMI range of ≥25 kg/m² (Figs. 1, 2 and Supplementary Fig. S3).
Fig. 1. Age- and sex-adjusted HRs across 11 categories of BMI for HCC incidence.
BMI categories (kg/m2; <17.5, 17.5–18.9 to 29.5–30.9 by 1.5, ≥31.0, with 23.5–24.9 as reference). The midpoint was used as a representative value of each BMI category, except for both ends (16.7 and 32.9) for which the median was used. HRs and 95% confidence intervals were calculated using Cox proportional hazards models with adjustment for sex (for all participants only) and age at baseline as a continuous variable. BMI body mass index, HR hazard ratio, HCC hepatocellular carcinoma.
Fig. 2. HRs across seven categories of BMI for HCC incidence according to age.
BMI categories (kg/m2; <18.5, 18.5–20.9, 21–22.9, 23–24.9 [reference], 25–27.4, 27.5–29.9, and ≥30). The midpoint was used as a representative value of each BMI category, except for both ends (17.6 and 31.9), for which the median was used. HRs and 95% confidence intervals were calculated using Cox proportional hazards models with adjustment for sex (for all participants only), age at baseline, household income, smoking status, alcohol use, and physical activity. BMI body mass index, HCC hepatocellular carcinoma, HR hazard ratio.
Linear analyses
Assuming linear associations, in the BMI range of ≥25 kg/m2, the multivariable-adjusted HR for each 5-kg/m2 increase in BMI was 1.60 for all participants, 1.60 for men, and 1.59 for women (P for interaction between sexes = 0.853; Table 2). The corresponding HRs were 1.56, 1.61 and 1.60 for individuals aged <45, 45–64 and ≥65 years, respectively. In the BMI range of <25 kg/m2, the results of linear analyses were in accordance with those of the categorical analyses; BMI was not positively associated with the risk of HCC. The multivariable-adjusted HR per 5-kg/m2 increase in BMI was 0.96 for all participants, 0.93 for men and 1.07 for women (Supplementary Table S3).
Table 2.
HRs per 5 kg/m2 increase in BMI for HCC incidence by age and sex in the BMI range of ≥25 kg/m2.
| Age group, | BMI ≥ 25 kg/m2 (n = 4,284,559) | |||||
|---|---|---|---|---|---|---|
| Sex group | Years | No. of HCC | P value | HR (95% CI) | Pinteraction (age) | Pinteraction (sex) |
| All participants | 18–99 | 17,992 | <0.001 | 1.60 (1.55–1.66) | ||
| 18–44 | 3384 | <0.001 | 1.56 (1.45–1.68) | 0.610 | ||
| 45–64 | 10,747 | <0.001 | 1.61 (1.54–1.68) | |||
| 65–99 | 3861 | <0.001 | 1.64 (1.52–1.76) | |||
| Men | 18–99 | 14,212 | <0.001 | 1.60 (1.54–1.67) | 0.853 | |
| 18–44 | 3166 | <0.001 | 1.59 (1.47–1.72) | 0.485 | 0.085 | |
| 45–64 | 8603 | <0.001 | 1.59 (1.51–1.68) | 0.591 | ||
| 65–99 | 2443 | <0.001 | 1.70 (1.54–1.88) | 0.322 | ||
| Women | 18–99 | 3780 | <0.001 | 1.59 (1.50–1.70) | ||
| 18–44 | 218 | 0.118 | 1.24 (0.95–1.63) | 0.166 | ||
| 45–64 | 2144 | <0.001 | 1.63 (1.51–1.77) | |||
| 65–99 | 1418 | <0.001 | 1.58 (1.42–1.76) | |||
BMI body mass index, CI confidence interval, HCC hepatocellular carcinoma, HR hazard ratio.
HRs were calculated using Cox models after adjusting for age at baseline, sex, household income, smoking status, alcohol use and physical activity.
Sensitivity analyses
In the spline analysis, the associations of BMI with HCC risk were generally similar to those observed in the categorical analysis, and non-linear associations were statistically confirmed for the overall analyses and each subgroup analysis (Supplementary Figs. S5 andS6, P for non-linearity <0.001 for each analysis).
