Abstract
Introduction
The ability to perform psoriasis skin assessments remotely through digital image-based psoriasis area and severity index (DIB-PASI) would be a valuable tool for psoriasis clinical trials. An ideal teledermatological assessment would be robust across patients of diverse skin tones as well as across assessors of varying experience levels. In this pilot study, we evaluated the reliability of face-to-face (FTF) versus DIB-PASI scores determined by trained clinical assessors with a spectrum of experience and with patients of different skin tones.
Methods
Fourteen subjects of varying skin tones with moderate-to-severe plaque psoriasis were treated with adalimumab. In-person PASI assessments and digital photography were performed in the clinic at weeks 0, 12, and 24. Photographs were reviewed by four independent assessors to derive a digital image-based PASI score. The concordance of face-to-face PASI (FTF-PASI) and DIB-PASI were analyzed across patient and assessor factors.
Results
Overall concordance between FTF-PASI and DIB-PASI was high (ICC 0.82, p < 0.0001), with good agreement across individual assessors. When analyzed by PASI score component or body region, digital assessors also demonstrated good agreement with the FTF assessor. Similarly, DIB-PASI showed high concordance with FTF-PASI for patients with light skin tones and patients with medium-to-dark skin tones, and across clinical training levels.
Conclusion
Overall, PASI scores derived from digital images showed good agreement with those determined in person. Importantly, these remote assessments were reliable for both light and medium-to-dark skin tones, and robust to training level of the assessor. The findings from this pilot study lay the foundation for expanding teledermatology-based clinical trials for patients with psoriasis and enabling accurate, remote monitoring of disease severity and therapy response.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-022-00750-w.
Keywords: Telemedicine, Teledermatology, Digital health, Psoriasis, Clinical research, PASI
Key Summary Points
| Why carry out this study? |
| Participation in clinical trials often requires time-consuming travel to academic centers or urban areas, which may lead to lower rates of participation and enrollment of less diverse subjects. |
| Teledermatology has the potential to bridge this gap; however, remote assessments should be reliable and robust across patients with different skin tones and clinical assessors of different experience levels. |
| This study sought to determine whether psoriasis severity could be reliably assessed remotely through digital images, and whether patient skin tone or the experience level of the assessor impacted this reliability. |
| What was learned from the study? |
| Overall, all digital image-based assessors showed good agreement with a face-to-face assessor when evaluating patients with psoriasis across a range of disease severity. |
| Remote and face-to-face assessments demonstrated good concordance regardless of patient skin tone or the training level of the assessors. |
| Our pilot study lays the groundwork for further expanding telehealth-based clinical trials for patients of varied different skin tones in underserved areas. |
Introduction
Telemedicine has the potential to expand clinical research access to new patient populations through enhanced recruitment, retention, and real-time data collection. Over the past two decades, these services have demonstrated value by helping improve patient–provider visit efficiency, reducing time to treatment, and allowing patients living in underserved areas to access dermatologic care and specialty consults [1–4]. Telehealth can also be used to augment traditional clinical trial methodologies by expanding access to new patient populations and enhancing recruitment, retention, and real-time data collection [5].
Psoriasis is a chronic, inflammatory skin disease that manifests as thick, red, scaly plaques on the body [6]. Treatment is often complex and involves visual, long-term monitoring of symptoms[7, 8]. The most widely used system for measuring psoriasis severity is the Psoriasis Area and Severity Index (PASI) score, which is a graded representation of involved body area across the scalp, upper limbs, trunk, and lower limbs [9]. In digital image-based PASI (DIB-PASI), digital photographs are used to score patients remotely. Previous research on the accuracy of DIB-PASI methods has shown good concordance between DIB-PASI and face-to-face PASI (FTF-PASI) scores [8, 10, 11]. Additionally, clinical outcomes did not differ between patients with psoriasis treated using an entirely online, collaborative connected-health model involving DIB-PASI and those who were managed in person [12].
Specifically, DIB-PASI services have the potential to improve the recruitment and retention of patients with psoriasis who do not live near academic medical centers and could not otherwise enroll. DIB-PASI can facilitate access to trials for these patients, while decreasing costs incurred by repeated visits and travel time [13–15]. By eliminating the need for on-site visits, DIB-PASI decreases the patient burden and could potentially mitigate the high drop-out rate seen in traditional trials [16]. Improved retention rates help avoid delays and can significantly minimize research costs [17].
