Abstract
Purpose of Review
Spondylolysis remains one of the most common causes of lower back pain in the pediatric and adolescent populations and is particularly prevalent in young sporting individuals. Despite this, approaches to diagnostic imaging and both conservative and surgical treatment vary widely among surgeons. The current review investigates recent literature on the etiology, clinical presentation, diagnosis, and treatment of spondylolysis. In particular, it interrogates the use of various advanced imaging modalities (CT, MRI, SPECT) in diagnosis as well as common surgical approaches to the condition.
Recent Findings
Recent data has provided more information on how pars defect laterality, stage, and presence or absence of bone marrow edema impact healing potential. Other studies have highlighted the advantages of using MRI for spondylolysis diagnosis. Other data has provided more clarity on which adults may benefit from direct pars repair, while other studies have compared the various techniques for direct repair of pars defects.
Summary
While the exact cause of spondylolysis remains unclear, there is growing understanding of the behavioral, genetic, and biomechanical risk factors that predispose individuals to the condition. MRI may be emerging as the advanced imaging modality of choice for diagnosis due to its lack of radiation and comparable sensitivity to other advanced imaging techniques. Conservative treatment remains the first step in management due to excellent outcomes in most patients, with surgical intervention rarely necessary. In patients that do require surgery, direct repair using a pedicle screw-based approach is preferred over spinal fusion and other direct repair techniques.
Keywords: Spondylolysis, Pars Defect, Spondylolisthesis, Spine surgery, Athletics
Introduction
Spondylolysis is a condition defined as a defect or fracture of the pars interarticularis, the portion of the vertebra between the superior and inferior articular processes. It occurs most commonly at the level of L5 (85–95%) and presents as either a unilateral or bilateral defect, which may lead to spondylolisthesis with anterior slippage of the vertebral body [1, 2]. Together, spondylolysis and spondylolisthesis are some of the most common causes of lower back pain in the pediatric and adolescent population, particularly in those involved in athletics [3]. The prevalence of spondylolysis in the general population is estimated to be 6%; however, this may vary widely due to a variety of factors that can enhance or reduce the development of pars fractures within the lumbar spine [4]. Specific associated factors that have been studied include age, ethnicity, sex, heredity, and involvement in sports [5–7]. Treatment tends to be conservative; however, there are surgical options available for patients who fail to respond to conservative treatment or develop sequelae including spondylolisthesis. Choices in diagnostic imaging and treatment may also differ depending on the patient population treated, with some imaging and treatment courses being more appropriate for the elite athlete than the general population. The primary aim of this article is to provide a current review of this condition’s etiology, natural history, clinical presentation, and imaging approach, as well as a comprehensive discussion of both conservative and surgical treatment.
Etiology
While its cause remains mostly unknown, spondylolysis likely occurs due to a combination of chronic mechanical stress and genetic predisposition. Symptoms often arise in adolescent athletes experiencing increased athletic demand and growth spurts [8]. Mechanical stress is a likely explanation, as spondylolysis has never been demonstrated in nonambulatory patients and is not found in newborns [4, 9]. Athletics involving axial stress combined with hyperextension such as gymnastics and weightlifting have been shown to predispose athletes to higher rates of spondylolysis as well, likely due to repetitive microtrauma induced by sports-specific maneuvers [10–12]. Mechanically, the pars is susceptible to chronic axial loading injury due to the high stress load it experiences during extension of the lumbar spine. These tensile stresses have been measured to be the greatest at the ventral-caudal aspect of the pars which is the location most commonly seen [13]. Congenitally narrow pars structure and uneven trabeculation in the lower lumbar vertebrae may also play a role [14]. Neural arch strength continues to increase up to the age of 50, increasing the risk of pars fractures in children and adolescents [15]. In patients with a higher pelvic incidence and sacral slope, there is greater shear force at the lumbosacral junction, generating higher stress in the pars and potentially increasing susceptibility to stress fracture [16–19]. While pars fractures most often occur as the result of chronic stress, these injuries can also occur acutely in a single overload injury [20].
Recently, evidence has emerged supporting a genetic component to this diagnosis. Wynne-Davies et al. demonstrated significantly increased incidence in first-degree relatives of patients with spondylolysis, an observation reported by multiple other investigators [7, 21–23]. Furthermore, in 2015 an autosomal dominant mutation of the diastrophic dysplasia sulfate transporter gene was found in patients with dysplastic spondylolysis [24]. Additional diagnoses such as spina bifida occulta and scoliosis have also been associated with spondylolysis, indicating a congenital predisposition in some patients [25•, 26–28].
Natural History
Spondylolytic defects exist on a spectrum, ranging from an early stress reaction to a terminal pseudarthrosis. Stress reactions in the pars precede fractures and likely represent a grey area of a physiologic stress response transitioning to a pathologic lysis. Morita et al. defined pars fractures into 3 stages: early, progressive, and terminal [29]. Early fractures are hairline defects with focal bony resorption, while progressive fractures have wider defects and often the presence of small fragments. Terminal stage fractures exhibit sclerotic change and are effectively a pseudarthrosis. Fractures that reach the terminal stage will never progress to union, impacting management [30]. Defects may progress from unilateral to bilateral, with 80% of defects bilateral and 20% unilateral. Adolescent athletes may present with a unilateral defect due to asymmetric axial loading. Once a unilateral defect occurs, stresses increase at the contralateral pars, likely explaining the greater proportion of bilateral cases [31]. L5 is the most common location for spondylolysis (85–95%), and the remaining cases are typically observed at L4 or L3 [32]. Defects can be found at more than one level in 4% of patients [29, 33]. Patients with bilateral pars defects are at risk for spondylolisthesis, while unilateral pars defects generally do not lead to slippage [33•, 34]. It is estimated that 75% of patients with bilateral spondylolysis will go on to develop some degree of spondylolisthesis [34–36]. A cross-sectional study by Aoki et al. in 2020 demonstrated that 51% of patients with spondylolysis exhibited spondylolisthesis on CT scan, with no unilateral defects exhibiting spondylolisthesis. The same authors also demonstrated an age-dependent increase in progression to spondylolisthesis, with 90% of bilateral spondylolysis patients greater than 60 years old exhibiting spondylolisthesis and only 8.3% of patients less than 60 years old showing spondylolisthesis [33•]. Several studies have demonstrated that progression of spondylolisthesis, previously thought to only occur during adolescence, can occur in older adults as well [37, 38]. Beutler et al. demonstrated that patients with bilateral pars defects typically follow a clinical course comparable to the general population and only a small percentage develop symptomatic slip progression [34].
Epidemiology
Age is a key factor in the development of spondylolysis, with an estimated prevalence of 4.4% in children growing to 6% by adulthood [4]. Sex also plays a role, with males 2–3 times more likely to develop spondylolysis than females [4, 39]. The role of ethnicity has been demonstrated in the USA: spondylolysis occurs in 6.4% of white men, 2.8% of black men, 2.3% of white women, and 1.1% of black women [40, 41]. Athletes are also more likely to develop spondylolysis, with an 8–15% prevalence in asymptomatic adolescent athletes and 47% prevalence in adolescent athletes with concomitant LBP [6, 8]. This rate in adolescent athletes with LBP can be contrasted with just a 5% prevalence in adult athletes with LBP [2]. Prevalence in specific sports has also been studied, with up to 15% of college football players and 11% of female gymnasts developing spondylolysis [42, 43]. Even higher rates have been observed in weightlifters (20–30%) and wrestlers (30–35%) [44]. Sport-specific rates likely vary due to differing levels of trunk rotation and hyperextension movements required in each sport. Iatrogenic spondylolysis is another less-known entity that can occur as a postoperative complication and has been described in up to 20% of patients after spinal fusion or decompression [45–47].
Clinical Presentation
Spondylolysis can present in multiple ways, including as an incidental finding on radiographs in an asymptomatic patient [48]. However, the key patient population to suspect spondylolysis in are adolescents involved in athletics who present with LBP, as almost half of this patient population will be diagnosed with spondylolysis [8, 20]. The mean age of presentation in the juvenile population is 15, aligning with the adolescent growth spurt [49]. While acute spondylolysis most often presents in adolescents or children, it has also been described in adult high-level athletes and should be considered in such patients presenting with acute LBP [50, 51]. It is important to gain a complete history from the patient including acuity, sports involvement, and any other physical activities. A family history of low back pain or spondylolytic defects may be helpful for diagnosis.
On physical exam, low back pain worsened by extension or repetitive movement may be seen, as well as weakness of the L5 myotome [52]. Pain with back extension has been shown to have a sensitivity of 81% in patients with spondylolysis [53]. Considering that some patients may have a congenital component to their spondylolysis, inspection of the spine for skin discoloration, dimples, patches, or tufts should also be performed. The neurologic exam is typically unremarkable; if positive neurologic signs are found then spondylolisthesis or alternate diagnoses should be considered. The one-legged hyperextension test remains the only test that has been specifically evaluated for its ability to diagnose lumbar spondylolysis and was shown by Masci et al. to have a low sensitivity of 50–55% in detecting SPECT-confirmed spondylolysis, translating to very little clinical utility in diagnosing patients [54, 55]. Conversely, spondylolisthesis may present with radiculopathy symptoms including numbness, tingling, and pain, typically in the lower limbs in the case of lumbar spondylolisthesis. Sudden worsening of pain in a patient with known spondylolysis may indicate progression to spondylolisthesis. Lumbar spinous process palpation was shown to have a sensitivity of 60% and specificity of 87% for diagnosis of spondylolisthesis [56]. Ultimately, physical exam findings can be used to increase the degree of suspicion for spondylolysis but when used by themselves have very poor sensitivity and diagnostic reliability and therefore should be used in combination with the correct choice of imaging.
