Abstract
Melatonin is an endogenous hormone with various biological functions and possesses anti‐tumor properties in multiple malignancies. Immune evasion is one of the most important hallmarks of head and neck squamous cell carcinoma (HNSCC) and is closely related to tumor progression. However, as an immune modulator under physiological conditions, the roles of melatonin in tumor immunity in HNSCC remains unclear. In this study, we found that the endogenous melatonin levels in patients with HNSCC were lower than those in patients with benign tumors in head and neck. Importantly, lower melatonin levels were related to lymph node metastasis among patients with HNSCC. Moreover, melatonin significantly suppressed programmed death‐ligand 1 (PD‐L1) expression and inhibited epithelial–mesenchymal transition (EMT) of HNSCC through the ERK1/2/FOSL1 pathway in vitro and in vivo. In SCC7/C3H syngeneic mouse models, anti‐programmed death‐1 (PD‐1) antibody combined with melatonin significantly inhibited tumor growth and modulated anti‐tumor immunity by increasing CD8+ T cell infiltration and decreasing the regulatory T cell (Treg) proportion in the tumor microenvironment. Taken together, melatonin inhibited EMT and downregulated PD‐L1 expression in HNSCC through the ERK1/2/FOSL1 pathway and exerted synergistic effects with anti‐PD‐1 antibody in vivo, which could provide promising strategies for HNSCC treatment.
Keywords: anti‐tumor immunity, epithelial–mesenchymal transition, head and neck squamous cell carcinoma, melatonin, PD‐L1
Endogenous melatonin levels were lower in head and neck squamous cell carcinoma (HNSCC) patients and related to lymph node metastasis. Moreover, melatonin significantly suppressed EMT and downregulated PD‐L1 expression in HNSCC through the ERK1/2/FOSL1 pathway in vitro and vivo. Anti‐PD‐1 antibody combined with melatonin significantly inhibited tumor growth and modulated anti‐tumor immunity in SCC7/C3H mouse models.
Abbreviations
- α‐PD‐1
anti‐PD‐1 antibody
- aMT6s
6‐sulfatoxymelatonin
- EMT
epithelial–mesenchymal transition
- FOSL1
Fos‐like 1
- HNSCC
head and neck squamous cell carcinoma
- HPLC‐MS
high performance liquid chromatography/mass spectrometry
- IFN‐γ
interferon‐γ
- PD‐1
programmed death‐1
- PD‐L1
programmed death‐ligand 1
- RNA‐seq
RNA sequencing
- TME
tumor microenvironment
- Treg
regulatory T cell
1. INTRODUCTION
Head and neck squamous cell carcinoma is the seventh most common malignancy worldwide and in 2020 nearly 930,000 patients were diagnosed along with 470,000 deaths. 1 Even though diagnosis and treatment skills have improved, the 5‐year survival rate of patients with advanced HNSCC remains low, mainly due to recurrence and metastasis. 2 , 3 Immune evasion is one of the critical hallmarks of HNSCC and is closely related to tumor development. 4 Recently, immune checkpoint inhibitors targeting the PD‐L1/PD‐1 axis have brought hope to patients with HNSCC. 2 , 5 , 6 However, the objective response rates of these inhibitors have still been limited, 6 , 7 calling for new strategies to improve their efficacy.
Mainly synthesized and secreted by the pineal gland, melatonin is a pleiotropic neuroendocrine hormone that participates in circadian rhythm regulation, free radical scavenging, immunoregulation, and even anti‐tumor processes. 8 , 9 , 10 , 11 Accumulating evidence has indicated that melatonin exerts anti‐tumor effects in multiple malignancies by inhibiting proliferation, angiogenesis and metastasis of tumor cells, and regulating tumor immunity. 11 , 12 , 13 , 14 However, the effects of melatonin on immunoregulation in HNSCC and its underlying mechanisms still remain unclear.
As an immune inhibitory molecule, PD‐L1 can induce exhaustion of CD8+ T cells by interacting with its receptor PD‐1, leading to immune evasion of tumor cells. 15 The mechanisms of PD‐L1 expression regulation in tumor cells are not fully understood. Recent studies have found that epithelial‐derived tumor cells acquiring mesenchymal phenotypes exhibited higher PD‐L1 expression levels in multiple malignancies, including HNSCC. 16 , 17 , 18 Epithelial–mesenchymal transition‐related transcription factors (EMT‐TFs), including the Snail, Twist, and ZEB families, participate in the regulation of PD‐L1. 16 These finding indicated that EMT might play important roles in tumor immunosuppression via the PD‐L1/PD‐1 axis. However, mechanisms of the correlation between EMT and PD‐L1 in HNSCC have not been well elucidated.
Fos‐like 1 (FOSL1), a member of the FOS family, has been reported to play important roles in tumor progression and metastasis. 19 By interacting with EMT‐TFs, FOSL1 regulates EMT of tumor cells and promotes metastasis of many solid tumors, including HNSCC. 19 , 20 Moreover, as a vital effector of multiple oncogenic pathways, FOSL1 regulates the expression of several important proteins such as PD‐L1. 21 , 22 However, the relationship between FOSL1 and PD‐L1 in HNSCC cells remains unknown and the effects of melatonin on FOSL1 has not been explored before.
In this study, we found that urinary 6‐sulfatoxymelatonin (aMT6s) levels decreased in patients with HNSCC and were associated with lymph node metastasis. Melatonin significantly inhibited the EMT of HNSCC cells and downregulated PD‐L1 expression in HNSCC through the ERK1/2/FOSL1 pathway. Moreover, in SCC7/C3H mouse models, the combination of melatonin and anti‐PD‐1 antibody markedly inhibited tumor growth and attenuated immunosuppression in the tumor microenvironment (TME) by increasing CD8+ effector T cells and decreasing FOXP3+ Treg infiltration. Collectively, melatonin regulated EMT and PD‐L1 expression in HNSCC cells and exerted synergistic effects with anti‐PD‐1 antibody in vivo. Our study offers new therapeutic strategies for HNSCC treatment.
2. MATERIALS AND METHODS
Details are clarified in Supplemental Materials and Methods.
3. RESULTS
3.1. Urinary aMT6s levels are lower in patients with HNSCC and associated with lymph node metastasis
To determine melatonin levels in patients with HNSCC, we collected first morning urine of 33 patients with HNSCC and 24 patients with benign tumors in the head and neck region, then measured urinary aMT6s using ELISA. As shown in Figure 1A, morning urinary aMT6s concentrations were significantly lower in patients with HNSCC (16.27 ± 1.963 ng/mg creatinine) than in patients with benign tumors (30.39 ± 3.247 ng/mg creatinine). Melatonin level generally decreases as age increases, 23 so the patients were divided into two age groups (≤50‐year old and >50‐year old). Strikingly, in both age groups, urinary aMT6s levels were lower in patients with HNSCC than in patients with benign tumors (Figure 1B,C). In the younger patient group (≤50‐year old), patients with HNSCC with lymph node metastasis had lower urinary aMT6s levels compared with patients with HNSCC without lymph node metastasis (Figure 1F). But there was no statistical difference in urinary aMT6s concentrations among patients with HNSCC with different tumor sizes and pathological grades (Figure 1D,E).
FIGURE 1.
