Abstract
Background
There is growing evidence suggesting that the occurrence of immune-related adverse events (irAEs) may be a predictor of immune checkpoint inhibitor efficacy. Whether this association extends to all irAEs or just those within particular organs/systems is yet to be resolved. As immune-related thyroid dysfunction (thyroid irAE) is one of the most commonly reported irAEs, this study aims to summarize the available data and determine if thyroid irAE is a surrogate marker for improved cancer outcomes during ICI therapy.
Methods
PubMed, EMBASE and Cochrane Library were searched up to July 1st 2021 for studies assessing the relationship between thyroid irAE development during ICI therapy and cancer outcomes. Outcome measures of interest include overall survival (OS) and progression free survival (PFS). Sub-group analyses based on cancer type and adjustment for immortal time bias (ITB) were also performed.
Results
Forty-seven studies were included in the systematic review. Twenty-one studies were included in the OS meta-analysis whilst 15 were included in the PFS meta-analysis. Development of thyroid irAE during ICI therapy was associated with improved OS and PFS (OS: HR 0.52, CI 0.43–0.62, p < 0.001; PFS: HR 0.58, CI 0.50–0.67, p < 0.001). Sub-group analyses involving non-small cell lung cancer populations and studies where ITB was accounted for, observed similar results (HR 0.37, CI 0.24–0.57, p < 0.001) and (HR 0.51, CI 0.39–0.69, p < 0.001), respectively.
Conclusion
Despite the heterogeneity and biases identified, the evidence does suggest that the development of thyroid irAE is associated with anti-tumor effects of ICIs and therefore, can be used as a surrogate marker for clinical response.
Keywords: Autoimmune thyroiditis, Autoimmune thyroid dysfunction, Overall survival, Progression free survival, Objective response rate
Introduction
Immune checkpoint inhibitors (ICI) such as inhibitors of programmed cell death receptor 1 (PD-1), programmed cell death ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), have been studied and shown to improve cancer outcomes in a variety of clinical settings, including in locally advanced and metastatic cancer [1–4]. However, by blocking the usual inhibitory signal to the immune system, immune-related adverse events (irAEs) are common. The thyroid has proven to be particularly vulnerable, with immune-related thyroid dysfunction (thyroid irAE) being one of the most frequently described irAEs [5, 6].
Whilst there is growing evidence suggesting that the development of irAEs signifies an enhanced.
T cell-mediated immunoreaction and therefore, a potentially more efficacious ICI response [7–10], it remains debated as to whether this relationship extends to all irAEs or only to those that develop within certain organs/systems. Results to date from studies investigating thyroid irAE and cancer outcomes are mixed, with significant heterogeneity between study design, study populations and the methods in which immortal time bias (ITB) is accounted for.
ITB is a key element in determining the effective association between clinical outcomes and a time-dependent variable [11]. It describes the phenomenon where patients who die or whose disease progresses earlier are less likely to develop an outcome, whilst those patients that stayed in the study for a longer time interval have a theoretically increased risk of experiencing an outcome, therefore resulting in a bias. Unfortunately, a significant proportion of the studies investigating the effects of thyroid irAE on cancer outcomes do not account for ITB. A robust review taking into consideration ITB, heterogeneity as well as other potential biases, is therefore required to thoroughly examine and evaluate these studies. We performed a systematic review and meta-analysis (including a sub-group analysis of studies that accounted for ITB) to determine if the presence of thyroid irAE in patients after the use of ICIs is associated with improved treatment efficacy and cancer outcomes.
Methods
Search strategy and inclusion criteria
This study is registered with the International Prospective Register of Systematic Reviews, number CRD42021259904. We followed the PRISMA guidelines and employed the Population-Intervention-Comparator-Outcome-Study Design framework to structure the research question and its corresponding literature search.
We searched PubMed, EMBASE and Cochrane Library for studies published up until the 1st of July 2021. The key terms included (“thyroid” “thyroid dysfunction” OR “hypothyroid” OR “hyperthyroid” OR “thyroiditis” OR “immune-related adverse event” OR “endocrine”) AND (“nivolumab” OR “pembrolizumab” OR “durvalumab” OR “avelumab” OR “atezolizumab” OR “ipilimumab” OR “tremelimumab” OR “cemiplimab” OR “camrelizumab” OR “sintilimab” OR “tislelizumab” OR “toripalimab” OR “PD-1” OR “PD-L1” OR “CTLA-4”) AND (“progression free survival” OR “objective response rate” OR “overall survival” OR “survival”).
Selection process
Two review authors (YC, OH) independently screened titles and abstracts identified using the above search strategy for eligible studies. The citations of relevant studies were also screened for additional eligible studies.
The predetermined inclusion criteria for the systematic review were:
Full text, peer reviewed articles
Articles in English
The reporting of the correlation between thyroid irAE or endocrine irAEs and treatment outcome during ICI therapy (at least one of: OS or PFS).
Studies that only evaluated endocrine irAEs must include a breakdown of the endocrinopathies developed so as to determine the proportionate contribution of thyroid dysfunction to overall cancer outcomes, or specify that thyroid irAE made up the majority of endocrine irAEs.
The additional inclusion criteria for the meta-analysis were:
The reporting of OS or PFS outcome data specific to thyroid irAE (as the meta-analysis examines the association of thyroid irAE with cancer outcomes, having outcome data for just immune-related endocrine adverse events was not sufficient to be included in the meta-analysis)
The reporting of both hazard ratio (HR) and confidence intervals (CI) for OS or PFS specific to thyroid irAE (studies that only reported an isolated p value, HR or CI were excluded as analyses were unable to be performed)
For both the systematic review and the meta-analysis we excluded reviews, case reports, guidelines, editorials and letters to the editor, and those published as conference abstracts only. Final eligibility and inclusion were determined by the agreement of both reviewers.
Studies included
The electronic literature search identified 2788 citations in PubMed, 789 in EMBASE and 170 in Cochrane Library. After removal of duplicate copies and studies that did not meet the requirements of the inclusion criteria, 101 were retrieved for more detailed, full text evaluation. Fifty-nine studies that passed the initial citation screening were then excluded after full text screening leaving 42 studies that satisfied the systematic review inclusion criteria. After manually reviewing the citations of relevant publications, a further five studies were included in the systematic review.
Twenty-one studies met the criteria to be included in the OS meta-analysis, whilst 15 fulfilled the inclusion criteria for the PFS meta-analysis (Fig. 1).
Fig. 1.
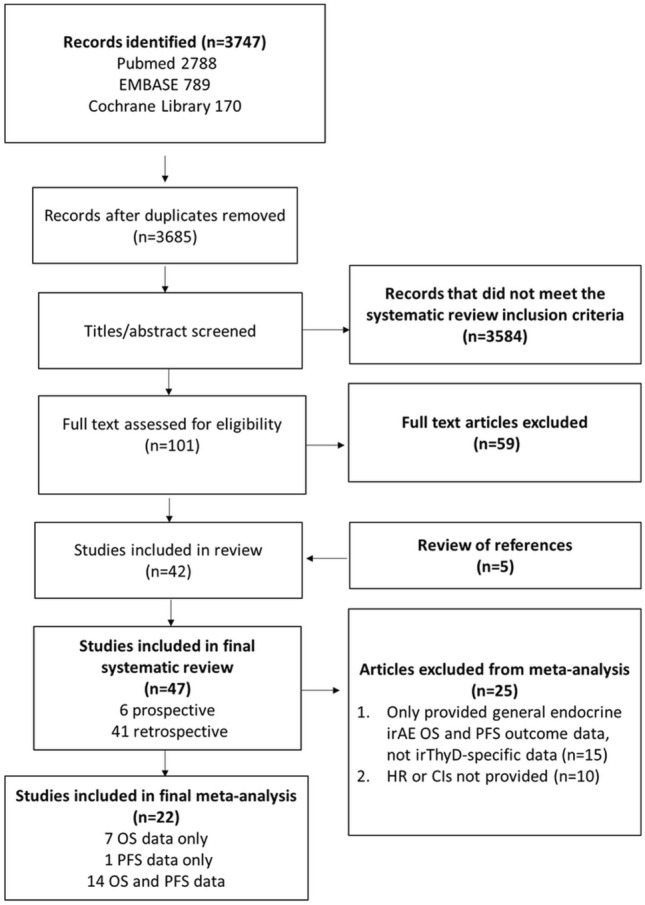
Study selection flowchart. Confidence interval (CI), hazard ratio (HR), immune-related adverse effects (irAE), immune-related thyroid dysfunction (thyroid irAE), number (n), odds ratio (OR), overall survival (OS), progression free survival (PFS)
Data extraction and collection
Data were extracted by the same two reviewers, and entered into a pre-designed data extraction form within Microsoft Excel version 2019 (Microsoft Corporation, Seattle, Washington, USA). Variables collected included: primary disease site, number of participants, study design, type of ICI, percentage of patients developing thyroid irAE, median time to onset of thyroid irAE, biochemical severity of thyroid irAE, number of deaths, OS and PFS in patients with and without thyroid irAE, and whether ITB was accounted for. Of note, there were no overlapping datasets within the studies included in the meta-analysis.
