Abstract
The tumor microenvironment (TME) is related to chronic inflammation and is currently identified as a risk factor for the occurrence and development of endometrial cancer (EC). Pyroptosis is a new proinflammatory form of programmed cell death that plays a critical role in the progression of multiple diseases. However, the important role of pyroptosis in high‐glucose (HG)‐related EC and the underlying molecular mechanisms remain elusive. In the present study, transcriptome high‐throughput sequencing revealed significantly higher hexokinase domain‐containing 1 (HKDC1) expression in EC patients with diabetes than in EC patients with normal glucose. Mechanistically, HKDC1 regulates HG‐induced cell pyroptosis by modulating the production of reactive oxygen species and pyroptosis‐induced cytokine release in EC. In addition, HKDC1 regulates TME formation by enhancing glycolysis, promoting a metabolic advantage in lactate‐rich environments to further accelerate EC progression. Subsequently, miR‐876‐5p was predicted to target the HKDC1 mRNA, and HOXC‐AS2 was identified to potentially inhibit the miR‐876‐5p/HKDC1 axis in regulating cell pyroptosis in HG‐related EC. Collectively, we elucidated the regulatory role of the HOXC‐AS2/miR‐876‐5p/HKDC1 signal transduction axis in EC cell pyroptosis at the molecular level, which may provide an effective therapeutic target for patients with diabetes who are diagnosed with EC.
Keywords: endometrial cancer, high glucose, HKDC1, pyroptosis, tumor microenvironment
We elucidated the regulatory role of the HOXC‐AS2/miR‐876‐5p/HKDC1 signal transduction axis in endometrial cancer (EC) cell pyroptosis at the molecular level, which may provide an effective therapeutic target for patients with diabetes who are diagnosed with EC.
Abbreviations
- ASC
PYD and CARD domain‐containing
- DM
diabetes mellitus
- EC
endometrial cancer
- ECAR
extracellular acidification rate
- EMT
epithelial‐mesenchymal transition
- GALE
UDP‐galactose‐4‐epimerase
- GALNT6
polypeptide N‐acetylgalactosaminyltransferase 6
- GCNT3
glucosaminyl (N‐acetyl) transferase 3
- GO
gene ontology
- HG
high glucose
- HKDC1
hexokinase domain‐containing 1
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LDH
lactate dehydrogenase
- LncRNA
long noncoding RNAs
- miRNA
microRNA
- NC
negative control
- NG
normal glucose
- ROS
reactive oxygen species
- siRNA
small interfering RNA
- TME
tumor microenvironment
1. INTRODUCTION
Endometrial cancer (EC) is the most common gynecological malignancy in Europe and North America. The incidence of EC in these areas has increased significantly in recent decades, driven mainly by the diabetes mellitus (DM) and obesity epidemic. 1 , 2 Tumor microenvironment (TME) has recently received increasing attention because it affects the occurrence and progression of cancer through various mechanisms. 3 , 4 DM and obesity are high‐energy, proinflammatory diseases that form a nutrient‐saturated environment by increasing the activity of a series of signal transduction pathways, which ultimately leads to the proliferation, migration, and invasion of EC cells. 5 According to several studies, the TME influences the progression of breast cancer, glioblastoma, and gastric cancer. 6 , 7 , 8 Current research on the biological relationship between the TME and hyperglycemia‐associated EC is limited, and an extremely meaningful approach is to explore more inflammatory pathways that will improve our understanding of EC pathogenesis.
Pyroptosis is a new proinflammatory form of programmed cell death activated by certain inflammasomes, resulting in the cleavage of gasdermin D and maturation of the proinflammatory cytokines IL‐18 and IL‐1β, which then cause a series of inflammatory cascades. 9 , 10 , 11 A large number of studies have provided evidence that pyroptosis is involved in the development of cardiovascular diseases, malignancy, neurological diseases, and infectious diseases. 12 , 13 , 14 , 15 Recently, pyroptosis has been reported to modulate the proliferation, migration, and invasion of colorectal pancreatic cancer cells. 16 For example, mammalian STE20‐like kinase 1 (MST1) suppresses pancreatic cancer progression via reactive oxygen species (ROS)‐induced pyroptosis. 17 Importantly, a recent study identified a classical pyroptosis pathway that occurs in EC. 18 However, the relationship between high glucose (HG) levels and pyroptosis remains inadequately investigated in EC.
Long noncoding RNAs (lncRNAs) and short noncoding RNAs (miRNAs) are associated with EC onset, metastasis, and prognosis. 19 , 20 For instance, lncRNA LOXL1‐AS1 promotes EC progression by sponging miR‐28‐5p to upregulate RAP1B expression. 21 LncRNA SNHG14 promotes EC cell proliferation by regulating microRNA‐655‐3p. 22 LncRNA RP11‐395G23.3 suppresses EC progression by regulating the microRNA‐205‐5p/PTEN axis. 23 Although some advances have been made in studying the biological function of lncRNAs in EC, researchers have not clearly reported whether or not lncRNAs are involved in regulating hyperglycemia‐induced pyroptosis in EC.
We therefore conducted the present study to identify new functional genes and clarify their roles in hyperglycemia‐related EC. Meanwhile, this information might help clinicians develop new, efficacious, nonsurgical alternatives for patients unsuitable for surgery, as well as for women with advanced and recurrent EC.
2. MATERIAL AND METHODS
Expanded Methods can be found in Appendix S1.
2.1. Samples from patients with endometrial cancer
All endometrial tissue samples used for RNA sequencing, immunohistochemistry, and qRT‐PCR validation were obtained from 60 patients with EC (30 with severe diabetes and 30 without diabetes) who had received operative treatment at the First Affiliated Hospital of Xiamen University (Table 1). The conditions of the obtained tissue samples were the same as those described previously. 24 The normal glucose level in human blood is 100 mg/dl, whereas the glucose levels of patients with severe diabetes are almost five times higher than the physiological level. None of these patients received radiotherapy or chemotherapy before surgery. Additionally, endometrial tissue was collected from 30 patients with uterine leiomyoma who underwent hysterectomy, and these specimens were pathologically confirmed to be normal endometrium. This study was approved by the Ethics Committee of the First Affiliated Hospital of Xiamen University (2022016), and all patients provided informed consent before the study began.
TABLE 1.
Clinicopathological characteristics of study subjects
| Clinicopathological data |
EC (n = 60) |
Control (n = 30) |
P |
|---|---|---|---|
| Age | |||
| <55 | 28 | 16 | >0.05 |
| ≥55 | 32 | 14 | |
| Surgical stage (FIGO 2009) | |||
| I | 34 | – | – |
| II | 17 | – | |
| III | 9 | – | |
| Histological grade | |||
| G1 | 20 | – | – |
| G2 | 30 | – | |
| G3 | 10 | – | |
| Myometrial invasion | |||
| ≤1/2 | 30 | – | – |
| >1/2 | 30 | – | |
| Lymph‐node metastasis | |||
| Positive | 7 | – | – |
| Negative | 53 | – | |
Abbreviations: Control, normal endometrium tissue from 30 patients with uterine leiomyoma were recruited as a control group; EC, endometrial cancer.