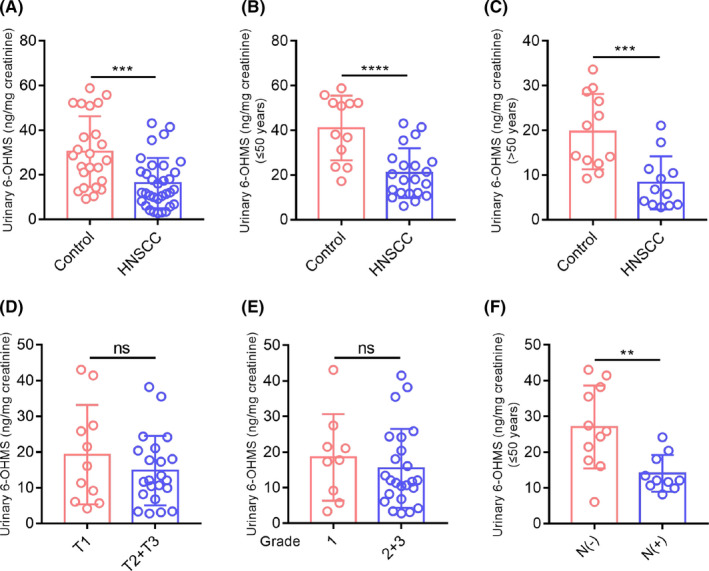
Urinary aMT6s (ng/mg creatinine) levels are lower in patients with HNSCC and are associated with lymph node metastasis. (A) Morning urinary aMT6s concentrations were significantly lower in patients with HNSCC (n = 33) than in patients with benign tumors (n = 24). (B, C) Morning urinary aMT6s concentrations of patients with HNSCC in the younger group (≤50 years old) and older group (>50 years old). (D, E) Morning urinary aMT6s levels of patients with HNSCC with different tumor sizes and different pathological grades. (F) Morning urinary aMT6s levels of patients with HNSCC in the younger group with or without lymph node metastasis. Student's t test, **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; ns, no significance
3.2. Melatonin inhibits EMT of HNSCC cells
First, we found that melatonin inhibited proliferation and induced apoptosis of CAL27 and SCC25 cells in a dose‐dependent manner (Figure S1A,B). As there were no prominent growth‐inhibiting and apoptosis‐inducing effects on both cells at less than 2 mM melatonin, we chose melatonin concentration of 0.5 and 1.0 mM for subsequent experiments. EMT plays important roles in tumor invasion, metastasis, and immune evasion. 24 As melatonin levels are related to lymph node metastasis in HNSCC, as mentioned above, the effects of melatonin on EMT of HNSCC cells were investigated. As shown in Figure 2A–C, melatonin significantly upregulated E‐cadherin expression and downregulated N‐cadherin, vimentin, and Slug levels in CAL27 and SCC25 cells in a dose‐dependent manner. In addition, melatonin transformed CAL27 and SCC25 cells into a cobblestone‐like epithelial morphology (Figure 2D). Wound healing assay and Matrigel invasion assay showed that melatonin markedly inhibited migration and invasion of both cells (Figure 2E–H). Moreover, melatonin significantly attenuated TGF‐β1‐induced EMT of CAL27 and SCC25 cells (Figure 2I). Collectively, these results suggested that melatonin could suppress the EMT of HNSCC cells.
FIGURE 2.
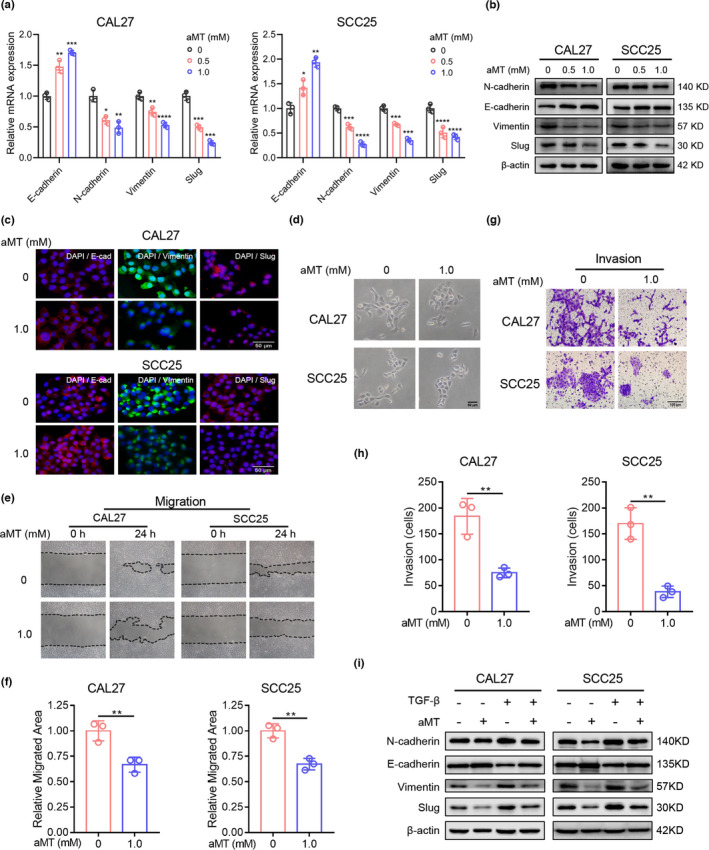
Melatonin inhibits the EMT of HNSCC cells. (A) RT‐qPCR analysis of EMT‐related markers. GAPDH was used as the internal reference gene. One‐way ANOVA. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. (B) Western blot analysis of EMT‐related markers, E‐cadherin, N‐cadherin, vimentin, and Slug in CAL27 and SCC25 cells treated with indicated concentrations of melatonin (aMT) for 24 h. β‐Actin was used as the loading control. (C) Cell immunofluorescence analysis of EMT‐related markers, E‐cadherin, vimentin, and Slug. Scale bar, 50 μm. (D) Representative photographs of the morphology of CAL27 and SCC25 cells treated with melatonin (1 mM) for 24 h. Scale bar, 50 μm. (E–H) Wound healing assay and Matrigel invasion assay were performed and analyzed to determine the migration and invasion abilities of CAL27 and SCC25 cells pretreated with melatonin (1 mM) for 24 h. Scale bar, 100 μm. Student's t test. (I) Western blot analysis of EMT‐related markers in CAL27 and SCC25 cells treated with TGF‐β (10 ng/ml) and melatonin (1 mM) for 48 h. Independent experiments were performed in triplicate. Values are represented as means ± SD
3.3. Melatonin downregulates PD‐L1 expression in HNSCC cells
PD‐L1 expression is related to EMT and plays critical roles in immune evasion in HNSCC. 18 Whether melatonin could affect PD‐L1 expression and PD‐L1‐induced immunosuppression in HNSCC was still unknown. In tissue microarray, we found that PD‐L1 levels were markedly higher in HNSCC tissue than in normal mucosa (Figure S2A,B). Melatonin significantly downregulated PD‐L1 expression in CAL27 and SCC25 cells in dose‐ and time‐dependent manners (Figure 3A–D). Moreover, melatonin markedly suppressed interferon (IFN)‐γ‐induced PD‐L1 upregulation in both cells (Figures 3E and S3A). Then T lymphocytes were isolated from the whole blood of healthy donors and pre‐activated with anti‐CD3/CD28 antibodies for 48 h. Next, we cocultured melatonin‐pretreated CAL27 and SCC25 cells for 48 h with the activated T cells that showed high PD‐1 expression (Figure S4A). In the supernatants of the coculture system, concentration of IFN‐γ, an indicator of T cell activity, significantly increased, indicating that melatonin relieved HNSCC‐induced T cell suppression in a dose‐dependent manner (Figure 3F). Furthermore, we found that TGF‐β1 upregulated PD‐L1 expression in CAL27 and SCC25 cells, and that melatonin markedly inhibited the TGF‐β1‐induced PD‐L1 increase (Figure 3G). In addition, IFN‐γ secretion from T cells decreased when cells were cocultured with TGF‐β1‐pretreated CAL27 and SCC25 cells, but melatonin reversed these effects (Figure 3H). Moreover, pretreated HNSCC cells with melatonin did not further enhance T cell activity when the PD‐1/PD‐L1 signaling was blocked by anti‐PD‐L1 antibody. In addition, the IFN‐γ‐ and TGF‐β1‐induced suppressive effect of HNSCC cells on T cells was relieved by PD‐L1 blocking (Figure S4B–D). Taken together, melatonin could downregulate PD‐L1 expression in HNSCC cells.
