Abstract
National guidelines recommend testing all cases of non-mucinous epithelial ovarian cancer (NMEOC) for germline (blood) and somatic (tumour) BRCA1/2 pathogenic variants (PVs). We performed paired germline and somatic BRCA1/2 testing in consecutive cases of NMEOC (n = 388) to validate guidelines. Thirty-four somatic BRCA1/2 (sBRCA) PVs (9.7%) were detected in 350 cases with germline BRCA1/2 (gBRCA) wild-type. All sBRCA PVs were detected in non-familial cases. By analysing our regional germline BRCA1/2 database there were 92/1114 (8.3%) gBRCA PVs detected in non-familial cases (only 3% ≥70 years old) and 245/641 (38.2%) in familial cases. Germline non-familial cases were dominated by BRCA2 in older women (8/271 ≥ 70 years old, all BRCA2). The ratio of sBRCA-to-gBRCA was ≤1.0 in women aged <70 years old, compared to 5.2 in women aged ≥70 years old (P = 0.005). The likelihood of missed germline BRCA1/2 PVs (copy-number variants missed on most somatic assays) by testing only tumour DNA was 0.4% in women aged ≥70 years old. We recommend reflex tumour BRCA1/2 testing in all NMEOC cases, and that gBRCA testing is not required for women aged ≥70 years old with no identifiable tumour BRCA1/2 PV and/or family history of breast, ovarian, prostate and/or pancreatic cancer.
Subject terms: Diagnostic markers, Molecular medicine, Gynaecological cancer
Background
Ovarian cancer is the most lethal gynaecological cancer [1]. Despite significant advancements in our understanding of the genetic hallmarks of ovarian cancer histological subtypes, only poly(ADP-ribose) polymerase-1/2 inhibitors (PARPi) are used as standard therapy for genetically stratified tumours, in BRCA1/2-mutant (germline [gBRCA] or somatic [sBRCA]) high-grade serous carcinoma [2–5].
Germline BRCA1/2 testing is now performed as standard practice following a diagnosis of high-grade serous carcinoma, where gBRCA pathogenic variants (PVs) account for ~15–20% of cases [6, 7]. Although the use of PARPi has expanded through oncology-led gBRCA testing services, additional tumour BRCA1/2 testing maximises the utility of PARPi by identifying actionable sBRCA PVs [8]. National guidelines recommend testing all cases of non-mucinous epithelial ovarian cancer (NMEOC) for gBRCA and sBRCA PVs [9–11]. We assessed our experience of paired germline (blood) and somatic (tumour) BRCA1/2 testing in consecutive NMEOC cases to validate guidelines.
Methodology
Women diagnosed with NMEOC underwent germline (September 1996 to August 2021) and more recently tumour BRCA1/2 testing (July 2017 to August 2021) using clinically validated assays in the Manchester Centre for Genomic Medicine. Between July 2017 and August 2021, paired germline and tumour BRCA1/2 testing could be requested by the treating physician.
The next-generation sequencing (NGS) tumour BRCA1/2 assay has been previously reported [12, 13]. Briefly, tumour DNA was extracted from formalin-fixed, paraffin-embedded blocks that contained ≥20% tumour content. Bioinformatic analysis used an in-house pipeline validated to detect tumour BRCA1/2 variants down to an allele frequency of ~4%. The NGS assay detects single nucleotide variants and small duplications, deletions and/or insertions ≤40 base pairs across the whole coding sequence of BRCA1/2 + /− 15 base pairs beyond exon–intron junctions. Variant allele frequency ≥4% has a call sensitivity >95% and specificity >99% after manual review. Germline BRCA1/2 testing was performed on DNA extracted from peripheral circulating lymphocytes. The NGS and multiplex ligation-dependent probe amplification (MLPA) assays used to detect gBRCA PVs have also been previously reported [14, 15]. The variant interpretation was performed as per published guidelines [16].
Women were considered as ‘non-familial’ (low familial risk) if they had no more than a single breast cancer themselves or in their family diagnosed ≥50 years old. Familial cases included those with more extensive personal and/or family histories of breast, ovarian, prostate and/or pancreatic cancer.
The data included in this study were collected as part of a continuous clinical audit. Clinical data were gathered at the time that the germline and/or tumour BRCA1/2 status was reported. All patients provided informed consent for blood (germline) and tumour (somatic) BRCA1/2 testing. Statistical tests used Fisher exact test (two-sided).
