Abstract
Interleukin-12 (IL-12) is a type I cytokine involved in both innate and adaptive immunity that stimulates T and natural killer cell activity and induces interferon gamma production. IL-12 has been identified as a potential immunotherapeutic component for combinatorial cancer treatments. While IL-12 has successfully been used to treat a variety of cancers in mice, it was associated with toxicity when administered systemically in cancer patients. In this review, we discuss the research findings and progress of IL-12 used in combination with other cancer treatment modalities. We describe different methods of IL-12 delivery, both systemic and local, and ultimately highlight the potential of an in situ vaccination approach for minimizing toxicities and providing antitumor efficacy. This review offers a basis for pursuing an in situ vaccine approach that may eventually allow IL-12 to be more readily integrated as an immunotherapy into the clinical treatment of cancers.
Keywords: Interleukin-12, In situ cancer vaccine, Cancer immunotherapy, Local delivery, Targeted delivery
Introduction
Originally characterized in the early 1990s [1], IL-12 is a pro-inflammatory type I cytokine known for its potent activation of CD8 + T and natural killer (NK) cells and role in the increased production of interferon gamma (IFN-γ) [1]. Primarily acting within the tumor microenvironment (TME) when tumor-targeted [2], IL-12 has also been found to synergize with other cytokines and immunotherapeutic treatments. While the biological and synergistic effects of IL-12 have been shown to augment antitumor activity, IL-12 has also been associated with severe toxic side effects largely due to excessive systemic IFN-γ [3]. The success of IL-12 in preclinical models has encouraged ongoing research efforts to mitigate its toxicity via targeted delivery and increased local concentration within the TME. This review provides a molecular description of IL-12 and its role in the innate and adaptive immune responses. We discuss ongoing research efforts and challenges associated with integrating IL-12 into combinatorial cancer treatment regimens, demonstrating that these efforts have allowed IL-12 to become an increasingly feasible immunotherapeutic treatment, particularly for its use in an in situ cancer vaccine. In an in situ vaccine, immunomodulating treatments are delivered in the tumor, thereby inducing local and systemic T cell responses against a patient’s tumor antigens.
Molecular and biological basis of IL-12: structure, expression, regulation, and immune mechanisms
IL-12 is a type I cytokine that serves as the ligand of a receptor containing two amino acid chains: IL-12R-β1 and IL-12R-β2 [4]. The IL-12 receptor (IL-12R) is expressed either constitutively or in an inducible manner in several types of immune cells [5]; most notably, NK cells and T and B lymphocytes express the IL-12R [4]. IL-12 is a heterodimer comprised of a 35-kDa light chain known as p35 or IL-12α and a 40-kDa heavy chain known as p40 or IL-12β [6].
IL-12 production is induced by the interaction between CD40 on antigen-presenting cells (APCs) and CD40 ligand (CD40L) expressed on activated T cells [7]. IL-12 is predominantly produced by activated phagocytic cells [8] and dendritic cells (DC) [7], but also neutrophils and microglia [6]. It is known to be an enhancer of the proliferation and cytotoxic activity of both CD8 + T cells and NK cells [8, 9]. Once produced, IL-12 induces production of IFN-γ in T and NK cells [8] via activation of their IL-12Rs. Amplifying signals such as IFN-γ, IL-15, or CD40-CD40L cell–cell interactions precede the optimal production of biologically active IL-12 [2]. Thus, the production of IFN-γ can be described as benefiting from a positive feedback loop in which the secretion of IFN-γ induces IL-12 production, and IL-12 further induces secretion of IFN-γ by APCs [2]. As opposed to IFN-γ or other amplifying signals, cytokines IL-10 and TGF-β1 have the opposite effect, downregulating IL-12 production [2].
Importantly, and to the interest of immunotherapeutic research, IL-12 connects innate and adaptive immunity (Fig. 1). Produced during the inflammatory innate response, IL-12 directs naive CD4 + T cells toward T helper (Th) 1 differentiation by augmenting IFN-γ production [6]. Thus, IL-12 is an important regulator of adaptive, cell-mediated immunity. IL-12 is also known to directly or indirectly (such as through IFN-γ) stimulate B cells, enhancing the production of IgG2a antibodies in mice [6].
Fig. 1.
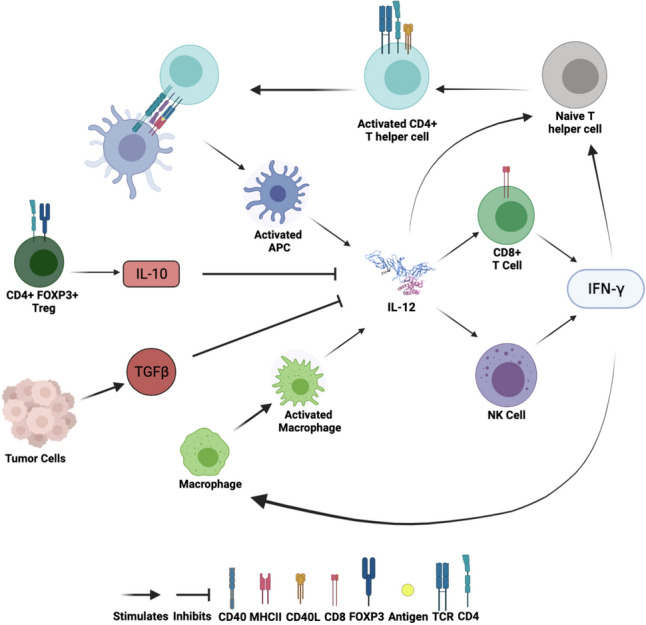
Role of IL-12 in innate and adaptive immunity. IL-12 is produced by activated APCs. IL-12 acts predominantly on lymphocytes such as T and NK cells. Stimulation by IL-12 causes these cells to increase their secretion of IFN-γ, further activating tumor-suppressing immune responses. IL-12 and IFN-γ amplifying signals enhance the cytotoxic activities of NK and CD8 + T cells and the cytokine response of CD4 + T cells. IL-10 and TGFβ produced in the TME inhibit secretion of IL-12. Created with BioRender.com
Synergistic and therapeutic effects of IL-12-based combination treatments
Synergy with immune molecules and other therapies
IL-12 has been shown to synergize in its biological activity with several other cytokines, including tumor necrosis factor α, IL-2, IL-15, IL-18, and granulocyte–macrophage colony-stimulating factor (GM-CSF) [4, 10, 11]. The potent synergistic effects observed in IL-12/IL-2 treatment combinations are especially well-studied. The interaction of these two cytokines involves the reciprocal upregulation of each other’s receptors using separate signaling pathways [11]. Alone, the two cytokines are each able to induce CD3 + T cell proliferation, IFN-γ production, and tumor cell death through NK and CD8 + T cell stimulation [11]. When used in combination, the synergistic effects of IL-12/IL-2 include the phosphorylation of signaling proteins and the augmentation of activated T cell response [11]. While IL-2-based immunotherapy is better studied and has achieved FDA approval for cancer immunotherapy, IL-12 appears to have greater potency in many systems [12, 13]. Thus, the two cytokines are well-positioned to be studied in tandem given their unique and shared biological effects.