FIGURE 3.
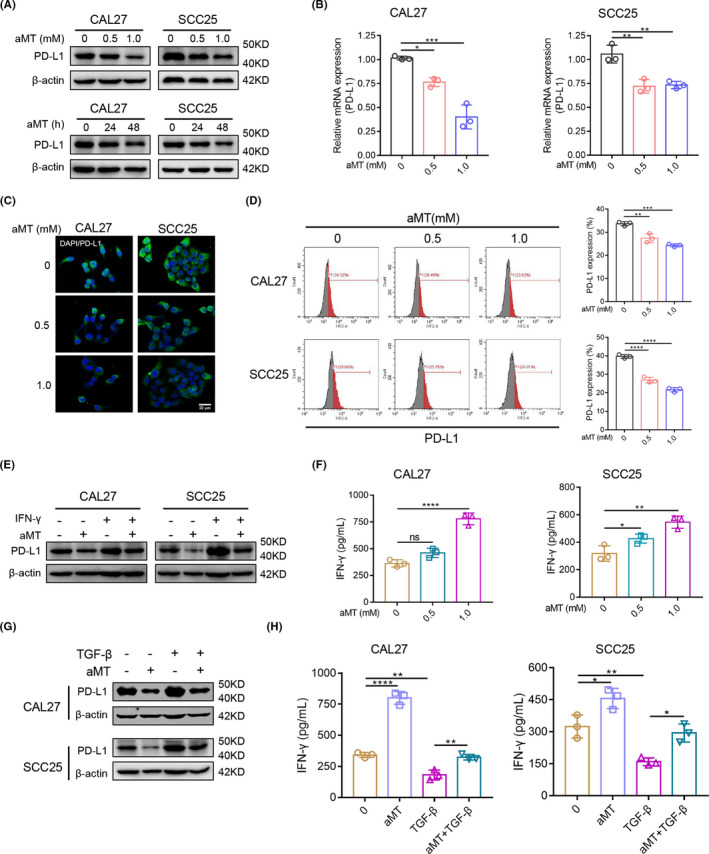
Melatonin downregulates PD‐L1 expression in HNSCC cells. (A) Western blot analysis of PD‐L1 expression in CAL27 and SCC25 cells treated with 0, 0.5, or 1.0 mM melatonin for 24 h, or treated with 1 mM melatonin for 24 or 48 h. (B) RT‐qPCR analysis of PD‐L1 expression in CAL27 and SCC25 cells treated with the indicated concentration of melatonin for 48 h. (C) Cell immunofluorescence was performed to detect PD‐L1 expression in CAL27 and SCC25 cells treated with the indicated concentration of melatonin for 48 h. Scale bar, 20 μm. (D) Flow cytometry analysis of PD‐L1 expression in CAL27 and SCC25 cells treated with the indicated concentration of melatonin for 48 h. (E) Western blot analysis of PD‐L1 expression in CAL27 and SCC25 cells treated with IFN‐γ (20 ng/mL) and melatonin (1 mM) for 48 h. (F) CAL27 and SCC25 cells were treated with melatonin (1 mM) for 48 h, before coculturing with pre‐activated T cells from healthy donors. After 48 h, supernatants were collected to measure IFN‐γ using ELISA. (G) Western blot analysis of PD‐L1 in CAL27 and SCC25 cells treated with TGF‐β (10 ng/ml) and melatonin (1 mM) for 48 h. (H) CAL27 and SCC25 cells were treated as described in Figure 3G before coculturing with pre‐activated T cells from healthy donors. After 48 h of coculture, supernatants were collected to measure IFN‐γ using ELISA. One‐way ANOVA. ns, no significance; *, p < 0.05; **, p < 0.01; ****, p < 0.0001. Independent experiments were performed in triplicate. Values are represented as means ± SD
3.4. Melatonin suppresses phosphorylation of ERK1/2 and FOSL1 expression in HNSCC cells
To investigate the mechanisms underlying the effects of melatonin on EMT and PD‐L1 expression in HNSCC cells, we performed RNA‐seq analysis of CAL27 and SCC25 cells treated with or without 1 mM melatonin for 24 h and obtained 472 differentially expressed genes (DEGs; Figure 4A). KEGG pathway enrichment analysis of DEGs indicated that focal adhesion pathway, metabolic pathways, MAPK signaling pathway, and p53 signaling pathway were mostly affected (Figure 4B). The top 20 identified DEGs (10 upregulated, 10 downregulated) are shown in Figures 4C and S3A. Among these DEGs, the FOSL1 level markedly decreased after melatonin treatment (log2 FC = −1.71430884 in CAL27, log2 FC = −2.600713661 in SCC25). According to the GEPIA database (http://gepia.cancer‐pku.cn/), FOSL1 was at a high level in HNSCC tissues and negatively correlated with the prognosis of patients with HNSCC (Figure S5B,C). However, the relationship between melatonin and FOSL1 had not been uncovered before. Here, western blotting, RT‐qPCR, and immunofluorescence confirmed that melatonin downregulated FOSL1 expression in CAL27 and SCC25 cells (Figure 4D–F). FOSL1 is a downstream effector of the MAPK/ERK1/2 pathway 22 and we found that melatonin mediated the MAPK signaling pathway in HNSCC cells (Figure 4B). The results of western blot analysis demonstrated that melatonin suppressed the phosphorylation of ERK1/2 and expression of FOSL1 in CAL27 and SCC25 cells (Figure 4E). Therefore, these findings suggested that melatonin could suppress the ERK1/2/FOSL1 pathway in HNSCC cells.
FIGURE 4.
