Abstract
Objectives
To synthesise evidence on incidence rates and risk factors for myocarditis and pericarditis after use of mRNA vaccination against covid-19, clinical presentation, short term and longer term outcomes of cases, and proposed mechanisms.
Design
Living evidence syntheses and review.
Data sources
Medline, Embase, and the Cochrane Library were searched from 6 October 2020 to 10 January 2022; reference lists and grey literature (to 13 January 2021). One reviewer completed screening and another verified 50% of exclusions, using a machine learning program to prioritise records. A second reviewer verified all exclusions at full text, extracted data, and (for incidence and risk factors) risk of bias assessments using modified Joanna Briggs Institute tools. Team consensus determined certainty of evidence ratings for incidence and risk factors using GRADE (Grading of Recommendations, Assessment, Development and Evaluation).
Eligibility criteria for selecting studies
Large (>10 000 participants) or population based or multisite observational studies and surveillance data (incidence and risk factors) reporting on confirmed myocarditis or pericarditis after covid-19 mRNA vaccination; case series (n≥5, presentation, short term clinical course and longer term outcomes); opinions, letters, reviews, and primary studies focused on describing or supporting hypothesised mechanisms.
Results
46 studies were included (14 on incidence, seven on risk factors, 11 on characteristics and short term course, three on longer term outcomes, and 21 on mechanisms). Incidence of myocarditis after mRNA vaccines was highest in male adolescents and male young adults (age 12-17 years, range 50-139 cases per million (low certainty); 18-29 years, 28-147 per million (moderate certainty)). For girls and boys aged 5-11 years and women aged 18-29 years, incidence of myocarditis after vaccination with BNT162b2 (Pfizer/BioNTech) could be fewer than 20 cases per million (low certainty). Incidence after a third dose of an mRNA vaccine had very low certainty evidence. For individuals of 18-29 years, incidence of myocarditis is probably higher after vaccination with mRNA-1273 (Moderna) compared with Pfizer (moderate certainty). Among individuals aged 12-17, 18-29, or 18-39 years, incidence of myocarditis or pericarditis after dose two of an mRNA vaccine for covid-19 might be lower when administered ≥31 days compared with ≤30 days after dose one (low certainty). Data specific to men aged 18-29 years indicated that the dosing interval might need to increase to ≥56 days to substantially drop myocarditis or pericarditis incidence. For clinical course and short term outcomes, only one small case series (n=8) was found for 5-11 year olds. In adolescents and adults, most (>90%) myocarditis cases involved men of a median 20-30 years of age and with symptom onset two to four days after a second dose (71-100%). Most people were admitted to hospital (≥84%) for a short duration (two to four days). For pericarditis, data were limited but more variation than myocarditis has been reported in patient age, sex, onset timing, and rate of admission to hospital. Three case series with longer term (3 months; n=38) follow-up suggested persistent echocardiogram abnormalities, as well as ongoing symptoms or a need for drug treatments or restriction from activities in >50% of patients. Sixteen hypothesised mechanisms were described, with little direct supporting or refuting evidence.
Conclusions
These findings indicate that adolescent and young adult men are at the highest risk of myocarditis after mRNA vaccination. Use of a Pfizer vaccine over a Moderna vaccine and waiting for more than 30 days between doses might be preferred for this population. Incidence of myocarditis in children aged 5-11 years is very rare but certainty was low. Data for clinical risk factors were very limited. A clinical course of mRNA related myocarditis appeared to be benign, although longer term follow-up data were limited. Prospective studies with appropriate testing (eg, biopsy and tissue morphology) will enhance understanding of mechanism.
Introduction
Case reports and surveillance signals1 2 3 4 of myocarditis (including myopericarditis) and pericarditis after covid-19 vaccination appeared as early as April 2021, leading to the surveillance of adverse events of special interest after vaccination with mRNA vaccines manufactured by Pfizer/BioNTech (BNT162b2) and Moderna (mRNA-1273). Estimated rates of myocarditis are 11 per 100 000 person years in the UK5 and 1-2 cases per 100 000 person years in the US,6 7 regardless of age. In the US, estimated background rates or expected rates after covid-19 vaccination of myocarditis is 0.2 per one million people and of pericarditis is 1.4 per one million people, after adjustment for a 7 day risk period where most cases appear.6 Historically, myocarditis has been more prevalent in male than female individuals, from childhood through young adulthood,8 and this tendency was reflected in early post-covid-19 vaccination case series.9 10
Myocarditis, an inflammatory disease of the myocardium, and pericarditis, an inflammatory disease of the pericardial sac, can occur in isolation, although some patients present with overlapping features. Viral causes are common for both disorders. Symptoms indicative of myocarditis or pericarditis typically include new onset and persisting chest pain, shortness of breath, palpitations, or a combination of these symptoms.11 Diagnosis of a probable case of myocarditis usually requires elevated troponin concentrations or findings from imaging (eg, echocardiography or magnetic resonance imaging) or other testing (eg, echocardiogram); histopathology for a definitive diagnosis is not usually done.12 Differential diagnoses, including covid-19 infection or other viruses, should be considered and ruled out.12 13 Most people with these conditions will fully recover. Myocarditis can lead to heart failure or asymptomatic left ventricular dysfunction, whereas long term consequences associated with pericarditis include one or more recurrences and, rarely, thickening of the pericardium and constrictive heart filling.14
This evidence synthesis included systematic reviews of incidence rates (in people aged 0-39 years) and risk factors for myocarditis and pericarditis after covid-19 mRNA vaccination, evidence reviews of initial presentation and clinical course and of longer term outcomes of cases, and a description of proposed mechanisms and their supporting evidence. We originally reviewed (in our literature search on 6 October 2021) incidence rates, risk factors, and case presentation and short term outcomes of myocarditis or pericarditis after covid-19 vaccination across all vaccines, ages, and sexes. Our results indicated that incident rates were very low to none after non-mRNA vaccines and in adults aged 40 years and older, that rates after mRNA vaccination varied by sex, age, and dose, and that the case characteristics and clinical course of myocarditis and pericarditis differed.15 At that time, no data were available for children aged <12 years or for people after receiving a third dose. For this review, we refined the questions to focus on gaps and priorities of the stakeholder or decision maker, specifically, mRNA vaccines, priority age and risk groups, and cases after a third vaccine dose. Where possible, we limited cases to those confirmed by medical record review and of myocarditis or myopericarditis, or pericarditis rather than in combination (when data were available separately). We also expanded the scope to include evidence on longer term outcomes. Finally, we added descriptions and studies of hypothesised mechanisms. This report focuses on the refined questions but also includes findings on characteristics and short term clinical course of myocarditis or pericarditis after any dose of the vaccine across all ages from the original review.
Review questions
We asked five main questions for the review. Firstly, what is the incidence of myocarditis and pericarditis after mRNA covid-19 vaccination? We used data stratified by age and sex, in individuals aged 0-4, 5-11, 12-17, or 18-29 years after their second dose, recipients of any age after a third dose, and individuals with a history of myocarditis after mRNA covid-19 vaccination.
Secondly, among individuals of a similar age and sex, what risk or protective factors exist for myocarditis and pericarditis after mRNA covid-19 vaccination? Examples of factors include pre-existing conditions (eg, cardiac diseases, immunocompromise), previous SARS-CoV-2 infection (symptomatic or asymptomatic) or other viral infections, pharmacotherapies (eg, hormones), type of vaccine product, length of vaccine dosing interval, and vaccine combinations for first v second v booster doses.
Thirdly, what are the characteristics and short term clinical course of myocarditis or pericarditis after covid-19 vaccination, in children younger than 12 years, recipients of any age after a third dose, and individuals with a history of myocarditis after mRNA covid-19 vaccination? From our original review, what are the characteristics and short term clinical course of myocarditis or pericarditis after covid-19 vaccination (ie, across all ages and after a second vaccine dose)?
The fourth question asked what the prognosis is in the longer term (≥4 weeks) among individuals of a similar age and sex who had myocarditis or pericarditis after mRNA covid-19 vaccination, and does this vary by patient characteristics or vaccine?
Finally, we asked what are the hypothesised mechanisms involved in myocarditis and pericarditis after vaccination with mRNA covid-19 vaccines, and do they vary by patient or vaccine characteristics?
Methods
We developed a protocol before screening began and synthesis of evidence. Knowledge users from the Public Health Agency of Canada contributed to scoping the reviews but did not participate in their conduct. We followed guidance for systematic reviews when conducting16 and reporting17 questions one and two. The reviews are now living and future updates will be posted on the COVID-END website at https://www.mcmasterforum.org/networks/covid-end.
Literature search
We worked with an experienced medical information specialist to develop the search strategy, which was peer reviewed (see acknowledgments).18 Searches combined concepts for covid-19, vaccines, and myocarditis, pericarditis, cardiovascular manifestations, adverse events, surveillance; each concept included various keyword and medical subject heading terms. We originally searched on 6 October 2021 and updated the search on 10 January 2022; the updated search (appendix 1) was slightly modified from the original search (eg, adding omicron, removing non-mRNA vaccine terms). We did not add limits for language, country, or study design, but had limits to exclude case reports and news or newspaper articles. Using the multifile option as well as the de-duplication tool in Ovid, we searched Ovid MEDLINE(R) and epub ahead of print, in-process, in-data-review and other non-indexed citations and daily from 1946 to 10 January 2022, inclusive, and Embase 1974 to 10 January 2022, inclusive. We searched grey literature by scanning 20 key national websites (eg, Public Health Agency of Canada, UK’s Medicines and Healthcare Products Regulatory Agency, Centers for Disease Control and Prevention) to identify unpublished data. On 13 January 2022, we searched L-OVE, CT.gov, Cochrane covid Reg, WHO Covid reg, and Google Scholar for additional grey literature. We scanned reference lists of included studies and systematic reviews and consulted our clinician authors (AM and IP) and an expert consultant (Bruce McManus; see acknowledgments) to identify potentially eligible studies on mechanisms. All studies and reports included in the original review were screened for eligibility for the revised questions in this update.
Eligibility criteria
Our inclusion criteria are outlined in box 1. Studies could be eligible for more than one question.
Box 1. Eligibility criteria for each question.
Population
People of any age; data for questions one and two (Q1 and Q2) must be reported using age categories (eg, 0-4, 5-11, 12-17, 18-29, 30-39, ≥40 years) and by dose. If incidence rates were very low and for question two on risk factors, we included data across sexes if no other study reported by sex.
Incident rates after a second dose of vaccine in Q1 were intended to be limited to people younger than 30 years; because several studies reported on an 18-39-year-old category, we included these data, as well as that for people aged 30-39 years, in a post hoc manner.
Intervention or exposure
Q1: mRNA vaccines approved in Canada: BNT162b2 RNA/PfizerBioNTech/Comirnaty, mRNA-1273/Moderna Spikevax (alternative manufacturers of same vaccine are eligible), by type of vaccine and dose.
Q2: Same as Q1, plus potential risk or protective factors: pre-existing conditions (eg, cardiac diseases, immunocompromise), previous SARS-CoV-2 infection (symptomatic or asymptomatic) or other viral infections, length of vaccine dosing interval, type of vaccine, vaccine combination for first versus second versus booster doses.
Q3-Q5: Confirmed myocarditis or pericarditis after mRNA covid-19 vaccination. For Q3, part 2, cases did not need to be confirmed.
At least one dose of the vaccine needed to be with an mRNA vaccine; one or more other doses might have been with a non-mRNA vaccine.
For Q3-Q5, we included exposure to myocarditis or pericarditis (combined) if no separate data were available.
Control or comparator
Q1: People previously vaccinated with mRNA covid-19 vaccine but no longer at risk for outcome, previously vaccinated with other vaccines (ie, controlling for confounders associated with vaccine uptake), or unvaccinated people; or no comparator.