Quality assessment
The Cochrane risk-of-bias tool for non-randomized trials was used to assess the design, conduct and reporting of the included studies [12]. Studies were classified as low, moderate, serious, critical or unclear risk.
Statistical analysis
Both fixed and random effects model meta-analyses were performed. The generic inverse-variance weighted method was used to estimate overall effect size from the final set of studies reporting OS and/or PFS data in patients with and without thyroid irAE. I2 statistics were used to estimate the proportion of the variability of the results attributed to heterogeneity rather than sampling error. I2 levels of 25% or less correspond to a low heterogeneity [13, 14]. Given the relatively small size of our final study set along with the moderate levels of heterogeneity identified between the studies included, we have chosen to report all our results based on the random effects model [15].
Begg funnel plots [16] and Egger’s test [17] were performed to detect publication bias. For both tests, significant publication bias was considered when p < 0.05.
Sub-group analyses were also performed to investigate the effects primary cancer type and ITB have on the development of thyroid irAE during ICI therapy and potential cancer treatment outcomes.
Results
Forty-seven studies were included in the systematic review, 21 studies were included in the OS meta-analysis and 15 were included in the PFS meta-analysis. All 47 studies included were in the setting of advanced or metastatic malignancies. The most common cancers studied were non-small cell lung cancer (NSCLC) followed by melanoma. PD-1 inhibitors were the most commonly prescribed ICI followed by combination therapy (PD-1/PD-L1 inhibitor with a CTLA-4 inhibitor). Forty-one of the 47 studies were retrospective in nature, whilst 6 were prospective. The pooled patient population was 19,115, with the population of individual studies ranging from 40 to 6596 participants. The incidence of thyroid irAE was between 1 and 37.5%. The specifics of each study are summarized in Table 1.
Table 1.
Systematic review of the studies evaluating the correlation between the development of immune-related thyroid dysfunction and clinical outcomes secondary to immune checkpoint inhibitors
| Number | Reference | Study type | Primary tumor site | Treatment | N = | ITB | irAE of interest | Outcomes (thyroid irAE versus no thyroid irAE) | Summary | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | PFS | Positive/Negative study | |||||||||
| 1 | Freeman-Keller et al. [54] | Retrospective | Melanoma | Anti-PD-1 ± peptide vaccination (nivolumab + peptide vaccine or nivolumab alone) | 148 | Yes | General, thyroid | NS | Not reported | Negative | Non-significant OS for endocrinopathies, including thyroid irAE |
| 2 | Kim et al. [18] | Retrospective | NSCLC | Anti-PD-1 (nivolumab or pembrolizumab) | 58 | No | Thyroid |
118 versus 71 days, (p = 0.025) Adjusted HR 0.11, (p = 0.041) |
118 versus 61 days, (p = 0.014) Adjusted HR 0.38, (p = 0.018) |
Positive | Thyroid irAE was an independent predictive factor for favorable outcome |
| 3 | Fujisawa et al. [63] | Retrospective | Melanoma | CTLA-4 post PD-1 (ipilimumab post nivolumab) | 60 | Yes | General, endocrine | Adjusted RR 0.22, (p = 0.015) | Not reported | Positive | Endocrine irAEs were significant factors associated with survival |
| 4 | Osorio et al. [20] | Prospective | NSCLC | Anti-PD-1 (pembrolizumab) | 51 | No | Thyroid |
40 versus 14 months, HR 0.29, (p = 0.029) |
NS | Positive | OS was significantly longer in patients who developed thyroid irAE |
| 5 | Grangeon et al. ([21] | Retrospective | NSCLC | Anti-PD-1 or anti-PD-L1 (not specified) | 270 | No | General, thyroid |
NR versus 18.2 months, HR 0.46, (p = 0.01) |
8.05 versus 2.59 months, HR 0.56, (p = 0.005) | Positive | Thyroid irAE was correlated with better clinical outcomes |
| 6 | Haratani et al. [22] | Retrospective | NSCLC | Anti-PD-1 (nivolumab) | 134 | Yes | General, endocrine | NS | Adjusted HR 0.237, (p = 0.02) | Positive | Endocrine irAEs were correlated with better PFS, but not OS |
| 7 | Owen et al. [23] | Retrospective | NSCLC | Anti-PD-1 or anti-PD-L1 (nivolumab, pembrolizumab, atezolizumab) | 91 | Yes | General, thyroid |
NR versus 6.5 months, (0.018) 3-month landmark NR versus 16.2 months, (p = 0.0296) |
Not reported | Positive | Thyroid irAE was correlated with longer OS |
| 8 | Ricciuti et al. [24] | Retrospective | NSCLC | Anti-PD-1 (nivolumab) | 195 | No | General, endocrine | Adjusted HR 0.45, (p = 0.001) | Adjusted HR 0.59, (p = 0.011) | Negative | Endocrine irAEs were correlated with better clinical outcomes |
| 9 | Ahn et al. [51] | Retrospective | NSCLC | Anti-PD-1 (nivolumab or pembrolizumab) | 155 | Yes | General, endocrine |
NR versus 12.58 months, (p = 0.037) Adjusted HR NS |
NS | Negative | Endocrine irAEs were not identified as significant positive predictive factors of better clinical outcomes |
| 10 | Campredon et al. [52] | Retrospective | NSCLC | Anti-PD-1 (nivolumab) | 105 | Yes | Thyroid | NS | NS | Positive | A non-statistically significant tendency towards improvement of the overall survival was observed in the thyroid irAE group |
| 11 | Cortellini et al. [25] | Retrospective | NSCLC | Anti-PD-1 (nivolumab or pembrolizumab) | 559 | Yes | General, endocrine | Adjusted HR 0.55, (p = 0.0044) | Adjusted HR 0.63, (p = 0.0084) | Positive | Endocrine irAEs correlated with improved ORR and PFS and improved OS |
| 12 | Funazo et al. [26] | Retrospective | NSCLC | Anti-PD-1 (nivolumab) | 111 | No | Thyroid |
Low fT4: NR versus 556 days HR 0.139, (p = 0.020) |
Low fT4: NR versus 67 days HR 0.297, (p = 0.010) |
Positive | In the patients with advanced NSCLC, low fT4 after nivolumab treatment was associated with significantly longer PFS and OS |
| 13 | Koyama et al. [27] | Retrospective | NSCLC | Anti-PD1 (nivolumab or pembrolizumab) | 132 | No | Thyroid | NR versus 14.1 months, (p = 0.011) | 9.8 versus 1.8 months, (p = 0.012) | Negative | Thyroid irAE was correlated with better OS and PFS in NSCLC patients |
| 14 | Ksienski et al. [53] | Retrospective | NSCLC | Anti-PD-1 (nivolumab or pembrolizumab) | 254 | Yes | General, thyroid | NS | Not reported | Positive | Thyroid irAE was not correlated with OS after nivolumab or pembrolizumab treatment |
| 15 | Lei et al. [28] | Retrospective | Melanoma, RCC, NSCLC | Anti-PD-1 (nivolumab or pembrolizumab) | 103 | No | Thyroid |
NR versus 12.9 months, HR 0.40, (p = 0.014) |
10.1 versus 3.7 months HR 0.45, (p = 0.002) | Positive | Thyroid irAE was correlated with better clinical outcomes OS, PFS and ORR |
| 16 | Maeda et al. [29] | Retrospective | Melanoma | Anti-PD-1 (nivolumab) | 73 | Yes | General, endocrine | 20-week landmark (p = 0.27) | 20-week landmark (p = 0.07) | Positive | Endocrine irAEs were correlated with better clinical outcomes |