The benefits of DIB-PASI are especially relevant for clinical trials concerning psoriasis, which requires regular follow-up to track progress and evaluate treatment response. In these patient populations, tele-assessments such as DIB-PASI have been shown to substantially decrease total patient turnaround time with no difference in efficacy compared with face-to-face consultations [18, 19]. In addition, visit compliance is significantly improved by associated reductions in patient travel time [10, 20]. In psoriatic populations, teledermatology applications have also been successfully used for high-need home monitoring and full-service online care management [11, 12]. Thus, DIB-PASI represents a cost-effective, practical way to include patients in geographically remote areas or disparate living conditions without compromising medical care or research quality. As a result, improved recruitment could allow for the study of diverse and previously underrepresented patients that are more representative of the disease population.
In this pilot study, we evaluate the reliability of digital photography to assess psoriasis severity. Our work expands on previous research to provide a more complete comparison of DIB-PASI and FTF-PASI scoring by examining clinical experience as well as evaluating the potential impact of patient skin tone on score reliability.
Methods
Study Design and Participants
Patients with plaque psoriasis were recruited from the Department of Dermatology, University of California, San Francisco for the clinical trial: “A Single-Center, Open-Label Study to Assess Improvement in Psychosocial and Occupational Dimensions with Adalimumab Treatment of Moderate-to-Severe Psoriasis evaluated with the Work Productivity and Activity Impairment-Specific Health Problem (WPAI-SHP), Psychological General Well-Being (PGWB), Psoriasis Quality of Life-12 (PQOL-12), and Dermatology Life Quality Index (DLQI).” Fourteen patients with moderate-to-severe plaque psoriasis were recruited and treated with adalimumab for 52 weeks. Eligibility criteria included being of age 18 or older and having physician-diagnosed plaque psoriasis.
Face-to-face visits were performed according to the study protocol. During each visit, FTF-PASI scores were assessed by a clinical research fellow with 2 years of clinical experience in PASI scoring. Simultaneously, digital images of all PASI-scored body sites excluding the scalp, which was obstructed by hair, were taken for DIB-PASI scoring. Each patient generally had six photos taken by a clinical research coordinator: full front, upper body front, lower body front, full back, upper body back, and lower body back. Patient skin tone was categorized as “light” (n = 8) for Fitzpatrick I–II phototypes, and “medium-to-dark” (n = 6) for Fitzpatrick III or greater. Participants with photos determined to be of sufficient quality for scoring were included in the study, on the basis of lighting, sharpness, and color clarity (Fig. 1). Of the 210 total sets of images, 198 (94.3%) were determined to be high quality and retained for our analysis. Images of these subjects from weeks 0, 12, and 24 were reviewed by four independent assessors, spanning the attending physician, two clinical fellows, and medical student training levels with 6, 3, 2, and 1 years of clinical experience in PASI scoring, respectively. For each week, all assessors used the images to score each participant’s upper limbs, trunk, and lower limbs across the four PASI components to derive a DIB-PASI score. All assessors completed the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) PASI score training prior to completing assessments for the study. Because no digital images were taken for the scalp body site, FTF-PASI scores for component were used in the calculation of the total DIB-PASI score.
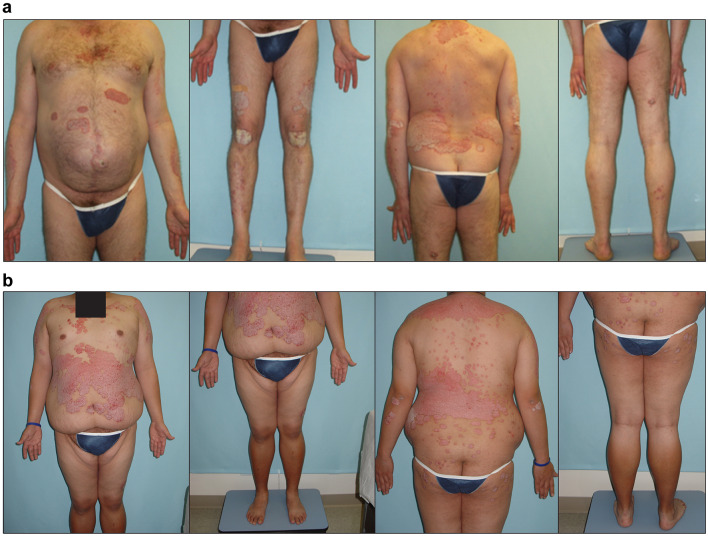
Fig. 1.