Q2: People vaccinated with mRNA covid-19 vaccine but without the risk or protective factor. Might be a different type of mRNA vaccine, interval between doses, or mixture of vaccines for different doses.
Q3: No comparator.
Q4: No comparator but will include data for any comparisons with people vaccinated and no myocarditis or pericarditis.
Q5: People previously vaccinated with mRNA covid-19 vaccine who did not have myocarditis or pericarditis; or no comparator.
Outcome
Q1: Incidence rate or cumulative risk of confirmed myocarditis (including myopericarditis) or pericarditis by dose. Effect measures: incidence rate or cumulative risk (might be risk difference if accounting for background rate in control group). Will include rates of myocarditis or pericarditis (reported collectively) if no data are available for these separately.
Q2: Ratio measures of incidence or reported events by risk or protective factor (eg, RR or odds ratios), adjusted for key confounders (eg, previous covid-19 illness and severity) when reported. Will include rates of myocarditis or pericarditis (reported collectively) if no data is available for these separately.
Q3: Characteristics of the patients (eg, age, sex, pre-existing conditions (eg, cardiac diseases) and infections (eg, recent or past SARS-CoV-2 infection), race and ethnicity) and case presentation (eg, timing, dose, type of vaccine; diagnostics; illness severity; treatments provided; short-term outcomes).
Q4: As in Q3, plus any outcomes measured ≥4 weeks after onset of myocarditis or pericarditis (eg, re-admission to hospital, functional capacity, chest pain).
Q5: Authors’ summaries of any hypotheses or findings after investigating potential mechanisms (eg, histology, experiments with viral spike glycoprotein of SARS-CoV-2 (encoded by mRNA vaccine)), gene panels, serology for innate and acquired immune system components, autoimmune antibodies).
Setting
Any setting and country.*
Study design
Q1: Large (>10 000 vaccinated people) sample or multisite or health system based observational studies; reports or databases of confirmed case using surveillance data.
Q2: Observational studies (including case control studies) with number of ≥10 with the risk or protective factor; data for subset of people with myocarditis or pericarditis may come from passive reporting systems.
Q3: Case series with five or more cases; data could come from medical record review of cases reported to active or passive surveillance systems (if reporting more than age, sex, and dose and type of vaccine); for Q3, part 2, we relied first on case series reported in systematic reviews and added data on cases from more recent case series or studies included for incident rates if they reported sufficient data on clinical course.
Q4: Case series with at least five cases; data might come from medical record review of cases reported to active or passive surveillance systems.
Q5: Any primary study, systematic review, or expert opinion article or letter on the topic.
Letters and commentaries will be included if they provide sufficient data.
Publication language
English full texts.
We cite those excluded based on language.
Publication year and status
From October 2020 onwards (vaccines were authorised in mid-September 2020).
Preprints are included.
*For Q1 and Q2, when reports collected data from similar or overlapping populations (eg, US national surveillance data), we first included the study with the most recent data and then included additional studies if they differed in their methods (eg, differing risk intervals (period after the vaccine where cases are counted)), differing age or sex stratification, use of unvaccinated or similar controls).
Study selection
All reviewers JP, LH, LB, LG, and AW undertaking screening conducted a pilot round in Excel using 300 records. We then conducted screening in DistillerSR (Evidence Partners) using structured forms. For title and abstract review, we applied the machine learning program Daisy AI, which continually reprioritises records during screening.19 Records were divided among reviewers with one reviewer screening titles and abstracts and another reviewer verifying exclusions for the first 50% of records. For full text selection, one reviewer reviewed all records, and a second reviewer verified all exclusions. Studies were further verified for inclusion during data extraction.
For question three part 2, we mapped the case series by country and data source to identify the most recent and comprehensive data source for each geographical region (ie, US, Canada, UK, Europe, and Israel). For regions with multiple reports, we prioritised data that were most recent, most comprehensive (largest numbers), and based on confirmed cases of myocarditis and pericarditis. We aimed to avoid overlap in data (ie, the same cases reported in different sources). However, we did include sources that reported on specific subgroups (eg, 12-17 year olds) to present more details on these groups of interest. We have noted in the tables where overlap might be present in cases between reports and we avoided aggregating events and counts across studies.
Data extraction
We extracted all data into a structured format in Microsoft Word and conducted a pilot exercise with two studies for each question. Thereafter, one reviewer extracted data and another verified study eligibility and all data. Discrepancies were resolved by discussion or by a review lead (JP or LH).
For question one, we extracted data that related to all elements of the eligibility criteria (box 1) and data used for risk of bias assessment. We focused on methods for identifying cases (ie, passive surveillance versus active surveillance or registry data), outcome ascertainment and confirmation or adjudication (including criteria for case definitions and classification), as well as the dosing interval and risk interval for which the events were captured. We preferred estimates of incidence compared with an unexposed group (ie, excess incidence or risk differences) over those without a control. For question two, we extracted data as for question one, along with associations or subgroup analyses based on pre-existing conditions, different vaccine types or products, dosing interval, and combinations of vaccines. We extracted rates in each group and (if reported) the relative effects between groups (eg, incidence rate ratio and odds ratios), adjusted for key confounders (ie, infection status, cardiac and immunodeficiency or autoimmune conditions) when reported.
For questions three and four, we based data extraction on the evidence table presented in an existing review9 because these findings covered most of the items specified under our outcomes in box 1. We added the following items: criteria for confirmation of cases, breakdown of cases by diagnosis (myocarditis, pericarditis, myopericarditis), data source, age included, percentage of patients with pre-existing conditions, percentage of patients admitted to an intensive care unit, percentage of patients admitted to hospital, and number of fatalities. For question four, we also added longer term investigations and outcomes.
For question five, we extracted verbatim authors’ summaries of any hypotheses and, where available, findings by the authors or cited works investigating potential mechanisms (eg, histology, gene panels, serology for innate and acquired immune system components, autoimmune antibodies, tissue biopsies, autopsy findings, etc). We checked references used to support statements made by authors in proposing or explaining hypotheses to identify whether they provided direct empirical evidence (ie, specific to covid-19 mRNA vaccination). Three content experts (AM, IP, BM) reviewed proposed mechanisms for comprehensiveness and interpretation.
Risk of bias assessment
For questions one and two, all reviewers involved in the risk of bias assessments (JP, LB, LG, and AW) piloted the risk of bias tool with two papers. We used the JBI (formerly Joanna Briggs Institute) checklist for cohort studies,20 with modifications mainly to ascertain valid risk factor measurement (appendix 2). We focused on valid and reliable case finding and confirmation, and, for question two, accounting for key confounders. Assessments were completed by one reviewer and verified by another. Discrepancies were resolved by discussion or by a review lead.
For questions three and four, we did not assess the risk of bias of the included studies. The intent of these questions was to characterise patients and their clinical course, rather than providing quantitative estimates, for example, of incidence. In this case, we judged the main risk of bias to come from case ascertainment: whether each report includes only cases confirmed or verified by clinicians. To deal with this potential bias, we limited inclusion to confirmed cases (question three part 1 and question four) or extracted this information and considered its potential effect when summarising the results (question three part 2).
Data synthesis and certainty assessments
For question one, we did not pool results due to heterogeneity in case finding (passive v active surveillance), dosing and risk intervals, age groupings, and, in some cases, some degree of overlap in cases expected between studies. We tabulated all results and compared and contrasted findings between studies based on the major differentiating population, vaccine, and methodological variables. We report the range in incidence rates across studies, or, when all rates are low, concluded that the incidence is fewer than 20 cases per million, which, based on our clinical investigator input, we considered very rare. Based on input from clinicians and our knowledge users from Public Health Agency of Canada, we developed the key age categories (0-4, 5-11, 12-17, and 18-29 years) to rely on when possible. If a study contributed more than one result within these (eg, 20-24 and 25-29, results for each mRNA vaccine) we calculated a weighted average (eg, by proportion of years in the age range) of the incident rates. When a study reported an incidence rate (or data to calculate this) and an incidence rate ratio compared with a control or background rate, but not the difference in incidence (excess incidence over background rate), we calculated the excess incidence (ie, crude incidence − (crude incidence/ incidence rate ratio)).
We assessed the certainty for each of our conclusion statements using Grading of Recommendations, Assessment, Development and Evaluation (GRADE) guidance.21 22 23 We did not apply a threshold for an important incidence rate, but in general, we considered fewer than 20 cases per million doses to be very rare. For question two, associations (odds ratio or relative risk) of 1.5 or more were considered clinically relevant (ie, odds ratio <1.5 shows little-to-no association). Observational studies for incident rates (question one) started at low certainty (ie, our confidence in the incident rate is limited; the true rate might be substantially different from the estimate) and studies on risk factors (question two) started at high certainty21 (ie, we are very confident that the true difference in rate between risk groups lies close to the estimate). We rated down for serious concerns about risk of bias, inconsistency or absence of consistency (single study), indirectness, imprecision, or reporting biases. We rated down for risk of bias when only studies having high risk for case ascertainment contributed to an outcome (eg, passive surveillance where we assume there is under-ascertainment). We also rated down for indirectness for comparisons across both sexes or if the age group reported did not match one of our groups of interest (eg, 13-39 year olds) and the incident rates could vary substantially among ages. We rated down for imprecision in groups with fewer people than would be expected for one event to occur based on estimated baseline incidence rates (eg, a subgroup with 8000 people but reporting zero events). We considered uprating when incidence rates were large (eg, twice or more than our 20 per million threshold) when no other major limitations were evident and we had moderate confidence in our conclusion, often reported as a range of incidences.24 In our conclusions, we have used the terms probably, might, and uncertain to reflect, respectively, moderate, low, or very low certainty of evidence based on GRADE. When we have very low certainty, we made no conclusions about specific incident rates or other outcomes.
For questions three and four, we present the details for each case series in an evidence table and provide a descriptive summary. For question five, we present a summary of the mechanisms and a more descriptive table.
Patient and public involvement
Two patient or public partners joined the research team (see acknowledgments). They were not involved in decisions about the questions or outcomes. The research leads met with these partners to present and discuss the findings and their implications. The partners co-developed with the leads key messages from their perspective.