IL-12 synergizes with certain biological molecules to potentiate the immune response. In an in vitro study that co-administered B7-transfected L cells or certain anti-CD28 antibodies with IL-12, a synergistic effect in activating human T cells was observed. The maximal levels of T cell proliferation obtained using IL-12 added to B7-transfected cells or certain anti-CD28 antibodies were higher than those obtained using IL-2 alone, even when IL-12 concentrations were 100 to 1000 times lower than effective concentrations of IL-2. [14]. Others demonstrated the synergistic relationship between OX40 and IL-12 in three different tumor models [15]. Specifically, IL-12 signaling was shown to be critical for OX40-mediated CD4 + T cell survival and function.
IL-12 was also shown to synergize with other forms of cancer treatment, i.e., radiotherapy (RT) as will be detailed below, and chemotherapy. In regard to chemotherapy, it was shown that IL-12 and cyclophosphamide can synergize to eradicate large immunogenic tumors [16] which involves activation of T cells and macrophages [17].
Antitumor effects of IL-12 as a vaccine adjuvant
A number of studies have combined IL-12 with cancer vaccines to mediate antitumor effects. One study using a combination-based immunotherapy regimen found that combining IL-12 with a cancer vaccine comprised of major histocompatibility complex (MHC) class II and CD80-transfected tumor cells elicited the highest degree of T and NK cell activation, IFN-γ production, and antitumor effect against B16-derived lung metastases in mice [18]. In a murine B78-H1 melanoma model, a vaccine containing JAWSII DCs (an immature DC line) plus IL-12 using three vaccination schedules was superior to treatments using DCs or IL-12 given separately [19]. One group evaluated the effectiveness of the combined therapy of tumor lysate-pulsed DC immunization and IL-12 in inducing antitumor immunity in a mouse hepatocellular carcinoma model [20]. The combined therapy, systemically administered, resulted in either tumor rejection or significantly inhibited tumor growth compared to mice treated with the lysate-pulsed DCs alone. The combination therapy was shown to be dependent on CD8 + and CD4 + T cells but not NK cells, indicating that IL-12 had potentiated the therapeutic effect of immunization against hepatocellular carcinoma in mice. In another study, mice challenged with either TSA mammary adenocarcinoma or C-26 colon adenocarcinoma cells engineered to release IL-2 received single or multiple intraperitoneal (i.p.) injections of recombinant IL-12 [21]. The combination of systemic recombinant IL-12 and locally released IL-2 led to a greater percentage of mice that later rejected these two different IL-2 gene-transduced tumors. Several cases of tumor eradication after vaccine combination with IL-12 also indicated the crucial role IL-12 may have in overcoming tumor immune evasion mechanisms [20, 22]. IL-12 has been found to inhibit tumors via IFN-γ and T cell-dependent pathways and via suppression of angiogenesis [23, 24].
Noguchi et al. examined whether a combination of antigenic peptide 234CM in adjuvant QS-21 immunization and i.p. IL-12 treatment could suppress the growth of established Meth A sarcoma tumors [25]. Results showed that the growth of Meth A was suppressed in IL-12-treated mice immunized with 234CM in QS-21 but not in IL-12-treated mice injected with either QS-21 or 234CM alone. Without IL-12 treatment, no tumor suppression was observed in mice vaccinated with 234CM in QS-21. However, it is important to note the authors reported multiple toxic side effects caused by IL-12 in the mice in this regimen. Likely due to its systemic administration, mice given injections of 500 ng or more of IL-12 experienced weight loss, piecemeal necrosis of the liver, hepatosplenomegaly, and elevation of multiple serum enzymes, the latter of which was observed to persist for up to 4 weeks post-IL-12 injections and was seen in mice treated with doses as low as 0.1 ng of IL-12. These toxic side effects are a primary concern related to the systemic administration of IL-12 across experimental models [26–28] and the clinic [3], suggesting the need to use IL-12 in ways that cause less systemic exposure of IL-12; one such strategy involves targeting IL-12 directly to the site(s) of tumor to maintain antitumor efficacy while minimizing systemic exposure and resultant toxicity.
Localized IL-12 increases the likelihood of antitumor efficacy via use of immune checkpoint blockade
Immune checkpoint blockade has emerged as a “common denominator” treatment approach to a variety of cancer types including melanoma, kidney, lung, prostate, and several other cancers [29]. Combined, seven types of anti-CTLA-4, anti-PD-1, and anti-PD-L1 monoclonal antibodies have been approved by the FDA [30] and represent some of the most practical and efficacious forms of cancer immunotherapy currently available. The improvement of response rates to immune checkpoint inhibitors could thus have important clinical impact. IL-12 has been identified as an immunotherapeutic agent that can augment the efficacy of checkpoint blockade therapy in several preclinical [31–36] and clinical trials [37, 38]. Garris et al. suggested the activation of antitumor effector T cells by anti-PD-1 is not direct but instead involves T cell:DC crosstalk and is licensed by IFN-γ and IL-12 [39]. In a phase II trial of intratumoral (i.t.) IL-12 DNA via plasmid electroporation combined with anti-PD-1 antibody pembrolizumab in patients with non-immune infiltrated, “cold” melanoma, correlative analyses showed that the combination made these tumors more responsive to pembrolizumab [37]. Saha et al. demonstrated that an i.t. injected oncolytic virus expressing IL-12 can act synergistically with anti-CTLA-4 and anti-PD1 to cure murine glioblastoma in a manner dependent on CD4 + and CD8 + T cells as well as macrophages [33]. Encouraging preclinical results were recently published following trial of an i.t. administered IL-12 mRNA-based therapy, suggesting the possible clinical benefit of human IL-12 mRNA as a novel treatment for patients otherwise unresponsive to immune checkpoint blockade [35]. A single dose of mouse IL-12 (mIL-12) mRNA induced IFN-γ and CD8 + T cell-dependent tumor regression in multiple mouse and tumor models [35]. I.t. mIL-12 mRNA increased PD-L1 expression on immune infiltrating cells in MC38-R tumors (a PD-L1 blockade monotherapy-resistant model); combination of this i.t. mIL-12 mRNA with anti-PD-L1 enhanced antitumor immunity, allowing most mice with complete responses to reject rechallenge of the same tumor [35]. In a GL-261 glioblastoma model, i.t. IL-12 protein combined with anti-CTLA-4 has been shown to elicit T cell-mediated tumor regression and strong immunologic memory. Monotherapy of i.t. IL-12 or i.p. anti-CTLA-4 alone resulted in only minor or no survival, respectively. When combined, i.t. IL-12 and systemic anti-CTLA-4 led to full remission in most mice [31].