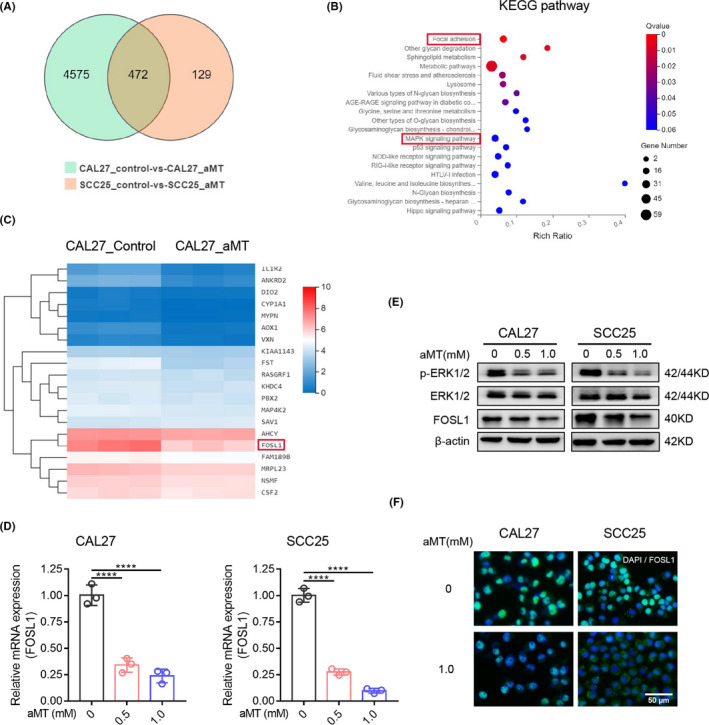
Melatonin suppresses the phosphorylation of ERK1/2 and decreases FOSL1 expression. (A) Venn diagram of differentially expressed genes (DEGs) from RNA‐seq of CAL27 and SCC25 cells treated with or without melatonin (1 mM) for 24 h. (B) KEGG pathway enrichment analysis of DEGs. The top 20 overrepresented pathways were provided. (C) Heat map of expression clustering of the top 20 DEGs identified in CAL27 cells treated with melatonin (1 mM) for 24 h. (D) Verification of FOSL1 expression by RT‐qPCR in CAL27 and SCC25 cells treated with the indicated concentration of melatonin for 24 h. GAPDH was used as the control. One‐way ANOVA. ****, p < 0.0001. (E) Western blot analysis of ERK1/2, p‐ERK1/2, and FOSL1 in CAL27 and SCC25 cells treated with the indicated concentration of melatonin for 48 h. β‐Actin was used as the loading control. (F) Cell immunofluorescence analysis of FOSL1 in CAL27 and SCC25 cells treated with or without melatonin (1 mM) for 48 h. Scale bar, 50 μm. Independent experiments were performed in triplicate. Values are represented as means ± SD
3.5. The ERK1/2/FOSL1 pathway mediates melatonin‐induced inhibition of EMT and PD‐L1 expression in HNSCC
FOSL1 is associated with EMT and metastasis of multiple malignancies and regulates PD‐L1 expression in premalignant human bronchial epithelial cells. 21 Therefore, we explored whether melatonin inhibited EMT and PD‐L1 expression in HNSCC cells through the ERK1/2/FOSL1 pathway. As shown in Figure 5A, MEK inhibitor U0126 significantly decreased p‐ERK1/2, FOSL1 and PD‐L1 levels in CAL27 and SCC25 cells. Furthermore, U0126 upregulated E‐cadherin expression, but downregulated N‐cadherin, vimentin, and Slug levels in both cells (Figure 5B). Then we used FOSL1 overexpression lentivirus (Lv‐FOSL1) to determine the effects of FOSL1 on PD‐L1 expression and EMT. CAL27 and SCC25 cells transfected with Lv‐FOSL1 exhibited significantly upregulated expression of FOSL1 and PD‐L1. Moreover, melatonin markedly suppressed the Lv‐FOSL1‐induced FOSL1 and PD‐L1 upregulation (Figure 5C). Furthermore, in the supernatants from the coculture system, IFN‐γ concentration decreased when T cells were cocultured with FOSL1‐overexpressed CAL27 and SCC25 cells, but increased when FOSL1‐overexpressed HNSCC cells were pretreated with melatonin (Figure 5D).
FIGURE 5.
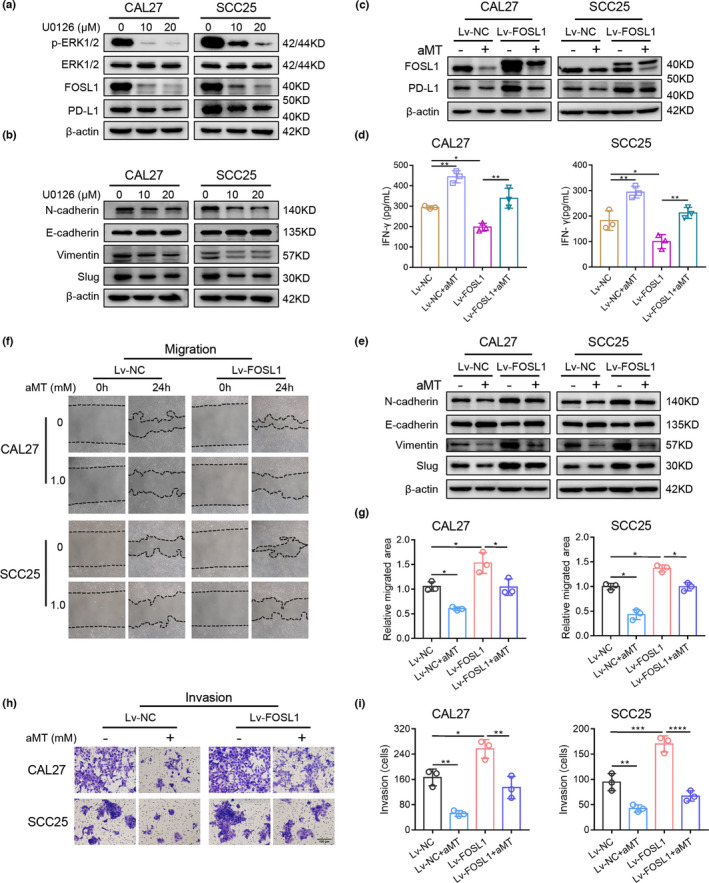
The ERK1/2/FOSL1 Pathway is involved in melatonin‐induced inhibition of EMT and PD‐L1 expression in HNSCC cells. (A) Western blot analysis of p‐ERK1/2, ERK1/2, FOSL1, and PD‐L1 in CAL27 and SCC25 cells treated with 10 and 20 μM MEK inhibitor (U0126) for 24 h. (B) Western blot analysis of EMT‐related markers, E‐cadherin, N‐cadherin, vimentin, and Slug in CAL27 and SCC25 cells treated with 10 or 20 μM U0126 for 24 h. β‐Actin was used as the loading control. (C) CAL27 and SCC25 cells were transfected with an empty carrier lentivirus (negative control, Lv‐NC) or a FOSL1 overexpression lentivirus (Lv‐FOSL1). Lv‐NC or Lv‐FOSL1 cells were treated with or without melatonin (1 mM) for 48 h, then the protein expression levels of FOSL1 and PD‐L1 were determined by western blotting. (D) FOSL1‐overexpressed CAL27 and SCC25 cells were treated as described in Figure 5C before coculturing with pre‐activated T cells from healthy donors. After 48 h of coculture, supernatants were collected to measure IFN‐γ levels using ELISA. One‐way ANOVA. (E) FOSL1‐overexpressed CAL27 and SCC25 cells were treated with or without melatonin (1 mM) for 48 h, then the expression levels of the EMT‐related markers were determined by western blot analysis. (F–I) Wound healing assay and Matrigel invasion assay were performed and analyzed to determine the migration and invasion abilities of FOSL1‐overexpressed CAL27 and SCC25 cells pretreated with melatonin (1 mM) for 24 h. One‐way ANOVA. Scale bar, 100 μm. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. Independent experiments were performed in triplicate. Values are represented as means ± SD
In FOSL1‐overexpressed CAL27 and SCC25 cells, E‐cadherin expression significantly decreased, but levels of N‐cadherin, vimentin, and Slug markedly increased. Interestingly, melatonin reversed the effects of FOSL1 overexpression (Figure 5E). Furthermore, melatonin pre‐treatment significantly suppressed the migration and invasion of both cells promoted by FOSL1 overexpression (Figure 5F–I).
To further investigate the role of FOSL1 in EMT and PD‐L1 expression, an immunodeficient mouse model was established. Lv‐NC and Lv‐FOSL1 CAL27 cells were injected subcutaneously into nude mice separately and treated with vehicle or melatonin (100 mg/kg). The melatonin concentrations in the circulating blood of melatonin‐treated mice were measured using the HPLC‐MS system. After 15 min of injection, the serum melatonin level of the nude mice was ~40 µg/ml (0.17 mM) (Figure S6A). As shown in Figure 6A–C, the untreated Lv‐FOSL1 group exhibited higher tumor volumes and weights than the untreated Lv‐NC group. Melatonin considerably inhibited the growth abilities of Lv‐NC and Lv‐FOSL1 CAL27 cells in vivo. Immunohistochemical staining indicated that the Lv‐FOSL1 group possessed higher PD‐L1 and vimentin expressions and lower E‐cadherin expression than the Lv‐NC group, while melatonin reduced PD‐L1 and vimentin expression and elevated E‐cadherin expression in both groups (Figure 6D,E). Taken together, melatonin suppressed PD‐L1 expression and the EMT of HNSCC through the ERK1/2/FOSL1 pathway.
