Abstract
We developed a PCR-based assay to quantify trichothecene-producing Fusarium based on primers derived from the trichodiene synthase gene (Tri5). The primers were tested against a range of fusarium head blight (FHB) (also known as scab) pathogens and found to amplify specifically a 260-bp product from 25 isolates belonging to six trichothecene-producing Fusarium species. Amounts of the trichothecene-producing Fusarium and the trichothecene mycotoxin deoxynivalenol (DON) in harvested grain from a field trial designed to test the efficacies of the fungicides metconazole, azoxystrobin, and tebuconazole to control FHB were quantified. No correlation was found between FHB severity and DON in harvested grain, but a good correlation existed between the amount of trichothecene-producing Fusarium and DON present within grain. Azoxystrobin did not affect levels of trichothecene-producing Fusarium compared with those of untreated controls. Metconazole and tebuconazole significantly reduced the amount of trichothecene-producing Fusarium in harvested grain. We hypothesize that the fungicides affected the relationship between FHB severity and the amount of DON in harvested grain by altering the proportion of trichothecene-producing Fusarium within the FHB disease complex and not by altering the rate of DON production. The Tri5 quantitative PCR assay will aid research directed towards reducing amounts of trichothecene mycotoxins in food and animal feed.
Fusarium head blight (FHB) (also known as scab) is a significant disease of small-grain cereals throughout Europe (33), the United States (28), Canada (15), South America (5), Asia (45), and Australia (4). Up to 17 causal organisms have been associated with the disease (33). However, Fusarium avenaceum, Fusarium culmorum, Fusarium graminearum (teleomorph, Gibberella zeae), Fusarium poae, and Microdochium nivale (teleomorph, Monographella nivalis) are the species most commonly associated with the disease. There is extensive prior work on the effect of FHB on grain yields of cereals. For example, several breeding programs for the selection of varieties resistant to FHB have observed losses ranging between 6 and 74% (37, 39, 40). In addition, in field trials, fungicides that successfully reduced FHB also increased yields between 17 and 80% (20, 47).
FHB also is of concern since several species that cause the disease can produce trichothecene mycotoxins. F. culmorum, F. graminearum, and F. poae produce type B trichothecenes such as nivalenol, deoxynivalenol (DON) (also known as vomitoxin), 3-acetyl-DON (3-ADON), 15-ADON, and fusarenon-X (35). These mycotoxins cause a wide range of acute and chronic effects in humans and animals (8). DON is the predominant trichothecene found in cereal grains in Europe and North America (35). The U.S. Food and Drug Administration advises a limit of less than 1 mg/kg for finished flour products (28). Grain samples from two epidemic years (1993 and 1994) in Minnesota had an average DON content of 8 mg/kg (22). At present there are two major approaches to reducing DON contamination of grain: (i) application of fungicides at anthesis to reduce FHB (20) and (ii) selective breeding of cultivars resistant to Fusarium pathogens (29).
FHB may be caused by a complex of ear blight pathogens (9, 32). Some of these species do not produce known mycotoxins, which means that mycotoxin contamination need not correlate with visual symptoms of disease severity (25, 26). Conflicting evidence exists regarding the effect of fungicides on the development of FHB and on the concentration of trichothecene mycotoxins in grain. For example, applications of propiconazole significantly reduced the incidence of FHB caused by F. graminearum but did not affect the DON concentration in harvested grain (26). In contrast, other work has shown that propiconazole (3), triadimefon (3), thiophanate-methyl (43), and tebuconazole (41) all could reduce the severity of FHB and the accumulation of DON. However, a formulation of tebuconazole plus triadimenol applied to ears of winter wheat artificially inoculated with F. culmorum reduced symptoms of FHB but increased the nivalenol content of harvested grain 16-fold (14).
The effects of fungicides on the relationship between the severity of FHB symptoms and the amount of DON in harvested grain may be due to the fungicides either (i) altering the proportion of trichothecene-producing Fusarium spp. within the FHB complex or (ii) altering the rate of DON synthesis. There is evidence from in vitro studies that fungicides do alter the rate of synthesis of mycotoxins by Fusarium spp., but the results are contradictory. For example, cultures of F. graminearum grown in the presence of dicloram (500 μg/ml) or vinclozolin (250 μg/ml) produced less diacetoxyscirpenol than if fungicides were absent (16). Production of 3-ADON by F. graminearum was inhibited when this pathogen was grown in the presence of tebuconazole (1 μg/ml), thiabendazole (1 μg/ml), benomyl (1 μg/ml), or prochloraz (5 ng/ml) (27). In contrast, the production of 3-ADON by cultures of F. culmorum increased fourfold when the fungus was grown in the presence of tebuconazole (0.1 μg/ml) and threefold when it was grown in the presence of difenoconazole (0.1 μg/ml) compared with when fungicides were absent (7). Cultures of Fusarium sporotrichioides exposed to sublethal doses of tridemorph (30 to 50 μg/ml) (30) or carbendazim (5 μg/ml) (34) increased T-2 toxin production fivefold.
