Abstract
Chondrosarcoma is the second most common primary malignant bone tumor. In this multicenter study, we sought to evaluate the disease‐specific survival (DSS) and disease‐free survival (DFS), and prognostic factors in patients with dedifferentiated chondrosarcoma (DDCS) or grade 3 chondrosarcoma (G3CS) in Japan. We retrospectively investigated the treatment outcomes and prognostic factors in 62 patients with DDCS and 19 patients with G3CS at 15 institutions participating in the Japanese Musculoskeletal Oncology Group. We also clarified significant clinicopathological factors for oncological outcomes. In surgery for primary lesions aimed at cure, a histologically negative margin (R0) was obtained in 93% (14/15) of patients with G3CS and 100% (49/49) of patients with DDCS. The 5‐year DSS was 18.5% in patients with DDCS and 41.7% in patients with G3CS (p = 0.13). Local control was obtained in 80% (12/15) and 79.6% (39/49) of patients with G3CS and DDCS in the primary lesion after surgery with a wide surgical margin, respectively. In multivariate analysis, stage and no treatment/palliative treatment for the primary lesion were independent prognostic factors for DSS of DDCS, and age and no treatment/palliative treatment for DSS of G3CS. The 5‐year DFS rate was 22.8% in 26 patients with DDCS who did not receive adjuvant chemotherapy, and 21.4% in 14 patients who received adjuvant chemotherapy. The prognosis of DDCS remains poor, although R0 resection was carried out in most cases. Effective and/or intensive chemotherapeutic regimens or agents should be considered or developed for patients with high‐grade chondrosarcoma, particularly for those with DDCS.
Keywords: dedifferentiated chondrosarcoma, grade 3 chondrosarcoma, multicenter study, prognosis, R0 resection
This study clarified the clinical outcomes of dedifferentiated chondrosarcoma (DDCS) and grade 3 chondrosarcoma (G3CS) by a nationwide multicenter study in Japan, which is the largest research report from Asian countries. Although all cases (100%) of DDCS and 93% of G3CS underwent surgery with a negative margin (R0), the 5‐year disease‐specific survival was 18.5% in DDCS and 41.7% in G3CS patients.
Abbreviations
- AWD
alive with disease
- CDDP
cisplatin
- CI
confidence interval
- CIR
carbon iron radiotherapy
- DDCS
dedifferentiated chondrosarcoma
- DFS
disease‐free survival
- DOD
died of disease
- DSS
disease‐specific survival
- DXR
doxorubicin
- G3CS
grade 3 chondrosarcoma
- HR
hazard ratio
- IFM
ifosfamide
- JMOG
Japanese Musculoskeletal Oncology Group
- MFH
malignant fibrous histiocytoma
- NED
no evidence of disease
- OAS
overall survival
- OS
osteosarcoma
- RFA
radiofrequency ablation
- RT
radiotherapy
- SCR
surgical complete remission
- UPS
undifferentiated pleomorphic sarcoma
1. INTRODUCTION
Chondrosarcoma is histologically classified as grade 1–3 according to the degree of malignancy, in addition, there are dedifferentiated and mesenchymal subtypes. Grade 3 chondrosarcoma is highly cellular with a mucomyxoid matrix and mitoses. Grade 3 chondrosarcoma tumors are mainly observed in adults, and most involve the pelvis, followed by the femur and humerus, and have a poor prognosis. 1 Grade 3 chondrosarcoma accounts for 3%–25% of central chondrosarcomas. 1 , 2 , 3 , 4 , 5 , 6 , 7
Dedifferentiated chondrosarcoma is a highly malignant variant of chondrosarcoma characterized by high‐grade nonchondrosarcoma, such as fibrosarcoma, OS, or undifferentiated pleomorphic sarcoma, immediately adjacent to a low‐grade chondroid neoplasm. 8 The incidence of DDCS in all chondrosarcomas is low (1.4%) 9 and represents 10%–15% of patients with central chondrosarcoma. 10 , 11 The prognosis of DDCS is poor, with early distant metastasis and 5‐year OAS rates of 6%–24%. 10 , 11 , 12 , 13 , 14 , 15 , 16
The mainstay of treatment for chondrosarcoma has been tumor resection with a wide surgical margin. However, in a significant number of cases, the disease affects the pelvis and trunk, making complete resection difficult. Moreover, invasive treatment is often impossible because a high proportion of affected patients are elderly. 15 , 17 Factors that adversely affect the prognosis include distant metastasis at the time of diagnosis (M1), trunk location, pathological fracture, positive surgical margin, age 60 years or older, histological subtype, and occupancy rate of dedifferentiated components. 15 , 17 Several reports have suggested that adjuvant chemotherapy improves the prognosis of DDCS, 10 , 14 , 17 but this remains to be verified.
It is thought that optimal treatment can be achieved by setting a standard for a safe surgical margin in surgery for bone and soft tissue sarcomas. Criteria for surgical margin that modify the concept of Enneking’s wide margin 18 have been established in Japan. 19 Specialty centers in Japan undertake resection of bone and soft tissue tumors nationwide according to these criteria.
Dedifferentiated chondrosarcoma and G3CS are rare, with an incidence of 0.44 and 0.4 per million, respectively, as indicated by a national cohort study 1 ; therefore, nationwide multicenter joint research is necessary to obtain clinically meaningful information. A multicenter joint research organization for bone and soft tissue sarcoma has been established in Japan, named the JMOG. In the current study, we collected clinicopathological and prognostic information of patients with DDCS and G3CS from participating specialized facilities of the JMOG. We aimed to determine: (i) the DSS and DFS in patients with DDCS and G3CS, and (ii) the prognostic factors in patients with DDCS and G3CS. The aim of this research was to deepen our understanding of this disease and thereby obtain information to improve the oncological outcomes of these patients.
2. PATIENTS AND METHODS
2.1. Study subjects
This multicenter joint research was approved by the JMOG Committee and the JMOG General Assembly. This study was also approved by the Institutional Review Board of Nagoya University (approval number: 2015‐0433). In this approval, the need for informed consent was waived due to the retrospective design of the study based on anonymous data. This research conformed to the provisions of the Declaration of Helsinki. Fifteen facilities in the JMOG participated in this study. The inclusion criteria were patients with primary bone tumors diagnosed with DDCS or G3CS and treated from 1990 to 2014. Anonymized patient information sheets were created to collect medical information for each facility. Details of 86 patients (DDCS, 66; G3CS, 20) were collected from 15 facilities. The exclusion criteria were patients who were only consulted at our facilities but not treated, and those with insufficient medical information. Patients who were followed up until death due to tumor, or patients with a follow‐up period of 3 months or more were included because short‐term survival in patients with G3CS and DDCS is considered meaningful due to the tumor aggressiveness. After excluding four patients with DDCS and one patient with G3CS, 81 patients (DDCS, 62; G3CS, 19) were subjected to the retrospective analyses for clinicopathological features and treatment outcomes, including patient information at the first visit, American Joint Committee on Cancer staging, 20 imaging features, histological subtype, treatment for primary lesion and recurrent lesions (distant metastasis/local recurrence), treatment of recurrent lesions, adjuvant chemotherapy/radiotherapy, and oncological outcomes.
