Abstract
Thirty-five Finnish Campylobacter jejuni strains with five SmaI/SacII pulsed-field gel electrophoresis (PFGE) genotypes selected among human and chicken isolates from 1997 and 1998 were used for comparison of their PFGE patterns, amplified fragment length polymorphism (AFLP) patterns, HaeIII ribotypes, and heat-stable (HS) serotypes. The discriminatory power of PFGE, AFLP, and ribotyping with HaeIII were shown to be at the same level for this selected set of strains, and these methods assigned the strains into the same groups. The PFGE and AFLP patterns within a genotype were highly similar, indicating genetic relatedness. The same HS serotypes were distributed among different genotypes, and different serotypes were identified within one genotype. HS serotype 12 was only associated with the combined genotype G1 (PFGE-AFLP-ribotype). These studies using polyphasic genotyping methods suggested that common Finnish C. jejuni genotypes form genetic lineages which colonize both humans and chickens.
Campylobacter jejuni is the leading cause of human bacterial gastroenteritis in developed countries (22, 29). Serious consequence of campylobacteriosis can be the development of the Guillain-Barré and Miller-Fisher syndromes (33). Most human infections are apparently sporadic, and the distribution of cases shows a seasonal variation. In the Northern hemisphere the human cases occur mostly from June to September (19, 29). C. jejuni is commonly found in the intestinal contents of many domestic and wild animals (27), and there may also be a seasonal variation in the infection rate of poultry (3, 18) and the fecal excretion of C. jejuni in cattle and calves. (28). Although in a few cases, the transmission routes from animal hosts and environmental sources to humans have not been determined, epidemiological studies and data from outbreaks indicate that contaminated drinking water, unpasteurized milk, and eating or handling contaminated poultry products are important risk factors associated with human infections (19, 29).
Subtyping of C. jejuni strains supports epidemiological studies for tracing sources and transmission routes of infections. Serotyping, phage typing, and molecular typing of Campylobacter isolates from human and animal sources have revealed that C. jejuni is a highly heterogeneous organism (7, 11, 23). For example, approximately 70 heat-stable and more than 100 heat-labile serotypes have been identified for C. jejuni and C. coli (22). Application of several typing techniques for comparison of strains obtained from humans and animals have revealed that there is an overlap of serotypes and phage types indicating either common infection sources or transmission of the organism from animal reservoirs to humans through food chains, drinking water, or direct animal contact (11, 21).
Genotyping techniques have shown distinct levels of discriminatory power when applied for studies on C. jejuni. One of the most discriminating techniques has been shown to be pulsed-field gel electrophoresis (PFGE), which uses rare-cutting restriction enzymes and shows sequence variation in restriction sites located over the whole genome (4, 20). However, with SmaI, an enzyme commonly used for PFGE of C. jejuni, only a limited number of fragments is generated, which limits the discriminatory power of this technique (9, 11). To increase the discrimatory power, KpnI (10) or SacII analysis (11) can be used in combination with SmaI. Ribotyping, based on restriction fragment length polymorphism (RFLP) analysis of ribosomal loci, is a less discriminatory method than PFGE for C. jejuni (4, 9) since C. jejuni only has three copies of ribosomal genes, which decreases the number of fragments obtained for a pattern (6). Amplified fragment length polymorphism (AFLP) is a rather new technique used for Campylobacter typing which, by combination of DNA restriction with one or more restriction enzymes and the use of a selective PCR, amplifies a subset of chromosomal fragments. AFLP has been recently applied to studies on C. jejuni strains from different sources and was shown to be a highly discriminatory technique for analysis of both C. jejuni and C. coli strains (5).
In the present study three genotyping methods—PFGE, AFLP, and ribotyping and serotyping—were applied to a set of selected C. jejuni strains. The selected strains represented five combined SmaI/SacII PFGE genotype groups that were commonly found in Finnish patients and chicken isolates in 1997 and 1998 (14). The interstrain relatedness within selected PFGE genotype groups was further studied with the use of other molecular typing methods and heat-stable serotyping.
MATERIALS AND METHODS
Bacterial strains.
Thirty-five C. jejuni strains were selected from a large collection of strains with known epidemiological backgrounds and whose SmaI/SacII PFGE genotypes had been determined (14). The strains were collected from human infections that were domestically acquired and from chicken fecal and meat samples in the summers of 1997 and 1998. The origins of the strains are presented in Table 1.
TABLE 1.