Current methods of quantifying FHB pathogens include (i) visual assessment of disease severity (20), (ii) counts of infected ears (23) or spikelets (17) at postanthesis, and (iii) counts of fusarium-damaged (22) or -infected (20) kernels at harvest. These techniques estimate pathogen populations indirectly based on disease expression or, in the case of plate counts, directly based on the incidence of the FHB pathogens. Identification of the species present can be determined only by subculturing onto agar plates and examining morphological characters. Recently, molecular techniques that can estimate the biomass of one or more FHB pathogens in grain samples have been developed. An immunoassay based on the exoantigens of F. sporotrichioides which allows the quantification of Fusarium spp. within grain samples has been described (2). A technique to quantify individual species of Fusarium within plant material using competitive PCR also has been described (10). Quantification of trichothecene-producing Fusarium spp. within harvested grain could be used to determine if fungicides were affecting the proportion of trichothecene-producing Fusarium spp. within the FHB complex and hence altering the relationship between FHB severity and the amount of DON in harvested grain. Such an assay would allow this important functional fungal group, with regard to grain quality, to be quantified as part of fungicide efficacy and variety resistance studies.
The Tri5 gene encodes trichodiene synthase (6), an enzyme that catalyzes the isomerization and cyclization of farnesyl phosphate to trichodiene (18), the first step in the biosynthetic pathway of trichothecenes. The development of Tri5-specific primers has allowed trichothecene-producing Fusarium spp. to be distinguished from nonproducing species using PCR-based assays (31). Similar primers have been used to develop a Tri5-specific reverse transcription-PCR assay to monitor the expression of Tri5 in vitro and in planta (11).
The objectives of this work were (i) to develop a competitive PCR assay to quantify the amounts of trichothecene-producing F. culmorum and F. graminearum within harvested grain; (ii) to evaluate the efficacy of fungicides to control FHB caused by a mixed inoculation of M. nivale, F. culmorum, and F. graminearum, to reduce colonization of grain by trichothecene-producing F. culmorum and F. graminearum and to reduce DON accumulation in grain; and (iii) to determine if there was a correlation between the amounts of trichothecene-producing F. culmorum and F. graminearum and DON levels in grain samples.
MATERIALS AND METHODS
Fungal material.
Isolates of FHB pathogens (Table 1) were maintained in the Harper Adams culture collection as spore suspensions in 10% glycerol at −80°C. Conidial inoculum was produced using methods described previously by Hilton et al. (17).
TABLE 1.
Amplification of a 260-bp Tri5 PCR product from FMB pathogens of wheat
| Species | Isolate(s) | Origin | Tri5 |
|---|---|---|---|
| F. culmorum | F95,b F41/1, Fu36, Fc53,ab Fc70ab | United Kingdom | + |
| 405/3, 405/11 | Germany | + | |
| 421/5, 421/14 | France | + | |
| F. gramineraum | F86, F507, Fg113,ab Fg173ab | United Kingdom | + |
| 405/1 | Germany | + | |
| 421/1 | France | + | |
| F. poae | F733, F62, F113/1 | United Kingdom | + |
| 421/11 | France | + | |
| F. crookwellense | 421/10, 421/19 | France | + |
| F. sporotrichioides | 421/4, 421/23 | France | + |
| F. sambucinum | 421/24, 421/26 | France | + |
| F. avenaceum | F402, F303, 24/1, 59/1 | United Kingdom | − |
| 405/16 | Germany | − | |
| 421/18 | France | − | |
| F. tricinctum | F308, 133/1 | United Kingdom | − |
| 421/3 | France | − | |
| M. nivale | 1/1,b 94/1,b 30/1b | United Kingdom | − |
Isolate supplied by Central Science Laboratory, York, United Kingdom.
Isolate used as inoculum for the field trial.
Field trial.
During the 1998 to 1999 growing season, 48 plots (10 by 2 m) of the winter wheat cultivar Equinox were established and maintained according to standard crop husbandry practices at Harper Adams University College, Shropshire, United Kingdom. Seven fungicide treatments plus a control treatment without spraying were allocated to plots according to a randomized block design with six replicates for each treatment. Fungicides (Table 2) were applied in 200 liters of water/ha using a knapsack sprayer when plants were at ear emergence (Zadoks growth stage [ZGS] 59) (48). When at midanthesis (ZGS 65), wheat ears were inoculated with a conidial suspension (total concentration of 105 spores/ml) of F. culmorum, F. graminearum, and M. nivale (1:1:1 ratio) (Table 1) at a rate of 33 ml/m2 using a knapsack sprayer. Plots were mist irrigated for 21 days as described previously (17) to produce conditions conducive to FHB. Plots were assessed for severity of head blight by counting the number of infected spikelets per ear for 50 arbitrarily chosen ears 28 days after inoculation. Plots were harvested when ripe (ZGS 92) using a Seedmaster Plot combine (Wintersteiger, Ried im Innkreis, Austria). Samples of grain from each plot were analyzed using the Tri5 PCR and DON assay described below.
TABLE 2.