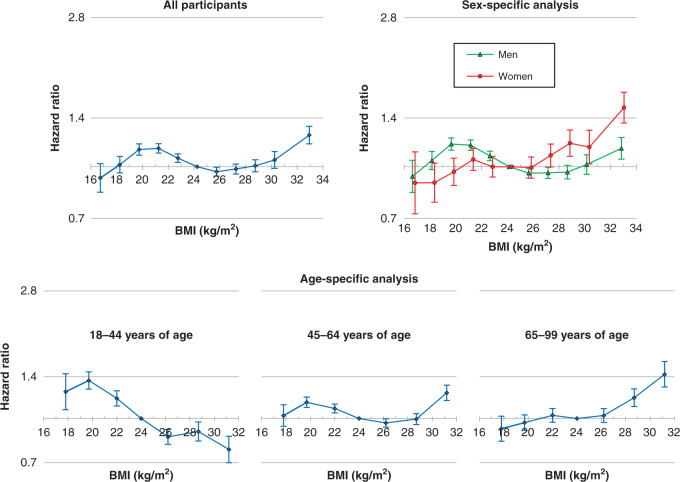
The overall shape of the associations of BMI with HCC was somewhat different between statistical models in which different variables, including confounders and mediators, were adjusted for (Fig. 3 and Supplementary Fig. S7). In particular, adjustment for ALT levels dramatically changed the overall associations. Higher risks of HCC associated with higher BMI were found to be substantially weakened, especially in men and younger adults (Fig. 3 and Supplementary Fig. S8), whereas high BMI-related HCC risks were found to be less attenuated in women and older adults.
Fig. 3. HRs across BMI categories for HCC incidence after further adjustment for mediators (sensitivity analysis).
Eleven categories, as shown in Fig. 1, for overall and sex-specific analyses and seven categories, as shown in Fig. 2, for age-specific analysis were used. HRs and 95% confidence intervals were calculated using Cox proportional hazards models with adjustment for sex (for all participants only), age at baseline, household income, smoking status, alcohol use, physical activity, diabetes status, total cholesterol level and log-transformed alanine transaminase level. BMI body mass index, HCC hepatocellular carcinoma, HR hazard ratio.
Discussion
In this study, BMI had a non-linear association with HCC risk; in the BMI range of ≥25 kg/m2, a higher BMI increased the risk of HCC, regardless of sex and age, while in the BMI range of <25 kg/m2, a lower BMI was generally not associated with decreased risk. Overweight participants, even those with a BMI of 26.5–27.9 kg/m2, were clearly at a higher risk of HCC. In individuals with BMI ≥ 25 kg/m2, the impact of higher BMI on HCC was generally similar between men and women and between the early adulthood (18–44 years), middle-aged (45–64 years), and older (≥65 years) populations. The effect of higher BMI on HCC was materially mediated through high ALT levels, especially in men and younger adults, but less in women and older adults.
Our study showed that the BMI range of ≥25 kg/m2 had positive graded associations with HCC. In previous studies on general or low-risk populations, the association of overweight status with HCC has been unclear [3–5, 9, 10, 16, 23–27]. Furthermore, the effects of obesity on HCC (or PLC) have also been less clear in Asian populations [3–5, 11, 16, 28, 29]. This study included 14.3 million Korean adults and provides compelling evidence that overweight status, even in those with a BMI range of 26.5–27.9 kg/m2, and obesity, increased the risk of HCC in the general population or low-risk population that had a low prevalence of obesity (2.8%).
Obesity was associated with an 89% increased risk of HCC in our study (Supplementary Table S3). Previous systematic reviews of prospective studies reported 83% [9] and 96% [5] increased risks of HCC/PLC associated with obesity. Despite the lower prevalence of obesity (2.8%), the magnitude of the effect size in our study was comparable to those of previous meta-analyses [5, 7, 9] and large-scale population-based cohort studies in Western populations [30], and it was substantially higher than that of studies conducted in Asian populations [3–5, 9–11]. Potential effect modification of prevalent liver diseases, such as stronger associations of higher BMI with HCC in individuals without liver diseases [18, 31, 32], may explain the stronger association of obesity with HCC in the present study than in previous studies among Asians. Our study excluded individuals with prevalent liver diseases. However, many previous studies on Asians, particularly the general population, included a substantial number of participants with liver diseases such as viral hepatitis and cirrhosis, owing to the high prevalence of viral hepatitis [4, 27], which may have contributed to the materially lower effect size of higher BMI. Adjustment for viral hepatitis and cirrhosis may not eliminate the potential effect modification of these liver diseases [4], and the exclusion of prevalent liver diseases may better capture the effect of higher BMI on HCC risk in low-risk populations.
In this study, men and women showed similar associations between high BMI and HCC. Previous meta-analyses and large-scale population-based cohort studies reported stronger associations of obesity in men than in women [5, 8, 9, 30]. A large-scale UK cohort study reported that the stronger association of obesity with HCC in men than in women was prominent in the BMI range of >35 kg/m2 [30]. The present study included only 0.2% of individuals with the BMI range of ≥35 kg/m2 which may partly explain the similar association in men and women. Early adulthood (18–44 years), middle-aged (45–64 years), and old age (≥65 years) populations showed generally similar associations, which were in line with the findings of a large-scale UK study [30].