2.2. Histological and radiological diagnosis
Histological diagnosis was determined by biopsy and excised specimens in resected cases and by biopsy tissue in biopsy‐only cases, including grading based on the WHO Classification of Soft Tissue and Bone Tumors. 21 Dedifferentiated chondrosarcoma was diagnosed by the presence of borderline to low‐grade chondrosarcoma, as well as the presence of nonchondromatous sarcoma in distinct areas, so‐called dedifferentiated components. The types of dedifferentiated components were pathologically divided into UPS‐like, OS‐like, fibrosarcoma‐like, and others. Chondrosarcoma was graded according to the areas demonstrating the highest grade. Grade 3 chondrosarcoma diagnosed as the highest grade was evaluated as grade 3. Diagnosis was determined histologically at each institute by experienced pathologists. Cases with previously resected chondrosarcoma that subsequently recurred as DDCS or G3CS were considered to have preceding lesions. The site of occurrence was divided into upper limbs (from the shoulder girdle to the hands), lower limbs (from the buttocks to the feet), chest wall (ribs and sternum), and trunk (pelvis and spine). The maximum diameter of the tumor was considered the tumor size, and the DDCS size was the diameter including the cartilaginous component and the high‐grade component. The follow‐up period was defined as extending from the date of pathological diagnosis to the date of final observation or death. Extension to soft tissue was defined as destroyed cortex coupled with evidence of tumor formation in the soft tissue. Pathological fractures were defined as conditions in which the presence of a tumor caused destruction of cortical bone and bone malalignment or crushing on imaging.
2.3. Treatment
At all participating facilities, the standard treatment strategy for DDCS and G3CS was wide/radical resection (amputation or disarticulation, hemipelvectomy). Even if the histological diagnosis was grade 1, wide resection with curative intent was carried out by comprehensively diagnosing it as a higher grade by adding image information. Carbon ion radiotherapy was considered for patients in whom wide resection was impossible. Local control with CIR was considered as SCR. Patients in whom it was difficult to perform CIR due to tumor size or location were treated with conventional radiotherapy (60 Gy) (considered as palliative radiotherapy) or other palliative treatment. Lesions that recurred from the resection area or resection margin were designated as local recurrence. Surgical margins were defined as microscopic negative (R0), microscopic positive (R1), or macroscopically positive (R2). Adjuvant chemotherapy was indicated at the discretion of the attending physician or institution, considering the patient’s performance status, age, and general condition. Local recurrence and distant metastases were regularly followed up by imaging at intervals of 3–6 months. Resection was considered for local recurrence considering the presence or absence of distant metastasis, and resection or RFA was considered for lung metastases when feasible. Palliative chemotherapy and palliative radiotherapy were performed for advanced stage tumors at the discretion of the attending physician, considering the type and number of metastatic organs, the patient’s general condition, and tumor pain.
2.4. Statistical analysis
We analyzed the association between various clinicopathological factors and the prognosis of DDCS and G3CS. The composition of the cohort is shown in Figure 1. We compared the prognosis of G3CS and DDCS in: (i) all patients (n = 81; DDCS 62, G3CS 19), (ii) patients with no distant metastasis at the time of diagnosis (M0, n = 59; DDCS 46, G3CS 13), and (iii) M0 patients with DDCS in whom the primary lesion was resected and SCR obtained.
FIGURE 1.
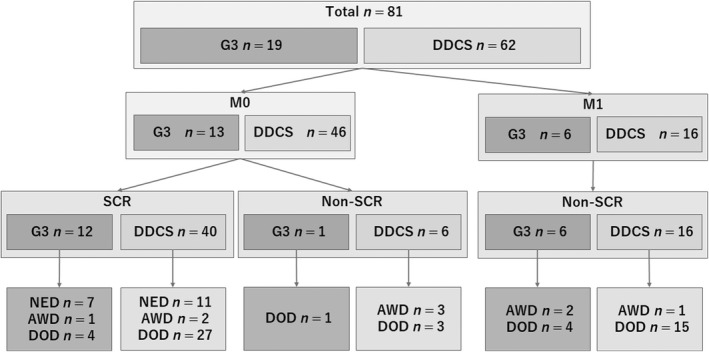
Cohort of the present study of Japanese patients with dedifferentiated chondrosarcoma (DDCS) or grade 3 chondrosarcoma (G3). AWD, alive with disease; DOD, died of disease; M0, no distant metastasis; M1, distant metastasis; NED, no evidence of disease; SCR, surgical complete remission
Fisher’s exact test was used to evaluate the differences between G3CS and DDCS groups for categorical variables of clinical characteristics. Survival probabilities over time were determined by the Kaplan–Meier method. The effect of each prognostic variable was analyzed using the log–rank test. The DSS was calculated from the date of pathological diagnosis until death or the last follow‐up visit; the DFS was defined as the period until the date of local or systemic disease recurrence. Multivariate analysis was undertaken using Cox’s proportional hazards method, with variables chosen using a forward conditional stepwise approach. Statistical analysis was undertaken using SPSS Statistics version 22.0 (SPSS Inc.). Confidence intervals of 95% were calculated for statistical parameters; p‐values of less than 0.05 were considered statistically significant.
3. RESULTS
3.1. Overall cohort analyzed in this study (n = 81)
The patient and disease characteristics of the 81 patients are shown in Table 1. Regarding the G3CS group, the mean follow‐up period for the 19 patients was 36 (range, 1–240 ) months and the mean age was 61 (range, 30–81) years. The site of occurrence in the upper limbs was the scapula in two patients, humerus in three patients, and forearm in one patient; in the lower limbs it was the femur in five patients and the tibia in one. In the chest wall, it was the ribs in two patients and the sternum in one; and in the trunk it was cervical spine in one patient and the iliac bones in three. Preceding lesions were present in three patients (two patients with recurrence after resection of G2CS and one patient with a preceding lesion of unknown grade). Distant metastases at the first visit were found in six patients. Two patients had pathological fractures, one each in the scapula and rib. Surgery with a wide surgical margin for the primary lesion was intended and performed in 15 patients, among whom 14 (93%) were evaluated as R0 and one as R2. Surgical treatment for the primary lesion was also performed in three patients with stage IV for palliative local control. No surgery was performed on the primary lesion in the remaining one patient. Radiotherapy was carried out in three patients.
TABLE 1.