Effects of three fungicides on severity of FHB and concentrations of Tri5 DNA and DON in harvested grain of winter wheat (cv. Equinox) inoculated at ZGS 65 with a conidial suspension of F. culmorum, F. graminearum, and M. nivale
| Fungicidea | Rate of application (g of AI/ha) | % of spikelets infectedb | Tri5 DNA (pg/ng of total DNA)b | DON (mg/kg)c |
|---|---|---|---|---|
| Metconazole | 90 | 19 ab | 1.2 a | 2.0 a |
| Azoxystrobin | 250 | 25 c | 10 f | 12 d |
| Tebuconazole | 250 | 17 a | 1.9 ab | 1.9 a |
| Metconazole | 45 | 21 b | 3.5 cd | 5.6 bc |
| Azoxystrobin | 125 | 21 b | 6.2 ef | 10 d |
| Tebuconazole | 125 | 20 ab | 2.5 bc | 3.8 ab |
| Metconazole + azoxystrobin | 45 + 125 | 11 | 5.1 de | 6.7 c |
| Unsprayed control | 30 | 7.6 ef | 11 d |
Metconazole (flowable concentrate) manufactured by BASF Plc (Cheadle, United Kingdom), azoxystrobin (flowable concentrate) manufactured by Syngenta (Whittlesford, United Kingdom), and tebuconazole (emulsion) manufactured by Bayer Plc (Bury St Edmunds, United Kingdom).
For statistical analysis (analysis of variance), the percentage of spikelets infected was arcsine transformed, and Tri5 DNA was logarithmically transformed. Back-transformed mean data are presented. Transformed means were not significantly different (df = 35; P > 0.05) for values followed by the same letter.
DON values did not require transformation; means were not significantly different (df = 35; P > 0.05) for treatments with the same letter.
DNA extraction.
DNA was extracted from fungal cultures using a modification of the method of Walsh et al. (44). Small pieces of aerial mycelium were removed from a potato dextrose agar (Merck KGaA, Darmstadt, Germany) plate culture and crushed against the wall of a 1.5-ml Eppendorf tube using a sterile pipette tip. Two hundred microliters of Chelex carbon buffer (5% Chelex 100 [Sigma, Poole, United Kingdom], 1% activated charcoal [C5260; Sigma] aqueous suspension) was added. Tubes were vortexed, boiled for 10 min, left to cool for 5 min, vortexed again, and then centrifuged (12,000 × g, 5 min). The DNA in 5 μl of supernatant was amplified within a 25-μl PCR mixture.
A hammer was used to crush 14 g of grain within an envelope made from a heat-sealed A4 acetate sheet. DNA was extracted from crushed grain samples using a cetyltrimethylammonium bromide (CTAB) buffer (sorbitol, 23 g; N-lauryl sarcosine, 10 g; CTAB, 8 g; sodium chloride, 87.7 g; polyvinylpolypyrrolidone, 10 g; and water to 1 liter). Thirty milliliters of CTAB buffer was added to a 10-g sample of crushed grain in a 50-ml centrifuge tube, mixed, and incubated at 65°C for 16 h. Ten milliliters of potassium acetate (5 M) was added and mixed, and the tubes were frozen for 1 h at −20°C. The tubes were thawed, and the contents were mixed and centrifuged (3,000 × g, 15 min). A 1.3-ml aliquot of supernatant was removed and added to 0.6 ml of chloroform in a 2-ml Eppendorf tube. The contents of the tubes were mixed by gentle inversion for 1 min and then centrifuged (12,000 × g, 15 min). A 1-ml aliquot of the aqueous phase was removed to a fresh tube containing 0.8 ml of 100% isopropanol. The contents of the tubes were mixed by gentle inversion for 1 min, and the tubes were incubated at 18°C for 30 min and then centrifuged (6,000 × g, 15 min). The resulting DNA pellets were washed twice with 44% isopropanol and then air dried. Pellets were resuspended in 200 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) at 65°C for 1 h before storage at 4°C. Total DNA was quantified by spectrophotometry (Beckman Instruments manual no. 517304B; Beckman Instruments Inc., Fullerton, Calif.) and diluted to DNA concentrations of 40 and 4 ng/μl.
DNA was extracted from F. culmorum for quantitative PCR using a modification of the method described above. A 7-day-old culture of F. culmorum in potato dextrose broth (Merck) (20°C, 100 rpm) was filtered through sterile muslin, freeze-dried, and shaken by hand to a powder in a 50-ml centrifuge tube with five 8-mm-diameter stainless steel ball bearings. Thirty milliliters of CTAB buffer was added and incubated at 65°C for 1 h. DNA was extracted and quantified as described above for grain and diluted to 1 ng/μl.
Primer design.
Primers for the Tri5 gene (HATri/F [CAGATGGAGAACTGGATGGT] and HATri/R [GCACAAGTGCCACGTGAC]) were derived from conserved regions of Tri5 from F. culmorum (38), F. poae (12) (EMBL accession no. U15658), F. sporotrichioides (18) (accession no. M27246), F. graminearum (36) (accession no. U22464), and F. sambucinum (19) (accession no. M64348) after sequence alignment using CLUSTAL W (42). The expected product size was 260 bp.
Diagnostic PCR.