Examples of digital images for DIB-PASI scoring. a Example digital photographs taken of a patient with psoriasis, front and back. b Example digital photographs taken of another patient with psoriasis, front and back. Identifying region redacted
Compliance with Ethics Guidelines
This study received approval from the UCSF IRB (#16-21005) and was performed in accordance with the Declaration of Helsinki 1964 and its later amendments. All subjects provided informed consent to participate in the study.
Analysis Methods
Statistical programming and analyses were performed using R 4.0.3. Results were considered statistically significant at p < 0.05.
The intraclass correlation coefficient (ICC) was used to evaluate the concordance of DIB-PASI scores with their corresponding FTF-PASI scores. The ICC measures score reliability by comparing the variability of different scores assigned to the same participant with the total variation across all scores and all participants. ICC values < 0.50 were categorized as “poor agreement,” 0.50 ≤ ICC < 0.75 as “moderate agreement,” 0.75 ≤ ICC < 0.90 as “good agreement,” and ICC ≥ 0.90 as “excellent agreement” [21–23]. ICC estimates and their 95% confidence intervals were calculated using an absolute-agreement, two-way mixed effect models with error adjustment for the number of raters compared in a given analysis, α = 0.05. Missing values and corresponding rows were omitted from analysis.
Results
Participants
We recruited patients and conducted study visits. A total of 14 eligible patients with plaque psoriasis, 10 male-identified and 4 female-identified, agreed to participate in the study. Age ranged from 26 to 53 years old with an average of 37.7 years. Age, gender identification, and baseline FTF-PASI scores at week 0 of each patient are described in Table 1. Digital images of sufficient quality for PASI scoring were available for 13, 12, and 14 patients for weeks 0, 12, and 24, respectively. All clinical assessors completed PASI assessments for each patient at each timepoint.
Table 1.
Description of the gender identification, age, and baseline FTF-PASI score for 14 recruited participants
| Patient | Gender identification | Age | Baseline PASI (FTF-PASI at week 0) |
|---|---|---|---|
| 1 | Male | 34 | 16.3 |
| 2 | Male | 30 | 19.6 |
| 3 | Male | 40 | 17.9 |
| 4 | Male | 38 | 12.1 |
| 5 | Male | 40 | 15.8 |
| 6 | Male | 53 | 7.8 |
| 7 | Male | 48 | 15.8 |
| 8 | Female | 26 | 13.8 |
| 9 | Female | 31 | 22.6 |
| 10 | Female | 34 | 8.5 |
| 11 | Male | 48 | 15.6 |
| 12 | Female | 30 | 16.4 |
| 13 | Male | 33 | 12.5 |
| 14 | Male | 43 | 20.1 |
Overall Trends and Concordance in PASI Scores
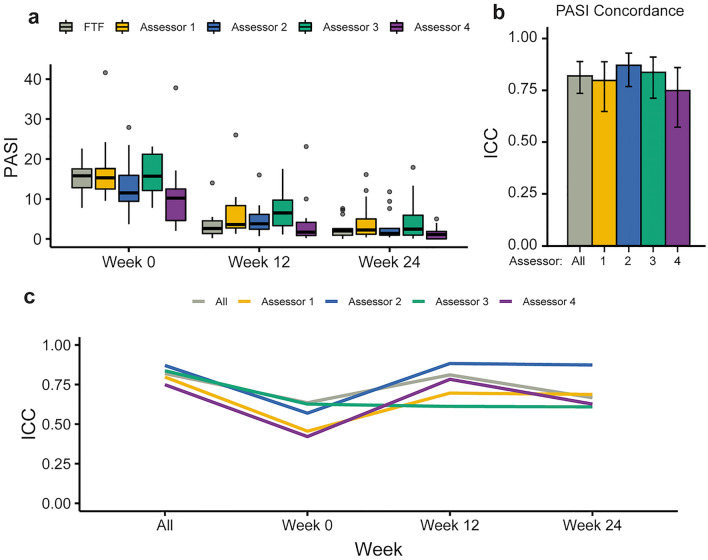
In general, the distribution of all PASI scores was similar (Fig. 2a) and median scores across DIB-PASI and FTF-PASI trended close together. The full distribution of PASI scores ranged from 0 to 41.6. The median PASI score across all patients was 13.8 at week 0, 3.5 at week 2, and 1.8 at week 24. As illustrated in Fig. 2, median PASI decreased over time, reflecting the clinical improvement that was consistent across assessors both in person and digitally.