Characteristics of Japanese patients with dedifferentiated chondrosarcoma (DDCS) or grade 3 chondrosarcoma (G3CS) (n = 81)
| Characteristic | G3CS (n = 19) (%) | DDCS (n = 62) (%) | p value | |
|---|---|---|---|---|
| Age, years | Mean (range) | 61 (30–81) | 65 (21–85) | 0.790 |
| <65 | 8 (42) | 30 (48) | 0.420 | |
| ≥65 | 11 (58) | 32 (52) | ||
| Sex | Male | 10 (53) | 37 (60) | 0.610 |
| Female | 9 (47) | 25 (40) | ||
| Location | Upper extremity | 6 (32) | 11 (18) | 0.420 |
| Lower extremity | 6 (32) | 30 (48) | ||
| Chest wall | 3 (16) | 6 (10) | ||
| Trunk | 4 (20) | 15 (24) | ||
| Size, cm | Mean (range) | 11 (4–32) | 10 (4–31) | 0.680 |
| ≤8 | 8 (42) | 10 (16) | 0.027 | |
| >8 | 10 (34) | 48 (77) | ||
| Unknown | 1 (5) | 4 (7) | ||
| Preceding lesion | Yes | 3 (16) | 6 (10) | 0.440 |
| No | 16 (84) | 55 (88) | ||
| Unknown | 0 (0) | 1 (2) | ||
| Pathological fracture | Yes | 2 (10) | 10 (16) | 0.720 |
| No | 15 (80) | 46 (74) | ||
| Unknown | 2 (10) | 6 (10) | ||
| Subtype (DDCS) | OS | NA | 10 (16) | NA |
| UPS | NA | 36 (58) | ||
| Fibrosarcoma | NA | 5 (8) | ||
| Other | NA | 11 (18) | ||
| AJCC stage | II | 12 (63) | 46 (74) | 0.160 |
| III | 1 (5) | 0 (0) | ||
| IV | 6 (32) | 16 (26) | ||
| Treatment for primary lesion | Surgery | 15 (80) | 49 (78) | 0.730 |
| CIR | 0 (0) | 3 (5) | ||
| Palliative a /no treatment | 3 a /1 (20) | 1 a /9 (17) | ||
| Surgical margin and other treatment | R0 | 14 (75) | 49 (78) | 0.230 |
| R1 | 0 (0) | 0 (0) | ||
| R2 | 1 (5) | 0 (0) | ||
| CIR | 0 (0) | 3 (5) | ||
| Palliative a /no treatment | 3 a /1 (20) | 1 a /9 (17) | ||
| Adjuvant (±neo) chemotherapy | Yes | 0 (0) | 14 (23) | 0.016 |
| No | 19 (100) | 48 (77) | ||
| Palliative radiotherapy | Yes | 3 (16) | 0 (0) | 0.001 |
| No | 16 (84) | 63 (100) | ||
| Local recurrence b | Yes | 3 (20) | 10 (20) | 0.640 |
| No | 12 (80) | 39 (80) | ||
| Outcome | NED | 7 (27) | 11 (18) | 0.130 |
| AWD | 3 (16) | 6 (10) | ||
| DOD | 9 (47) | 45 (72) |
Abbreviations: AJCC, American Joint Committee on Cancer; AWD, alive with disease; CIR, carbon ion radiotherapy; DOD, died of disease; NA, not available; NED, no evidence of disease; OS, osteosarcoma; Surgery, surgery with wide surgical margin; UPS, undifferentiated pleomorphic sarcoma.
Palliative treatment.
Excluding patients with palliative surgical treatment and no treatment.
Regarding DDCS, the mean follow‐up period was 28 (range, 1–245) months, and the mean age was 65 (range, 21–85) years. The development site in the upper limbs was humerus in nine patients, the finger in one, and the scapula in one; in the lower limbs, the development site was the femur in 27 patients and the tibia in three. In the chest wall, ribs in four, and the sternum in two; in the trunk, cervical spine in one, pelvis in 13, and unknown in one. Six patients with preceding lesions included four and one patient with recurrence of G2 and G1 chondrosarcomas, respectively, and one patient with unknown grade. Ten patients with pathological fractures were observed, the femur in five, the humerus in four, and the rib in one. The most common subtype of the dedifferentiated component was UPS‐like (36 [58%] patients). Definitive treatment for the primary tumor was undertaken in 52 patients, including surgery with a wide surgical margin in 49 patients and CIR in three. All 49 patients receiving surgical treatment were histologically evaluated as R0 (100%). Treatment for the primary lesion was not undertaken in 10 patients; of them, nine patients did not receive treatment, and palliative surgical fixation was carried out in one.
Comparing the clinicopathological factors between the G3CS and DDCS groups (Table 1), no difference in tumor size was noted between the two. However, the frequency of tumors larger than 8 cm was 34% in the G3CS group and 77% in the DDCS group (p = 0.027). Adjuvant chemotherapy was administered to 14 patients with DDCS but no patients with G3CS (p = 0.016). Patients with DDCS did not receive palliative radiotherapy (p = 0.001). No significant difference was found between the two with regard to any other factors.
The results of the relationship between various factors and DSS in patients with G3CS and DDCS by univariate analysis are shown in Table 2. The 5‐year DSS of patients with G3CS (41.7%) was better than that of those with DDCS (18.5%), although this difference was not significant (p = 0.13, Figure 2). By univariate analysis for G3CS, age, stage, and definitive treatment (surgery with wide surgical margin and CIR) for the primary lesion (p = 0.005, 0.013, and 0.011, respectively) were significant prognostic factors. In multivariate analysis (Table 3), patient age ≥65 years (p = 0.018, HR 18.33; 95% CI, 1.64–205.03) and no treatment/palliative treatment for the primary lesion (p = 0.023, HR 17.75; 95% CI, 1.49–211.57) were independent prognostic factors for patients with G3CS. Regarding patients with DDCS, univariate analysis indicated that stage (p = 0.001) and definitive treatment for the primary lesion (p = 0.006) were significant prognostic factors (Table 2, Figure 3A,B). When classified according to the subtype of the dedifferentiated component, the 5‐year DSS rate was 45% in OS‐like and 14.7% in UPS‐ and fibrosarcoma‐like (p = 0.044). The OS‐like component was a significant good prognostic factor (Table 2, Figure 3C). In the multivariate analysis, stage (p = 0.016, HR 1.71; 95% CI, 1.11–2.65) and no treatment/palliative treatment for the primary lesion (p = 0.043, HR 3.77; 95% CI, 1.04–13.64) were independent prognostic factors in patients with DDCS (Table 3).
TABLE 2.
Prognostic factors for disease‐specific survival in patients with grade 3 chondrosarcoma (G3CS) or dedifferentiated chondrosarcoma (DDCS) by univariate analysis
| G3CS | DDCS | ||||||
|---|---|---|---|---|---|---|---|
| n | 5 y (%) | p value | n | 5 y (%) | p value | ||
| 19 | 41.7 | 62 | 18.5 | ||||
| Age, years | <65 | 8 | 62.5 | 0.005 | 30 | 22.5 | 0.590 |
| ≥65 | 11 | 19.5 | 32 | 13.2 | |||
| Sex | Male | 10 | 37.5 | 0.700 | 37 | 42.2 | 0.230 |
| Female | 9 | 50.0 | 25 | 46.5 | |||
| Location | Upper extremity | 6 | 40.0 | 0.270 | 11 | 26.7 | 0.390 |
| Lower extremity | 6 | 41.7 | 30 | 12.3 | |||
| Chest wall | 3 | 0.0 | 6 | 53.3 | |||
| Axial | 4 | 50.0 | 15 | 13.3 | |||
| Stage | Ⅱ(+Ⅲ) | 13 | 57.1 | 0.013 | 46 | 25.5 | 0.001 |
| Ⅳ | 6 | 0.0 | 16 | 0.0 | |||
| Size, cm | ≤8 | 8 | 0.0 | 0.310 | 10 | 22.2 | 0.920 |
| >8 | 10 | 33.8 | 48 | 19.6 | |||
| Unknown | 1 | NA | 4 | NA | |||
| Preceding lesion | Yes | 16 | 28.8 | 0.054 | 55 | 18.7 | 0.910 |
| No | 3 | 0.0 | 6 | 20.0 | |||
| Unknown | 0 | NA | 1 | NA | |||
| Pathological fracture | Yes | 2 | 0.0 | 0.100 | 10 | 26.3 | 0.830 |
| No | 15 | 54.3 | 46 | 17.7 | |||
| Unknown | 2 | 0.5 | 6 | 0.0 | |||
| Subtype | |||||||
| (DDCS) | OS | NA | NA | 10 | 45.0 | 0.044 | |
| UPS + fibrosarcoma | NA | NA | 41 | 14.7 | |||
| Other | NA | NA | 11 | 0.0 | |||
| Treatment for primary lesion | Definitive treatment (surgery + CIR) | 15 | 52.7 | 0.011 | 52(3) | 21.2 | 0.006 |
| Palliative a /no treatment | 3 a /1 | 0.0 | 1 a /9 | 0.0 | |||
| Surgical margin | R0 | 14 | 52.7 | NA | 49 | 23.1 | NA |
| R2 | 1 | NA | 0 | NA | |||
| CIR | 0 | NA | 3 | NA | |||
| Palliative a /no treatment | 3 a /1 | NA | 1 a /9 | NA | |||
| Adjuvant (±neo) chemotherapy | Yes | 0 | NA | NA | 14 | 28.1 | 0. 260 |
| No | 19 | 41.7 | 48 | 14.0 | |||
| Palliative radiotherapy | Yes | 3 | 0.0 | 0.240 | 0 | NA | NA |
| No | 16 | 56.0 | 62 | 18.5 | |||
| Local recurrence b | Yes | 3 | NA | NA | 10 | 0.0 | 0.290 |
| No | 12 | 35.0 | 39 | 30.2 | |||
P (univariate analysis with log–rank test).