HATri primers were tested against a range of FHB pathogens (Table 1). PCR mixtures (25 μl) contained a 100 μM concentration of each nucleotide, 100 nM HATri/F and HATri/R, 20 U of Red Hot Taq polymerase (ABgene, Epsom, United Kingdom) per ml, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 50 mM KCl, 100 μg of gelatin per ml, 0.5 mg of Tween 20 (Sigma) per ml, 0.5 mg of Nonidet P-40 (Sigma) per ml, and 5 μl of DNA sample. Negative controls contained 5 μl of water instead of DNA sample. The program used had 35 temperature cycles of 94°C for 15 s, 62°C for 15 s, and 72°C for 45 s. The first cycle had an extra 75 s at 94°C, and the final cycle had an extra 4 min 15 s at 72°C. PCR products were observed after electrophoresis through agarose gels (2% [wt/vol] agarose containing 0.5 μg of ethidium bromide per ml) in TAE buffer (40 mM Tris-acetate, 1 mM EDTA, pH 8.0).
Internal standard construction.
An internal standard (HATriIS) was produced from a 1.2-kb fragment of the alliinase gene from onion (EMBL accession no. L48614) based on the method of Förster (13). This fragment was amplified using primers ONI/F (TGCTCTGCTGATGTTGCCAG) and ONI/R (TACATGGGGATGGAGGTCTC) with the same PCR conditions as described above except that the annealing temperature was 58°C. The 1.2-kb PCR product was excised from the gel after electrophoresis and placed in 1 ml of TE buffer. The gel slice was incubated at 4°C for 16 h. The DNA in a 5-μl aliquot of gel slice solution was amplified with the linker primers (HATri/FL [ACTGGATGGTAGGAAATGCAGCGG] and HATri/RL [GCCACGTGACAAGTGCCCTCCGTG]) with a Touchup PCR program. This program is the same as that described above except that it consisted of 10 cycles with an annealing temperature of 38°C, followed by 20 cycles with an annealing temperature of 55°C. The resulting 410-bp PCR product was excised from the gel after electrophoresis, placed in 1 ml of TE buffer, and incubated for 16 h at 4°C. This linker product consisted of 390 bp of alliinase gene bordered by the first 10 bp of the HATri primer sites. The DNA in a 5-μl aliquot of gel slice solution was amplified with HATri primers using the Touchup program. A 430-bp PCR product was excised from the gel after electrophoresis and purified using a Wizard PCR Prep Kit (Promega, Southampton, United Kingdom). This PCR product consisted of 390 bp of alliinase gene bordered by the complete HATri primer sites. Purified PCR products were quantified on a gel and then ligated into a pGEM-T Vector (Promega) and transformed into Escherichia coli JM109. White colonies were screened by amplification with the HATri primers using the conditions described above for diagnostic PCR. A selection of positive clones were grown overnight in LB broth (Merck) before plasmid DNA was extracted using a Wizard Plus SV Miniprep Kit (Promega). Internal standard plasmid DNA (HATriIS) and DNA from F. culmorum were diluted and amplified together with HATri primers as described above to determine the optimum concentration of HATriIS. This concentration provided a wide quantification range (65-fold) and the greatest sensitivity. Once this was determined, working stocks of HATriIS (7.4 fg/μl) and F. culmorum DNA standards (4 to 260 pg/μl) were prepared in the presence of 10 ng of herring sperm DNA per μl and stored at −20°C. Storing low concentrations of DNA in the presence of herring sperm DNA increased the stability of the DNA.
Quantitative PCR.
Quantitative PCRs were as described above for diagnostic PCR except that a 50-μl total volume was used, with 10 μl of DNA sample and 10 μl of HATriIS working stock. Ten-microliter F. culmorum standards (4 to 260 pg/μl) also were amplified with 10 μl of HATriIS in the same experiment to produce a standard curve. Three controls were used: HATriIS alone, F. culmorum alone, and water. Total DNA stocks of field trial samples at 4 and 40 ng/μl were amplified. Following gel electrophoresis, gels were viewed under UV light on a Gel Doc 1000 fluorescent gel documentation system (Bio-Rad Laboratories Ltd., Hemel Hempstead, United Kingdom), and unsaturated gel images were analyzed using Molecular Analyst software (Bio-Rad). PCR product ratios were determined for each standard and sample by dividing the band intensity of the Tri5 gene product (260 bp) by that of the HATriIS product (430 bp). Since the standard curve was generated using genomic DNA of F. culmorum F36, the unit of DNA quantified is the amount of Tri5 DNA present within 1 pg of F. culmorum F36 genomic DNA.
DON analysis.
Winter wheat cultivar Equinox seed (8 g) was autoclaved at 121°C for 20 min with 3 ml of distilled water in glass universal bottles. The seed was inoculated with two mycelial plugs of an isolate of F. culmorum or F. graminearum used in the field trial or an isolate of F. avenaceum (Table 1). A control bottle was not inoculated. Bottles were incubated at 20°C for 2 weeks. Grain was removed from each bottle, freeze-dried, crushed as described for DNA extraction, and analyzed for DON using a Ridascreen DON enzyme immunoassay (Digen Ltd., Oxford, United Kingdom) according to the manufacturer's instructions. Grain samples from the field trial were analyzed for DON using a Ridascreen DON Fast enzyme immunoassay (Digen) according to the manufacturer's instructions.
Statistical analysis.
