Abstract
Concurrent anxiety is frequent in major depressive disorder and a shared pathophysiological mechanism between anxiety and other depressive symptoms is plausible. The serotonin 4 receptor (5-HT4R) has been implicated in both depression and anxiety. This is the first study to investigate the association between the cerebral 5-HT4R binding and anxiety in patients with depression before and after antidepressant treatment and the association to treatment response. Ninety-one drug-free patients with depression were positron emission tomography scanned with the 5-HT4R ligand [11C]-SB207145. Depression severity and concurrent anxiety was measured at baseline and throughout 8 weeks of antidepressant treatment. Anxiety measures included four domains: anxiety/somatization factor score; Generalized Anxiety Disorder 10-items (GAD-10) score; anxiety/somatization factor score ≥7 (anxious depression) and syndromal anxious depression. Forty patients were rescanned at week 8. At baseline, we found a negative association between global 5-HT4R binding and both GAD-10 score (p < 0.01) and anxiety/somatization factor score (p = 0.06). Further, remitters had a higher baseline anxiety/somatization factor score compared with non-responders (p = 0.04). At rescan, patients with syndromal anxious depression had a greater change in binding relative to patients with non-syndromal depression (p = 0.04). Concurrent anxiety in patients with depression measured by GAD-10 score and anxiety/somatization factor score is negatively associated with cerebral 5-HT4R binding. A lower binding may represent a subtype with reduced natural resilience against anxiety in a depressed state, and concurrent anxiety may influence the effect on the 5-HT4R from serotonergic antidepressants. The 5-HT4R is a promising neuroreceptor for further understanding the underpinnings of concurrent anxiety in patients with depression.
Subject terms: Predictive markers, Molecular neuroscience
Introduction
While anxiety is not a formal ICD/DSM symptom of depression, it is commonly observed in depressed patients and has prognostic importance [1]. Hence, it is weighted high and included in standard rating scales for depression, e.g. the Hamilton Depression Rating Scale (HAMD) [2]. While anxiety disorders have a prevalence of 284 million people worldwide [3], comorbidity of anxiety disorders or significant levels of anxious symptoms are present in up to 85% of patients with Major Depressive Disorder (MDD) [1, 4–7]. The presence of concurrent anxiety in depression has been associated with more and worse side effects to antidepressant medication, longer time to remission, greater severity and poorer treatment outcome [8, 9]. The underlying mechanisms are not fully understood, but serotonin (5-HT) is assumed to play a key role and serotonergic-acting drugs are first-line pharmacotherapeutic options for treatment of both depression and anxiety disorders. Previous positron emission tomography (PET) studies of the serotonin system in patients with concurrent anxious depression are scarce and have mainly focused on the serotonin transporter (SERT) and the 5-HT1A receptor [10, 11]. One study found that low SERT binding in thalamus was correlated with higher anxiety symptoms in a small sample of ten unmedicated patients with MDD [11] which was later replicated (n = 10) [12]. Another group confirmed a negative correlation between somatic anxiety and SERT binding in the thalamus, midbrain and amygdala, while psychic anxiety was positively correlated with midbrain SERT binding [10].
There is growing evidence for involvement of the 5-HT 4 receptor (5-HT4R) in anxiety and MDD, which is a Gs-protein coupled receptor abundant in neostriatum, the limbic region and prefrontal cortex [13–15]. Studies have demonstrated that 5-HT4R modulation is associated with anxiolytic-like behaviour in rodents, e.g., 5-HT4R knockout mice show attenuated novelty seeking behaviour [16] and both acute and (sub)chronic 5-HT4R stimulation elicits anxiolytic and antidepressant-like behaviour [17, 18]. Further, chronic 5-HT4R agonism treatment was found to prevent depressive- and anxiety-like behaviour [19]. Due to the observed antidepressant and anxiolytic properties, 5-HT4R agonists have been proposed as a new/add-on pharmacological target in anxiety and MDD [19, 20]. To our knowledge, only one study has investigated the effect of 5-HT4R agents in humans; a single oral administration of the partial 5-HT4R agonist prucalopride exerted pro-cognitive effects in healthy individuals, but had little effect on emotional processing related to an antidepressant profile [21]. Interestingly, preclinical and clinical evidence suggest that the 5-HT4R may serve as an inverse biomarker of the cerebral serotonin tonus [22–24], which makes it a relevant candidate when studying conditions with a presumed serotonergic involvement. The implications of 5-HT4R in MDD have been recognized [25] and recently, we showed that antidepressant-free patients with MDD had lower 5-HT4R binding compared with healthy controls, especially those responding well to escitalopram [26].
We here aim, for the first time, to investigate the association between 5-HT4R PET binding and concurrent anxious symptomatology in patients with MDD. We also investigate the association between concurrent anxious depression and antidepressant treatment response. Based on the previous findings of increased anxiety- and depressive-like behaviour in 5-HT4R knock-out mice and the anxiolytic effects from 5-HT4R stimulation in rodents [18, 27], we hypothesized that higher anxiety symptoms at baseline would be associated with lower 5-HT4R binding in patients with MDD. We also hypothesized that having concurrent anxiety at baseline would be associated with worse treatment response, that a change in 5-HT4R binding after 8 weeks of serotonergic antidepressant treatment would be associated with higher baseline anxiety, and that a change in binding would be associated with a change in anxiety score.
Methods and materials
The present paper is part of the NeuroPharm-1 study; a first-time, non-randomized, open-label clinical trial conducted between August 2016 to April 2019. Recruitment took place at a referral centre within the Mental Health System or at collaborating primary care centres in the capital region of Denmark. All participants provided written informed consent before participation. The study was pre-registered at clinicaltrials.gov (ID: NCT02869035) and complied with regulations from the Committees on Health Research Ethics in the Capital Region of Denmark (ID: H-15017713), the Danish Medicines Agency (ID: NeuroPharm-NP1) and the Danish Data Protection Agency (ID: 04711/RH-2016-163). The study is detailed in the trial protocol [28]. The participants were also included in a study of the association between 5-HT4R and MDD as primary outcome [26], whereas the anxiety measures presented in this study served as exploratory outcomes.
