Abstract
Hexokinase II is an enzyme central to glucose metabolism and glucose repression in the yeast Saccharomyces cerevisiae. Deletion of HXK2, the gene which encodes hexokinase II, dramatically changed the physiology of S. cerevisiae. The hxk2-null mutant strain displayed fully oxidative growth at high glucose concentrations in early exponential batch cultures, resulting in an initial absence of fermentative products such as ethanol, a postponed and shortened diauxic shift, and higher biomass yields. Several intracellular changes were associated with the deletion of hexokinase II. The hxk2 mutant had a higher mitochondrial H+-ATPase activity and a lower pyruvate decarboxylase activity, which coincided with an intracellular accumulation of pyruvate in the hxk2 mutant. The concentrations of adenine nucleotides, glucose-6-phosphate, and fructose-6-phosphate are comparable in the wild type and the hxk2 mutant. In contrast, the concentration of fructose-1,6-bisphosphate, an allosteric activator of pyruvate kinase, is clearly lower in the hxk2 mutant than in the wild type. The results suggest a redirection of carbon flux in the hxk2 mutant to the production of biomass as a consequence of reduced glucose repression.
Glycolysis plays a central role in glucose metabolism in the yeast Saccharomyces cerevisiae. It is the root for many different pathway branches which lead primarily to the production of biomass, ethanol, and CO2. The first step in glycolysis is the transport of glucose across the cell membrane by members of the hexose transporter family (6, 19, 33). Subsequently, intracellular glucose is phosphorylated to glucose-6-phosphate. In the yeast S. cerevisiae, there are three isozymes that phosphorylate glucose: glucokinase (encoded by GLK1), hexokinase I (encoded by HXK1), and hexokinase II (encoded by HXK2). These isozymes have different affinities for glucose and ATP and different specificities toward other sugars, such as fructose and mannose (14, 21). Furthermore, there are differences in the transcriptional regulation of the genes that encode these hexose-phosphorylating enzymes, depending on the source and the amount of carbon (16). In contrast to hexokinases from other organisms, S. cerevisiae hexose kinases are not inhibited by their product glucose-6-phosphate (for a review, see reference 7). Instead, the inhibition of hexokinase II activity by trehalose-6-phosphate (5) may be involved in the regulation of the sugar influx into glycolysis by trehalose-6-phosphate synthase (40, 41) and thereby in the regulation of intracellular metabolite pools. Furthermore, from previous studies it is known that hexokinase II is involved in glucose repression (for reviews, see references 8 and 13).
Glucose repression is a mechanism that adapts yeast cells for the fermentation of glucose, the preferred carbon source (for recent reviews, see references 8 and 13), by repressing a large number of genes at the level of transcription. Transcription of SUC2 (encoding invertase), GAL genes (encoding proteins involved in galactose metabolism), MAL genes (encoding proteins involved in maltose metabolism), HXK1 (encoding hexokinase I), and genes encoding enzymes of the glyoxylate shunt, the tricarboxylic acid (TCA) cycle, and gluconeogenesis are all repressed in the presence of glucose. In addition, genes involved in respiration and other mitochondrial activities are repressed by glucose. In S. cerevisiae, glucose repression leads to the occurrence of diauxic growth on glucose.
The mechanism governing glucose repression is not yet well understood, and several regulatory pathways seem to be involved. A central role for hexokinase II is apparent (13). The absence of hexokinase II causes derepression of high-affinity glucose transport (26, 37) encoded by at least HXT7 (20, 28). Also, the synthesis of hexokinase I (11), invertase (encoded by SUC2), maltase, malate dehydrogenase (12), galactokinase, cytochrome c reductase, and cytochrome c oxidase (27) is no longer repressed by glucose in hxk2 mutants.
Several properties of hexokinase II may be involved in glucose repression. Previously, the sugar-phosphorylating activity of hexokinase II was suggested to be directly correlated to the extent of glucose repression (24). Overproduction of hexokinase I (encoded by HXK1) restored glucose repression in a hxk2 mutant; however, overexpression of GLK1 (encoding glucokinase) did not (35). Hexokinase II is a phosphoprotein in vivo (44), which suggests a regulatory function. Both hexokinase I and hexokinase II exist in two isoforms in vitro, a monomeric form and a dimeric form, which have different affinities for glucose (1). Phosphorylation at serine-15 converts hexokinase II to the monomeric form (1, 18), which seems to be essential for glucose repression (32). Furthermore, a dual cytosolic-nuclear localization of hexokinase II has been demonstrated (31). In the nucleus, the hexokinase II protein participates in a regulatory DNA-protein complex necessary for glucose repression of the SUC2 gene (17). Thus, there are strong connections between glucose repression and hexokinase II, in terms of both metabolic and regulatory activity.