C. jejuni strains, their sources, PFGE patterns, ribotypes, AFLP types, and HS serotypes
| Strain (n = 35) | Source dataa | PFGE pattern (SmaI/SacII) | Ribotype (HaeIII) | AFLP type | Combined genotype | Serotype (HS)b |
|---|---|---|---|---|---|---|
| 5423F | Patient, Pori, 98-07 | I/K | A | AF1 | G1 | 12 |
| 4593 | Chicken, retail shop, Helsinki, producent A, 98-08 | I/K | A | AF1 | G1 | 12 |
| 4772 | Chicken, retail shop, Helsinki, producent B, 98-08 | I/K | A | AF1 | G1 | 12 |
| FB3886 | Patient, Helsinki, 98-07 | I/K | A | AF1 | G1 | 1,44 |
| FB4287 | Patient, Helsinki, 98-07 | I/K | A | AF1 | G1 | 1,44 |
| 25A | Chicken fecal sample, 98-07 | I/Kc | B | AF1 | G2 | 57 |
| 5768 | Chicken, retail shop, Helsinki, producent C, 98-09 | I/K | A | AF1 | G1 | 12 |
| 5483 | Chicken, retail shop, Helsinki, producent A, 98-09 | I/Ka | Aa | AF2 | G3 | 15 |
| 40A | Chicken, fecal sample, 98-11 | I/K | A | AF3 | G4 | 6,7 |
| 35A | Chicken, fecal sample, 98-11 | IV | C | AF4 | G5 | 1,44 |
| 37A | Chicken, fecal sample, 98-11 | I/Kc | B | AF4 | G6 | 57 |
| 28A | Chicken, fecal sample, 98-08 | I/Ka | Aa | AF5 | G7 | 27 |
| BK116 | Chicken, retail shop, Helsinki, producent C, 97-08 | I/K | A | AF5 | G8 | 27 |
| 5862 | Chicken, retail shop, Helsinki, 98-09 | VII | E | AF6 | G9 | NS |
| FB5241 | Patient, Helsinki, 98-08 | VIa | D | AF7 | G10 | 1,44 |
| FB5519 | Patient, Helsinki, 98-08 | VIc | D | AF7 | G10 | 1,44 |
| FB4619 | Patient, Helsinki, 98-07 | VIa | D | AF7 | G10 | 1,44 |
| 4859 | Chicken, retail shop, Helsinki, 98-08 | VIb | D | AF7 | G10 | NS |
| FB4700 | Patient, Helsinki, 98-07 | VIa | D | AF7 | G10 | 1,44 |
| 23OO4 | Patient, Pori, 98-07 | VIa | D | AF7 | G10 | NS |
| 88055 | Patient, Pori, 98-07 | VIa | D | AF7 | G10 | NS |
| FB4877 | Patient, Helsinki, 98-07 | VIc | D | AF7 | G10 | NS |
| BK292 | Chicken, retail shop, Helsinki, 98-08 | VIa | D | AF7 | G10 | 4 |
| 4854 | Patient, Helsinki, 98-07 | VIc | D | AF7 | G10 | NS |
| 81209 | Patient, Pori, 98-07 | VIc | D | AF7 | G10 | 4 |
| BR170 | Chicken, retail shop, Helsinki, producent A, 98-08 | VIb | D | AF7 | G10 | NS |
| 5259 | Chicken, retail shop, 98-08, Helsinki, producent B | VIc | D | AF7 | G10 | NS |
| FB6271 | Patient, Helsinki, 97-07 | T101a | F | AF8 | G11 | 1,44 |
| 456 | Patient, Helsinki, 97-07 | T101a | F | AF8 | G11 | 4 |
| BR77 | Chicken, retail shop, Helsinki, 97-07 | T101a | F | AF8 | G11 | 4 |
| 4180 | Chicken, retail shop, Helsinki, 98-07 | T101b | Fa | AF9 | G12 | 4 |
| BR100 | Chicken, retail shop, Helsinki, 97-07 | IV | C | AF10 | G13 | 1,44 |
| 2475 | Chicken, retail shop, Helsinki, 98-05 | IV | C | AF10 | G13 | 1,44 |
| FB287 | Patient, Helsinki, 98-06 | IV | C | AF10 | G13 | 1,44 |
| FB8164 | Patient, Helsinki, 97-08 | IV | Ca | AF10 | G13 | 1,44 |
C. jejuni strains were obtained from chicken and human (patient) sources in the cities of Helsinki and Pori, as indicated, on the specified dates (year-month).
HS, heat stable; NS, nonserotypeable.
Typing C. jejuni isolates by PFGE.