Participants
One hundred patients were included to reach statistical power of 0.8, for detection of a 7% difference in BPND between remitters and non-responders in the primary study [26, 28]. The expected drop-out rate was 20%. Ninety-one out of the recruited 100 planned patients completed a PET scan at baseline. All patients had unipolar, moderate to severe MDD according to DSM-IV criteria [29]. Depression severity was assessed with the HAMD-17 items (HAMD17) where all patients had a baseline score >17. Patients were 18–65 years and medically untreated for their depression for at least 2 months prior to inclusion. The duration of the current depressive episode did not exceed 2 years. Exclusion criteria were psychotic manifestations; acute suicidal ideations; alcohol abuse/substance use disorder; another primary axis I psychiatric diagnosis; previous non-response to an selective serotonin reuptake inhibitor (SSRI); central acting drugs that could not be washed out prior to scanning; pregnancy/breast feeding; post-concussion syndrome and severe somatic comorbidity.
Baseline assessments
Patients filled out the questionnaire of Generalized Anxiety Disorder 10 items (GAD-10) [30] and were interviewed with the Mini International Neuropsychiatric Interview (MINI) [31]. Baseline assessments further included medical history; somatic examination; routine bloodwork; urine pregnancy and toxicology tests; blood test for genetic variation of the serotonin transporter-linked polymorphic region (5-HTTLPR); and magnetic resonance imaging (MRI)- and PET scans.
Treatment programme
After baseline assessments, patients started antidepressant treatment with escitalopram at flexible doses between 10–20 mg daily, individually adjusted depending on side effects and clinical response. Face to face clinical follow-up sessions were conducted after 1, 2, 4, 8 and 12 weeks. Additional visits were accepted if clinically justified. Treatment response was monitored at each follow-up session using the HAMD17 and the subscale of 6 items (HAMD6), collected by a trained physician or study assistant. Co-ratings for HAMD calibration was held monthly. Drug compliance was assessed by pill-count at each visit and trough serum blood samples at week 8. Short acting sleeping pills and anxiolytics (benzodiazepines) were accepted to reduce initial side effects, but not 72 h prior to PET-scans. If patients had excessive side effects or insufficient response to escitalopram after 4 weeks, they were offered to switch to duloxetine. Duloxetine is a standard second line antidepressant treatment and has negligible affinity to the 5-HT4R [32]. After 8 weeks, a subgroup of patients (n = 43) was rescanned.
Characterization of anxious depression
Concurrent anxious depression was measured by (A) the HAMD17 anxiety/somatization factor score (here referred to as “factor score”) [8]. The factor score was derived from a HAMD17 factor analysis by Cleary and Guy [33] and included six items: item 10 (psychic anxiety), 11 (somatic anxiety), 12 (gastrointestinal somatic symptoms), 13 (general somatic symptoms), 15 (hypochondriasis) and 17 (insight). We also used (B) the GAD-10 questionnaire (“GAD-10 score”) which is an inventory for generalized anxiety distress symptoms [30]. For the categorical outcomes, we used (C), a previously established definition of high levels of anxiety in depressed patients, where “anxious depression” was defined as a HAMD17 somatization/anxiety factor score ≥7 and “non-anxious depression” as a score <7 [8]. Lastly, (D) “syndromal anxious depression” was defined as having at least one concurrent anxiety diagnosis identified by the M.I.N.I. interview [34, 35], including panic disorder, generalized/not generalized social phobia, agoraphobia and generalized anxiety (associated with the depressed episode). Correspondingly, patients with “non-syndromal” anxious depression had no additional anxiety diagnosis verified by the M.I.N.I. interview. GAD-10 was a pre-registered outcome, whereas the other anxiety measures were exploratory and chosen because they are previous established outcomes [8, 34, 35].
Definitions of clinical treatment outcome
The clinical treatment outcome was based on changes in HAMD6 from baseline. HAMD6 was chosen because of its superior properties in monitoring treatment response compared to HAMD17 [36]. The primary treatment outcome was composed of two response categories at week 8: patients were categorized as remitters if they had an early response of ≥50% reduction in HAMD6 at week 4 and a HAMD6 < 5 at week 8; non-responders had an early non-response of <25% reduction in HAMD6 at week 4 and <50% HAMD6 reduction at week 8. Additionally, patients in between these categories were referred to as intermediate responders at week 8. This construct was applied to capture early (week 4) and sustained (week 8) treatment response. The secondary treatment outcome measure was a continuous outcome defined as percentage change in HAMD6 at week 8 relative to baseline (r∆HAMD6).
Brain regions of interest
Regions of interest (ROI) were set to neocortex, neostriatum and hippocampus. These regions display high density of 5-HT4R [13], are thought to be involved in MDD and were chosen to align with several studies of the serotonin system in MDD [22, 28, 37, 38].
PET acquisition and kinetic modelling
The PET acquisition and quantification has been described in detail elsewhere [28]. Briefly, all patients were scanned with a High-resolution Research Tomography (HRRT) PET scanner (CTI/Siemens, Knoxville, TN, USA) for 120 min after an intravenous 20 s bolus injection of [11C]-SB207145, and a 6 min transmission scan. The PET data was reconstructed into 38 time frames (6 × 5 s, 10 × 15 s, 4 × 30 s, 5 × 2 min, 5 × 5 min, and 8 × 10 min) and motion correction was performed with Air.5.2.5 [39], aligning each PET frame to the first 5 min frame (frame 26). All patients were MR-scanned using a Siemens 3-Tesla Prisma scanner, and T1 weighted MRI images were co-registered with the PET images to acquire anatomical information using SPM8. ROIs were automatically extracted using the pvelab software package [40] and delineated on each subject’s MRI. Correct co-registration of PET and MR images and ROI placement were visually quality checked in three planes by a trained investigator. Mean time activity curves for hemisphere weighted grey matter volumes were used in the kinetic modelling and the simplified reference tissue model with cerebellum (excluding vermis) as reference region [41, 42] yielded non-displaceable binding potential (BPND) as outcome measure.
Statistical analysis
Association between baseline 5-HT4R binding and concurrent anxiety
A latent variable model (LVM) [43] was used to jointly model the 5-HT4R BPND across brain regions and concurrent anxious depression at baseline. A separate LVM was used for each of the four anxiety measures. It included a single latent variable representing the three brain region’s 5-HT4R binding with neocortex as a reference (“global BPND”) and its relation to anxiety measures. Score tests were used to detect model misspecifications in the covariance structure and additional parameters were included until no misspecification could be detected.
5-HT4R BPND values in the LVM were log-transformed. In the primary baseline analyses, we tested for an association between global BPND and (A) factor score and (B) GAD-10 score. In a secondary analysis, we tested for a difference in global BPND between (a) anxious vs. non-anxious depression and (b) syndromal vs. non-syndromal anxious depression. If an effect was found, the association between 5-HT4R BPND in each brain region and the anxiety-measure was tested.