| 17 | Peiro et al. [30] | Prospective | Majority NSCLC, melanoma, lymphoma | Anti-PD-1 (nivolumab) | 73 | No | Thyroid | NSCLC & TD: HR, 0.4, (p = 0.035) | Not reported | Positive | In patients with NSCLC, nivolumab-induced thyroid dysfunction appears to be correlated with better OS |
| 18 | Sakakida et al. [31] | Retrospective | Majority NSCLC, melanoma, lymphoma, RCC, head and neck, gastric, urothelial | Anti-PD-1 (nivolumab or pembrolizumab) | 174 | Yes | Thyroid |
156 versus 59 weeks, HR 0.34, (p = 0.01) Adjusted HR 0.42, (p = 0.04) |
66 versus 27 weeks, HR 0.50, (p = 0.02) Adjusted HR NS |
Positive | Thyroid irAE was an independent prognostic factor for longer OS |
| 19 | Verzoni et al. [32] | Prospective | RCC | Anti-PD-1 (nivolumab) | 398 | Yes | General, endocrine | 1-year OS 92.3% (p = 0.001) | Not reported | Negative | Endocrine irAEs were correlated with improved OS |
| 20 | Yamauchi et al. [5] | Retrospective | Lung, melanoma, others | Anti-PD-1 (nivolumab) | 200 | Yes | Thyroid |
16.1 versus 13.6 months, HR 0.61, (p = 0.022) Lung & TD: NR versus14.2 months, HR 0.51 CI 0.27–0.92, (p = 0.025) Melanoma & TD: NS |
4.9 versus 2.9 months, HR 0.66 (p = 0.023) Lung &TD: 5.8 versus2.3 months, HR 0.55 CI 0.33–0.88, (p = 0.012) Melanoma & TD: 3.3 versus 4.1 months, HR 0.94 CI 0.41–2.00, (p = 0.885) |
Positive | Thyroid irAE related to good prognosis in lung cancer but might be inconclusive in melanoma |
| 21 | Al Mushref et al. [56] | Retrospective | Melanoma | Anti-PD-1 or CTLA-4 (ipilimumab, pembrolizumab or nivolumab) | 186 | No | Thyroid | NS | Not reported | Positive | Thyroid irAEs did not appear to be associated with change in survival |
| 22 | Basak et al. [33] | Prospective | Melanoma, NSCLC, RCC | Anti-PD-1 (nivolumab or pembrolizumab) | 168 | Yes | Thyroid |
1-year OS rates 94 versus 59%, HR 0.18, (p = 0.020) |
1-year PFS rates 64 versus 34%, HR 0.39, (p = 0.050) |
Positive | Thyroid irAE is associated with improved OS and PFS |
| 23 | Cortellini et al. [64] | Retrospective | NSCLC w PD-L1 expression > 50% | Anti-PD-1 (pembrolizumab) | 1010 | Yes | General, thyroid | Adjusted HR 0.30, (p < 0.0001) | Adjusted HR 0.40, (p < 0.0001) | Positive | Endocrine irAEs were significantly related to improved OS, PFS and ORR |
| 24 | Economopoulou et al. [35] | Retrospective | Head and neck | Anti-PD-1 (nivolumab) | 89 | No | General, endocrine | (p = 0.014) | Not reported | Positive | The development of endocrine irAEs is a predictor of improved survival in patients with advanced HNSCC treated with nivolumab |
| 25 | Eggermont et al. [36] | RCT (secondary analysis) | Melanoma | Anti-PD-1 (Pembrolizumab) | 1011 | Yes | General, endocrine | Not reported | HR 0.34, (p = 0.03) | Positive | Occurrence of endocrine irAEs were associated with a longer PFS in the pembrolizumab arm |
| 26 | Espana et al. [37] | Retrospective | NSCLC, melanoma, urothelial | Anti-PD-1 ± anti-CTLA-4 or anti-CTLA-4 (pembrolizumab, nivolumab, atezolizumab, ipilimumab or combination therapy) | 188 | No | Endocrine |
NR versus 31.4 months (p = 0.001) Adjusted HR 0.42, (p = 0.008) |
56.7 versus 27.7 months, (p = 0.008) | Positive | Endocrine irAEs were significantly associated with improved OS and PFS |
| 27 | Lima-Ferreira et al. [38] | Retrospective | Melanoma, NSCLC, lymphoma, urothelial and head and neck | Anti-PD-1 or anti-CTLA-4 (pembrolizumab, nivolumab or ipilimumab) | 161 | No | Thyroid | 3.26 versus 1.76 years, (p = 0.030) | Not reported | Positive | Primary and central thyroid dysfunction can be a predictive clinical biomarker of a better response to ICI across several neoplasms |
| 28 | Kelly et al. [39] | Prospective | Majority NSCLC | Anti-PD-L1 (avelumab) | 1783 | Yes | General, thyroid | HR 0.53, CI 0.39–0.71 | Not reported | Positive | Thyroid irAE was significantly associated with improved OS |
| 29 | Kijima et al. [40] | Retrospective | Urothelial | Anti-PD-1 (pembrolizumab) | 97 | No | General, endocrine | (p = 0.04) | Not reported | Positive | Endocrine irAEs were associated with increased ORR and longer OS |
| 30 | Kotwal et al. [41] | Retrospective | Lung (85%), Uroepithelial, Merkel cell, prostate, penis | Anti-PD-L1 (atezolizumab or avelumab) | 91 | No | Thyroid |
NR versus 9.8 months, (p = 0.027) Adjusted HR 0.49, CI 0.25–0.99, (p = 0.034) |
Not reported | Negative | Thyroid irAE appears to be associated with improved OS |
| 31 | Maillet et al. [42] | Retrospective | Melanoma, NSCLC, RCC, urothelial | Anti-PD-L1 or anti-CTLA-4 (not specified) | 410 | Yes | General, thyroid |
HR 0.53, CI 0.3–0.94 |
HR 0.5, CI 0.3–0.84 |
Negative | Thyroid irAE is correlated with better OS and PFS |
| 32 | Matsuo et al. [55] | Retrospective | Head and neck squamous cell | Anti-PD-1 (nivolumab) | 108 | No | General, endocrine | Not reported | NS | Positive | No correlation between endocrine irAEs and clinical outcomes |
| 33 | Shankar et al. [6] | Retrospective | NSCLC | Anti-PD-1, anti-PD-L1 (not specified) | 623 | No | General, thyroid | NS | NS | Positive | No correlation between thyroid irAE and clinical outcomes observed |
| 34 | Al Ashi et al. [50] | Retrospective | NSCLC, melanoma, RCC, bladder | Anti-PD-1 anti-PD-L1 or anti-CTLA-4 (nivolumab, pembrolizumab, atezolizumab, durvalumab, avelumab or ipilimumab) | 551 | No | Endocrine | Adjusted HR 0.56, CI 0.42–0.76, p < 0.001 | Not reported | Negative | The development of endocrine irAEs was associated with a longer OS |
| 35 | Bai et al. [43] | Retrospective | Lung, melanoma, esophageal, urothelial, gastric | Anti-PD-1, anti-PD-L1 or combination therapy (not specified) | 103 | No | General, endocrine | Not reported | 13.3 versus 4.13 months (p = 0.01) | Positive | Endocrine irAEs were associated with better PFS |
| 36 | D’Aiello et al. [57] | Retrospective | NSCLC, SCLC | Anti-PD-1 or anti-PD-L1 (pembrolizumab, nivolumab, durvalumab or atezolizumab) | 205 | Yes | Thyroid | Not reported | NS | Negative | There were no observed differences in PFS between those that developed thyroid irAE and those that did not |
| 37 | Frelau et al. [44] | Retrospective | Melanoma | Anti-PD-1 ± CTLA-4 or CTLA-4 (pembrolizumab or nivolumab or combination therapy) | 110 | No | General, thyroid |
43.9 versus 9.8 months, (p = 0.0021) Adjusted HR 0.4, 95% CI 0.21–0.76, (p = 0.005) |
18.1 versus 3.9 months, (p = 0.0085) NS |
Negative | Thyroid irAE appeared to be associated with better OS |
| 38 | Holstead et al. [62] | Retrospective | Melanoma | Anti-PD-1 (nivolumab or pembrolizumab) | 87 | Yes | General, thyroid | NS | Not reported | Positive | There appeared to be a trend towards better OS in individuals with endocrine irAEs |
| 39 | Lui et al. [61] | Retrospective | HCC, lung, breast, melanoma, RCC, CC pancreas, colorectal, gastric, NET | Anti-PD-1/anti-CTLA-4 combination therapy (nivolumab/pembrolizumab and ipilimumab) | 103 | Yes | Endocrine |
17.9 versus 5.7 months (p < 0.001) Adjusted HR 0.34, 95% CI 0.17–0.71, (p = 0.004) 3-month landmark: HR 0.42, CI 0.13–1.36, (p = 0.135) |
Not reported | Negative | Thyroid irAE may have prognostic significance in individuals with advanced cancer and combination therapy |