For PFGE analysis, the isolates were grown on brucella blood agar (Oxoid, Ltd., Basingstoke, Hampshire, England) for 2 days at 37°C in a microaerobic atmosphere. The bacterial cells were harvested and treated with formaldehyde to inactivate endogeneous nucleases (8). Otherwise, DNA was prepared as described by Maslow et al. (20). The DNA fragments were separated with GeneNavigator (Pharmacia LKB Biotechnology AB, Uppsala, Sweden) in 1% agarose gel in 0.5× TBE (45 mmol Tris, 45 mmol boric acid, 1 mmol EDTA) at 200 V. SmaI and SacII fragments were separated with ramped pulses of 1 to 30 s for 20 h and of 1 to 20 s for 18 h, respectively. A combined SmaI/SacII pattern was designated as a PFGE genotype. If strains had one to five differing fragments in their SmaI and SacII patterns, they were designated as subtypes and marked with a letter (for example, genotypes VIa, VIb, VIc, etc.).
AFLP analysis.
The AFLP analysis was performed by using a protocol adapted from the AFLP microbial fingerprinting protocol of PE Applied Biosystems (Perkin-Elmer, Norwalk, Conn.). A more detailed description of the used procedure has been published earlier (5). AFLP data were analyzed using GelCompar (Applied Maths, Kortrijk, Belgium), and a similarity matrix was created with the use of the Pearson product-moment correlation coefficient (r). The unweighted pair group method using average linkage was used to cluster the patterns (30).
Ribotyping.
Purified chromosomal DNA in agar plugs prepared for PFGE was used for ribotyping. In brief, a 2-mm slide was cut from an agar plug, washed two times with the restriction buffer, and transferred into a tube with restriction buffer. DNA was digested with HaeIII (6) according to the instructions of the manufacturer (Boehringer Mannheim, Mannheim, Germany). The digests were electrophoresed in 1.2% agarose gels (SeaKem ME Agarose; FMC BioProducts, Rockland, Maine) with TBE (45 mM Tris, 1 mM EDTA [pH adjusted to 8.0 with boric acid]) as running buffer. DNA transfer and probing were performed as described earlier (13).
Serotyping.
A commercially available serotyping kit (Campylobacter Antisera Seiken Set; Denka, Seiken, Japan) based on Penner's heat-stable serogroups was used as described earlier (26).
RESULTS
PFGE patterns.
A total of 35 strains that belonged to five different PFGE genotype groups were selected on the basis of their SmaI and SacII patterns. The distribution of the strains within PFGE types is shown in Table 1.
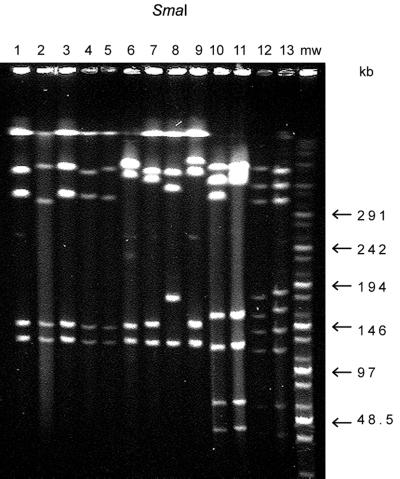
PFGE genotype I/K included eight strains, isolated from patients and chickens in the summer of 1998 (Table 1), which showed identical PFGE patterns (Fig. 1 and 2, lanes 1 and 4; partial digestion seen in Fig. 2, lane 1). In addition, two strains with the highly related PFGE patterns I/Ka and I/Kb differed from pattern I/K by four fragments in only their SacII profiles (Fig. 1 and 2, lanes 3 and 5, respectively). Two more strains were of the related PFGE type I/Kc and had a SacII pattern which differed from the pattern K by five fragments (Fig. 1 and 2, lane 2).
FIG. 1.
SmaI PFGE patterns of C. jejuni strains. Lanes 1 to 5, SmaI pattern I, strains 4772 (lane 1), 25A (lane 2), 5483 (lane 3), 40A (lane 4), and 28A (lane 5); lanes 6 to 9, pattern VII, strains 5862 (lane 6), FB5241 (lane 7), 4859 (lane 8), and FB5519 (lane 9); lanes 10 and 11, pattern T101, strain FB6271 (lane 10) and 4180 (lane 11); lanes 12 and 13, pattern IV, strains FB287 (lane 12) and strain 35A (lane 13); mw, molecular size marker.
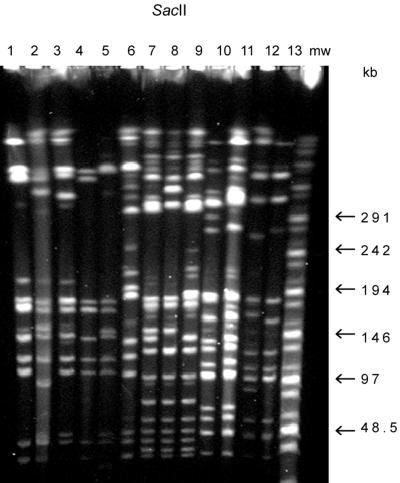
FIG. 2.