Abbreviations: CIR, carbon ion radiotherapy; NA, not available; OS, osteosarcoma; Surgery, surgery with wide surgical margin; UPS, undifferentiated pleomorphic sarcoma.
Palliative treatment.
Excluding patients with palliative surgical treatment and no treatment.
FIGURE 2.
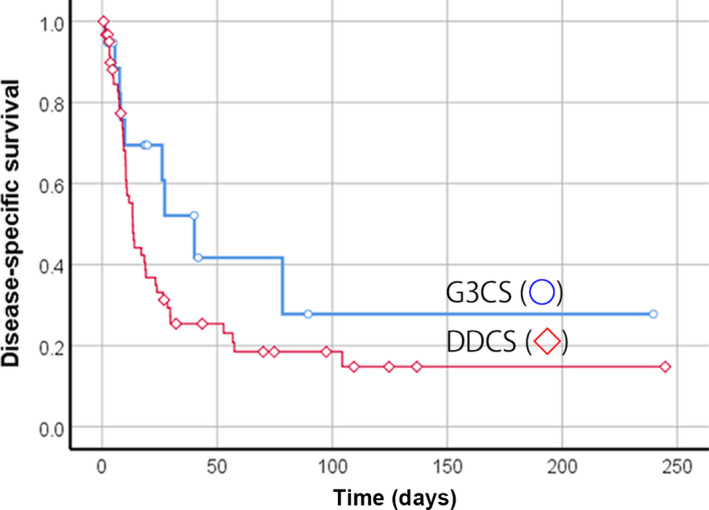
Disease‐specific survival of 81 Japanese patients with grade 3 chondrosarcoma (G3CS; n = 19) or dedifferentiated chondrosarcoma (DDCS; n = 62). Survival curves with Kaplan–Meier estimates were plotted. G3CS (○) and DDCS (◇) patients (p = 0.13)
TABLE 3.
Multivariate analysis for disease‐specific survival in Japanese patients with grade 3 chondrosarcoma (G3CS) (n = 19) or dedifferentiated chondrosarcoma (DDCS) (n = 62)
| Variable | G3CS | DDCS | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age <65 years | Ref. | – | Ref. | – |
| Age ≥65 years | 18.33 (1.64–205.03) | 0.018 | 1.18 (0.57–2.41) | 0.660 |
| Definitive treatment (surgery + CIR) | Ref. | – | Ref. | – |
| Palliative a /no treatment | 17.75 (1.49–211.57) | 0.023 | 3.77 (1.04–13.64) | 0.043 |
| Stage Ⅱ(+Ⅲ) | Ref. | – | Ref. | – |
| Stage Ⅳ | 1.58 (0.56–4.50) | 0.390 | 1.71 (1.11–2.65) | 0.016 |
| Osteosarcoma | NA | NA | Ref. | – |
| Fibrosarcoma + UPS | NA | 1.90 (0.71–5.11) | 0.200 | |
Abbreviations: –, not applicable; CI, confidence interval; CIR, carbon ion radiotherapy; HR, hazard ratio; NA, not available; Ref., reference; Surgery, surgery with wide surgical margin; UPS, undifferentiated pleomorphic sarcoma.
Palliative treatment.
FIGURE 3.
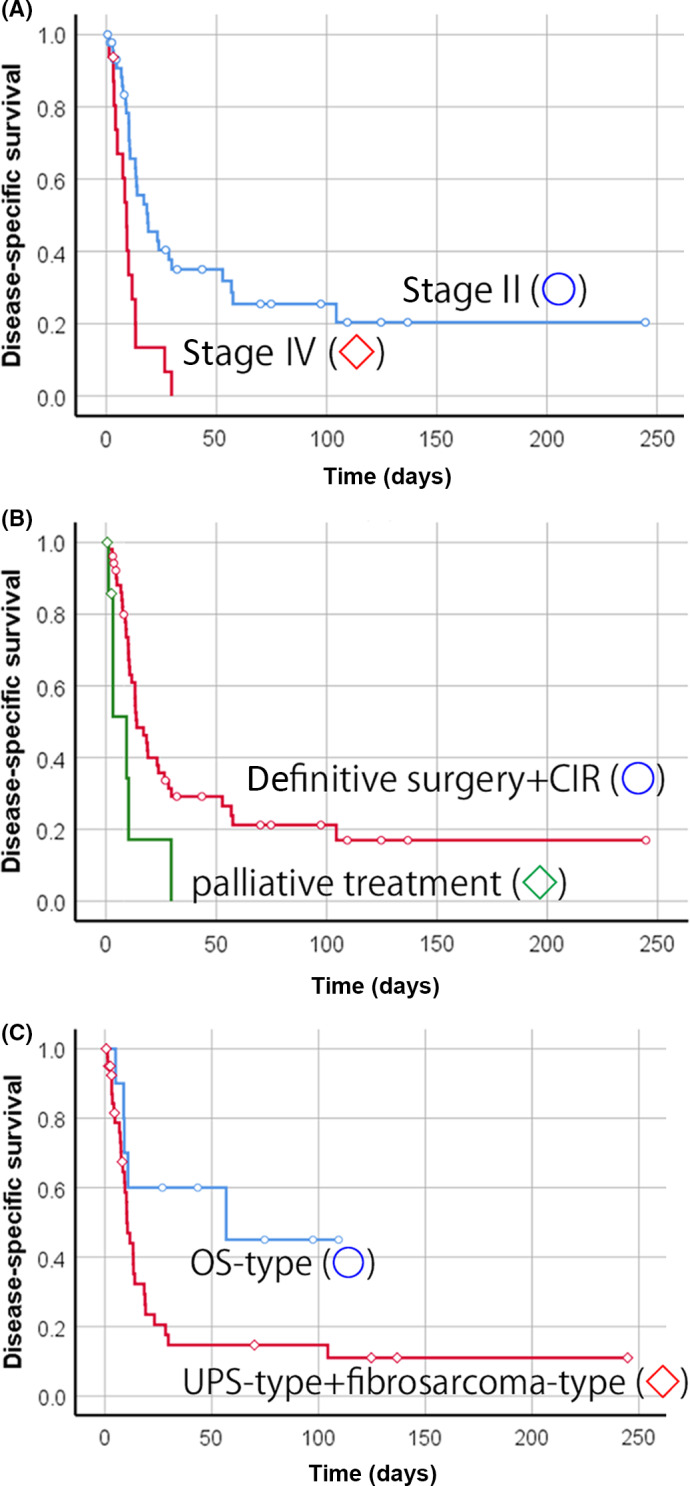
Disease‐specific survival of 62 Japanese patients with dedifferentiated chondrosarcoma. (A) Survival curves of patients with stage II (○) or stage IV (◇) disease (p = 0.001). (B) Survival curves of patients receiving treatment with curative intent or palliative treatment. Definitive treatment (surgery with wide surgical margin/carbon ion radiotherapy [CIR]) (○) and palliative treatment for primary lesion (◇) (p = 0.006). (C) Survival curves of patients with osteosarcoma (OS)‐type (○) or undifferentiated pleomorphic sarcoma (UPS)‐type + fibrosarcoma‐type (◇) dedifferentiated component (p = 0.044)
The results of the histological diagnosis at the time of biopsy and after excision are shown in Table 4. Of the final G3CS diagnoses, only three (16%) patients were diagnosed with G3 at the time of biopsy. Of the final DDCS diagnosis, only 17 (27%) patients were diagnosed with DDCS at the time of biopsy. For G3CS or DDCS patients diagnosed with G1 by biopsy, intensive analyses of imaging data suggested features of higher grade tumors and therefore they were treated adequately with a wide surgical margin.