2.2. Immunohistochemical staining
Immunohistochemical staining and the quantitative evaluation were performed as described in our previous study. 25 Briefly, the tissue sections were incubated with antibodies (anti‐NLRP3 1:50, anti‐HKDC1 1:50, anti‐IL18 1:50) overnight at 4 °C. After appropriate secondary antibodies were reacted with the sections for 15 min at 37 °C, 3,3‐diaminobenzidine (DAB) and hematoxylin were used for counterstaining. Immunoreactivity of HKDC1, NLRP3, and IL18 in EC tissue was evaluated by the H‐score method, which incorporates both the positive intensity of staining and the percentage of positive‐stained cells. 24
2.3. Statistical analysis
Data are presented as the means ± SD of at least three independent experiments. GraphPad Prism 8 software (GraphPad Software Inc.) was used for statistical analyses. Statistical analyses were performed with Student's t‐test or one‐way ANOVA followed by Tukey's test, where appropriate. A statistically significant difference was considered as P < 0.05.
3. RESULTS
3.1. The role of high‐glucose‐induced pyroptosis in EC
Normal endometrium and tumor tissues from patients with EC presenting normal blood glucose levels and complicated with DM were collected to determine whether pyroptosis occurs in HG‐related EC. Immunohistochemistry was used to monitor the expression of the pyroptosis‐related proteins NLRP3 and IL18 in vivo. As shown in Figure 1A, NLRP3 and IL18 immunoreactivity in the tumor tissues of patients with EC complicated with DM was significantly higher than that in normal endometrium and tissues from patients with EC presenting normal blood glucose levels. Moreover, Ishikawa and Hec‐1‐B cells were treated with medium (no glucose, NO), low glucose (1 mM, LG), normal glucose (5 mM, NG), and high glucose (25 mM, HG) to elucidate the role of pyroptosis in HG‐related EC in vitro. Glucose levels of 5 and 25 mM are equivalent to the normal physiological level in human blood (100 mg/dl) and the level of a patient with uncontrolled severe diabetes, respectively. 26 The results from western blot assay show that the expression of Caspase1 (classical pyroptosis pathway) but not Caspase4 (nonclassical pyroptosis pathway) was increased by glucose in a dose‐dependent manner (Figure S1A). Increased PI positive cells was found in Ishikawa and Hec‐1‐B cells treated with high glucose compared with those for the control cells without glucose incubation (Figure S1B). HG‐treated cells were exposed to VX‐765, a specific inhibitor of pyroptosis. Increased proliferation and migration were observed in HG‐treated cells, changes that were eliminated by VX‐765 (Figure 1B,C). These results indicate that pyroptosis is increased in patients with EC complicated with DM and that HG‐induced pyroptosis may affect the proliferation and migration of EC cells.
FIGURE 1.
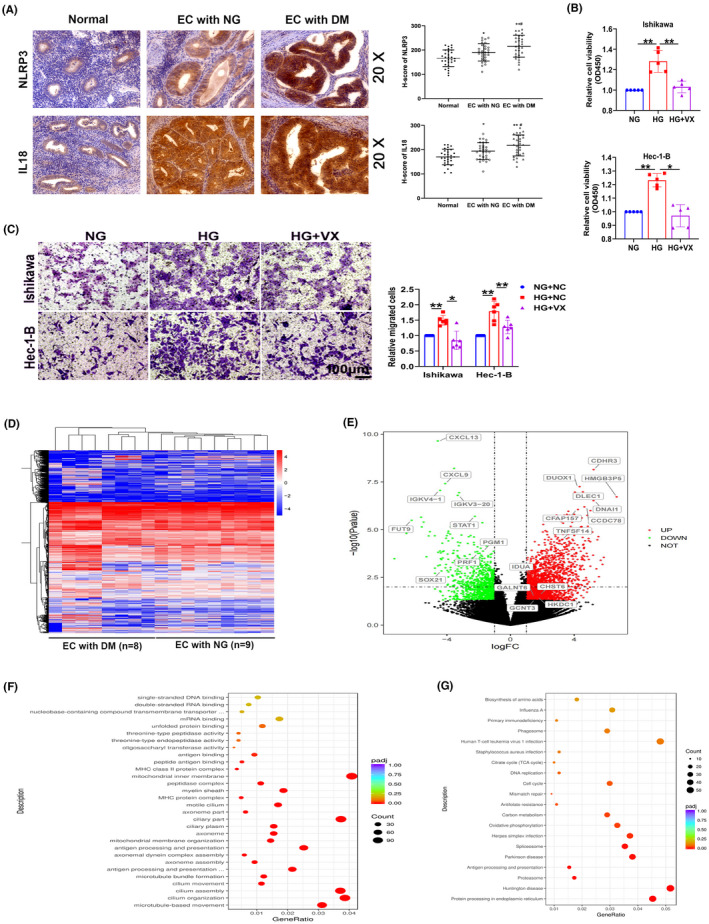
Pyroptosis is involved in EC progression. (A) Representative images of immunohistochemical staining for NLRP3 and IL18 in normal endometrial tissues (normal), tumor tissues from patients with EC presenting normal blood glucose levels (EC with NG), and tumor tissues from patients with EC complicated with diabetes mellitus (EC with DM), (n = 30). (B, C) CCK‐8 and transwell assays were used to detect the proliferation and migration capacity of Ishikawa and Hec‐1‐B cells treated with the pyroptosis inhibitor VX‐765 (VX), (n = 5–6). (D) RNA sequencing data from EC tissues from patients (n = 17). (E) Volcano map of RNA expression in EC tissues. (F‐G) Gene Ontology (GO) and Kyoto Gene and Genomic Encyclopedia (KEGG) analyses of differentially expressed genes. All values are presented as the means ± SD, * P < 0.05 and ** P < 0.01 compared with normal, # P < 0.05 compared with EC with NG (A), * P < 0.05 and ** P < 0.01 (B, C). EC, endometrial cancer; HG, high glucose; NG, normal glucose
3.2. Hexokinase domain‐containing protein 1 is associated with HG‐related endometrial cancer
High‐throughput transcriptome sequencing was performed to profile differentially expressed genes in tumor tissues from patients with EC presenting normal blood glucose levels and complicated with DM (Figure 1D). As shown in Figure 1E, 2742 genes were upregulated and 1830 genes were downregulated (P < 0.05). Subsequently, the correlations of significantly differentially expressed genes with HG‐related EC were analyzed by performing GO and KEGG analyses (Figure 1F,G). Furthermore, among the significantly upregulated genes, four glycometabolism‐related genes were first studied: hexokinase domain‐containing protein 1 (HKDC1), polypeptide N‐acetylgalactosaminyltransferase 6 (GALNT6), glucosaminyl (N‐acetyl) transferase 3 (GCNT3), and UDP‐galactose‐4‐epimerase (GALE). We detected their expression levels in tissues from patients with EC. Results showed the significant upregulation of HKDC1, GALNT6, and GALE expression in tumor tissues from patients with EC complicated with diabetes, consistent with the RNA sequencing results (Figure 2A). Next, the expression of HKDC1, GALNT6, and GALE mRNA was measured in Ishikawa and Hec‐1‐B cells, and the results confirmed the upregulation of HKDC1 but not GALNT6 and GALE in the HG group (Figure 2B). Increased expression of the HKDC1 protein in Ishikawa and Hec‐1‐B cells was also observed after HG treatment (Figure 2C). Immunohistochemistry was performed to obtain insights into the expression of HKDC1 in normal endometrium and EC tissues. We found higher HKDC1 immunoreactivity in the tissues from patients with EC complicated with DM than in the other groups, as shown in Figure 2D. All of these results revealed that HKDC1 expression was increased in HG‐related EC.