Diagnostic Imaging
Radiographs
The initial assessment of suspected spondylolysis starts with standing AP, lateral, and flexion/extension lumbar radiographs. Historically, oblique plain radiographs have been described for initial assessment but are no longer routinely used as they do not provide any additional increase in sensitivity or specificity for spondylolysis detection compared with AP or lateral radiographs [57]. One scenario in which oblique radiographs may be useful is in identification of upper lumbar spondylolysis [58]. AP and lateral standing radiographs have been shown to have a sensitivity of 75% compared to a 90% sensitivity provided by CT scans [3]. Surgeons must therefore have a high degree of suspicion in adolescent athletes presenting with LBP when evaluating radiographs [59]. Plain radiographs are a reasonable first step in diagnosis due to high availability and cost effectiveness. Advanced imaging studies such as CT, SPECT, or MRI should be pursued in the case of patients with persistent lower back pain and negative radiographs. Need for an expedited diagnosis in elite athletes also typically warrants advanced imaging in tandem with radiographs.
Computed Tomography
Computed tomography (CT) scans provide improved sensitivity and specificity compared to plain radiography in diagnosis of spondylolysis [3]. Advantages of CT include high resolution of bony anatomy and ability to properly stage spondylolysis, as well as assessment of healing in chronic defects [60–62, 63••]. Multiple studies have identified CT as the best modality for tracking healing following initial diagnosis [63••, 64•]. CT scans better visualize cortical integrity compared to MRI, allowing CT to identify chronic fractures with sclerotic margins with very high fidelity [65]. It has also been suggested that CT be used as the primary imaging modality in patients with dysplastic or abnormal pars anatomy due to higher false-negative and false-positives rates on MRI in this patient population [63••]. For detection of early-stage pars defects on CT, sagittal reconstructed CT may be best due to improved visualization of defects isolated to the ventral-caudal aspect of the pars, as well as better differentiation between the pars defect and facet joints [13, 30, 64•].
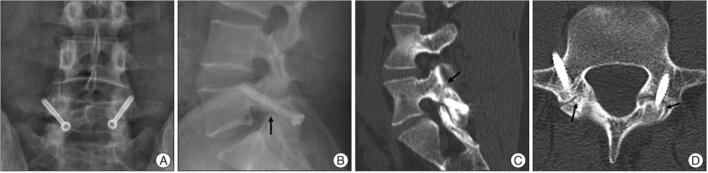
Although CT is less reliable for distinguishing active fractures from chronic non-unions, it can still provide valuable information for prognostic purposes: wide sclerotic margins indicate a chronic non-union that will not heal with conservative treatment, while a narrow pars defect with non-corticated margins indicates an acute fracture that may heal with activity reduction and other conservative measures [63••, 64•]. Early, pre-lysis lesions without fracture are often occult on CT, limiting potential for earlier diagnoses [65]. A principal disadvantage of this modality is increased radiation exposure, which is particularly relevant in the choice of imaging for juveniles with suspected spondylolysis. Lifetime cancer mortality risks attributable to the radiation from a pediatric CT examination are estimated to be much higher than for adults [66]. A 2013 study of 680,000 individuals exposed to CT scans in childhood or adolescence found an incidence rate ratio of 1.13 for cancers of all types from spine CT scans [67]. In 2015, Fadell et al. found that z-axis limitation and CT protocol optimization were able to reduce radiation exposure to even less than standard radiographs in some cases while maintaining the enhanced diagnostic utility of CT scans (Fig. 1) [68]. Overall, CT is an excellent option for proper staging and visualization of pars defects but generates significant radiation exposure that must be weighed carefully, particularly when selecting advanced imaging for juveniles.
Fig. 1.
Sagittal limited z-axis CT scan, optimized to decrease radiation dose, demonstrating a complete fracture of the left-sided L5 pars interarticularis. Courtesy of Fadell et al. 68. Published with permission from Springer Nature Inc.
Magnetic Resonance Imaging
While early investigations of magnetic resonance imaging (MRI) for detection of spondylolysis reported poor sensitivity and positive predictive value, recent reports have provided support for use of MRI as the advanced imaging modality of choice in spondylolysis evaluation [60, 62, 63••, 65, 69–72]. Development of thin-slice MRI protocols specifically designed for evaluation of the pars interarticularis have likely improved performance, with reports of sensitivity as high as 92–98% for detection of spondylolysis [30, 64•, 71]. Specifically, fat-suppressed fast-spin T2-weighted images in the sagittal plane are key for making the diagnosis using MRI [71•, 73]. Higher field strength (3-tesla MRI) generates a higher signal-to-noise ratio and may be preferred by some surgeons [74, 75]. Ancillary findings on MRI have also been documented that may help increase test sensitivity, including widened sagittal diameter of the spinal canal, wedging of the posterior aspect of the vertebral body at the level of spondylolysis, and marrow changes in the adjacent pedicle [69, 76]. MRI has demonstrated high negative predictive value (97%) for spondylolysis when marrow is seen across the pars region, providing valuable rule-out ability for initial diagnostic evaluation [77].
In 2002, Hollenberg et al. devised a classification scale for pars defects on MRI with high intraobserver and interobserver agreement: grade 0: normal; grade 1: stress reaction; grade 2: incomplete fracture; grade 3: acute complete fracture; grade 4: chronic complete fracture [78]. Campbell et al. performed a prospective analysis comparing MRI vs. CT vs. SPECT, concluding that MRI was highly accurate for grade 0, 3, and 4 defects, with limitation in distinguishing stress reactions from incomplete defects [63••]. Despite this potential limitation, multiple investigators have performed studies comparing MRI, CT, and SPECT using this scale and concluded that MRI should be used as the advanced imaging modality of choice because of its risk-benefit ratio [63••, 64•, 71•]. One key advantage MRI has over other imaging techniques is the ability to pick up on stress reactions and early fractures that may be missed on CT or plain radiography [65]. Cortical edema (high signal change, HSC) in the pedicle adjacent to the pars on MRI is one of the earliest indicators of spondylolysis and is a positive predictor of bony healing: HSC-positive defects have a higher rate of healing than HSC-negative defects [49, 79]. Early recognition of these stress reactions and pre-lysis lesions on MRI may enable earlier initiation of conservative treatment, preventing progression to chronic non-union and avoiding surgical intervention [71•, 80]. Other principal advantages include the absence of ionizing radiation and superior assessment of concomitant pathology including nerve root compression, disc degeneration and tissue within the pars defect [64, 72]. The combination of these advantages with sensitivity comparable to SPECT and CT as well as potential prognostic information not offered by other imaging modalities makes MRI an attractive option as the primary advanced imaging choice in spondylolysis evaluation.
SPECT/Bone Scan
Single-photon emission computed tomography (SPECT) is a nuclear imaging technique which produces a 3-dimensional image showing distribution of a radiotracer taken up by certain tissues [81]. SPECT is extremely sensitive and will pick up on stress reactions and acute lesions that may be missed on CT or plain radiographs [82, 83]. It may also provide information on the ability of a pars lesion to heal, similar to the presence or absence of HSC in adjacent pedicles on MRI [52]. However, SPECT has significant downsides that must be considered. Radiation exposure is a concern, with an estimated dose of 5 mSv from SPECT alone and 10 mSv with combined CT-SPECT testing [52, 64•]. SPECT imaging cannot reliably distinguish between stress reactions and overt pars fractures and detects only 17% of chronic lesions [63••, 84]. A positive SPECT result for spondylolysis is increased radiotracer uptake in the pars region, which may be mimicked by facet joint arthritis or unilateral lesions including infection and osteoid osteoma [64•]. This produces a high false-positive rate which is compounded by false-negatives from missed chronic lesions and non-unions. It has thus been suggested that SPECT can essentially be abandoned in the general population, except in cases where MRI may be contraindicated or nondiagnostic [63••]. However, SPECT may still have a role in expedited diagnosis of elite athletes with lower back pain for more than 3 weeks. If SPECT is positive, a CT scan can be performed to better delineate the pathology; SPECT and CT can also be merged for definitive diagnosis and prognosis. A positive SPECT without fracture on CT is a stress reaction and has a favorable prognosis, while a negative SPECT with a positive pars fracture on CT is likely a chronic non-union and will likely not heal. It has been estimated that in 8–10% of cases SPECT is positive and MRI is nondiagnostic, indicating slightly higher sensitivity on SPECT than MRI [74]. This should be interpreted with caution however, as positive SPECT imaging has been documented in asymptomatic athletes, and may be picking up on a physiologic stress reaction that would not naturally progress to spondylolysis [83].