Moreover, the potent effects of IL-12 in combination with checkpoint blockade have been studied within the context of novel fusion proteins, or immunocytokines (ICs), which will be discussed in more detail below. In one study, the activity of a novel IC consisting of murine IL-12 and L19 anti-fibronectin mAb (L19-mIL12) was potentiated by either CTLA-4 or PD-1 blockade, and the combination of L19-mIL12 with checkpoint blockade was potently active against murine CT26 carcinomas and WEHI-164 sarcomas [40]. The use of L19-mIL12 in this study demonstrated the importance of both targeted delivery and the synergistic potential of IL-12 with checkpoint blockade.
I.t. IL-12 combined with RT induces local and systemic antitumor immunity
An important barrier to achieving higher complete response rates in patients receiving checkpoint blockade therapy is the immunosuppressive TME [41]. The delivery of ionizing RT to tumors not only induces cancer cell death through DNA damage [42] but has also been shown to promote the ability of DCs to cross-present tumor cell antigens to T cells [43]. Specifically, RT induces immunogenic cell death, upon which the release of antigens may drive the activation and migration of tumor-specific CD8 + T cells to both the irradiated tumor site and to distant, untreated tumors (known as the “abscopal effect”) [43].
Growing evidence suggest that the combination of localized IL-12 and RT may contribute toward an in situ vaccine effect [44–47]. The combination of stereotactic body RT with i.t. IL-12 microsphere immunotherapy was found to markedly reduce, and even cure, multiple preclinical murine models of pancreatic ductal adenocarcinoma [47]. The study found that an increase in IFN-γ production within the TME following administration of RT/IL-12 initiated suppressor cell reprogramming and an increase in CD8 + T cell activation. In KCKO-luc pancreatic tumors, the treatment was found to result in systemic tumor immunity capable of eradicating distant metastases after rechallenge [47]. In a hepatocellular carcinoma mouse model, i.t. injection of an adenoviral vector encoding IL-12 (Ad/IL-12) combined with RT was found to be highly effective in reducing or eliminating large subcutaneous or orthotopic tumors; increased expression of MHC class II and co-stimulatory molecules CD40 and CD86 on tumor-infiltrating DCs suggested improved antigen presentation activity [46]. Additionally, the treatment significantly reduced the number of myeloid-derived suppressor cells in the TME, allowing for greater CD8 + T cell activation. Using CT26 tumors, the same Ad/IL-12 + RT treatment combination, but not Ad/IL-12 or RT alone, was able to completely suppress the growth of distant tumors in all mice [46]. Others demonstrated that the combination of murine IL-2, murine IL-12 gene therapy, and RT confers a potent antitumor immune response against head and neck squamous cell carcinoma in mice with evidence of CD8 + T cell infiltration in the TME [44].
Targeted delivery and in situ tumor vaccination as strategies to reduce IL-12 toxicity
As discussed above, the clinical use of systemically administered IL-12 has been curtailed by its dose-dependent toxic side effects. Although the combination of lower doses of systemic IL-12 with other cytokines and therapeutic modalities has been found to achieve synergistic and reproducible antitumor immune responses, some of these treatment regimens still result in high levels of systemic IFN-γ and can create a dangerous level of toxicity [2]. In humans, toxic side effects related to IL-12 include fatigue, dyspnea, stomatitis, acidosis, and gastrointestinal hemorrhage [3]. Serious adverse events, including deaths, have been associated with systemic administration of recombinant human IL-12 (rhIL-12) [3]. While synergistic treatment combinations, targeted delivery methods, and in situ vaccination offer the potential for lower systemic IL-12 exposure with the possibility for clinical benefit and safety, the importance of the IL-12 dosing regimen must also be noted. In a Phase 2 trial of rhIL-12, two patient deaths were associated with the schedule of their rhIL-12 administration, which had been modified so that they received daily rhIL-12 doses without the single injection of rhIL-12 2 weeks prior that was employed in the Phase 1 trial [3]. These results revealed that a single primer injection of IL-12 has a major abrogating effect on subsequent IL-12-induced toxicity, although it also likely diminishes its antitumor efficacy.
The amount of IL-12 available at the tumor site is known to be critical in controlling the type and number of infiltrating leukocytes that can promote tumor regression [48]. Additionally, systemically administered IL-12 and its associated toxicity highlight the potential benefit of localized IL-12 treatment. Furthermore, the relative instability and short half-life of IL-12 further support the potential benefit of localized IL-12 delivery directly to sites of tumor [2]. Thus, more attention is being focused on novel, tumor-targeted delivery methods of IL-12 to the TME (Fig. 2) to improve the antitumor effect and reduce toxicity [49]. Because tumor-targeted IL-12 primarily acts within the TME as opposed to inducing systemic immune activation [2], it has been identified as a promising therapeutic approach for the treatment of cancer. Others have experimented with in situ vaccination as a method for local IL-12 delivery to tumors. In situ tumor vaccination is a therapeutic strategy in which immunoenhancing agents are delivered locally to one site of a tumor, thereby activating a local T cell immune response that then stimulates a systemic antitumor immune response [50–53]. Locally delivered IL-12 in the form of gene therapy has been shown to be effective against several tumor types [54], to be superior to several other cytokines [12], and to reduce the toxicity of IL-12 [55].