FIGURE 2.
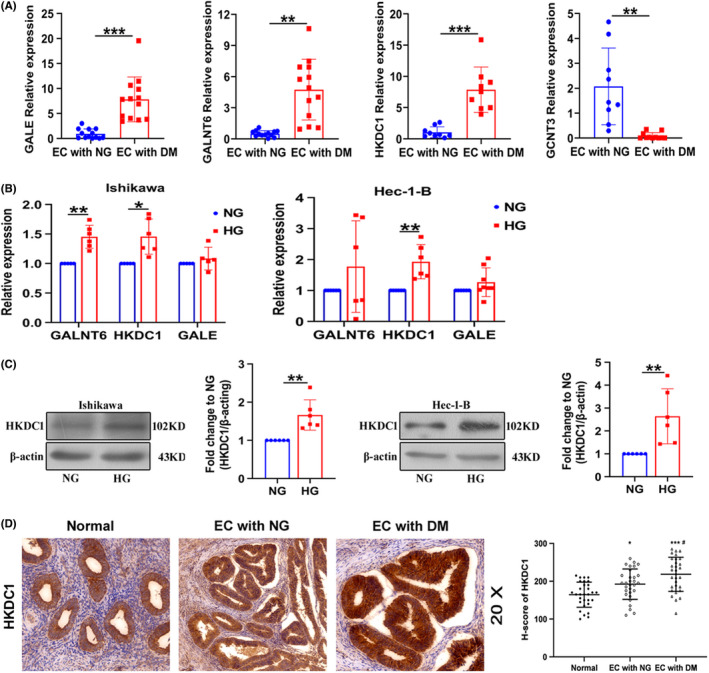
Aberrant expression of HKDC1 in vivo and in vitro. (A) qRT‐PCR analysis of GALE, GALNT6, HKDC1, and GCNT3 expression in tumor tissues from patients with EC presenting normal blood glucose levels (NG) and patients with EC complicated with diabetes mellitus (DM; n = 9–13). (B) qRT‐PCR analysis of GALE, GALNT6, and HKDC1 expression in EC cells (n = 6). (C) Western blot analysis was used to detect HKDC1 levels in EC cells treated with high glucose (n = 6). (D) Representative images of immunohistochemical staining for HKDC1 in endometrial tissues (n = 30). All values are presented as the means ± SD, * P < 0.05, ** P < 0.01, and *** P < 0.001 (A‐C), * P < 0.05 and *** P < 0.001 compared with normal, # P < 0.05 compared with EC with NG (D). EC, endometrial cancer; HG, high glucose; NG, normal glucose
3.3. Hexokinase domain‐containing protein 1 mediates HG‐induced pyroptosis
Hexokinase domain‐containing protein 1 protein expression was knocked down by using specific siRNAs in EC cells to determine the role of HKDC1 in HG‐induced pyroptosis (Figure 3A). We then measured the expression of pyroptosis‐related proteins in cells transfected with HKDC1 siRNAs and found that si‐HKDC1 decreased the upregulation of NLRP3, Caspase1, pro‐Caspase1, IL‐1β, and PYD and CARD domain‐containing (ASC) in HG‐treated cells, as shown in Figure 3B. In addition, increased PI uptake and LDH release further confirmed that pyroptosis was induced by HG, which was reversed by HKDC1 silencing (Figure 3C,D). The metabolic patterns of glycolysis were monitored in real time using a Seahorse XFe24 extracellular flux analyzer to determine the role of HKDC1 in glucose metabolism in EC. HG activated glycolytic stress, as evidenced by increased extracellular acidification rates, which were blocked by siHKCD1‐1 and siHKCD1‐3 (Figure 3E). These results provide the first evidence that HKDC1 is involved in the mechanism regulating EC cell pyroptosis and glycolysis induced by HG, and the increased IL‐1β levels and extracellular acidification rate may contribute to the formation of a suitable proinflammatory and acidic TME for EC cell growth.
FIGURE 3.
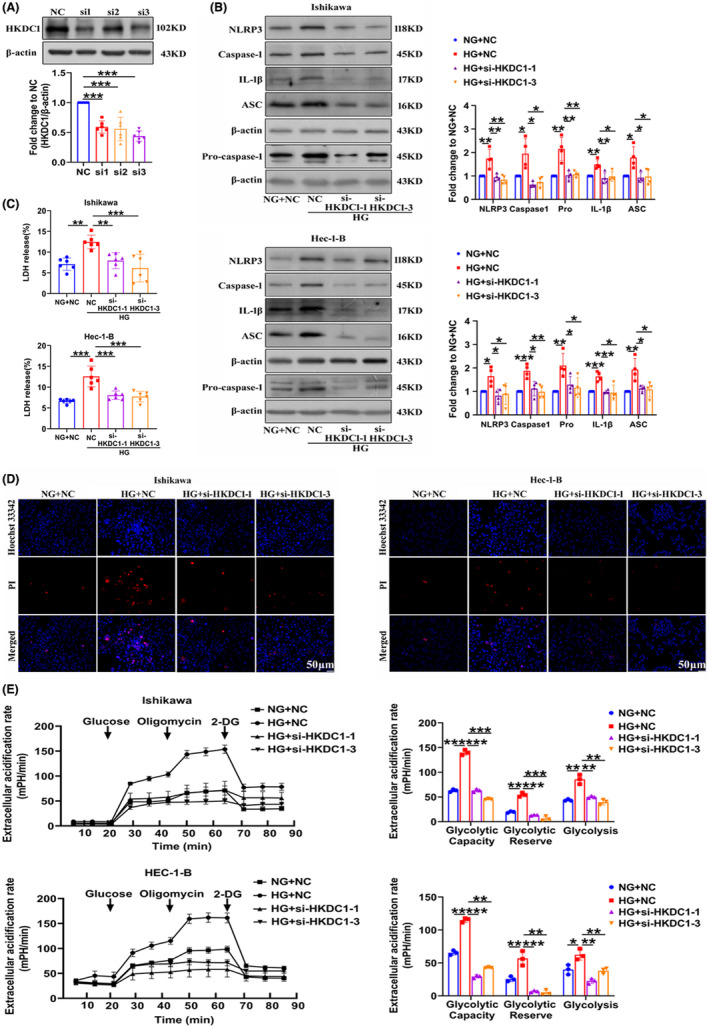
HKDC1 regulates pyroptosis and glycolysis in Ishikawa and Hec‐1‐B cells exposed to HG. (A) The interference efficiency of the HKDC1 siRNAs (n = 6). (B) The expression of pyroptosis‐related proteins in EC cells transfected with NC and HKDC1 siRNAs cultured under high and normal glucose conditions (n = 4). (C) The changes in LDH release from EC cells transfected with NC and HKDC1 siRNAs were measured (n = 6). (D) Representative cells staining with PI (red) and Hoechst 33342 (blue), (n = 3), scale bars = 50 µm. (E) Glycolysis, glycolytic reserve, and nonglycolytic acidification in EC cells were measured using a Seahorse XFe24 extracellular flux analyzer (n = 3). All values are presented as the means ± SD, * P < 0.05, ** P < 0.01, and *** P < 0.001. EC, endometrial cancer; HG, high glucose; NC, negative control; NG, normal glucose; si‐HKDC1, small interfering RNA targeting HKDC1
3.4. Hexokinase domain‐containing protein 1 inhibits HG‐induced EC cell pyroptosis by targeting the ROS pathway
Given that HKDC1 is localized in mitochondria, we hypothesized that HKDC1 interacts with pyroptotic genes indirectly. 27 According to previous studies, mitochondrial ROS are the major factors promoting NLRP3 inflammasome‐dependent pyroptotic cell death. 28 Thus, we proceeded to elucidate whether HKDC1‐mediated pyroptosis in HG‐induced EC cells was depended on ROS. Increased ROS levels were detected in HG‐treated EC cells, which were inhibited by HKDC1 silencing (Figure 4A). Then, we incubated Ishikawa and Hec‐1‐B cells with the ROS‐specific inhibitor TEMPOL to confirm the role of ROS in HG‐induced cell pyroptosis. As shown in Figure 4B, TEMPOL reduced the expression of NLRP3, Caspase1, pro‐Caspase1, IL‐1β, and ASC in HG‐treated EC cells. At the same time, the results of PI staining and LDH release analyses showed that pyroptosis was obviously inhibited in the HG + TEMPOL group compared to the HG group (Figure 4C,D), suggesting that the pyroptosis of HG‐treated EC cells was depended on ROS. Finally, we found that the expression of HKDC1 was unchanged after treated cells with TEMPOL compared with HG group, suggesting that HKDC1 is not regulated by ROS and HKDC1 may be the upstream of ROS (Figure 4E).
