Abstract
The treatment for lymph node involvement (LNI) after radical prostatectomy (RP) has not been established. This study aimed to reveal the outcomes of various management strategies among patients with LNI after RP. Retrospectively, 561 patients with LNI after pelvic lymph node dissection (PLND) with RP treated between 2006 and 2019 at 33 institutions participating in the Japanese Urological Oncology Group were investigated. Metastasis‐free survival (MFS) was the primary outcome. Patients were stratified by prostate‐specific antigen (PSA) persistence after RP. Cox regression models were used to analyze the relationships between clinicopathological characteristics and survival. Survival analyses were conducted using the Kaplan‐Meier method and log‐rank test with or without propensity score matching. Prognoses, including MFS and overall survival, were prominently inferior among patients with persistent PSA compared with those without persistent PSA. In multivariate analysis, androgen deprivation therapy (ADT) plus radiotherapy (RT) was associated with better MFS than ADT alone among patients with persistent PSA (hazard ratio = 0.37; 95% confidence interval = 0.15‐0.93; p = 0.034). Similarly, MFS and overall survival were significantly better for ADT plus RT than for ADT alone among patients with persistent PSA after propensity score matching. This study indicated that PSA persistence in LNI prostate cancer increased the risk of poor prognoses, and intensive treatment featuring the addition of RT to ADT might improve survival.
Keywords: androgen deprivation therapy, lymph node involvement, PSA persistence, radical prostatectomy, radiotherapy
The treatment for lymph node involvement (LNI) after radical prostatectomy (RP) among patients with persistent prostate‐specific antigen (PSA) after RP has not been established. Here, the outcomes of various management strategies among patients with LNI after RP were investigated. This study indicated that PSA persistence in LNI prostate cancer increased the risk of poor prognoses, and intensive treatment featuring the addition of RT to androgen deprivation therapy (ADT) might improve survival.

Abbreviations
- ADT
androgen deprivation therapy
- BCR
biochemical recurrence
- CRFS
castration resistance–free survival
- CSS
cancer‐specific survival
- DFS
disease‐free survival
- HR
hazard ratio
- LNI
lymph node involvement
- MFS
metastasis‐free survival
- OS
overall survival
- PLND
pelvic lymph node dissection
- RP
radical prostatectomy
- RT
radiotherapy
1. INTRODUCTION
Although the therapeutic benefit of pelvic lymph node dissection (PLND) during radical prostatectomy (RP) has not been demonstrated, PLND remains a gold standard procedure for nodal staging to identify patients who would benefit from additional treatment. 1 , 2 Currently, extended PLND is recommended by most guidelines for high‐risk patients with lymph node involvement (LNI). 3 , 4
For LNI after RP, adjuvant androgen deprivation therapy (ADT) is a standard treatment supported by prospective randomized control trial data detailing significant improvements of cancer‐specific survival (CSS) and overall survival (OS). 5 However, this trial was conducted from 1988 to 1993, and medical managements including PLND have changed. Most critically, salvage treatment performed as a control was triggered by distant metastases or symptomatic recurrences but not by biochemical recurrence (BCR). In addition, approximately 20% of patients had prostate‐specific antigen (PSA) persistence, but they did not receive immediate treatment in the control arm. Therefore, the evidence obtained from the study by Messing et al. may not fit the current status of the management for prostate cancer with LNI.
The favorable impact of adjuvant radiotherapy (RT) on survival in patients with LNI compared with adjuvant ADT alone and salvage RT was suggested in retrospective studies. 6 , 7 , 8 , 9 , 10 However, the survival benefit of adjuvant RT may be achieved in a limited population with moderate risk, and patient selection may be important. 11 , 12 , 13
Recently, PSA persistence in prostate cancer with LNI has been recognized to be robustly associated with unfavorable outcomes including survival, and most patients with persistent PSA should be managed with immediate intensive treatment. 14 , 15 , 16 However, to our knowledge, there is no evidence of the therapeutic impact of adding RT to ADT for PSA persistence in patients with LNI. Meanwhile, a subset of patients with LNI does not experience recurrence or require additional treatment. Therefore, adjuvant treatment for all patients with LNI may result in overtreatment and increase the physiological and economic burden. Accordingly, the European Association of Urology (EAU) guideline recommends the use of adjuvant ADT with or without RT or observation if a patient has only one or two positive lymph nodes and no PSA persistence (PSA < 0.1 ng/ml) after RP with extended PLND. 3 However, the strength of this recommendation is weak, and the treatment strategy for patients with LNI has not been well established. Therefore, we aimed to reveal the outcomes of various management strategies among patients with LNI in a Japanese multi‐institutional retrospective study.
2. MATERIALS AND METHODS
2.1. Patients
This study retrospectively enrolled patients who were pathologically diagnosed with LNI after PLND during RP conducted between 2006 and 2019 at 33 institutions participating in the Japanese Urological Oncology Group. The study was approved by the institutional review board of each institute. Patients at least 20 years old were included. Patients with a prior history of treatment for prostate cancer before RP, evidence of distant metastasis, or no performance of RP were excluded. Of 572 eligible patients, we excluded 11 patients for whom (i) the pathological diagnosis using the RP specimen was nonadenocarcinoma and/or (ii) their PSA level was not examined after RP (Figure 1). Finally, the data of 561 patients were analyzed.
FIGURE 1.