Analysis of variance and regression analysis were performed on all data by using Genstat 5 (release 4.1 [PC/Windows NT]; Lawes Agricultural Trust, Harpenden, United Kingdom). Data were transformed to give a normal distribution where necessary. Individual treatments were compared using the least significant difference test (df = 35, P = 0.05).
RESULTS
Visual assessment.
All fungicide treatments significantly reduced the severity of FHB compared to the control treatment without spraying (Table 2). Of the fungicide treatments tested, a mixture of metconazole (45 g of active ingredient [AI]/ha) and azoxystrobin (125 g of AI/ha) provided the most effective control of FHB and was significantly better than all other treatments. Halving the recommended field dose rate of either metconazole, azoxystrobin, or tebuconazole did not significantly affect the control of FHB achieved by these fungicides. Of these three fungicides, azoxystrobin alone was the least effective at controlling disease.
Diagnostic PCR.
HATri primers amplified a single PCR product of 260 bp from genomic DNAs of our isolates of F. culmorum, F. graminearum, F. poae, F. sambucinum, F. sporotrichioides, and F. crookwellense but not from our isolates of F. avenaceum, F. tricinctum, and M. nivale (Table 1).
Quantitative PCR.
Most grain samples amplified at the 40-ng/μl concentration resulted in PCR product ratios greater than those of the F. culmorum standards. All samples amplified at the 4-ng/μl concentration resulted in PCR product ratios within the range of the F. culmorum DNA standards, so we quantified the Tri5 gene at this concentration. A log-log plot of PCR product ratio against Tri5 DNA concentration for the F. culmorum DNA standards gave a linear regression of y = 1.15 + 0.69x (r2 = 0.97). PCR product ratios were converted to DNA concentrations (picograms per nanogram of total DNA) using the above formula (Table 2). Azoxystrobin at both concentrations and the metconazole-azoxystrobin mixture had no significant effect on the Tri5 DNA concentration within harvested grain. The most effective treatments at reducing Tri5 DNA levels were metconazole and tebuconazole at the higher rates of application.
DON analysis.
The DON immunoassay kits quantify the combined concentrations of DON, 3-ADON, and 15-ADON. All of the isolates of F. culmorum and F. graminearum that we tested were producers of DON. The amounts of DON produced after 2 weeks of incubation ranged from 2.5 to 25 mg/kg. DON was not detected in grain inoculated with isolates of F. avenaceum or the uninoculated control. The assay's detection limit was 12.5 μg of DON per kg. Table 2 shows the DON concentration within harvested grain samples for each fungicide treatment. Azoxystrobin at both concentrations had no significant effect on the DON concentration. The most effective treatments for reducing DON levels were metconazole and tebuconazole at the higher rates of application.
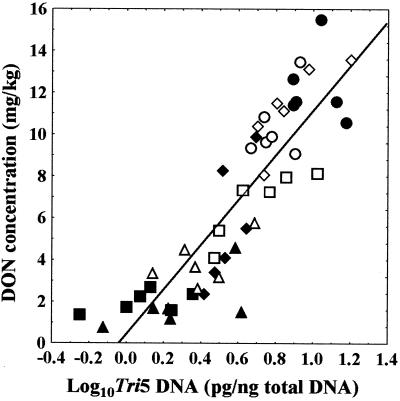
Regression analysis of all transformed data sets showed no correlation (P > 0.05) between either the percentage of spikelets infected and Tri5 DNA concentration or the percentage of spikelets infected and DON concentration. However, a highly significant correlation (P < 0.001) did exist between the log of the Tri5 DNA concentration and the DON concentration (Fig. 1). Individual treatments are indicated on the regression plot.
FIG. 1.
Relationship between Tri5 DNA and DON concentration in grain of winter wheat (cv. Equinox) harvested from field plots sprayed with metconazole (90 g of AI/ha) (▪), azoxystrobin (250 g of AI/ha) (●), tebuconazole (250 g of AI/ha) (▴), metconazole (45 g of AI/ha) (♦), azoxystrobin (125 g of AI/ha) (○), tebuconazole (125 g of AI/ha) (▵), or metconazole (45 g of AI/ha) plus azoxystrobin (125 g of AI/ha) (□) at ZGS 59 or from an unsprayed control plot (⋄) . Regression analysis indicated a strong correlation (y = 11x + 0.44; r2 = 0.76; P < 0.001).
DISCUSSION
We developed a quantitative PCR assay for the trichodiene synthase gene (Tri5) of trichothecene-producing Fusarium. The developed assay allowed us to accurately assess the ability of fungicides to control trichothecene-producing F. culmorum and F. graminearum within a head blight complex containing M. nivale (which does not produce trichothecene). The Tri5 primers (HATri/F and HATri/R) were designed within two highly conserved regions of the Tri5 gene of Fusarium spp. (31). The primer sites are positioned at bases 1106 to 1123 and 1346 to 1363 of the Gibberella pulicaris Tri5 sequence (19). The HATri primers amplified all trichothecene-producing Fusarium spp. tested to produce a single PCR product of 260 bp. F. avenaceum and F. tricinctum are usually considered trichothecene nonproducers (31, 38). All of the F. avenaceum isolates that we tested were negative in the Tri5 PCR assay and did not produce DON in culture when tested. However, there are some reports of F. avenaceum isolates that produce DON (1, 21).