Fig. 2.
Overall PASI score distribution and assessor concordance. a Box plots showing distribution and range of PASI scores recorded by the face-to-face (FTF, gray) and four image-based assessors (numbered 1–4, colored). b Bar plots showing overall intraclass correlation (ICC, a measure of concordance) for each of the DIB-PASI assessors with the in-person assessor (colored bars), as well as a combined group concordance (gray bar). Error bars show 95% confidence interval. c Line plot showing ICC values across all time points, with colors corresponding to each of the assessors
To analyze the concordance between FTF-PASI and DIB-PASI scores, we calculated the intraclass correlation coefficient, a measure of agreement between observers that has been used in other studies of remote monitoring of psoriasis [21–23] The concordance of all scores was high, with an ICC value of 0.82 (Fig. 2b, gray bar; p < 0.0001), indicating good agreement between FTF-PASI and DIB-PASI scores from all assessors. When analyzed at the level of individual assessors, we found that all DIB assessors also demonstrated good agreement with the FTF assessor. Concordance was lowest at week 0 (ICC 0.64) when the distribution of PASI scores was widest, which was still considered moderate agreement.
Concordance by PASI Component and Region
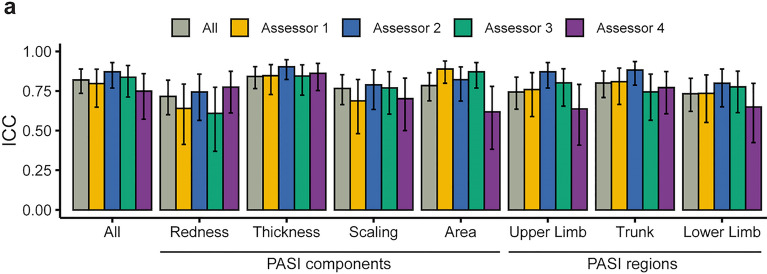
The PASI is scored on four clinical components: erythema (redness), induration (thickness), desquamation (scaling), and degree of involvement for each affected body region (area). To understand the relative contribution of each component to concordance as previously observed, we evaluated their individual ICC values between FTF-PASI and DIB-PASI.
Scores of the four components showed good agreement for induration (ICC 0.84, p < 0.0001), desquamation (ICC 0.77, p < 0.0001), and area (ICC 0.78, p < 0.0001), and moderate agreement for erythema (ICC 0.72, p < 0.0001). ICC values were further analyzed across all assessors (Fig. 3a, colored bars), which all showed moderate or good agreement.
Fig. 3.
Concordance of PASI scores by clinical component and body region. a ICC values of PASI components (redness, thickness, scaling, area) and body regions (upper limb, trunk, lower limb; scalp excluded, see Methods). Gray bars denote combined concordance of all DIB-PASI assessors compared with the FTF-PASI assessor, while colored bars denote individual assessors. Error bars denote 95% confidence intervals
The measurements of the clinical PASI components are taken at four body regions: the scalp, upper extremities, trunk, and lower extremities [20]. We similarly evaluated the ICC values of each region between FTF-PASI and DIB-PASI scores. We found consistently good to moderate agreement across each body region: trunk (ICC 0.80, p < 0.0001), upper extremities (ICC 0.74, p < 0.0001), and lower extremities (ICC 0.73, p < 0.0001). A one-way between-subjects analysis of variance (ANOVA) found that different body areas were not significantly associated with differences in mean concordance values (F = 4.066, p = 0.393).