Association between baseline anxiety and treatment outcome (longitudinal analyses)
In the primary longitudinal analyses, we used a univariate linear regression model to test for an association between either (I) baseline factor score or (II) baseline GAD-10 score and categorical treatment outcome at week 8. Secondary, we tested for a difference in r∆HAMD6 in (III) anxious vs. non-anxious depression and (IV) syndromal vs. non-syndromal anxious depression at baseline.
Anxiety as a prognostic biomarker of treatment response (prediction analyses)
A receiver operating characteristic (ROC) curve was used to visualize the ability of baseline anxiety to discriminate between treatment remitters and non-responders from “all other” patients (i.e., intermediate responders combined with either non-responders or remitters respectively). The predictive ability of baseline anxiety was then summarized using area under the curve (AUC). On top of the clinical covariates, we tested the added value of anxiety by comparing AUCs between the predicted probabilities of a logistic model with and without anxiety. No cross-validation was used to correct the optimism in the estimated AUC because the optimism was expected to be small when assessing the anxiety effect (only 1 more degree of freedom when including this variable).
Association between change in 5-HT4R binding and concurrent baseline anxiety
In the rescan analyses, we used an LVM where we primarily tested if a change in BPND was associated with concurrent baseline anxiety; (1) syndromal vs. non-syndromal anxious depression and (2) anxious vs. non-anxious depression. Secondary, we tested if a change in global BPND was associated with a change in (3) factor score or (4) GAD-10 score from baseline to week 8.
An LVM has the advantage of no need for adjustment of multiple comparison at a global level since all regions are tested jointly. Regionally, p-values and 95% confidence intervals (CI) were adjusted for multiple comparisons using the single-step Dunnett procedure [44] denoted “p.adj”, unless otherwise specified. All tests were two-sided. Baseline analyses were adjusted for sex, age, injected tracer (mass/kg), 5-HTTLPR genotype status (LALA or non-LALA) [37, 45–47] and HAMD17/6 item 1 + 2 (depressed mood and feelings of guilt, i.e. non-anxious core depression scores). For the longitudinal and prediction analyses, we corrected for age, sex, HAMD17 item 1 + 2 and for use of benzodiazepines. The rescan analyses were corrected for the difference in injected tracer (mass/kg) and for the difference in HAMD17 item 1 + 2 between baseline and rescan. Missing data from the baseline PET-scan was considered missing completely at random. The longitudinal analyses were performed as complete case analyses and patients with non-compliance at week 8 were not included in these analyses. Violations of normality assumptions were inspected with Q–Q plots and all BPND values were log-transformed and at last back-transformed.
Results
Demographics and patient-characteristics are shown in Table 1. One hundred patients entered the study, nine of these did not complete the baseline PET-scan and two patients failed to fill in the baseline GAD-10 questionnaire. Seventy-eight patients (13 non-responders, 43 intermediate responders and 22 remitters) were included in the longitudinal analyses. One non-responder did not return the baseline GAD-10 questionnaire; 9 patients were excluded for various reasons (spontaneous remission (n = 1), acute psychosis (n = 1), intolerable side effects (n = 1), self-reported non-compliance (n = 1), withdrawal of consent (n = 5)), and four patients were excluded due to undetectable serum drug levels at week 8. Six patients were switched to duloxetine before week 8. Due to side-effects, one patient (remitter) maintained a therapeutic dose of 5 mg escitalopram and had a serum escitalopram level within one standard deviation from the mean of the group at week 8. Six patients received short-term benzodiazepines before week 8. Forty-three patients were allocated to the rescan-programme, three of those were excluded from the analyses because of non-compliance (n = 2) and PET-scan failure (n = 1). See Fig. S1 (CONSORT-diagram) for an overview. No serious adverse event occurred.
Table 1.
Demographics and clinical profile of patients at baseline.
| N | % | ||
|---|---|---|---|
| Sex | Female | 65 | 71.4 |
| Male | 26 | 28.6 | |
| 5-HTTLPR genotype | LALA | 26 | 28.6 |
| Non-LALA | 65 | 71.4 | |
| Syndromal anxious depression | Yes | 42 | 46.2 |
| No | 49 | 53.8 | |
| Anxious depression | Yes | 75 | 82.4 |
| No | 16 | 17.6 | |
| Severity of MDD | Severe | 32 | 35 |
| Moderate | 59 | 65 | |
| Mean (SD) | n | Range | |
| Age (years) | 27.1 (8.2) | 91 | 18.3–57.3 |
| Factor score | 7.9 (2.0) | 91 | 3–15 |
| GAD-10 score | 24.0 (9.3) | 89 | 7–47 |
| HAMD17 score | 22.9 (3.4) | 91 | 18–31 |
| HAMD6 score | 12.3 (1.6) | 91 | 7–17 |
| Years of education | 11.6 (1.1) | 76 | 5–12 |
| Body mass index (kg/m2) | 24.5 (5.6) | 91 | 17–45 |
| Injected dose (MBq) | 577.4 (56) | 91 | 263–615 |
| Injected mass/kg (µg/kg) | 0.013 (0.015) | 91 | 0.004–0.082 |
| Cerebellum, area under curve (kBq/ml) | 10.3 (2.6) | 91 | 3.9–17.8 |
5-HTTLPR; serotonin transporter-linked polymorphic region. MDD; Major depressive disorder. Factor score; total score from HAMD17-items 10- 13, 15 and 17. GAD-10; Generalized anxiety disorder 10 items. HAMD17+6; Hamilton depression rating scale 17 and 6 items.
Baseline outcomes
The LVM identified a negative association between the global 5-HT4R BPND and anxiety factor score (p = 0.046). At a regional level, there was a negative association between baseline BPND and factor score, spanning between 1.45% to 1.76% lower binding per anxiety factor score in neocortex, hippocampus and neostriatum (p.adj = 0.06) (Fig. 1A). A significant negative association was found between baseline global BPND and GAD-10 score (p < 0.01) (Fig. 1B). Regionally, there was between 0.45% to 0.57% lower 5-HT4R binding per GAD-10 item score across all tested regions (p.adj < 0.01) (Fig. 2 shows neocortex as a representative example). We found no evidence for a difference in global BPND in patients depending on anxious depression (n = 75, p = 0.52) or syndromal anxious depression (n = 42, p = 0.11).
Fig. 1. LVM of Baseline Scores.