FIGURE 4.
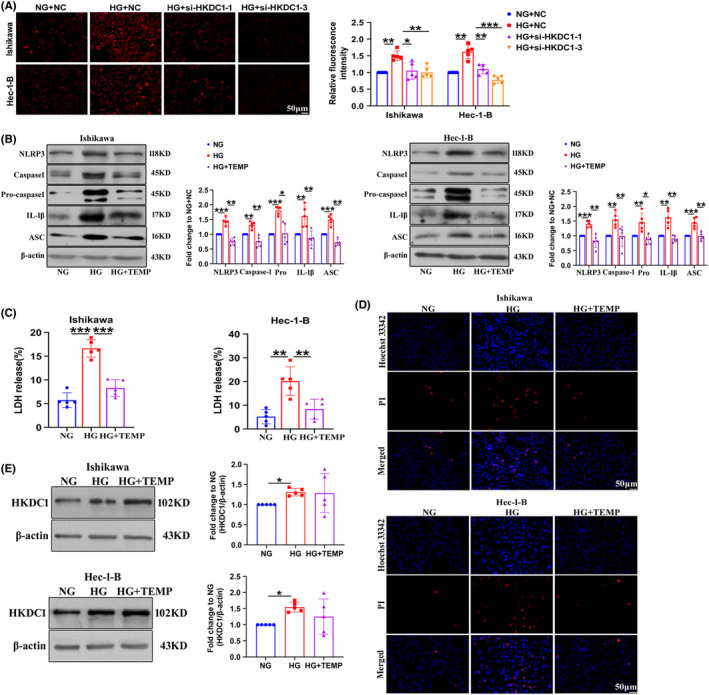
TEMPOL prevents the HG‐induced pyroptosis of Ishikawa and Hec‐1‐B cells. (A) Mito‐SOX Red (1 µM), a mitochondrial superoxide indicator, was used to detect mitochondrial‐derived ROS production in EC cells (n = 6). (B) The expression of pyroptosis‐related proteins in EC cells treated with TEMPOL (n = 5). (C) LDH release from EC cells treated with TEMPOL (n = 5). (D) TEMPOL reduced the PI‐positive staining (red) induced by HG in EC cells (n = 3). Scale bars = 50 µm. (E) The expression of HKDC1 was determined in EC cell lines after exposure to HG for 24 h. All values are presented as the means ± SD, * P < 0.05, ** P < 0.01, and *** P < 0.001. EC, endometrial cancer; HG, high glucose; NC, negative control; NG, normal glucose; si‐HKDC1, small interfering RNA targeting HKDC1; TEMP, TEMPOL
3.5. MiR‐876‐5p targets HKDC1
MiRNAs are able to regulate downstream gene expression and thus play an important role in the development of many diseases, including cancer and cardiovascular diseases. 29 We first predicted upstream miRNAs associated with HKDC1 through TargetScan (http://www.targetscan.org/). MiR‐3167 and miR‐876‐5p were found to potentially target HKDC1, and the representative putative target binding sites are shown in Figure 5A. The expression of miR‐876‐5p was remarkably downregulated by HG and the differential expression of miR‐3167 was not detected (Figure 5B). Moreover, we found that miR‐876‐5p expression was significantly decreased in tumor tissues from patients complicated with DM (Figure 5C). A space structure docking model between HKDC1 and miR‐876‐5p was predicted using the HDOCK Server (http://huanglab.phys.hust.edu.cn/; Figure 5D). As shown in Figure 5E, miR‐876‐5p agomir (mimics) had a negative effect on HKDC1 protein expression in HG‐treated Ishikawa and Hec‐1‐B cells. Luciferase reporter gene assays were performed using HKDC1 3'‐UTR sequence fragments containing the predicted miR‐876‐5p binding site and a mutated site to verify the relationship between miR‐876‐5p and HKDC1. The luciferase activity of the wild‐type HKDC1 3'‐UTR was significantly decreased compared with that of the control, whereas that of the mutant HKDC1 3'‐UTR did not change significantly (Figure 5F). Based on these results, there is a great possibility that miR‐876‐5p interacts with HKDC1 in EC cells.
FIGURE 5.