SacII patterns of same strains as in Fig. 1. Lanes 1 and 4, pattern K; lane 2, pattern Kc; lane 3, pattern Ka; lane 5, pattern Kb; lane 6, pattern VII; lane 7, pattern VIa; lane 8, pattern VIb; lane 9, pattern VIc; lane 10, pattern T101a; lane 11, pattern T101b; lanes 12 and 13, pattern IV. mw, molecular size marker.
Thirteen strains represented the genotype VI with three closely related groups designated VIa, VIb, and VIc (Table 1). Their SmaI and SacII patterns differed from each other by two to five fragments (Fig. 1 and 2, lanes 7, 8, and 9). Strain 5862 was assigned to type VII. It showed a closely related SmaI pattern (Fig. 1, lane 6) with the group VI strains, but the SacII pattern differed by more than 10 fragments from the other patterns of this group (Fig. 2, lane 6).
PFGE genotype IV included five strains (Table 1; Fig. 1 and 2, lanes 12 and 13), and PFGE genotype T101 had two subtypes, a and b (Fig. 1 and 2, lanes 10 and 11; Table 1), that differed by one fragment in their SmaI profiles (double band on T101b) and by one fragment in their SacII profiles.
AFLP.
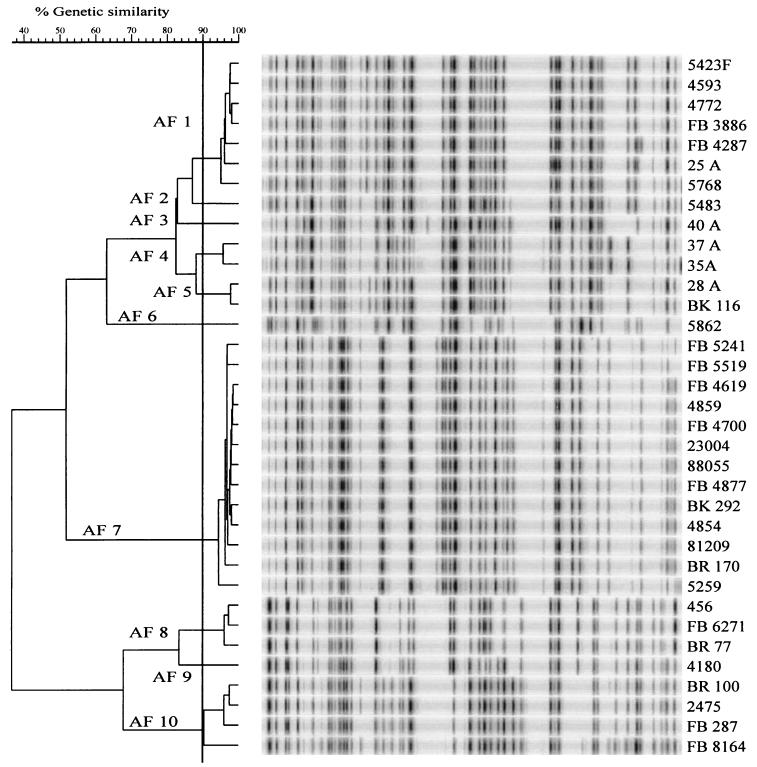
AFLP analysis subdivided the 35 C. jejuni strains into 10 AFLP types (AF1 to AF10). AFLP fingerprints were identified as distinct types when the banding patterns shared less than 90% homology, as has been shown by Duim et al. (5). Cluster analysis of AFLP patterns clearly separated distinct PFGE types and thus produced in most cases congruent results between the PFGE and AFLP analyses. The only exception was strain 35A (PFGE IV), which clustered into the AF4 type (Table 1; Fig. 1 and 2, lane 13, and Fig. 3).
FIG. 3.
AFLP patterns of 35 C. jejuni strains selected for the study.