Results
Population
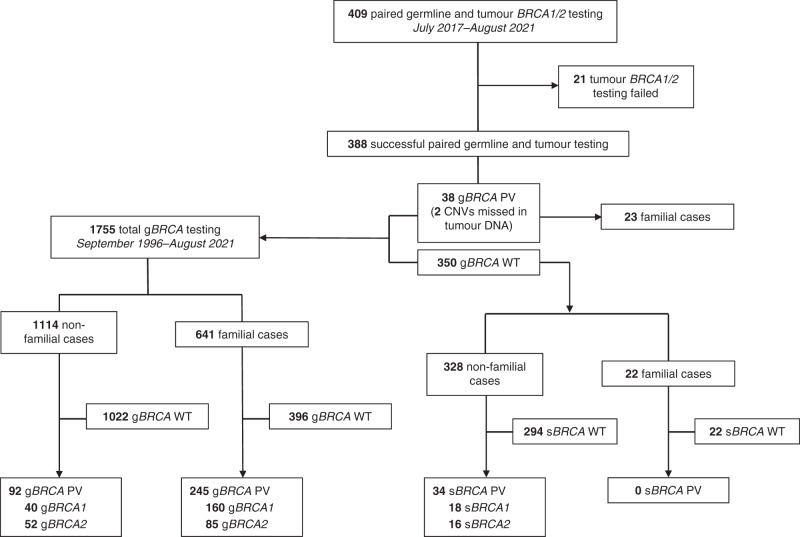
One-thousand-seven-hundred-fifty-five women underwent germline testing and 337 were diagnosed with a gBRCA PV (gBRCA1 = 200, gBRCA2 = 137; prevalence 19.2%); not exclusively in high-grade serous carcinoma (Table 1). Three-hundred eighty-eight women (out of 409; 94.9%) had successful germline and tumour BRCA1/2 testing (n = 21 insufficient material and/or sample failed) (Fig. 1). Initially, samples were tested sequentially with germline BRCA1/2 testing first (n = 209; no tumour testing reported if a gBRCA PV was detected) and then testing was carried out either simultaneously or tumour first (n = 200).
Table 1.
Germline and somatic BRCA1/2 pathogenic variants by age and familial situation with germline miss rates and somatic-to-germline ratios.
| Non-familial OC (low familial risk) | Familial OC | Combined total | HGSC | CCC | EOC | Carcinosarcoma | Other | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Total | ||||||||||||
| <30 | 30–49 | 50–59 | 60–69 | 70 + | |||||||||
| Full germline BRCA1/2 testing database (N = 1755) | |||||||||||||
| Germline BRCA1/2 tested (no.) | 18 | 199 | 319 | 325 | 271 | 1114 | 641 | 1755 | 1512 | 61 | 108 | 23 | 51d |
| gBRCA1/2 wild-type (no.) | 18 | 177 | 285 | 297 | 263 | 1022 | 396 | 1418 | 1197 | 55 | 97 | 18 | 51d |
| gBRCA1 PV (no.) | 0 | 12 | 19 | 9 | 0 | 40 | 160 | 200 | 188 | 4 | 7 | 1 | 0 |
| %a | 0.0% | 6.0% | 6.0% | 2.8% | 0.0% | 3.6% | 25.0% | 11.4% | 12.4% | 6.6% | 6.5% | 4.3% | 0.0% |
| gBRCA2 PV (no.) | 0 | 10 | 15 | 19 | 8 | 52 | 85 | 137 | 127 | 2 | 4 | 4 | 0 |
| %a | 0.0% | 5.6% | 5.3% | 6.4% | 3.0% | 5.1% | 21.5% | 9.7% | 10.6% | 3.6% | 4.1% | 22.2% | 0.0% |
| Combined gBRCA1/2 PV (no.) | 0 | 22 | 34 | 28 | 8 | 92 | 245 | 337 | 315 | 6 | 11 | 5 | 0 |
| % Combined gBRCA1/2 PVa | 0.0% | 11.1% | 10.7% | 8.6% | 3.0% | 8.3% | 38.2% | 19.2% | 20.8% | 9.8% | 10.2% | 21.7% | 0.0% |
| Successful paired tumour and germline BRCA1/2 testing cohort (N = 388) | |||||||||||||
| BRCA1/2 tested (no.) | 0 | 42 | 82 | 107 | 112 | 343 | 45 | 388 | 361 | 9 | 15 | 1 | 2 |
| gBRCA1/2 PV confirmed (no.) | 0 | 5 | 3 | 6 | 1 | 15 | 23 | 38 | 32 | 1 | 3 | 0 | 0 |
| gBRCA1/2 wild-type (no.) | 0 | 37 | 79 | 101 | 111 | 328 | 22 | 350 | 327 | 8 | 12 | 1 | 2 |
| gBRCA1/2 PV missed in tumour DNA (no.) | 0 | 0 | 0 | 2b | 0 | 2b | 0 | 2b | 2b | 0 | 0 | 0 | 0 |
| % gBRCA1/2 PV Missed | 0.0% | 0.0% | 0.0% | 33.3% | 0.0% | 13.3% | 0.0% | 5.3% | 5.9% | 0.0% | 0.0% | 0.0% | 0.0% |
| sBRCA1 PV (no.) | 0 | 3 | 3 | 6 | 6 | 18 | 0 | 18 | 18 | 0 | 0 | 0 | 0 |
| %c | 0.0% | 8.1% | 3.8% | 5.9% | 5.4% | 5.5% | 0.0% | 5.1% | 5.5% | 0.0% | 0.0% | 0.0% | 0.0% |