For the BMI range of <25 kg/m2, BMI increment was not associated with a higher HCC risk, especially in men. These results are in accordance with those of previous studies with >1000 HCC/PLC cases [4, 10, 25, 30, 32]. Overall, our study suggests that in individuals with normal weight and underweight, a lower BMI may not guarantee a lower risk of HCC.
A higher BMI was associated with higher levels of fasting glucose, TC, and ALT. These potential mediators of the effect of high BMIs were adjusted for in the sensitivity analyses (Supplementary Figs. S7 andS8). Adjustment for diabetes status modestly weakened the association between higher BMI and HCC risk. After additional adjustments for TC, similar associations were found in the main analysis. However, after further adjustment for log-transformed ALT levels, the impact of high BMI on HCC was found to be substantially weakened, especially in men and particularly in youngest adults. The hypothesis that the potential mechanisms by which higher BMI causes HCC to involve the increment of ALT is not new because obesity could cause HCC through obesity-induced liver damage such as non-alcoholic fatty liver disease (NAFLD). However, the finding that the impact of high BMI was mediated by ALT levels to a lesser extent in women and older adults than in men and younger adults, despite a similar magnitude of effect on HCC between sexes and age groups, is novel. This may partly explain the ALT levels observed in NAFLD patients according to sex and age. In NAFLD patients, a higher number of women had normal ALT values than men [33], and ALT values progressively declined with advancing age for both with and without advanced fibrosis [34]. These results suggest that although progressive liver damage may be a key component of the causal pathway linking a high BMI to HCC, liver damage associated with high BMI may not entirely result in high ALT levels, especially in women and older adults. High BMI-induced liver damage can progress to HCC. However, our results imply that ALT levels, especially when measured only once, may not accurately predict such damage, particularly in women and older adults.
To the best of our knowledge, this study included the largest number of HCC cases in the studies on the association of adiposity with HCC in the general population, which enabled high statistical power, the use of detailed BMI categories, and analyses of various subgroups, which is a strength of the study. Careful consideration of the most important risk factors and mediators, including diabetes, alcohol use, lipids, and ALT level, is another strength. As a prospective cohort study, recall and selection biases related to the retrospective design were minimised. Nearly complete follow-up through record linkage to a national database was another strength of this study.
However, our study had several limitations. As BMI was the only measure of obesity in our study, the role of abdominal obesity or fat distribution, which may further provide mechanistic insight into the association between obesity and HCC, was not examined. The study population was homogeneous and comprised only Koreans. Because of the very low proportion of individuals with a BMI of ≥35 kg/m2 in the Korean population, obesity in the current study mainly represents grade I obesity [18], and the estimated risks associated with obesity probably underestimate the effect of obesity in other populations that have substantially more individuals with grade II obesity or above. Despite the exclusion of individuals with prevalent liver diseases, individuals with liver diseases, including chronic HBV infection, who were not diagnosed within 1 year before, and 2 months after the health examination date were included in the study. This may have induced further underestimation of the impact of higher BMI. Thus, the exact effect size in the association between BMI and HCC may differ by region and ethnicity owing to the varying distribution of HCC aetiology. However, the effect size associated with high BMI on HCC risk was consistent across sexes and age groups, which can confer varying levels of HCC risk and aetiology. These results enhance the generalisability of our findings.
In conclusion, our findings showed that overweight and obesity increased the risk of HCC in the Korean population. In the BMI range of ≥25 kg/m2, a higher BMI increased the risk of HCC, regardless of sex and age, but not in the BMI range of <25 kg/m2. The impact of high BMI on HCC was consistent between men and women and between young, middle-aged, and older adults. The impact of high BMI on HCC development was substantially mediated by high ALT levels, especially in men and younger adults, but to a lesser extent in women and older adults.
Supplementary information
Revised supplementary Figures and Tables
Author contributions
BGJ and SWY conceived the study concept and design. SWY acquired the data and performed statistical analyses. BGJ, MK and SWY wrote the first draft. HSS and JJY searched literature. BGJ, MK, SWY, HSS and JJY analysed and interpreted the data. All authors contributed to the critical revision of the manuscript. All authors have read and approved the final submitted version of the manuscript. SWY is the guarantor of this study, and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This research was supported by Medical Research Promotion Program through the Gangneung Asan Hospital funded by the Asan Foundation (2021IB005).