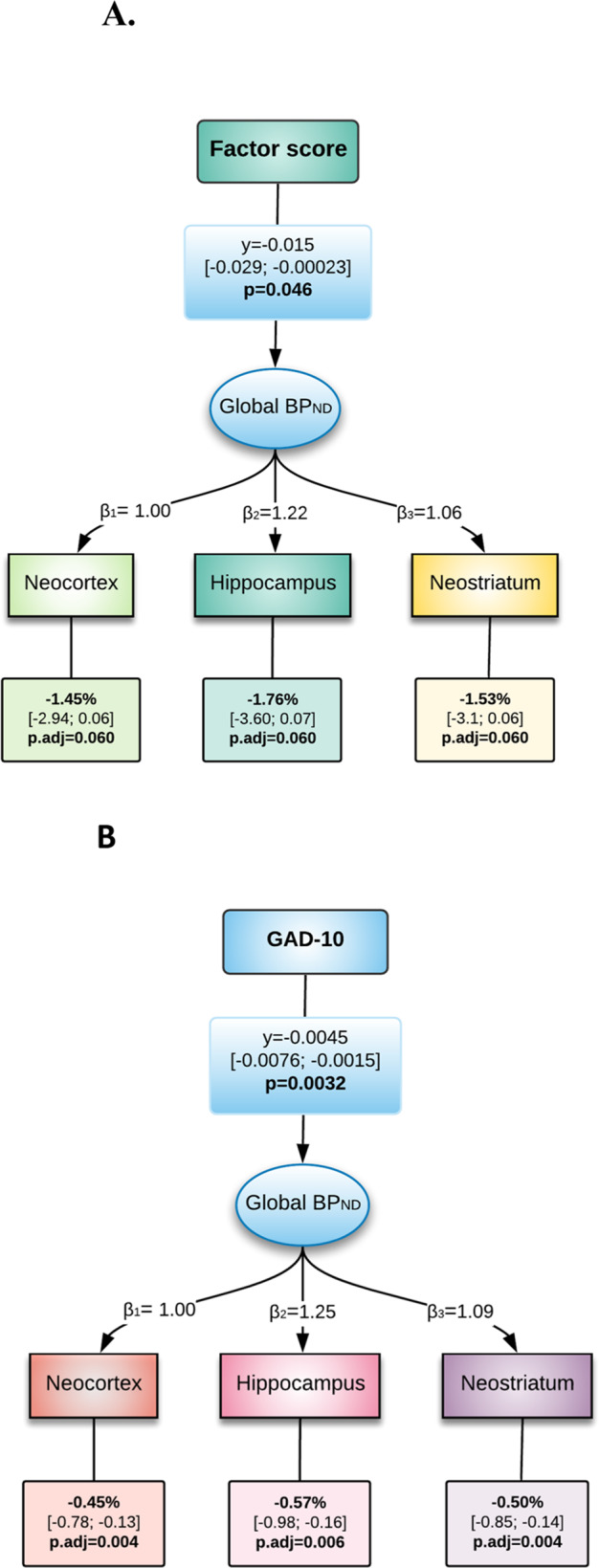
A Latent variable model of log-transformed baseline 5-HT4R BPND and factor score. B Latent variable model of log-transformed baseline 5-HT4R BPND and GAD-10. Factor score: Anxiety/somatization factor score. GAD-10: Generalized Anxiety Disorder 10 items. The lower boxes contain the percent change in regional binding for each unit increase in factor-score and GAD-10 score. β and γ correspond to the effects on the log-transformed binding.
Fig. 2. Partial residual plot showing the association between baseline 5-HT4R BPND in neocortex and GAD-10 (adjusted for covariates).
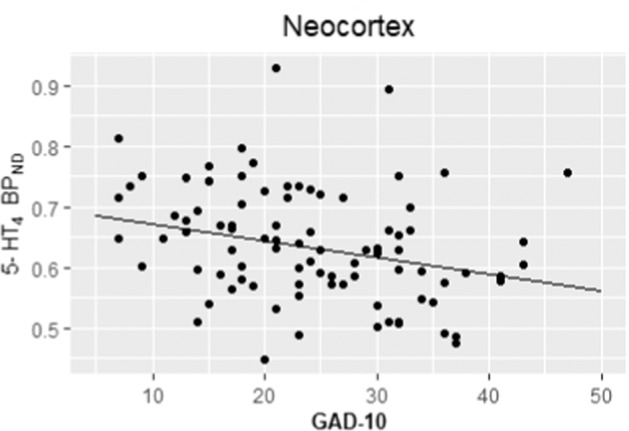
Adjustment is performed using a linear regression on the log-transformed binding, subtracting the covariate effects compared to a female subject, age 25, non-LALA, injected mass/kg of 0.0085 and a HAMD item 1 + 2 score of 5 as reference.
Longitudinal outcomes
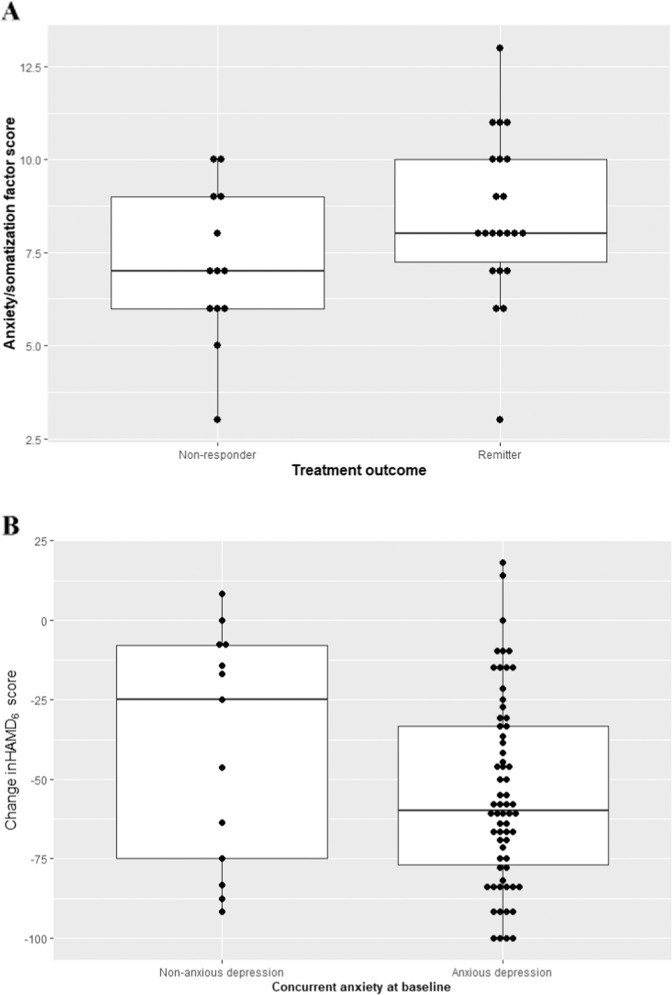
The baseline factor score was 1.6 point higher ([0.07–3.14], p = 0.04) in week 8 remitters compared to non-responders (Fig. 3A). One remitter was an outlier, and exclusion of this observation led to similar results (+1.76 point [0.19–3.32], p = 0.029). We did not find an association between baseline GAD-10 score and treatment response (regression coefficient 2.34 [−3.59 to 8.27], p = 0.43). We estimated a greater reduction in r∆HAMD6 of 18.3 points ([−37.3–0.79], p = 0.06) for anxious vs. non-anxious depression (Fig. 3B). Syndromal anxious depression at baseline was not associated with change in HAMD (−2.18 [−16.8–12.4], p = 0.77).