| sBRCA2 PV (no.) | 0 | 1 | 1 | 3 | 11 | 16 | 0 | 16 | 15 | 1 | 0 | 0 | 0 |
| %c | 0.0% | 2.7% | 1.3% | 3.0% | 9.9% | 4.9% | 0.0% | 4.6% | 4.6% | 12.5% | 0.0% | 0.0% | 0.0% |
| Combined sBRCA1/2 PV (no.) | 0 | 4 | 4 | 9 | 17 | 34 | 0 | 34 | 33 | 1 | 0 | 0 | 0 |
| % Combined sBRCA1/2 PVc | 0.0% | 10.8% | 5.1% | 8.9% | 15.3% | 10.4% | 0.0% | 9.7% | 10.1% | 12.5% | 0.0% | 0.0% | 0.0% |
| sBRCA-to-gBRCA ratio | 0.0 | 1.0 | 0.5 | 1.0 | 5.2 | 1.3 | 0.0 | 0.5 | 0.5 | 1.3 | 0.0 | 0.0 | 0.0 |
| Missed rate gBRCA1/2 CNVs | 0.0% | 1.3% | 1.2% | 0.7% | 0.4% | 0.9% | 5.0% | 2.4% | 2.7% | 1.8% | 1.3% | 0.9% | 0.0% |
CCC clear cell carcinoma, CNVs copy-number variants, EOC endometrioid ovarian carcinoma, gBRCA germline BRCA1/2, HGSC high-grade serous carcinoma, no. number, OC ovarian cancer, PV pathogenic variant, sBRCA somatic BRCA1/2.
aThe denominator is the number of cases tested for germline BRCA1/2 pathogenic variants.
bTwo cases of non-mucinous epithelial ovarian cancers had a copy-number variant detected in germline DNA but not in tumour DNA.
cThe denominator is the number of cases tested for tumour BRCA1/2 pathogenic variants with confirmed germline BRCA1/2 wild-type.
dIncludes n = 11 and n = 2 low-grade serous carcinoma cases who underwent blood (germline) and tumour (somatic) testing, respectively, as well as adenocarcinoma otherwise specified (NOS).
Fig. 1. Flow chart for germline and tumour BRCA1/2 testing.
Paired germline and tumour testing was requested in 409 patients. In total, 1755 patients underwent germline testing.
Thirty-four sBRCA PVs were identified in tumour DNA from 350 patients with germline BRCA1/2 wild-type (sBRCA1 = 18, sBRCA2 = 16; prevalence 9.7%; Table 1). All 34 sBRCA PVs were detected in non-familial cases (Fig. 1).
Thirty-six gBRCA PVs were confirmed in tumour DNA, but two copy-number variants (CNVs) found using MLPA were not identified in tumour DNA, including BRCA1 Exon 13 duplication (n = 1) and BRCA2 Exons 1–2 deletion (n = 1; Table 1). Fifteen (out of 38; 39.4%) gBRCA PVs were found in non-familial cases (Fig. 1).
Somatic versus germline BRCA1/2 pathogenic variants
We used the full germline (n = 1755) and tumour (n = 388) BRCA1/2 testing databases to compare the age-specific prevalence of sBRCA versus gBRCA PVs (Table 1). The most striking difference was found in women aged ≥70 years old, where 17/111 (15.3%) had a sBRCA PV (sBRCA1 = 6, sBRCA2 = 11) but only 8/271 (3.0%) had a gBRCA PV (all gBRCA2; Table 1). The sBRCA-to-gBRCA ratio of 5.2 in women diagnosed with non-familial NMEOC aged ≥70 years old contrasted all other ratios (≤1.0) in women aged <70 years old (P = 0.005; Table 1).