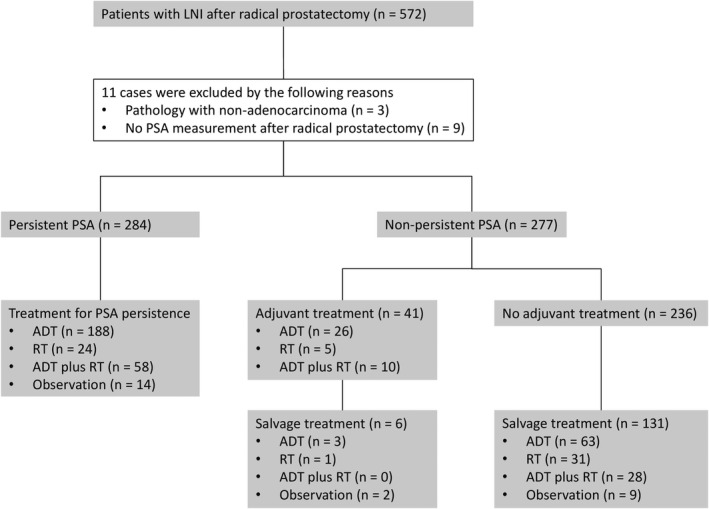
CONSORT diagram illustrating the distribution of patients and their management. The analyzed patients were stratified by prostate‐specific antigen (PSA) persistence and adjuvant treatment in patients without PSA persistence. ADT, androgen deprivation therapy; LNI, lymph node involvement; RT, radiotherapy
2.2. Methods
Clinicopathological and survival data were obtained from patients’ medical records. Clinical staging was determined using the unified TNM criteria. 17 International Society of Urological Pathology (ISUP) grade groups were categorized as follows: <3 + 4 = 7 (I); 3 + 4 (II); 4 + 3 (III); 4 + 4 (IV); or 9‐10 (V). Gleason scores 3 + 5 and 5 + 3 were included in category IV according to a previous report. 18
The indication and extent of PLND were determined at the physician's discretion. The patients were stratified by the presence or absence of persistent PSA (≥0.1 ng/ml at the initial assessment of PSA levels after RP). 16 The timing of postoperative PSA measurement was determined at the physician's discretion (median, 34 days; interquartile range [IQR] = 29‐45 days).
2.3. Treatment
All treatments were performed at the physician's discretion. Adjuvant and salvage treatments were defined as treatments provided before and after postoperative disease recurrence, respectively. When patients had PSA persistence after RP, any treatment was defined as salvage treatment. ADT consisted of castration alone, antiandrogen monotherapy, or combined androgen blockade (CAB; surgical or medical castration plus an antiandrogen). Radiotherapy was administered to the prostate and seminal vesicle bed with or without pelvic lymph node area. Three‐dimensional conformal RT or intensity‐modulated RT using linear accelerators with high‐energy photon beams was performed, and 50‐76 Gy were delivered at 1.8‐2.0 Gy per fraction. Median 66 Gy (IQR, 64.8‐70 Gy) was irradiated to the prostate bed alone (20.1%) or prostate bed with pelvis (79.9%).
2.4. Follow‐up
Biochemical recurrence was defined as PSA ≥0.2 ng/ml after RP with or without adjuvant treatment. Biochemical recurrence after salvage treatment was defined as PSA ≥0.2 ng/ml if PSA declined by <0.2 ng/mL. If PSA did not decline by <0.2 ng/ml after salvage treatment, the starting date of salvage treatment was defined as the date of BCR. Disease recurrence was determined by BCR, radiographic recurrence, or physician's judgment. Castration resistance was defined as an increase of PSA levels of 25% and 1.0 ng/ml, radiographic progression by RECIST version 1.1, or by physician's judgment. 19 Metastasis was detected by imaging modalities such as computed tomography and bone scan.
To analyze disease‐free survival (DFS), castration resistance–free survival (CRFS), and metastasis‐free survival (MFS), disease recurrence, castration resistance, and the presence of metastasis in addition to death attributable to any cause were defined as the end events, respectively. To analyze CSS and OS, deaths attributable to prostate cancer and all‐cause mortality were defined as the end events, respectively. Patients with none of these events were censored at the last follow‐up visit. Follow‐up started on the date of RP and ended on the date of last follow‐up or the date of the event. Metastasis‐free survival was set as primary outcome because it has been identified as a robust surrogate marker for OS in prostate cancer. 20 , 21 , 22
2.5. Statistical analysis
All analyses were performed using JMP16 software (SAS Institute). Continuous and categorical data were described as the median with IQR and numbers with percentages, respectively. Continuous and categorical data were analyzed using Wilcoxon's rank sum and Pearson's chi‐squared tests, respectively. Survival analyses were conducted using the Kaplan‐Meier method and log‐rank test. A Cox proportional hazards model was used to estimate hazard ratios (HRs). The propensity score, which reflects the probability of survival, was calculated using a logistic regression model in which potential confounders such as the percentage of positive biopsy core, clinical N‐stage, ISUP grade group at RP specimen, pathological T‐stage, and number of positive lymph nodes were used as independent variables and treatment method was used as the dependent variable. One‐to‐one propensity score–matched pairs were selected from the two groups by nearest neighbor matching. All P values were two‐sided, and p < 0.05 was considered significant.