Treatment
The vast majority of patients with acute symptomatic spondylolysis can be treated successfully with conservative measures, while asymptomatic and incidentally discovered cases do not require treatment at all. Treatment is always conservative initially, while surgical intervention is reserved for refractory cases in patients with debilitating pain for more than 6–12 months. Positive predictors of pars defects spontaneously healing include unilateral defects, earlier fracture stage, and lumbar level other than L5 [85]. While good clinical outcomes with conservative treatment do not necessarily depend on the bony healing of the lesion, osseous union should still be an objective because this reduces the risk of progression to spondylolisthesis [29, 62, 77, 86]. It is important to differentiate acute from chronic pars defects when selecting management: acute lesions include stress reactions and early/progressive fractures, while chronic defects are non-unions that will not heal spontaneously. Pain mechanism likely differs between acute and chronic defects, with inflammation from the acute fracture causing pain while chronic defects generate pain due to communicating synovitis from the pars pseudarthrosis [30]. The goal in treatment of early and progressive defects is therefore to achieve bony union through immobilization, while in terminal defects, the goal is to limit pain from synovitis and ultimately achieve bony union through surgical means. Spondylolysis in adult athletes tends to be chronic, so pain relief is key. In the rare case of acute pars fractures in an adult elite athlete, immobilization and conservative treatment is preferred; however, this must be weighed against the ability of the athlete to miss training for prolonged periods of time (3–6 months).
Conservative/Non-operative
Conservative measures are often able to achieve favorable outcomes in both the general population and athletes. A prospective study by Lee et al. compared 145 young patients with spondylolysis treated surgically and conservatively and found that at 12 months there was no significant difference in pain and Oswestry Disability Index between the two groups [87•]. Similar results have been reported in athletes, with conservative treatment successful in 85% of athletes versus 87.7% in athletes treated surgically [88]. A 2016 review found that athletes return to play at 3.7 months on average when treated conservatively [89]. Interestingly, non-bony union of the defect may not negatively impact overall short-term outcome or sports resumption: prospective cohort studies by Sys and Iwamoto respectively found that athletes without bony union still had excellent subjective outcomes and rates of return to play [90, 91]. This may be due to fibrous, non-bony union stabilizing the defect and preventing pain [10, 12].
Union rate with conservative measures is highly dependent on fracture stage: a retrospective study by Sakai et al. in 2017 demonstrated 100% bony healing rate in “very early,” 93.8% in “early,” and 80% in “progressive” stage defects with no attempt made for conservative measures in terminal defects [92•]. Diagnosis of each stage is based on a combination of plain X-ray, CT, and MRI findings: “very early” stage defects have a stress reaction on MRI with no visible fracture line on CT, “early” stage have a visible hairline fracture, “progressive” stage have an obvious fracture with a gap, while “terminal” stage defects are a pseudarthrosis. A similar prospective study by Sairyo et al. demonstrated the impact that presence or absence of pedicle edema (HSC) on MRI has on healing: 64% of progressive defects with pedicle edema healed by 3 months compared to 27% of lesions without pedicle edema [80].
Conservative treatment typically consists of a combination of bracing, activity restriction, pain control, and physical therapy. Evidence for bracing is mixed, and positive results from bracing are likely a product of the activity restriction that bracing enforces versus the actual bracing itself. The 3 predominant brace types that have been studied are TSLO, LSO, and non-rigid braces [89]. Studies comparing patients treated with or without a brace have found no significant difference in clinical outcomes and demonstrated that pain relief and functional recovery can be achieved without bracing [77, 93]. Physical therapy is an integral component of treatment: the patient should stop all pain-producing activity for at least 6 weeks, followed by initiation of a back exercise physical therapy program, which may help lower the rate of refracture after union [92•]. Oral nonsteroidal anti-inflammatory drugs (NSAIDs) may reduce pain; local anesthetic/corticosteroid combination (e.g., lidocaine/methylprednisolone) injections infiltrating the pars defect have been reported to produce significant temporary pain relief as well [94, 95]. Teriparatide is occasionally used in professional athletes due to reports of faster recovery of osteoporotic fractures and increased bony union in spine fusion but has not specifically been shown to improve spondylolysis healing [96, 97].
Low-intensity pulsed ultrasound (LIPUS) is a technique that has recently emerged with promising results for increasing bony union rates, particularly in progressive stage pars fractures. In one clinical study, LIPUS increased bony union percentage in progressive fractures with HSC on MRI from 10% in a conventional treatment group to 67% in the LIPUS-treated group [86]. Another prospective study in athletes with spondylolysis demonstrated that LIPUS reduced time required to return to sport by 72%, from a mean of 167 days to 61 days [98]. This therapy may become a key component in conservative treatment of early and progressive spondylolysis defects.
Operative Intervention
While rare, there are patients who do not have symptom improvement with conservative treatment and continue to have severe pain interfering with quality of life or ability to return to sports. A total of 5% of athletes fail conservative treatment and require surgery [99]. A systematic review by Scheepers et al. found that in adult athletes who fail conservative management, surgery is likely to enable return to sport, reduce pain, and improve overall function [100]. Indications for surgery include persistent pain after 6–12 months of conservative treatment, neurologic deficits, and worsening symptomatic spondylolisthesis. Factors that may positively influence clinical outcomes after surgery include unilateral defects and younger patient age [101]. There are 2 principal surgical methods that can be used for spondylolysis: fusion or direct repair. Posterolateral fusion was once the only method of choice until the introduction of direct repair by Buck in 1970 [102]. Spinal fusion for spondylolysis without spondylolisthesis has become less popular with time largely due to loss of range of motion at the fused level and the potential for adjacent segment disease (ASD). Regardless of the surgical intervention, thorough debridement of the pars defect is crucial to remove fibrous or cartilaginous tissue as well as the sclerotic bone margins. Bone grafting and use of alternative sources such as bone morphogenetic protein may improve bony healing rates and should be strongly considered, particularly in fractures without any metabolic activity evident on SPECT/MRI [95, 103].
Spinal Fusion
While no longer the preferred method for surgical treatment of spondylolysis, there remain scenarios in which spinal fusion is indicated instead of direct pars repair. Spondylolisthesis Meyerding grade I or greater, significant degenerative disc disease and dysplastic pars anatomy preclude direct pars repair, leaving spinal fusion as the primary surgical option in patients with refractory pain [74, 95]. Spinal fusion increases stability and reduces translation in the sagittal plane [104]. In the case of a bilateral L5 spondylolysis causing anterolisthesis of L5, an L5-S1 in situ fusion with autogenous iliac crest bone graft historically has led to excellent clinical outcomes approaching 90% [105]. More recently, lumbar interbody fusion has been utilized during surgical treatment in hopes of increasing bony fusion rates and restoring lordosis, particularly at the L5-S1 level. Transforaminal lumbar interbody fusion (TLIF) possesses advantages compared to posterior lumbar interbody fusion (PLIF) including reduced complication rate, blood loss, and operation duration [106–110]. While spinal fusion provides excellent stability, its intersegmental fixation causes loss of natural range of motion and carries significant risk of ASD: 36% of patients who undergo posterior lumbar fusion will require additional surgery at 10 years postoperatively due to ASD [111]. Additionally, spinal fusion should be avoided in professional athletes if possible due to lower chances of returning to prior athletic level as well as necessity to take a prolonged period of time off from athletics [74]. These downsides are significant and are reduced if not obviated in the alternative method of direct pars repair.
Direct Repair
Since its introduction in 1970 by Buck, direct repair of the pars has become the preferred intervention for pars defects after conservative measures fail [102]. Direct repair does not restrict range of motion in the involved segments, can restore normal anatomy, and has good clinical functional outcomes [112]. Contraindications for direct repair include facet joint arthropathy, significant degenerative disk disease, and associated spondylolisthesis [113]. Pfirrmann grade III disc degeneration may be an acceptable limit to offer pars repair [114]. Spondylolisthesis greater than Meyerding grade 1 is a contraindication, although the actual amount of slippage that requires fusion vs. direct repair is controversial. In patients with lower extremity symptoms such as neurogenic claudication and associated stenosis, direct repair alone is insufficient and a decompressive laminotomy or laminectomy should be considered [95]. Anesthetic infiltration of the pars is useful for identification of patients most likely to benefit from surgical treatment, as it can confirm the pars defect as the pain-generating source [115]. It has previously been suggested that direct repair should be reserved for patients younger than 20 due to reduced union rates and potentially worse clinical outcomes in older patients [116, 117]. Despite this, a 2021 systematic review by Kumar et al. demonstrated that in adults aged 18–45 with a positive pars infiltration test and no signs of degenerative disc changes, direct repair is still highly successful [118••]. The evidence-based literature suggests excellent clinical results from direct repairs regardless of level [119, 120, 121•].
There are a variety of methods for performing direct repair, including the Buck repair (direct pars lag screw, Fig. 2), Scott repair (segmental wire fixation, Fig. 3), Morscher repair (screw hook, Fig. 4), and pedicle screw-based techniques (Fig. 5) [95, 102, 122–127]. A 2018 meta-analysis by Mohammed et al. compared these four direct repair methods and assessed overall pooled fusion, complications, and positive outcome rate. Pedicle screw techniques had the highest fusion rate (90.21%) and lowest complication rate (12.8%), with the Buck repair providing the second-best results. Positive outcome rates were high with all methods, with positive outcomes seen in 84.33% of Buck repair-treated patients and 80.1% of pedicle screw-treated patients. These results indicate that the Buck repair and pedicle screw-based methods are highly comparable in fusion and complication rates and both provide a high rate of good clinical outcomes [128••]. Biomechanical data support these two methods as well, with cadaveric studies demonstrating that Buck and pedicle screw-hook systems have the least amount of displacement and greatest stability during flexion/extension [113, 129].
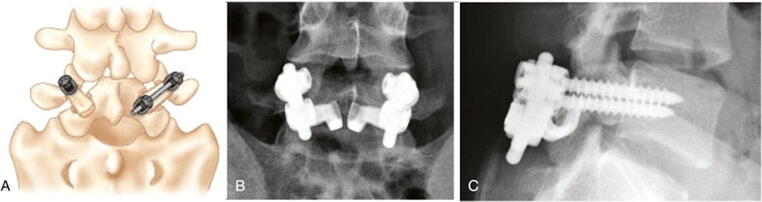
Fig. 2.