Interestingly, there was also a reversal of the gBRCA2-to-gBRCA1 ratio in non-familial NMEOC of 0.8 (25:31) aged <60 years old to 3.0 (27:9) aged ≥60 years old (P = 0.005; Table 1).
Miss rate by upfront tumour BRCA1/2 testing
To assess the potential miss rate of not universally testing all NMEOC cases for germline BRCA1/2 PVs, we used the full gBRCA testing database (n = 1755), which included all familial cases. Forty (out of 200; 20%) gBRCA1 PVs were CNVs, compared to only 5/137 (3.6%) gBRCA2 PVs. The data suggested only 0.4% of non-familial gBRCA-positive CNVs would be missed by testing only tumour DNA in women diagnosed with NMEOC aged >70 years old, but would result in a 1.3% miss rate in those aged <60 years old and 5.0% miss rate in familial cases (Table 1).
Discussion
Tumour BRCA1/2 analysis of NMEOC cases generally results in gBRCA and sBRCA PV rates of 15–20% and 5–7%, respectively [6, 15, 17–19]. However, germline rates in non-familial NMEOC, even in high-grade serous carcinoma, are much lower, particularly in those diagnosed >60 years old [14, 20]. Indeed, age ≥70 years old in one study found only 1/86 of unselected cases of NMEOC had a gBRCA PV [20]. Our rate of 3% (8/271) in women diagnosed ≥70 years old was made up entirely of gBRCA2 PVs, which contrasted with the age of onset with gBRCA1 PVs [21].
Thus far, the age of onset of sBRCA has not been correlated, but we found an almost double rate of somatic BRCA1/2 PVs diagnosed ≥70 years old. This means that only one in six BRCA1/2 PVs found on tumour analysis in non-familial NMEOC cases ≥70 years old will be germline. Whilst national guidelines recommend initial gBRCA testing, a more practical approach would be to start with reflex tumour BRCA1/2 testing (by pathologists) of all cases of NMEOC, particularly in those women diagnosed ≥70 years old. This would enable a timely result to facilitate PARPi maintenance therapy, thereby avoiding the delays/misses/refused cases with germline BRCA1/2 testing. Indeed, the tumour BRCA1/2 testing assay described in this study has a turnaround time of 21 to 28 days, and testing can be requested immediately following histological diagnosis. Moreover, tumour BRCA1/2 testing may not require specific consent, and even if consent is required this should not entail a detailed discussion about personal and/or familial risks. In practice, discussions about inherited risk can be particularly worrying for women who are simultaneously trying to deal with the diagnosis and treatment of ovarian cancer.
The high ratio of sBRCA-to-gBRCA PVs in non-familial cases aged >70 years old is an interesting finding from our study. The likelihood of somatic variants in oncogenes and/or tumour suppressor genes increases with age, potentially due to faltering DNA repair mechanisms. Therefore, more sBRCA PVs are likely to be detected in older age groups. Moreover, the increase in sBRCA1/2 PVs is offset by a lower prevalence of gBRCA1/2 PVs. Indeed, the risk of hereditary ovarian cancer does not increase with age >50 years old, and cases of gBRCA-mutant NMEOC aged >70 years are reduced by the competing mortality from breast cancer and/or risk-reducing bilateral salpingo-oophorectomy in younger heterozygotes.
Given the extremely low chance of missing a gBRCA PV on tumour BRCA1/2 testing in women diagnosed ≥70 years old (0.4%; in UK costs approximately £500,000 per CNV) it is arguable whether a germline (blood) sample is required unless a tumour BRCA1/2 PV is detected and/or tumour testing fails. However, in younger women and especially those with a family history, miss rates could be as high as 5.0% if CNVs are not reliably detected through tumour BRCA1/2 testing [8, 19, 22–24]. It is therefore essential that germline BRCA1/2 testing is carried out on all familial cases and those diagnosed <60 years old. Rates of germline BRCA1/2 CNVs vary from 3–5% in BRCA2 to 10–20% in BRCA1, reflecting the sensitivity of detection.