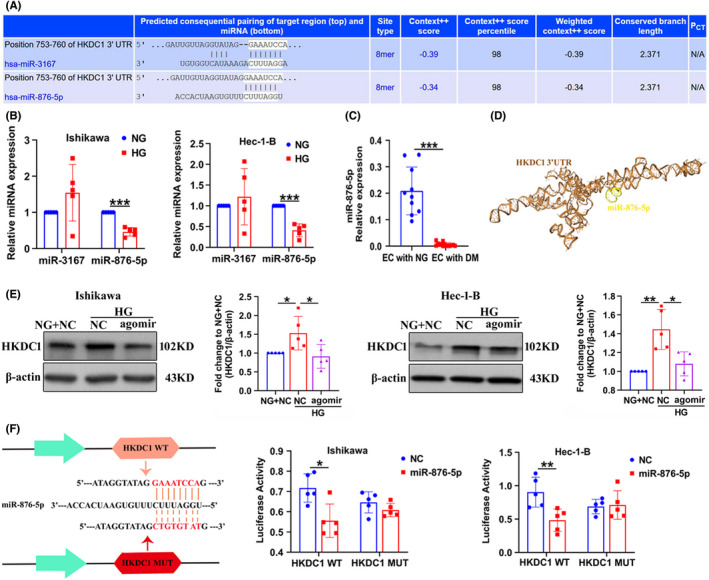
HOXC‐AS2 binds to miR‐876‐5p and regulates HKDC1 expression. (A) MiRNAs targeting HKDC1 were predicted by the TargetScan database. (B) qRT‐PCR analysis of miR‐3176 and miR‐876‐5p expression in EC cells treated with NG and HG (n = 5). (C) qRT‐PCR analysis of miR‐876‐5p expression in tumor tissues from patients with EC presenting normal blood glucose levels (NG) and tumor tissues from patients with EC complicated with diabetes mellitus (DM; n = 10). (D) Space structure docking model of HKDC1 and miR‐876‐5p, as predicted by the HDOCK server. (E) Western blot analysis of HKDC1 expression in Ishikawa and Hec‐1‐B cells after miR‐876‐5p agomir (mimics) was introduced (n = 5). (F) EC cells were cotransfected with a luciferase reporter construct carrying wild‐type (WT) or MUT HKDC1 and miR‐876‐5p mimics or NC and the luciferase activities were detected (n = 5). All values are presented as the mean ± SD, * P < 0.05, ** P < 0.01, and *** P < 0.001. EC, endometrial cancer; NC, negative control; HG, high glucose; MUT, mutant; NG, normal glucose; WT, wild‐type
3.6. Association of miR‐876‐5p and HOXC‐AS2
LncRNAs have become important participants in gene regulation and potentially function as competing endogenous RNAs (ceRNAs) that serve as molecular sponges for miRNAs. 30 We predicted that miR‐876‐5p would interact with lncRNA‐HOXC‐AS2 (HOXC cluster antisense RNA 2) using computational approaches, including LncBase Predicted v.2 (http://carolina.imis.athena‐innovation.gr/diana_tools/web/index.php?r=lncbasev2%2Findex‐predicted) and RNAhybrid (https://bibiserv.cebitec.uni‐bielefeld.de/rnahybrid/). We found that HOXC‐AS2 expression was increased in tumor tissues from patients complicated with DM compared with NG (Figure 6A). Meanwhile, HG exposure increased HOXC‐AS2 expression in Ishikawa and Hec‐1‐B cells (Figure 6B). FISH and cytoplasm and nuclear RNA isolation assay showed that HOXC‐AS2 was distributed in both of the cytoplasm and nucleus of EC cells, and that was increased by HG (Figure 6C,D). A space structure docking model between HOXC‐AS2 and miR‐876‐5p was simulated using the HDOCK Server (Figure 6E). An anti‐AGO2 antibody was utilized for the RNA immunoprecipitation analysis, and the results showed that miR‐876‐5p but not miR‐3167 mimics increased the enrichment of HOXC‐AS2 with Ago2 (Figure 6F). In addition, miR‐876‐5p reduced the luciferase activity of wild‐type HOXC‐AS2 but had no significant effect on that of mutant HOXC‐AS2 (Figure 6G). Taken together, HOXC‐AS2 may directly bind to miR‐876‐5p and regulate its levels and activity.
FIGURE 6.
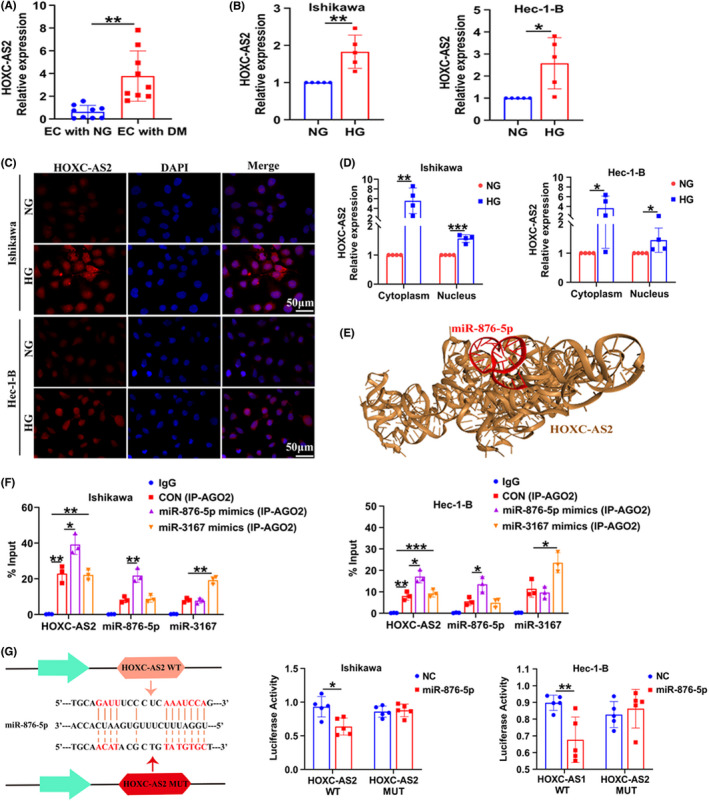
HOXC‐AS2 regulates HKDC1 expression through miR‐876‐5p. (A) qRT‐PCR analysis of HOXC‐AS2 expression in tumor tissues from patients with EC (n = 9). (B) qRT‐PCR analysis of HOXC‐AS2 expression in EC cells (n = 5). (C, D) The subcellular localization of HOXC‐AS2 was detected using RNA‐FISH and qRT‐PCR after cytoplasm and nuclear RNA isolation in EC cells (n = 4). Scale bar = 50 μm. (E) Space structure docking model of HOXC‐AS2 and miR‐876‐5p, as predicted by the HDOCK Server. (F) The interaction of HOXC‐AS2 and miR‐876‐5p was determined by RNA immunoprecipitation (RIP) enrichment, and the amount of RNA immunoprecipitated with the AGO2 antibody relative to an input control was calculated (n = 3). (G) EC cells were cotransfected with a luciferase reporter construct carrying wild‐type (WT) or MUT HOXC‐AS2 and miR‐876‐5p mimics or NC (n = 5). All values are presented as the mean ± SD, * P < 0.05, ** P < 0.01, and *** P < 0.001. EC, endometrial cancer; HG, high glucose; NC, negative control; NG, normal glucose; MUT, mutant; WT, wild‐type
3.7. HOXC‐AS2 mediates HG‐induced pyroptosis in EC cells through the miR‐876‐5p/HKDC1 axis
Cells were transfected with si‐HOXC‐AS2 to elucidate the mechanisms by which HOXC‐AS2 regulates HKDC1 expression and cell pyroptosis. The transfection efficiency of si‐HOXC‐AS2 was measured, and two valid siRNAs against HOXC‐AS2 were used in the subsequent experiments (Figures 7A and S2A). Compared with the HG group, HKDCI expression was reduced by HOXC‐AS2 siRNAs and miR‐876‐5p agomir; this effect was reversed after cotransfection of the miR‐876‐5p antagomir (antisense oligonucleotides, inhibitors) through the silencing of HOXC‐AS2 (Figures 7B and S2B,C). Moreover, rescue experiments were performed in HG‐treated Ishikawa and Hec‐1‐B cells to further verify the relationship between HOXC‐AS2/miR‐876‐5p and pyroptosis. As shown in Figure 7C, HG‐induced EC cell pyroptosis was decreased in the si‐HOXC‐AS2 and miR‐876‐5p agomir groups, whereas the miR‐876‐5p antagomir exerted the opposite effect. Similar results were obtained with PI staining (Figure 7D). Thus, HOXC‐AS2 interacted with the miR‐876‐5p/HKDC1 pathway to control pyroptosis under HG conditions.
FIGURE 7.