Buck method of direct repair using a direct pars lag screw. A,B Postoperative AP (A) and lateral (B) plain X-rays showing placement of the pars screw through the pars defect (black arrow). C,D Sagittal (C) and axial (D) CT scans depicting a well fused pars defect 26 months after the operation (black arrows). Figure 2 labeled for reuse according to Creative Commons Attribution Non-Commercial 3.0 Unported License (From Shin MH, Ryu KS, Rathi NK, Park CK. Direct pars repair surgery using two different surgical methods: a pedicle screw with universal hook system and direct pars screw fixation in symptomatic lumbar spondylolysis patients. J Korean Neurosurg Soc. 2013. 51(1): p. 14-9, https://dx.doi.org/10.3340%2Fjkns.2012.51.1.14)
Fig. 3.
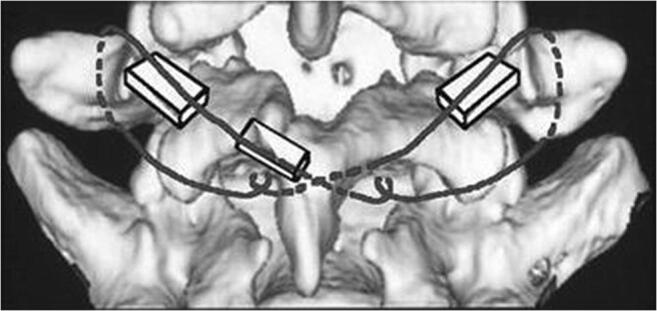
Scott method of direct repair using segmental wire fixation. Courtesy of Yamamoto et al. 125. Published with permission from Springer Nature Inc.
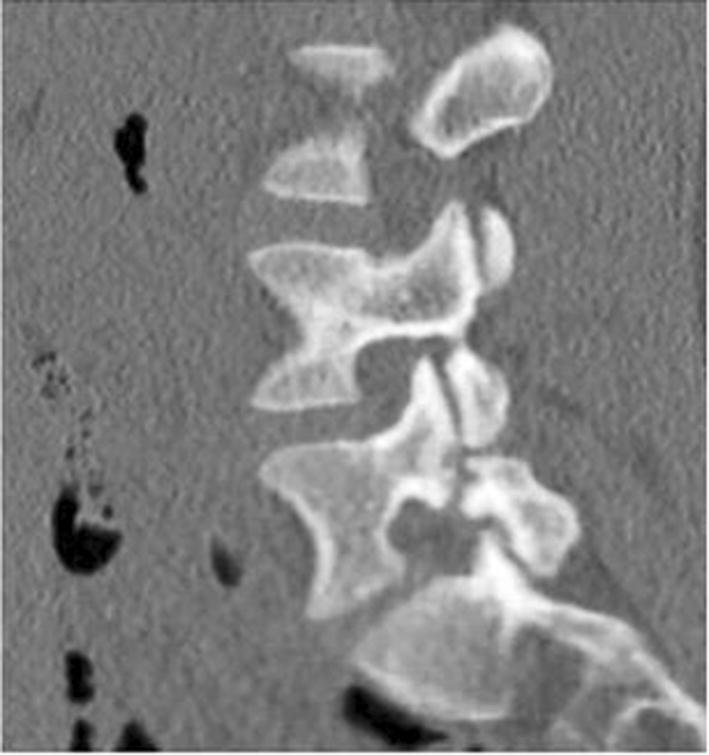
Fig. 4.
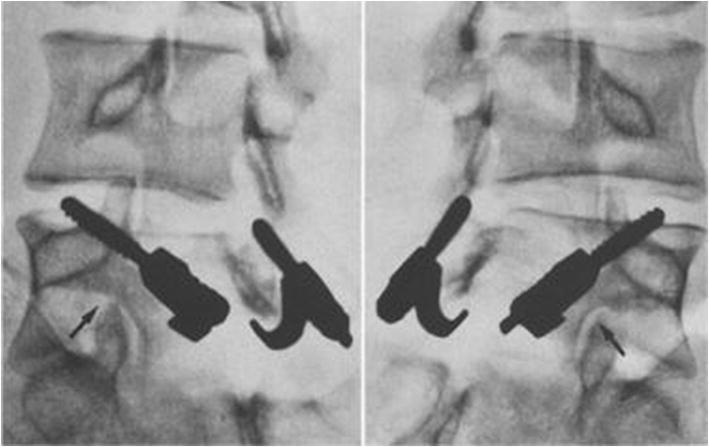
Morscher method of direct repair using a screw hook. Courtesy of Hefti et al. 126. Published with permission from Springer Nature Inc.
Fig. 5.
Pedicle screw-hook-rod method of direct repair depicted in A schematic view, B AP plain X-ray, and C lateral plain X-ray. (Adapted with permission from Elsevier: Spondylolysis repair, in Vaccaro A, Baron E [eds]: Spine Surgery. Third edition. Philadelphia, PA: Elsevier, 2018. pp 322-328.)
Challenges associated with the direct screw technique may include difficult screw placement due to narrow area of the lamina as well as decreased area available for bone graft placement due to the screw [130, 131]. Laminae may also fracture upon screw placement [132]. Conversely, challenges with the pedicle screw-hook method may include difficult hook placement on dysplastic or abnormal lamina, as well as applying enough compression to approximate the defect [124]. Keys to success with the pedicle screw-hook method include use of polyaxial pedicle screws and selection of a sublaminar hook that fits well on the lamina [95]. Overall, the pedicle screw-based direct repair is preferred due to higher fusion rates and lower complication rates, followed by the Buck repair. Postoperatively, the patient should be placed in a rigid lumbosacral brace for 3 months and perform daily isometric core-strengthening exercises. A gradually escalating physiotherapy program can be begun at 1 month postoperatively. Athletes are typically permitted to return to play within 1 year of surgery, but should not return until pain free [133].
Conclusion
Spondylolysis and spondylolisthesis are extremely common causes of lower back pain in the pediatric and adolescent population. It is particularly important for surgeons to have a high degree of suspicion in young athletes presenting with low back pain, especially those involved in gymnastics, wrestling, and weightlifting. While etiology remains unclear, pars defects likely occur due to repetitive microtrauma in a genetically susceptible individual. Multi-sport involvement may help interrupt this cycle of microtrauma and should be encouraged in young athletes. Appropriate diagnostic imaging is crucial due to the poor sensitivity of isolated physical exams: MRI is a highly sensitive tool for diagnosis of spondylolysis that avoids radiation exposure and provides information about the ability of a lesion to heal naturally. Treatment is almost always conservative and consists of a combination of physiotherapy, activity restriction, and bracing, with the key component being cessation of activities causing pain. Surgical intervention is rarely required, but in patients with refractory pain interfering with daily activities or sports, direct repair of the pars offers effective pain relief and excellent clinical outcomes. Various methods may be utilized for direct pars repair, but pedicle screw-based systems and percutaneous placement of a direct pars screw appear to perform best. Continued development of minimally invasive approaches will likely help reduce postoperative pain and hospitalization time after surgery for spondylolysis.
Author Contributions
AL—idea generation, manuscript research, writing and preparation. Dr. WH—idea generation, manuscript editing.
Funding
Not applicable
Data Availability
Not applicable
Code Availability
Not applicable
Declarations
Competing Interests
Alexander Linton—No conflicts. Dr. Wellington Hsu—Advisory board member of Stryker, Medtronic, Asahi, Bioventus.