FIGURE 6.
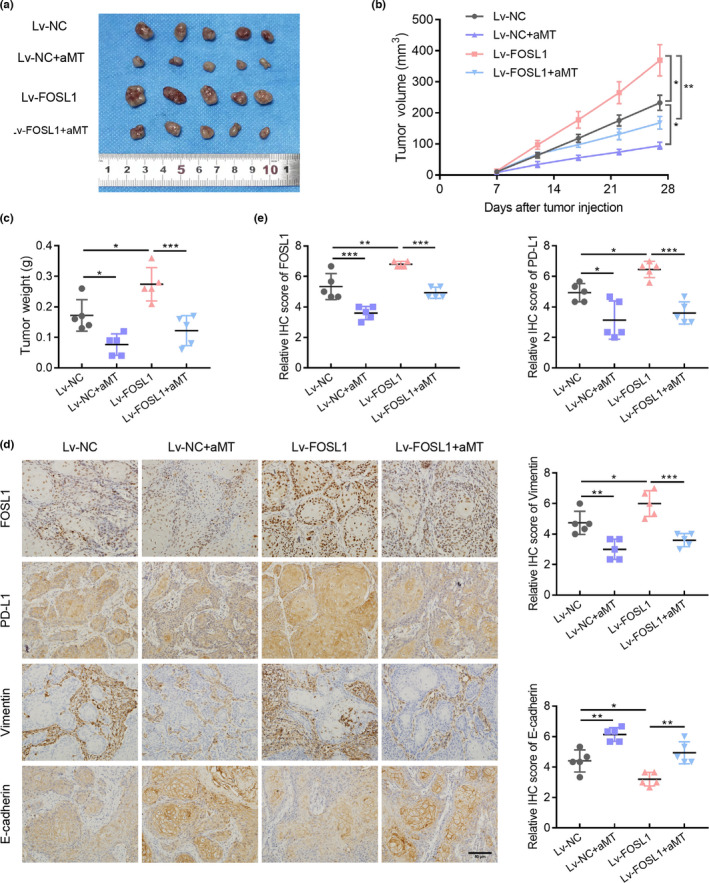
FOSL1 mediates the effects of melatonin on EMT and PD‐L1 expression of HNSCC in vivo. (A) Images of xenograft tumors excised from nude mice. (B) Growth curves of tumors. Tumor volumes were measured every 4 days. n = 5. (C) Weights of tumors excised from the nude mice were measured and analyzed using one‐way ANOVA. (D) Representative immunohistochemistry staining of FOSL1, PD‐L1, vimentin, and E‐cadherin in tumor tissues. Scale bar, 50 μm. (E) Quantification analysis of FOSL1, PD‐L1, vimentin, and E‐cadherin staining. n = 5, one‐way ANOVA. Values are represented as means ± SD, *, p < 0.05; **, p < 0.01; ***, p < 0.001
3.6. Melatonin improves the suppressive effects of anti‐PD‐1 antibody on tumor growth and enhances anti‐tumor immunity in vivo
As we found that melatonin also suppressed PD‐L1 expression in SCC7 cells through the ERK1/2/FOSL1 pathway (Figure S7A,B), whether it exerted synergistic effects on PD‐1 blockade deserved further investigation. Therefore, syngeneic animal models were established by injecting SCC7 cells into the right flanks of C3H mice. Next, we treated these mice with melatonin and anti‐PD‐1 antibody (α‐PD‐1) at different doses following the procedure in Figure 7A. As shown in Figure 7B,C, treatment with melatonin plus low‐dose (5 mg/kg) and normal‐dose (10 mg/kg) α‐PD‐1 considerably reduced tumor volumes, compared with treatment with melatonin alone or α‐PD‐1 alone or vehicle. Moreover, the combination therapy group treated with melatonin plus normal‐dose α‐PD‐1 exhibited the least tumor weight (Figure 7D). Immunohistochemical staining revealed that p‐ERK1/2 and FOSL1 expression decreased in groups that had been treated with melatonin alone or melatonin plus α‐PD‐1 compared with groups treated with vehicle or α‐PD‐1 alone. Interestingly, compared with the control group, the expression of PD‐L1 increased in the remaining four groups. In addition, the melatonin alone and melatonin plus α‐PD‐1 groups displayed lower expression levels of PD‐L1 than the α‐PD‐1 alone group (Figure 7E,F), but there was no statistical significance. Altogether, melatonin could exert synergistic effects with α‐PD‐1 on tumor growth suppression and suppress p‐ERK1/2 and FOSL1 expression in vivo.
FIGURE 7.
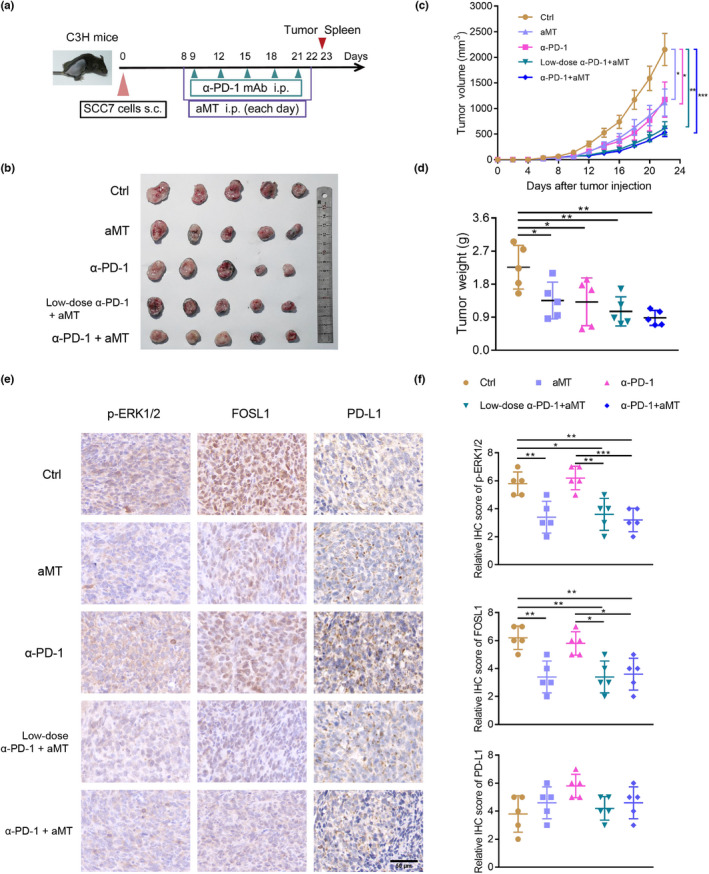
Combination therapy with melatonin and anti‐PD‐1 antibody suppresses tumor growth in vivo. (A) Procedure of animal experiment. In total, 2 × 105 SCC7 cells were injected subcutaneously into the right flanks of 6‐week‐old female C3H mice. The tumor‐bearing mice were divided randomly into 5 groups (n = 5) and administrated with vehicle (5% DMSO + 40% PEG300 + 55% saline), melatonin alone (100 mg/kg), α‐PD‐1 (10 mg/kg) alone, melatonin plus low‐dose α‐PD‐1 (5 mg/kg) and melatonin plus normal‐dose α‐PD‐1 (10 mg/kg), respectively. Vehicle and melatonin administered i.p. was initiated on day 8 and ended on day 22 after SCC7 cell inoculation. Anti‐PD‐1 mAb was administered i.p. on days 9, 12, 15, 18, and 21. All mice were euthanized on day 23. Tumors were excised, weighed, and photographed. Spleens of mice were used for subsequent experiments. (B) Images of SCC7 tumors excised from C3H mice. (C) Growth curves of tumors. Tumor volumes were measured every 2 days. Volumes of different groups on day 22 were analyzed using one‐way ANOVA. (D) Tumor weights were measured and analyzed using one‐way ANOVA. (E) Representative immunohistochemistry staining of p‐ERK1/2, FOSL1, and PD‐L1 in tumor tissues. Scale bar, 50 μm. (F) Quantification analysis of p‐ERK1/2, FOSL1, and PD‐L1 staining. n = 5, one‐way ANOVA. Values are represented as means ± SD, *, p < 0.05; **, p < 0.01; ***, p < 0.001
HNSCC is an immunosuppressive disease with impaired tumor‐infiltrating T lymphocyte functions and the accumulation of suppressive Tregs in TME. 4 To investigate the effects of melatonin on tumor immunity in vivo, we detected the CD8+ T cell proportion in spleens and evaluated the infiltration of CD8+ T cells and FOXP3+ Tregs in the TME of the SCC7/C3H mouse models. We found that the population of CD45+CD3+CD8+ T cells in spleens of tumor‐bearing mice significantly increased in the groups treated with melatonin plus α‐PD‐1 and melatonin alone (Figure 8A,B). Moreover, the number of tumor‐infiltrating CD3+CD8+ T cells in TME increased, while CD4+FOXP3+ Tregs decreased in combined treatment groups (Figure 8C,D). These results suggested that melatonin improved anti‐tumor immunity by promoting effector T cell infiltration and inhibiting regulatory T cell accumulation in the TME of HNSCC.