3. RESULTS
Patients’ characteristics are presented in Table 1. As expected, the PSA level at diagnosis, percentage of positive biopsy core, ISUP grade group at biopsy and RP specimens, clinical and pathological T‐stages, and rate of positive resection margin were high. Concerning the surgical approach, 181 (32.3%), 46 (8.2%), and 334 patients (59.5%) were treated with open, laparoscopic, and robot‐assisted RP, respectively. The median numbers of positive lymph nodes and excised lymph nodes were 1 and 14, respectively. During a median follow‐up of 4.8 years (IQR, 2.6‐8.0 years), castration resistance, local recurrence, regional lymph node metastasis, distant metastasis, mortality from prostate cancer, and mortality from other causes were observed in 81 (14.4%), 10 (1.8%), 7 (1.2%), 50 (8.9%), 21 (3.7%), and 12 patients (2.1%), respectively. Among 50 patients who presented with distant metastasis, the sites of metastasis were nonregional lymph node in 17 patients, bone in 39 patients, lungs in 7 patients, liver in 2 patients, and other sites in 4 patients. The CRFS, MFS, CSS, and OS rates were 86.8%, 89.9%, 97.7%, and 96.7%, respectively, at 5 years and 75.3%, 79.5%, 91.4%, and 88.5%, respectively, at 10 years (Figure S1). When the prognostic factors for MFS were analyzed, the percentage of positive biopsy core, clinical N‐stage, ISUP grade group at RP specimen, pathological T‐stage, and number of positive lymph nodes, and PSA persistence after RP were prognostic for MFS (Table 2).
TABLE 1.
Patient characteristics stratified by PSA persistence or adjuvant treatment for patients without PSA persistence
| Variable | All (n = 561) | PSA persistence | Adjuvant treatment in no PSA persistence | ||||
|---|---|---|---|---|---|---|---|
| Presence (n = 284) | Absence (n = 277) | P value | Performed (n = 41) | Not performed (n = 236) | P value | ||
| Age at diagnosis, years (IQR) | 68 (64–72) | 68 (63–72) | 68 (65–71) | 0.16 | 68 (65–72) | 68 (65–71) | 0.70 |
| NA | 0 | 0 | 0 | 0 | 0 | ||
| PSA value at diagnosis, ng/ml (IQR) | 13.5 (8.4–22.6) | 16.2 (9.3–27.5) | 11.5 (7.6–17.9) | <0.0001* | 10.4 (6.6–15.6) | 11.5 (7.8–18.5) | 0.16 |
| NA | 0 | 0 | 0 | 0 | 0 | ||
| Percentage of positive biopsy core, % (IQR) | 50 (33–69) | 50 (33–75) | 50 (30–62) | 0.018* | 50 (38–70) | 50 (29–60) | 0.17 |
| NA | 1 | 1 | 0 | 0 | 0 | ||
| Biopsy ISUP grade group, n (%) | |||||||
| Group I | 12 (2.1%) | 5 (1.8%) | 7 (2.5%) | 2 (4.9%) | 5 (2.1%) | ||
| Group II | 46 (8.2%) | 21 (7.4%) | 25 (9.0%) | 5 (12.2%) | 20 (8.5%) | ||
| Group III | 102 (18.2%) | 42 (14.9%) | 60 (21.7%) | 7 (17.1%) | 53 (22.5%) | ||
| Group IV | 162 (29.0%) | 86 (30.5%) | 76 (27.4%) | 10 (24.4%) | 66 (28.0%) | ||
| Group V | 237 (42.4%) | 128 (45.4%) | 109 (39.4%) | 0.20 | 17 (41.5%) | 92 (39.0%) | 0.69 |
| NA | 2 | 2 | 0 | 0 | 0 | ||
| Clinical T‐stage, n (%) | |||||||
| T1 | 113 (20.2%) | 51 (18.0%) | 62 (22.4%) | 9 (22.0%) | 53 (22.5%) | ||
| T2a | 297 (53.0%) | 155 (54.8%) | 142 (51.3%) | 23 (56.1%) | 119 (50.4%) | ||
| T2b | 5 (0.9%) | 2 (0.7%) | 3 (1.1%) | 0 (0%) | 3 (1.3%) | ||
| T2c | 15 (2.7%) | 4 (1.4%) | 11 (4.0%) | 1 (2.4%) | 10 (4.2%) | ||
| T3/4 | 130 (23.2%) | 71 (25.1%) | 59 (21.3%) | 0.19 | 8 (19.5%) | 51 (21.6%) | 0.89 |
| NA | 1 | 1 | 0 | 0 | 0 | ||
| Clinical N‐stage, n (%) | |||||||
| N0 | 526 (94.8%) | 264 (94.3%) | 262 (95.3%) | 37 (92.5%) | 225 (95.7%) | ||