Results
Study selection
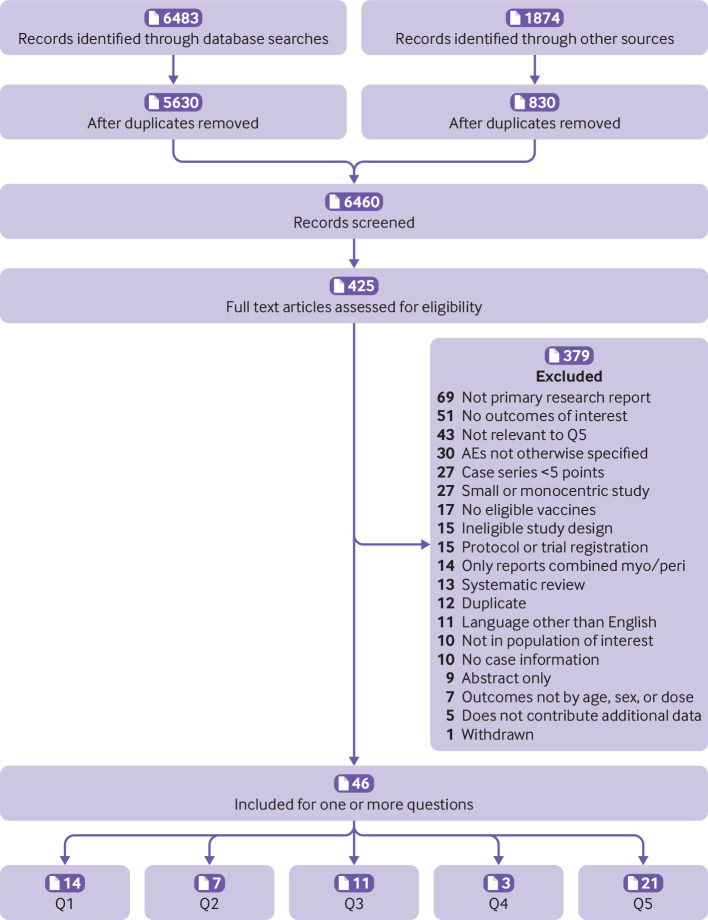
After removing duplicates, we retrieved 3439 citations from our database search on 6 October 2021 and 2191 citations from our search on 10 January 2022; other sources identified 830 citations. After title and abstract screening, we retrieved and screened full texts of 159 and 266 citations during each search, respectively. Forty-six studies were included in this update (fig 1) (question (Q)1=14; Q2=7; Q3=11; Q4=3; Q5=21; 10 studies contributed data to more than one question). Findings from 12 studies from the original review15 were carried forward (Q1=5,25 26 27 28 29 Q2=2,29 30 Q3 part 2=1025 26 27 28 31 32 33 34 35 36). From the search update, we identified 34 new reports across all questions (Q1=9,37 38 39 40 41 42 43 44 45 Q2=5,37 41 45 46 47 Q3 part 1=1,48 Q4=3,39 49 50 Q5=219 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70). We excluded five studies that only reported on combined myocarditis and pericarditis (as we located studies reporting these separately),71 72 73 74 75 eight studies that did not report on confirmed cases,76 77 78 79 80 81 82 83 and three studies84 85 86 that did not contribute additional data to other included studies on the same cases. Tables of study characteristics, with all data for results, and risk of bias ratings for questions one and two are in appendix 2.
Fig 1.
Study flow. Q=question
Question 1: incidence of myocarditis and pericarditis after covid-19 vaccination
We included 14 studies (reported in publications, presentations, online reports, accessible data; all are considered here as studies for simplicity), with eight that used active surveillance data sources (eg, medical records or registries) and six that used passive reporting systems.25 26 27 28 29 37 38 39 40 41 42 43 44 45 Data came from the US, Canada, the UK, Israel, Denmark, Singapore, and internationally (Moderna Global Safety Database). Four studies used the US Vaccine Adverse Events Reporting System for cases, and populations overlapped in analyses for young people aged 5-1143 44 and 12-1725 29 years in which two reports were included for each group because they applied different risk intervals (seven days and any duration). Four studies included a comparator group or background rate to estimate excess incidence rates.27 38 39 41 All studies on myocarditis reported on incident rates by age and sex; the only study reporting on pericarditis by age (5-11 years) reported data for both sexes.39 Risk of bias was rated as some concerns (n=238 39) and high (n=626 27 28 40 41 45), mostly from no adjustment for variables other than age and sex) for studies using data from active surveillance systems and rated as high for all six studies using passive surveillance data.
Table 1 includes the results by study, the conclusions for each age and sex category, and the GRADE certainty assessments. We refer to studies by their source of adverse event data and the date of data collection; references for each study using this format are in appendix 3.
Table 1.
Summary of findings for incident rates after receipt of Pfizer or Moderna vaccine (question one)
| Sex | Age | Data source of study, date; country*† | Risk interval | Incidence rates per million doses after dose two of either mRNA vaccine, unless otherwise stated‡§¶ | Conclusions after vaccination | Certainty about conclusions using GRADE |
|---|---|---|---|---|---|---|
| Myocarditis (after dose two) | ||||||
| Male | 5-11 years | Vaccine Adverse Events Reporting System,* 19 December; US† | 7 days | 2.3 to 4.1¶ (Pfizer) | Incidence of myocarditis after Pfizer vaccine might be fewer than 20 cases per million | Low |
| Vaccine Adverse Events Reporting System,* 9 December; US† | 12 days | 2.98 (both sexes; Pfizer) | ||||
| Vaccine Safety Datalink, 30 December; US |
21 days | No events (both sexes; myocarditis or pericarditis; Pfizer) | ||||
| 12-17 years | Vaccine Adverse Events Reporting System,* June 18; US† | Any | 139.5‡ (Pfizer) | Incidence myocarditis after Pfizer vaccine might be between 50 and 139 cases per million | Low††,‡‡ | |
| COVaxON,* 4 September; Canada | 7 days | 88.1 (Pfizer) | ||||
| Vaccine Adverse Events Reporting System,* 6 October; US† | 7 days | 49.6‡ (Pfizer) | ||||
| 12-39 years | DVR/DPR, 5 October; Denmark | 14 days | 87.7§ | We are uncertain about incidence of myocarditis with an mRNA vaccine | Very low§§ | |
| 18-29 years | Singapore Military, 24 July; Singapore | Any | 71.4 | Incidence of myocarditis with an mRNA vaccine is probably between 28 to 147 cases per million | Moderate‡‡ | |
| COVaxON* September 4; Canada | 7 days | 147.2§ (18-24 years) | ||||
| Israel Defence Forces, 7 March; Israel, | 7days | 50.7 (Pfizer; 18-24 years) | ||||
| Moderna Global Safety Database,* 30 September; worldwide | 7 days | 27.9ঠ(Moderna; 18-24 years) | ||||
| Vaccine Adverse Events Reporting System,* 6 October; USA | 7 days | 27.8‡ | ||||
| Israel Ministry of Health, 31 May; Israel** | 30 days | 82.0ঠ(Pfizer) | ||||
| 18-39 years | Singapore Military, 24 July; Singapore | Any | 60.2‡ | Incidence of myocarditis with an mRNA vaccine might be between 25 and 80 cases per million | Low | |
| US Military, 30 April; US | 4 days (all cases) | 44 (median 25 years (IQR 20-51)) | ||||
| Moderna Global Safety Database,* 30 September; worldwide | 7 days | 25.4‡ (Moderna) | ||||
| COVaxON,* 4 September; Canada | 7 day | 82.2ठ| ||||
| 30-39 years | Vaccine Adverse Events Reporting System,* 6 October; US | 7 days | 5.7§¶ | We are uncertain about the incidence of myocarditis with an mRNA vaccine | Very low††,§§ | |
| Female | 5-11 years | Vaccine Adverse Events Reporting System,* 19 December; US† | 7 days | 0-1.8¶ (Pfizer) | Incidence of myocarditis with the Pfizer vaccine might be fewer than 20 cases per million | Low |
| Vaccine Adverse Events Reporting System,* 9 December; US† | 12 days | 2.98 (both sexes; Pfizer) | ||||
| Vaccine Safety Datalink, 30 December; US | 21 days | 2.3‡ (both sexes; myocarditis or pericarditis; Pfizer) | ||||
| 12-17 years | Vaccine Adverse Events Reporting System,* June 18; US† | Any | 13.1‡ (Pfizer) | We are uncertain if the incidence of myocarditis with the Pfizer vaccine is fewer than 20 cases per million | Very low†† | |
| COVaxON,* 4 September; Canada | 7 days | 9.7 (Pfizer) | ||||
| Vaccine Adverse Events Reporting System,* 6 October; US‡ | 7 days | 5.2‡ (Pfizer) | ||||
| 18-29 years | Vaccine Adverse Events Reporting System,* 6 October; US | 7 days | 3.8ঠ| Incidence of presenting with myocarditis with an mRNA vaccine might be fewer than 20 cases per million | Low | |
| Moderna Global Safety Database,* 30 September; worldwide | 7 days | 0ঠ(ages 18-24 years; Moderna) | ||||
| COVaxON,* 4 September; Canada | 7 days | 34.6§ | ||||
| Israel Ministry of Health, 31 May; Israel** | 30 days | 8.9ঠ(16-29 years; Pfizer) | ||||
| 18-39 years | COVaxON,* 4 September; Canada | 7 days | 22.8‡§ | We are uncertain about the incidence of myocarditis with an mRNA vaccine | Very low§§ | |
| Moderna Global Safety Database,* 30 September; worldwide | 7 days | 2.7‡ (Moderna) | ||||
| 30-39 years | Vaccine Adverse Events Reporting System,* 6 October; US | 7 days | 0.6§¶ | We are uncertain about the incidence of myocarditis with an mRNA vaccine | Very low††,§§ | |
| Myocarditis (after dose three) | ||||||
| Male | 13-39 years | NIMS/NHS, 15 November; UK | 28 days | 13¶ (Pfizer) 0 events/8856 (Moderna) |
We are uncertain about the incidence of myocarditis with mRNA vaccines | Very low§§,¶¶,*** |
| 7 days | 0§¶ | |||||
| ≥40 years | NIMS/NHS, 15 November; UK | 28 days | 3¶ (Pfizer) 0 events/143 066 (Moderna) |
Incidence of myocarditis with mRNA vaccines might be fewer than 20 cases per million | Low††† | |
| 7 days | 0§¶ | |||||
| Female | 13-39 years | NIMS/NHS, 15 November; UK | 28 days | 0§¶ | We are uncertain about the incidence of myocarditis with mRNA vaccines | Very low§§,¶¶ |
| 7 days | 0§¶ | |||||
| ≥40 years | NIMS/NHS, 15 November; UK | 28 days | 0§¶ | We are uncertain about the incidence of myocarditis with mRNA vaccines | Very low§§,¶¶ | |
| 7 days | 0§¶ | |||||
| Mayo Clinic, 17 October; US | 14d | 41.5§ | ||||
| Pericarditis | ||||||
| Male | 5-11 years | Vaccine Safety Datalink, 30 December; US | 21 days | 2.3 (both sexes; Pfizer) | We are uncertain about the incidence of pericarditis after vaccination with Pfizer | Very low§§,*** |
| Female | 5-11 years | Vaccine Safety Datalink, 30 December; US | 21 days | 2.3 (both sexes; Pfizer) | We are uncertain about the incidence of pericarditis after vaccination with Pfizer | Very low§§,*** |
Explanations for Grading of Recommendations, Assessment, Development and Evaluation (GRADE): in the plain language conclusions, we have used “probably,” “might be,” and “uncertain” to reflect level of certainty in the evidence based on GRADE of moderate, low, or very low, respectively. DPR=Danish National Patient Register; DVR=Danish Vaccination Register; IQR=interquartile range; NHS=National Health Services; NIMS=NHS Immunisation Management Service.
Passive surveillance.
Some overlap in cases with another source for this age group.
Weighted average across age groups.
Weighted average across vaccine products.
Excess incidence.
Crude incident rates were converted to excess incidence rates using the estimated adjusted incidence rate ratios (aIRRs) from the study (excess=crude incidence – (crude incidence÷aIRR); for men: 16-19 years, aIRR 8.96 (95% confidence interval 4.50 to 17.83); 20-24 years, 6.13 (3.16 to 11.88); 25-29 years, 3.58 (1.82 to 7.01); for women: 16-19 years, 2.95 (0.42 to 20.91), 20-24 years, 7.56 (1.47 to 38.96), 25-29 years, 0 (only one event).
Rated down for potential risk of bias because all data from passive reporting systems. Incidence rates might be underestimated.
Rated up for estimated incidence likely to be more than twice our clinically important threshold of 20 cases per million, highly unlikely to be seen by chance and credible to be higher than for other age categories.24
Rated down for inconsistency for only one study or for a large incidence range within one category for age or sex.
Rated down for all contributing studies as being at high risk of bias.
Rated down for indirectness of findings to entire population, based on large differences in estimates in analyses for male individuals across age groups indicating one estimate (or even a range of estimates) for all ages (for both sexes or male patients) is not credible.
All contributing studies rated at high risk of bias, however potential biases unlikely to change conclusion so did not rate down.