Footnotes
This article is part of the Topical Collection on Updates in Spine Surgery - Techniques, Biologics, and Non-Operative Management
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. Instr Course Lect. 2008;57:431–445. [PubMed] [Google Scholar]
- 2.Standaert CJ, Herring SA, Halpern B, King O. Spondylolysis. Phys Med Rehabil Clin N Am. 2000;4(11):785–803. doi: 10.1016/S1047-9651(18)30102-5. [DOI] [PubMed] [Google Scholar]
- 3.Miller R, Beck NA, Sampson NR, Zhu X, Flynn JM, Drummond D. Imaging modalities for low back pain in children: a review of spondyloysis and undiagnosed mechanical back pain. J Pediatr Orthop. 2013;3(33):282–288. doi: 10.1097/BPO.0b013e318287fffb. [DOI] [PubMed] [Google Scholar]
- 4.Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984;5(66):699–707. doi: 10.2106/00004623-198466050-00008. [DOI] [PubMed] [Google Scholar]
- 5.Lemoine T, Fournier J, Odent T, Sembély-Taveau C, Merenda P, Sirinelli D, Morel B. The prevalence of lumbar spondylolysis in young children: a retrospective analysis using CT. Eur Spine J. 2018;27(5):1067–1072. doi: 10.1007/s00586-017-5339-5. [DOI] [PubMed] [Google Scholar]
- 6.Rossi F. Spondylolysis, spondylolisthesis and sports. J Sports Med Phys Fitness. 1978;4(18):317–340. [PubMed] [Google Scholar]
- 7.Wynne-Davies R, Scott JH. Inheritance and spondylolisthesis: a radiographic family survey. J Bone Joint Surg Br. 1979;3(61-b):301–305. doi: 10.1302/0301-620x.61b3.383720. [DOI] [PubMed] [Google Scholar]
- 8.Micheli LJ, Wood R. Back pain in young athletes. Significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med. 1995;149(1):15–18. doi: 10.1001/archpedi.1995.02170130017004. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg NJ, Bargar WL, Friedman B. The incidence of spondylolysis and spondylolisthesis in nonambulatory patients. Spine (Phila Pa 1976) 1981;1(6):35–38. doi: 10.1097/00007632-198101000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Jackson DW, Wiltse LL, Cirincoine RJ. Spondylolysis in the female gymnast. Clin Orthop Relat Res. 1976;117:68–73. [PubMed] [Google Scholar]
- 11.Aggrawal ND, Kaur R, Kumar S, Mathur DN. A study of changes in the spine in weight lifters and other athletes. Br J Sports Med. 1979;2(13):58–61. doi: 10.1136/bjsm.13.2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciullo JV, Jackson DW. Pars interarticularis stress reaction, spondylolysis, and spondylolisthesis in gymnasts. Clin Sports Med. 1985;1(4):95–110. doi: 10.1016/S0278-5919(20)31264-3. [DOI] [PubMed] [Google Scholar]
- 13.Terai T, Sairyo K, Goel VK, Ebraheim N, Biyani A, Faizan A, Sakai T, Yasui N. Spondylolysis originates in the ventral aspect of the pars interarticularis: a clinical and biomechanical study. J Bone Joint Surg Br. 2010;8(92):1123–1127. doi: 10.1302/0301-620x.92b8.22883. [DOI] [PubMed] [Google Scholar]
- 14.Mansfield JT, Wroten M. Pars Interarticularis Defect. Treasure Island (FL): StatPearls; 2021. https://www.ncbi.nlm.nih.gov/pubmed/30855876. Accessed 20 Jun 2021. [PubMed]
- 15.Cyron BM, Hutton WC. The fatigue strength of the lumbar neural arch in spondylolysis. J Bone Joint Surg Br. 1978;2(60-b):234–238. doi: 10.1302/0301-620x.60b2.659472. [DOI] [PubMed] [Google Scholar]
- 16.Sterba M, Arnoux PJ, Labelle H, Warner WC, Aubin C. Biomechanical analysis of spino-pelvic postural configurations in spondylolysis subjected to various sport-related dynamic loading conditions. Eur Spine J. 2018;8(27):2044–2052. doi: 10.1007/s00586-018-5667-0. [DOI] [PubMed] [Google Scholar]
- 17.Sunami T, Kotani T, Aoki Y, Sakuma T, Nakayama K, Iijima Y, Akazawa T, Minami S, Ohtori S, Yamazaki M. Large lumbar lordosis is a risk factor for lumbar spondylolysis in patients with adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2021; 10.1097/brs.0000000000004192. [DOI] [PubMed]
- 18.Labelle H, Roussouly P, Berthonnaud E, Transfeldt E, O'Brien M, Chopin D, Hresko T, Dimnet J. Spondylolisthesis, pelvic incidence, and spinopelvic balance: a correlation study. Spine (Phila Pa 1976) 2004;18(29):2049–2054. doi: 10.1097/01.brs.0000138279.53439.cc. [DOI] [PubMed] [Google Scholar]
- 19.Yin J, Peng BG, Li YC, Zhang NY, Yang L, Li DM. Differences of sagittal lumbosacral parameters between patients with lumbar spondylolysis and normal adults. Chin Med J (Engl) 2016;10(129):1166–1170. doi: 10.4103/0366-6999.181972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logroscino G, Mazza O, Aulisa G, Pitta L, Pola E, Aulisa L. Spondylolysis and spondylolisthesis in the pediatric and adolescent population. Childs Nerv Syst. 2001;11(17):644–655. doi: 10.1007/s003810100495. [DOI] [PubMed] [Google Scholar]
- 21.Haukipuro K, Keränen N, Koivisto E, Lindholm R, Norio R, Punto L. Familial occurrence of lumbar spondylolysis and spondylolisthesis. Clin Genet. 1978;6(13):471–476. doi: 10.1111/j.1399-0004.1978.tb01200.x. [DOI] [PubMed] [Google Scholar]
- 22.Yamada A, Sairyo K, Shibuya I, Kato K, Dezawa A, Sakai T. Lumbar spondylolysis in juveniles from the same family: a report of three cases and a review of the literature. Case Rep Orthop. 2013;272514(2013) 10.1155/2013/272514. [DOI] [PMC free article] [PubMed]
- 23.Kato K, Hakozaki M, Mashiko R, Konno SI. Familial development of lumbar spondylolysis: a familial case report of 7- and 4-year-old brothers and their father. J Int Med Res. 2021;5(49) 10.1177/03000605211015559. [DOI] [PMC free article] [PubMed]
- 24.Cai T, Yang L, Cai W, Guo S, Yu P, Li J, Hu X, Yan M, Shao Q, Jin Y, Sun ZS, Luo ZJ. Dysplastic spondylolysis is caused by mutations in the diastrophic dysplasia sulfate transporter gene. Proc Natl Acad Sci U S A. 2015;112(26):8064–8069. doi: 10.1073/pnas.1502454112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.•.Sakai T, Goda Y, Tezuka F, Takata Y, Higashino K, Sato M, Mase Y, Nagamachi A, Sairyo K. Characteristics of lumbar spondylolysis in elementary school age children. Eur Spine J. 2016;2(25):602–606. doi: 10.1007/s00586-015-4029-4. [DOI] [PubMed] [Google Scholar]
- 26.Seitsalo S, Osterman K, Poussa M. Scoliosis associated with lumbar spondylolisthesis. A clinical survey of 190 young patients. Spine (Phila Pa 1976) 1988;8(13):899–904. doi: 10.1097/00007632-198808000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Fisk JR, Moe JH, Winter RB. Scoliosis, spondylolysis, and spondylolisthesis. Their relationship as reviewed in 539 patients. Spine (Phila Pa 1976) 1978;3(3):234–245. doi: 10.1097/00007632-197809000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Tsukagoshi Y, Kamegaya M, Tatsumura M, Tomaru Y, Kamada H, Morita M, Saisu T, Nomura S, Ikezawa Y, Yamazaki M. Characteristics and diagnostic factors associated with fresh lumbar spondylolysis in elementary school-aged children. Eur Spine J. 2020;10(29):2465–2469. doi: 10.1007/s00586-020-06553-x. [DOI] [PubMed] [Google Scholar]
- 29.Morita T, Ikata T, Katoh S, Miyake R. Lumbar spondylolysis in children and adolescents. J Bone Joint Surg Br. 1995;4(77):620–625. doi: 10.1302/0301-620X.77B4.7615609. [DOI] [PubMed] [Google Scholar]
- 30.Koichi Sairyo TS, Takata Y, Yamashita K, Tezuka F. Hiroaki Manabe. Spondylolysis and spondylolisthesis in athletes. In: Wellington K, Hsu TJJ, editors. Spinal Conditions in the Athlete. Springer; 2020. pp. 235–247. [Google Scholar]
- 31.Sairyo K, Katoh S, Sasa T, Yasui N, Goel VK, Vadapalli S, Masuda A, Biyani A, Ebraheim N. Athletes with unilateral spondylolysis are at risk of stress fracture at the contralateral pedicle and pars interarticularis: a clinical and biomechanical study. Am J Sports Med. 2005;4(33):583–590. doi: 10.1177/0363546504269035. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama T, Ehara S. Spondylolytic spondylolisthesis: various imaging features and natural courses. Jpn J Radiol. 2015;1(33):3–12. doi: 10.1007/s11604-014-0371-4. [DOI] [PubMed] [Google Scholar]
- 33.•.Aoki Y, Takahashi H, Nakajima A, Kubota G, Watanabe A, Nakajima T, Eguchi Y, Orita S, Fukuchi H, Yanagawa N, Nakagawa K, Ohtori S. Prevalence of lumbar spondylolysis and spondylolisthesis in patients with degenerative spinal disease. Sci Rep. 2020;1(10):6739. doi: 10.1038/s41598-020-63784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beutler WJ, Fredrickson BE, Murtland A, Sweeney CA, Grant WD, Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine (Phila Pa 1976). 2003;10(28):1027–35. 10.1097/01.Brs.0000061992.98108.A0. [DOI] [PubMed]