Fig. 2.
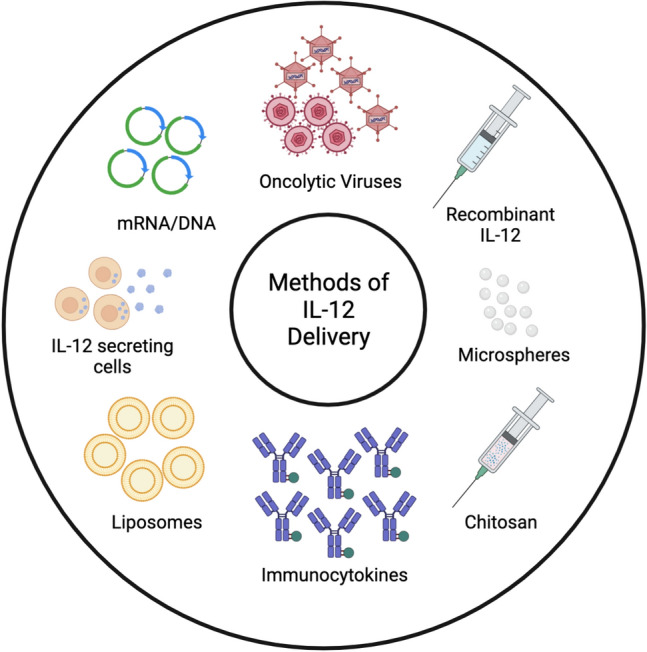
Methods of IL-12 delivery. Schematic representation of targeted delivery and/or in situ tumor vaccination strategies that have been used to deliver IL-12 to the TME with the potential to reduce IL-12-related toxicity. Created with BioRender.com
Immunocytokines
ICs are bioengineered molecules that link tumor-reactive monoclonal antibodies to cytokines that are able to activate immune cells in TME [56]. IC-based delivery of IL-12 has been found to achieve increased local concentration at the TME and thus circumvent the potentially toxic or deadly dose-limiting side effects caused by systemic administration of IL-12 [40]. One example of targeted IL-12 is the L19-mIL-12 IC, made of IL-12 fused to a human antibody fragment specific to the oncofetal ED-B domain of fibronectin [57]. In a mouse lung metastasis model and two other subcutaneous tumor models, the intravenously (i.v.) administered IC was found to have antitumor activity significantly superior to that of untargeted IL-12 [57]. I.v. L19-mIL12 was well tolerated in mice and more efficacious against CT26 tumors than KSF-mIL12, an IC of irrelevant specificity used as a negative control [40].
Combining the technology of targeted delivery with the advantages of in situ vaccination, others have also studied the efficacy of i.t. injected ICs. An example of an antibody-targeted bifunctional IC is KS-IL12/IL2, which combines regions of the anti-EpCAM antibody KS with both IL-12 and IL-2 [58]. This IC was designed to utilize the synergistic potential of the individual cytokines IL-12 and IL-2 [58]. The KS-IL12/IL2 IC was able to induce the complete regression of bulky LLC lung tumors expressing EpCAM when injected i.t.—a more favorable outcome than that of using a combination of KS-IL12 and KS-IL2 [58]. Moreover, the authors found that i.t. injected KS-IL12/IL2 led to demonstrably greater tumor regression than i.v. administration. Importantly, this study also showed that i.t. injection induced a level of systemic IFN-γ approximately 10 times lower than that induced by i.v. administration despite the fact that i.t. injection demonstrated significantly greater antitumor efficacy. This study provides compelling evidence to suggest the benefits of tumor-targeted in situ vaccination as a method of IL-12 delivery. Particularly when combined with other treatments in a manner that may confer a synergistic effect, limitations of untargeted IL-12 administration may be overcome with the fusion of cytokines to tumor-targeted antibodies. Thus, a first-in-human phase I trial of an NHS-IL12 IC was recently conducted and showed no toxicity, which suggests combining it next with checkpoint blockade for better antitumor efficacy [59].
Oncolytic Viruses
The utility of combining IL-12 with other immunomodulatory agents into a single immunotherapy product has also been explored in oncolytic viruses to aid in generating a more potent in situ immune response [60]. Kim et al. tested an oncolytic virus-based cancer vaccine in which IL-12 and GM-CSF were co-expressed in Herpes simplex virus 1 (HSV-1) [61]. I.t. administration of the novel oncolytic HSV-1 in a B16-F10 murine melanoma model inhibited tumor growth and prolonged survival compared to treatment with HSV-1 expressing either cytokine (IL-12 or GM-CSF) alone, demonstrating the synergistic effect of the combinatorial treatment. When administered together, IL-12 and GM-CSF were found to increase antigen presentation and T-cell activation. GM-CSF, a hematopoietic growth factor which reduces hematological toxicity, proved to be crucial in attenuating IL-12-associated toxicity [61]. In another study, Nakao et al. showed that i.t. injection of a tumor-selective oncolytic vaccinia virus encoding IL-7 and IL-12 (hIL-7/mIL-12-VV) activated an inflammatory immune response in several poorly immunogenic murine tumor models and resulted in complete tumor regression even at distant tumor sites [62]. It is of clinical interest that hIL-7/mIL-12-VV was found to upregulate PD-L1 expression on tumor cells, priming and sensitizing both directly treated and distant tumors to anti-PD-1 and anti-CTLA4 immune checkpoint blockade antibodies [62]. Combination treatments of hIL-7/mIL-12-VV and immune checkpoint blockade were tolerated in these preclinical studies without indications of cytokine storm despite the extent of tumor regression, likely due in part to local viral delivery via i.t. administration.