AFLP patterns of six strains with the the PFGE genotype I/K were clustered at a >90% similarity level (AF1), but patterns of two strains of this PFGE group were clustered only with an 82% similarity level with other strains of the I/K group (AF3 and AF5; Fig. 3). Strains 25A and 37A, with PFGE types I/Kc (Fig. 1, lane 2), were clustered in the AFLP analysis into two clusters, AF1 and AF4, respectively (Fig. 3). In the AFLP pattern analysis, all PFGE genotype VI strains were clustered into the same group AF7 with highly similar profiles (Fig. 3). The pattern of strain 5862 (AF6) clustered between AF7 and AF1 to AF5, being only distantly related to the AF7 strains, thus further confirming that this strain does not belong to the same lineage as the other strains in this group. Three T101a genotype strains from humans and chickens had similar AFLP patterns (AF8), and the AFLP pattern of PFGE genotype T101b was related with a similarity level of 82% with the genotype T101a (Fig. 3, AF9).
Ribotyping.
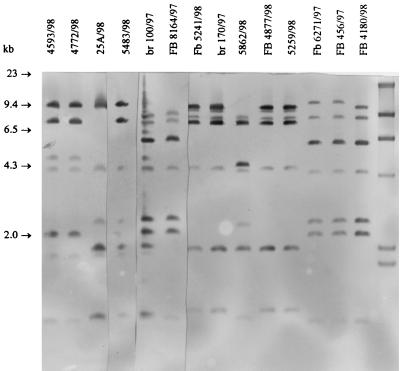
HaeIII ribotypes of the strains are shown in Fig. 4 and Table 1. Ribotypes of eight strains of PFGE/AFLP genotypes I/K/AF1, I/K/AF3, and I/K/AF5 were identical (ribotype A; Fig. 4, lanes 1 and 2), whereas two strains (5483 and 28A) had a slightly different ribotype (ribotype Aa, Fig. 4, lane 4). Also, the PFGE types (I/Ka and IKb) of these two strains were slightly different from the pattern I/K (Fig. 1 and 2, lanes 1, 3, and 5). Two strains with PFGE genotypes I/Kc and AFLP genotypes AF1 and AF4 were of ribotype B (Fig. 4, lane 3). All strains of PFGE type VI and AFLP type AF7 had the identical ribotype D (Fig. 4, lanes 7, 8, 10, and 11; Table 1). The ribotype of the strain 5862 (PFGE/AFLP genotype VII/AF6) was E (Fig. 4, lane 9; Table 1). All three strains of PFGE/AFLP genotype IV/AF10 had highly similar ribotypes C and Ca (Fig. 4, lanes 5 and 6). Three strains of PFGE/AFLP genotypes T101a/AF8 and T101b/AF9 had highly similar ribotypes F and Fa, respectively (Fig. 4, lanes 12, 13 and 14; Table 1).
FIG. 4.
HaeIII ribopattern types of C. jejuni strains selected for studies. Lane 1, strain 4593, type A; lane 2, strain 4772, type A; lane 3, strain 25A, type B; lane 4, strain 5483, type Aa; lane 5, strain BR100, type C; lane 6, strain FB8164, type Ca; lane 7, strain FB5241, type D; lane 8, strain BR170, type D; lane 9, strain 5862, type E; lane 10, strain FB4877, type D; lane 11, strain 5259, type D; lane 12, FB6271, type F; lane 13, strain FB456, type F; lane 14, strain FB4180, type Fa; lane 15, molecular size marker (2.0, 2.3, 4.3, 6.5, 9.4, and 23 kb).
Combined genotypes.
Data from PFGE, AFLP, and ribotypes were combined and designated as combined genotypes, G1, G2, etc. (Table 1). A total of 13 combined genotypes were identified.
Serotypes.
Seven serotypes were identified among the strains studied, and eight strains remained untypeable (Table 1, NS). Heat-stable serotype 1,44 was identified among five different combined genotypes: G1, G5, G10, G11, and G13. Serotype 4 was identified among the combined genotypes G10, G11, and G12. Serotype 12 was associated with the G1 genotype, and two PFGE genotype I/Kc strains were of serotype 57. The strains with related patterns of combined genotype of G7 and G8 had the same serotype 27.
DISCUSSION
The results of comparative analysis of PFGE and AFLP patterns of C. jejuni showed that both methods produced congruent results in most cases, thus having similar levels of sensitivity. In one group, AFLP subdivided PFGE type I/K strains into three subclusters (AF1, AF3, and AF5). In the group PFGE VI, however, PFGE analysis was more discriminatory than AFLP because PFGE subdivided the strains into three subtypes and AFLP analysis showed a high relatedness of the patterns. An explanation for the high discriminatory power of AFLP is the large number of fragments used in the analysis. Ribotype analysis was shown to have a level of discriminatory power similar to that of the other genetic methods used. Other ribotyping studies have revealed that ribotyping was less discriminatory than PFGE (4, 9) or AFLP (4). In these studies a highly diverse collection of C. jejuni strains was used, whereas in the present study the strains represented a restricted set of PFGE genotypes, which may explain the difference in discrimination by ribotyping.