It is notable that our study only reports the incidence of BRCA1/2 PVs in NMEOC cases. Indeed, consideration should be given for extended panel testing to detect germline PVs in moderate-to-low penetrance genes associated with hereditary ovarian cancer (e.g., RAD51C/D, BRIP1, PALB2) in BRCA1/2 wild-type cases with a family history of cancer. Although we are not recommending extended panel testing for all NMEOC cases with BRCA1/2 wild-type, we do recommend referral to Clinical Genetics for those women who have a first-degree or second-degree relative with ovarian cancer.
It is also noteworthy that there may be a degree of selection bias in our study. Firstly, for those women in whom a gBRCA PV was detected prior to completion of tumour testing (i.e., sequential or simultaneous germline/tumour testing versus tumour first), if a germline PV was reported first, it would halt additional unnecessary reporting of tumour variants. Therefore, it is likely that more than 409 cases of NMEOC had paired germline/tumour testing requested. This factor explains the relatively low prevalence of gBRCA PV detected in the paired testing cohort (9.3%; 38/409) versus the full germline database (19.2%; 337/1755). Secondly, if tumour tissue was not available; for example, due to cytological diagnosis and/or low tumour cell content following diagnostic workup, then only germline BRCA1/2 testing could take place, meaning the somatic status of some gBRCA wild-type cases remains unknown. This factor may explain the relatively high prevalence of sBRCA PVs detected in the paired testing cohort (9.7%; 34/350).
In conclusion, we report the detection rate of sBRCA PVs with paired blood (germline) and tumour (somatic) DNA testing in a large cohort of NMEOC cases. We recommend starting with tumour BRCA testing, and that germline testing is probably not indicated after confirming tumour BRCA1/2 wild-type in women diagnosed with NMEOC aged ≥70 years and no family history of breast, ovarian, prostate and/or pancreatic cancer.
Acknowledgements
ERW and DGRE are supported by the Manchester National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (IS-BRC-1215-20007). EJC is supported by a NIHR Advanced Fellowship (NIHR300650). NF is supported by CRUK via the funding to Cancer Research UK Manchester Centre: [C147/A18083] and [C147/A25254].
Author contributions
RDM and DGRE designed and initiated the study. RDM, NF, ARC, JH, CLM, ZS, ERW, FL, EJC, RJE, GCJ and DGRE gained consent for germline and tumour BRCA1/2 testing. GJB, MB, PS and AJW reported all germline and tumour BRCA1/2 results. All authors interpreted the data and reviewed the final version of the manuscript.
Funding
The tumour BRCA1/2 testing service was funded by AstraZeneca and Merck Sharp & Dohme, who gave permission to publish the data. Neither company had any influence in the writing of the manuscript or the views expressed by the authors.
Competing interests
RDM, GJB, NF, MB, PS, JH, CLM, ZS, ERW, FL, EJC, RJE, AJW and DGRE declare no competing interests. GCJ declares research funding from AstraZeneca for investigator-led clinical trials. ARC declares research funding and advisory boards fees from AstraZeneca, and speaker and advisory board fees from Clovis Oncology.
Ethics approval and consent to participate
All women included in this study provided informed consent to undergo germline and tumour BRCA1/2 testing. The germline BRCA1/2 database is approved by North Manchester Research Ethics Committee (08/H1006/77). The Genetic Variants in Gynaecological Cancer database is approved by the Christie NHS Foundation Trust (59).