TABLE 4.
Comparison of histological diagnosis of dedifferentiated chondrosarcoma (DDCS) and grade 3 chondrosarcoma (G3CS) at the time of biopsy and after excision
| Biopsy | Definitive diagnosis | |
|---|---|---|
| G3CS (n = 19), n (%) | DDCS (n = 62), n (%) | |
| DDCS | 0 (0) | 17 (27) |
| CS grade 1–2 | 3 (16) | 12 (20) |
| CS grade 3 | 3 (16) | 0 (0) |
| CS unknown | 8 (42) | 10 (16) |
| OS | 1 (5) | 4 (7) |
| UPS | 1 (5) | 9 (14) |
| Uncertain | 3 (16) | 10 (16) |
Abbreviations: CS, chondrosarcoma; OS, osteosarcoma; UPS, undifferentiated pleomorphic sarcoma.
3.2. Cohort with distant metastases during the first visit (M1, n = 22)
The treatment and outcomes in stage IV patients with G3CS and DDCS at the time of referral are shown in Table S1. Surgery with a wide surgical margin or palliative RT was carried out for the primary lesion in three and two patients, respectively, for those with M1 G3CS. The primary lesion of the remaining patient was not treated. There was no local recurrence of the primary lesion after surgery with a wide surgical margin. Chemotherapy was performed in four patients, RFA in one patient with lung metastasis, and conventional RT in one patient with bone metastasis. The oncological outcomes were DOD in four patients and AWD in two. Patients with G3CS with distant metastasis (M1, n = 6) had a 5‐year DSS of 0% and a 1‐year DSS of 27.8%.
In the 16 patients with DDCS with distant metastasis, surgery with a wide surgical margin for the primary lesion was undertaken in 10 patients, CIR was performed in two patients, and four patients were untreated (Table S1). After definitive treatment for primary tumors, local recurrence occurred in two patients (17%, one surgery, and one CIR). Regarding treatment for distant metastasis in patients with DDCS, two patients were irradiated and eight underwent chemotherapy. The oncological outcomes were DOD in 15 patients and AWD in one. The 5‐year DSS was 0%, and the 1‐year DSS was 26.8%, which was not significantly different from the corresponding values in patients with G3CS.
3.3. Cohort without metastases at diagnosis (M0, n = 59)
We next undertook subgroup analysis of M0 patients (Table S2). Of the 59 patients with M0, 13 had G3CS and 46 had DDCS. The primary lesion could not be treated in seven patients (G3CS, 1; DDCS, 6) with curative intent due to advanced age (four patients) and unresectable location (three patients), while the remaining 52 achieved SCR (G3CS, 12; DDCS, 40).
Regarding the patients with G3CS, in 12 with SCR, 11 were evaluated as R0 and one patient as R2. Neither adjuvant chemotherapy nor adjuvant RT was given to patients with M0 G3CS. Local recurrence occurred in three patients after treatment for the primary tumor; among them, amputation (or disarticulation) was carried out in two and repeat wide resection in one. As a result, local control was obtained in all three patients. Palliative RT was administered to one patient who did not undergo definitive treatment for the primary lesion. Progression of the primary lesion did not occur in this patient. Distant metastases after treatment were observed in six patients, three of whom underwent resection. One of the remaining three patients without metastasectomy received chemotherapy, one was irradiated, and one was not treated.
Regarding patients with DDCS with M0, treatment with curative intent for the primary lesion was carried out in 40 patients, surgery with wide surgical margin was performed in 39, and CIR was performed in one. Among patients with SCR with DDCS (n = 40), local recurrence occurred in 10 patients (25%), and repeat wide resection (or amputation) was carried out in four. The oncological outcome of all 10 local recurrence patients was DOD. Surgery with curative intent for the primary lesion was not undertaken in six (13%) of 46 patients because of difficulty with the procedure. New distant metastases occurred in 30 patients (65%), eight of whom received metastasectomy, and among them, four underwent adjuvant chemotherapy after metastasectomy. Seven of the 30 patients without metastasectomy received chemotherapy and 15 were untreated. The oncological outcome of the M0 DDCS patients was NED in 11 patients, AWD in five, and DOD in 30. The 5‐year DSS for patients with M0 G3CS was better (57.1%) than that of those with DDCS (25.5%), albeit with no significant difference (p = 0.11) (Figure S1).
The prognosis was also analyzed for M0 patients at initial referral who achieved SCR by surgery or CIR. The results of correlated factors with the prognosis of DDCS with SCR patients are shown in Table S3. The subtype of dedifferentiation with OS was better than the 5‐year DFS (p = 0.032). A comparison of the prognosis of patients with DDCS and G3CS with SCR indicated that the 5‐year DSS of 40 patients with DDCS who obtained SCR was 27.4%, which tended to be a worse outcome than that of the 12 patients with G3CS who obtained SCR, albeit without significance (62.3%; p = 0.081) (Figure S2A). The 5‐year DFS of 40 patients with DDCS who achieved SCR was 21.7%, which was not significantly different from that of the 12 patients with G3CS who achieved SCR (32.4%; p = 0.24) (Figure S2B). The results of a comparison of 5‐year DSS and DFS in the M0, M1, and M0 + SCR cohort of patients with G3CS and DDCS are presented in Table S4.
Regarding the effect of chemotherapy on the prognosis of patients with DDCS with M0 and SCR, the 5‐year DSS of the 26 patients not treated with adjuvant chemotherapy was 25.0%, which was not significantly different from that of the 14 patients who received adjuvant chemotherapy (28.6%; p = 0.79) (Figure S3A). The 5‐year DFS of those not treated with adjuvant chemotherapy was 22.8%, while that of the 14 patients who received adjuvant chemotherapy was 21.4% (p = 1.0) (Figure S3B). Of the 14 patients who received adjuvant chemotherapy after primary tumor treatment, all received DXR, 12 received an IFM and DXR‐based regimen, six received CDDP, and three received methotrexate (Table S5).
The 5‐year DSS and DFS of the OS‐like subtype were 50.0% and 55.6%, respectively, whereas those of the combined UPS‐ and fibrosarcoma‐like subtypes were 24.2% and 13%, respectively (p = 0.13 and p = 0.032, respectively).
4. DISCUSSION
This study is the largest retrospective study of DDCS and G3CS cases in the Asian population. Northamerican and European countries have reported that both DDCS and G3CS have poor prognoses, but only a few reports have compared the two (Table 5). One report indicated that the mortality rate does not differ significantly between patients with G3CS and DDCS, although the mean time to death did differ significantly between them. 2 Tsuda et al. 22 compared G3CS versus G2CS and DDCS versus G2CS for sarcoma‐specific survival and reported that the HR was higher in DDCS, suggesting a worse prognosis. Thorkildsen et al. used data from the National Cancer Registry in Norway to analyze chondrosarcoma. 1 For 5‐year DSS, DDCS had a worse prognosis than G3CS, but no direct comparative statistical analysis was carried out. In this study, the proportion of distant metastases at the time of diagnosis of DDCS was 26%, which was approximately the same as the 21%–36% noted in previous reports, 1 , 16 , 23 and 32% in high‐grade G3CS, which was not reported in previous reports. There was no significant difference in the prognosis between G3CS and DDCS in this study. However, in the analysis of all (M0 + M1), M0, and SCR cohorts, patients with DDCS had a relatively poor outcome (p = 0.13, p = 0.11, and p = 0.081, respectively). It seems that this difference will become even more significant when increased numbers of patients are analyzed.