The C. jejuni strains were systematically collected after human infections that were domestically acquired in two geographic areas and from chicken samples between 1995 to 1998 in Finland (14). We determined the genotype diversity among these C. jejuni strains, which PFGE genotypes were commonly found each year, and how persistent the genotypes were during the study period. On the basis of these data, representatives of five common PFGE genotypes found in 1997 and 1998 were chosen for AFLP analysis, ribotyping, and serotyping. The present extensive genetic analysis revealed that the five chosen genotypes differed from each other by all of the genotyping methods used, and in most cases the majority of strains within one PFGE genotype shared fragments in the AFLP and HaeIII ribotype patterns. This indicated that PFGE genotype groups I/K, IV, VI, and T101 represent genetic lineages among highly diverse genotypes of C. jejuni isolated during a period of 1 year and that these genotypes seemed to persist from 1 year to another. The strain 5862 of PFGE genotype VII was related to PFGE genotype VI but was shown by polyphasic genotype analysis to be only distantly related to genotype VI. Polyphasic genetic analysis of predominant genotypes is recommended because this approach gives information on the relatedness of assigned genotypes and on the homogeneity within a genotype and helps to choose the most applicable genotyping method(s) for future monitoring studies.
Heat-stable serotyping revealed that identical serotypes were distributed among different genotypes and on the opposite several serotypes were identified within one genotype, as has been noted earlier (23, 26). Extensive serotyping data on Finnish strains is not available, but heat-stable serotypes 1, 4, and 6 complexes have been predominant in England (7, 23), Denmark (21), and the United States (25). In the present study serotypes 1,44 and 4 were distributed among most of the selected common Finnish genotypes. Penner serotype 12 consisted only of combined genotype G1, which suggests that this serotype belongs to a stable genotype, similar to that seen for the heat-labile serotypes 4 and 7 (17) and the heat-stable serotype 55 (12). When a more extensive international database for C. jejuni genotypes and serotypes becomes available, the comparison of typing data from different countries will be possible and information on common genotypes and serotypes occurring in different countries will be provided.
Population genetic analysis using multilocus enzyme electrophoresis has suggested a heterogenic stucture for C. jejuni (2). Certain strains with shared genotypes and phenotypes, however, may become locally predominant and form temporary clonal groupings, probably due to specific characteristics that are advantageous for their colonization in animals or for their environmental transmission and pathogenicity for humans. C. jejuni has been shown to be naturally transformable (31). For the flagellin locus recombination by intra- and interstrain transfer of DNA has been described (15). Recent analysis of the whole genome sequence of the C. jejuni strain NCTC 11168 has revealed that the strain has 23 hypervariable homopolymeric tracts within the chromosomal DNA. These sequences can be sensitive to slipped-strand mispairing during genome replication of C. jejuni (24). Slipped-strand mispairing, as well as recombination or large-scale genomic rearrangements (plasticity), may be useful in the adaptation of the organism for colonization and survival in the gut of a variety of hosts. Slightly changed fragment patterns in the PFGE and AFLP genotypes with otherwise highly related patterns may result from single nucleotide changes in the restriction site or from large-scale genome rearrangements. These mechanisms may contribute to the observed small variation in the number and size of fragments, as was noted in all selected genotypes with otherwise-similar PFGE or AFLP patterns. This minor genomic variability, however, may lead to overestimation of genetic diversity, as recently shown for Helicobacter pylori with in silico comparison of PFGE patterns of two H. pylori strains with known whole genome sequences. Minor sequence variation was mainly caused by silent nucleotide variation in genes which accounted for the most verified differences in the PFGE patterns of two H. pylori strains J199 and 26995 (1). We have shown earlier that at least certain C. jejuni strains may change their genotypes after experimental infections in chickens (12) and Wassenaar et al. (32) noted genomic changes in a set of highly related strains from a batch of meat. The present selection of strains may represent natural variation occurring in a genetic lineage after isolation from various hosts.
In conclusion, our study on selected C. jejuni strains isolated during the same time period from humans and chickens indicates that five predominant Finnish genotypes shared PFGE, AFLP, and ribotypes and formed genetic lineages which seemed to persist for 1 year. PFGE and AFLP analyses were shown to have a high level of discriminatory power, although in some cases AFLP was able to further distinguish strains with identical PFGE patterns. In one case AFLP patterns of the strains were highly similar, but PFGE patterns showed differences. Ribotyping allotted the strains into the same genotyping groups as PFGE and AFLP. Identical serotypes were distributed among different genotypes, suggesting that serotyping alone cannot be used for strain identification. In epidemiological studies combined serotyping and genotyping could provide the most relevant data for the identification of strains.