Controlled-release polymers
The use of biodegradable controlled-release polymers in in situ vaccinations has previously been described [63, 64]. One study examined the feasibility of biodegradable polymer microspheres as a clinically viable alternative to systemic IL-12 therapy and IL-12 gene-modified cell vaccines for the treatment of cancer [63]. The authors found that i.t. injection of murine IL-12-loaded biodegradable polymer microspheres, but not human polyethylene glycol-IL-2 nor murine GM-CSF-loaded microspheres, promoted tumor regression, inhibited spontaneous metastasis, and contributed to the development of tumor-specific immunity in a weakly immunogenic murine lung alveolar cell carcinoma model. This study demonstrated the importance of local and sustained IL-12 release, finding that 53% of tumors regressed completely after i.t. microsphere delivery, while none of the tumors regressed when microspheres were injected on the contralateral flank of the tumor-bearing mice. Additionally, the IL-12-loaded microspheres were shown to be the superior delivery method to bolus injections of free IL-12, since a single i.t. injection of free IL-12 at a dose equal to that delivered by the microspheres resulted in the regression of only 20% of tumors. In the same study, systemic/i.p. delivery of free IL-12 resulted in an even worse outcome, where no tumors regressed.
Another group experimented with i.t. injection of IL-12 co-formulated with the biodegradable polysaccharide chitosan [64]. The co-formulation not only increased local retention of IL-12 by up to 4 days but also increased complete tumor regression from ≤ 10% (weekly i.t. injections of IL-12 alone) of established MC32a and Panc02 tumors to 80% and 100%, respectively. Furthermore, this in situ chitosan/IL-12 immunotherapy was well tolerated and generated systemic tumor-specific immunity, where more than 80% of cured mice were at least partially protected from tumor rechallenge. I.t. injection of IL-12 co-formulated with chitosan seems especially feasible as a clinical alternative to systemic injections of IL-12, which have proven to be toxic in both preclinical [65, 66] and clinical studies [3, 67]. Given the use and safety of chitosan-based antiviral vaccines in the clinic [68], chitosan/IL-12 is one example of an in situ vaccination approach that holds promise for clinical translation in cancer patients.
Conclusions and perspectives
Due to the potentially lethal and dose-limiting toxicities associated with its systemic administration, recombinant IL-12 is not FDA-approved for the treatment of cancers. Controlled and tumor-targeted delivery is a major focus of IL-12-related research that offers a potential path to a less toxic and more effective IL-12-facilitated antitumor response [2]. Several of these approaches have already advanced to clinical trials [69–71]. Specifically, in recent clinical trials, IL-12 was delivered as NHS-IL-12 fusion protein for targeting to the necrotic part of metastatic solid tumors [59] or as a recombinant cytokine injected locally in combination with cetuximab in patients with head and neck tumors [72]; in both of these phase I and I/II clinical trials, acceptable tolerability and encouraging antitumor results were observed. In several other recent clinical trials, IL-12 was used in plasmids: as local regulatable gene therapy for treatment of glioma [73], i.t. via electroporation (Tavo) in patients with melanoma [74], and as adjuvant with a tumor vaccine [75]. Importantly, it was shown with i.t. delivery via electroporation that IL-12 functioned as an in situ vaccine inducing a systemic immune response [74, 76] and achieved complete responses in 17.9% of patients [76]. Adaptive immune resistance (increased expression of PD-L1) limited the responses to IL-12 in that study [76]. Subsequently, the same group showed a benefit of combining this same IL-12 approach with PD-1 blockade (pembrolizumab) achieving complete responses in 36% of melanoma patients [37].
In designing better methods of delivery, achieving high local concentration of IL-12, and reducing IL-12-related toxicity, some have innovated IL-12-linked ICs, or fusion proteins [57, 58]. Through genetic engineering, bifunctional fusion proteins are also able to take advantage of synergistic relationships between cytokines and may eventually make IL-12 administration a more viable option in the clinic [58]. I.t. injection of IL-12-containing IC has been shown to induce significantly less systemic IFN-γ production than i.v. administered IL-12 IC [58], suggesting the benefit of an in situ vaccine approach in minimizing toxicities. The success of several other IL-12 in situ tumor vaccination strategies, including oncolytic viruses and in combination with RT, provides further rationale for the benefit of an in situ vaccine approach in delivering IL-12 to the TME. Other research involves i.t. injected IL-12 co-formulations that enhance antitumor response by increasing local retention of IL-12 in the TME and generating systemic tumor-specific immunity [63, 64]. Several sustained, local release delivery methods, including IL-12 encapsulation in polymeric microspheres [47, 63] and IL-12 incorporation into liposomes [77], have been investigated. Given the clear role of IL-12 in inducing tumor regression and antitumor immunity, and its role in enhancing immune checkpoint blockade therapy in mice [31, 33, 35, 37] and cancer patients [37], the distinct advantages of tumor-targeted delivery and/or in situ vaccination should be further explored to mitigate the current toxicities associated with systemic administration that limit the clinical use of IL-12.
Author contributions
EC and AR conceptualized the review. PS oversaw the project. EC, AR, and NT performed the literature search. The first draft of the manuscript was written by EC. NT created the figures. All authors contributed to the editing and revision of the review and approved the final version.
Funding
This work was supported by Midwest Athletes Against Childhood Cancer; Stand Up 2 Cancer; the St. Baldrick’s Foundation; the Crawdaddy Foundation; and the University of Wisconsin Carbone Cancer Center. This research was also supported in part by public health service grants TR002373, U54-CA232568, R35-CA197078, U01-CA233102, P30-CA01452, P01 CA250972 from the National Cancer Institute; the National Institutes of Health and the Department of Health and Human Services.