Concordance by Patient Skin Tone and Assessor Experience
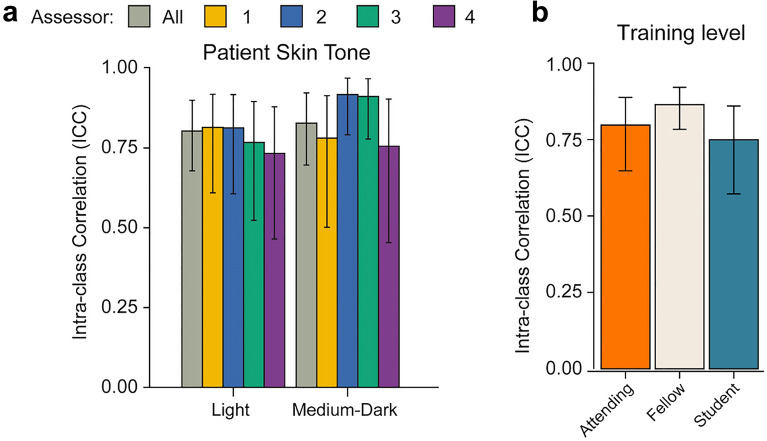
The presentation of psoriasis has been shown to vary between ethnic groups, often with important implications for the treatment and management of nonwhite individuals [24]. To determine if the concordance between FTF-PASI and DIB-PASI scores was affected by skin tone, patients were classified as having a light or medium-to-dark skin tone by an experienced clinical assessor based on Fitzpatrick phototype. Of the 14 participants, 8 had light skin tones (Fitzpatrick I–II) and 6 had medium-to-dark skin tones (Fitzpatrick III and above). Both groups showed good agreement between FTF-PASI and DIB-PASI, with ICC values of 0.80 and 0.83 respectively. A one-way between-subjects ANOVA showed no significant differences in concordance associated with either skin tone group (F = 4.572, p = 0.1) (Fig. 4).
Fig. 4.
PASI concordance by patient skin tone and assessor training level. a ICC values grouped by patient skin tone (light and medium dark). Gray bars denote combined concordance of all DIB-PASI assessors compared with the FTF-PASI assessor, while colored bars denote individual assessors. Error bars denote 95% confidence intervals. b ICC values by training level (attending, fellow, student). Error bars denote 95% confidence intervals
Finally, we analyzed ICC values for concordance across levels of clinical training. The fellow level of training showed the highest concordance with the in-person assessor (ICC 0.86), followed by the attending (ICC 0.80), and student (ICC 0.75) training levels. These ICC values indicate that the high concordance between FTF-PASI and DIB-PASI holds at the individual assessor level as well as the overall group level.
Discussion
To our knowledge, we have for the first time assessed the level of concordance between PASI scores measured during face-to-face visits and those determined using digital images across different levels of clinical experience and patient skin tones. Overall, we demonstrate a high level of concordance between a face-to-face assessment compared with assessments by digital images alone. In particular, good agreement was achieved regardless of patient skin tone and clinical training.
Erythema was the only PASI component that showed moderate agreement instead of good agreement (defined by ICC ≥ 0.75). Additionally, when evaluating each component at the individual assessor level, the least experienced assessor showed moderate instead of good agreement for area. Given the use of area as a multiplicative factor in the calculation of PASI, even a small difference in measurement can have a relatively large effect on overall scores. While there was no difference observed between groups in this study, future implementation of DIB-PASI may benefit from implementing clear protocols or automated computer algorithms in evaluating area.
DIB-PASI also showed good agreement with FTF-PASI across the range of clinical experience, The fellow level of training showed the highest concordance with the in-person assessor (ICC 0.86), followed by attending (0.80), and student (0.75) training levels. Even though the higher training levels (i.e., attending and fellow) did correspond to greater concordance, good agreement was still obtained at the student level. These results suggest that even 1 year of PASI scoring experience could lead to good agreement between digital and face-to-face assessments, and that the reliability of digital image-based scores do not have to be solely obtained by highly experienced physicians.