ACKNOWLEDGMENTS
We thank Urszula Hirvi and Alan Rigter for technical assistance.
REFERENCES
- 1.Alm R A, Ling L-S L, Moir D, King D T, et al. Genomic sequence comparison of two unrelated isolates of the gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 2.Aschenbacher M, Piffaretti J-C. Population genetics of human and animal enteric Campylobacter strains. Infect Immun. 1989;57:1432–1437. doi: 10.1128/iai.57.5.1432-1437.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berndtson E, Emanuelsson U, Danielsson-Tham M-L, Engvall A. One year epidemiological study of campylobacters in eighteen Swedish chicken farms. Prevent Vet Med. 1996;26:167–185. [Google Scholar]
- 4.de Boer P, Duim B, Rigter A, van der Plas J, Jacobs-Reitsma W F, Wagenaar J A. Computer-assisted analysis and epidemiological value of genotyping methods for Campylobacter jejuni and Campylobacter coli. J Clin Microbiol. 2000;38:1940–1946. doi: 10.1128/jcm.38.5.1940-1946.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duim B, Wassenaar T, Rigter A, Wagenaar J. High-resolution genotyping of Campylobacter strains isolated from poultry and humans with amplified fragment length polymorphism fingerprinting. Appl Environ Microbiol. 1999;65:2369–2375. doi: 10.1128/aem.65.6.2369-2375.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald C, Owen R J, Stanley J. Comprehensive ribotyping scheme for heat-stable serotypes of Campylobacter jejuni. J Clin Microbiol. 1996;34:265–269. doi: 10.1128/jcm.34.2.265-269.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frost J A, Oza A N, Thwaites R T, Rowe B. Serotyping scheme for Campylobacter jejuni and Campylobacter coli based on direct agglutination of heat-stable antigens. J Clin Microbiol. 1998;36:335–339. doi: 10.1128/jcm.36.2.335-339.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson J R, Sutherland K, Owen R J. Inhibition of DNase activity in PFGE analysis of Campylobacter jejuni. Lett Appl Microbiol. 1994;19:357–358. doi: 10.1111/j.1472-765x.1994.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 9.Gibson J R, Fitzgerald C, Owen R J. Comparison of PFGE, ribotyping and phage-typing in the epidemiological analysis of Campylobacter jejuni serotype H2 infections. Epidemiol Infect. 1995;11:215–225. doi: 10.1017/s0950268800058349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson J, Lorenz E, Owen R J. Lineages within Campylobacter jejuni defined by numerical analysis of pulsed-field gel electrophoretic DNA profiles. J Med Microbiol. 1997;46:157–163. doi: 10.1099/00222615-46-2-157. [DOI] [PubMed] [Google Scholar]
- 11.Hänninen M-L, Pajarre S, Klossner M-L, Rautelin H. Typing of Campylobacter jejuni isolates in Finland by pulsed-field gel electrophoresis. J Clin Microbiol. 1998;36:1787–1789. doi: 10.1128/jcm.36.6.1787-1789.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hänninen M-L, Hakkinen M, Rautelin H. Genomic stability of related macrorestriction patterns of Campylobacter jejuni studied by pulsed-field gel electrophoresis. Appl Environ Microbiol. 1999;65:2272–2275. doi: 10.1128/aem.65.5.2272-2275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hänninen M-L, Salmi S, Mattila L, Taipalinen R, Siitonen A. Association of Aeromonas spp. with traveller's diarrhoea in Finland. J Med Microbiol. 1995;42:26–31. doi: 10.1099/00222615-42-1-26. [DOI] [PubMed] [Google Scholar]
- 14.Hänninen M-L, Perko-Mäkelä P, Pitkala A, Rautelin H. A three-year study of Campylobacter jejuni genotypes in humans with domestically acquired infections and in chicken samples from the Helsinki area. J Clin Microbiol. 2000;38:1998–2000. doi: 10.1128/jcm.38.5.1998-2000.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrington C S, Carter F M, Carter P E. Evidence for recombination in the flagellin locus of Campylobacter jejuni. Implications for the flagellin gene typing scheme. J Clin Microbiol. 1997;35:2386–2392. doi: 10.1128/jcm.35.9.2386-2392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrington C S, Thomson-Carter F M, Carter P E. Molecular epidemiological investigation of an outbreak of Campylobacter jejuni identifies a dominant clonal line within Scottish serotype HS55 populations. Epidemiol Infect. 