Declarations
Conflict of interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, et al. Natural killer cell stimulatory factor (NKSF/IL-12) induces Th1-type specific immune responses and inhibits the development of IL-4 producing Th cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tugues S, Burkhard SH, Ohs I, Vrohlings M, Nussbaum K, et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015;22:237–246. doi: 10.1038/cdd.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, et al. Effects of single-dose interleukin-12 exposure on interleukin-12–associated toxicity and interferon-γ production. Blood. 1997;90:2541–2548. doi: 10.1182/blood.V90.7.2541. [DOI] [PubMed] [Google Scholar]
- 4.Lasek W, Zagożdżon R, Jakobisiak M. Interleukin 12: still a promising candidate for tumor immunotherapy? Cancer Immunol Immunother. 2014;63:419–435. doi: 10.1007/s00262-014-1523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pistoia V, Cocco C, Airoldi I. Interleukin-12 receptor β2: from cytokine receptor to gatekeeper gene in human B-cell malignancies. J Clin Oncol. 2009;27:4809–4816. doi: 10.1200/JCO.2008.21.3579. [DOI] [PubMed] [Google Scholar]
- 6.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 7.Snijders A, Kalinski P, Hilkens CM, Kapsenberg ML. High-level IL-12 production by human dendritic cells requires two signals. Int Immunol. 1998;10:1593–1598. doi: 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]
- 8.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 9.Briukhovetska D, Dörr J, Endres S, Libby P, Dinarello CA, et al. Interleukins in cancer: from biology to therapy. Nat Rev Cancer. 2021 doi: 10.1038/s41568-021-00363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oshikawa K, Shi F, Rakhmilevich AL, Sondel PM, Mahvi DM, et al. Synergistic inhibition of tumor growth in a murine mammary adenocarcinoma model by combinational gene therapy using IL-12, pro-IL-18, and IL-1β converting enzyme cDNA. Proc Natl Acad Sci USA. 1999;96:13351–13356. doi: 10.1073/pnas.96.23.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss JM, Subleski JJ, Wigginton JM, Wiltrout RH. Immunotherapy of cancer by IL-12-based cytokine combinations. Expert Opin Biol Ther. 2007;7:1705–1721. doi: 10.1517/14712598.7.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rakhmilevich AL, Janssen K, Turner J, Culp J, Yang NS. Cytokine gene therapy of cancer using gene gun technology: superior antitumor activity of interleukin-12. Hum Gene Ther. 1997;8:1303–1311. doi: 10.1089/hum.1997.8.11-1303. [DOI] [PubMed] [Google Scholar]
- 13.Gillies SD, Lan Y, Wesolowski JS, Qian X, Reisfeld RA, et al. Antibody-IL-12 fusion proteins are effective in SCID mouse models of prostate and colon carcinoma metastases. J Immunol. 1998;160:6195–6203. [PubMed] [Google Scholar]
- 14.Kubin M, Kamoun M, Trinchieri G. Interleukin 12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J Exp Med. 1994;180:211–222. doi: 10.1084/jem.180.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruby CE, Montler R, Zheng R, Shu S, Weinberg AD. IL-12 is required for anti-OX40-mediated CD4 T cell survival. J Immunol. 2008;180:2140–2148. doi: 10.4049/jimmunol.180.4.2140. [DOI] [PubMed] [Google Scholar]
- 16.Tsung K, Meko JB, Tsung YL, Peplinski GR, Norton JA. Immune response against large tumors eradicated by treatment with cyclophosphamide and IL-12. J Immunol. 1998;160:1369–1377. [PubMed] [Google Scholar]
- 17.Tsung K, Dolan JP, Tsung YL, Norton JA. Macrophages as effector cells in interleukin 12-induced T cell-dependent tumor rejection. Cancer Res. 2002;62:5069–5075. [PubMed] [Google Scholar]
- 18.Pulaski BA, Clements VK, Pipeling MR, Ostrand-Rosenberg S. Immunotherapy with vaccines combining MHC class II/CD80+ tumor cells with interleukin-12 reduces established metastatic disease and stimulates immune effectors and monokine induced by interferon γ. Cancer Immunol Immunother. 2000;49:34–45. doi: 10.1007/s002620050024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zapała Ł, Wolny R, Wachowska M, Jakóbisiak M, Lasek W. Synergistic antitumor effect of JAWSII dendritic cells and interleukin 12 in a melanoma mouse model. Oncol Rep. 2013;29:1208–1214. doi: 10.3892/or.2012.2193. [DOI] [PubMed] [Google Scholar]
- 20.Tatsumi T, Takehara T, Kanto T, Miyagi T, Kuzushita N, et al. Administration of interleukin-12 enhances the therapeutic efficacy of dendritic cell-based tumor vaccines in mouse hepatocellular carcinoma. Cancer Res. 2001;61:7563–7567. [PubMed] [Google Scholar]
- 21.Vagliani M, Rodolfo M, Cavallo F, Parenza M, Melani C, et al. Interleukin 12 potentiates the curative effect of a vaccine based on interleukin 2-transduced tumor cells. Cancer Res. 1996;56:467–470. [PubMed] [Google Scholar]
- 22.Fallarino F, Uyttenhove C, Boon T, Gajewski TF. Improved efficacy of dendritic cell vaccines and successful immunization with tumor antigen peptide-pulsed peripheral blood mononuclear cells by coadministration of recombinant murine interleukin-12. Int J Cancer. 1999;80:324–333. doi: 10.1002/(sici)1097-0215(19990118)80:2<324::aid-ijc25>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 23.Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994;153:1697–1706. [PubMed] [Google Scholar]
- 24.Brunda MJ, Luistro L, Rumennik L, Wright RB, Dvorozniak M, et al. Antitumor activity of interleukin 12 in preclinical models. Cancer Chemother Pharmacol. 1996;38:S16–S21. doi: 10.1007/s002800051031. [DOI] [PubMed] [Google Scholar]
- 25.Noguchi Y, Richards EC, Chen YT, Old LJ. Influence of interleukin 12 on p53 peptide vaccination against established Meth A sarcoma. Proc Natl Acad Sci USA. 1995;92:2219–2223. doi: 10.1073/pnas.92.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motzer RJ, Rakhit A, Schwartz LH, Olencki T, Malone TM, et al. Phase I trial of subcutaneous recombinant human interleukin-12 in patients with advanced renal cell carcinoma. Clin Cancer Res. 1998;4:1183–1191. [PubMed] [Google Scholar]
- 27.Car BD, Eng VM, Lipman JM, Anderson TD. The toxicology of interleukin-12: a review. Toxicol Pathol. 1999;27:58–63. doi: 10.1177/019262339902700112. [DOI] [PubMed] [Google Scholar]