- 35.Toueg CW, Mac-Thiong JM, Grimard G, Parent S, Poitras B, Labelle H. Prevalence of spondylolisthesis in a population of gymnasts. Stud Health Technol Inform. 2010;(158):132–7. [PubMed]
- 36.Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine (Phila Pa 1976) 2009;2(34):199–205. doi: 10.1097/BRS.0b013e31818edcfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Floman Y. Progression of lumbosacral isthmic spondylolisthesis in adults. Spine (Phila Pa 1976) 2000;3(25):342–347. doi: 10.1097/00007632-200002010-00014. [DOI] [PubMed] [Google Scholar]
- 38.Wáng YXJ, Deng M, Griffith JF, Kwok AWL, Leung JC, Ahuja AT, Kwok T, Leung PC. Lumbar spondylolisthesis progression and de novo spondylolisthesis in elderly Chinese men and women: a year-4 follow-up study. Spine (Phila Pa 1976) 2016;13(41):1096–1103. doi: 10.1097/brs.0000000000001507. [DOI] [PubMed] [Google Scholar]
- 39.Sakai T, Sairyo K, Takao S, Nishitani H, Yasui N. Incidence of lumbar spondylolysis in the general population in Japan based on multidetector computed tomography scans from two thousand subjects. Spine (Phila Pa 1976) 2009;34(21):2346–2350. doi: 10.1097/BRS.0b013e3181b4abbe. [DOI] [PubMed] [Google Scholar]
- 40.Merbs CF. Incomplete spondylolysis and healing. A study of ancient Canadian Eskimo skeletons. Spine (Phila Pa 1976) 1995;21(20):2328–2334. doi: 10.1097/00007632-199511000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Rowe GG, Roche MB. The etiology of separate neural arch. J Bone Joint Surg Am. 1953;1(35-a):102–110. doi: 10.2106/00004623-195335010-00010. [DOI] [PubMed] [Google Scholar]
- 42.McCarroll JR, Miller JM, Ritter MA. Lumbar spondylolysis and spondylolisthesis in college football players. A prospective study. Am J Sports Med. 1986;5(14):404–406. doi: 10.1177/036354658601400513. [DOI] [PubMed] [Google Scholar]
- 43.Jackson DW. Low back pain in young athletes: evaluation of stress reaction and discogenic problems. Am J Sports Med. 1979;6(7):364–366. doi: 10.1177/036354657900700614. [DOI] [PubMed] [Google Scholar]
- 44.Stanitski C. Spondylolysis and spondylolisthesis in athletes. Oper Tech Sports Med. 2006;14:141–146. doi: 10.1053/j.otsm.2006.04.008. [DOI] [Google Scholar]
- 45.Maurer SG, Wright KE, Bendo JA. Iatrogenic spondylolysis leading to contralateral pedicular stress fracture and unstable spondylolisthesis: a case report. Spine (Phila Pa 1976) 2000;25(7):895–898. doi: 10.1097/00007632-200004010-00022. [DOI] [PubMed] [Google Scholar]
- 46.König MA, Ebrahimi FV, Nitulescu A, Behrbalk E, Boszczyk BM. Early results of stand-alone anterior lumbar interbody fusion in iatrogenic spondylolisthesis patients. Eur Spine J. 2013;12(22):2876–2883. doi: 10.1007/s00586-013-2970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki K, Ishida Y, Ohmori K. Spondylolysis after posterior decompression of the lumbar spine. 35 patients followed for 3-9 years. Acta Orthop Scand. 1993;1(64):17–21. doi: 10.3109/17453679308994519. [DOI] [PubMed] [Google Scholar]
- 48.Randall RM, Silverstein M, Goodwin R. Review of pediatric spondylolysis and spondylolisthesis. Sports Med Arthrosc Rev. 2016;24(4):184–187. doi: 10.1097/jsa.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 49.Sairyo K, Katoh S, Takata Y, Terai T, Yasui N, Goel VK, Masuda A, Vadapalli S, Biyani A, Ebraheim N. MRI signal changes of the pedicle as an indicator for early diagnosis of spondylolysis in children and adolescents: a clinical and biomechanical study. Spine (Phila Pa 1976) 2006;2(31):206–211. doi: 10.1097/01.brs.0000195161.60549.67. [DOI] [PubMed] [Google Scholar]
- 50.Tezuka F, Sairyo K, Sakai T, Dezawa A. Etiology of adult-onset stress fracture in the lumbar spine. Clin Spine Surg. 2017;3(30):E233–e238. doi: 10.1097/bsd.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 51.Sutton JH, Guin PD, Theiss SM. Acute lumbar spondylolysis in intercollegiate athletes. J Spinal Disord Tech. 2012;8(25):422–425. doi: 10.1097/BSD.0b013e318236ba6c. [DOI] [PubMed] [Google Scholar]
- 52.Plantz MA, Selverian S, Jenkins TJ, Watkins III RG, Watkins IV RG, Hecht AC, Hsu WK. Evidence-Based Management of Spinal Conditons in the Elite Athlete. In: Khanuja HS, Strauss EJ, editors. AAOS Instructonal Course Lectures, Volume 70. American Academy of Orthopaedic Surgeons; 2021. Chapter 20. [PubMed]
- 53.Hirano A, Takebayashi T, Yoshimoto M, Ida K, Yamashita T, Nakano K Characteristics of clinical and imaging findings in adolescent lumbar spondylolysis associated with sports activities. J Spine. 2012;(1):124. 10.4172/2165-7939.1000124.
- 54.Masci L, Pike J, Malara F, Phillips B, Bennell K, Brukner P. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;11(40):940–946. doi: 10.1136/bjsm.2006.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alqarni AM, Schneiders AG, Cook CE, Hendrick PA. Clinical tests to diagnose lumbar spondylolysis and spondylolisthesis: a systematic review. Phys Ther Sport. 2015;3(16):268–275. doi: 10.1016/j.ptsp.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 56.Collaer JW, McKeough DM, Boissonnault WG. Lumbar isthmic spondylolisthesis detection with palpation: interrater reliability and concurrent criterion-related validity. J Man Manip Ther. 2006;1(14):22–29. doi: 10.1179/106698106790820917. [DOI] [Google Scholar]
- 57.Beck NA, Miller R, Baldwin K, Zhu X, Spiegel D, Drummond D, Sankar WN, Flynn JM. Do oblique views add value in the diagnosis of spondylolysis in adolescents? J Bone Joint Surg Am. 2013;10(95):e65. doi: 10.2106/jbjs.L.00824. [DOI] [PubMed] [Google Scholar]
- 58.Lim MR, Yoon SC, Green DW. Symptomatic spondylolysis: diagnosis and treatment. Curr Opin Pediatr. 2004;16(1):37–46. doi: 10.1097/00008480-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 59.McCleary MD, Congeni JA. Current concepts in the diagnosis and treatment of spondylolysis in young athletes. Curr Sports Med Rep. 2007;6(1):62–66. doi: 10.1007/s11932-007-0014-y. [DOI] [PubMed] [Google Scholar]
- 60.Gould HP, Winkelman RD, Tanenbaum JE, Hu E, Haines CM, Hsu WK, Kalfas IH, Savage JW, Schickendantz MS, Mroz TE. Epidemiology, treatment, and performance-based outcomes in american professional baseball players with symptomatic spondylolysis and isthmic spondylolisthesis. Am J Sports Med. 2020;11(48):2765–2773. doi: 10.1177/0363546520945727. [DOI] [PubMed] [Google Scholar]
- 61.Rothman SL, Glenn WV., Jr CT multiplanar reconstruction in 253 cases of lumbar spondylolysis. AJNR Am J Neuroradiol. 1984;1(5):81–90. [PMC free article] [PubMed] [Google Scholar]
- 62.Kobayashi A, Kobayashi T, Kato K, Higuchi H, Takagishi K. Diagnosis of radiographically occult lumbar spondylolysis in young athletes by magnetic resonance imaging. Am J Sports Med. 2013;1(41):169–176. doi: 10.1177/0363546512464946. [DOI] [PubMed] [Google Scholar]
- 63.••.Campbell RS, Grainger AJ, Hide IG, Papastefanou S, Greenough CG. Juvenile spondylolysis: a comparative analysis of CT, SPECT and MRI. Skeletal Radiol. 2005;2(34):63–73. doi: 10.1007/s00256-004-0878-3. [DOI] [PubMed] [Google Scholar]
- 64.•.Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal Radiol. 2011;6(40):683–700. doi: 10.1007/s00256-010-0942-0. [DOI] [PubMed] [Google Scholar]
- 65.West AM, d'Hemecourt PA, Bono OJ, Micheli LJ, Sugimoto D. Diagnostic accuracy of magnetic resonance imaging and computed tomography scan in young athletes with spondylolysis. Clin Pediatr (Phila) 2019;6(58):671–676. doi: 10.1177/0009922819832643. [DOI] [PubMed] [Google Scholar]
- 66.Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;2(176):289–296. doi: 10.2214/ajr.176.2.1760289. [DOI] [PubMed] [Google Scholar]
- 67.Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, Giles GG, Wallace AB, Anderson PR, Guiver TA, McGale P, Cain TM, Dowty JG, Bickerstaffe AC, Darby SC. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;f2360(346) 10.1136/bmj.f2360. [DOI] [PMC free article] [PubMed]
- 68.Fadell MF, Gralla J, Bercha I, Stewart JR, Harned RK, Ingram JD, Miller AL, Strain JD, Weinman JP. CT outperforms radiographs at a comparable radiation dose in the assessment for spondylolysis. Pediatr Radiol. 2015;45(7):1026–1030. doi: 10.1007/s00247-015-3278-z. [DOI] [PubMed] [Google Scholar]
- 69.Saifuddin A, Burnett SJ. The value of lumbar spine MRI in the assessment of the pars interarticularis. Clin Radiol. 1997;9(52):666–671. doi: 10.1016/s0009-9260(97)80029-3. [DOI] [PubMed] [Google Scholar]