1999;122:367–375. doi: 10.1017/s0950268899002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson C J, Fox A F, Jones D M, Wareing D R A, Hutchinson D N. Association between heat-stable (O) and heat-labile (HL) serogroup antigens of Campylobacter jejuni: evidence for interstrain relationships within three O/HL serovars. J Clin Microbiol. 1998;36:2223–2228. doi: 10.1128/jcm.36.8.2223-2228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs-Reitsma W F, van de Giessen A W, Bolder N M, Mulder R W A W. Epidemiology of Campylobacter spp. at two Dutch broiler farms. Epidemiol Infect. 1995;114:413–421. doi: 10.1017/s0950268800052122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapperud G, Skjerve E, Bean N H, Ostroff S M, Lassen J. Risk factors for sporadic Campylobacter infections: results of a case-control study in southern Norway. J Clin Microbiol. 1992;30:3117–3121. doi: 10.1128/jcm.30.12.3117-3121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maslow J N, Slutsky A M, Arbeit R D. Application of pulsed-field gel electrophoresis to molecular epidemiology. In: Pershing D H, Smith T F, White T J, editors. Diagnostic molecular microbiology: principles and applications. Washington, D.C.: American Society for Microbiology; 1993. pp. 563–572. [Google Scholar]
- 21.Moller Nielsen E, Engberg J, Madsen M. Distribution of serotypes of Campylobacter jejuni and C. coli from Danish patients, poultry, cattle and swine. FEMS Immunol Med Microbiol. 1997;19:47–56. doi: 10.1111/j.1574-695X.1997.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 22.Nachamkin I. Campylobacter jejuni. In: Doyle M P, Beuchat L R, Montville T J, editors. Food microbiology: fundamentals and frontiers. Washington, D.C.: American Society for Microbiology; 1997. pp. 159–170. [Google Scholar]
- 23.Owen R J, Slater E, Telford D, Donovan T, Barnham M. Subtypes of Campylobacter jejuni from sporadic cases of diarrhoeal disease at different locations in England are highly diverse. Eur J Epidemiol. 1997;13:837–840. doi: 10.1023/a:1007497005152. [DOI] [PubMed] [Google Scholar]
- 24.Parkhill J, Wren B W, Mungall K, Ketley J M, Churcher C, Basham D, Chillingworth T, Davies R M, Feltwell T, Holroyd S, et al. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 25.Patton C M, Wachsmuth I K, Evins G M, Keilbauch J A, Plikaytis B D, Troup N, Tomkins L, Lior H. Evaluation of ten methods to distinguish epidemic-associated Campylobacter strains. J Clin Microbiol. 1991;29:680–688. doi: 10.1128/jcm.29.4.680-688.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rautelin H, Hänninen M-L. Commercial test for serotyping heat-stable antigens of Campylobacter jejuni as compared with genotyping with pulsed-field gel electrophoresis. J Med Microbiol. 1999;48:617–621. doi: 10.1099/00222615-48-7-617. [DOI] [PubMed] [Google Scholar]
- 27.Skirrow M B. Diseases due to Campylobacter, Helicobacter and related bacteria. J Comp Pathol. 1994;111:113–149. doi: 10.1016/s0021-9975(05)80046-5. [DOI] [PubMed] [Google Scholar]
- 28.Stanley K N, Wallace J S, Currie J E, Diggle P J, Jones K. The seasonal variation of thermophilic campylobacters in beef cattle, dairy cattle and calves. J Appl Microbiol. 1998;85:472–480. doi: 10.1046/j.1365-2672.1998.853511.x. [DOI] [PubMed] [Google Scholar]
- 29.Tauxe R V. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. In: Nachamkin I, Blaser M J, Tomkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C.: American Society for Microbiology; 1992. pp. 9–19. [Google Scholar]
- 30.Vauterin L A, Vauterin P. Computer-aided objective comparison of electrophoresis patterns for grouping and identification of microorganisms. Eur Microbiol. 1992;1:37–41. [Google Scholar]
- 31.Wang Y, Taylor D E. Natural transformation in Campylobacter species. J Bacteriol. 1990;172:949–945. doi: 10.1128/jb.172.2.949-955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wassenaar T M, Geilhausen B, Newell D G. Evidence of genomic instability in Campylobacter jejuni isolated from poultry. Appl Environ Microbiol. 1998;65:1816–1821. doi: 10.1128/aem.64.5.1816-1821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuki N, Miyatake T. Guillain-Barre syndrome and Miller-Fisher's syndrome following Campylobacter jejuni infection. Ann N Y Acad Sci. 1998;845:330–340. doi: 10.1111/j.1749-6632.1998.tb09685.x. [DOI] [PubMed] [Google Scholar]