FIGURE 8.
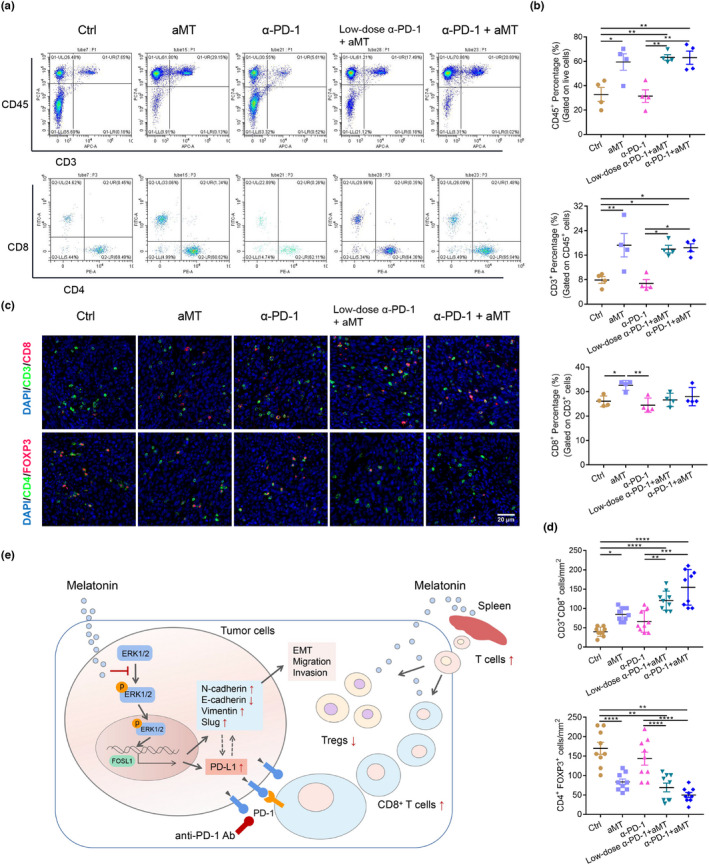
Combination therapy with melatonin and anti‐PD‐1 antibody enhances anti‐tumor immunity. (A) Flow cytometry was performed to determined the T cell population in spleens of tumor‐bearing C3H mice. (B) Percentages of CD45+, CD4+ and CD8+ T cells in spleens were calculated and analyzed using one‐way ANOVA. (C) Representative immunofluorescence images of the tumors (day 23) stained with CD8 (red), CD3 (green), and DAPI (blue); CD4 (green), FOXP3 (red), and DAPI (blue). Scale bar, 20 μm. (D) Numbers of CD3+CD8+ T cells and CD4+FOXP3+ cells per tumor area (mm2) in three randomly selected fields in each tumor section, n = 3 mice. One‐way ANOVA, *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. (E) Schematic diagram illustrates the roles of melatonin in HNSCC. Melatonin inhibits EMT and downregulates PD‐L1 expression through the ERK1/2/FOSL1 pathway. Furthermore, melatonin exerts synergistic effects with anti‐PD‐1 antibody by increasing the CD8+ T cell proportion in spleens and the tumor microenvironment and by reducing Treg infiltration
4. DISCUSSION
HNSCC, the most common malignancy in the head and neck, is an immunosuppressive disease. Immune checkpoint inhibitors, including PD‐1 antibodies, display great benefits in advanced metastatic HNSCC treatment, but low response rates and acquired resistance remain big challenges. 6 , 7 In this study, we found that endogenous melatonin levels decreased in patients with HNSCC and were related to lymph node metastasis. Melatonin markedly inhibited EMT and downregulated PD‐L1 expression in HNSCC through the ERK1/2/FOSL1 pathway in vitro and in vivo. Moreover, melatonin inhibited tumor growth in SCC7/C3H syngeneic mouse models and alleviated immunosuppression by promoting CD8+ T cell infiltration and reducing the Treg proportion, therefore exerting synergistic effects with anti‐PD‐1 antibody. Taken together, our study provided new evidence that melatonin can regulate the EMT and anti‐tumor immunity of HNSCC and enhance the efficacy of antibodies targeting the PD‐L1/PD‐1 axis.
aMT6s is a major urinary metabolite of melatonin and morning urinary aMT6s reflect the nocturnal plasma level of melatonin. 25 , 26 Previous studies have indicated that higher urinary aMT6s levels were correlated with lower risks of breast and prostate cancers. 27 , 28 Ovarian cancer patients retain lower serum melatonin levels compared with healthy women. 29 Moreover, lower serum melatonin concentrations were related to tumor size, invasion depth, and lymph node metastasis, and might predict the severity of oral squamous cell carcinoma. 30 In this study, we found that morning urinary aMT6s levels of patients with HNSCC were relatively low and were associated with lymph node metastasis. However, no statistical difference was observed in urinary aMT6s concentrations among patients with HNSCC with different tumor sizes. The sample size needs to be expanded in the future to obtain more information about the relationship between melatonin levels and tumor size of patients with HNSCC. Collectively, endogenous melatonin levels might be related to HNSCC development and be a biomarker for patients with HNSCC.