| N1 | 29 (5.2%) | 16 (5.7%) | 13 (4.7%) | 0.27 | 3 (7.5%) | 10 (4.3%) | 0.37 |
| NA | 6 | 4 | 2 | 1 | 1 | ||
| Year of operation, n (%) | |||||||
| 2006‐2012 | 187 (33.3%) | 97 (34.2%) | 90 (32.5%) | 23 (56.1%) | 67 (28.4%) | ||
| 2013‐2019 | 374 (66.7%) | 187 (65.8%) | 187 (67.5%) | 0.68 | 18 (43.9%) | 169 (71.6%) | 0.0005* |
| NA | 0 | 0 | 0 | 0 | 0 | ||
| Operation approach, n (%) | |||||||
| Open RP | 181 (32.3%) | 97 (34.2%) | 84 (30.3%) | 22 (53.7%) | 62 (26.3%) | ||
| Laparoscopic RP | 46 (8.2%) | 27 (9.5%) | 19 (6.9%) | 4 (9.8%) | 15 (6.4%) | ||
| Robot‐assisted RP | 334 (59.5%) | 160 (56.3%) | 174 (62.8%) | 0.24 | 15 (36.6%) | 159 (67.4%) | 0.0007* |
| NA | 0 | 0 | 0 | 0 | 0 | ||
| RP ISUP grade group, n (%) | |||||||
| Group I | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Group II | 48 (8.6%) | 14 (4.9%) | 34 (12.4%) | 4 (9.8%) | 30 (12.8%) | ||
| Group III | 131 (23.4%) | 56 (19.8%) | 75 (27.3%) | 11 (26.8%) | 64 (27.4%) | ||
| Group IV | 78 (14.0%) | 48 (17.0%) | 30 (10.9%) | 3 (7.3%) | 27 (11.5%) | ||
| Group V | 301 (53.8%) | 165 (58.3%) | 136 (49.5%) | 0.0005* | 23 (56.1%) | 113 (48.3%) | 0.74 |
| NA | 3 | 1 | 2 | 0 | 2 | ||
| Pathological T‐stage, n (%) | |||||||
| T2 | 91 (16.3%) | 27 (9.5%) | 64 (23.2%) | 6 (14.6%) | 58 (24.7%) | ||
| T3a | 175 (31.3%) | 81 (28.6%) | 94 (34.1%) | 11 (26.8%) | 83 (35.3%) | ||
| T3b | 282 (50.4%) | 167 (59.0%) | 115 (41.7%) | 22 (53.7%) | 93 (39.6%) | ||
| T4 | 11 (2.0%) | 8 (2.8%) | 3 (1.1%) | <0.0001* | 2 (4.9%) | 1 (0.4%) | 0.016* |
| NA | 2 | 1 | 1 | 0 | 1 | ||
| Resection margin, n (%) | |||||||
| Negative | 252 (45.2%) | 107 (37.8%) | 145 (52.7%) | 17 (41.5%) | 128 (54.7%) | ||
| Positive | 306 (54.8%) | 176 (62.2%) | 130 (47.3%) | 0.0004* | 24 (58.5%) | 106 (45.3%) | 0.12 |
| NA | 3 | 1 | 2 | 0 | 2 | ||
| Number of positive lymph nodes (IQR) | 1 (1–2) | 1 (1–3) | 1 (1–2) | <0.0001* | 1 (1–2) | 1 (1–2) | 0.057 |
| NA | 0 | 0 | 0 | 0 | 0 | ||
| Number of removed lymph nodes (IQR) | 14 (9–21) | 13 (9–19) | 15 (9–23) | 0.0075* | 10 (7–17) | 17 (10–25) | 0.0011* |
| NA | 0 | 0 | 0 | 0 | 0 | ||
Abbreviations: IQR, interquartile range; ISUP, International Society of Urological Pathology; NA, not available; PSA, prostate‐specific antigen; RP, radical prostatectomy.
*Statistically significant.
TABLE 2.
Univariate analysis of the associations between clinicopathological parameters and metastasis‐free survival
| Variable | All (n = 561) | PSA persistence (n = 284) | No PSA persistence (n = 277) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P– value | |
| Age at diagnosis, | |||||||||
| <60 years | ref | – | – | ref | – | – | ref | – | – |
| 60–69 years | 0.91 | 0.45–1.83 | 0.79 | 0.93 | 0.40–2.12 | 0.85 | 1.03 | 0.28–3.83 | 0.96 |
| ≥70 years | 1.13 | 0.56–2.29 | 0.73 | 1.65 | 0.73–3.71 | 0.23 | 0.91 | 0.14–2.62 | 0.51 |
| PSA value at diagnosis | |||||||||
| <10 ng/ml | ref | – | – | ref | – | – | ref | – | – |
| ≥10, <20 ng/ml | 0.72 | 0.39–1.32 | 0.29 | 0.57 | 0.28–1.15 | 0.12 | 0.65 | 0.19–2.23 | 0.49 |
| ≥20 ng/ml | 1.23 | 0.71–2.15 | 0.46 | 0.63 | 0.32–1.24 | 0.18 | 2.32 | 0.84–6.40 | 0.11 |
| Percentage of positive biopsy core | |||||||||
| <50% | ref | – | – | ref | – | – | ref | – | – |
| ≥50% | 2.40 | 1.40–4.11 | 0.0014* | 1.75 | 0.97–3.18 | 0.065 | 7.61 | 1.75–33.0 | 0.0067* |
| Clinical T‐stage | |||||||||