Previous work has shown that visual disease assessments of the FHB and DON contents of grain may (3, 24) or may not (25, 26) be closely correlated. We found no correlation between visual assessments of FHB and the concentration of trichothecene-producing F. culmorum and F. graminearum or DON content within harvested grain. Azoxystrobin is known to be active against M. nivale (S. J. E. West, personal communication), and metconazole and tebuconazole were shown in this trial to be active against trichothecene-producing F. culmorum and F. graminearum. These differences in fungicidal activity against members of the FHB complex probably altered the proportion of trichothecene-producing Fusarium within the complex and could have resulted in the absence of a relationship between FHB severity and the amount of DON in harvested grain. Visual assessments showed that the combination of azoxystrobin and metconazole was the most effective treatment at reducing FHB symptoms, probably because this mixture controls both M. nivale and Fusarium spp.
Regression analysis of trichothecene-producing Fusarium and DON in harvested grain showed a good correlation between biomass and the amount of DON produced. There is no obvious cluster of an individual treatment above or below the regression line. If an individual treatment resulted in a cluster above or below the regression line, this would indicate that this treatment increased or decreased, respectively, the amount of DON produced per Tri5 copy present. The lack of any obvious cluster indicates that in the field, these fungicides did not influence the DON concentration within grain other than by altering the amount of trichothecene-producing Fusarium present. This conclusion is contrary to the results of several in vitro studies (7, 16, 27, 30, 34) which have shown that various fungicides, including tebuconazole, alter the amount of mycotoxin produced per unit of fungal biomass. For example, Doohan et al. (11) showed that expression of Tri5 increased 1.7-fold in the presence of 8 μg of tebuconazole per ml. However, a small increase in synthesis rate over what may be a short time is unlikely to cause a significant increase in DON concentration relative to the amount of trichothecene-producing Fusarium when grain is harvested.
In our study, the correlation between Tri5 DNA and DON concentrations occurred in an inoculated-field trial. This relationship will probably be weaker for field samples, which may differ in the trichothecene-producing isolates present. Isolates may differ in their ability to produce DON or, in the case of F. graminearum, in the relative amounts of DON and nivalenol that they produce. Despite these potential problems, good correlations were found between Fusarium exoantigens and DON in harvested grain from field samples (2). However, different correlations existed for hard red spring wheat in Manitoba, Canada, and soft white winter wheat in Ontario, Canada, indicating that the relationship between the amounts of Fusarium spp. and DON in harvested grain may be affected by host and/or environmental conditions.
Azoxystrobin is recommended as a control for late ear diseases of wheat in the United Kingdom (46). Azoxystrobin and trifloxystrobin are known to control M. nivale and sooty molds, but they have very limited activity against Fusarium spp. (S. J. E. West, personal communication). We recommend that these strobilurins be used only in combination with fungicides that do control Fusarium spp. if DON reduction is an objective. The Tri5 PCR assay showed that the most effective fungicides against trichothecene-producing F. culmorum and F. graminearum were metconazole and tebuconazole.
Trichothecene mycotoxins are important to human and animal health. The Tri5 PCR assay provides a valuable tool in the selection of cultivars and fungicides that are more effective against trichothecene-producing Fusarium and thus should benefit research aimed at reducing trichothecene mycotoxins within food and animal feed.
ACKNOWLEDGMENTS
S.R.P. was supported by Cyanamid UK Ltd.
We thank Digen Ltd., Oxford, United Kingdom, for donating the Ridascreen DON and DON Fast enzyme immunoassay kits used in this study and Phil Jennings, Central Science Laboratory, York, United Kingdom, for providing Fusarium isolates.