Fig. 3. Longitudinal outcomes in anxious and remitters.
A Baseline factor score in MDD non-responders and remitters as defined after 8 weeks of treatment. B Change in HAMD score from baseline to week 8 in patients with concurrent anxious vs. non-anxious depression at baseline.
Prediction of treatment outcome
AUC ranged between 0.64 and 0.67; inclusion of baseline anxiety measures resulted in an increase in AUC between 0 and 0.14. The best predictive value was found for non-responders versus all others when including baseline anxious depression as predictor; AUC with 0.79 [0.68–0.89] versus without 0.65 [0.49–0.81] anxious depression at baseline, although not significant p = 0.12 (not adjusted) (Table S1 and Fig. S2).
Rescan
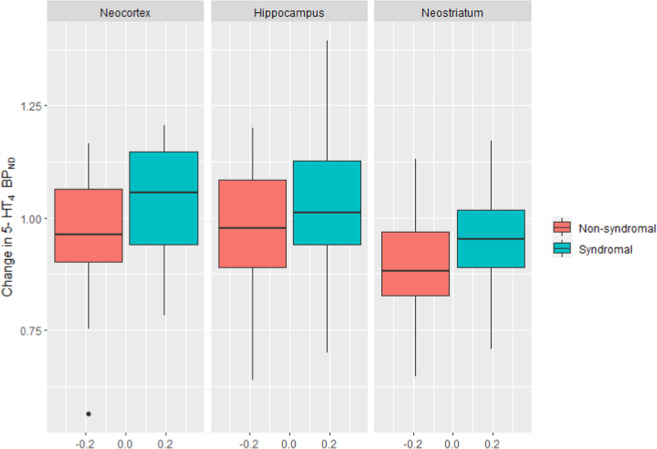
After 8 weeks of treatment, we found an association between the change in global BPND and syndromal anxious depression at baseline (p = 0.034). The distribution of the change in binding according to syndromal anxious depression is visualized in Fig. 4. In neocortex, patients with baseline syndromal anxious depression had a change in BPND of +3.5% vs. −4.7% in patients with non-syndromal depression, leading to a ratio of (1 + 3.5%)/(1–4.7%) = 8.5% (95%CI [−0.037 to 17.8], p.adj = 0.051) between groups. Correspondingly, in hippocampus there was a change in binding of +7.1% vs. −2.9%, and a ratio of 10.3%, (95%CI [−0.069 to 21.7], p.adj = 0.052), and for neostriatum −3.9% vs. −10.9, and a ratio of 7.8% (95%CI [0.04 to 16.1], p.adj = 0.048). One observation was an outlier and exclusion of this patient led to a slight decrease of the global effect from 8.51% to 5.54% (p = 0.068). We found no evidence for an association between change in BPND and anxious or non-anxious depression (p = 0.62), or any of the secondary outcomes (change in factor score (p = 0.46), change in GAD-10 score (p = 0.67)). In a post-hoc analysis, adjustment for duloxetine- or escitalopram treatment did not significantly influence the results.
Fig. 4. Change in 5-HT4R BPND in patients with/without syndromal anxious depression at baseline after 8 weeks of serotonergic antidepressant treatment.
Regions of interest: neocortex, hippocampus and neostriatum. 1.00 on the y-axis represents no change in binding between baseline and week 8. Boxplot shows median bars and are not adjusted for covariates.
Discussion
In line with our hypothesis, a higher level of baseline concurrent anxiety symptoms was associated with lower 5-HT4R BPND across brain regions, both according to the self-rating questionnaire GAD-10 and the interview-based assessment of HAMD. The difference in BPND per one GAD-10 score was small (~0.5%), but a more clinically meaningful measure e.g. per ten GAD-10 score would generate a ~5% difference in BPND, which is comparable to the magnitude of SSRI induced change seen in healthy men after 3 weeks of SSRI-intervention [22].
In line with rodent litterature [16–19], lower 5-HT4R agonistic capacity in humans may represent an endophenotype which is more prone to anxiety. We cannot, however, firmly establish whether low 5-HT4R binding is a trait or a state marker for anxiety in depression. Intriguingly, if 5-HT4R is an inverse proxy for cerebral 5-HT levels [22, 38], our observations suggest that high anxiety levels in depression are associated with higher-than-normal brain 5-HT levels and may correspond to the observation of serotonin-increase caused engagement of anxiety and fear-promoting circuit in the brain [48] and the well-known transiently increased anxiety following initiation of SSRI treatment. In line with our previous study [26], we propose that in the depressed or anxious state, higher brain 5-HT levels could reflect a compensatory mechanism to gain anxiolytic and antidepressant effects.
However, it is not possible in this study to establish whether the binding to 5-HT4R should be understood as an inverse proxy for 5-HT level and/or as a direct 5-HT4R effect.
We did not find that baseline anxiety was associated with worse treatment outcome; remitters had even a slightly higher baseline factor score than non-responders. The effect size, however, was small and the clinical importance of this observation is uncertain, especially since there was no difference in GAD-10 baseline score between non-responders and remitters (p = 0.43). Others have found that higher baseline anxiety score was associated with worse treatment response [49], although this observation might have been drug-specific since patients with higher baseline anxiety had better effect of escitalopram than another SSRI. In the STAR*D trial [8], patients with anxious depression were less likely to obtain remission. It is possible that the relatively young age (27, SD 8.2 years) of our patients, as age is known to increase the likelihood of a favourable outcome of SSRI’s [50–52], could influence this difference.