| T1 | ref | – | – | ref | – | – | ref | – | – |
| T2 | 1.30 | 0.68–2.49 | 0.43 | 0.91 | 0.45–1.85 | 0.80 | 6.02 | 0.78–46.4 | 0.085 |
| T3/4 | 1.41 | 0.68–2.93 | 0.35 | 1.03 | 0.46–2.31 | 0.93 | 5.32 | 0.62–45.6 | 0.13 |
| Clinical N‐stage | |||||||||
| N0 | ref | – | – | ref | – | – | ref | – | – |
| N1 | 4.49 | 2.21–9.13 | <0.0001* | 4.40 | 1.84–10.5 | 0.0008* | 4.84 | 1.39–16.9 | 0.013* |
| Year of operation | |||||||||
| 2006‐2012 | ref | – | – | ref | – | – | ref | – | – |
| 2013‐2019 | 1.28 | 0.74–2.22 | 0.38 | 1.07 | 0.56–2.04 | 0.83 | 2.34 | 0.73–7.48 | 0.15 |
| Operation approach | |||||||||
| Open RP | ref | – | – | ref | – | – | ref | – | – |
| Laparoscopic RP | 0.47 | 0.14–1.53 | 0.21 | 0.19 | 0.025–1.38 | 0.10 | 1.79 | 0.36–8.84 | 0.48 |
| Robot‐assisted RP | 1.15 | 0.66–1.99 | 0.62 | 1.06 | 0.56–2.02 | 0.85 | 1.77 | 0.57–5.51 | 0.32 |
| RP ISUP grade group | |||||||||
| Group ≤III | ref | – | – | ref | – | – | ref | – | – |
| Group IV | 3.02 | 1.25–7.29 | 0.014* | 2.02 | 0.68–6.01 | 0.21 | 4.45 | 0.99–20.1 | 0.052 |
| Group V | 3.63 | 1.78–7.40 | 0.0004* | 3.30 | 1.39–7.82 | 0.0068* | 3.28 | 0.92–11.6 | 0.066 |
| Pathological T‐stage | |||||||||
| T2/3a | ref | – | – | ref | – | – | ref | – | – |
| T3b | 2.81 | 1.60–4.97 | 0.0004* | 2.20 | 1.12–4.35 | 0.023* | 3.16 | 1.12–8.92 | 0.029* |
| T4 | 14.3 | 5.55–37.1 | <0.0001* | 9.00 | 3.09–26.2 | <0.0001* | 23.8 | 2.72–209 | 0.0042* |
| Resection margin | |||||||||
| Negative | ref | – | – | ref | – | – | ref | – | – |
| Positive | 1.61 | 0.98–2.66 | 0.062 | 1.48 | 0.81–2.70 | 0.21 | 1.37 | 0.55–3.40 | 0.50 |
| Number of positive lymph nodes | |||||||||
| 1 | ref | – | – | ref | – | – | ref | – | – |
| 2 | 1.29 | 0.68–2.46 | 0.44 | 1.04 | 0.93 | 0.44 | 1.21 | 0.33–4.42 | 0.77 |
| ≥3 | 3.25 | 1.90–5.56 | <0.0001* | 1.97 | 1.05–3.70 | 0.034* | 5.66 | 2.02–15.8 | 0.0010* |
| Number of removed lymph nodes | |||||||||
| <10 | ref | – | – | ref | – | – | ref | – | – |
| ≥10, <20 | 1.32 | 0.74–2.38 | 0.35 | 1.82 | 0.93–3.55 | 0.081 | 0.54 | 0.15–2.03 | 0.36 |
| ≥20 | 1.58 | 0.84–2.98 | 0.16 | 1.70 | 0.76–3.84 | 0.20 | 1.91 | 0.64–5.65 | 0.24 |
| PSA persistence | |||||||||
| Absence | ref | – | – | – | – | – | – | – | – |
| Presence | 2.71 | 1.60–4.59 | 0.0002* | – | – | – | – | – | – |
Abbreviations: CI, confidence interval; HR, hazard ratio; ISUP, International Society of Urological Pathology; PSA, prostate‐specific antigen; RP, radical prostatectomy.
*Statistically significant.
Of the 561 patients analyzed, 284 (50.6%) had PSA persistence, whereas 277 (49.4%) did not have PSA persistence. When patient characteristics were compared between patients with and without PSA persistence, the PSA level at diagnosis, percentage of positive biopsy cores, ISUP grade group at RP, pathological T‐stage, and resection margin status were more adverse in patients with PSA persistence (Table 1). In addition, the number of positive lymph nodes and the number of excised lymph nodes were higher and lower, respectively, in patients with persistent PSA (Table 1). The survival rates were prominently worse among patients with persistent PSA than among those without persistent PSA (5 and 10 years: CRFS, 81.0% and 62.2% vs 92.8% and 88.9%; MFS, 86.2% and 69.2% vs 93.8% and 90.4%; CSS, 97.1% and 85.8% vs 98.4% and 97.5%; and OS, 96.8% and 81.8% vs 96.7% and 95.8%; Figure 2).
FIGURE 2.