Myocarditis after dose two
For girls and boys aged 5-11 years, the incidence of myocarditis after vaccination with Pfizer might be fewer than 20 cases per million in both groups (low certainty). The incidence of myocarditis after mRNA vaccines is highest in male adolescents and male young adults (12-17 years, range 50-139 cases per million (low certainty); 18-29 years, 28-147 per million (moderate certainty)). For 18-39 year old male individuals, the incidence of myocarditis might be between 25 and 82 cases per million (low certainty). Among female individuals 18-29 years of age, the incidence of myocarditis after vaccination might be less than 20 cases per million (low certainty). We are very uncertain about the incidence of myocarditis after vaccination with mRNA vaccines in girls aged 12-17, people aged 30-39 years, and in women aged 18-39 years (very low certainty from risk of bias and inconsistency).
Myocarditis after dose three
Among men who are 40 years or older, the incidence of myocarditis after a third dose of an mRNA vaccines might be fewer than 20 cases per million (low certainty). For individuals aged 13-39 years and women 40 years or older, we are uncertain about the incidence of myocarditis after a third dose of an mRNA vaccine due to concerns about imprecision and inconsistency from multiple studies (very low certainty).
Pericarditis
Based on one study only reporting across both sexes, we are uncertain about the incidence of pericarditis after Pfizer vaccination in children aged 5-11 years old (very low certainty).
Question 2: risk factors
In this question, we assessed the relative differences in outcomes across subgroups. It is important to note, however, that these relative results must be taken in context with question one findings reporting on incidence; the relative differences in subgroups in female patients and older age groups should be given less weight in policy decision-making, based on the very low-to-no incidence of myocarditis after mRNA vaccination in these groups. Table 2 and appendix 4 contain the results by study (including absolute numbers), the conclusions for risk factor by age and (when available) sex, and the GRADE certainty assessments.
Table 2.
Summary of findings for myocarditis after Moderna versus Pfizer mRNA vaccination (question 2)
| Sex | Age (years) | Data source, date; country* | Risk interval; confirmed cases (yes/no) | Incidence or reporting rate per million doses after dose two (95% CI)†¶ | Relative measures (95% CI) | Conclusion following vaccination | Certainty about conclusions using GRADE |
|---|---|---|---|---|---|---|---|
| Moderna v Pfizer (ref), dose two | |||||||
| Male | 18-29 | Vaccine Adverse Events Reporting System, 6 October;* US | 7 days | Moderna: 23.9†; Pfizer: 26.0† | — | Probably a higher incidence of myocarditis with Moderna compared with Pfizer | Moderate§,‡‡ |
| COVaxON, 4 September;* Canada | Any | Moderna: 299.5 (171.2 to 486.4); Pfizer: 35.5 (7.3 to 103.7) | — | ||||
| 18-39 | Vaccine Adverse Events Reporting System, 6 October;* US | 7 days | Moderna: 19.2†; Pfizer:16.5† | — | Probably a higher incidence of myocarditis with Moderna than with Pfizer | Moderate‡‡ | |
| Vaccine Safety Datalink, 9 October; US | 7 days | — | RD: 19.1; aRR: 2.14 (0.93 to 4.98) | ||||
| Singapore Military, 24 July; Singapore | Any | Moderna: 135.3†; Pfizer: 0 events/27 632 | — | ||||
| COVaxON, 4 September;* Canada | Any | Moderna: 144.5†; Pfizer: 19.9† | — | ||||
| 30-39 | Vaccine Adverse Events Reporting System, 6 October;* US | 7 days | Moderna: 6.7; Pfizer: 5.2 | — | Might be a little-to-no difference in the incidence of myocarditis with Moderna than with Pfizer | Low‡‡,§§ | |
| 12-39 | NIMS, 15 November,‡ UK | 7 days | Moderna: IRR=54.65 (29.74 to 100.40); Pfizer: IRR=8.05 (5.37 to 12.06) | — | Might be a higher incidence of myocarditis with Moderna than with Pfizer | Low**,§§ | |
| NIMS, 15 November;1 UK | 28 days | Moderna: IRR=16.52 (9.10 to 30.00); Pfizer: IRR=3.41 (2.44 to 4.78) | — | ||||
| ≥40 | Vaccine Adverse Events Reporting System, 6 October;* US | 7 days | Moderna: 1.52† (40-64 years); Pfizer: 0.98† (40-64 years ) | — | Probably little-to-no difference in risk of myocarditis with Moderna compared with Pfizer | Moderate§§ | |
| NIMS, 15 November;‡ UK | 7 days | Moderna: 0 events; Pfizer: IRR=0.65 (0.27 to 1.59) | — | ||||
| NIMS, 15 November;‡ UK | 28 days | Moderna: 0 events; Pfizer: IRR=0.79 (0.51 to 1.23) | — | ||||
| COVaxON, 4 September;* Canada |
Any | Moderna: 0.0 (0.0 to 35.6); Pfizer: 0.0 (0.0 to 23.3) | — | ||||
| Female | 18-29 | COVaxON, 4 September* Canada |
Any | Moderna: 69.1 (14.2 to 201.9) (18-24 years); Pfizer: 0.0 (0.0 to 50.5) (18-24 years) | — | Probably higher incidence of myocarditis with Moderna than with Pfizer | Moderate§§ |
| Vaccine Adverse Events Reporting System, 6 October;* US | 7 days | Moderna: 5.5†; Pfizer: 2.0† | — | ||||
| 18-39 | Vaccine Adverse Events Reporting System, 6 October;* US | 7 days | Moderna: 3.1†; Pfizer: 1.4† | — | Might be higher incidence of myocarditis with Moderna than with Pfizer | Low**,§§ |
|
| COVaxON, 4 September * Canada |
Any | Moderna:36.8†; Pfizer: 8.9† | — | ||||
| 30-39 | Vaccine Adverse Events Reporting System, 6 October* US |
7 days | Moderna: 0.4; Pfizer: 0.7 | — | Little-to-no difference in incidence of myocarditis with Moderna than with Pfizer | Low‡‡,§§ |
|
| 12-39 | NIMS, 15 November; UK | 7 days | Moderna: IRR=28.49 (6.22 to 130.41); Pfizer: IRR=3.11 (1.23 to 7.86) | — | Might be a higher incidence of myocarditis with Moderna than with Pfizer | Low**,‡‡ | |
| NIMS, 15 November; UK | 28 days | Moderna: IRR=7.55 (1.67 to 34.12); Pfizer: 1.37 (0.67 to 2.80) | — | ||||
| ≥40 | COVaxON, 4 September;* Canada | Any | Moderna: 0.0 (0.0 to 40.9); Pfizer: 0.0 (0.0 to 23.5) | — | Probably little-to-no difference in the incidence of myocarditis with Moderna than with Pfizer | Moderate§§ |
|
| NIMS, 15 November; UK | 7 days | Moderna: 0 events; Pfizer: IRR=0.80 (0.33 to 1.97) | — | ||||
| NIMS, 15 November; UK | 28 days | Moderna: 0 events; Pfizer: IRR=1.00 (0.64 to 1.55) | — | ||||
| Vaccine Adverse Events Reporting System, 6 October;* US | 7 days | Moderna: 0.8† (40-64 years); Pfizer: 0.74† (40-64 years) | — | ||||
Explanations for Grading of Recommendations, Assessment, Development and Evaluation (GRADE): in the plain language conclusions, we have used “probably,” “might be,” and “uncertain” to reflect level of certainty in the evidence based on GRADE of moderate, low, or very low, respectively. aRR=adjusted risk ratio. RD=risk difference.
Passive surveillance.
Weighted average across multiple age groups.
This study reported incidence rate ratios (IRRs) calculated using a self-controlled case series design. In this study design, individuals serve as their own controls and risk estimates in pre-intervention and post-intervention intervals are calculated within individuals, rather than across individuals.
Because of the large overlap in data between male patients aged 18-29 years and 18-39 years, we only downrated 18-29 years once for inconsistency despite the large differences in effects reported between studies.
Weighted averages across age groups were calculated based on contribution of each age to the review level age category.
Rated down for indirectness to whole population, based on large differences in estimates in analyses for male individuals across age groups indicating one estimate (or even a range of estimates) for all ages (for both sexes or male patients) is not credible.
Rated down for risk of bias from use of passive surveillance and lack of case verification
Rated down for inconsistency due to only one study providing estimates
Rated down for imprecision for small sample size (<10 000 per group) or very low event rate.
Myocarditis
For Moderna versus Pfizer, after dose two, in people aged 18-29 years, and men aged 18-39 years, the incidence of myocarditis is probably higher after vaccination with Moderna compared with Pfizer (moderate certainty; table 2). For women aged 18-39 years, the incidence of myocarditis might be higher after vaccination with Moderna compared with Pfizer (low certainty). For people aged 30-39 years, little to no difference might be expected in the incidence of myocarditis after vaccination with Moderna compared with Pfizer (low certainty). Among individuals aged ≥40 years, myocarditis after vaccination with Moderna compared with Pfizer is probably little to no difference (moderate certainty).
Myocarditis or pericarditis
For homologous versus heterologous vaccine for dose two, among adults aged 18-29 and 18-39 years and men aged 18-29 years, little to no difference is predicted in the incidence of myocarditis or pericarditis after mRNA vaccination with a heterologous dose two (mixed Moderna and Pfizer with either for dose two) compared with homologous dose two (low certainty). For adults ≥40 years old, we are uncertain about any difference in the incidence of myocarditis/pericarditis after mRNA vaccination with a heterologous dose two compared with homologous dose two (very low certainty).
Considering dose intervals, among individuals aged 12-17, 18-29, and 18-39 years, the incidence of myocarditis or pericarditis after dose two of an mRNA vaccine might be lower when administered 31 days or more compared with 30 days or less after dose one (low certainty). Data specific to men aged 18-29 indicated that the dosing interval might need to increase to 56 days or more to substantially drop incidence. For 18-29 year olds, the proportional decrease in incidence of myocarditis or pericarditis after dose two of an mRNA vaccine when administered 31 days or more compared with 30 days or less after dose one might be similar for Moderna and Pfizer (low certainty). This proportional decrease might be smaller with Moderna compared with Pfizer for 18-39 year olds (low certainty). Among people aged 40 years or older, incidence of myocarditis or pericarditis after dose two of an mRNA vaccine might be higher when administered 31 days or more compared with 30 days or less after dose one (low certainty). In this group, the proportional increase in incidence of myocarditis or pericarditis after dose two when administered 31 days or more compared with 30 days or less after dose one might be greater for Moderna compared with Pfizer.
We are uncertain if people with immunocompromised or inflammatory conditions have a different risk of myocarditis after mRNA vaccination (very low certainty from single studies using passive reporting systems without case confirmation and having inadequate sample sizes).
Question 3: case characteristics and short term clinical course
Part 1: Individuals younger than 12 years, after a third dose, or with a history of myocarditis following mRNA covid-19 vaccination
For this update question, we included one study (also included for question one) reporting data for children aged 5-11 years with confirmed myocarditis.43 No study was found that reported on case presentation after a third dose or for those with a previous experience of myocarditis after a covid-19 vaccine. In a case series of 5-11 year olds who received Pfizer (n=8), 50% were male and the median age was nine years (range 6-11). Race and ethnic group was not reported. Six children presented after their second dose and median time to present was three days. Seven presented with chest pain; of six tested three had abnormal echocardiogram findings; and of five tested, one had an abnormal echocardiogram. Of six with known outcomes, five had complete resolution of symptoms (appendix 4).