| 40 | Luongo et al. [45] | Retrospective | NSCLC, melanoma, RCC | Anti-PD-1 (nivolumab or pembrolizumab) | 96 | No | Thyroid | HR 0.41, CI 0.2–0.87, p = 0.0197 | NS | Positive | Thyroid irAE was associated with an improved 2-year OS when compared to euthyroid patients |
| 41 | Morimoto et al. [60] | Retrospective | NSCLC | Anti-PD-1 or anti-PD-L1 combined with chemotherapy | 70 | No | General, thyroid | NS | HR 0.46, CI 0.17–1.29, (p = 0.14) | Negative | Endocrine irAEs were associated with a trend towards improved PFS |
| 42 | Muir et al. [46] | Retrospective | Melanoma | Anti-PD-1 ± CTLA-4 or CTLA-4 (ipilimumab, pembrolizumab or nivolumab or combination therapy) | 1246 | No | Thyroid |
Adjusted HR 0.57, 95% CI 0.39–0.84, (p = 0.005) |
Adjusted HR 0.68, 95% CI 0.49–0.94, (p = 0.02) |
Negative | Overt thyrotoxicosis appeared to be associated with better OS and PFS. No association was observed for hypothyroidism |
| 43 | Paderi et al. [59] | Retrospective | RCC | Anti-PD-1 ± CTLA-4 (nivolumab or combination therapy) | 43 | Yes | General, thyroid | Not reported |
Adjusted HR 0.34 CI 0.13–0.87, (p = 0.025) 16-week landmark (p = 0.160) |
Positive | At the 16-week landmark analysis, thyroid irAE showed a trend towards improved PFS |
| 44 | Rubino et al. [58] | Retrospective | NSCLC, melanoma | Anti-PD-1 (pembrolizumab or nivolumab) | 251 | No | General, endocrine | NS | NS | Positive | Endocrine irAEs were not associated with OS or PFS |
| 45 | Street et al. [47] | Retrospective | Melanoma, breast, gastrointestinal, genitourinary, head and neck, hematologic, neurologic, thoracic | Anti-PD-1, anti-PD-L1 or CTLA-4 or combination therapy (not specified) | 6596 | Yes | Thyroid | Adjusted HR 0.8, CI 0.71–0.89), (p = < 0.001) | Not reported | Positive | Thyroid irAE was associated with improved OS even after accounting for immortal time biases |
| 46 | Thuillier et al. [48] | Retrospective | NSCLC | Anti-PD-1 (nivolumab) | 194 | No | Thyroid |
29.8 versus 8.1 months, (p < 0.001) Adjusted HR 0.32, (p < 0.001) |
8.7 versus 1.7 months, (p < 0.001) Adjusted HR = 0.36 (p < 0.001) |
Positive | Thyroid irAE appeared to be correlated with better OS, PFS and ORR |
| 47 | Zhou et al. [49] | Retrospective | NSCLC | Anti-PD-1 (pembrolizumab or nivolumab) | 191 | No | General, thyroid | 16.8 versus 11.1 months, (p < 0.001) | 10.4 versus 5.5 months, (p < 0.001) | Positive | Thyroid irAE appeared to be correlated with better OS and PFS |
CC Cholangiocarcinoma, CR complete response, CTLA-4 cytotoxic T-lymphocyte-associated antigen 4, HR hazards ratio, HNSCC head and neck squamous cell carcinoma, HCC hepatocellular carcinoma, ICI immune checkpoint inhibitor, irAE immune-related adverse event, thyroid irAE immune-related thyroid dysfunction, N number, NSCLC non-small cell lung cancer, NR not reached, NS not significant, OS overall survival, PD-1 programmed cell death receptor 1, PD-L1 programmed cell death ligand, PFS progression free survival, RCC renal cell carcinoma, SCLC small cell lung cancer, CI 95% confidence interval, TD thyroid dysfunction
Significant results (p-values below 0.05 or HR with confidence intervals that do not cross 1) are highlighted in bold
Systematic review
Data review according to outcome
Of the 47 studies, 34 were positive [5, 18–50] meaning they observed a correlation between thyroid irAE/endocrine irAEs and either a longer OS or PFS. Thirteen studies did not find a statistically significant association between the development of thyroid irAE/endocrine irAEs and ICI efficacy [6, 51–62].
Thirty-one studies observed a significant association between the occurrence of thyroid irAE/endocrine irAE and a longer OS [5, 18, 20, 21, 23–33, 35, 38–42, 44–50, 57, 63, 64], whilst eleven did not [6, 22, 51–54, 56, 58, 60–62]. Five studies did not include correlations between immune-related endocrine or thyroid dysfunction and OS as an endpoint [36, 43, 55, 57, 59].
Nineteen studies were positive for a longer PFS [5, 18, 21, 22, 24–29, 33, 36, 42, 43, 46, 48, 49, 55, 64], whilst eleven were negative [6, 20, 31, 44, 45, 51, 52, 55, 57, 59, 60]. Seventeen studies did not report correlations between endocrine irAE/thyroid irAE and PFS as an endpoint [23, 30, 32, 35, 38–41, 47, 50, 53–56, 61–63].
Data review according to type of irAE
Eighteen studies evaluated thyroid irAE exclusively [5, 18, 20, 26–28, 30, 31, 33, 38, 41, 45–48, 52, 56, 57]. Thirteen studies evaluated irAEs in general, but also provided details on thyroid irAE [6, 21, 23, 39, 42, 44, 49, 53, 54, 59, 60, 62, 64]. Sixteen studies evaluated endocrine irAEs where the majority of adverse events were due to thyroid dysfunction [22, 24, 25, 29, 32, 35–37, 40, 43, 50, 51, 55, 58, 60, 61, 63]. In these 16 studies, 57–91% of the endocrine irAEs were reported as thyroid-related.
Twenty-two [5, 18, 20, 21, 23, 26–28, 30, 31, 33, 38, 39, 41, 42, 44–49, 64] of the 31 studies that specifically included details of thyroid irAE were positive [5, 18, 20, 21, 23, 26–28, 30, 31, 33, 38, 39, 41, 42, 44–49, 52, 54, 56, 57, 59, 60, 62, 64], and seven were negative [6, 52–54, 56, 57, 65] Of those that reported on endocrine irAEs, twelve out of 16 studies were positive [22, 24, 25, 29, 32, 35–37, 40, 43, 50, 63] and four were negative [51, 55, 58, 61].
Data review according to primary tumor
Thirty-three studies evaluated NSCLC/lung populations [5, 6, 18, 20–28, 30, 31, 33, 37–39, 41–43, 45, 47–49, 51–53, 57, 58, 60, 61, 64]. Seventeen studies evaluated populations containing only NSCLC [6, 18, 20–27, 48, 49, 51–53, 60, 64] (n = 4300). Twelve of these 17 studies were positive [18, 20–27, 48, 49, 64], while five were negative [6, 51–53, 60]. Sixteen studies evaluated NSCLC/lung together along with a mix of other cancers [5, 28, 30, 31, 33, 37–39, 41–43, 45, 47, 57, 58, 61]. Fourteen of these 16 studies were positive while two were negative [5, 28, 30, 31, 33, 37–39, 41–43, 45, 47, 57, 58]. For the majority of these “mixed cancer” studies, NSCLC remained the predominant primary cancer type.
Thirteen of the 47 studies did not include NSCLC/lung participants as part of their cohort.
Eight studies evaluated participants exclusively with a diagnosis of melanoma (n = 2921) [29, 36, 44, 46, 54, 56, 62, 63], Of these eight studies, three had negative findings [54, 56, 62], and five had positive associations [19, 29, 36, 44, 46] with either OS or PFS. Two studies exclusively evaluated participants with renal cell carcinoma (n = 441) [32, 59], while one study exclusively evaluated participants with urothelial cancer [40] (n = 97). All three studies were associated with positive findings. The remaining two studies exclusively investigated populations with head and neck cancers [35, 55] (n = 197). One was associated with a longer OS [35] whilst the other was a negative study [55].
The reported incidence of thyroid irAE during ICI monotherapy in the studies comprising non-NSCLC/non-lung populations when compared to studies comprising NSCLC/lung populations were 1–22.6% and 6.2–32.7%, respectively.