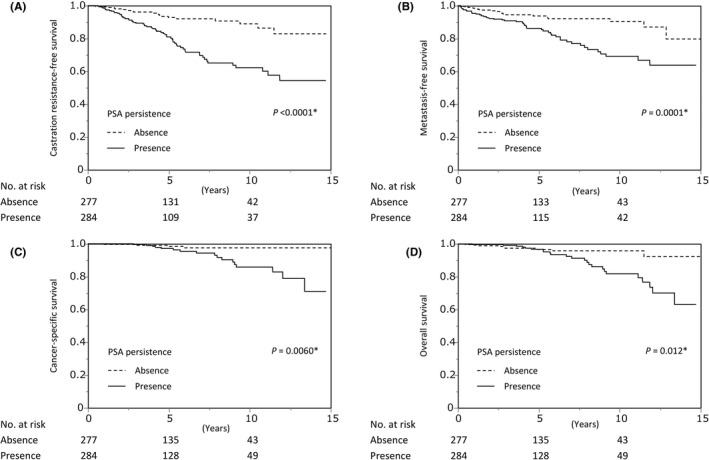
Kaplan‐Meier analysis of prognosis stratified by prostate‐specific antigen (PSA) persistence. Castration resistance–free survival (A), metastasis‐free survival (B), cancer‐specific survival (C), and overall survival (D) stratified by PSA persistence
When the prognostic factors for MFS were analyzed among patients with persistent PSA, clinical N‐stage, ISUP grade group at RP specimen, pathological T‐stage, and the number of positive lymph nodes were prognostic for MFS (Table 2). Among 284 patients with persistent PSA, 188, 24, and 58 were treated with ADT, RT, and ADT plus RT, respectively. Radiotherapy was associated with inferior DFS than ADT (HR, 3.54; 95% confidence interval [CI], 2.02‐6.21; p < 0.0001), whereas ADT plus RT was linked to better DFS than ADT alone, albeit without statistical significance (HR, 0.63; 95% CI, 0.35‐1.12; p = 0.11; Figure 3A). Castration resistance–free survival, MFS (Figure 3B), CSS, and OS were similar among the treatments for PSA persistence in univariate analysis (Figure S2A). However, when adjusted by prognostic factors for MFS including clinical N‐stage, ISUP grade group at RP specimen, pathological T‐stage, and the number of positive lymph nodes, ADT plus RT was associated with significantly better MFS than ADT alone (HR, 0.37; 95% CI, 0.15‐0.93; p = 0.034; Table 3). However, ADT plus RT was more frequently administered to patients with younger age, treated with minimum‐invasive surgery, and positive resection margin (Table S1). Then, propensity score matching was performed to adjust for the imbalanced backgrounds between the groups as much as possible. Excluding the year of operation and surgical approach, background characteristics were balanced after propensity score matching (Table S2). When the prognosis of patients was compared between the ADT and ADT plus RT groups, DFS (Figure 3C), MFS (Figure 3D), and OS (Figure S2B) were significantly better in the ADT plus RT group, whereas statistical significance was not reached for CRFS and CSS (Figure S2B).
FIGURE 3.
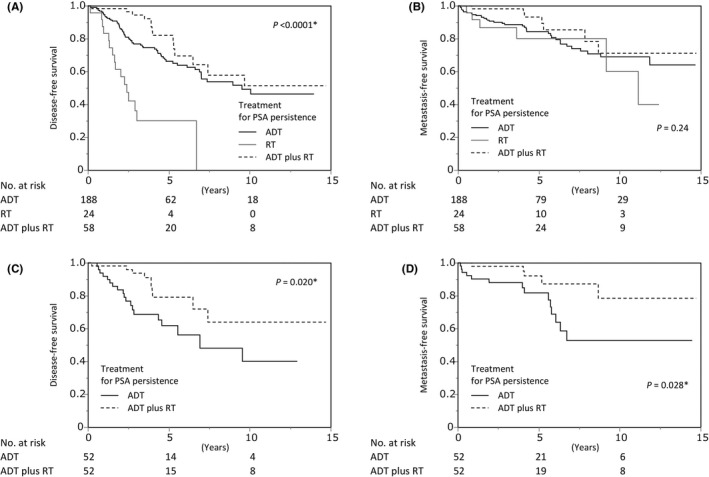
Kaplan‐Meier analysis of prognosis among patients with prostate‐specific antigen (PSA) persistence. Disease‐free survival (A) and metastasis‐free survival (B) stratified by treatment for PSA persistence. Disease‐free survival (C) and metastasis‐free survival (D) stratified by treatment for PSA persistence after propensity score matching. ADT, androgen deprivation therapy; RT, radiotherapy
TABLE 3.
Multivariate analysis of the associations between clinicopathological parameters and metastasis‐free survival among patients with PSA persistence
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Treatment for PSA persistence | |||
| ADT | ref | ‐ | ‐ |
| RT | 1.67 | 0.67–4.16 | 0.27 |
| ADT plus RT | 0.37 | 0.15–0.93 | 0.034* |
| Clinical N‐stage | |||
| N0 | ref | ‐ | ‐ |
| N1 | 5.09 | 2.04–12.7 | 0.0005* |
| RP ISUP grade group | |||
| Group ≤III | ref | ‐ | ‐ |
| Group IV | 2.65 | 0.82–8.62 | 0.10 |
| Group V | 3.30 | 1.26–8.63 | 0.015* |
| Pathological T‐stage | |||
| T2/3a | ref | ‐ | ‐ |
| T3b | 1.94 | 0.96–3.92 | 0.066 |
| T4 | 11.6 | 3.33–40.2 | 0.0001* |
| Number of positive lymph nodes | |||
| 1 | ref | ‐ | ‐ |
| 2 | 0.90 | 0.42–1.95 | 0.79 |
| ≥3 | 1.19 | 0.58–2.41 | 0.64 |
Abbreviations: ADT, androgen deprivation therapy; CI, confidence interval; HR, hazard ratio; ISUP, International Society of Urological Pathology; PSA, prostate‐specific antigen; RP, radical prostatectomy; RT, radiotherapy.
*Statistically significant.
Among 277 patients without PSA persistence, 41 and 236 patients were treated with and without adjuvant treatment, respectively (Figure 1). When the prognostic factors for MFS were analyzed among patients without PSA persistence, the percentage of positive biopsy core, clinical N‐stage, pathological T‐stage, and number of positive lymph nodes were prognostic for MFS (Table 2). The assessment of patient characteristics indicated that more patients with operation in former years, open RP, high pathological T‐stage, and a small number of excised lymph nodes were treated with adjuvant treatment (Table 1). Disease‐free survival was better among patients treated with adjuvant treatment than among those assigned to observation (Figure S3). Meanwhile, CRFS, MFS, CSS, and OS were comparable between adjuvant treatment and observation (Figure S3). When adjusted by prognostic factors for MFS including the percentage of positive biopsy core, clinical N‐stage, pathological T‐stage, and number of positive lymph nodes, MFS was inferior for observation than for adjuvant treatment, although statistical significance was not reached (HR, 3.03; 95% CI, 0.48‐19.1; p = 0.24; Table S3).