Part 3: After the first or second dose in people of any age
From the original review, we report on 10 case series (six published as case series31 32 33 34 35 36 and four included in Q1 but having relevant data on cases25 26 27 28) reporting on a total of 10 264 cases (median 22, range 6-5611) of myocarditis or pericarditis. All reports except one using EudraVigilance data from the European Economic Area included cases confirmed by a clinician (appendix 4).
Most case series focused on myocarditis (10 reports, n=5955). Across the case series, most cases (often >90%) were men with a reported median age most often between 20 and 29 years; confirmed cases ranged from 12 to 56 years. Race and ethnic group were not reported in any case series. Time between last vaccine and symptom onset was on average two to four days, and most people (71-100% across reports) presented symptoms after a second dose. Most cases presented with chest pain or pressure, and troponin elevation; when tested, a minority (14-29% across studies) showed left ventricular dysfunction (ie, left ventricular ejection fraction (LVEF) <50%).
Most (≥84%) patients with myocarditis in the case series were admitted to hospital with few admitted to intensive care unit (five of 49 patients with data); the average length of hospital stay was two to four days in four series (n=47) reporting on this. Non-steroidal anti-inflammatory drugs were most often used as treatment; other interventions included bisoprolol, ramipril, colchicine, famotidine, steroids (for myocarditis), and intravenous immune globulin (for myopericarditis). Among the series of confirmed cases of myocarditis that reported on fatalities (n=220), one fatality was reported in Israel in a 22 year old with fulminant myocarditis. The unconfirmed series from the EudraVigilance data reported 84 fatalities among 5611 cases (1.5%), although cause of death was not confirmed.
Three reports provided data for pericarditis (n=4309; >98% unconfirmed). Most involved male patients (54.4%); however, variation was noted across reports (54-91%). Median age (59 years) was only reported in one small series (n=37). The same series reported a median interval of 20 days between the last vaccine given and symptom onset, with 60% presenting after the second vaccination. Hospital admission rates varied (35% and 73% reported in two series with a total of 59 cases); one series (n=37) reported 3% admitted to intensive care unit and one day for median length of stay. One series (n=37) reported no fatalities, and a larger study of unconfirmed cases (n=4250) reported 15 deaths (0.4%).
Question 4: longer term outcomes
Three reports39 49 50 reported on 38 cases with follow-up of about 90 days after diagnosis of myocarditis following vaccination with an mRNA covid-19 vaccine (table 3). Among 14 patients admitted to hospital for myocarditis after vaccination in the two smaller case series (n=14), patients were male individuals aged 13-19 years and followed up for 90-105 days after diagnosis. In the case series of five patients,49 repeat cardiac magnetic resonance imaging (MRI) was undertaken in two patients, with both showing persistent but decreased late gadolinium enhancement similar to the distribution of the initial MRI but no new abnormalities.
Table 3.
Case series of longer term follow-up of myocarditis or pericarditis after mRNA covid-19 vaccination (question four)
| Case series (country) | Chelala 2021 (US) | Patel 2021 (US) | Klein 2022 (US) |
|---|---|---|---|
| Date of cases | 14 June 2021 | May 2021-June 2021 | 25 December 2021 |
| No of cases | 5 | 9 | 43 |
| Confirmed cases | Clinically confirmed through review of medical records, results of biochemical laboratory testing, ECG results, and findings from echocardiography, cardiac MRI; met 2018 Lake Louise criteria | Diagnoses reviewed and met the CDC case definition and troponin elevation | ICD-10 used then diagnoses confirmed by medical record review |
| Case source | Single medical centre in US | Single medical centre in Atlanta, US | Kaiser Permanente in Colorado, Oregon, California, and Washington; HealthPartners Institute Minnesota; Denver Health |
| Myocarditis (No (%)) | 5 (100) | 9 (100) | 23 (53) |
| Pericarditis (No (%)) | 0 | 0 | 2 (5) |
| Myopericarditis (No (%)) | 0 | 0 | 18 (42) |
| Male (No (%)); female (No (%)) | 5 (100); 0 (0) | 9 (100); 0 (0) | 37 (86); 5 (14) |
| Age (years) | Mean 17 (range 16-19) | 15.7 (IQR 14.5-16.6) | 29 (67%) 12-15 years; 14 (33%) 16-17 years |
| Vaccine product (No (%)) | 4 (80) BNT162b2 (Pfizer); 1 (20) mRNA-1273 (Moderna) | 9 (100) mRNA vaccine | 43 (100) BNT162b2 (Pfizer) |
| In intensive care unit (No (%)) | NR | 2 (22) | 11 (26) |
| Admitted to hospital (No (%)) | 5 (100) | 9 (100) | 28 (65) |
| Presenting after second vaccination (No (%)) | 5 (100) | 8 (89) | NR |
| History of covid-19 (No (%)) | 0 | NR | 2 (5) |
| Positive result on polymerase chain reaction test for covid-19 (No (%)) | 0 | NR | NR |
| Patients with covid nucleocapsid antibody present (among tested) (No (%)) | NR | NR | NR |
| Patients with SARS-CoV-2 spike antibody (No (%)) | NR | NR | NR |
| Previous myocarditis or pericarditis history (No (%)) | None reported | NR | 2 (5%) |
| Median time between last vaccine and symptom onset, days, (range) | 4 (3-4) | 3 between 2nd vaccination and hospital admission | 2 (0-20) |
| Chest pain on presentation (No (%)) | 5 (100) | 9 (100) | NR |
| Other symptoms (eg, myalgia, fatigue, fever; (No (%)) | NR | 4 (44) dyspnoea | NR |
| Troponin elevation (among tested; No (%)) | 5 (100, all tested) | NR | NR |
| Median time to troponin peak after vaccination (days) | NR | NR | NR |
| BNP or N-terminal-proBNP elevation (among tested; No (%)) | 0; normal (4/4 tested) | NR | NR |
| CRP elevation (among tested; No (%)) | 4 (80%, 5/5 tested) | NR | NR |
| Patients with eosinophilia (among tested) | NR | NR | NR |
| Abnormal ECG (among tested; No (%)) | 2 (40, 5/5 tested); ST segment elevation (1 patient), sinus bradycardia (1 patient), normal (3 patients) | 6 (67, 9/9 tested); repolarisation abnormalities (6 patients), normal (3 patients) | NR |
| Abnormal cardiac MRI (among tested; No (%)) | 5 (100, 5/5 tested); no segmental wall motions abnormalities, and basilar and mid-cavity involvement; early and late gadolinium enhancement | NR | NR |
| Abnormal ECG (among tested; No (%)) | 2 (40, 5/5 tested); LVEF mildly to moderately decreased and associated with global hypokinesis (1 patient), ectasia of right coronary artery and left anterior descending artery (1 patient), normal (3 patients) |
Median (IQR) LVEF at presentation=60 (58-67) | NR |
| Patients with LVEF <50% (among tested; No (%)) | 1 (20) | 2 (22) with 30-55% LVEF at presentation; 7 (78) with >55% LVEF at presentation | NR |
| Symptoms resolved (No (%)) | 5 (100) | NR | 43 (100) discharged home |
| Fatalities (No (%)) | 0 | 0 | 0 |
| Median (range) length of stay in hospital (days) | 3 (3-9) | NR | 2 (0-7) |
| Treated with medications for myocarditis (No (%)) | Prescribed at discharge: 1 (20) colchicine and metoprolol; 1 (20) metoprolol; 1 (20) NSAID; 1 (20) aspirin | 8 (89) other NSAID if no aspirin; 2 (22) vasopressors; 1 (11) IVIG; 1 (11) aspirin; 0 steroids | NR |
| Patients with follow-up data (No (%)) | 5/5 (100) | 9/9 (100) | 24/43 (56) |
| Mean (range) length of clinical follow-up (days) | 95 (92-104) | 90 (NR) | 88.5 (28-153) |
| Repeat cardiac MRI (No (%)) | 2 (40) | NR | 1 (4) |
| Characteristics of repeat cardiac MRI | 2 performed, both stable biventricular size and function; persistent, but decreased, LGE that was similar in distribution to the initial MRI; and an absence of new areas of abnormality | NR | Normal findings |
| Symptoms such as chest pain (No (%)) | 3 (60); mild intermittent self-resolving chest pain after discharge; in one patient recurrent symptoms occurred after discontinuation of the NSAID prescribed at discharge | NR | 9 (38) chest pain; 3 (13) shortness of breath; 3 (13) palpitations; 1 (4) fatigue; 3 (13) other (eg, orthostatic hypotension, dizziness) |
| Medical visits following discharge (No (%)) | 3 (60); recurrent symptoms resulted in an emergency department visit | ECG findings at clinic follow-up (1-2 weeks after discharge): 1 of 6/9 tested (17) repolarisation abnormalities, 5 of 6/9 tested (83) normal | 18 (75) electrocardiogram, with abnormal findings in 9 (50) patients; 17 (71) ECG, with abnormal findings in 2 (12) patients |
| Continued treatment with medications (No (%)) | NR | 0 on heart failure medication | 2 (8) still on medication (eg, NSAIDs, colchicine) |
| Recovered with no symptoms (No (%)) | NR | NR | 11 (46) no symptoms, medications, or exercise restrictions |
BNP=B type natriuretic peptide; CDC=Centers for Disease Control and Prevention; CRP=C reactive protein; ECG=echocardiogram; ICD=International Classification of Diseases; ICU=intensive care unit; IQR=interquartile range; IVIG=intravenous immune globulin; LGE=late gadolinium enhancement; LVEF=left ventricular ejection fraction; MRI=magnetic resonance imaging; NA=not applicable; NR=not reported; NSAID=non-steroidal anti-inflammatory drugs; PHAC=Public Health Agency of Canada.
Furthermore, three patients had self-resolving mild intermittent chest pain after discharge, one had recurrent chest pain after discontinuing the non-steroidal anti-inflammatory drugs prescribed at discharge, and three had recurrent symptoms that prompted emergency department visits after discharge. In the series of nine patients,50 none was taking heart failure drug treatment at 90 day follow-up. In the larger case series (n=43),39 among cases of myocarditis or pericarditis (n=2) with long term follow-up (n=24, mean follow-up time 89 days), most were male patients aged 12-17 years. Nine (50%) of 18 patients who underwent echocardiogram had abnormal findings; two (12%) of 17 with an echocardiogram showed abnormalities. Few (8%) patients were on drug treatments such as non-steroidal anti-inflammatory drugs and colchicine after discharge, and 46% had no symptoms, drug treatments, or exercise restrictions at follow-up.
Question 5: hypothesised mechanisms
We included 21 papers,9 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 including narrative reviews, opinion pieces, letters to the editor, case reports, case series, a retrospective study, and a protocol for a prospective observational study. Across the included papers, we identified 16 hypotheses that are presented in table 4. Additional details for each hypothesis are available in appendix 4. All hypotheses related to myocarditis rather than pericarditis. The most commonly discussed hypotheses were hyperimmune or inflammatory response; autoimmunity triggered by molecular mimicry or other mechanism; delayed hypersensitivity (serum sickness); eosinophilic myocarditis; and hypersensitivity to vaccine vehicle components (eg, polyethylene glycol and tromethamine; lipid nanoparticle sheath). Various novel hypotheses were put forward by single papers, such as low residual levels of double strand RNA, hyperviscosity inducing cardiac problems, and strenuous exercise induced secretion of proinflammatory interleukin 6. Some papers discussed observed differences in incidence by sex that could be attributed to sex steroid hormones or underdiagnosis in female patients.
Table 4.