For the prediction analyses, we investigated the added value of concurrent anxiety at baseline to identify non-responders or remitters from all others but found no evidence for such a discriminative power. This may be due to the limited sample size. The largest added value was observed when using baseline anxious depression, with an improvement in AUC of 0.14, which is (while not statistically significant in our study) not negligible.
Even though we found no clear evidence for a difference in 5-HT4R binding between syndromal- and non-syndromal anxious depression at baseline (p = 0.11), patients with syndromal anxious depression had higher 5-HT4R binding after 8 weeks of treatment compared with their counterpart. Further, non-syndromal anxious patients had a decrease in 5-HT4R binding across all brain regions at rescan, while syndromal anxious patients showed lowered 5-HT4R binding in neostriatum only. This suggests that concurrent anxiety before drug treatment may influence the drug effect on the 5-HT4R.
Some limitations should be mentioned. First; without a control group, we cannot determine if anxiety score is negatively associated with 5-HT4R binding in general. Studies addressing this matter are needed. Second; the choice of treatment response criteria for non-responders and remitters can be debated. Our choice reduced the group sample size, potentially leading to a type-II error in the longitudinal- and rescan analyses. On the other hand, the rather restricted definitions allow for a more mechanistic interpretation of the results, where only the extreme response outcomes are included which was the á priori intention of this classification [28].
Third; the HAMD17 anxiety/somatization factor score has been criticized for not being stable across studies and to be weakly correlated to specific anxiety scales [53, 54]. Here, we included the factor score to allow for comparisons with other studies of anxious depression in addition to specific anxiety measures including GAD-10 score and syndromal anxious depression [34, 35] which provides diagnostic consistency across studies [31]. It is possible that other anxiety measures would have yielded different results, but we believe that this study covers a wide range of anxiety measures, both subjective and objective, which enhances the validity of the results. Fourth; results are not adjusted for analyses made for outcomes in other domains included in the main trial [28], and some p-values presented here were borderline and therefore with limited evidence. However, we only consider four outcomes (PET in three brain regions and concurrent anxiety). PET measurements were summarized using a latent variable, such that a single parameter was used to relate PET and anxiety with no need for adjustment at the global level. Fifth; for the rescan results, we cannot exclude the possibility that the effects occurred because of multiple factors contributing to 5-HT4R binding. It is expected that a change in binding may have several causes but even though we only assess associations here, anxiety seems to be a favourable candidate for future investigations.
In conclusion, we found that concurrent anxiety in patients with depression was negatively associated with cerebral 5-HT4R binding. We also found that remitting patients had higher baseline anxiety compared to those who did not respond to treatment. We did not find conclusive evidence for baseline anxiety as a useful predictor of treatment outcome in depression. At rescan, we found that concurrent syndromal anxious depression was associated with a global change in 5-HT4R binding after serotonergic antidepressant treatment. Overall, the 5-HT4R appears to be a promising neuroreceptor for further understanding the biological underpinnings of concurrent anxiety in patients with MDD.
Supplementary information
Acknowledgements
Our gratitude goes to the participants and relatives who took part in the study, all investigators and collaborating recruiting sites within the Mental Health Services in the capital region of Denmark. We would like to recognize and thank the personnel involved in laboratory work and technical assistance, and especially; Lone Ibsgaard Freyr, Gerda Thomsen, Svitlana Olsen, Agnete Dyssegaard, Arafat Nasser, Bente Dall, Peter Steen Jensen, Dorthe Givard, Ida Marie Brandt and Anna Maria Florescu. Financial support was granted from various foundations: the Research Fund of the Mental Health Services—Capital Region of Denmark, the Innovation Fund Denmark and H. Lundbeck A/S (GrantID: 5189-00087 A), the Independent Research Fund Denmark (GrantID: DFF-6120-00038), the Lundbeck Foundation alliance BrainDrugs (GrantID: R279-2018-1145), the Research Council of Rigshospitalet, the Augustinus Foundation (GrantID: 16-0058), the G.J. Foundation and Savværksejer Jeppe Juhl og hustru Ovita Juhls Mindelegat. The fundings were given as unrestricted grants and did not involve decisions regarding the design of the study, data collection, analyses, interpretation, or writing.
Author contributions
KK-F, BO, AJ, GMK, VGF and MBJ contributed to the conception of the study. KK-F, ASP, EBL, SVL, VHD, CTI, AJ, VGF and MBJ helped design the study. BO, KK-F, MBJ, CS contributed to the analysis. All authors contributed to interpretation of data and critically and carefully read, revised and approved the final manuscript.
Code availability
All analyses were performed in R Studio (Version 1.4.1717). The code is available upon request.
Competing interests
MBJ has given talks sponsored by H. Lundbeck and Boehringer Ingelheim. GMK has served as expert consultant for SAGE Therapeutics and Sanos. VGF has served as consultant for SAGE Therapeutics. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-022-02034-5.