Disparities in dermatological diagnosis and treatment of patients with nonwhite skin are well documented [24–28]. For example, skin of color remains greatly underrepresented in teaching images, and nearly half of dermatologists in one study felt their training was inadequate for confident diagnosis in patients with skin of color [25]. As PASI scoring is widely used for tracking disease severity and serves as a key metric in clinical trials to assess treatment efficacy [29], it is vital that digital image-based PASI scoring is reliable for patients with nonwhite skin. In our study, FTF-PASI was concordant with DIB-PASI for patients with light skin tones as well as those with medium-to-dark skin tones, suggesting that 2D images can be reliably assessed for patients of different skin tones. It is important to note, however, that the accuracy of FTF-PASI scoring may still be impacted by patient’s skin tone, as PASI components—such as erythema—can present differently in skin of color. Although current datasets for artificial intelligence in dermatology lack diversity [28], the advantage of digital images is that future advances in these algorithms may enable more accurate assessment of psoriasis severity in skin of color.
Limitations
Our pilot study is limited by a sample size of 14 patients, which was relatively small. However, as this study was longitudinal with each patient analyzed by five clinical assessors across multiple PASI subcomponents, the total number of observations analyzed exceeded 1000. This pilot study thus provides a stepping stone for conducting larger studies with a larger cohort of patients as well as a greater number of clinical assessors. The overall number of quality images in our study was high (94.3%), but the lack of high-quality images of the scalp region also restricts our conclusions to PASI scoring over the upper limbs, trunk, and lower limbs. Additionally, we used standardized photographs taken in the clinic. We did not assess mobile photography taken by patients at home, which are typically of more variable quality. However, developments in machine learning and artificial intelligence algorithms can help identify low-quality images and assist patients in obtaining high-quality images [30].
Conclusions
Clinical trials in dermatology are not diverse, with substantial underrepresentation of nonwhite patients [26]. Additionally, the burden of traveling to academic centers is a major obstacle for clinical trial recruitment of patients from underrepresented or marginalized communities. Remote DIB-PASI services can provide a cost-effective, accessible alternative to face-to-face visits needed for clinical research to improve recruitment and retention in clinical trials for chronic conditions such as psoriasis.
To effectively reduce these disparities, DIB-PASI scoring should agree with in-person assessments, be applicable to different skin tones, and be robust to varying experience level of assessors. Our results are consistent with previous research showing that PASI scores can be determined with a high level of accuracy by clinical assessors using digital images [8, 11, 31]. Notably, our pilot study takes a step further to demonstrate that a high level of concordance can be achieved across different patient skin tones and the clinical experience level of the assessor.
Reliable remote assessment is a vital step toward addressing the disparities in dermatology. Our pilot study used in-person digital photography in the office as a proof-of-concept, but advances in mobile phone capabilities and artificial intelligence algorithms [30] will soon enable patients with limited in-person access to dermatologists to obtain accurate images from their own homes for clinical assessment [32]. These advances lay the groundwork for reliable and high-quality teledermatological care and clinical research for diverse and remote patient populations.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: Wilson Liao; Methodology: Wilson Liao, David Wu; Formal analysis and investigation: David Wu, Wilson Liao, Xueyan Lu, Mio Nakamura, Sahil Sekhon, Caleb Jeon, Tina Bhutani; Writing – original draft preparation: Xueyan Lu, David Wu, Wilson Liao; Writing – review and editing: David Wu, Wilson Liao; Resources: Wilson Liao; Supervision: Wilson Liao.
Disclosures
Tina Bhutani has received research funding from Abbvie, Celgene, Galderma, Janssen, Pfizer, Regeneron, and Sun; and has served as an advisor for Abbvie, Boehringer-Ingelheim, Bristol Myers Squibb, Pfizer, Leo, Lilly, and Novartis. Wilson Liao has received research grant funding from Abbvie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. David Wu, Xueyan Lu, Mio Nakamura, Sahil Sekhon and Caleb Jeon have nothing to disclose.
Compliance with Ethics Guidelines
This study received approval from the UCSF IRB (#16–21,005) and was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All subjects provided informed consent to participate in the study.