- 28.Bortolanza S, Bunuales M, Otano I, Gonzalez-Aseguinolaza G, Ortiz-de-Solorzano C, et al. Treatment of pancreatic cancer with an oncolytic adenovirus expressing interleukin-12 in Syrian hamsters. Mol Ther. 2009;17:614–622. doi: 10.1038/mt.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers. 2020;12:738. doi: 10.3390/cancers12030738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vom Berg J, Vrohlings M, Haller S, Haimovici A, Kulig P, et al. Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell–mediated glioma rejection. J Exp Med. 2013;210:2803–2811. doi: 10.1084/jem.20130678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bozeman EN, He S, Shafizadeh Y, Selvaraj P. Therapeutic efficacy of PD-L1 blockade in a breast cancer model is enhanced by cellular vaccines expressing B7–1 and glycolipid-anchored IL-12. Hum Vaccin Immunother. 2016;12:421–430. doi: 10.1080/21645515.2015.1076953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saha D, Martuza RL, Rabkin SD. Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell. 2017;32:253–267. doi: 10.1016/j.ccell.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fallon JK, Vandeveer AJ, Schlom J, Greiner JW. Enhanced antitumor effects by combining an IL-12/anti-DNA fusion protein with avelumab, an anti-PD-L1 antibody. Oncotarget. 2017;8(13):20558–20571. doi: 10.18632/oncotarget.16137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hewitt SL, Bailey D, Zielinski J, Apte A, Musenge F, et al. Intratumoral IL12 mRNA therapy promotes TH1 transformation of the tumor microenvironment. Clin Cancer Res. 2020;26:6284–6298. doi: 10.1158/1078-0432.CCR-20-0472. [DOI] [PubMed] [Google Scholar]
- 36.Mansurov A, Ishihara J, Hosseinchi P, Potin L, Marchell TM, et al. Collagen-binding IL-12 enhances tumour inflammation and drives the complete remission of established immunologically cold mouse tumours. Nat Biomed Eng. 2020;4:531–543. doi: 10.1038/s41551-020-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Algazi AP, Twitty CG, Tsai KK, Le M, Pierce R, et al. Phase II trial of IL-12 plasmid transfection and PD-1 blockade in immunologically quiescent melanoma. Clin Cancer Res. 2020;26:2827–2837. doi: 10.1158/1078-0432.CCR-19-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiocca EA, Lukas RV, Chen CC, Rao G, Amidei C, et al. Controlled IL-12 in combination with a PD-1 inhibitor subjects with recurrent glioblastoma. J Clin Oncol. 2020;38:2510. doi: 10.1200/JCO.2020.38.15_suppl.2510. [DOI] [Google Scholar]
- 39.Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity. 2018;49:1148–1161. doi: 10.1016/j.immuni.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puca E, Probst P, Stringhini M, Murer P, Pellegrini G, et al. The antibody-based delivery of interleukin-12 to solid tumors boosts NK and CD8+ T cell activity and synergizes with immune checkpoint inhibitors. Int J Cancer. 2020;146:2518–2530. doi: 10.1002/ijc.32603. [DOI] [PubMed] [Google Scholar]
- 41.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1:1325–1332. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- 44.Xian J, Yang H, Lin Y, Liu S. Combination nonviral murine interleukin 2 and interleukin 12 gene therapy and radiotherapy for head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2005;131:1079–1085. doi: 10.1001/archotol.131.12.1079. [DOI] [PubMed] [Google Scholar]
- 45.Deplanque G, Shabafrouz K, Obeid M. Can local radiotherapy and IL-12 synergise to overcome the immunosuppressive tumor microenvironment and allow “in situ tumor vaccination”? Cancer Immunol Immunother. 2017;66:833–840. doi: 10.1007/s00262-017-2000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu CJ, Tsai YT, Lee IJ, Wu PY, Lu LS, et al. Combination of radiation and interleukin 12 eradicates large orthotopic hepatocellular carcinoma through immunomodulation of tumor microenvironment. Oncoimmunology. 2018;7:e1477459. doi: 10.1080/2162402X.2018.1477459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mills BN, Connolly KA, Ye J, Murphy JD, Uccello TP, et al. Stereotactic body radiation and interleukin-12 combination therapy eradicates pancreatic tumors by repolarizing the immune microenvironment. Cell Rep. 2019;29:406–421. doi: 10.1016/j.celrep.2019.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colombo MP, Vagliani M, Spreafico F, Parenza M, Chiodoni C, et al. Amount of interleukin 12 available at the tumor site is critical for tumor regression. Cancer Res. 1996;56:2531–2534. [PubMed] [Google Scholar]
- 49.Nguyen KG, Vrabel MR, Mantooth SM, Hopkins JJ, Wagner ES, et al. Localized interleukin-12 for cancer immunotherapy. Front Immunol. 2020;11:575597. doi: 10.3389/fimmu.2020.575597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marabelle A, Kohrt H, Caux C, Levy R. Intratumoral immunization: a new paradigm for cancer therapy. Clin Cancer Res. 2014;20:1747–1756. doi: 10.1158/1078-0432.CCR-13-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris ZS, Guy EI, Francis DM, Gressett MM, Werner LR, et al. In situ tumor vaccination by combining local radiation and tumor-specific antibody or immunocytokine treatments. Cancer Res. 2016;76:3929–3941. doi: 10.1158/0008-5472.CAN-15-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sagiv-Barfi I, Czerwinski DK, Levy S, Alam IS, Mayer AT, et al. Eradication of spontaneous malignancy by local immunotherapy. Sci Transl Med. 2018 doi: 10.1126/scitranslmed.aan4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baniel CC, Heinze CM, Hoefges A, Sumiec EG, Hank JA, et al. In situ vaccine plus checkpoint blockade induces memory humoral response. Front Immunol. 2020;11:1610. doi: 10.3389/fimmu.2020.01610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rakhmilevich AL, Turner J, Ford MJ, McCabe D, Sun WH, et al. Gene gun-mediated skin transfection with interleukin 12 gene results in regression of established primary and metastatic murine tumors. Proc Natl Acad Sci USA. 1996;93:6291–6296. doi: 10.1073/pnas.93.13.6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rakhmilevich AL, Timmins JG, Janssen K, Pohlmann EL, Sheehy MJ, et al. Gene gun-mediated IL-12 gene therapy induces antitumor effects in the absence of toxicity: a direct comparison with systemic IL-12 protein therapy. J Immunother. 1999;22:135–144. doi: 10.1097/00002371-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Sondel PM, Gillies SD. Current and potential uses of immunocytokines as cancer immunotherapy. Antibodies. 2012;1:149–171. doi: 10.3390/antib1020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Halin C, Rondini S, Nilsson F, Berndt A, Kosmehl H, et al. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat Biotechnol. 2002;20:264–269. doi: 10.1038/nbt0302-264. [DOI] [PubMed] [Google Scholar]