Melatonin is a pleiotropic molecule and possesses anti‐tumor abilities. Previous studies have demonstrated that melatonin could suppress tumor proliferation, angiogenesis, invasion, and metastasis. 11 In addition, melatonin enhanced the anti‐tumor effects of cisplatin and rapamycin on HNSCC by regulating mitochondrial function. 31 , 32 However, the roles and mechanisms of melatonin in anti‐tumor immunity have not been well elucidated. Here, we found that melatonin significantly suppressed PD‐L1 expression in HNSCC cells and tumor‐bearing nude mice. Moreover, melatonin exerted synergetic effects with anti‐PD‐1 antibody and enhanced anti‐tumor immunity by promoting CD8+ T cell infiltration and reducing the Treg proportion in TME of SCC7/C3H mouse models. However, compared with the control group, in the remaining four groups the expression of PD‐L1 in tumor tissues slightly increased, which might result from increased CD8+ T cell infiltration. 33 , 34 The melatonin alone and melatonin plus α‐PD‐1 groups displayed lower expression levels of PD‐L1 compared with the α‐PD‐1 alone group, which indicated that melatonin might suppress PD‐L1 expression in SCC7 cells in vivo, but these results need further investigation. PD‐L1 is a critical immune checkpoint that facilitates the immune evasion of tumor cells by exhausting CD8+ cytotoxic T lymphocytes in the TME. 15 Recently, Cheng et al. found that melatonin‐treated hepatocarcinoma cells secreted exosomes to downregulate PD‐L1 expression in macrophages through the STAT3 pathway. 35 Also, melatonin could downregulate PD‐L1 expression in KRAS‐mutant non–small‐cell lung cancer cells through the Hippo pathway. 36 Taken together, our results revealed the immunoregulatory properties of melatonin in HNSCC and provided evidence for melatonin as an adjuvant of PD‐L1/PD‐1 axis‐targeted immunotherapy.
EMT plays vital roles in tumor metastasis. 37 Melatonin can modulate the EMT in multiple solid tumors. 38 , 39 However, the effects of melatonin on the EMT of HNSCC have not been explored. In our study, we found that melatonin could inhibit EMT and suppress the migration and invasion of HNSCC cells in vitro and regulate EMT‐related markers in vivo. Interestingly, accumulating evidence has suggested that the EMT is associated with immunosuppression and PD‐L1 expression in multiple malignancies. 16 , 24 For example, mesenchymal carcinoma cell lines of breast cancer expressed high levels of PD‐L1 and formed an immunosuppressive TME in vivo. 40 Mesenchymal cancer cells in HNSCC tissue exhibited elevated PD‐L1 expression. 17 , 18 Chen et al. found that CMTM6, a vital regulator of PD‐L1, could modulate stemness and the EMT of HNSCC. 41 However, the mechanisms underlying the correlation between EMT and PD‐L1 regulated by melatonin in HNSCC have not been elucidated. Here, through RNA‐seq analysis of HNSCC cells treated with or without melatonin, we found that the transcription factor FOSL1 was the key mediator of EMT and PD‐L1 expression. Melatonin inhibited EMT and downregulated PD‐L1 expression simultaneously through the ERK1/2/FOSL1 pathway. Our study uncovered a new regulatory network behind EMT and PD‐L1 expression in HNSCC.
FOSL1 is an oncogenic transcription factor. Recently, studies have suggested that FOSL1 regulated the EMT in multiple tumors. 19 For instance, SIRT1 promoted the EMT and metastasis of colorectal carcinoma in a FOSL1‐dependent manner. 42 FOSL1 regulated ZEB1/2 and TGF‐β1 expression to induce the EMT of mouse mammary epithelial cells. 43 Furthermore, the KRAS mutation increased PD‐L1 expression through the MEK–ERK–FOSL1 pathway in premalignant human bronchial epithelial cells. 21 However, whether melatonin regulated FOSL1 to alter EMT status and PD‐L1 expression in HNSCC remains unknown. In this study, we found that melatonin significantly suppressed FOSL1 expression by inhibiting the phosphorylation of ERK1/2. Overexpression of FOSL1 induced EMT and upregulated PD‐L1 expression in HNSCC cells and tumor‐bearing nude mice. Altogether, our study revealed that the ERK1/2/FOSL1 pathway mediated the melatonin‐induced inhibition of EMT and PD‐L1 expression in HNSCC.
However, there were some caveats in this study. First, although previous studies have suggested that morning urinary aMT6s concentration could reflect plasma levels of melatonin, collection of urine for more than 24 hours could provide more reliable data about the plasma melatonin levels. 44 Second, there was no statistical difference for tumor volumes and weights between combination therapy and melatonin alone or α‐PD‐1 alone. The reasons for this difference might be the small number of animals and the differences within the groups. Last, the mechanisms behind the effects of melatonin on CD8+ T cells and Treg infiltration in TME need further investigation.
In conclusion, our study demonstrated that melatonin significantly inhibited EMT and downregulated PD‐L1 expression through the ERK1/2/FOSL1 pathway in HNSCC. Melatonin exerted synergistic effects with anti‐PD‐1 antibody and enhanced anti‐tumor immunity in SCC7/C3H mouse models by increasing CD8+ T cell infiltration and decreasing the Treg proportion in TME. Our study elucidated the roles of melatonin in EMT and anti‐tumor immunity, and could provide new strategies for HNSCC therapy.
DISCLOSURE
The authors declare no conflict of interests.
Supporting information
Figures S1‐S7
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge the great help from Dr. Xin Liu and Dr. Qichao Yang (Wuhan University). This work was supported by the National Natural Science Foundation of China (Grant number: 81972547) and the Fundamental Research Funds for the Central Universities (Grant number: 2042020kf0176).
Luo X, Chen Y, Tang H, et al. Melatonin inhibits EMT and PD‐L1 expression through the ERK1/2/FOSL1 pathway and regulates anti‐tumor immunity in HNSCC. Cancer Sci. 2022;113:2232–2245. doi: 10.1111/cas.15338
Contributor Information
Xiaocheng Zhou, Email: zhouxiaocheng@whu.edu.cn.