Data review according to ICI type
The majority of studies included patients treated with PD-1 inhibitors. Eleven studies evaluated patients treated with nivolumab alone, [5, 22, 24, 26, 29, 30, 32, 35, 48, 52, 55] one evaluated patients with nivolumab ± peptide vaccination [54], five evaluated patients treated with pembrolizumab alone [20, 36, 40, 64, 66], thirteen evaluated patients treated with either nivolumab or pembrolizumab [6, 18, 25, 27, 28, 31, 33, 45, 49, 51, 53, 58, 62] and eight included patients treated with a mix of PD-1/PD-L1 inhibitors or ipilimumab [21, 23, 37, 38, 42, 50, 56, 57]. Seven studies included patients where a PD-1/PD-L1 was used in combination with a CTLA-4 inhibitor (combination therapy) [37, 43, 44, 46, 47, 59, 61], two studies evaluated patients treated exclusively with PD-L1 inhibitors [39, 41] and one study included patients treated with a combination of PD-1/PD-L1 combined with chemotherapy [60]. Finally, one study included participants treated with ipilimumab after a course of nivolumab [63]. Of the studies where treatment included PD-1 inhibitor monotherapy, 22 were positive and six were negative [51–53, 55, 58, 62]. The two studies [39, 41] that evaluated patients treated exclusively with PD-L1 inhibitors were both positive. All seven studies that included patients treated with combination therapy were either positive, or observed a longer OS/PFS that did not reach statistical significance [43, 44, 46, 47, 59–61]. The study that evaluated patients treated with nivolumab ± peptide vaccination [54] along with the study that included patients treated with PD-1/PD-L1 combined with chemotherapy [60], were both negative.
Data review according to biochemical severity
Six studies [5, 30, 33, 46, 48, 49] conducted sub-group analyses on thyroid irAE based on biochemical severity (subclinical vs. overt hyperthyroidism and hypothyroidism, where overt is defined as biochemical evidence of an abnormal thyroid stimulating hormone [TSH] and free triiodothyronine [fT3] or free thyroxine [fT4] levels whilst subclinical is defined as an abnormal TSH with normal fT3 and fT4 levels). All six studies did not observe a statistically significant correlation between the development of subclinical thyroid states and improved clinical outcomes.
Data review according time to onset of thyroid irAE
Twenty-one studies reported the time to onset of thyroid irAE post ICI treatment [18, 20, 26, 28–31, 33, 38, 41, 44–49, 52, 54, 56, 57, 61]. The earliest median time to onset of thyroid irAE reported was 3.3 weeks whilst the latest was 30 weeks [52]. Ten studies reported time to onset based on the type of thyroid irAE (ie hyperthyroidism vs. hypothyroidism) [20, 28, 30, 31, 41, 45, 46, 49, 57, 61] and five differentiated between subclinical and overt thyroid irAE [28, 33, 46, 49, 61]. Overt disease along with thyrotoxicosis/hyperthyroidism appeared to occur earlier, whilst subclinical thyroid irAE and hypothyroidism appeared to develop later.
Data review according to ITB
Twenty-five of the 47 studies addressed the confounding effects of ITB [5, 22–25, 30–33, 36, 39, 42, 51, 53, 54, 57, 59–61, 63, 64]. Eighteen of these 25 studies employed landmark analyses [5, 22–25, 29, 32, 47, 51, 53, 54, 57, 59, 60, 62–64] whilst 7 utilized an extended cox model with time-varying covariates [30, 31, 33, 36, 39, 42, 47]. Two studies performed both landmark and an extended cox model with time-varying covariates as part of their analyses [47, 54]. After excluding the 22 studies that did not account for ITB, 17 studies were positive [5, 22–25, 30–33, 36, 39, 42, 47, 59, 60, 63, 64] and eight were negative [51–54, 57, 59, 61, 62].
Meta-analysis
Overall survival
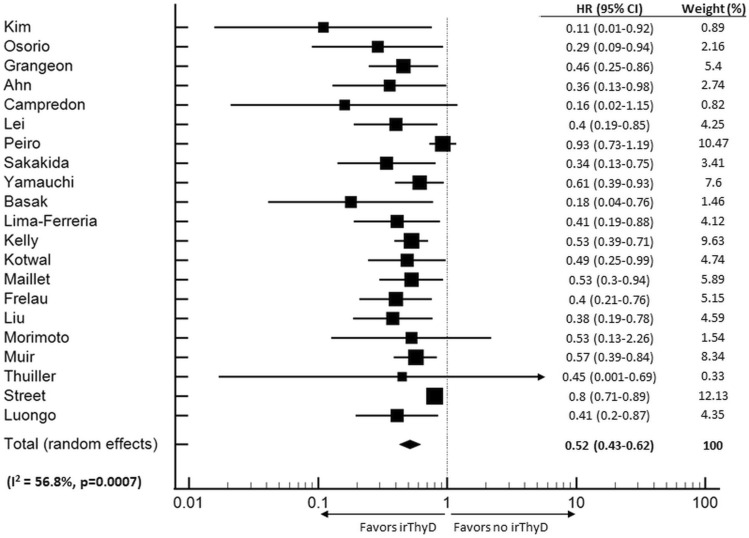
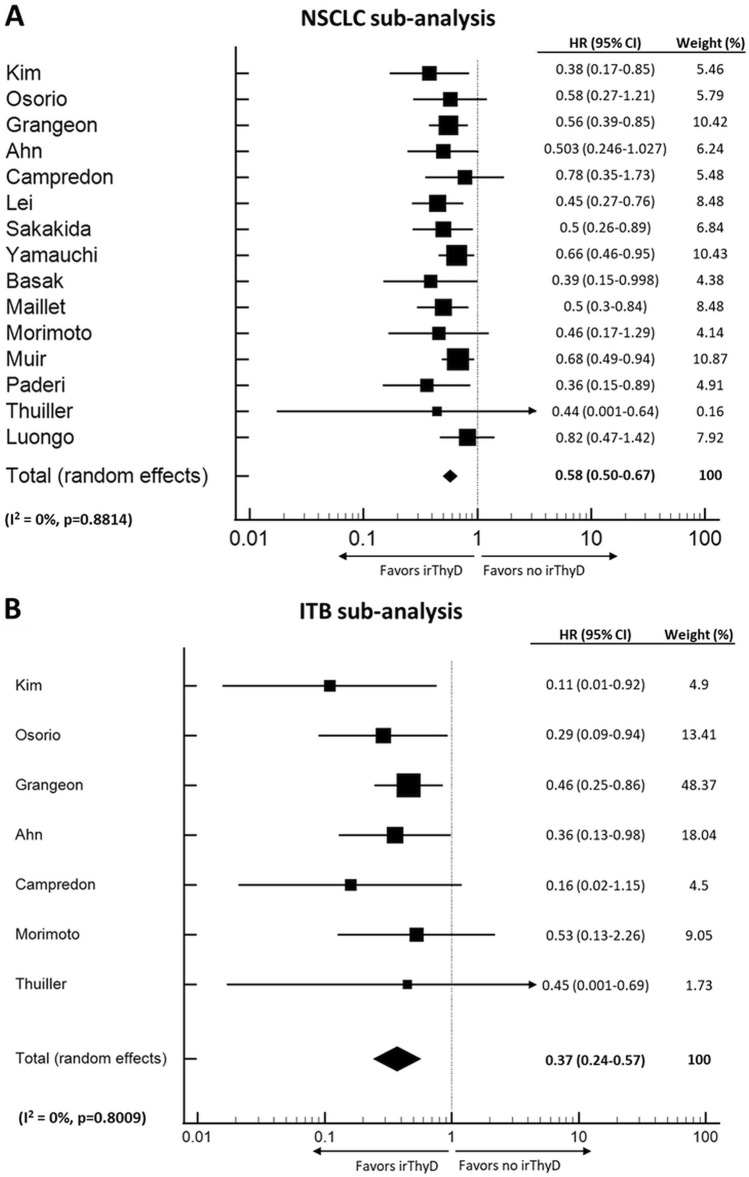
Twenty-one studies (n = 12,158) fulfilled the criteria for inclusion in the OS meta-analysis [5, 18, 20, 21, 28, 30, 31, 33, 38, 39, 41, 42, 44–48, 51, 52, 60, 61]. Thyroid irAE occurrence was significantly associated with longer OS (HR 0.52, CI 0.43–0.62, p < 0.001) (Fig. 2). Moderate level heterogeneity was detected (I2 = 56.8%, p = 0.0007). Further sub-group analyses were conducted based on primary cancer type and whether ITB was accounted for. In the seven studies where NSCLC was the only cancer evaluated, patients that developed thyroid irAE appeared to have longer OS (HR 0.37, CI 0.24–0.57, p < 0.001) than individuals that did not develop thyroid irAE. Heterogeneity between studies was low (I2 = 0%, p = 0.8009) (Fig. 3a). There were too few studies assessing other cancer types to perform sub-group analyses. Finally, nine studies that accounted for ITB had sufficient data provided to perform a sub-group analysis. In these studies, thyroid irAE was again, associated with longer OS (HR, 0.51, CI 0.39–0.69, p < 0.001), (I2 = 64.2%, p = 0.0044) (Fig. 3b).