Consent to publish
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–87. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N. Engl J Med. 2019;385:2403–15. doi: 10.1056/NEJMoa1909707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949–61. doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Martin A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 5.Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl J Med. 2018;379:2495–505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 6.Hauke J, Hahnen E, Schneider S, Reuss A, Richters L, Kommoss S, et al. Deleterious somatic variants in 473 consecutive individuals with ovarian cancer: results of the observational AGO-TR1 study ( NCT02222883) J Med Genet. 2019;56:574–80. doi: 10.1136/jmedgenet-2018-105930. [DOI] [PubMed] [Google Scholar]
- 7.Huang KL, Mashl RJ, Wu Y, Ritter DI, Wang J, Oh C, et al. Pathogenic germline variants in 10,389 adult cancers. Cell. 2018;173:355–70. doi: 10.1016/j.cell.2018.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hennessy BT, Timms KM, Carey MS, Gutin A, Meyer LA, Flake DD, 2nd, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol. 2010;28:3570–6. doi: 10.1200/JCO.2009.27.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pujol P, Barberis M, Beer P, Friedman E, Piulats JM, Capoluongo ED, et al. Clinical practice guidelines for BRCA1 and BRCA2 genetic testing. Eur J Cancer. 2021;146:30–47. doi: 10.1016/j.ejca.2020.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Konstantinopoulos PA, Norquist B, Lacchetti C, Armstrong D, Grisham RN, Goodfellow PJ, et al. Germline and somatic tumor testing in epithelial ovarian cancer: ASCO guideline. J Clin Oncol. 2020;38:1222–45. doi: 10.1200/JCO.19.02960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundar S, Manchanda R, Gourley C, George A, Wallace A, Balega J, et al. British Gynaecological Cancer Society/British Association of Gynaecological Pathology consensus for germline and tumor testing for BRCA1/2 variants in ovarian cancer in the United Kingdom. Int J Gynecol Cancer. 2021;31:272–8. doi: 10.1136/ijgc-2020-002112. [DOI] [PubMed] [Google Scholar]
- 12.Ellison G, Huang S, Carr H, Wallace A, Ahdesmaki M, Bhaskar S, et al. A reliable method for the detection of BRCA1 and BRCA2 mutations in fixed tumour tissue utilising multiplex PCR-based targeted next generation sequencing. BMC Clin Pathol. 2015;15:5. doi: 10.1186/s12907-015-0004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan RD, Bulman M, Clamp AR, MacMohon S, Thompson L, Ribeiro S, et al. Incidence of tumour BRCA1/2 variants in relapsed, platinum-sensitive ovarian, fallopian tube and primary peritoneal cancer. Ann Oncol. 2019;30:v403–v434. doi: 10.1093/annonc/mdz250.017. [DOI] [Google Scholar]
- 14.Morgan RD, Burghel GJ, Flaum N, Bulman M, Clamp AR, Hasan J, et al. Prevalence of germline pathogenic BRCA1/2 variants in sequential epithelial ovarian cancer cases. J Med Genet. 2019;56:301–7. doi: 10.1136/jmedgenet-2018-105792. [DOI] [PubMed] [Google Scholar]
- 15.Flaum N, Morgan RD, Burghel GJ, Bulman M, Clamp AR, Hasan J, et al. Mainstreaming germline BRCA1/2 testing in non-mucinous epithelial ovarian cancer in the North West of England. Eur J Hum Genet. 2020;28:1541–7. doi: 10.1038/s41431-020-0692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–63. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunningham JM, Cicek MS, Larson NB, Davila J, Wang C, Larson MC, et al. Clinical characteristics of ovarian cancer classified by BRCA1, BRCA2, and RAD51C status. Sci Rep. 2014;4:4026. doi: 10.1038/srep04026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frugtniet B, Morgan S, Murray A, Palmer-Smith S, White R, Jones R, et al. The detection of germline and somatic BRCA1/2 genetic variants through parallel testing of patients with high-grade serous ovarian cancer: a national retrospective audit. BJOG. 2022;129:433–42. doi: 10.1111/1471-0528.16975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plaskocinska I, Shipman H, Drummond J, Thompson E, Buchanan V, Newcombe B, et al. New paradigms for BRCA1/BRCA2 testing in women with ovarian cancer: results of the Genetic Testing in Epithelial Ovarian Cancer (GTEOC) study. J Med Genet. 2016;53:655–61. doi: 10.1136/jmedgenet-2016-103902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flaum N, Crosbie EJ, Woodward ER, Lalloo F, Gareth Evans D. Challenging the believed proportion of ovarian cancer attributable to BRCA2 versus BRCA1 pathogenic variants. Eur J Cancer. 2020;124:88–90. doi: 10.1016/j.ejca.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Hodgson DR, Brown JS, Dearden SP, Lai Z, Elks CE, Milenkova T, et al. Concordance of BRCA mutation detection in tumor versus blood, and frequency of bi-allelic loss of BRCA in tumors from patients in the phase III SOLO2 trial. Gynecol Oncol. 2021;163:563–8. doi: 10.1016/j.ygyno.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Callens C, Vaur D, Soubeyran I, Rouleau E, Just PA, Guillerm E, et al. Concordance between tumor and germline BRCA status in high-grade ovarian carcinoma patients in the phase III PAOLA-1/ENGOT-ov25 trial. J Natl Cancer Inst. 2021;113:917–23. doi: 10.1093/jnci/djaa193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fumagalli C, Tomao F, Betella I, Rappa A, Calvello M, Bonanni B, et al. Tumor BRCA test for patients with epithelial ovarian cancer: the role of molecular pathology in the era of PARP inhibitor therapy. Cancers. 2019;11:1641. [DOI] [PMC free article] [PubMed]