TABLE 5.
Comparison with previous reports of patients with grade 3 chondrosarcoma (G3CS) or dedifferentiated chondrosarcoma (DDCS)
| First author | Year | n | M1 (%) | Age (years) | OAS | p value | |
|---|---|---|---|---|---|---|---|
| Lee FY 2 | 1999 | G3CS | 18 | NR | NR | 32 ± 22.8 months | <0.001 |
| DDCS | 20 | NR | NR | 5 ± 3.7 months | |||
| Thorkildsen J 1 | 2019 | G3CS a | 21 | NR | NR | 66% (DSS) | NR |
| G3CS b | 28 | NR | NR | 51% (DSS) | NR | ||
| DDCS | 39 | 14 (36) | 63 | 17% (DSS) | NR | ||
| Tsuda Y 22 | 2020 | G3CS | 78 | NR | NR | G2CS vs. G3CS HR 2.35 (CISSD) | <0.001 |
| DDCS | 48 | NR | NR |
G2CS vs. DDCS HR 5.77 (CISSD) |
<0.001 | ||
| This study | G3CS | 19 | 6 (32) | 61 | 41.7% (DSS) | 0.130 | |
| DDCS | 62 | 16 (26) | 65 | 18.5% (DSS) | |||
Abbreviations: CISSD, cumulative incidence sarcoma‐specific death; DSS, 5‐year disease‐specific survival; G2CS, grade 2 chondrosarcoma; HR, hazard ratio; M1, distant metastasis; NR, not reported; OAS, overall survival.
Extremity.
Axial.
Thus, G3CS seems to have a better prognosis than DDCS. However, G3CS has a poorer prognosis than G1CS and G2CS. 7 , 24 , 25 The 5‐year DSS of G3CS in this study was 41.7%, which is roughly equivalent to the 31%–66% reported by others. 1 , 7 , 24 , 25 Few reports have analyzed the prognostic factors for G3CS independently. In this study, age and SCR were factors significantly related to prognosis. Previous studies analyzing chondrosarcoma, including G1–G3, reported that sex, 9 age, 26 surgical margin, 2 , 24 the presence of pathological fractures, 27 and primary or secondary peripheral tumors were associated with prognosis. 28 , 29 As we found in this study, G3CS is rarer than DDCS, making it even more difficult to extract significant prognostic factors. Adjuvant chemotherapy was given to 14 patients with DDCS but not to any patients with G3CS. Adjuvant chemotherapy for G3CS is generally considered ineffective, although it is expected that chemotherapy, including molecular targeted therapy, based on gene mutation profiling of G3CS, will be developed in the future.
Regarding the treatment results of DDCS, Table 6 summarizes reports of more than 50 patients with DDCS published within the past 20 years. The 5‐year OAS was reported to be 18%–39%, 11 , 16 , 23 , 30 , 31 which is similar to the results of this study (DSS, 18.5%). Factors reported to promote a poor outcome of DDCS include pelvic development, 14 , 16 , 23 , 32 positive margin, old age, 16 , 33 tumor size, 23 , 30 pathological fracture, 16 , 23 extraosseous extension, 1 , 23 tissue type of dedifferentiated component, 11 , 23 and proportion of dedifferentiated tissue component. 11 In this study, stage IV and inability to undergo treatment with curative intent for the primary lesion (including three patients with CIR) were significant adverse prognostic factors. Of the 49 patients who underwent surgery on the primary lesion, all had a wide surgical margin in this study; thus, if a wide surgical margin is achieved in cases with pathological fracture, a favorable outcome could be obtained. This might explain why pathological fractures and surgical margins were not extracted as prognostic factors in this study. In other words, stage and unresectability remained prognostic factors for patients with G3CS or DDCS who received surgery with a wide surgical margin.
TABLE 6.
Comparison with previous reports of patients with dedifferentiated chondrosarcoma
| First author, year | n | M1 (%) | Age (years) | Extremity (%) | OAS (%) | Factors |
|---|---|---|---|---|---|---|
| Staals EL, 2006 11 | 123 | 27 (24.3) | 59.2 | 87 (70.7) | 24 | M1, MFH, dedifferentiated component % |
| Grimer RJ, 2007 16 | 337 | 71 (21) | 59 | 167 (50.1) | 24 | Fx, pelvic, age, margins |
| Strotman PK, 2017 30 | 159 | Stage III/IV (22) | 65.2 | 118 (74) | 18 |
Size (>8 cm), M1, surgical resection chest wall tumor (positive factor) |
| Miao R, 2019 23 | 72 | 23 (31.9) | 60.5 | 47 (65) | 19.2 | M1, surgical resection, size, Fx, LN involvement, extraosseous extension, UPS, Cht |
| Hompland I, 2021 31 | 57 | 23 (40) | 52 | 37 (65) | 39 | Surgical complete remission |
| This study | 62 | 16 (26) | 65 | 41 (66) | 18.5 (DSS) | Stage, curative treatment for primary lesions |
All studies were published after 2000, with N > 50.
Abbreviations: Cht, chemotherapy; DSS, 5‐year disease‐specific survival; Fx, fracture; LN, lymph node; M1, distant metastasis at first visit; MFH, malignant fibrous histiocytoma; OAS, 5‐year overall survival; UPS, undifferentiated pleomorphic sarcoma.
Pathological fractures in DDCS occurred in 16% of patients in this study, with previous reports of 13%–44.4%. 23 , 28 , 32 Pathological fracture has been reported as a poor prognostic factor because of the difficulty in achieving a wide resection due to bleeding from the fracture and dissemination of the tumor. 16 , 23 A strict resection margin including the seeded area settings is necessary to ensure wide resection. Pathological fractures of DDCS occurred only in locations where a wide resection was likely to be carried out (femur, 5; humerus, 4; and rib, 1) in this study. Although only four patients required amputation in this study, the 5‐year DSS was even better in patients with pathological fracture, suggesting that good preoperative planning for wide resection reduces the adverse influence of pathological fractures on prognosis.