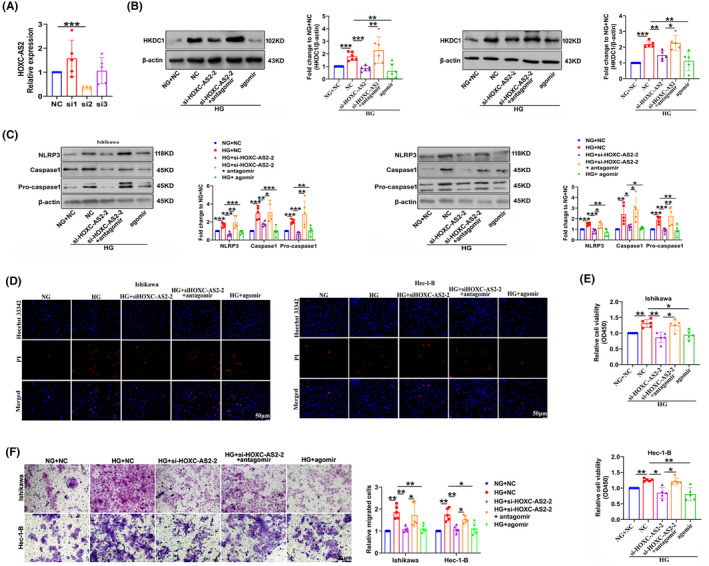
The HOXC‐AS2/miR‐876‐5p/HKDC1 axis mediates EC cell pyroptosis, proliferation, and migration in response to HG. (A) Si‐HOXC‐AS2‐2 had the highest silencing efficiency (n = 5). (B) The expression of HKDC1 in Ishikawa and Hec‐1‐B cells transfected with si‐HOXC‐AS2, miR‐876‐5p agomir, and miR‐876‐5p antagomir (n = 5). (C) Expression of pyroptosis‐related proteins, including NLRP3, Caspase1, and pro‐Caspase1 (n = 5). (D) PI staining was performed to detect pyroptosis in different groups. Scale bar = 50 μm. (E, F) CCK‐8 and transwell assays were conducted to detect the proliferation and migration of EC cells (n = 5). All values are presented as the means ± SD, * P < 0.05, ** P < 0.01, and *** P < 0.001. agomir, miR‐876‐5p mimics; antagomir, miR‐876‐5p inhibitor; EC, endometrial cancer; HG, high glucose; NC, negative control; NG, normal glucose; si‐HOXC‐AS2, small interfering RNA targeting HOXC‐AS2
3.8. The HOXC‐AS2/miR‐876‐5p/HKDC1 axis regulates HG‐related EC cell function
Finally, CCK‐8 and migration assays were performed to confirm the role of the HOXC‐AS2/miR‐876‐5p/HKDC1 axis in EC cell homeostasis. Ishikawa and Hec‐1‐B cells transfected with si‐HOXC‐AS2 and miR‐876‐5p agomir exhibited significant reductions in HG‐induced cell proliferation and migration, and cotransfection with si‐HOXC‐AS2 and miR‐876‐5p antagomir reversed these effects (Figure 7E,F). Overall, HOXC‐AS2‐mediated downregulation of miR‐876‐5p leading to increased expression of HKDC1, contributing to EC cell proliferation and migration under HG conditions.
4. DISCUSSION
Diabetes is associated with an increased risk of EC and a disturbance in glucose metabolism is a prerequisite for the development of EC. 31 In the present study, we presented evidence for a critical role for the upregulation of the glycolysis‐related gene HKDC1 in HG‐related EC. HKDC1 promoted pyroptosis by inducing ROS production and contributed to glycolysis activation, enabling a metabolic advantage in lactate‐rich environments to form a suitable inflammatory and acidic TME that resulted in EC cell proliferation and migration. In addition, miR‐876‐5p was responsible for the regulation of HKDC1. Further analyses indicated that HOXC‐AS2 interacted with the miR‐876‐5p/HKDC1 axis to control pyroptosis under HG conditions (Figure 8).
FIGURE 8.
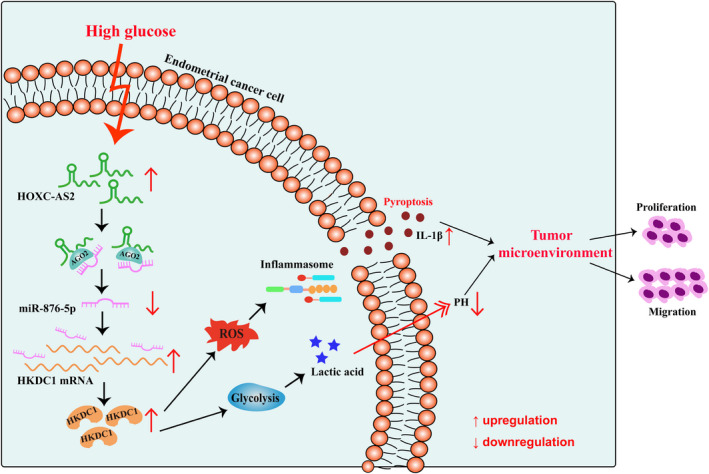
Graphical models. HOXC‐AS2, a competing endogenous RNA, bound to miR‐876‐5p via a lncRNA sponging mechanism and abolished the suppression of HKDC1 expression caused by miR‐876‐5p. This process led to increased pyroptosis and glycolysis, subsequently to induce the production of inflammatory factors and lactic acid release into the TME, finally promoting EC cell proliferation and migration. EC, endometrial cancer; TME, tumor microenvironment
Hyperglycemia, increased insulin resistance, and hyperinsulinemian have been implicated as the pathogenic factors for the development of EC. 32 Due to the positive correlation of EC with the HG‐related gene set, we performed RNA sequencing in clinical samples to profile critical factors for diabetes‐associated EC and proposed the significantly upregulated genes HKDC1, GALNT6, GALE, and GCNT3. Among them, the increased expression of HKDC1, GALNT6, and GALE, but not GCNT3, was verified by qRT‐PCR in tissues from patients with EC complicated with DM. The technologies of RNA sequencing and qRT‐PCR are different in principle and method, and there may be some probability of inconsistent results. However, the understanding of GCNT3 in EC is still deficient and further validation will be carried out by expanding clinical sample size. Another limitation of the study is that we only confirmed the increased expression of HKDC1 in HG‐treated Ishikawa and Hec‐1‐B cells, while we cannot rule out the involvement of GALNT6 and GALE in the development of EC. The expression and function of GALNT6 and GALE in more human EC cells, including HEC‐1‐A, AN3CA, and ECC‐1, need further study to clarify them.
Studies have shown that HKDC1 is a novel fifth hexokinase and a key glucose metabolism‐related gene that maintains whole‐body glucose homeostasis and the mitochondrial membrane potential in hepatocytes. 27 , 33 HKDC1 is required for breast cancer cell proliferation in vitro and tumor growth and metastasis in vivo. 34 HKDC1 functions as an oncogene by regulating the AMPK/mTOR signaling pathway in lung adenocarcinoma. 35 However, to date, the role of HKDC1 in EC is still unknown. Our present study suggested significantly increased HKDC1 expression in HG‐related EC tissues and cells. Knockdown of HKDC1 decreased HG‐induced pyroptosis by inhibiting mitochondrial ROS production in EC cells. Pyroptosis is a process of programmed and inflammatory cell death that is closely associated with the activation of the NLRP3 inflammasome. 36 , 37 Emerging evidence indicates that pyroptosis is an important trigger and endogenous regulator of inflammatory diseases, including diabetes and cancer. 38 Additionally, the NLRP3 inflammasome has been shown to promote the migration and invasion of gastric cancer cells. 39 In the present study, pyroptosis‐related proteins, including NLRP3, ASC, Caspase1, pro‐Caspase1, and IL‐1β, were substantially upregulated by increasing ROS production in the HG environment. Our results further indicate that HG‐induced proliferation and migration of EC cells is reversed by VX‐765, a specific inhibitor of pyroptosis. Taken together, these findings imply that the abnormal occurrence of pyroptosis contributes to HG‐related EC development.