Hypothesised mechanisms for myocarditis following mRNA covid-19 vaccination and direct (ie, on myocarditis after covid-19 vaccine) supporting or refuting empirical evidence (question 5)
| Hypothesis | Citations | Direct empirical evidence | ||
|---|---|---|---|---|
| Supporting | Refuting | |||
| 1 | Hyper immune or inflammatory response, via exposure to spike protein, mRNA strand, or unknown trigger | n=99 52 55 58 59 64 65 68 69 | 3 case reports; multiple case series or reports reporting highest incidence in youth who have higher immunogenicity and reactogenicity from vaccines | 2 case reports; 1 case series |
| 2 | Delayed hypersensitivity (serum sickness) | n=59 53 54 58 68 | 1 case report; 1 case series | 6 case reports; 6 case series |
| 3 | Eosinophilic myocarditis | n=49 54 58 66 | 1 case report | 6 case reports; 6 case series |
| 4 | Hypersensitivity to vaccine vehicle components (eg, polyethylene glycol) and tromethamine; lipid nanoparticle sheath) | n=49 61 62 68 | 4 case reports (1 case with biopsy in series); 1 cohort study | 6 several case reports; 6 case series |
| 5 | Response to mRNA vaccine lipid nanoparticles (direct deleterious effect; not delayed—see hypothesis 4 above) | n=260 68 | 1 cohort | None |
| 6 | Autoimmunity triggered by molecular mimicry or other mechanism | n=99 53 54 56 58 59 64 65 68 | Molecular mimicry: 2 case reports, 1 case series, 1 in vitro study; other autoimmune: 1 case report | Molecular mimicry: 3 cohorts or registry, 2 case reports; other autoimmune: direct findings indicated but not cited |
| 7 | Low residual levels of double strand RNA | n=14 | None | None |
| 8 | Dysregulated micro-RNA response | n=151 | None | None |
| 9 | Production of anti-idiotype antibodies against immunogenic regions of antigen-specific antibodies | n=168 | None | None |
| 10 | Trigger of pre-existing dysregulated immune pathways in certain individuals with predispositions (eg, resulting in a polyclonal B cell expansion, immune complex formation, and inflammation9) | n=29 65 | None | For specific predispositions: 1 case report; 1 case series |
| 11 | Antibody-dependent enhancement of immunity or other forms of immune enhancement with re-exposure to virus after vaccine | n=19 | None | Multiple case reports and series reviewed and tabulated, having no evidence of acute covid-19 infections after vaccine when presenting with myocarditis |
| 12 | Direct cell invasion via the spike protein interacting with the angiotensin converting enzyme 2 (ACE2) widely expressed and prevalent in cardiomyocytes53 | n=253 65 | None | 2 cases |
| 13 | Cardiac pericyte expression of ACE2 with immobilized immune complex on the surface of pericytes activation of the complement system | n=160 | None | None |
| 14 | Spike-activated neutrophils (expressing ACE2) augmenting inflammatory response | n=260 87 | 1 case report | None |
| 15 | Hyperviscosity induced cardiac problem | n=170 | None | None |
| 16 | Strenuous exercise induced secretion of proinflammatory interleukin 6 | n=17 | None | None |
| Observation | ||||
| — | Differences in incidence by sex could be due to sex steroid hormones or underdiagnosis of condition in female patients | n=59 53 59 64 68 | Sex hormones: none; underdiagnosis in women: 2 reports (not studies) | Sex hormones: 1 cohort; underdiagnosis in women: none |
Discussion
Principal findings
Male adolescents and male young adults are at the highest risk of myocarditis after mRNA vaccination for covid-19, and the incidence of myocarditis might be as high as 140 cases per million (1.4 per 10 000). For girls and boys aged 5-11 years and females aged 18-29 years, the incidence of myocarditis after vaccination with Pfizer might be fewer than 20 cases per million although the evidence is of low certainty. Data for incidence rates after a third vaccine dose is limited. For individuals aged 18-29 years, incidence of myocarditis is probably higher after vaccination with Moderna than with Pfizer (moderate certainty). Among individuals aged 12-17, 18-29, and 18-39 years, incidence of myocarditis or pericarditis after dose two of an mRNA vaccine might be lower when administered ≥31 days compared with ≤30 days after dose one (low certainty). Data specific to males aged 18-29 years indicated that the dosing interval might have needed to increase to ≥56 days to substantially drop incidence of these diseases (low certainty). Data for other potential risk factors were very limited. The clinical course of myocarditis in children 5-11 years, for those after a third dose, and for those with previous myocarditis after mRNA vaccination is largely unknown. Although the short term course has consistently shown to be quite mild and self-limiting, more data for longer term prognosis is needed.
Hypothesised mechanisms
Several mechanisms have been hypothesised to account for covid-19 mRNA vaccine associated myocarditis. The hyperimmune or inflammatory response hypothesis raises the question of whether the response is a systemic process or local to the heart. Multi-organ injury is commonly reported in systemic inflammation; however, an isolated cardiac insult is more likely given that most patients manifest chest pain symptoms and measurable changes in cardiac biomarkers and imaging. Although autoimmunity triggered by molecular mimicry or other mechanism is among the more commonly discussed hypothesis, the observed response timing after the second vaccine dose (one to five days) is considered early for this type of mechanism. If mRNA vaccine related myocarditis occurs from exposure to partial antigens (epitopes of SARS-CoV-2 spike protein), then this mechanism should also account for myocarditis after covid-19 infection. Additionally, vaccines using adenoviral vector based platforms produce the spike protein but have not been implicated in acquired myocarditis.
Moreover, these putative mechanisms would not explain the similar incidence of myocarditis associated with other vaccines (eg, smallpox).88 Delayed hypersensitivity reaction has been studied as a potential cause for myocarditis with other viruses (eg, coxsackieviruses and echoviruses) and has produced late onset reactions at the injection site following mRNA covid-19 vaccination. Neither hypersensitivity (to the mRNA or other vaccine components) or eosinophilic myocarditis is likely to be a major cause of cardiac inflammation after vaccination because allergic reactions to the vaccines have been very rare.
The different incidence seen across the sexes also suggests a non-allergic reaction predominating. The mechanism might be similar to that for myocarditis with covid-19 infection but at a lower incidence owing to the smaller quantity of spike protein exposure. Therefore, exploring some of the mechanisms in the covid-19 myocarditis literature might be valuable. An alternate hypothesis not described in the examined articles relates to microvessel partial or complete thrombosis with multi-focal ischaemic injury related to expression of endothelial angiotensin converting enzyme 2 and fibrin-platelet interactions in susceptible individuals. The rapid response to anti-inflammatory treatment would, although, make an ischaemic injury quite unlikely.
Several limitations exist in the mechanistic literature. Firstly, little direct empirical evidence to support or refute the proposed hypotheses; where direct empirical evidence was available, it often derived from case reports or small series. Secondly, when assessing laboratory findings in case reports, series, and retrospective studies, whether any differences seen (eg, increases in natural killer cells, autoantibodies) reflect a causal pathological immune response or reactive adaptive responses to the myocardial inflammation is unclear. Thirdly, new studies refuting previous assertions could emerge, for example, earlier reports stating no myocardial eosinophilia are later contradicted by more recent studies finding evidence of this. Furthermore, there has been a scarcity of invasive investigations (eg, endomyocardial tissue sampling), which is understandable given the typically mild course of affected patients.
Finally, confirmation of a causal link from the vaccine is difficult; for example, an important proportion of observed myocarditis events might not be vaccine related and this will contribute to the heterogeneity of presentations, clinical characteristics, and resulting hypotheses. Choi et al87 described a patient with myocarditis who died after mRNA vaccination, and compared that patient with to another who died, as reported by Verma et al.69 Both patients had had comprehensive clinicopathological analysis. The two cases were remarkably different, with Choi et al suggesting that “myocarditis after covid-19 mRNA vaccination is heterogeneous, both clinically and histologically.”87 Moreover, these data support the concept that myocarditis related to covid-19 vaccination can arise from different mechanisms.
Key messages from patient partners
Several key messages were co-developed with our patient partners to reflect their perspectives. Firstly, the highest risk for myocarditis after covid-19 vaccination exists for male adolescents and male young adults (age 12-29 years), although the risk is likely to be small and cases are generally mild. This population probably has some benefit in receiving Pfizer rather than Moderna and prolonging the dosing interval might be of value. Secondly, clear and effective communication of the risks (rates of myocarditis and likely clinical course) and benefits from vaccination, and the availability of good alternatives (eg, non-mRNA vaccines) will be crucial for young male patients and their parents. Finally, more research into children and what personal risk factors (eg, pre-existing conditions) might put someone at higher risk is urgently needed.
Strengths and limitations of the review
This review had several strengths. We used a comprehensive, peer reviewed search strategy and included grey literature to capture recent data. A second reviewer screened the most relevant citations (based on machine learning) and verified all data and risk of bias assessments. GRADE assessments were based on team consensus including clinical experts. Patient partners reviewed the evidence and developed interpretations from the patient perspective.
The main limitation of our review was that the covid-19 literature base is evolving rapidly with new evidence constantly emerging; nevertheless, some of our review findings were associated with moderate certainty evidence. Furthermore, one or more updates are planned and will be made publicly accessible via the COVID-END website. Because of apparent differences in presentation between myocarditis and pericarditis after vaccination seen in our original review and different long term sequelae, we analysed these outcomes separately where possible. Although myocarditis and pericarditis often coexist, the former is more easily diagnosed due to the presence of elevated cardiac troponin or imaging findings on cardiac MRI. Conversely, pericarditis is often diagnosed based on chest pain symptoms and findings on echocardiogram, and given this subjectivity; related data are likely less robust.
In some cases, authors clearly considered the term myopericarditis to include myocarditis, pericarditis, or both; whereas in others this distinction was not transparent and use of these data for rates of myocarditis and myopericarditis might overestimate the incidence to a small degree. Furthermore, our eligibility criterium of requiring case confirmation was not limited to use of any particular criteria and, in some cases, the clinician diagnosis might not have been based on current definitions. Therefore, these criteria could lead to some over or under ascertainment. None of the studies performed cardiac MRI or biopsy on all patients and, as such, most cases are considered probable (v definitive) at best and any subclinical cases would not have been identified. We are unaware of any other comprehensive examination, and critique, of existing descriptions of the potential mechanisms that might be involved in myocarditis associated with covid-19 vaccination. We might have missed some mechanistic studies because we added this question for this update, after the original review was completed.
Conclusions
Male adolescents and male young adults are at the highest risk of myocarditis after an mRNA vaccination. Pfizer over Moderna and extending dosing interval to more than 30 days might be preferred for this population. As the incidence of myocarditis after mRNA vaccination is a rare adverse event, the findings must be considered alongside the overall benefits of vaccination and with detailed risk-benefit analyses to support policy recommendations for optimal dosing intervals and vaccine products for different populations. As the covid-19 pandemic enters its third year, continued surveillance of myocarditis after mRNA vaccines, especially in younger ages, after dose three (and subsequent doses) and in previous cases is needed to support continued decision making for covid-19 boosters. Additional monitoring of populations with clinical comorbidities of interest (eg, cardiac conditions, previous myocarditis, immunocompromised, etc) is also needed to inform on best practices. Long term follow-up of patients with myocarditis is needed to better understand the natural history including disease recurrence. Finally, multicentre prospective studies with appropriate testing (eg, biopsy, tissue morphology) will enhance understanding of the mechanism or mechanisms of myocarditis and pericarditis after vaccination. This understanding will help to identify and guide recommendations for those who might be at higher risk.