REFERENCES
- 1.Abramson D, Clear R M, Smith D M. Trichothecene production by Fusarium spp. isolated from Manitoba grain. Can J Plant Pathol. 1993;15:147–152. [Google Scholar]
- 2.Abramson D, Gan Z, Clear R M, Gilbert J, Marquardt R R. Relationships among deoxynivalenol, ergosterol and the Fusarium exoantigens in Canadian hard and soft wheat. Int J Food Microbiol. 1998;45:217–224. doi: 10.1016/s0168-1605(98)00164-0. [DOI] [PubMed] [Google Scholar]
- 3.Boyacioglu D, Hettiarachchy N S, Stack R W. Effect of three systemic fungicides on deoxynivalenol (vomitoxin) production by Fusarium graminearum in wheat. Can J Plant Sci. 1992;72:93–101. [Google Scholar]
- 4.Burgess L W, Klein T A, Bryden W L, Tobin N F. Head blight of wheat caused by Fusarium graminearum Group 1 in NSW in 1983. Aust J Plant Pathol. 1987;16:72–78. [Google Scholar]
- 5.de Galich M T V. Fusarium head blight in Argentina. In: Dubin H J, Gilchrist L, Reeves J, McNab A, editors. Fusarium head scab: global status and future prospects. Mexico City, Mexico: International Maize and Wheat Improvement Center; 1996. pp. 19–28. [Google Scholar]
- 6.Desjardins A E, Hohn T M, McCormick S. Effect of gene disruption of trichodiene synthase on the virulence of Gibberella pulicaris. Mol Plant-Microbe Interact. 1992;5:214–222. doi: 10.1094/mpmi-5-249. [DOI] [PubMed] [Google Scholar]
- 7.D'Mello J P F, Macdonald A M C, Postel D, Dijksma W T P, Dujardin A, Placinta C M. Pesticide use and mycotoxin production in Fusarium and Aspergillus phytopathogens. Eur J Plant Pathol. 1998;104:741–751. [Google Scholar]
- 8.D'Mello J P F, Placinta C M, Macdonald A M C. Fusarium mycotoxins: a review of global implications for animal health, welfare and productivity. Anim Feed Sci Tech. 1999;80:183–205. [Google Scholar]
- 9.Doohan F M, Parry D W, Jenkinson P, Nicholson P. The use of species-specific PCR-based assays to analyse Fusarium ear blight of wheat. Plant Pathol. 1998;47:197–205. [Google Scholar]
- 10.Doohan F M, Parry D W, Nicholson P. Fusarium ear blight of wheat: the use of quantitative PCR and visual disease assessment in studies of disease control. Plant Pathol. 1999;48:209–217. [Google Scholar]
- 11.Doohan F M, Weston G, Rezanoor H N, Parry D W, Nicholson P. Development and use of a reverse transcription-PCR assay to study expression of Tri5 by Fusarium species in vitro and in planta. Appl Environ Microbiol. 1999;65:3850–3854. doi: 10.1128/aem.65.9.3850-3854.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fekete C, Logrieco A, Giczey G, Hornok L. Screening of fungi for the presence of trichodiene synthase encoding sequence by hybridization to the Tri5 gene cloned from Fusarium poae. Mycopathologia. 1997;138:91–97. doi: 10.1023/a:1006882704594. [DOI] [PubMed] [Google Scholar]
- 13.Förster E. An improved general method to generate internal standards for competitive PCR. BioTechniques. 1994;16:18–20. [PubMed] [Google Scholar]
- 14.Gareis M, Ceynowa J. Influence of the fungicide Matador (tebuconazole and triadimenol) on mycotoxin production by Fusarium culmorum. Z Lebensm Unters Forsch. 1994;198:244–248. doi: 10.1007/BF01192603. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert J, Tekauz A. Recent developments in research in fusarium head blight of wheat in Canada. Can J Plant Pathol. 2000;22:1–8. [Google Scholar]
- 16.Hasan H A H. Fungicide inhibition of aflatoxins, diacetoxyscirpenol and zearalenone production. Folia Microbiol. 1993;38:295–298. doi: 10.1007/BF02898597. [DOI] [PubMed] [Google Scholar]
- 17.Hilton A J, Jenkinson P, Hollins T W, Parry D W. Relationship between cultivar height and severity of Fusarium ear blight in wheat. Plant Pathol. 1999;48:202–208. [Google Scholar]
- 18.Hohn T M, Beremand P D. Isolation and nucleotide sequence of sesquiterpene cyclase gene from the trichothecene-producing fungus Fusarium sporotrichioides. Gene. 1989;79:131–138. doi: 10.1016/0378-1119(89)90098-x. [DOI] [PubMed] [Google Scholar]
- 19.Hohn T M, Desjardins A E, McCormick S P. Analysis of Tox5 gene expression in Gibberella pulicaris strains from different trichothecene production phenotypes. Appl Environ Microbiol. 1993;59:2359–2363. doi: 10.1128/aem.59.8.2359-2363.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hormdock S, Fehrmann H, Beck R. Effects of field application of tebuconazole on yield, yield components and the mycotoxin content of Fusarium-infected wheat grain. J Phytopathol. 2000;148:1–6. [Google Scholar]
- 21.Hussein H M, Baxter M, Andrew I G, Franich R A. Mycotoxin production by Fusarium species isolated from New Zealand maize fields. Mycopathologia. 1991;113:506–511. doi: 10.1007/BF00436385. [DOI] [PubMed] [Google Scholar]
- 22.Jones R K, Mirocha C J. Quality parameters in small grains from Minnesota affected by fusarium head blight. Plant Dis. 1999;83:506–511. doi: 10.1094/PDIS.1999.83.6.506. [DOI] [PubMed] [Google Scholar]
- 23.Lacy J, Bateman G L, Mirocha C J. Effects of infection time and moisture on development of ear blight and deoxynivalenol production by Fusarium spp. in wheat. Ann Appl Biol. 1999;134:277–283. [Google Scholar]