Fig. 2.
Forest Plot (random effects model) of the association between thyroid irAE development and overall survival. The size of the squares indicates the weight of each study. irThyD: Immune-related thyroid dysfunction.
Fig. 3.
Forest Plots (random effects model) between thyroid irAE development and overall survival in individuals with NSCLC (panel a) and when ITB is accounted for (panel b). The size of the squares indicates the weight of each study. irThyD: Immune-related thyroid dysfunction.
Progression-free survival
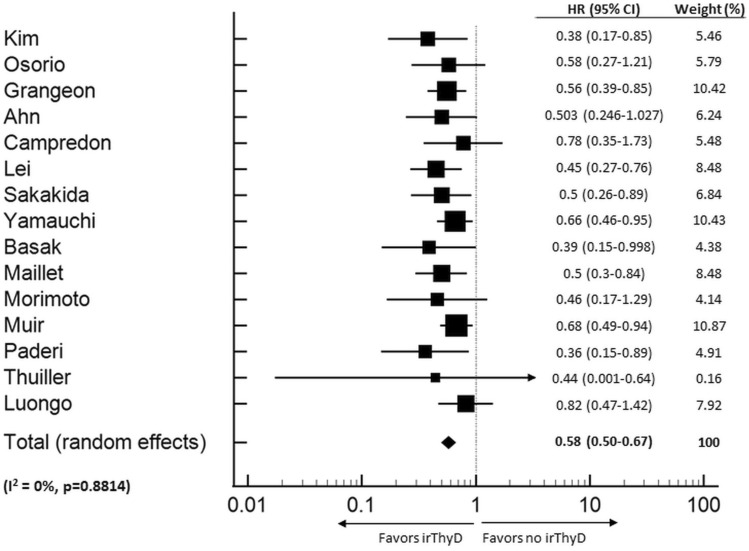
Fifteen studies (n = 3284) fulfilled the inclusion criteria to be included in the PFS meta-analysis [5, 18, 20, 21, 28, 31, 33, 42, 45, 46, 48, 51, 52, 59, 60]. Thyroid irAE occurrence was significantly associated with longer PFS (HR 0.58, CI 0.50–0.67, p < 0.001) (Fig. 4) and heterogeneity between studies was low (I2 = 0%, p = 0.881). Sub-group analyses were not performed due to the small number of studies available for analysis.
Fig. 4.
Forest Plot (random effects model) of the association between thyroid irAE development and progression free survival. The size of the squares indicates the weight of each study. irThyD: Immune-related thyroid dysfunction.
Publication bias
Using the Cochrane risk-of-bias tool, the risk of bias within studies was primarily adjudicated as being “moderate or serious” due to the limitations inherent to a retrospective design. The majority of studies did conduct multivariate analyses, but only 53% accounted for ITB.
Publication bias was also assessed using the Begg funnel plot and Egger’s test. The funnel plot for OS did not display evident asymmetry (p = 0.13). However, Egger’s test was significant for publication bias (p < 0.001). The funnel plot for PFS on the other hand, did not show asymmetry (p = 0.151) and Egger’s test was also not suggestive of publication bias (p = 0.06).
As small-sized studies can commonly contribute to publication bias, we performed a sub-analysis where only OS studies with population sizes ≥ 100 were included. The results of the Begg funnel plot (p = 0.40) and Egger’s test (p < 0.0001) remained consistent with publication bias despite the exclusion of these smaller studies.
Discussion
While there have been multiple systematic reviews and meta-analyses assessing the incidence of irAEs and their associations with clinical outcomes [7, 67–71], to our knowledge, this is the first to specifically review and evaluate the potential correlations between the development of thyroid irAE and cancer outcomes.
The results of our meta-analysis suggests that the presence of thyroid irAE appears to be inversely associated with the oncological benefits of ICI therapy, where a net benefit in OS and PFS is observed in spite of developing an irAE.
The studies that did not observe a correlation between cancer outcomes and the development of thyroid irAE were often performed in small [51, 52, 60] or populations with high levels of comorbidities (older age ≥ 70 years, Charlson Comorbidity Index score ≥ 3 and Eastern Cooperative Oncology Group Performance Status ≥ 2) [53]. This raises the possibility that population size as well as high mortality rates may be contributing factors to these negative studies.
In addition, a number of the negative studies were performed in mixed [57–59] or non-NSCLC/lung cohorts [54–56, 59, 62], suggesting that the effects of thyroid irAE on cancer outcomes may also be potentially dependent on the primary cancer type. This is supported by the findings of two mixed cancer cohort studies where longer OS was observed in individuals that developed thyroid irAE, but this association was then lost in a sub-group analysis of individuals with melanoma. [5, 47] It is unknown why thyroid irAEs would be associated with survival in only some cancers. One possible explanation would be the presence of shared antigens between certain cancers and the thyroid, although this has not been proven. Alternatively, there may be sex-associated molecular differences in the immune components of cancers. Studies have shown a divergent sex-bias of immune features between lung cancers and melanoma (i.e., higher tumor mutation burden and neoantigen load in males with melanoma vs. higher stimulatory/inhibitory immune checkpoints in females with lung cancer) [72], which could lead to an apparent association with cancers that are more or less common in men versus women. Further studies investigating the sex-associated molecular differences in immunotherapy response; however, are required.
The correlation between the development of thyroid irAE and improved cancer outcomes; however, appeared to only be significant in individuals who develop overt rather than subclinical thyroid dysfunction. Although we acknowledge that this correlation is based on a small number of studies, this is not a surprising finding as severity of irAEs has previously been described as an independent favorable predictor of OS and PFS [40].
Furthermore, given the large number of studies included in our review that did not account for ITB, it is possible that an artificial inflation of the correlation between thyroid irAE and clinical outcomes is observed. However, our sub-group analysis (involving only studies that accounted for ITB) did observe a significant association between thyroid irAE and longer OS, suggesting a legitimate association.
The association between thyroid irAE and cancer outcomes has potential relevant clinical implications. Unlike most other irAEs which commonly result in serious sequelae, ICI interruption and cessation, thyroid irAE is considered a relatively safe irAE. Furthermore, current guidelines recommend and encourage the continuation of ICI therapy in the setting of thyroid irAEs [73]. The majority of patients tend to present with mild symptoms and can be managed with close monitoring and where appropriate, levothyroxine therapy [74]. Thyroid irAE is therefore more likely to have continued clinical benefits when compared to other irAEs. Also, although most thyroid irAE tend to occur around or after the time of when the tumors are first evaluated for response to ICI therapy via computed tomography scans (6–8 weeks post ICI initiation), overt thyroid irAE can occur as early as 3 weeks after ICI commencement. In these circumstances, it would be somewhat reassuring if thyroid function tests (TFTs) already demonstrate overt thyroid irAE. TFTs should therefore be considered in all patients undergoing ICI therapy due to the high rates of thyroid irAE in this population, but can also be used to complement other clinical findings in assessing the likelihood of clinical response.
Our study has several limitations. Firstly, the majority of data collected were from retrospective studies, which can lead to various biases including information, selection as well potential biases in outcome measurements. Secondly, heterogeneity was detected between studies. While this is not surprising given endocrine/thyroid irAE were not the primary outcomes of interest for many of the included studies, significant heterogeneity can inherently impact the reliability of comparisons made between studies. Two studies in our systematic review reported results which were undoubtedly outside of the expected ranges (i.e., a thyroid irAE incidence of 1% and a median time to isolated overt hypothyroidism of 30 weeks), and could therefore also contribute to heterogeneity. However, only one study [52] was included in our meta-analysis, and it had a relatively small contribution to the overall effect size for both the OS (weight 0.82%) and PFS (weight 1.89%) meta-analyses. Similarly, while we included the one study which had the unique treatment regimen of PD-1/PD-L1 therapy in combination of chemotherapy as part of our meta-analysis, this study also had a relatively small impact on the overall effect size for both the OS (weight 1.54%) and PFS (weight 4.14%) meta-analyses.
Publication bias was also identified and is likely a consequence of reporting bias. There appeared to be selective outcome reporting with an evidently larger number of published studies reporting on a positive association between thyroid irAE and OS compared with a negative association. Selective analysis reporting was also present and contributed to a number of negative studies being excluded from our meta-analysis due to insufficient reporting of data (i.e., HR and CI). These biases can lead to a potential overestimation of the association between thyroid irAE and clinical outcomes. Further prospective studies that report on both the positive and negative associations between thyroid irAE and ICI therapy outcomes are therefore required to validate our findings. Additionally, ICI doses were not evaluated in this review. This is because the ICI regimens within studies were complex and could not be summarized without the risk of over-simplification and the introduction of further biases.