What is already known about this topic
Case reports and surveillance signals of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the two layered sac surrounding the heart) after covid-19 vaccination appeared as early as April 2021
These reports have prompted ongoing surveillance and research of these complications to investigate their incidence, possible attribution to the vaccines, and clinical course
What this study adds
The presentation and clinical course of more than 8000 reported cases of myocarditis and pericarditis is described and some initial reports of longer term outcomes; further, many possible mechanisms are outlined and discussed
Incidence of myocarditis is probably the highest in males aged 12-29 years and more likely with Moderna than with Pfizer mRNA vaccines; longer dosing intervals might be beneficial
Most cases of myocarditis are mild and self-limiting, but data on children and some severe cases are limited
Acknowledgments
We thank Bruce McManus (University of British Columbia) for his insight on the proposed mechanisms and the patient/public partners Linda Wilhelm (New Brunswick) and Natasha Trehan (Toronto, Ontario) for contributing key message from their perspective; Susanna Ogunnaike-Cooke and Natalia Abraham (Knowledge Users; Public Health Agency of Canada) and Andrea Tricco (Unity Health Toronto, Ontario and SPOR Evidence Alliance) for reviewing the protocol and draft report from which this article stems; Becky Skidmore, (St Joseph’s Healthcare Hamilton/McMaster University) for drafting and running the searches, and Kaitryn Campbell (St Joseph’s Healthcare Hamilton/McMaster University) for peer reviewing the search strategy.
Web extra.
Extra material supplied by authors
Web appendix: Online appendix
Contributors: JP, LH, AM, and IP designed the study. JP, LH, LB, LG, and AW screened citations for inclusion and were involved in data extraction and interpretation. JP, LB, LG, and AW were involved is risk of bias assessments. All authors were involved in interpretation of the data. JP wrote the draft manuscript with input from all authors. All authors approved the final version of the manuscript. LH and JP are the guarantors of this manuscript and accept full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This project was funded by the Canadian Institutes of Health Research (CIHR) through the covid-19 Evidence Network to support Decision-making (COVID-END) at McMaster University, the SPOR Evidence Alliance, and the Public Health Agency of Canada. The funders provided input on the design of the study, but did not take part in the collection, analysis, or interpretation of data. They reviewed a report generated from the findings but did not make decisions about submission of the article for publication. The opinions, results, and conclusions are those of the team that prepared the living evidence synthesis, and independent of the Government of Canada, CIHR and the Public Health Agency of Canada. No endorsement by the Government of Canada, CIHR or Public Health Agency of Canada is intended or should be inferred. This review was developed through the analysis, interpretation and synthesis of scientific research and systematic reviews published in peer reviewed journals, institutional websites and other distribution channels. This document might not fully reflect all the scientific evidence available at the time this report was prepared. Other relevant scientific findings might have been reported since completion of this synthesis report. SPOR Evidence Alliance, COVID-END, and the project team at ARCHE make no warranty, express or implied, nor assume any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, data, product, or process disclosed in this report. Conclusions drawn from, or actions undertaken on the basis of, information included in this report are the sole responsibility of the user. LH is supported by a Canada Research Chair in knowledge synthesis and translation. LH and ASM are distinguished researchers with the Stollery Science Laboratory supported by the Stollery Children’s Hospital Foundation.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the Canadian Institutes of Health Research, SPOR Evidence Alliance, and Public Health Agency of Canada for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead authors of this work, JP and LH, affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned in the protocol have been explained.
Dissemination to participants and related patient and public communities: To disseminate our results, we aim to target a broad audience, including health professionals, scientists, and members of the public through presentations and the COVID-END website and communications.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
This research did not involve human research participants and thus did not require ethics approval.
Data availability statement
All of the data extracted for this review are included in the manuscript and associated supplementary files.
References
- 1. Bautista García J, Peña Ortega P, Bonilla Fernández JA, Cárdenes León A, Ramírez Burgos L, Caballero Dorta E. [Acute myocarditis after administration of the BNT162b2 vaccine against COVID-19]. Rev Esp Cardiol 2021;74:812-4. 10.1016/j.recesp.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Israel Ministry of Health. Press release: surveillance of myocarditis (inflammation of the heart muscle) cases between December 2020 and May 2021 (Including). 2021. https://www.gov.il/en/departments/news/01062021-03 (accessed 28 October 2021).
- 3. Marshall M, Ferguson ID, Lewis P, et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer-BioNTech COVID-19 vaccination. Pediatrics 2021;148:e2021052478. 10.1542/peds.2021-052478. [DOI] [PubMed] [Google Scholar]
- 4.Reuters. Israel examining heart inflammation cases in people who received Pfizer COVID shot. 2021 Apr 25, 2021. https://www.reuters.com/world/middle-east/israel-examining-heart-inflammation-cases-people-who-received-pfizer-covid-shot-2021-04-25/ (accessed Oct 28, 2021).
- 5.Willame C, Sturkenboom M, Weibel D. ACCESS Background rates of adverse events of special interest for monitoring COVID-19 vaccine. 2021. https://www.encepp.eu/documents/DraftReport.pdf (accessed 28 October 2021).
- 6. Abara WE, Gee J, Mu Y, et al. Expected rates of select adverse events following immunization for COVID-19 Vvccine safety monitoring. medRxiv 2021. 10.1101/2021.08.31.21262919 [DOI] [PMC free article] [PubMed]
- 7. Gubernot D, Jazwa A, Niu M, et al. U.S. Population-Based background incidence rates of medical conditions for use in safety assessment of COVID-19 vaccines. Vaccine 2021;39:3666-77. 10.1016/j.vaccine.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kytö V, Sipilä J, Rautava P. The effects of gender and age on occurrence of clinically suspected myocarditis in adulthood. Heart 2013;99:1681-4. 10.1136/heartjnl-2013-304449. [DOI] [PubMed] [Google Scholar]
- 9. Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021;144:471-84. 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep 2021;70:977-82. 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cooper LT, Jr. Myocarditis. N Engl J Med 2009;360:1526-38. 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fung G, Luo H, Qiu Y, Yang D, McManus B. Myocarditis. Circ Res 2016;118:496-514. 10.1161/CIRCRESAHA.115.306573. [DOI] [PubMed] [Google Scholar]
- 13. Chiabrando JG, Bonaventura A, Vecchié A, et al. Management of acute and recurrent pericarditis: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;75:76-92. 10.1016/j.jacc.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 14. Imazio M, Brucato A, Barbieri A, et al. Good prognosis for pericarditis with and without myocardial involvement: results from a multicenter, prospective cohort study. Circulation 2013;128:42-9. 10.1161/CIRCULATIONAHA.113.001531. [DOI] [PubMed] [Google Scholar]
- 15. Pillay J, Bialy L, Gaudet L, et al. Myocarditis and pericarditis following COVID-19 vaccination: rapid systematic Review of incidence, risk factors, and clinical course. medRxiv 2021. 10.1101/2021.11.19.21266605 [DOI]
- 16. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 2016;75:40-6. 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 19. Hamel C, Kelly SE, Thavorn K, Rice DB, Wells GA, Hutton B. An evaluation of DistillerSR’s machine learning-based prioritization tool for title/abstract screening - impact on reviewer-relevant outcomes. BMC Med Res Methodol 2020;20:256. 10.1186/s12874-020-01129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joanna Briggs Institute (JBI). Checklist for Cohort Studies 2017. https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Cohort_Studies2017_0.pdf (accessed 12 May 2022).
- 21. Foroutan F, Guyatt G, Zuk V, et al. GRADE Guidelines 28: Use of GRADE for the assessment of evidence about prognostic factors: rating certainty in identification of groups of patients with different absolute risks. J Clin Epidemiol 2020;121:62-70. 10.1016/j.jclinepi.2019.12.023. [DOI] [PubMed] [Google Scholar]
- 22. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011;64:380-2. 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 23. Hultcrantz M, Rind D, Akl EA, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol 2017;87:4-13. 10.1016/j.jclinepi.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guyatt GH, Oxman AD, Sultan S, et al. GRADE Working Group . GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol 2011;64:1311-6. 10.1016/j.jclinepi.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 25. Høeg TB, Krug A, Stevenson J, Mandrola J. SARS-CoV-2 mRNA vaccination-associated myocarditis in children ages 12-17: a stratified national database analysis. medRxiv 2021. 10.1101/2021.08.30.21262866 [DOI]
- 26. Levin D, Shimon G, Fadlon-Derai M, et al. Myocarditis following COVID-19 vaccination - A case series. Vaccine 2021;39:6195-200. 10.1016/j.vaccine.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N Engl J Med 2021;385:2140-9. 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol 2021;6:1202-6. 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su JR. Myopericarditis following COVID-19 vaccination: updates from the Vaccine Adverse Event Reporting System (VAERS). Presentation 21 October 2021 to U.S. Advisory Committee on Immunization Practices. 2021. https://www.cdc.gov/vaccines/acip/meetings/index.html (accessed May 12, 2022)
- 30.Klein NP. Myocarditis analysis in the Vaccine Safety Datalink: rapid cycle analyses and “head-to-head” product comparisons. Presentation Oct 2021 to US Advisory Committee on Immunization Practices. 2021. https://www.cdc.gov/vaccines/acip/meetings/index.html (accessed 12 May 2022)
- 31. Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA 2021;326:1210-2. 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dickey JB, Albert E, Badr M, et al. A series of patients with myocarditis following SARS-CoV-2 vaccination with mRNA-1279 and BNT162b2. JACC Cardiovasc Imaging 2021;14:1862-3. 10.1016/j.jcmg.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.European Medicines Agency. EudraVigilance - European database of suspected adverse drug reaction reports Amsterdam, the Netherlands: European Medicines Agency. 2021. https://www.adrreports.eu/en/eudravigilance.html (accessed 26 October 2021)
- 34. Larson KF, Ammirati E, Adler ED, et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation 2021;144:506-8. 10.1161/CIRCULATIONAHA.121.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosner CM, Genovese L, Tehrani BN, et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation 2021;144:502-5. 10.1161/CIRCULATIONAHA.121.055891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schauer J, Buddhe S, Colyer J, et al. Myopericarditis After the Pfizer Messenger Ribonucleic Acid Coronavirus Disease Vaccine in Adolescents. J Pediatr 2021;238:317-20. 10.1016/j.jpeds.2021.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv 2021. 10.1101/2021.12.02.21267156 [DOI] [PMC free article] [PubMed]
- 38. Husby A, Hansen JV, Fosbøl E, et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ 2021;375:e068665. 10.1136/bmj-2021-068665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein N. Vaccine Safety Datalink rapid cycle analyses: uptake and safety of COVID-19 vaccines in 5-11 and 12-17-year-olds. Presentation 5 January 2022 to U.S. Advisory Committee on Immunization Practices., 2022. https://www.cdc.gov/vaccines/acip/meetings/index.html (accessed 12 May 2022)