Data Availability
All data generated or analyzed during this study are included as supplementary information files.
References
- 1.Burg G. Telemedicine and teledermatology. New York: Karger; 2003. [Google Scholar]
- 2.Ford AR, Gibbons CM, Torres J, Kornmehl HA, Singh S, Young PM, et al. Access to dermatological care with an innovative online model for psoriasis management: results from a randomized controlled trial. Telemed E-health. 2019;25:619–627. doi: 10.1089/tmj.2018.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warshaw EM, Hillman YJ, Greer NL, Hagel EM, MacDonald R, Rutks IR, et al. Teledermatology for diagnosis and management of skin conditions: a systematic review. J Am Acad Dermatol. 2011;64:759–772.e21. doi: 10.1016/j.jaad.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Dekio I, Hanada E, Chinuki Y, Akaki T, Kitani M, Shiraishi Y, et al. Usefulness and economic evaluation of ADSL-based live interactive teledermatology in areas with shortage of dermatologists: ADSL-based interactive teledermatology. Int J Dermatol. 2010;49:1272–1275. doi: 10.1111/j.1365-4632.2010.04572.x. [DOI] [PubMed] [Google Scholar]
- 5.Laggis CW, Williams VL, Yang X, Kovarik CL. Research techniques made simple: teledermatology in clinical trials. J Invest Dermatol. 2019;139:1626–1633.e1. doi: 10.1016/j.jid.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Parisi R, Symmons DPM, Griffiths CEM, Ashcroft DM, team on behalf of the I and M of P and AC (IMPACT) project. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–85. [DOI] [PubMed]
- 7.Armstrong AW, Siegel MP, Bagel J, Boh EE, Buell M, Cooper KD, et al. From the medical board of the national psoriasis foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290–298. doi: 10.1016/j.jaad.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Singh P, Soyer HP, Wu J, Salmhofer W, Gilmore S. Tele-assessment of psoriasis area and severity index: a study of the accuracy of digital image capture. Australas J Dermatol. 2011;52:259–263. doi: 10.1111/j.1440-0960.2011.00800.x. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt J, Wozel G. The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatology. 2005;210:194–199. doi: 10.1159/000083509. [DOI] [PubMed] [Google Scholar]
- 10.Frühauf J, Schwantzer G, Ambros-Rudolph CM, Weger W, Ahlgrimm-Siess V, Salmhofer W, et al. Pilot study on the acceptance of mobile teledermatology for the home monitoring of high-need patients with psoriasis. Australas J Dermatol. 2012;53:41–46. doi: 10.1111/j.1440-0960.2011.00852.x. [DOI] [PubMed] [Google Scholar]
- 11.Koller S, Hofmann-Wellenhof R, Hayn D, Weger W, Kastner P, Schreier G, et al. Teledermatological monitoring of psoriasis patients on biologic therapy. Acta Derm Venereol. 2011;91:680–685. doi: 10.2340/00015555-1148. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong AW, Chambers CJ, Maverakis E, Cheng MY, Dunnick CA, Chren M-M, et al. Effectiveness of online vs in-person care for adults with psoriasis: a randomized clinical trial. Jama Netw Open. 2018;1:e183062. doi: 10.1001/jamanetworkopen.2018.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang GD, Bull J, McKee KJ, Mahon E, Harper B, Roberts JN, et al. Clinical trials recruitment planning: a proposed framework from the clinical trials transformation initiative. Contemp Clin Trials. 2018;66:74–79. doi: 10.1016/j.cct.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Barbieri JS, Kovarik CL. Cost analysis of a store-and-forward teledermatology consult system in Philadelphia. J Am Acad Dermatol. 2019;81:758–764. doi: 10.1016/j.jaad.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 15.Sertkaya A, Birkenbach A, Berlind A, Eyraud J. Examination of clinical trial costs and barriers for drug development. U.S. Department of Health and Human Services. 2014.
- 16.Lamberti MJ, Getz K. Profiles of new approaches to improving the efficiency and performance of pharmaceutical drug development. Tufts Center for the Study of Drug Development. 2015.