Finally, our study population was heavily skewed towards patients treated for advanced NSCLC. Similarly, the vast majority of the patients were treated with PD-1 inhibitors, thus it would be difficult to generalize our study’s findings to the general cancer population treated with any ICIs.
Conclusions
As the use of ICIs continue to expand, thyroid irAE will be increasingly encountered in clinical practice. Although deficiencies and biases remain within the current literature, the evidence does suggest that the development of thyroid irAE is associated with anti-tumor effects of ICIs and therefore, can be used as a surrogate marker for clinical response. Additional prospective studies are needed to further validate the correlation between thyroid irAE and clinical outcomes, particularly in different primary cancer sites and ICI types, as well as the role biomarkers such as TFTs may have in clinical practice.
Author contributions
Y-MMC had substantial contribution in the conception and design of the manuscript, along with its methodology. She also conducted the statistical analyses and was primarily responsible for drafting the manuscript. She was one of two reviewers responsible for the review and selection of studies for both the systematic review and meta-analysis, as well as for the extracting of data from the selected studies. WW had substantial contribution in overseeing and supervising all statistical analyses performed for the meta-analysis. She also contributed to the revisions of the manuscript. BM had substantial contribution in the drafting and revisions of the manuscript. O-PRH had substantial contribution in the conception and design of the manuscript, along with its methodology. He also contributed to the drafting and revisions of the manuscript. He was one of two reviewers responsible for the review and selection of studies for both the systematic review and meta-analysis, as well as for the extracting of data from the selected studies. He also provided supervision and oversight of the project. All authors have given approval for this version of the manuscript to be published.
Funding
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or National Institutes of Health.
Declarations
Conflict of interest
The authors (YC, BM, WW and OH) do not have any disclosures or conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2018;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamauchi I, Yasoda A, Matsumoto S, et al. Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS One. 2019;14:e0216954. doi: 10.1371/journal.pone.0216954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shankar B, Zhang J, Naqash AR, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. 2020;6:1952–1956. doi: 10.1001/jamaoncol.2020.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou X, Yao Z, Yang H, et al. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020;18:87. doi: 10.1186/s12916-020-01549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussaini S, Chehade R, Boldt RG, et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors: a systematic review and meta-analysis. Cancer Treat Rev. 2020;92:102134. doi: 10.1016/j.ctrv.2020.102134. [DOI] [PubMed] [Google Scholar]
- 9.Cortellini A, Buti S, Agostinelli V, et al. A systematic review on the emerging association between the occurrence of immune-related adverse events and clinical outcomes with checkpoint inhibitors in advanced cancer patients. Semin Oncol. 2019;45:362–371. doi: 10.1053/j.seminoncol.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Fujii T, Naing A, Rolfo C, et al. Biomarkers of response to immune checkpoint blockade in cancer treatment. Crit Rev Oncol Hematol. 2018;130:108–120. doi: 10.1016/j.critrevonc.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Gleiss A, Oberbauer R, Heinze G. An unjustified benefit: immortal time bias in the analysis of time-dependent events. Transpl Int. 2018;31:125–213. doi: 10.1111/tri.13081. [DOI] [PubMed] [Google Scholar]
- 12.Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HI, Kim M, Lee SH, et al. Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. Oncoimmunology. 2017;7:e1375642. doi: 10.1080/2162402X.2017.1375642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujisawa Y, Yoshino K, Otsuka A, et al. Fluctuations in routine blood count might signal severe immune-related adverse events in melanoma patients treated with nivolumab. J Dermatol Sci. 2017;88:225–231. doi: 10.1016/j.jdermsci.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28:583–589. doi: 10.1093/annonc/mdw640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grangeon M, Tomasini P, Chaleat S, et al. Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non-small-cell lung cancer. Clin Lung Cancer. 2019;30:201–207. doi: 10.1016/j.cllc.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4:374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owen DH, Wei L, Bertino EM, et al. Incidence, risk factors, and effect on survival of immune-related adverse events in patients with non-small-cell lung cancer. Clin Lung Cancer. 2018;19:e893–e900. doi: 10.1016/j.cllc.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricciuti B, Genova C, De Giglio A, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145:479–485. doi: 10.1007/s00432-018-2805-3. [DOI] [PubMed] [Google Scholar]
- 25.Cortellini A, Chiari R, Ricciuti B, et al. Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin Lung Cancer. 2019;20:237–247.e1. doi: 10.1016/j.cllc.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Funazo TY, Nomizo T, Ozasa H, et al. Clinical impact of low serum free T4 in patients with non-small cell lung cancer treated with nivolumab. Sci Rep. 2019;9:17085. doi: 10.1038/s41598-019-53327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koyama J, Horiike A, Yoshizawa T, et al. Correlation between thyroid transcription factor-1 expression, immune-related thyroid dysfunction, and efficacy of anti-programmed cell death protein-1 treatment in non-small cell lung cancer. J Thorac Dis. 2019;11:1919–1928. doi: 10.21037/jtd.2019.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lei M, Michael A, Patel S, et al. Evaluation of the impact of thyroiditis development in patients receiving immunotherapy with programmed cell death-1 inhibitors. J Oncol Pharm Pract. 2019;25:1402–1411. doi: 10.1177/1078155219829813. [DOI] [PubMed] [Google Scholar]
- 29.Maeda T, Yoshino K, Nagai K, et al. Development of endocrine immune-related adverse events and improved survival in advanced melanoma patients treated with nivolumab monotherapy. Eur J Cancer. 2019;115:13–16. doi: 10.1016/j.ejca.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Peiró I, Palmero R, Iglesias P, et al. Thyroid dysfunction induced by nivolumab: searching for disease patterns and outcomes. Endocrine. 2019;64:605–613. doi: 10.1007/s12020-019-01871-7. [DOI] [PubMed] [Google Scholar]
- 31.Sakakida T, Ishikawa T, Uchino J, et al. Clinical features of immune-related thyroid dysfunction and its association with outcomes in patients with advanced malignancies treated by PD-1 blockade. Oncol Lett. 2019;18:2140–2147. doi: 10.3892/ol.2019.10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verzoni E, Cartenì G, Cortesi E, et al. Real-world efficacy and safety of nivolumab in previously-treated metastatic renal cell carcinoma, and association between immune-related adverse events and survival: the Italian expanded access program. J Immunother Cancer. 2019;7:99. doi: 10.1186/s40425-019-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basak EA, van der Meer JWM, Hurkmans DP, et al. Overt thyroid dysfunction and anti-thyroid antibodies predict response to anti-PD-1 immunotherapy in cancer patients. Thyroid. 2020;30(7):966–973. doi: 10.1089/thy.2019.0726. [DOI] [PubMed] [Google Scholar]
- 34.Cortellini A, Friedlaender A, Banna GL, et al. Immune-related adverse events of pembrolizumab in a large real-world cohort of patients With NSCLC with a PD-L1 expression ≥ 50% and their relationship with clinical outcomes. Clin Lung Cancer. 2020;21:498–508.e2. doi: 10.1016/j.cllc.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Economopoulou P, Kotsantis I, Papaxoinis G, et al. Association of autoimmunity with survival in patients with recurrent/metastatic head and neck squamous cell carcinoma treated with nivolumab. Oral Oncol. 2020;111:105013. doi: 10.1016/j.oraloncology.2020.105013. [DOI] [PubMed] [Google Scholar]