4. DISCUSSION
In data from the US National Cancer Data Base, approximately 63% of patients with LNI after RP were initially managed with observation, whereas approximately 20%, 5%, and 13% were treated with ADT alone, RT alone, and ADT plus RT, respectively. 23 Similarly, 42% of patients were initially managed with observation in this study, whereas 38%, 5%, and 12% were initially treated with ADT alone, RT alone, and ADT plus RT, respectively. The lower percentage of patients assigned to observation may be attributable to the high rate of PSA persistence in this study, although data on PSA persistence are unavailable in the US National Cancer Data Base. 23
When the PSA level after RP does not decline to <0.1 ng/mL, almost all patients will experience BCR. Therefore, the immediate initiation of additional treatment is recommended for such patients. 14 Based on the study by Messing et al., add‐on ADT is a standard treatment for patients with LNI after RP. However, as revealed in this study, the prognoses of such patients were not satisfactory, and improvement of oncological outcomes is required. Previously, adjuvant RT for LNI after RP was reported to be associated with improved OS compared with adjuvant ADT alone and salvage RT in retrospective studies. 6 , 7 , 8 , 9 , 10 Consistently, adding RT to ADT was associated with better DFS, MFS, and OS after RP even for patients with prostate cancer with LNI and PSA persistence. Addition of RT is thought to eradicate or decrease residual tumor in prostate and pelvis after operation, leading to improved outcomes. Actually, the patients with positive resection margin were more frequently treated with ADT plus RT in this study, consistently with a previous study. 7 To the best of our knowledge, this is the first study to demonstrate benefits including improved survival for adding RT to ADT in patients with LNI and PSA persistence after RP. Previously, a subgroup of patients with LNI was suggested to have benefited from adjuvant RT. 11 , 12 , 13 Based on the finding in this study, patients with PSA persistence are also candidates for adjuvant RT in addition to ADT even though their clinicopathological features do not fit the criteria for adjuvant RT. PSA persistence can be recognized before physicians recommend adjuvant treatment. Thus, this finding is important in clinical decision‐making for selecting adjuvant ADT with RT, indicating the need for prospective studies comparing outcomes between ADT alone and ADT plus RT.
Contrary to the findings for patients with PSA persistence, the prognoses among most patients without PSA persistence were favorable. Meanwhile, high‐risk patients might benefit from adjuvant treatment, as supported by the favorable HR for adjuvant treatment among patients without PSA persistence. However, definitive finding was not obtained because of the limited statistical power of the study. Then, as recommended by the EAU guideline, adjuvant ADT with or without RT or observation is considered appropriate if patients have one or two positive lymph nodes and PSA <0.1 ng/ml after RP with extended PLND. 3
The present study had several limitations. The study design was retrospective, and the study cohort consisted mostly of Japanese patients. Although this study enrolled patients from multiple institutions to increase the number of cases with the relatively rare entity of LNI after RP, the number of cases was not sufficient in some subgroups, such as ADT plus RT for patients with PSA persistence. Also, an analysis by dividing into subcategories is statistically less powerful. The pathological diagnosis was assessed in each institution, and central review was not performed. The features of the follow‐up protocol, such as the test interval and modality, including PSA and imaging, and the treatment protocol, including the strategy for PLND, differed among patients. Detailed information on the imaging performed for each patient and toxicity related to treatment was unavailable.
In conclusion, the results of this study indicated that PSA persistence in patients with prostate cancer with LNI carries a high risk of poor prognosis, and it should be managed by intensive treatment, as adding RT to ADT might improve survival. Thus, this study warrants further investigation on the role of ADT plus RT for patients with prostate cancer with LNI and PSA persistence.
DISCLOSURE
Masaki Shiota received honoraria from Astellas, AstraZeneca, Bayer, Janssen, Sanofi, and Takeda and research funding from Daiichi‐Sankyo. Takahiro Kimura is a paid consultant/advisor of Astellas, Bayer, Janssen, and Sanofi. Shintaro Narita received honoraria from Janssen. Shusuke Akamatsu received honoraria from Janssen, AstraZeneca, Astellas, Sanofi, Takeda, and Chugai and research grants from AstraZeneca, Astellas, and Tosoh. Masatoshi Eto is an Associate Editor of Cancer Science. All other authors do not have any conflict of interest.
Supporting information
Fig S1
Fig S2
Fig S3
Table S1
Table S2
Table S3
ACKNOWLEDGMENTS
We thank Joe Barber Jr., PhD, from Edanz (https://www.edanz.com/ac) for editing a draft of this manuscript.
Shiota M, Takamatsu D, Kimura T, et al; the Japanese Urological Oncology Group . Radiotherapy plus androgen deprivation therapy for prostate‐specific antigen persistence in lymph node–positive prostate cancer. Cancer Sci. 2022;113:2386–2396. doi: 10.1111/cas.15383
Funding information
None.