- 24.Lemmens M, Josephs R, Schumacher R, Grausgruber H, Buerstmayr H, Ruckenbauer P, Neuhold G, Fidesser M, Krska R. Head blight (Fusarium spp.) on wheat: investigations on the relationship between disease symptoms and mycotoxin content. Cereal Res Commun. 1997;25:459–465. [Google Scholar]
- 25.Liu W Z, Langseth W, Skinnes H, Elen O N, Sundheim L. Comparison of visual head blight ratings, seed infection levels, and deoxynivalenol production for assessment of resistance in cereals inoculated with Fusarium culmorum. Eur J Plant Pathol. 1997;103:589–595. [Google Scholar]
- 26.Martin R A, Johnston H W. Effects and control of Fusarium diseases of cereal grains in the Atlantic Provinces. Can J Plant Pathol. 1982;4:210–216. [Google Scholar]
- 27.Matthies A, Buchenauer H. Investigations on the action of different active ingredients on the biosynthesis of mycotoxins in Fusarium culmorum and Fusarium graminearum. In: Lyr H, Russell P E, Sisler H D, editors. Modern fungicides and antifungal compounds. Andover, United Kingdom: Intercept Ltd.; 1996. pp. 199–204. [Google Scholar]
- 28.McMullen M, Jones R, Gallenberg D. Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Dis. 1997;81:1340–1348. doi: 10.1094/PDIS.1997.81.12.1340. [DOI] [PubMed] [Google Scholar]
- 29.Mesterhazy A, Bartok T, Mirocha C G, Komoroczy R. Nature of wheat resistance to Fusarium head blight and the role of deoxynivalenol for breeding. Plant Breeding. 1999;118:97–110. [Google Scholar]
- 30.Moss M O, Frank J M. Influence of the fungicide tridemorph on T2 toxin production of Fusarium sporotrichioides. Trans Br Mycol Soc. 1985;84:585–590. [Google Scholar]
- 31.Niessen L M, Vogel R F. Group specific PCR-detection of potential trichothecene-producing Fusarium-species in pure culture and cereal samples. Syst Appl Microbiol. 1998;21:618–631. doi: 10.1016/S0723-2020(98)80075-1. [DOI] [PubMed] [Google Scholar]
- 32.Parry D W, Pettitt T R, Jenkinson P, Lees A K. The cereal Fusarium complex. In: Blakeman P, Williamson B, editors. Ecology of plant pathogens. Wallingford, United Kingdom: CAB International; 1994. pp. 301–320. [Google Scholar]
- 33.Parry D W, Jenkinson P, McLeod L. Fusarium ear blight (scab) in small grain cereals—a review. Plant Pathol. 1995;44:207–238. [Google Scholar]
- 34.Placinta C M, Macdonald A M C, D'Mello J B F, Harling R. Proceedings of the Brighton Crop Protection Conference 1996: Pests and Diseases. Farnham, United Kingdom: British Crop Protection Council; 1996. The influence of carbendazim on mycotoxin production in Fusarium sporotrichioides; pp. 415–416. [Google Scholar]
- 35.Placinta C M, D'Mello J B F, Macdonald A M C. A review of world contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim Feed Sci Technol. 1999;78:21–37. [Google Scholar]
- 36.Proctor R H, Hohn T M, McCormick S P. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol Plant-Microbe Interact. 1995;8:593–601. doi: 10.1094/mpmi-8-0593. [DOI] [PubMed] [Google Scholar]
- 37.Saur L. Sources of resistance to headblight caused by Fusarium culmorum in bread wheat and related species. Agronomie. 1991;11:535–541. [Google Scholar]
- 38.Smith P. Ph.D. thesis. Trichodiene synthase and the role of trichothecenes in Fusarium spp. Norwich, United Kingdom: University of East Anglia; 1997. [Google Scholar]
- 39.Snijders C H A. Fusarium head blight and mycotoxin contamination of wheat, a review. Neth J Plant Pathol. 1990;96:187–198. [Google Scholar]
- 40.Snijders C H A, Perkowski J. Effects of headblight caused by Fusarium culmorum on toxin content and weight of wheat kernels. Phytopathology. 1990;80:566–570. [Google Scholar]
- 41.Suty A, Mauler-Machnik A, Courbon R. Proceedings of the Brighton Crop Protection Conference 1996: Pests and Diseases. Farnham, United Kingdom: British Crop Protection Council; 1996. New findings on the epidemiology of Fusarium ear blight on wheat and its control with tebuconazole; pp. 511–516. [Google Scholar]
- 42.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ueda S, Yoshizawa T. Effect of fungicides on abakabi-byo and trichothecene production in wheat and barley. Proc Jpn Assoc Mycotoxicol. 1988;28(Suppl. 1):234–235. [Google Scholar]
- 44.Walsh P S, Metzger D A, Higuchi R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques. 1991;10:506–513. [PubMed] [Google Scholar]
- 45.Wang Y Z. Epidemiology and management of wheat scab in China. In: Dubin H J, Gilchrist L, Reeves J, McNab A, editors. Fusarium head scab: global status and future prospects. Mexico City, Mexico: International Maize and Wheat Improvement Center; 1996. pp. 97–105. [Google Scholar]
- 46.Whitehead R. The United Kingdom pesticide guide 1999. Wallingford, United Kingdom: CAB International; 1999. [Google Scholar]
- 47.Wong L S L, Tekauz A, Leisle D, Abramson D, McKenzie R I H. Prevalence, distribution and importance of Fusarium headblight in wheat in Manitoba. Can J Plant Pathol. 1992;14:233–238. [Google Scholar]
- 48.Zadoks J C, Chang T T, Konzak C F. A decimal code for the growth stages of cereals. Weed Res. 1974;14:415–421. [Google Scholar]