- 58.Gillies SD, Lan Y, Brunkhorst B, Wong WK, Li Y, et al. Bi-functional cytokine fusion proteins for gene therapy and antibody-targeted treatment of cancer. Cancer Immunol Immunother. 2002;51:449–460. doi: 10.1007/s00262-002-0302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strauss J, Heery CR, Kim JW, Jochems C, Donahue RN, et al. First-in-human phase I trial of a tumor-targeted cytokine (NHS-IL12) in subjects with metastatic solid tumors. Clin Cancer Res. 2019;25:99–109. doi: 10.1158/1078-0432.CCR-18-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melero I, Castanon E, Alvarez M, Champiat S, Marabelle A. Intratumoural administration and tumour tissue targeting of cancer immunotherapies. Nat Rev Clin Oncol. 2021;18:558–576. doi: 10.1038/s41571-021-00507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim KJ, Moon D, Kong SJ, Lee YS, Yoo Y, et al. Antitumor effects of IL-12 and GM-CSF co-expressed in an engineered oncolytic HSV-1. Gene Ther. 2021;28:186–198. doi: 10.1038/s41434-020-00205-x. [DOI] [PubMed] [Google Scholar]
- 62.Nakao S, Arai Y, Tasaki M, Yamashita M, Murakami R, et al. Intratumoral expression of IL-7 and IL-12 using an oncolytic virus increases systemic sensitivity to immune checkpoint blockade. Sci Transl Med. 2020 doi: 10.1126/scitranslmed.aax7992. [DOI] [PubMed] [Google Scholar]
- 63.Egilmez NK, Jong YS, Sabel MS, Jacob JS, Mathiowitz E, et al. In situ tumor vaccination with interleukin-12-encapsulated biodegradable microspheres: induction of tumor regression and potent antitumor immunity. Cancer Res. 2000;60:3832–3837. [PubMed] [Google Scholar]
- 64.Zaharoff DA, Hance KW, Rogers CJ, Schlom J, Greiner J. Intratumoral immunotherapy of established solid tumors with chitosan/IL-12. J Immunother. 2010;33:697–705. doi: 10.1097/CJI.0b013e3181eb826d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orange JS, Salazar-Mather TP, Opal SM, Spencer RL, Miller AH, et al. Mechanism of interleukin 12-mediated toxicities during experimental viral infections: role of tumor necrosis factor and glucocorticoids. J Exp Med. 1995;181:901–914. doi: 10.1084/jem.181.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaufman HL, Swartout BG, Hörig H, Lubensky I. Combination interleukin-2 and interleukin-12 induces severe gastrointestinal toxicity and epithelial cell apoptosis in mice. Cytokine. 2002;17:43–52. doi: 10.1006/cyto.2001.0986. [DOI] [PubMed] [Google Scholar]
- 67.Cohen J. IL-12 deaths: explanation and a puzzle. Science. 1995;270:908. doi: 10.1126/science.270.5238.908a. [DOI] [PubMed] [Google Scholar]
- 68.Neimert-Andersson T, Binnmyr J, Enoksson M, Langebäck J, Zettergren L, et al. Evaluation of safety and efficacy as an adjuvant for the chitosan-based vaccine delivery vehicle ViscoGel in a single-blind randomised Phase I/IIa clinical trial. Vaccine. 2014;32:5967–5974. doi: 10.1016/j.vaccine.2014.08.057. [DOI] [PubMed] [Google Scholar]
- 69.Anwer K, Barnes MN, Fewell J, Lewis DH, Alvarez RD. Phase-I clinical trial of IL-12 plasmid/lipopolymer complexes for the treatment of recurrent ovarian cancer. Gene Ther. 2010;17:360–369. doi: 10.1038/gt.2009.159. [DOI] [PubMed] [Google Scholar]
- 70.Alvarez RD, Sill MW, Davidson SA, Muller CY, Bender DP, et al. A phase II trial of intraperitoneal EGEN-001, an IL-12 plasmid formulated with PEG–PEI–cholesterol lipopolymer in the treatment of persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2014;133:433–438. doi: 10.1016/j.ygyno.2014.03.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fallon J, Tighe R, Kradjian G, Guzman W, Bernhardt A, et al. The immunocytokine NHS-IL12 as a potential cancer therapeutic. Oncotarget. 2014;5(7):1869–1884. doi: 10.18632/oncotarget.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McMichael EL, Benner B, Atwal LS, Courtney NB, Mo X, et al. A Phase I/II trial of cetuximab in combination with interleukin-12 administered to patients with unresectable primary or recurrent head and neck squamous cell carcinoma. Clin Cancer Res. 2019;25:4955–4965. doi: 10.1158/1078-0432.CCR-18-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chiocca EA, Yu JS, Lukas RV, Solomon IH, Ligon KL, et al. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: results of a phase 1 trial. Sci Transl Med. 2019 doi: 10.1126/scitranslmed.aaw5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greaney SK, Algazi AP, Tsai KK, Takamura KT, Chen L, et al. Intratumoral plasmid IL12 electroporation therapy in patients with advanced melanoma induces systemic and intratumoral T-cell responses. Cancer Immunol Res. 2020;8:246–254. doi: 10.1158/2326-6066.CIR-19-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vonderheide RH, Kraynyak KA, Shields AF, McRee AJ, Johnson JM, et al. Phase 1 study of safety, tolerability and immunogenicity of the human telomerase (hTERT)-encoded DNA plasmids INO-1400 and INO-1401 with or without IL-12 DNA plasmid INO-9012 in adult patients with solid tumors. J Immunother Cancer. 2021;9:e003019. doi: 10.1136/jitc-2021-003019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Algazi A, Bhatia S, Agarwala S, Molina M, Lewis K, et al. Intratumoral delivery of tavokinogene telseplasmid yields systemic immune responses in metastatic melanoma patients. Ann Oncol. 2020;31:532–540. doi: 10.1016/j.annonc.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 77.Simpson-Abelson MR, Purohit VS, Pang WM, Iyer V, Odunsi K, et al. IL-12 delivered intratumorally by multilamellar liposomes reactivates memory T cells in human tumor microenvironments. Clin Immunol. 2009;132:71–82. doi: 10.1016/j.clim.2009.03.516. [DOI] [PMC free article] [PubMed] [Google Scholar]