Many previous studies have been dedicated to the role of hexokinase II in glucose repression; we present here the first comprehensive physiological characterization of S. cerevisiae cells deleted in hexokinase II during aerobic batch growth with glucose as a carbon source, from exponential growth on glucose to the diauxic shift and subsequent growth on ethanol.
We have shown that the deletion of HXK2 in S. cerevisiae results in a Crabtree-negative or Crabtree-diminished phenotype: the strain displayed completely oxidative growth during aerobic batch cultivation on glucose, with a high biomass yield. Ethanol production begins only after continued growth on glucose. Overproduction of the separate enzymes of glycolysis does not increase the glycolytic flux (36). We think that changing regulatory pathways rather than overexpressing particular enzymes, may be a more fruitful approach to alter yeast physiology and to divert glucose metabolism into desired pathways, e.g., the production of biomass or the production of heterologous proteins in the presence of abundant glucose.
MATERIALS AND METHODS
Strain and growth conditions.
S. cerevisiae wild-type strain CEN.PK113-7D (MATa MAL2-8c SUC2) provided by P. Kötter (Frankfurt, Germany) was used for a PCR-based gene disruption of HXK2. Primers described in this study were constructed by Isogen BV (Maarssen, The Netherlands). The HXK2 gene was replaced by a kanMX cassette in CEN.PK113-7D to create strain KY116 as follows: using primer AK53 (GTTGTAGGAATATAATTCTCCACACATAATAAGTACGCTAATTCGTACGCTGCAGGTCGAC; the underlined nucleotides correspond to the DNA immediately 5′ of the HXK2 open reading frame) and primer AK54 (AAAAGGGCACCTTCTTGTTGTTCAAACTTAATTTACAAATTAAGTATCGATGAATTCGAGCTCG; the underlined nucleotides correspond to the DNA 3′ of the HXK2 open reading frame) the kanMX cassette of plasmid pFA6a-kanMX4 (45) was amplified using the Expand PCR kit as recommended by the manufacturer (Roche Diagnostics, Mannheim, Germany). The resulting PCR product was transformed into competent CEN.PK113-7D as previously described (15). After 2 h of cultivation in YEPD medium (1% yeast extract, 2% peptone, 2% glucose), the transformed cells were plated on solid YEPD medium (2% agar) containing geneticin at 200 μg ml−1 (G418; Roche Diagnostics) and incubated at 30°C. G418-resistant isolates were tested for the proper integration of the kanMX cassette at the HXK2 locus by analytical PCR using the TaqPlus Long PCR kit with the primers AK60 (GACGAAATACGCGATCGCTGT) and AK61 (GCCGAACATTTCAAAGTCAACC) as recommended by the manufacturer (Stratagene, La Jolla, Calif.).
Aerobic batch cultivations were performed in a 2-liter bioreactor (working volume, 1.5 liter) at 30°C at a stirring speed of 1,000 rpm and an aeration rate of 1 volume of air per vessel volume per min. The minimal medium contained 1% (wt/vol) glucose, 0.17% (wt/vol) yeast nitrogen base (YNB) without amino acids or (NH4)2SO4 (Difco, Detroit, Mich.), 0.5% (NH4)2SO4 (wt/vol), and 0.1 M potassium phthalate at pH 5.0. Samples were taken for analysis of extracellular metabolites, intracellular metabolites, dry weight, protein, optical density, and enzyme activities. O2 consumption and CO2 production were measured by online mass spectrometry of the exhaust gas. The optical density of the culture was measured at 600 nm in a spectrophotometer (l = 1 cm; Novaspec II; Amersham-Pharmacia, Buckinghamshire, United Kingdom). Throughout this report data are shown from a single representative experiment. Variations between experiments were <15% and within each experiment each data point had an error of <5%.
Sample extraction.
For the determination of the protein concentration, 1.2 ml of the culture was centrifuged for 1 min at 14,000 × g. The pellet was resuspended in 1.2 ml of 1 M NaOH.
Samples for the determination of extracellular metabolites were prepared by adding 100 μl of 35% (vol/vol) perchloric acid (PCA) to 1 ml of the culture supernatant. Samples were neutralized before analysis with 55 μl of 7 M KOH. After centrifugation (1 min at 14,000 × g) the supernatant was filtered through 0.45 μm (pore-size) nylon syringe filters (Alltech, Deerfield, Ill.).
Samples for the determination of intracellular metabolites were prepared by addition of 100 μl of 35% (vol/vol) PCA to 600 μl of culture and put on ice. Samples were neutralized within an hour after extraction with 145 μl of 2 M K2CO3.