References
- 1.Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, et al. Article difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008. www.star-d.org. [DOI] [PubMed]
- 2.Hamilton M. A rating scale for depression. J Neurol, Neurosurg, Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018. https://github.com/ihmeuw/. [DOI] [PMC free article] [PubMed]
- 4.Lamers F, van Oppen P, Comijs HC, Smit JH, Spinhoven P, van Balkom AJ, et al. Comorbidity patterns of anxiety and depressive disorders in a large cohort study: the Netherlands Study of Depression and Anxiety (NESDA) J Clin psychiatry. 2011;72:341–8. doi: 10.4088/JCP.10m06176blu. [DOI] [PubMed] [Google Scholar]
- 5.Fava M, Rankin MA, Wright EC, Alpert JE, Nierenberg AA, Pava J, et al. Anxiety disorders in major depression. Compr Psychiatry. 2000;41:97–102. [DOI] [PubMed]
- 6.Tiller JWG. Depression and anxiety. Med J Aust. 2012;1:28–32. doi: 10.5694/mjao12.10628. [DOI] [Google Scholar]
- 7.Gorman JM. Comorbid depression and anxiety spectrum disorders. Depress Anxiety. 1996–1997;4:160–8. [DOI] [PubMed]
- 8.Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008. 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed]
- 9.Nelson JC. Anxious depression and response to treatment. Am J Psychiatry. 2008. 10.1176/appi.ajp.2007.07121927. [DOI] [PubMed]
- 10.Iscan Z, Rakesh G, Rossano S, Yang J, Zhang M, Miller J, et al. A positron emission tomography study of the serotonergic system in relation to anxiety in depression. European Neuropsychopharmacol. 2017. 10.1016/j.euroneuro.2017.07.009. [DOI] [PMC free article] [PubMed]
- 11.Reimold M, Batra A, Knobel A, Smolka MN, Zimmer A, Mann K, et al. Anxiety is associated with reduced central serotonin transporter availability in unmedicated patients with unipolar major depression: a [11C]DASB PET study. Mol. Psychiatry. 2008. 10.1038/sj.mp.4002149. [DOI] [PubMed]
- 12.Reimold M, Knobel A, Rapp MA, Batra A, Wiedemann K, Ströhle A, et al. Central serotonin transporter levels are associated with stress hormone response and anxiety. Psychopharmacology. 2011. 10.1007/s00213-010-1903-y. [DOI] [PMC free article] [PubMed]
- 13.Beliveau V, Ganz M, Feng L, Ozenne B, Højgaard L, Fisher PM, et al. A high-resolution in vivo atlas of the human brain’s serotonin system. J Neurosci. 2017. 10.1523/JNEUROSCI.2830-16.2016. [DOI] [PMC free article] [PubMed]
- 14.Bonaventure P, Hall H, Gommeren W, Cras P, Langlois X, Jurzak M, et al. Mapping of serotonin 5-HT4 receptor mRNA and ligand binding sites in the post-mortem human brain. Synapse. 2000. 10.1002/(SICI)1098-2396(200004)36. [DOI] [PubMed]
- 15.Bockaert J, Claeysen S, Compan V, Dumuis A. 5-HT4 receptors. Curr Drug Targets CNS Neurol Disord. 2004. 10.2174/1568007043482615. [DOI] [PubMed]
- 16.Compan V, Zhou M, Grailhe R, Gazzara RA, Martin R, Gingrich J, et al. Attenuated response to stress and novelty and hypersensitivity to seizures in 5-HT4 receptor knock-out mice. J. Neurosci. 2004. 10.1523/JNEUROSCI.2806-03.2004. [DOI] [PMC free article] [PubMed]
- 17.Faye C, Hen R, Guiard BP, Denny CA, Gardier AM, Mendez-David I, et al. Rapid anxiolytic effects of RS67333, a serotonin type 4 receptor agonist, and diazepam, a benzodiazepine, are mediated by projections from the prefrontal cortex to the dorsal raphe nucleus. Biol Psychiatry. 2020. 10.1016/j.biopsych.2019.08.009. [DOI] [PubMed]
- 18.Mendez-David I, David DJ, Darcet F, Wu MV, Kerdine-Römer S, Gardier AM, et al. Rapid anxiolytic effects of a 5-HT4 receptor agonist are mediated by a neurogenesis-independent mechanism. Neuropsychopharmacology. 2014. 10.1038/npp.2013.332. [DOI] [PMC free article] [PubMed]
- 19.Chen BK, Mendez-David I, Luna VM, Faye C, Gardier AM, David DJ, et al. Prophylactic efficacy of 5-HT4R agonists against stress. Neuropsychopharmacology. 2019. 10.1038/s41386-019-0540-3. [DOI] [PMC free article] [PubMed]
- 20.Lucas G, Rymar VV, Du J, Mnie-Filali O, Bisgaard C, Manta S, et al. Serotonin(4) (5-HT(4)) receptor agonists are putative antidepressants with a rapid onset of action. Neuron. 2007. 10.1016/j.neuron.2007.07.041. [DOI] [PubMed]
- 21.Murphy SE, Wright LC, Browning M, Cowen PJ, Harmer CJ. A role for 5-HT4 receptors in human learning and memory. Psychol Med. Published 2019. 10.1017/S0033291719002836. [DOI] [PubMed]
- 22.Haahr ME, Fisher PM, Jensen CG, Frokjaer VG, Mahon BM, Madsen K, et al. Central 5-HT4 receptor binding as biomarker of serotonergic tonus in humans: A [11C]SB207145 PET study. Mol Psychiatry. 2014. 10.1038/mp.2013.147. [DOI] [PubMed]
- 23.Licht CL, Marcussen AB, Wegener G, Overstreet DH, Aznar S, Knudsen GM. The brain 5-HT4 receptor binding is down-regulated in the Flinders Sensitive Line depression model and in response to paroxetine administration. J. Neurochemistry. 2009. 10.1111/j.1471-4159.2009.06050.x. [DOI] [PubMed]
- 24.Vidal R, Valdizán EM, Mostany R, Pazos A, Castro E Long-term treatment with fluoxetine induces desensitization of 5-HT 4 receptor-dependent signalling and functionality in rat brain. J. Neurochemistry. 2009. 10.1111/j.1471-4159.2009.06210.x. [DOI] [PubMed]
- 25.Rebholz H, Friedman E, Castello J. Alterations of expression of the serotonin 5-HT4 receptor in brain disorders. Int J Mol Sci. 2018. 10.3390/ijms19113581. [DOI] [PMC free article] [PubMed]
- 26.Köhler-Forsberg K, Ozenne B, Landman EB, Larsen SV, Poulsen AS, Dam VH, et al. Evidence for a serotonergic subtype of major depressive disorder: A NeuroPharm-1 study. medRxiv.10.1101/2021.06.17.21258740.
- 27.Amigó J, Díaz A, Pilar-Cuéllar F, Vidal R, Martín A, Compan V, et al. The absence of 5-HT4 receptors modulates depression- and anxiety-like responses and influences the response of fluoxetine in olfactory bulbectomised mice: adaptive changes in hippocampal neuroplasticity markers and 5-HT1A autoreceptor. Neuropharmacology. 2016. 10.1016/j.neuropharm.2016.08.037. [DOI] [PubMed]
- 28.Köhler-Forsberg K, Jorgensen A, Dam VH, Stenbæk DS, Fisher PM, Ip CT, et al. Predicting treatment outcome in major depressive disorder using serotonin 4 receptor PET brain imaging, functional MRI, cognitive-, EEG-based, and peripheral biomarkers: a NeuroPharm open label clinical trial Protocol. Front Psychiatry. 2020. 10.3389/fpsyt.2020.00641. [DOI] [PMC free article] [PubMed]
- 29.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR (text revision). Wiley. 2000. 10.1002/9780470479216.corpsy0271.