- 40. Niesen MJM, Pawlowski C, O’Horo JC, et al. Three doses of COVID-19 mRNA vaccination are safe based on adverse events reported in electronic health records. medRxiv 2021;09. 10.1101/2021.11.05.21265961 [DOI]
- 41. Patone M, Mei WX, Handunnetthi L, et al. Risk of myocarditis following sequential COVID-19 vaccinations by age and sex. medRxiv 2021. 10.1101/2021.12.23.21268276 [DOI]
- 42. Straus W, Urdaneta V, Esposito DB, et al. Myocarditis after mRNA-1273 vaccination: a population-based analysis of 151 million vaccine recipients worldwide. medRxiv 2021;12. 10.1101/2021.11.11.21265536 [DOI]
- 43.Su JR. Adverse events among children ages 5-11 years after COVID-19 vaccination: updates from v-safe and the Vaccine Adverse Event Reporting System (VAERS). Presentation Dec 16 2022 to U.S. Advisory Committee on Immunization Practices. CDC Stacks, 2021. https://www.cdc.gov/vaccines/acip/meetings/index.html (accessed 12 May 2022)
- 44.Su JR. COVID-19 vaccine safety updates: Primary series in children and adolescents ages 5-11 and 12-15 years, and booster doses in adolescents ages 16-24 years. Presentation Jan 5, 2022 to U.S. Advisory Committee on Immunization Practices, 2022. https://www.cdc.gov/vaccines/acip/meetings/index.html (accessed 12 May 2022)
- 45. Tan JTC, Tan C, Teoh J, et al. Adverse reactions and safety profile of the mRNA COVID-19 vaccines among Asian military personnel. Ann Acad Med Singap 2021;50:827-37. 10.47102/annals-acadmedsg.2021345. [DOI] [PubMed] [Google Scholar]
- 46. Lane S, Yeomans A, Shakir S. Systematic review of spontaneous reports of myocarditis and pericarditis in transplant recipients and immunocompromised patients following COVID-19 mRNA vaccination. MedRxiv 2021. https://medrxiv.org/cgi/content/short/2021.12.20.21268102 [DOI] [PMC free article] [PubMed]
- 47. Machado PM, Lawson-Tovey S, Strangfeld A, et al. Safety of vaccination against SARS-CoV-2 in people with rheumatic and musculoskeletal diseases: results from the EULAR Coronavirus Vaccine (COVAX) physician-reported registry. Ann Rheum Dis 2022;81:695-709. 10.1136/annrheumdis-2021-221490. [DOI] [PubMed] [Google Scholar]
- 48.Su J. Adverse events among children ages 5-11 years after COVID-19 vaccination: updates from v-safe and the Vaccine Adverse Event Reporting System (VAERS). Presentation Dec 16 222 to U.S. Advisory Committee on Immunization Practices. CDC Stacks, 2021. https://www.cdc.gov/vaccines/acip/meetings/index.html (accessed 12 May 2022)
- 49. Chelala L, Jeudy J, Hossain R, Rosenthal G, Peitris N, White C. Cardiac MRI findings of myocarditis after COVID-19 mRNA vaccination in adolescents. AJR Am J Roentgenol 2022;218:651-7. 10.2214/AJR.21.26853. [DOI] [PubMed] [Google Scholar]
- 50. Patel T, Kelleman M, West Z, et al. Comparison of MIS-C related myocarditis, classic viral myocarditis, and COVID-19 vaccine related myocarditis in children. MedRxiv 2021. https://medrxiv.org/cgi/content/short/2021.10.05.21264581 [DOI] [PMC free article] [PubMed]
- 51.AbdelMassih A, Shershaby M, Gaber H, et al. Can sarcopenia index serve as a predictor of myocarditis from mRNA based COVID-19 vaccine, insights from clustered cases and potential involvement of micro-RNAs in its pathogenesis (preprint). EuropePMC 2021. https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/ppcovidwho-292375 (accessed 12 May 2022)
- 52. Boursier C, Chevalier E, Filippetti L, et al. 68Ga-DOTATOC digital-PET imaging of inflammatory cell infiltrates in myocarditis following COVID-19 vaccination. Eur J Nucl Med Mol Imaging 2022;49:1433-4. 10.1007/s00259-021-05609-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chouchana L, Blet A, Al-Khalaf M, et al. Features of Inflammatory Heart Reactions Following mRNA COVID-19 Vaccination at a Global Level. Clin Pharmacol Ther 2022;111:605-13. 10.1002/cpt.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. D’Angelo T, Cattafi A, Carerj ML, et al. Myocarditis after SARS-CoV-2 vaccination: a vaccine-induced reaction? Can J Cardiol 2021;37:1665-7. 10.1016/j.cjca.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Das BB, Kohli U, Ramachandran P, et al. Myopericarditis after messenger RNA Coronavirus disease 2019 vaccination in adolescents 12 to 18 Years of Age. J Pediatr 2021;238:26-32.e1. 10.1016/j.jpeds.2021.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ehrlich P, Klingel K, Ohlmann-Knafo S, et al. Biopsy-proven lymphocytic myocarditis following first mRNA COVID-19 vaccination in a 40-year-old male: case report. Clin Res Cardiol 2021;110:1855-9. 10.1007/s00392-021-01936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elkazzaz MR, Alshuwaier G, Ahmed AK. Crosstalk among strenuous exercise, IL-6 and S-Protein Based Vaccines for COVID-19 may explain the rare adverse effects of myocarditis and thrombosis in recently vaccinated young people. A prospective observational study. Protocol Exchange 2022. 10.21203/rs.3.pex-1744/v1 (accessed 25 January 2022). [DOI]
- 58. Hajra A, Gupta M, Ghosh B, et al. Proposed pathogenesis, characteristics, and management of COVID-19 mRNA vaccine-related myopericarditis. Am J Cardiovasc Drugs 2022;22:9-26. 10.1007/s40256-021-00511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol 2022;19:75-7. 10.1038/s41569-021-00662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kadkhoda K. Post RNA-based COVID vaccines myocarditis: Proposed mechanisms. Vaccine 2022;40:406-7. 10.1016/j.vaccine.2021.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kounis NG, Koniari I, Mplani V, Velissaris D, Tsigkas G. The pathogenesis of potential myocarditis induced by COVID-19 vaccine. Am J Emerg Med 2022;56:382-3. 10.1016/j.ajem.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kounis NG, Mplani V, Koniari I, Velissaris D. Hypersensitivity myocarditis and COVID-19 vaccines. Kardiol Pol 2022;80:109-10. 10.33963/KP.a2021.0166. [DOI] [PubMed] [Google Scholar]
- 63. Milano G, Gal J, Creisson A, Chamorey E. Myocarditis and COVID-19 mRNA vaccines: a mechanistic hypothesis involving dsRNA. Future Virol 2021; published online 6 December. 10.2217/fvl-2021-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Parra-Lucares A, Toro L, Weitz-Muñoz S, Ramos C. Cardiomyopathy associated with anti-SARS-CoV-2 vaccination: what do we know? Viruses 2021;13:2493. 10.3390/v13122493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Switzer C, Loeb M. Evaluating the relationship between myocarditis and mRNA vaccination. Expert Rev Vaccines 2022;21:83-9. 10.1080/14760584.2022.2002690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Takeda M, Ishio N, Shoji T, Mori N, Matsumoto M, Shikamaet N. Eosinophilic myocarditis following coronavirus disease 2019 (COVID-19) vaccination. Circ J 2022;86:1020. 10.1253/circj.CJ-21-0935. [DOI] [PubMed] [Google Scholar]
- 67. Truong DT, Dionne A, Muniz JC, et al. Clinically Suspected Myocarditis Temporally Related to COVID-19 Vaccination in Adolescents and Young Adults: Suspected Myocarditis After COVID-19 Vaccination. Circulation 2022;145:345-56. 10.1161/CIRCULATIONAHA.121.056583. [DOI] [PubMed] [Google Scholar]
- 68. Tsilingiris D, Vallianou NG, Karampela I, Liu J, Dalamaga M. Potential implications of lipid nanoparticles in the pathogenesis of myocarditis associated with the use of mRNA vaccines against SARS-CoV-2. Metabol Open 2022;13:100159. 10.1016/j.metop.2021.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Verma AK, Lavine KJ, Lin C-Y. Myocarditis after Covid-19 mRNA Vaccination. N Engl J Med 2021;385:1332-4. 10.1056/NEJMc2109975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mungmunpuntipantip R, Wiwanitkit V. Response to case report on myocarditis and pericarditis after COVID-19 vaccination. J Am Coll Emerg Physicians Open 2021;2:e12559. 10.1002/emp2.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chua GT, Kwan MYW, Chui CSL, et al. Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following Comirnaty vaccination. Clin Infect Dis 2021;28:ciab989. 10.1093/cid/ciab989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Farahmand R, Trottier CA, Kannam JP, Ho KKL. Incidence of myopericarditis and myocardial injury in coronavirus disease 2019 vaccinated subjects. Am J Cardiol 2022;164:123-30. 10.1016/j.amjcard.2021.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fleming-Nouri A, Haimovich AD, Yang D, Schulz WL, Coppi A, Taylor RA. Myopericarditis in young adults presenting to the emergency department after receiving a second COVID-19 mRNA vaccine. Acad Emerg Med 2021;28:802-5. 10.1111/acem.14307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abraham N, Spruin S, Rossi T, et al. Myocarditis and/or pericarditis risk after mRNA COVID-19 vaccination: a Canadian head to head comparison of BNT162b2 and mRNA-1273 vaccines. SSRN 2021; JVAC-D-21-03106. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3988612 (accessed 12 May 2022) [DOI] [PMC free article] [PubMed]
- 75. Katie S, David MD, Jodi LL, Eric SJ, Paul FL. Risk of myopericarditis following COVID-19 mRNA vaccination in a large integrated health system: a comparison of completeness and timeliness of two methods. medRxiv 2021. https://medrxiv.org/cgi/content/short/2021.12.21.21268209 [DOI] [PMC free article] [PubMed]
- 76. Foltran D, Delmas C, Flumian C, et al. Myocarditis and pericarditis in adolescents after first and second doses of mRNA COVID-19 vaccines. Eur Heart J Qual Care Clin Outcomes 2022;8:99-103. 10.1093/ehjqcco/qcab090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hannah GR, Julianne MG, Ruiling L, et al. Safety monitoring of mRNA vaccines administered during the initial 6 months of the U.S. COVID-19 vaccination program: reports to Vaccine Adverse Events Reporting System (VAERS) and v-safe, 2021. medRxiv. 2021. https://medrxiv.org/cgi/content/short/2021.10.26.21265261
- 78. Choe YJ, Yi S, Hwang I, et al. safety and effectiveness of BNT162b2 mRNA Covid-19 vaccine in adolescents. Vaccine 2022;40:691-94. 10.1016/j.vaccine.2021.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lane S, Yeomans A, Shakir S. Reports of myocarditis and pericarditis following mRNA COVID-19 vaccines: a systematic review of spontaneously reported data from the UK, Europe, and the US and of the literature. medRxiv 2021;14 10.1101/2021.09.09.21263342 [DOI] [PMC free article] [PubMed]
- 80. Laurent C, Alice B, Mohammad A-K, et al. Cardiac inflammation after COVID-19 mRNA vaccines: a global pharmacovigilance analysis. medRxiv 2021. 10.1101/2021.08.12.21261955 [DOI]
- 81. Li M, Yuan J, Lv G, Brown J, Jiang X, Lu ZK. Myocarditis and pericarditis following COVID-19 vaccination: inequalities in age and vaccine types. J Pers Med 2021;11:1106. 10.3390/jpm11111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Prajwal MP, Changye L, Zhoutian S, Michael JR. Comparison of adverse events between COVID-19 and Flu vaccines, 2021. medRxiv 2021. https://medrxiv.org/cgi/content/short/2021.09.22.21263711
- 83. Singh A, Khillan R, Mishra Y, Khurana S. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control 2022;50:15-9. 10.1016/j.ajic.2021.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hause AM, Baggs J, Marquez P, et al. COVID-19 vaccine safety in children aged 5-11 years - United States, November 3-December 19, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1755-60. 10.15585/mmwr.mm705152a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dagan N, Barda N, Balicer RD. Adverse effects after BNT162b2 vaccine and SARS-CoV-2 infection, according to age and sex. N Engl J Med 2021;385:2299. 10.1056/NEJMc2115045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med 2022;28:410-22. 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Choi S, Lee S, Seo JW, et al. Myocarditis-induced sudden death after BNT162b2 mRNA COVID-19 vaccination in Korea: case report focusing on histopathological findings. J Korean Med Sci 2021;36:e286. 10.3346/jkms.2021.36.e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ling RR, Ramanathan K, Tan FL, et al. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: a systematic review and meta-analysis. Lancet Respir Med 2022. 10.1016/s2213-2600(22)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Online appendix
Data Availability Statement
All of the data extracted for this review are included in the manuscript and associated supplementary files.