Regarding the histological type of dedifferentiated component, the UPS component tended to have a poor prognosis in this study, but there was no significant difference in the multivariate analysis. Staals et al. 11 and Miao et al. 23 reported the histology of MFH/UPS to be a poor prognostic factor, and Mercuri et al. 34 reported that the MFH‐like pattern of the noncartilaginous component correlates with the rate of lung metastasis. In these reports, the MFH/UPS type accounted for 7.3%, 11 36.1%, 34 and 51.0%, 23 respectively, which is different from that in our study (58.0%). Although other studies have reported no significant difference in prognosis between subtypes of dedifferentiated components, 16 , 35 patients with UPS components might require novel and active intervention. However, the OS‐like subtype with dedifferentiated components had a 5‐year DSS of 45% in this study, which is similar to the prognosis of elderly high‐grade OS (5‐year OAS, 42.8%). 36
No effectiveness of adjuvant chemotherapy on DDCS was shown in this study, and previous reports did not conclude whether chemotherapy improves the outcome. Several studies have been unable to document any beneficial effects of adjuvant chemotherapy. 15 , 16 , 33 , 37 In contrast, relatively older studies revealed that the prognosis improves when surgery and chemotherapy are combined. 10 , 14 Regarding the impact of different drugs, a regimen including IFM improved the prognosis, with a 5‐year OAS of 32%. 17 Monotherapy with DXR had a significantly better PFS (p = 0.042) in 34 unresectable cases. 38 The combination of DXR and CDDP was significantly associated with prolonged PFS. 23 Our study was carried out in multiple centers and the diversity of regimens and agents used could explain why no difference was found in efficacy. Hompland et al. 31 recently reported the results of a noncontrolled trial by a multicenter European study regarding adjuvant chemotherapy for DDCS. They reported a 5‐year OAS of 39% in patients with DDCS who received chemotherapy (DXR + IFO + CDDP). This OAS was better than the previously reported 5‐year OAS of 18%–24%. However, the median age was 52 years in that study, whereas it was 65 years in our study. Although this difference might have been reflected in the difference in prognosis, the results of Hompland et al.’s study support the aggressive implementation of chemotherapy for DDCS.
The usefulness of adjuvant radiation therapy has not yet been proven. 11 , 16 Due to its poor sensitivity, palliative radiation therapy was only given to three patients with G3CS and none with DDCS in this study.
Regarding the difference between the diagnosis of biopsy and that of excised specimen, only 27% (17/62) of patients with DDCS were diagnosed with DDCS at the time of biopsy in this study. Incorrect comprehensive diagnosis on biopsy alone can lead to a poor outcome. Liu et al. 32 reported that 43% of patients with DDCS received an incorrect diagnosis at the time of biopsy. In that study, the group with an inaccurate diagnosis on biopsy had a significantly worse prognosis than the group with an accurate diagnosis (p = 0.022). If a low‐grade malignancy is preoperatively diagnosed and a resection margin planned, the margin may become closer to the tumor and affect prognosis. Thus, early recognition of characteristic radiographic features, adequate histological sampling, and wide surgical margins are necessary for satisfactory management of the highly malignant DDCS. 34 Mercuri et al. 34 classified the imaging features of central DDCS into three types. It is important to make an accurate preoperative diagnosis by examining and comparing images, planning the excision, and carrying out resection with curative intent. The presence of pathological fracture could also explain why it is difficult to make an accurate diagnosis by biopsy.
Several previous studies reported the prognosis of DDCS from China. Liu et al. 32 reported the prognosis in 23 patients with DDCS. Cao et al. 39 investigated the clinical, imaging, and pathological features in 25 cases of DDCS. Gong et al. 40 analyzed the clinical, histopathological, and immunohistochemical features of 57 cases of DDCS. However, prognostic factors were not analyzed in this report. Moreover, this study has the largest cohort of DDCS from Asian countries.
However, this study has several limitations. First, the treatment methods differed between institutions, particularly the indications for surgery and chemotherapy regimens. Physicians may have performed surgery with curative intent only on patients who appeared to have good clinical outcomes, which could lead to selection biases, distorting the results. However, wide resection was undertaken in almost all patients according to the standards set by the participating facilities, and, other than the margin of resection, factors that affect prognosis may be more clearly identified. Second, pathological diagnosis of chondrosarcoma was carried out at each institution. However, all participating institutions are specialized facilities, in which the pathological diagnosis is carefully performed by specialized pathologists, and so bias in diagnosis is minimized. As the resected specimen is sufficiently large, the final diagnosis seems appropriate. Third, the number of patients with G3CS was small, making it difficult to draw definitive conclusions. Finally, although this is the largest series of such cases from the Asian region, the number is still too small to be accurately analyzed. International collaboration will be required in the future to validate our findings.
We reported the results of the first multicenter study on G3CS and DDCS in Asia. The prognosis for high‐grade chondrosarcoma, particularly DDCS, remains poor. Although treatment with curative intent for the primary lesion was extracted as a good prognostic factor, the outcome remained poor in our cohort, in which surgery with a wide surgical margin was achieved in almost all patients. No benefit of adjuvant chemotherapy was observed, most likely due to differences in indications and regimens, and the small number of cases. As high‐quality surgery did not effectively mitigate the poor prognoses of DDCS and G3CS, it is imperative that effective and/or novel chemotherapeutic regimens and agents be considered and developed for managing high‐grade chondrosarcoma, particularly of DDCS.
DISCLOSURE
Yoshihiro Nishida is an associate editor of Cancer Science. The other authors declare no competing interests regarding this study.
Supporting information
Figure S1
Figure S2
Figure S3
Table S1‐S5
ACKNOWLEDGMENTS
This work was supported in part by the National Cancer Center Research and Development Fund (29‐A‐3). We thank all the staff at participating institutions who prepared and sent the information from anonymized medical records.
Kozawa E, Nishida Y, Kawai A, et al. Clinical features and treatment outcomes of dedifferentiated and grade 3 chondrosarcoma: A multi‐institutional study. Cancer Sci. 2022;113:2397–2408. doi: 10.1111/cas.15382
Funding information
National Cancer Center Research and Development Fund, Grant/Award Number: 29‐A‐3
Contributor Information
Eiji Kozawa, Email: ekozawae@gmail.com.
Yoshihiro Nishida, Email: ynishida@med.nagoya-u.ac.jp.
REFERENCES
- 1. Thorkildsen J, Taksdal I, Bjerkehagen B, et al. Chondrosarcoma in Norway 1990–2013; an epidemiological and prognostic observational study of a complete national cohort. Acta Oncol. 2019;58:273‐282. [DOI] [PubMed] [Google Scholar]
- 2. Lee FY, Mankin HJ, Fondren G, et al. Chondrosarcoma of bone: an assessment of outcome. J Bone Joint Surg Am. 1999;81:326‐338. [DOI] [PubMed] [Google Scholar]