REFERENCES
- 1. Fujimoto N, Shiota M, Tomisaki I, Minato A, Yahara K. Reconsideration on clinical benefit of pelvic lymph node dissection during radical prostatectomy for clinically localized prostate cancer. Urol Int. 2019;103:125‐136. [DOI] [PubMed] [Google Scholar]
- 2. Lestingi JFP, Guglielmetti GB, Trinh QD, et al. Extended versus limited pelvic lymph node dissection during radical prostatectomy for intermediate‐ and high‐risk prostate cancer: early oncological outcomes from a randomized phase 3 trial. Eur Urol. 2021;79:595‐604. [DOI] [PubMed] [Google Scholar]
- 3. Mottet N, van den Bergh RCN, Briers E, et al. EAU‐EANM‐ESTRO‐ESUR‐SIOG guidelines on prostate cancer‐2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79:243‐262. [DOI] [PubMed] [Google Scholar]
- 4. Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:479‐505. [DOI] [PubMed] [Google Scholar]
- 5. Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node‐positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472‐479. [DOI] [PubMed] [Google Scholar]
- 6. Abdollah F, Karnes RJ, Suardi N, et al. Predicting survival of patients with node‐positive prostate cancer following multimodal treatment. Eur Urol. 2014;65:554‐562. [DOI] [PubMed] [Google Scholar]
- 7. Jegadeesh N, Liu Y, Zhang C, et al. The role of adjuvant radiotherapy in pathologically lymph node‐positive prostate cancer. Cancer. 2017;123:512‐520. [DOI] [PubMed] [Google Scholar]
- 8. Rusthoven CG, Carlson JA, Waxweiler TV, et al. The impact of definitive local therapy for lymph node‐positive prostate cancer: a population‐based study. Int J Radiat Oncol Biol Phys. 2014;88:1064‐1073. [DOI] [PubMed] [Google Scholar]
- 9. Tilki D, Preisser F, Tennstedt P, et al. Adjuvant radiation therapy is associated with better oncological outcome compared with salvage radiation therapy in patients with pN1 prostate cancer treated with radical prostatectomy. BJU Int. 2017;119:717‐723. [DOI] [PubMed] [Google Scholar]
- 10. Tilki D, Chen MH, Wu J, et al. Adjuvant versus early salvage radiation therapy for men at high risk for recurrence following radical prostatectomy for prostate cancer and the risk of death. J Clin Oncol. 2021;39:2284‐2293. [DOI] [PubMed] [Google Scholar]
- 11. Abdollah F, Karnes RJ, Suardi N, et al. Impact of adjuvant radiotherapy on survival of patients with node‐positive prostate cancer. J Clin Oncol. 2014;32:3939‐3947. [DOI] [PubMed] [Google Scholar]
- 12. Abdollah F, Dalela D, Sood A, et al. Impact of adjuvant radiotherapy in node‐positive prostate cancer patients: the importance of patient selection. Eur Urol. 2018;74:253‐256. [DOI] [PubMed] [Google Scholar]
- 13. Gupta M, Patel HD, Schwen ZR, Tran PT, Partin AW. Adjuvant radiation with androgen‐deprivation therapy for men with lymph node metastases after radical prostatectomy: identifying men who benefit. BJU Int. 2019;123:252‐260. [DOI] [PubMed] [Google Scholar]
- 14. Bianchi L, Nini A, Bianchi M, et al. The role of prostate‐specific antigen persistence after radical prostatectomy for the prediction of clinical progression and cancer‐specific mortality in node‐positive prostate cancer patients. Eur Urol. 2016;69:1142‐1148. [DOI] [PubMed] [Google Scholar]
- 15. Kim JK, Jeong CW, Ku JH, Kim HH, Kwak C. Prostate specific antigen (PSA) persistence 6 weeks after radical prostatectomy and pelvic lymph node dissection as predictive factor of radiographic progression in node‐positive prostate cancer patients. J Cancer. 2019;10:2237‐2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ploussard G, Fossati N, Wiegel T, et al. Management of persistently elevated prostate‐specific antigen after radical prostatectomy: a systematic review of the literature. Eur Urol Oncol. 2021;4:150‐169. [DOI] [PubMed] [Google Scholar]
- 17. Sobin LH, Gospodarowicz MK, Wittekind CH. TNM Classification of Malignant Tumours (7th ed.). Wiley‐Blackwell; 2009. [Google Scholar]
- 18. Epstein JI, Zelefsky MJ, Sjoberg DD, et al. A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur Urol. 2016;69:428‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration‐resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mori A, Hashimoto K, Koroki Y, Wu DB, Masumori N. The correlation between metastasis‐free survival and overall survival in non‐metastatic castration resistant prostate cancer patients from the Medical Data Vision claims database in Japan. Curr Med Res Opin. 2019;35:1745‐1750. [DOI] [PubMed] [Google Scholar]
- 21. Smith MR, Mehra M, Nair S, Lawson J, Small EJ. Relationship between metastasis‐free survival and overall survival in patients with nonmetastatic castration‐resistant prostate cancer. Clin Genitourin Cancer. 2020;18:e180‐e189. [DOI] [PubMed] [Google Scholar]
- 22. Xie W, Regan MM, Buyse M, et al. Metastasis‐free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol. 2017;35:3097‐3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zareba P, Eastham J, Scardino PT, Touijer K. Contemporary patterns of care and outcomes of men found to have lymph node metastases at the time of radical prostatectomy. J Urol. 2017;198:1077‐1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Table S1
Table S2
Table S3