Data availability
The data underlying the results presented in the study are available from the National Health Insurance Service (https://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do).
Code availability
All data analyses were performed using SAS, version 9.4 (SAS Institute, Inc., Cary, NC). The code used to perform the analysis is available on request to the authors.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Board of Catholic Kwandong University. Informed consent was waived because the anonymized data were used that was provided by NHIS according to strong confidentiality protocol.
Consent to publish
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Baek Gyu Jun, Moonho Kim.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-01771-0.
References
- 1.Petrick JL, Braunlin M, Laversanne M, Valery PC, Bray F, McGlynn KA. International trends in liver cancer incidence, overall and by histologic subtype, 1978-2007. Int J Cancer. 2016;139:1534–45. doi: 10.1002/ijc.30211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Parr CL, Batty GD, Lam TH, Barzi F, Fang X, Ho SC, et al. Body-mass index and cancer mortality in the Asia-Pacific Cohort Studies Collaboration: pooled analyses of 424 519 participants. Lancet Oncol. 2010;11:741–52. doi: 10.1016/S1470-2045(10)70141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yi SW, Choi JS, Yi JJ, Lee YH, Han KJ. Risk factors for hepatocellular carcinoma by age, sex, and liver disorder status: a prospective cohort study in Korea. Cancer. 2018;124:2748–57. doi: 10.1002/cncr.31406. [DOI] [PubMed] [Google Scholar]
- 5.Gupta A, Das A, Majumder K, Arora N, Mayo HG, Singh PP, et al. Obesity is independently associated with increased risk of hepatocellular cancer-related mortality: a systematic review and meta-analysis. Am J Clin Oncol. 2018;41:874. doi: 10.1097/COC.0000000000000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N. Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Ju W, Huo C, Zhang S, Wang X, Huang K. Overweight and obesity as independent factors for increased risk of hepatocellular cancer-related mortality: a meta-analysis. J Am Coll Nutr. 2021;40:287–93. doi: 10.1080/07315724.2020.1751007. [DOI] [PubMed] [Google Scholar]
- 8.Setiawan VW, Lim U, Lipworth L, Lu SC, Shepherd J, Ernst T, et al. Sex and ethnic differences in the association of obesity with risk of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2016;14:309–16. doi: 10.1016/j.cgh.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Wang X, Wang J, Yan Z, Luo J. Excess body weight and the risk of primary liver cancer: an updated meta-analysis of prospective studies. Eur J Cancer. 2012;48:2137–45. doi: 10.1016/j.ejca.2012.02.063. [DOI] [PubMed] [Google Scholar]
- 10.Oh SW, Yoon YS, Shin S-A. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005;23:4742–54. doi: 10.1200/JCO.2005.11.726. [DOI] [PubMed] [Google Scholar]
- 11.Batty GD, Barzi F, Huxley R, Chang CY, Jee SH, Jamrozik K, et al. Obesity and liver cancer mortality in Asia: the Asia Pacific cohort studies collaboration. Cancer Epidemiol. 2009;33:469–72. doi: 10.1016/j.canep.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nam GE, Kim YH, Han K, Jung JH, Rhee EJ, Lee WY, et al. Obesity fact sheet in Korea, 2020: prevalence of obesity by obesity class from 2009 to 2018. J Obes Metab Syndr. 2021;30:141–8. doi: 10.7570/jomes21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020;(360):1–8. [PubMed]
- 14.Wong MCS, Huang JLW, George J, Huang J, Leung C, Eslam M, et al. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol. 2019;16:57–73. doi: 10.1038/s41575-018-0055-0. [DOI] [PubMed] [Google Scholar]
- 15.Chiang CH, Lee LT, Hung SH, Lin WY, Hung HF, Yang WS, et al. Opposite association between diabetes, dyslipidemia, and hepatocellular carcinoma mortality in the middle-aged and elderly. Hepatology. 2014;59:2207–15. doi: 10.1002/hep.27014. [DOI] [PubMed] [Google Scholar]
- 16.Loomba R, Yang H-I, Su J, Brenner D, Barrett-Connor E, Iloeje U, et al. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. Am J Epidemiol. 2013;177:333–42. doi: 10.1093/aje/kws252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nderitu P, Bosco C, Garmo H, Holmberg L, Malmstrom H, Hammar N, et al. The association between individual metabolic syndrome components, primary liver cancer and cirrhosis: a study in the Swedish AMORIS cohort. Int J Cancer. 2017;141:1148–60. doi: 10.1002/ijc.30818. [DOI] [PubMed] [Google Scholar]