The role of pyroptosis caused by inflammasome activation in human diseases is still a matter of debate and is a double‐edged sword. On the one hand, inflammasomes inhibit tumor growth by promoting tumor cell pyroptosis, which is a mechanism of antitumor immunity. 40 During tumor treatment, appropriate chemotherapeutic drugs can be selected according to the expression levels of DFNA5/GSDME, thereby increasing sensitivity to chemotherapeutic drugs and reducing drug resistance, suggesting that induced pyroptosis is essential for cancer treatment. 41 On the other hand, as a type of proinflammatory death pathway, pyroptosis can promote tumor growth. In gastric cancer, activation of pyroptosis may be associated with the induction of gastric cancer metastasis. 39 Consistent with this result, we found that pyroptosis is activated by HKDC1 under HG conditions, resulting in increased proliferation and migration of EC cells. One possibility is that the cumulative effect of the NLRP3 inflammasome and IL1β activated by ROS form a suitable microenvironment for EC cell growth and thus promote the development of EC. Pyroptosis has long been considered as a contributor to tumorigenesis through the effects of the inflammasome and the release of inflammatory factors, which leads to the formation of an inflammatory local TME that supports oncogenic mutations and genomic instability. 42 , 43 Obesity and obesity‐associated insulin resistance are significantly associated with the creation of a low‐grade chronic inflammatory state and promote a microenvironment that favors the development of cancer. 44 In addition, a second possibility may be related to the complexity of the regulatory mechanism of HG‐induced EC cell growth. Our previous study showed that the imbalance of mitochondrial homeostasis mediated by dynamin‐related protein 1 resulted in dysfunction in HG‐treated EC cells, including increased epithelial‐mesenchymal transition (EMT), migration, and invasion. 24 In the present study, HKDC1 increased the activation of glycolytic stress by HG. In this way, lactate released from glycolytic tumor cells leading to the formation of a highly acidic microenvironment may alter immune cell infiltration, ultimately contributing to immune escape and cancer progression. 45 , 46 Of course, these possibilities have yet to be further confirmed in subsequent experiments, and further studies are needed to determine whether the HOXC‐AS2/miR‐876‐5p/HKDC1 axis participates in the pyroptosis and glycolysis of HG‐related innate and adaptive immune cells within the TME.
The mechanism by which HG regulates HKDC1 expression was further explored in this study. CeRNA mechanisms play important roles in regulating biological behaviors in many diseases. 47 , 48 Therefore, we predicted physical interactions between HKDC1, miRNAs, and lncRNAs using a computational approach, and the HOXC‐AS2/miR‐876‐5p/HKDC1 axis was considered as a potential pathway. A previous study in glioma found that the HOXC‐AS2/miR‐876‐5p/ZEB1 axis regulates the EMT. 49 Consistent with this report, our results suggest that HOXC‐AS2 functions as an endogenous sponge for miR‐876‐5p, thereby regulating HKDC1 expression, which increases pyroptosis of EC cells under HG conditions. Accordingly, we speculated that the identification of ceRNA mechanisms that regulate pyroptosis may fill in the gap between unknown aspects of cell biology and the pathogenesis of HG‐related EC.
In conclusion, HG‐induced pyroptosis results in abnormal EC cell proliferation and migration. More importantly, the HOXC‐AS2/miR‐876‐5p/HKDC1 axis plays a key regulatory role in HG‐related EC progression. Thus, the HOXC‐AS2/miR‐876‐5p/HKDC1 axis might be regarded as a potential therapeutic strategy for patients with EC, especially those with diabetes.
ACKNOWLEDGEMENTS
This work was supported by the National Major Scientific and Technological Special Project “Significant New Drugs Development” (2020ZX09201005), the Sanming Project of Medicine in Shenzhen (SZSM201812075), Taishan Scholars (ts201511073), the Shenzhen Healthcare Research Project (SZLY2017030), and the Special Fund for Scientific Talents of the First Affiliated Hospital of Xiamen University (ZLYY201906), the National Natural Science Foundation of China (NSFC81672591), the Fujian Natural Science Foundation (Youth Innovation) Project (2020J05308), and the Xiamen Medical and Health Guiding Project Fund Project (3502Z20214ZD1020).
AUTHOR CONTRIBUTIONS
X.S. and J.G. designed the study and produced the draft of the manuscript. J.G., F.Y., W.X., X.Z. and R.Z. performed all in vitro experiments and collected the data. W.S. and Y.M. revised the manuscript. All authors have read and approved the final submitted manuscript.
DISCLOSURE
The authors have no conflict of interest.
ETHICS STATEMENT
This study was approved by the Ethics Committee of First Affiliated Hospital of Xiamen University (2022016), and all patients provided informed consent before the study began. Registry and the registration no. of the study/trial: N/A. Animal studies: N/A.
Supporting information
Appendix S1
Guo J, Ye F, Xie W, et al. The HOXC‐AS2/miR‐876‐5p/HKDC1 axis regulates endometrial cancer progression in a high glucose‐related tumor microenvironment. Cancer Sci. 2022;113:2297–2310. doi: 10.1111/cas.15384
Jing Guo, Feng Ye and Wenli Xie contributed equally to this work.