- 70.Yamaguchi KT, Jr, Skaggs DL, Acevedo DC, Myung KS, Choi P, Andras L. Spondylolysis is frequently missed by MRI in adolescents with back pain. J Child Orthop. 2012;3(6):237–240. doi: 10.1007/s11832-012-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.•.Rush JK, Astur N, Scott S, Kelly DM, Sawyer JR, Warner WC., Jr Use of magnetic resonance imaging in the evaluation of spondylolysis. J Pediatr Orthop. 2015;3(35):271–275. doi: 10.1097/bpo.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 72.Hsu WK, Jenkins TJ. Management of lumbar conditions in the elite athlete. JAAOS - J Am Acad of Orthopaedic Surgeons. 2017;7(25):489–498. doi: 10.5435/jaaos-d-16-00135. [DOI] [PubMed] [Google Scholar]
- 73.Dunn AJ, Campbell RS, Mayor PE, Rees D. Radiological findings and healing patterns of incomplete stress fractures of the pars interarticularis. Skeletal Radiol. 2008;5(37):443–450. doi: 10.1007/s00256-008-0449-0. [DOI] [PubMed] [Google Scholar]
- 74.Andrew C, Hecht ARV, Hsu W, Watkins RG, Dossett A. Spine and sports: a roundtable discussion. In: Hecht AC, editor. Spine Injuries in Athletes. LWW; 2017. pp. 278–284. [Google Scholar]
- 75.Schmitt F, Grosu D, Mohr C, Purdy D, Salem K, Scott KT, Stoeckel B. 3 Tesla MRI: successful results with higher field strengths. Radiologe. 2004;1(44):31–47. doi: 10.1007/s00117-003-1000-x. [DOI] [PubMed] [Google Scholar]
- 76.Ulmer JL, Mathews VP, Elster AD, Mark LP, Daniels DL, Mueller W. MR imaging of lumbar spondylolysis: the importance of ancillary observations. AJR Am J Roentgenol. 1997;1(169):233–239. doi: 10.2214/ajr.169.1.9207531. [DOI] [PubMed] [Google Scholar]
- 77.Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;2(29):146–156. doi: 10.1097/BPO.0b013e3181977fc5. [DOI] [PubMed] [Google Scholar]
- 78.Hollenberg GM, Beattie PF, Meyers SP, Weinberg EP, Adams MJ. Stress reactions of the lumbar pars interarticularis: the development of a new MRI classification system. Spine (Phila Pa 1976) 2002;2(27):181–186. doi: 10.1097/00007632-200201150-00012. [DOI] [PubMed] [Google Scholar]
- 79.Sakai T, Sairyo K, Mima S, Yasui N. Significance of magnetic resonance imaging signal change in the pedicle in the management of pediatric lumbar spondylolysis. Spine (Phila Pa 1976) 2010;14(35):E641–E645. doi: 10.1097/BRS.0b013e3181c9f2a2. [DOI] [PubMed] [Google Scholar]
- 80.Sairyo K, Sakai T, Yasui N, Dezawa A. Conservative treatment for pediatric lumbar spondylolysis to achieve bone healing using a hard brace: what type and how long?: Clinical article. J Neurosurg Spine. 2012;6(16):610–614. doi: 10.3171/2012.2.Spine10914. [DOI] [PubMed] [Google Scholar]
- 81.Yandrapalli S, Puckett Y. SPECT Imaging. In: editors. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- 82.Collier BD, Johnson RP, Carrera GF, Meyer GA, Schwab JP, Flatley TJ, Isitman AT, Hellman RS, Zielonka JS, Knobel J. Painful spondylolysis or spondylolisthesis studied by radiography and single-photon emission computed tomography. Radiology. 1985;1(154):207–211. doi: 10.1148/radiology.154.1.3155479. [DOI] [PubMed] [Google Scholar]
- 83.Papanicolaou N, Wilkinson RH, Emans JB, Treves S, Micheli LJ. Bone scintigraphy and radiography in young athletes with low back pain. AJR Am J Roentgenol. 1985;5(145):1039–1044. doi: 10.2214/ajr.145.5.1039. [DOI] [PubMed] [Google Scholar]
- 84.Pennell RG, Maurer AH, Bonakdarpour A. Stress injuries of the pars interarticularis: radiologic classification and indications for scintigraphy. AJR Am J Roentgenol. 1985;4(145):763–766. doi: 10.2214/ajr.145.4.763#. [DOI] [PubMed] [Google Scholar]
- 85.Tatsumura M, Gamada H, Okuwaki S, Eto F, Nagashima K, Ogawa T, Mammoto T, Hirano A, Koda M, Yamazaki M. Factors associated with failure of bony union after conservative treatment of acute cases of unilateral lumbar spondylolysis. BMC Musculoskelet Disord. 2021;1(22):75. doi: 10.1186/s12891-020-03940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arima H, Suzuki Y, Togawa D, Mihara Y, Murata H, Matsuyama Y. Low-intensity pulsed ultrasound is effective for progressive-stage lumbar spondylolysis with MRI high-signal change. Eur Spine J. 2017;26(12):3122–3128. doi: 10.1007/s00586-017-5081-z. [DOI] [PubMed] [Google Scholar]
- 87.•.Lee GW, Lee SM, Ahn MW, Kim HJ, Yeom JS. Comparison of surgical treatment with direct repair versus conservative treatment in young patients with spondylolysis: a prospective, comparative, clinical trial. Spine J. 2015;15(7):1545–1553. doi: 10.1016/j.spinee.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 88.Bouras T, Korovessis P. Management of spondylolysis and low-grade spondylolisthesis in fine athletes. A comprehensive review. Eur J Orthop Surg Traumatol. 2015;25(Suppl 1):S167–S175. doi: 10.1007/s00590-014-1560-7. [DOI] [PubMed] [Google Scholar]
- 89.Panteliadis P, Nagra NS, Edwards KL, Behrbalk E, Boszczyk B. Athletic population with spondylolysis: review of outcomes following surgical repair or conservative management. Global Spine J. 2016;6(6):615–625. doi: 10.1055/s-0036-1586743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sys J, Michielsen J, Bracke P, Martens M, Verstreken J. Nonoperative treatment of active spondylolysis in elite athletes with normal X-ray findings: literature review and results of conservative treatment. Eur Spine J. 2001;10(6):498–504. doi: 10.1007/s005860100326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iwamoto J, Takeda T, Wakano K. Returning athletes with severe low back pain and spondylolysis to original sporting activities with conservative treatment. Scand J Med Sci Sports. 2004;14(6):346–351. doi: 10.1111/j.1600-0838.2004.00379.x. [DOI] [PubMed] [Google Scholar]
- 92.•.Sakai T, Tezuka F, Yamashita K, Takata Y, Higashino K, Nagamachi A, Sairyo K. Conservative treatment for bony healing in pediatric lumbar spondylolysis. Spine (Phila Pa 1976). 2017;42(12):E716–e720. doi: 10.1097/brs.0000000000001931. [DOI] [PubMed] [Google Scholar]
- 93.Boyd ED, Mundluru SN, Feldman DS. Outcome of conservative management in the treatment of symptomatic spondylolysis and grade I spondylolisthesis. Bull Hosp Jt Dis (2013). 2019;77(3):172–182. [PubMed] [Google Scholar]
- 94.Berger AA, Hasoon J, Urits I, Viswanath O, Lee A. Alleviation of chronic low back pain due to bilateral traumatic L4 pars interarticularis fractures relieved with steroid injections. Cureus. 2020;12(8):e9821. doi: 10.7759/cureus.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wellington K, Hsu JKW. Pars Repair. In: Wang JC, editor. Advanced reconstruction. Spine. Rosemont, III: American Academy of Orthopaedic Surgeons; 2011. pp. 543–548. [Google Scholar]
- 96.Ohtori S, Orita S, Yamauchi K, Eguchi Y, Ochiai N, Kuniyoshi K, Aoki Y, Nakamura J, Miyagi M, Suzuki M, Kubota G, Inage K, Sainoh T, Sato J, Shiga Y, Abe K, Fujimoto K, Kanamoto H, Inoue G, Takahashi K. More than 6 months of teriparatide treatment was more effective for bone union than shorter treatment following lumbar posterolateral fusion surgery. Asian Spine J. 2015;9(4):573–580. doi: 10.4184/asj.2015.9.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang TW, Chuang PY, Lin SJ, Lee CY, Huang KC, Shih HN, Lee MS, Hsu RW, Shen WJ. Teriparatide improves fracture healing and early functional recovery in treatment of osteoporotic intertrochanteric fractures. Medicine (Baltimore). 2016;95(19):e3626. doi: 10.1097/md.0000000000003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsukada M, Takiuchi T, Watanabe K. Low-intensity pulsed ultrasound for early-stage lumbar spondylolysis in young athletes. Clin J Sport Med. 2019;29(4):262–266. doi: 10.1097/jsm.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 99.Tawfik S, Phan K, Mobbs RJ, Rao PJ. The incidence of pars interarticularis defects in athletes. Global Spine J. 2020;10(1):89–101. doi: 10.1177/2192568218823695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Scheepers MS, Streak Gomersall J, Munn Z. The effectiveness of surgical versus conservative treatment for symptomatic unilateral spondylolysis of the lumbar spine in athletes: a systematic review. JBI Database System Rev Implement Rep. 2015;13(3):137–173. doi: 10.11124/jbisrir-2015-1926. [DOI] [PubMed] [Google Scholar]
- 101.Debnath UK, Scammell BE, Freeman BJC, McConnell JR. Predictive factors for the outcome of surgical treatment of lumbar spondylolysis in young sporting individuals. Global Spine J. 2018;8(2):121–128. doi: 10.1177/2192568217713008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Buck J. Direct repair of the defect in spondylolisthesis. Preliminary report. J Bone Joint Surg Br. 1970;52(3):432–437. doi: 10.1302/0301-620X.52B3.432. [DOI] [PubMed] [Google Scholar]