- 30.Bech P, Lunde M, Undén M. An inventory for the measurement of generalised anxiety distress symptoms, the GAD-10 Inventory. Acta Psychiatrica Belg. 2005;105:111–8.
- 31.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed]
- 32.Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG, Shaw JL, Thompson L, Nelson DL, et al. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology. 2001. 10.1016/S0893-133X(01)00298-6. [DOI] [PubMed]
- 33.Cleary P, Guy W. Factor analysis of the hamilton depression scale. Drugs Exp Clin Res. 1977;1:115–20. [Google Scholar]
- 34.Ionescu DF, Niciu MJ, Henter ID, Zarate CA. Defining anxious depression: a review of the literature. CNS Spectrums. 2013. 10.1017/S1092852913000114. [DOI] [PMC free article] [PubMed]
- 35.Braund TA, Palmer DM, Williams LM, Harris AWF. Characterising anxiety in major depressive disorder and its use in predicting antidepressant treatment outcome: An iSPOT-D report. Aust N Z J Psychiatry. 2019. 10.1177/0004867419835933. [DOI] [PubMed]
- 36.Timmerby N, Andersen JH, Søndergaard S, Østergaard SD, Bech P. A systematic review of the clinimetric properties of the 6-item version of the Hamilton Depression Rating Scale (HAM-D6). Psychother Psychosom. 2017. 10.1159/000457131. [DOI] [PubMed]
- 37.Fisher PM, Holst KK, Mc Mahon B, Haahr ME, Madsen K, Gillings N, et al. 5-HTTLPR status predictive of neocortical 5-HT 4 binding assessed with [11C]SB207145 PET in humans. NeuroImage. 2012. 10.1016/j.neuroimage.2012.05.013. [DOI] [PubMed]
- 38.Madsen K, Torstensen E, Holst KK, Haahr ME, Knorr U, Frokjaer VG, et al. Familial risk for major depression is associated with lower striatal 5-HT<inf>4</inf> receptor binding. Int J Neuropsychopharmacol. 2015. 10.1093/ijnp/pyu034. [DOI] [PMC free article] [PubMed]
- 39.Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing pet images. J Comput Assist Tomogr. 1992. 10.1097/00004728-199207000-00024. [DOI] [PubMed]
- 40.Svarer C, Madsen K, Hasselbalch SG, Pinborg LH, Haugbøl S, Frøkjar VG, et al. MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. NeuroImage. 2005. 10.1016/j.neuroimage.2004.10.017. [DOI] [PubMed]
- 41.Marner L, Gillings N, Comley RA, Baaré WF, Rabiner EA, Wilson AA, et al. Kinetic modeling of 11C-SB207145 binding to 5-HT4 receptors in the human brain in vivo. J Nucl Med. 2009. 10.2967/jnumed.108.058552. [DOI] [PubMed]
- 42.Marner L, Gillings N, Madsen K, Erritzoe D, Baaré WF, Svarer C, et al. Brain imaging of serotonin 4 receptors in humans with [11C]SB207145-PET. NeuroImage. 2010. 10.1016/j.neuroimage.2010.01.054. [DOI] [PubMed]
- 43.Holst KK, Budtz-Jørgensen E. Linear latent variable models: the lava-package. Comput Stat. 2013. 10.1007/s00180-012-0344-y.
- 44.Dmitrienko A, D’Agostino Sr R. Traditional multiplicity adjustment methods in clinical trials. Stat Med. 2013;32:5172–218. doi: 10.1002/sim.5990. [DOI] [PubMed] [Google Scholar]
- 45.Fisher PM, Holst KK, Adamsen D, Klein AB, Frokjaer VG, Jensen PS, et al. BDNF Val66met and 5-HTTLPR polymorphisms predict a human in vivo marker for brain serotonin levels. Hum Brain Mapp. 2015. 10.1002/hbm.22630. [DOI] [PMC free article] [PubMed]
- 46.Madsen K, Haahr MT, Marner L, Keller SH, Baaré WF, Svarer C, et al. Age and sex effects on 5-HT 4 receptors in the human brain: A 11 CSB207145 PET study. J Cereb Blood Flow Metab. 2011. 10.1038/jcbfm.2011.11. [DOI] [PMC free article] [PubMed]
- 47.Madsen K, Marner L, Haahr M, Gillings N, Knudsen GM. Mass dose effects and in vivo affinity in brain PET receptor studies - a study of cerebral 5-HT 4 receptor binding with [11C]SB207145. Nucl Med Biol. 2011. 10.1016/j.nucmedbio.2011.04.006. [DOI] [PubMed]
- 48.Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF, et al. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature. 2016;537:97–101. doi: 10.1038/nature19318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boulenger JP, Hermes A, Huusom AKT, Weiller E. Baseline anxiety effect on outcome of SSRI treatment in patients with severe depression: Escitalopram vs paroxetine. Curr Med Res Opin. 2010. 10.1185/03007990903482467. [DOI] [PubMed]
- 50.Joyce PR, Mulder RT, Luty SE, McKenzie JM, Rae AM. A differential response to nortriptyline and fluoxetine in melancholic depression: The importance of age and gender. Acta Psychiatr Scandinavica. 2003;108:20–23. doi: 10.1034/j.1600-0447.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- 51.Mulder RT, Watkins WGA, Joyce PR, Luty SE. Age may affect response to antidepressants with serotonergic and noradrenergic actions. J Affect Disord. 2003;76:143–9. doi: 10.1016/S0165-0327(02)00083-6. [DOI] [PubMed] [Google Scholar]
- 52.Parker G. Differential effectiveness of newer and older antidepressants appears mediated by an age effect on the phenotypic expression of depression. Acta Psychiatr Scandinavica. 2002;106:168–70. doi: 10.1034/j.1600-0447.2002.02432.x. [DOI] [PubMed] [Google Scholar]
- 53.Goldberger C, Guelfi JD, Sheehan DV. Assessment of anxiety in clinical trials with depressed patients using the Hamilton depression rating scale. Psychopharmacology Bulletin. 2011;44(3):34-–50. [PMC free article] [PubMed]
- 54.Broen MP, Moonen AJ, Kuijf ML, Dujardin K, Marsh L, Richard IH, et al. Factor analysis of the Hamilton depression rating scale in Parkinson’s disease. Parkinsonism and Relat Disord. 2015. 10.1016/j.parkreldis.2014.11.016. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All analyses were performed in R Studio (Version 1.4.1717). The code is available upon request.