REFERENCES
- 1. Insin P, Prueksaritanond N. Therapeutic use of metformin in diabetes and survival outcomes in endometrial cancer patients with diabetes. Asian Pac J Cancer Prev. 2018;19:1295‐1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arima R, Marttila M, Hautakoski A, et al. Antidiabetic medication, statins and the risk and prognosis of non‐endometrioid endometrial cancer in women with type 2 diabetes. Anticancer Res. 2018;38:4169‐4178. [DOI] [PubMed] [Google Scholar]
- 3. Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79:4557‐4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jarosz‐Biej M, Smolarczyk R, Cichon T, Kulach N. Tumor microenvironment as a "Game Changer" in cancer radiotherapy. Int J Mol Sci. 2019;20(13):3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dann SG, Selvaraj A, Thomas G. mTOR Complex1‐S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252‐259. [DOI] [PubMed] [Google Scholar]
- 6. DeCordova S, Shastri A, Tsolaki AG, et al. Molecular heterogeneity and immunosuppressive microenvironment in glioblastoma. Front Immunol. 2020;11:1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yuan K, Ye J, Liu Z, et al. Complement C3 overexpression activates JAK2/STAT3 pathway and correlates with gastric cancer progression. J Exp Clin Cancer Res. 2020;39:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ma B, Cheng H, Mu C, et al. The SIAH2‐NRF1 axis spatially regulates tumor microenvironment remodeling for tumor progression. Nat Commun. 2019;10:1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shi J, Gao W, Shao F. Pyroptosis: gasdermin‐mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42:245‐254. [DOI] [PubMed] [Google Scholar]
- 10. Miao EA, Rajan JV, Aderem A. Caspase‐1‐induced pyroptotic cell death. Immunol Rev. 2011;243:206‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277:61‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McKenzie BA, Dixit VM, Power C. Fiery cell death: pyroptosis in the central nervous system. Trends Neurosci. 2020;43:55‐73. [DOI] [PubMed] [Google Scholar]
- 14. Fang Y, Tian S, Pan Y, et al. Pyroptosis: a new frontier in cancer. Biomed Pharmacother. 2020;121:109595. [DOI] [PubMed] [Google Scholar]
- 15. Jia C, Chen H, Zhang J, et al. Role of pyroptosis in cardiovascular diseases. Int Immunopharmacol. 2019;67:311‐318. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, Li F, Wang L, Lou Y. A438079 affects colorectal cancer cell proliferation, migration, apoptosis, and pyroptosis by inhibiting the P2X7 receptor. Biochem Biophys Res Commun. 2021;558:147‐153. [DOI] [PubMed] [Google Scholar]
- 17. Cui J, Zhou Z, Yang H, et al. MST1 suppresses pancreatic cancer progression via ROS‐induced pyroptosis. Mol Cancer Res. 2019;17:1316‐1325. [DOI] [PubMed] [Google Scholar]
- 18. Yang Y, Liu PY, Bao W, Chen SJ, Wu FS, Zhu PY. Hydrogen inhibits endometrial cancer growth via a ROS/NLRP3/caspase‐1/GSDMD‐mediated pyroptotic pathway. BMC Cancer. 2020;20:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu H, Wan J, Chu J. Long non‐coding RNAs and endometrial cancer. Biomed Pharmacother. 2019;119:109396. [DOI] [PubMed] [Google Scholar]
- 20. Wang Q, Xu K, Tong Y, et al. Novel miRNA markers for the diagnosis and prognosis of endometrial cancer. J Cell Mol Med. 2020;24:4533‐4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang X, Xing G, Liu S, Li B, He Y, Wang F. LncRNA LOXL1‐AS1 promotes endometrial cancer progression by sponging miR‐28‐5p to upregulate RAP1B expression. Biomed Pharmacother. 2020;125:109839. [DOI] [PubMed] [Google Scholar]
- 22. Wang GF, Wen LN. LncRNA SNHG14 promotes proliferation of endometrial cancer through regulating microRNA‐655‐3p. Eur Rev Med Pharmacol Sci. 2020;24:10410‐10418. [DOI] [PubMed] [Google Scholar]
- 23. Xin W, Gao X, Zhao S, et al. LncRNA RP11‐395G23.3 suppresses the endometrial cancer progression via regulating microRNA‐205‐5p/PTEN axis. Am J Transl Res. 2020;12:4422‐4433. [PMC free article] [PubMed] [Google Scholar]
- 24. Guo J, Ye F, Jiang X, et al. Drp1 mediates high glucose‐induced mitochondrial dysfunction and epithelial‐mesenchymal transition in endometrial cancer cells. Exp Cell Res. 2020;389:111880. [DOI] [PubMed] [Google Scholar]
- 25. Zhang Y, Zhang W, Li X, et al. Prognostic factors and genes associated with endometrial cancer based on gene expression profiling by bioinformatics analysis. Arch Gynecol Obstet. 2016;293:1287‐1295. [DOI] [PubMed] [Google Scholar]
- 26. Han J, Zhang L, Guo H, et al. Glucose promotes cell proliferation, glucose uptake and invasion in endometrial cancer cells via AMPK/mTOR/S6 and MAPK signaling. Gynecol Oncol. 2015;138:668‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pusec CM, De Jesus A, Khan MW, et al. Hepatic HKDC1 expression contributes to liver metabolism. Endocrinology. 2019;160:313‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y, Shi P, Chen Q, et al. Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J Mol Cell Biol. 2019;11:1069‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Daoud AZ, Mulholland EJ, Cole G, McCarthy HO. MicroRNAs in pancreatic cancer: biomarkers, prognostic, and therapeutic modulators. BMC Cancer. 2019;19:1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang L, Cho KB, Li Y, Tao G, Xie Z, Guo B. Long noncoding RNA (lncRNA)‐mediated competing endogenous RNA networks provide novel potential biomarkers and therapeutic targets for colorectal cancer. Int J Mol Sci. 2019;20(22):5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saed L, Varse F, Baradaran HR, et al. The effect of diabetes on the risk of endometrial cancer: an updated a systematic review and meta‐analysis. BMC Cancer. 2019;19:527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Onstad MA, Schmandt RE, Lu KH. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J Clin Oncol. 2016;34:4225‐4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ludvik AE, Pusec CM, Priyadarshini M, et al. HKDC1 Is a novel hexokinase involved in whole‐body glucose use. Endocrinology. 2016;157:3452‐3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen X, Lv Y, Sun Y, et al. PGC1beta regulates breast tumor growth and metastasis by SREBP1‐mediated HKDC1 expression. Front Oncol. 2019;9:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang X, Shi B, Zhao Y, et al. HKDC1 promotes the tumorigenesis and glycolysis in lung adenocarcinoma via regulating AMPK/mTOR signaling pathway. Cancer Cell Int. 2020;20:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang S, Yuan YH, Chen NH, Wang HB. The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in Parkinson's disease. Int Immunopharmacol. 2019;67:458‐464. [DOI] [PubMed] [Google Scholar]
- 37. He Y, Hara H, Nunez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41:1012‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu ZW, Zhang J, Li X, Wang Y, Fu YH, Gao XY. A new research hot spot: The role of NLRP3 inflammasome activation, a key step in pyroptosis, in diabetes and diabetic complications. Life Sci. 2020;240:117138. [DOI] [PubMed] [Google Scholar]
- 39. Xu Y, Li H, Chen W, et al. Mycoplasma hyorhinis activates the NLRP3 inflammasome and promotes migration and invasion of gastric cancer cells. PLoS One. 2013;8:e77955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang R, Chen J, Mao L, et al. Nobiletin triggers reactive oxygen species‐mediated pyroptosis through regulating autophagy in ovarian cancer cells. J Agric Food Chem. 2020;68:1326‐1336. [DOI] [PubMed] [Google Scholar]
- 41. Xia X, Wang X, Cheng Z, et al. The role of pyroptosis in cancer: pro‐cancer or pro‐"host"? Cell Death Dis. 2019;10:650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van Gorp H, Lamkanfi M. The emerging roles of inflammasome‐dependent cytokines in cancer development. EMBO Rep. 2019;20:e47575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li L, Jiang M, Qi L, et al. Pyroptosis, a new bridge to tumor immunity. Cancer Sci. 2021;112:3979‐3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ahechu P, Zozaya G, Marti P, et al. NLRP3 inflammasome: a possible link between obesity‐associated low‐grade chronic inflammation and colorectal cancer development. Front Immunol. 2018;9:2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li W, Xu M, Li Y, et al. Comprehensive analysis of the association between tumor glycolysis and immune/inflammation function in breast cancer. J Transl Med. 2020;18:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen B, Gao A, Tu B, et al. Metabolic modulation via mTOR pathway and anti‐angiogenesis remodels tumor microenvironment using PD‐L1‐targeting codelivery. Biomaterials. 2020;255:120187. [DOI] [PubMed] [Google Scholar]
- 47. Wang Y, Hou J, He D, et al. The emerging function and mechanism of ceRNAs in cancer. Trends Genet. 2016;32:211‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moreno‐Garcia L, Lopez‐Royo T, Calvo AC, et al. Competing endogenous RNA networks as biomarkers in neurodegenerative diseases. Int J Mol Sci. 2020;21:9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dong N, Guo J, Han S, Bao L, Diao Y, Lin Z. Positive feedback loop of lncRNA HOXC‐AS2/miR‐876‐5p/ZEB1 to regulate EMT in glioma. Onco Targets Ther. 2019;12:7601‐7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