- 103.Hsu WK, Wang JC. The use of bone morphogenetic protein in spine fusion. Spine J. 2008;8(3):419–425. doi: 10.1016/j.spinee.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 104.Lauber S, Schulte TL, Liljenqvist U, Halm H, Hackenberg L. Clinical and radiologic 2-4-year results of transforaminal lumbar interbody fusion in degenerative and isthmic spondylolisthesis grades 1 and 2. Spine (Phila Pa 1976). 2006;31(15):1693–1698. doi: 10.1097/01.brs.0000224530.08481.4e. [DOI] [PubMed] [Google Scholar]
- 105.Radcliff KE, Kalantar SB, Reitman CA. Surgical management of spondylolysis and spondylolisthesis in athletes: indications and return to play. Curr Sports Med Rep. 2009;8(1):35–40. doi: 10.1249/JSR.0b013e318194f89e. [DOI] [PubMed] [Google Scholar]
- 106.de Kunder SL, van Kuijk SMJ, Rijkers K, Caelers I, van Hemert WLW, de Bie RA, van Santbrink H. Transforaminal lumbar interbody fusion (TLIF) versus posterior lumbar interbody fusion (PLIF) in lumbar spondylolisthesis: a systematic review and meta-analysis. Spine J. 2017;17(11):1712–1721. doi: 10.1016/j.spinee.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 107.Sebastian AS, Dalton D, Slaven SE, Welch-Phillips A, Fredericks DR, Jr, Ahern DP, Butler JS. What is the optimal surgical treatment for low-grade isthmic spondylolisthesis? ALIF or TLIF? Clin Spine Surg. 2020;33(10):389–392. doi: 10.1097/bsd.0000000000000926. [DOI] [PubMed] [Google Scholar]
- 108.Derman PB, Albert TJ. Interbody fusion techniques in the surgical management of degenerative lumbar spondylolisthesis. Curr Rev Musculoskelet Med. 2017;10(4):530–538. doi: 10.1007/s12178-017-9443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Noorian S, Sorensen K, Cho W. A systematic review of clinical outcomes in surgical treatment of adult isthmic spondylolisthesis. Spine J. 2018;18(8):1441–1454. doi: 10.1016/j.spinee.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 110.Lightsey HM, Pisano AJ, Striano BM, Crawford AM, Xiong GX, Hershman S, Schoenfeld AJ, Simpson AK. ALIF versus TLIF for L5-S1 isthmic spondylolisthesis: ALIF demonstrates superior segmental and regional radiographic outcomes and clinical improvements across more patient-reported outcome measures domains. Spine (Phila Pa 1976). 2022; 10.1097/brs.0000000000004333. [DOI] [PubMed]
- 111.Ghiselli G, Wang JC, Bhatia NN, Hsu WK, Dawson EG. Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am. 2004;86(7):1497–1503. doi: 10.2106/00004623-200407000-00020. [DOI] [PubMed] [Google Scholar]
- 112.Tarpada SP, Kim D, Levine NL, Morris MT, Cho W. Comparing surgical treatments for spondylolysis: review on current research. Clin Spine Surg. 2021;34(8):276–285. doi: 10.1097/bsd.0000000000001115. [DOI] [PubMed] [Google Scholar]
- 113.Ulibarri JA, Anderson PA, Escarcega T, Mann D, Noonan KJ. Biomechanical and clinical evaluation of a novel technique for surgical repair of spondylolysis in adolescents. Spine (Phila Pa 1976). 2006;31(18):2067–2072. doi: 10.1097/01.brs.0000231777.24270.2b. [DOI] [PubMed] [Google Scholar]
- 114.Debusscher F, Troussel S. Direct repair of defects in lumbar spondylolysis with a new pedicle screw hook fixation: clinical, functional and Ct-assessed study. Eur Spine J. 2007;16(10):1650–1658. doi: 10.1007/s00586-007-0392-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Suh PB, Esses SI, Kostuik JP. Repair of pars interarticularis defect. The prognostic value of pars infiltration. Spine (Phila Pa 1976). 1991;16(8 Suppl):S445–S448. [PubMed] [Google Scholar]
- 116.Ivanic GM, Pink TP, Achatz W, Ward JC, Homann NC, May M. Direct stabilization of lumbar spondylolysis with a hook screw: mean 11-year follow-up period for 113 patients. Spine (Phila Pa 1976). 2003;28(3):255–259. doi: 10.1097/01.Brs.0000042251.62696.A5. [DOI] [PubMed] [Google Scholar]
- 117.Lee GW, Lee SM, Suh BG. Direct repair surgery with screw fixation for young patients with lumbar spondylolysis: patient-reported outcomes and fusion rate in a prospective interventional study. Spine (Phila Pa 1976). 2015;40(4):E234–E241. doi: 10.1097/brs.0000000000000714. [DOI] [PubMed] [Google Scholar]
- 118.••.Kumar N, Madhu S, Pandita N, Ramos MRD, BWL T, Lopez KG, Alathur Ramakrishnan S, Jonathan P, Nolan CP, Shree Kumar D. Is there a place for surgical repair in adults with spondylolysis or grade-I spondylolisthesis-a systematic review and treatment algorithm. Spine J. 2021;21(8):1268–1285. doi: 10.1016/j.spinee.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 119.Mobbs RJ, Choy WJ, Singh T, Cassar L, Davidoff C, Harris L, Phan K, Fiechter M. Three-dimensional planning and patient-specific drill guides for repair of spondylolysis/L5 pars defect. World Neurosurg. 2019;132:75–80. doi: 10.1016/j.wneu.2019.08.112. [DOI] [PubMed] [Google Scholar]
- 120.Rajasekaran S, Subbiah M, Shetty AP. Direct repair of lumbar spondylolysis by Buck’s technique. Indian J Orthop. 2011;45(2):136–140. doi: 10.4103/0019-5413.77133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.•.Karatas AF, Dede O, Atanda AA, Holmes L, Jr, Rogers K, Gabos P, Shah SA. Comparison of direct pars repair techniques of spondylolysis in pediatric and adolescent patients: pars compression screw versus pedicle screw-rod-hook. Clin Spine Surg. 2016;29(7):272–280. doi: 10.1097/BSD.0b013e318277cb7d. [DOI] [PubMed] [Google Scholar]
- 122.Askar Z, Wardlaw D, Koti M. Scott wiring for direct repair of lumbar spondylolysis. Spine (Phila Pa 1976). 2003;28(4):354–357. doi: 10.1097/01.Brs.0000048496.55167.22. [DOI] [PubMed] [Google Scholar]
- 123.Morscher E, Gerber B, Fasel J. Surgical treatment of spondylolisthesis by bone grafting and direct stabilization of spondylolysis by means of a hook screw. Arch Orthop Trauma Surg. 1984;103(3):175–178. doi: 10.1007/bf00435550. [DOI] [PubMed] [Google Scholar]
- 124.Shin MH, Ryu KS, Rathi NK, Park CK. Direct pars repair surgery using two different surgical methods : pedicle screw with universal hook system and direct pars screw fixation in symptomatic lumbar spondylosis patients. J Korean Neurosurg Soc. 2012;51(1):14–19. doi: 10.3340/jkns.2012.51.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yamamoto T, Iinuma N, Miyamoto K, Sugiyama S, Nozawa S, Hosoe H, Shimizu K. Segmental wire fixation for lumbar spondylolysis associated with spina bifida occulta. Arch Orthop Trauma Surg. 2008;128(10):1177–1182. doi: 10.1007/s00402-007-0521-6. [DOI] [PubMed] [Google Scholar]
- 126.Hefti F, Seelig W, Morscher E. Repair of lumbar spondylolysis with a hook-screw. Int Orthop. 1992;16(1):81–85. doi: 10.1007/bf00182992. [DOI] [PubMed] [Google Scholar]
- 127.Manish K, Kasliwal RGF, Vincent C. Traynelis. In: Alexander R. Vaccaro EMB, editor. Spine Surgery: Operative Techniques. Philadelphia: Elsevier; 2018. pp. 322–328. [Google Scholar]
- 128.••.Mohammed N, Patra DP, Narayan V, Savardekar AR, Dossani RH, Bollam P, Bir S, Nanda A. A comparison of the techniques of direct pars interarticularis repairs for spondylolysis and low-grade spondylolisthesis: a meta-analysis. Neurosurg Focus. 2018;44(1):E10. doi: 10.3171/2017.11.Focus17581. [DOI] [PubMed] [Google Scholar]
- 129.Fan J, Yu GR, Liu F, Zhao J, Zhao WD. A biomechanical study on the direct repair of spondylolysis by different techniques of fixation. Orthop Surg. 2010;2(1):46–51. doi: 10.1111/j.1757-7861.2009.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lu J, Ebraheim NA, Biyani A, Yang H. Screw placement in the lumbar vertebral isthmus. Clin Orthop Relat Res. 1997;338:227–230. doi: 10.1097/00003086-199705000-00030. [DOI] [PubMed] [Google Scholar]
- 131.Gagnet P, Kern K, Andrews K, Elgafy H, Ebraheim N. Spondylolysis and spondylolisthesis: a review of the literature. J Orthop. 2018;15(2):404–407. doi: 10.1016/j.jor.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sairyo K, Sakai T, Yasui N. Minimally invasive technique for direct repair of pars interarticularis defects in adults using a percutaneous pedicle screw and hook-rod system. J Neurosurg Spine. 2009;10(5):492–495. doi: 10.3171/2009.2.Spine08594. [DOI] [PubMed] [Google Scholar]
- 133.Reitman CA, Esses SI. Direct repair of spondylolytic defects in young competitive athletes. Spine J. 2002;2(2):142–144. doi: 10.1016/s1529-9430(02)00179-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable
Not applicable