For the preparation of enzyme extracts 10 ml of culture was centrifuged for 5 min at 4,000 × g at 4°C. The pellet was resuspended in 0.6 ml of 20 mM potassium phosphate buffer (pH 7) and extracted by vigorous shaking for 15 min with 0.5 g glass beads (diameter of ca. 0.45 × 10−3 m) at 4°C. To inhibit serine protease activity, 1 μM phenylmethylsulfonyl fluoride (PMSF; dissolved in dimethylsulfoxide [DMSO]) was added.
For the determination of mitochondrial H+-ATPase activity, 10 ml of culture was centrifuged for 5 min at 4,000 × g at 4°C. The pellet was resuspended in 100 μl of buffer containing 500 mM mannitol, 1 mM ATP, 2 mM EDTA, 0.2% (wt/vol) bovine serum albumin, 10% methanol, and 10 mM ɛ-aminocaproic acid in 0.1 M Tris-HCl (pH 7.5) and then extracted by vigorous shaking for 30 min with 0.2-g glass beads (diameter of ca. 0.45 × 10−3 m) at 4°C (42). To inhibit protease activity, 1 μM PMSF (in DMSO) was added. ATPase activity was measured in the complete extract.
Analyses.
Protein concentrations were determined by the method of Lowry et al. (23) using fatty-acid-free bovine serum albumin (Sigma, St. Louis, Mo.) as a standard. Extracellular metabolites were determined by means of high-performance liquid chromatography (LKB, Bromma, Sweden) with a Rezex organic acid analysis column with an 8-μm particle size, 8% cross-linking, and a hydrogen ionic form (Phenomex, Torrance, Calif.) at a temperature of 45°C and with 7.2 mM H2SO4 as the eluent. Detection was done by using an RI-1530 refractive index detector (Jasco, Tokyo, Japan). Peak integration and data processing were done with Borwin (Le Fontanil, France) chromatography software. Intracellular metabolites were determined by NAD(P)H-coupled enzymatic reactions (2). Intracellular concentrations were calculated by assuming that 1 mg of protein corresponds to 3.75 μl of intracellular volume (9, 34, 39). Enzyme activities were determined at 30°C and pH 7.0 by NAD(P)H-coupled enzymatic reactions (38). Mitochondrial ATPase activity (azide-sensitive ATP hydrolysis) was determined by subtracting the azide-insensitive ATPase activity from the total ATPase activity. Total ATPase activity was measured at 30°C at pH 8.0 with 0.5 mM phosphoenolpyruvate, 6 mM MgCl2 · 6H2O, 85 mM sucrose, 5 mM ATP, 35 mM Tris-HCl, 0.3 mM NADH, 50 μM antimycin A, 5 U of pyruvate kinase per ml, and 5 U of lactate dehydrogenase per ml (42). The reaction was started with crude enzyme extract. The azide-insensitive ATPase activity was measured with the same reaction mixture in the presence of 5 mM NaN3. Protein, intracellular metabolites, and enzyme activities were measured on a COBAS-FARA automatic analyzer (Roche Diagnostics).
RESULTS
Physiological changes.
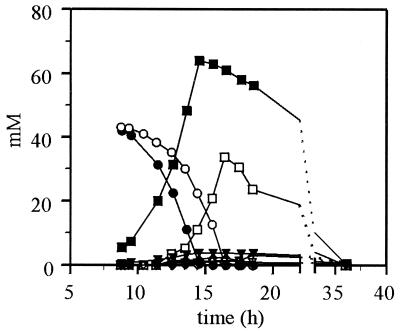
During aerobic batch growth on 1% glucose, distinct growth phases could be distinguished in wild-type S. cerevisiae (Fig. 1; for a description of wild-type yeast batch growth, see reference 22). In a first exponential growth phase, the glucose was metabolized predominantly to ethanol and CO2, with the minor products of fermentation being glycerol, acetate, and pyruvate (Fig. 2 and 3). Both the protein concentration and the optical density of the culture increased exponentially at a rate of approximately 0.38 h−1. The specific CO2 evolution rate was 800 nmo1 min−1 mg of protein−1, and the specific O2 consumption rate was 65 nmol min−1 mg of protein−1, resulting in a respiratory quotient (RQ, where RQ = CO2/O2) of approximately 12 during exponential growth (Fig. 3). This RQ is indicative of respirofermentative growth. Only a small part of the glucose was respired, whereas the rest was fermented primarily to ethanol. As a consequence of the production of ethanol, which has a relatively high energy content, the growth yield was low. On a C-molar basis, 71% of the glucose was converted to ethanol and CO2, 2% was converted to CO2 via the TCA cycle, 13% was converted to biomass, 5% was converted to glycerol, 2% was converted to acetate, and 0.5% was converted to pyruvate; which gives an incomplete carbon recovery of 93.5%. At least a part of the missing carbon can be accounted for by ethanol evaporation (i.e., substantial amounts of ethanol were measured in the off-gas by means of a cold trap).