- 18.Yi SW, Ohrr H, Shin SA, Yi JJ. Sex-age-specific association of body mass index with all-cause mortality among 12.8 million Korean adults: a prospective cohort study. Int J Epidemiol. 2015;44:1696–705. doi: 10.1093/ije/dyv138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016;48:436–50. doi: 10.4143/crt.2016.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seo HJ, Oh IH, Yoon SJ. A comparison of the cancer incidence rates between the national cancer registry and insurance claims data in Korea. Asian Pac J Cancer Prev. 2012;13:6163–8. doi: 10.7314/APJCP.2012.13.12.6163. [DOI] [PubMed] [Google Scholar]
- 21.Lee EY, Lee YH, Yi SW, Shin SA, Yi JJ. BMI and all-cause mortality in normoglycemia, impaired fasting glucose, newly diagnosed diabetes, and prevalent diabetes: a cohort study. Diabetes Care. 2017;40:1026–33. doi: 10.2337/dc16-1458. [DOI] [PubMed] [Google Scholar]
- 22.Yi SW, Kim SH, Han KJ, Yi JJ, Ohrr H. Higher cholesterol levels, not statin use, are associated with a lower risk of hepatocellular carcinoma. Br J Cancer. 2020;122:630–3. doi: 10.1038/s41416-019-0691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borena W, Strohmaier S, Lukanova A, Bjørge T, Lindkvist B, Hallmans G, et al. Metabolic risk factors and primary liver cancer in a prospective study of 578,700 adults. Int J Cancer. 2012;131:193–200. doi: 10.1002/ijc.26338. [DOI] [PubMed] [Google Scholar]
- 24.Hassan MM, Abdel-Wahab R, Kaseb A, Shalaby A, Phan AT, El-Serag HB, et al. Obesity early in adulthood increases risk but does not affect outcomes of hepatocellular carcinoma. Gastroenterology. 2015;149:119–29. doi: 10.1053/j.gastro.2015.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jee SH, Yun JE, Park EJ, Cho ER, Park IS, Sull JW, et al. Body mass index and cancer risk in Korean men and women. Int J Cancer. 2008;123:1892–6. doi: 10.1002/ijc.23719. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Warren Andersen S, Wen W, Gao YT, Lan Q, Rothman N, et al. Prospective cohort study of general and central obesity, weight change trajectory and risk of major cancers among Chinese women. Int J Cancer. 2016;139:1461–70. doi: 10.1002/ijc.30187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111–21. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 28.Duan D, Xu J, Feng X, Astell-Burt T, Xu G, Lu N, et al. Does body mass index and adult height influence cancer incidence among Chinese living with incident type 2 diabetes? Cancer Epidemiol. 2018;53:187–94. doi: 10.1016/j.canep.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Chen C-T, Chen J-Y, Wang J-H, Chang K-C, Tseng P-L, Kee K-M, et al. Diabetes mellitus, metabolic syndrome and obesity are not significant risk factors for hepatocellular carcinoma in an HBV- and HCV-endemic area of Southern Taiwan. Kaohsiung J Med Sci. 2013;29:451–9. doi: 10.1016/j.kjms.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5· 24 million UK adults. Lancet. 2014;384:755–65. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Yatsuya H, Yamagishi K, Wakai K, Tamakoshi A, Iso H, et al. Body mass index and weight change during adulthood are associated with increased mortality from liver cancer: the JACC Study. J Epidemiol. 2013;23:219–26. doi: 10.2188/jea.JE20120199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell PT, Newton CC, Freedman ND, Koshiol J, Alavanja MC, Freeman LEB, et al. Body mass index, waist circumference, diabetes, and risk of liver cancer for US adults. Cancer Res. 2016;76:6076–83. doi: 10.1158/0008-5472.CAN-16-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma X, Liu S, Zhang J, Dong M, Wang Y, Wang M, et al. Proportion of NAFLD patients with normal ALT value in overall NAFLD patients: a systematic review and meta-analysis. BMC Gastroenterol. 2020;20:10. doi: 10.1186/s12876-020-1165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goh GB, Pagadala MR, Dasarathy J, Unalp-Arida A, Pai RK, Yerian L, et al. Age impacts ability of aspartate-alanine aminotransferase ratio to predict advanced fibrosis in nonalcoholic fatty liver disease. Dig Dis Sci. 2015;60:1825–31. doi: 10.1007/s10620-015-3529-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Revised supplementary Figures and Tables
Data Availability Statement
The data underlying the results presented in the study are available from the National Health Insurance Service (https://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do).
All data analyses were performed using SAS, version 9.4 (SAS Institute, Inc., Cary, NC). The code used to perform the analysis is available on request to the authors.