- 36.Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6:519–527. doi: 10.1001/jamaoncol.2019.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.España S, Montes Pérez, de Oca A, Marques-Pamies M, et al. Endocrine adverse events related to immune-oncology agents: retrospective experience of a single institution. Transl Lung Cancer Res. 2020;9:103–110. doi: 10.21037/tlcr.2019.12.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lima Ferreira J, Costa C, Marques B, et al. Improved survival in patients with thyroid function test abnormalities secondary to immune-checkpoint inhibitors. Cancer Immunol Immunother. 2020 doi: 10.1007/s00262-020-02664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly K, Manitz J, Patel MR, et al. Efficacy and immune-related adverse event associations in avelumab-treated patients. J Immunother Cancer. 2020;8:e001427. doi: 10.1136/jitc-2020-001427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kijima T, Fukushima H, Kusuhara S, et al. Association between the occurrence and spectrum of immune-related adverse events and efficacy of pembrolizumab in Asian patients with advanced urothelial cancer: multicenter retrospective analyses and systematic literature review. Clin Genitourin Cancer. 2020;S1558–7673:30163–30164. doi: 10.1016/j.clgc.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Kotwal A, Kottschade L, Ryder M. PD-l1 inhibitor-induced thyroiditis is associated with better overall survival in cancer patients. Thyroid. 2020;30:177–184. doi: 10.1089/thy.2019.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maillet D, Corbaux P, Stelmes JJ, et al. Association between immune-related adverse events and long-term survival outcomes in patients treated with immune checkpoint inhibitors. Eur J Cancer. 2020;132:61–70. doi: 10.1016/j.ejca.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 43.Bai R, Li L, Chen X, et al. Correlation of peripheral blood parameters and immune-related adverse events with the efficacy of immune checkpoint inhibitors. J Oncol. 2021;10:9935076. doi: 10.1155/2021/9935076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frelau A, Jali E, Campillo-Gimenez B, et al. Prognostic impact of thyroid dysfunctions on progression-free survival in patients with metastatic melanoma treated with anti-PD-1 antibodies. Melanoma Res. 2021;31:208–217. doi: 10.1097/CMR.0000000000000739. [DOI] [PubMed] [Google Scholar]
- 45.Luongo C, Morra R, Gambale C, et al. Higher baseline TSH levels predict early hypothyroidism during cancer immunotherapy. J Endocrinol Invest. 2021;44:1927–1933. doi: 10.1007/s40618-021-01508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muir CA, Clifton-Bligh RJ, Long GV, et al. Thyroid immune-related adverse events following immune checkpoint inhibitor treatment. J Clin Endocrinol Metab. 2021 doi: 10.1210/clinem/dgab263. [DOI] [PubMed] [Google Scholar]
- 47.Street S, Chute D, Strohbehn I, et al. The positive effect of immune checkpoint inhibitor-induced thyroiditis on overall survival accounting for immortal time bias: a retrospective cohort study of 6596 patients. Ann Oncol. 2021;32:1050–1051. doi: 10.1016/j.annonc.2021.05.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thuillier P, Joly C, Alavi Z, et al. Thyroid dysfunction induced by immune checkpoint inhibitors is associated with a better progression-free survival and overall survival in non-small cell lung cancer: an original cohort study. Cancer Immunol Immunother. 2021 doi: 10.1007/s00262-020-02802-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Y, Xia R, Xiao H, et al. Thyroid function abnormality induced by PD-1 inhibitors have a positive impact on survival in patients with non-small cell lung cancer. Int Immunopharmacol. 2020;91:107296. doi: 10.1016/j.intimp.2020.107296. [DOI] [PubMed] [Google Scholar]
- 50.Al Ashi SI, Thapa B, Flores M, et al. Endocrine toxicity and outcomes in patients with metastatic malignancies treated with immune checkpoint inhibitors. J Endocr Soc. 2021;5:bvab100. doi: 10.1210/jendso/bvab100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahn BC, Pyo KH, Xin CF, et al. Comprehensive analysis of the characteristics and treatment outcomes of patients with non-small cell lung cancer treated with anti-PD-1 therapy in real-world practice. J Cancer Res Clin Oncol. 2019;145:1613–1623. doi: 10.1007/s00432-019-02899-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campredon P, Mouly C, Lusque A, et al. Incidence of thyroid dysfunctions during treatment with nivolumab for non-small cell lung cancer: retrospective study of 105 patients. Presse Med. 2019;48:e199–e207. doi: 10.1016/j.lpm.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 53.Ksienski D, Wai ES, Croteau N, et al. Efficacy of nivolumab and pembrolizumab in patients with advanced non-small-cell lung cancer needing treatment interruption because of adverse events: a retrospective multicenter analysis. Clin Lung Cancer. 2019;20:e97–e106. doi: 10.1016/j.cllc.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886–894. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsuo M, Yasumatsu R, Masuda M, et al. Relationship between immune-related adverse events and the long-term outcomes in recurrent/metastatic head and neck squamous cell carcinoma treated with nivolumab. Oral Oncol. 2020;101:104525. doi: 10.1016/j.oraloncology.2019.104525. [DOI] [PubMed] [Google Scholar]
- 56.Al Mushref M, Guido PA, Collichio FA, et al. Thyroid dysfunction, recovery, and prognosis in melanoma patients treated with immune checkpoint inhibitors: a retrospective review. Endocr Pract. 2020;26:36–42. doi: 10.4158/EP-2019-0244. [DOI] [PubMed] [Google Scholar]
- 57.D'Aiello A, Lin J, Gucalp R, et al. Thyroid dysfunction in lung cancer patients treated with immune checkpoint inhibitors (ICIs): outcomes in a multiethnic urban cohort. Cancers (Basel) 2021;13:1464. doi: 10.3390/cancers13061464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubino R, Marini A, Roviello G, et al. Endocrine-related adverse events in a large series of cancer patients treated with anti-PD1 therapy. Endocrine. 2021 doi: 10.1007/s12020-021-02750-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paderi A, Giorgione R, Giommoni E, et al. Association between immune related adverse events and outcome in patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors. Cancers (Basel) 2021;13:860. doi: 10.3390/cancers13040860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morimoto K, Yamada T, Takumi C, et al. Immune-related adverse events are associated with clinical benefit in patients with non-small-cell lung cancer treated with immunotherapy plus chemotherapy: a retrospective study. Front Oncol. 2021 doi: 10.3389/fonc.2021.630136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lui DTW, Lee CH, Tang V, et al (2021) Thyroid immune-related adverse events in patients with cancer treated with anti-PD1/anti-CTLA4 immune checkpoint inhibitor combination: clinical course and outcomes. Endocr Pract S1530-891X(21)00030-6 [DOI] [PubMed]
- 62.Holstead RG, Kartolo BA, Hopman WM, et al. Impact of the development of immune related adverse events in metastatic melanoma treated with PD -1 inhibitors. Melanoma Res. 2021;31:258–263. doi: 10.1097/CMR.0000000000000736. [DOI] [PubMed] [Google Scholar]
- 63.Fujisawa Y, Yoshino K, Otsuka A, et al. Retrospective study of advanced melanoma patients treated with ipilimumab after nivolumab: Analysis of 60 Japanese patients. J Dermatol Sci. 2018;89:60–66. doi: 10.1016/j.jdermsci.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 64.Cortellini A, Friedlaender A, Banna GL, et al. Immune-related adverse events of pembrolizumab in a large real-world cohort of patients with NSCLC with a PD-L1 expression ≥ 50% and their relationship with clinical outcomes. Clin Lung Cancer. 2020;21:498.e2–508. doi: 10.1016/j.cllc.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 65.Paderi A, Giorgione R, Giommoni E, et al. Association between immune related adverse events and outcome in patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors. Cancers (Basel) 2021;13:860. doi: 10.3390/cancers13040860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lisberg A, Tucker DA, Goldman JW, et al. Treatment-related adverse events predict improved clinical outcome in NSCLC patients on KEYNOTE-001 at a single center. Cancer Immunol Res. 2018;6:288–294. doi: 10.1158/2326-6066.CIR-17-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Filette J, Andreescu CE, Cools F, et al. A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm Metab Res. 2019;51:145–156. doi: 10.1055/a-0843-3366. [DOI] [PubMed] [Google Scholar]
- 68.Park R, Lopes L, Saeed A. Anti-PD-1/L1-associated immune-related adverse events as harbinger of favorable clinical outcome: systematic review and meta-analysis. Clin Transl Oncol. 2021;23:100–109. doi: 10.1007/s12094-020-02397-5. [DOI] [PubMed] [Google Scholar]
- 69.Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4:173–182. doi: 10.1001/jamaoncol.2017.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hussaini S, Chehade R, Boldt RG, et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors: a systematic review and meta-analysis. Cancer Treat Rev. 2021;92:102134. doi: 10.1016/j.ctrv.2020.102134. [DOI] [PubMed] [Google Scholar]
- 71.Cortellini A, Buti S, Agostinelli V, et al. A systematic review on the emerging association between the occurrence of immune-related adverse events and clinical outcomes with checkpoint inhibitors in advanced cancer patients. Semin Oncol. 2019;46:362–371. doi: 10.1053/j.seminoncol.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 72.Ye Y, Jing Y, Li L, et al. Sex-associated molecular differences for cancer immunotherapy. Nat Commun. 2020;11:1779. doi: 10.1038/s41467-020-15679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Network NCC (2021) NCCN guidelines version 4.2021 management of immune checkpoint inhibitor-related toxicity https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf
- 74.Chang LS, Barroso-Sousa R, Tolaney SM, et al. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev. 2019;40:17–65. doi: 10.1210/er.2018-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]