- 17.Hirsch IB, Martinez J, Dorsey ER, Finken G, Fleming A, Gropp C, et al. Incorporating site-less clinical trials into drug development: a framework for action. Clin Ther. 2017;39:1064–1076. doi: 10.1016/j.clinthera.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Seghers AC, Seng KH, Chio MT, Chia E, Ng SK, Tang MB. A prospective study on the use of teledermatology in psychiatric patients with chronic skin diseases: teledermatology. Australas J Dermatol. 2015;56:170–174. doi: 10.1111/ajd.12297. [DOI] [PubMed] [Google Scholar]
- 19.Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253–260. doi: 10.1007/s40257-017-0317-6. [DOI] [PubMed] [Google Scholar]
- 20.Balato N, Megna M, Costanzo LD, Balato A, Ayala F. Educational and motivational support service: a pilot study for mobile-phone-based interventions in patients with psoriasis. Brit J Dermatol. 2013;168:201–205. doi: 10.1111/j.1365-2133.2012.11205.x. [DOI] [PubMed] [Google Scholar]
- 21.Fink C, Alt C, Uhlmann L, Klose C, Enk A, Haenssle HA. Intra- and interobserver variability of image-based PASI assessments in 120 patients suffering from plaque-type psoriasis. J Eur Acad Dermatol. 2018;32:1314–1319. doi: 10.1111/jdv.14960. [DOI] [PubMed] [Google Scholar]
- 22.Fink C, Alt C, Uhlmann L, Klose C, Enk A, Haenssle HA. Precision and reproducibility of automated computer-guided Psoriasis Area and Severity Index measurements in comparison with trained physicians. Brit J Dermatol. 2019;180:390–396. doi: 10.1111/bjd.17200. [DOI] [PubMed] [Google Scholar]
- 23.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1:30–46. doi: 10.1037/1082-989X.1.1.30. [DOI] [Google Scholar]
- 24.Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405–423. doi: 10.1007/s40257-017-0332-7. [DOI] [PubMed] [Google Scholar]
- 25.Lester JC, Taylor SC, Chren M-M. Under-representation of skin of colour in dermatology images: not just an educational issue. Brit J Dermatol. 2019;180:1521–1522. doi: 10.1111/bjd.17608. [DOI] [PubMed] [Google Scholar]
- 26.Reddy VD, Myers BA, Chan SY, Thibodeaux QG, Brownstone ND, Bhutani T, et al. A review of current phase III clinical trials of plaque psoriasis: under-representation of nonwhite participants and need for reform. Brit J Dermatol. 2021;184:348–350. doi: 10.1111/bjd.19468. [DOI] [PubMed] [Google Scholar]
- 27.Bell MA, Whang KA, Thomas J, Aguh C, Kwatra SG. Racial and ethnic disparities in access to emerging and frontline therapies in common dermatological conditions: a cross-sectional study. J Natl Med Assoc. 2020;112:650–653. doi: 10.1016/j.jnma.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Daneshjou R, Smith MP, Sun MD, Rotemberg V, Zou J. Lack of Transparency and potential bias in artificial intelligence data sets and algorithms: a scoping review. Jama Dermatol. 2021;157:1362–1369. doi: 10.1001/jamadermatol.2021.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005;64:ii65. doi: 10.1136/ard.2004.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vodrahalli K, Daneshjou R, Novoa RA, Chiou A, Ko JM, Zou J. TrueImage: A machine learning algorithm to improve the quality of telehealth photos. Arxiv. 2020. [PubMed]
- 31.Frühauf J, Schwantzer G, Ambros-Rudolph CM, Weger W, Ahlgrimm-Siess V, Salmhofer W, et al. Pilot study using teledermatology to manage high-need patients with psoriasis. Arch Dermatol. 2010;146:200–201. doi: 10.1001/archdermatol.2009.375. [DOI] [PubMed] [Google Scholar]
- 32.Hadeler E, Hong J, Mosca M, Hakimi M, Brownstone N, Bhutani T, et al. Perspectives on the future development of mobile applications for dermatology clinical research. Dermatol Ther. 2021;11:1451–1456. doi: 10.1007/s13555-021-00604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included as supplementary information files.