Zhengjun Shang, Email: shangzhengjun@whu.edu.cn.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chow LQM. Head and Neck Cancer. N Engl J Med. 2020;382:60‐72. [DOI] [PubMed] [Google Scholar]
- 4. Ferris RL. Immunology and Immunotherapy of Head and Neck Cancer. J Clin Oncol. 2015;33:3293‐3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carlisle JW, Steuer CE, Owonikoko TK, Saba NF. An update on the immune landscape in lung and head and neck cancers. CA Cancer J Clin. 2020;70:505‐517. [DOI] [PubMed] [Google Scholar]
- 6. Ward FJ, Dahal LN, Abu‐Eid R. On the road to immunotherapy‐prospects for treating head and neck cancers with checkpoint inhibitor antibodies. Front Immunol. 2018;9:2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kok VC. Current understanding of the mechanisms underlying immune evasion from PD‐1/PD‐L1 immune checkpoint blockade in head and neck cancer. Front Oncol. 2020;10:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pandi‐Perumal SR, Srinivasan V, Maestroni GJM, Cardinali DP, Poeggeler B, Hardeland R. Melatonin: Nature's most versatile biological signal? FEBS J. 2006;273:2813‐2838. [DOI] [PubMed] [Google Scholar]
- 9. Hardeland R. Melatonin and inflammation‐Story of a double‐edged blade. J Pineal Res. 2018;65:e12525. [DOI] [PubMed] [Google Scholar]
- 10. Reiter RJ, Mayo JC, Tan D‐X, Sainz RM, Alatorre‐Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016;61:253‐278. [DOI] [PubMed] [Google Scholar]
- 11. Reiter RJ, Rosales‐Corral SA, Tan D‐X, et al. Melatonin, a Full Service Anti‐Cancer Agent: Inhibition of Initiation, Progression and Metastasis. Int J Mol Sci. 2017;18(4):843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma Q, Reiter RJ, Chen Y. Role of melatonin in controlling angiogenesis under physiological and pathological conditions. Angiogenesis. 2020;23(2):91‐104. [DOI] [PubMed] [Google Scholar]
- 13. Su S‐C, Hsieh M‐J, Yang W‐E, Chung W‐H, Reiter RJ. Cancer metastasis: mechanisms of inhibition by melatonin. J Pineal Res. 2017;62(1):e12370. [DOI] [PubMed] [Google Scholar]
- 14. Moradkhani F, Moloudizargari M, Fallah M, Asghari N, Heidari Khoei H, Asghari MH. Immunoregulatory role of melatonin in cancer. J Cell Physiol. 2020;235:745‐757. [DOI] [PubMed] [Google Scholar]
- 15. Boussiotis VA. Molecular and biochemical aspects of the PD‐1 checkpoint pathway. N Engl J Med. 2016;375:1767‐1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang Y, Zhan H. Communication between EMT and PD‐L1 signaling: New insights into tumor immune evasion. Cancer Lett. 2020;468:72‐81. [DOI] [PubMed] [Google Scholar]
- 17. Jung AR, Jung C‐H, Noh JK, Lee YC, Eun Y‐G. Epithelial‐mesenchymal transition gene signature is associated with prognosis and tumor microenvironment in head and neck squamous cell carcinoma. Sci Rep. 2020;10:3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ock C‐Y, Kim S, Keam B, et al. PD‐L1 expression is associated with epithelial‐mesenchymal transition in head and neck squamous cell carcinoma. Oncotarget. 2016;7:15901‐15914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Talotta F, Casalino L, Verde P. The nuclear oncoprotein Fra‐1: a transcription factor knocking on therapeutic applications’ door. Oncogene. 2020;39:4491‐4506. [DOI] [PubMed] [Google Scholar]
- 20. Zhang M, Hoyle RG, Ma Z, et al. FOSL1 promotes metastasis of head and neck squamous cell carcinoma through super‐enhancer‐driven transcription program. Mol Ther. 2021;29:2583‐2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee M‐H, Yanagawa J, Tran L, et al. FRA1 contributes to MEK‐ERK pathway‐dependent PD‐L1 upregulation by KRAS mutation in premalignant human bronchial epithelial cells. Am J Transl Res. 2020;12:409‐427. [PMC free article] [PubMed] [Google Scholar]
- 22. Dhillon AS, Tulchinsky E. FRA‐1 as a driver of tumour heterogeneity: a nexus between oncogenes and embryonic signalling pathways in cancer. Oncogene. 2015;34:4421‐4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schernhammer ES, Kroenke CH, Dowsett M, Folkerd E, Hankinson SE. Urinary 6‐sulfatoxymelatonin levels and their correlations with lifestyle factors and steroid hormone levels. J Pineal Res. 2006;40:116‐124. [DOI] [PubMed] [Google Scholar]
- 24. Terry S, Savagner P, Ortiz‐Cuaran S, et al. New insights into the role of EMT in tumor immune escape. Mol Oncol. 2017;11:824‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Graham C, Cook MR, Kavet R, Sastre A, Smith DK. Prediction of nocturnal plasma melatonin from morning urinary measures. J Pineal Res. 1998;24:230‐238. [DOI] [PubMed] [Google Scholar]
- 26. Cook MR, Graham C, Kavet R, Stevens RG, Davis S, Kheifets L. Morning urinary assessment of nocturnal melatonin secretion in older women. J Pineal Res. 2000;28:41‐47. [DOI] [PubMed] [Google Scholar]
- 27. Kubatka P, Zubor P, Busselberg D, et al. Melatonin and breast cancer: evidences from preclinical and human studies. Crit Rev Oncol Hematol. 2018;122:133‐143. [DOI] [PubMed] [Google Scholar]
- 28. Sigurdardottir LG, Markt SC, Rider JR, et al. Urinary melatonin levels, sleep disruption, and risk of prostate cancer in elderly men. Eur Urol. 2015;67:191‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao M, Wan J, Zeng K, et al. The Reduction in Circulating Melatonin Level May Contribute to the Pathogenesis of Ovarian Cancer: A Retrospective Study. J Cancer. 2016;7:831‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stanciu AE, Zamfir‐Chiru‐Anton A, Stanciu MM, et al. Clinical significance of serum melatonin in predicting the severity of oral squamous cell carcinoma. Oncol Lett. 2020;19:1537‐1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shen Y‐Q, Guerra‐Librero A, Fernandez‐Gil BI, et al. Combination of melatonin and rapamycin for head and neck cancer therapy: Suppression of AKT/mTOR pathway activation, and activation of mitophagy and apoptosis via mitochondrial function regulation. J Pineal Res. 2018;64(3):e12461. [DOI] [PubMed] [Google Scholar]
- 32. Fernandez‐Gil BI, Guerra‐Librero A, Shen Y‐Q, et al. Melatonin enhances cisplatin and radiation cytotoxicity in head and neck squamous cell carcinoma by stimulating mitochondrial ROS generation, apoptosis, and autophagy. Oxid Med Cell Longev. 2019;2019:7187128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spranger S, Spaapen RM, Zha Y, et al. Up‐regulation of PD‐L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abiko K, Matsumura N, Hamanishi J, et al. IFN‐γ from lymphocytes induces PD‐L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112:1501‐1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheng L, Liu J, Liu Q, et al. Exosomes from Melatonin Treated Hepatocellularcarcinoma Cells Alter the Immunosupression Status through STAT3 Pathway in Macrophages. Int J Biol Sci. 2017;13:723‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chao Y‐C, Lee K‐Y, Wu S‐M, Kuo D‐Y, Shueng P‐W, Lin C‐W. Melatonin Downregulates PD‐L1 expression and modulates tumor immunity in KRAS‐mutant non‐small cell lung cancer. Int J Mol Sci. 2021;22(11):5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lu W, Kang Y. Epithelial‐mesenchymal plasticity in cancer progression and metastasis. Dev Cell. 2019;49:361‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang X, Wang B, Xie J, Hou D, Zhang H, Huang H. Melatonin inhibits epithelial‐to‐mesenchymal transition in gastric cancer cells via attenuation of IL‐1β/NF‐κB/MMP2/MMP9 signaling. Int J Mol Med. 2018;42:2221‐2228. [DOI] [PubMed] [Google Scholar]
- 39. Chao C‐C, Chen P‐C, Chiou P‐C, et al. Melatonin suppresses lung cancer metastasis by inhibition of epithelial‐mesenchymal transition through targeting to Twist. Clin Sci (Lond). 2019;133:709‐722. [DOI] [PubMed] [Google Scholar]
- 40. Dongre A, Rashidian M, Reinhardt F, et al. Epithelial‐to‐mesenchymal transition contributes to immunosuppression in breast carcinomas. Cancer Res. 2017;77:3982‐3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen L, Yang Q‐C, Li Y‐C, et al. Targeting CMTM6 suppresses stem cell‐like properties and enhances antitumor immunity in head and neck squamous cell carcinoma. Cancer Immunol Res. 2020;8:179‐191. [DOI] [PubMed] [Google Scholar]
- 42. Cheng F, Su L, Yao C, et al. SIRT1 promotes epithelial‐mesenchymal transition and metastasis in colorectal cancer by regulating Fra‐1 expression. Cancer Lett. 2016;375:274‐283. [DOI] [PubMed] [Google Scholar]
- 43. Bakiri L, Macho‐Maschler S, Custic I, et al. Fra‐1/AP‐1 induces EMT in mammary epithelial cells by modulating Zeb1/2 and TGFβ expression. Cell Death Differ. 2015;22:336‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lockley SW. Journal of Pineal Research guideline for authors: Measuring melatonin in humans. J Pineal Res. 2020;69:e12664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1‐S7
Supplementary Material