- 3. Pritchard DJ, Lunke RJ, Taylor WF, Dahlin DC, Medley BF. Chondrosarcoma: a clinicopathologic and statistical analysis. Cancer. 1980;45:149‐157. [DOI] [PubMed] [Google Scholar]
- 4. Björnsson J, McLeod RA, Unni KK, Ilstrup DM, Pritchard DJ. Primary chondrosarcoma of long bones and limb girdles. Cancer. 1998;83:2105‐2119. [PubMed] [Google Scholar]
- 5. Welkerling H, Werner M, Delling G. Histologic grading of chondrosarcoma. A qualitative and quantitative analysis of 74 cases of the Hamburg bone tumor register. Der Pathologe. 1996;17:18‐25. [DOI] [PubMed] [Google Scholar]
- 6. Gitelis S, Bertoni F, Picci P, Campanacci M. Chondrosarcoma of bone. The experience at the Istituto Ortopedico Rizzoli. J Bone Joint Surg Am. 1981;63:1248‐1257. [PubMed] [Google Scholar]
- 7. van Praag VM, Rueten‐Budde AJ, Ho V, et al. Incidence, outcomes and prognostic factors during 25 years of treatment of chondrosarcomas. Surg Oncol. 2018;27:402‐408. [DOI] [PubMed] [Google Scholar]
- 8. Dahlin DC, Beabout JW. Dedifferentiation of low‐grade chondrosarcomas. Cancer. 1971;28:461‐466. [DOI] [PubMed] [Google Scholar]
- 9. Giuffrida AY, Burgueno JE, Koniaris LG, Gutierrez JC, Duncan R, Scully SP. Chondrosarcoma in the United States (1973 to 2003): an analysis of 2890 cases from the SEER database. J Bone Joint Surg Am. 2009;91:1063‐1072. [DOI] [PubMed] [Google Scholar]
- 10. Frassica FJ, Unni KK, Beabout JW, Sim FH. Dedifferentiated chondrosarcoma. A report of the clinicopathological features and treatment of seventy‐eight cases. J Bone Joint Surg Am. 1986;68:1197‐1205. [PubMed] [Google Scholar]
- 11. Staals EL, Bacchini P, Bertoni F. Dedifferentiated central chondrosarcoma. Cancer. 2006;106:2682‐2691. [DOI] [PubMed] [Google Scholar]
- 12. Johnson S, Tětu B, Ayala AG, Chawla SP. Chondrosarcoma with an additional mesenchymal component (dedifferentiated chondrosarcoma). I. A clinicopathologic study of 26 cases. Cancer. 1986;58:278‐286. [DOI] [PubMed] [Google Scholar]
- 13. Capanna R, Bertoni F, Bettelli G, et al. Dedifferentiated chondrosarcoma. J Bone Joint Surg Am. 1988;70:60‐69. [PubMed] [Google Scholar]
- 14. Mitchell AD, Ayoub K, Mangham DC, Grimer RJ, Carter SR, Tillman RM. Experience in the treatment of dedifferentiated chondrosarcoma. J Bone Joint Surg Br. 2000;82:55‐61. [DOI] [PubMed] [Google Scholar]
- 15. Dickey ID, Rose PS, Fuchs B, et al. Dedifferentiated chondrosarcoma: the role of chemotherapy with updated outcomes. J Bone Joint Surg Am. 2004;86:2412‐2418. [PubMed] [Google Scholar]
- 16. Grimer RJ, Gosheger G, Taminiau A, et al. Dedifferentiated chondrosarcoma: prognostic factors and outcome from a European group. Eur J Cancer. 2007;43:2060‐2065. [DOI] [PubMed] [Google Scholar]
- 17. Kawaguchi S, Sun T, Lin PP, Deavers M, Harun N, Lewis VO. Does Ifosfamide therapy improve survival of patients with dedifferentiated chondrosarcoma? Clin Orthop Relat Res. 2014;472:983‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106‐120. [PubMed] [Google Scholar]
- 19. Kawaguchi N, Ahmed AR, Matsumoto S, Manabe J, Matsushita Y. The concept of curative margin in surgery of bone and soft tissue sarcoma. Clin Orthop Relat Res. 2004;419:165‐172. [DOI] [PubMed] [Google Scholar]
- 20. Amin MB, Edge SB, Greene FL. AJSS Cancer Staging Manual, 8th ed. Springer; 2017. [Google Scholar]
- 21. Fletcher C, Bridge JA, Antonescu C, Mertens F. WHO Classification of Tumours: Soft Tissue and Bone Tumours, 5th ed. IARC Press; 2013. [Google Scholar]
- 22. Tsuda Y, Tsoi K, Stevenson JD, et al. Development and external validation of nomograms to predict sarcoma‐specific death and disease progression after surgical resection of localized high‐grade conventional primary central chondrosarcoma and dedifferentiated chondrosarcoma. Bone Joint J. 2020;102:1752‐1759. [DOI] [PubMed] [Google Scholar]
- 23. Miao R, Choy E, Raskin KA, et al. Prognostic factors in dedifferentiated chondrosarcoma: a retrospective analysis of a large series treated at a single institution. Sarcoma. 2019;2019:9069272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fiorenza F, Abudu A, Grimer RJ, et al. Risk factors for survival and local control in chondrosarcoma of bone. J Bone Joint Surg Br. 2002;84:93‐99. [DOI] [PubMed] [Google Scholar]
- 25. Andreou D, Ruppin S, Fehlberg S, Pink D, Werner M, Tunn PU. Survival and prognostic factors in chondrosarcoma: results in 115 patients with long‐term follow‐up. Acta Orthop. 2011;82:749‐755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fromm J, Klein A, Baur‐Melnyk A, et al. Survival and prognostic factors in conventional central chondrosarcoma. BMC Cancer. 2018;18:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Albergo JI, Gaston CL, Jeys LM, et al. Management and prognostic significance of pathological fractures through chondrosarcoma of the femur. Int Orthop. 2015;39:943‐946. [DOI] [PubMed] [Google Scholar]
- 28. Bruns J, Elbracht M, Niggemeyer O. Chondrosarcoma of bone: an oncological and functional follow‐up study. Ann Oncol. 2001;12:859‐864. [DOI] [PubMed] [Google Scholar]
- 29. Donati D, El Ghoneimy A, Bertoni F, Di Bella C, Mercuri M. Surgical treatment and outcome of conventional pelvic chondrosarcoma. J Bone Joint Surg Br. 2005;87:1527‐1530. [DOI] [PubMed] [Google Scholar]
- 30. Strotman PK, Reif TJ, Kliethermes SA, Sandhu JK, Nystrom LM. Dedifferentiated chondrosarcoma: a survival analysis of 159 cases from the SEER database (2001–2011). J Surg Oncol. 2017;116:252‐257. [DOI] [PubMed] [Google Scholar]
- 31. Hompland I, Ferrari S, Bielack S, et al. Outcome in dedifferentiated chondrosarcoma for patients treated with multimodal therapy: Results from the EUROpean bone over 40 sarcoma study. Eur J Cancer. 2021;151:150‐158. [DOI] [PubMed] [Google Scholar]
- 32. Liu C, Xi Y, Li M, et al. Dedifferentiated chondrosarcoma: Radiological features, prognostic factors and survival statistics in 23 patients. PLoS One. 2017;12:e0173665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sambri A, Tuzzato G, Donati DM, De Paolis M, Bianchi G. Pathological fracture does not affect prognosis in dedifferentiated chondrosarcoma of the limbs. J Orthop Sci. 2021;26:473‐477. [DOI] [PubMed] [Google Scholar]
- 34. Mercuri M, Picci P, Campanacci L, Rulli E. Dedifferentiated chondrosarcoma. Skeletal Radiol. 1995;24:409‐416. [DOI] [PubMed] [Google Scholar]
- 35. Dornauer K, Söder S, Inwards CY, Bovee JVMG, Aigner T. Matrix biochemistry and cell biology of dedifferentiated chondrosarcomas. Pathol Int. 2010;60:365‐372. [DOI] [PubMed] [Google Scholar]
- 36. Nishida Y, Isu K, Ueda T, et al. Osteosarcoma in the elderly over 60 years: a multicenter study by the Japanese musculoskeletal oncology group. J Surg Oncol. 2009;100:48‐54. [DOI] [PubMed] [Google Scholar]
- 37. Nooij MA, Whelan J, Bramwell VHC, et al. Doxorubicin and cisplatin chemotherapy in high‐grade spindle cell sarcomas of the bone, other than osteosarcoma or malignant fibrous histiocytoma: a European Osteosarcoma Intergroup Study. Eur J Cancer. 2005;41:225‐230. [DOI] [PubMed] [Google Scholar]
- 38. van Maldegem A, Conley AP, Rutkowski P, et al. Outcome of first‐line systemic treatment for unresectable conventional, dedifferentiated, mesenchymal, and clear cell chondrosarcoma. Oncologist. 2019;24:110‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cao L, Wu Y, Han SM, et al. Dedifferentiated chondrsarcoma: a clinicopathologic analysis of 25 cases. BMC Musculoskelet Disord. 2021;22:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gong LH, Su YB, Zhang W, et al. Dedifferentiated central chondrosarcoma: a clinical, histopathological, and immunohistochemical analysis of 57 cases. Front Med. 2021;8:746909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Table S1‐S5