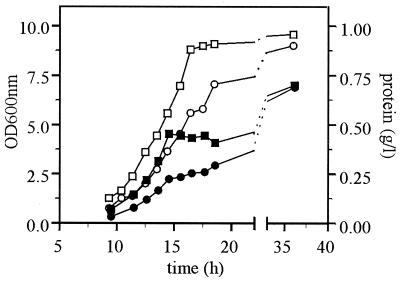
FIG. 1.
Influence on growth characteristics of the HXK2 deletion. The wild-type strain (solid symbols) and the hxk2 mutant strain (open symbols) were grown on YNB–1% glucose. Growth was monitored by measuring the optical density at 600 nm (circles) and the total protein concentration (boxes) of the culture. Errors are <5%. Data are shown from a representative experiment.
FIG. 2.
Changes in the external metabolite pattern as a consequence of a HXK2 deletion. The wild-type strain (solid symbols) and the hxk2 mutant strain (open symbols) were grown on YNB–1% glucose. Glucose (circles), ethanol (boxes), glycerol (triangles down), acetate (triangles up), and pyruvate (diamonds) were determined in the supernatant of the culture. Errors are <5%. Data are shown from a representative experiment.
FIG. 3.
Specific CO2 production, O2 consumption, and RQ during growth. The wild-type strain (solid symbols) and the hxk2 mutant strain (open symbols) were grown on YNB–1% glucose. CO2 production (circles) and O2 consumption (boxes) were measured continuously from the off-gas and are expressed in micromoles per minute per milligram of total cell protein. Errors are <5%. Data are shown from a representative experiment.
After glucose was exhausted, growth was arrested in the wild-type yeast cells for at least 4 to 5 h. The depletion of glucose coincided with a sudden drop in CO2 production and O2 consumption. Both CO2 production and O2 consumption resumed before growth did (compare Fig. 1 and 3). After the lag-phase the wild-type yeast consumed the ethanol and other fermentative products that were produced during growth on glucose. An RQ of approximately 0.6 was measured (Fig. 3) which is characteristic for growth on ethanol (22). During growth on ethanol the optical density increased relatively more than the protein concentration (Fig. 1). At the end of growth there was a small increase in RQ (Fig. 3), characteristic for the metabolism of acetate (22).
The deletion of the HXK2 gene altered the growth characteristics at high glucose concentrations (Fig. 1). Compared to the wild-type yeast the rate of exponential increase in protein and optical density was decreased (μ = 0.33 h−1), and it slowed further to a rate of 0.22 h−1 at later stages of growth on glucose. The protein content per optical density unit was lower in the hxk2 mutant during exponential growth on glucose (between 5 and 25% more protein per optical density unit in the wild-type yeast), possibly as a consequence of differences in the cellular makeup between the wildtype and hxk2 mutant (e.g., storage carbohydrates). Most striking, during early exponential growth the hxk2 mutant consumed much less glucose than the wild-type strain, and no products of fermentation could be detected in the culture supernatant (Fig. 2). During this period glucose was only converted into biomass and CO2 in contrast to the wild type, which produced ethanol during exponential growth on glucose. In the hxk2 mutant glucose was completely converted by oxidative metabolism, as evidenced by an RQ of 1 (Fig. 3).
The hxk2 mutant decreased O2 uptake rate and increased CO2 evolution, resulting in an increase in the RQ to a maximum of 2.5, which is still much lower than for the wild type during fermentative growth. These changes in gas metabolism coincided with the production of fermentative products such as ethanol, acetate, glycerol, and pyruvate (Fig. 2). On a C-molar basis, 25% of the glucose was converted to ethanol and CO2, 14% was converted to CO2 via the TCA cycle, 48% was converted to biomass, 6% was converted to glycerol, and 6% was converted to acetate (carbon recovery of 99%).
After glucose exhaustion and a lag period, which is shorter than for the wild type (Fig. 1 and 3), the hxk2 mutant metabolized and grew on the fermentative products of glucose metabolism. As in the wild type, this process occurred with an RQ of ca. 0.6. The growth on fermentative products was shorter than for the wild-type cells since the amount of fermentative products produced was lower during the preceding exponential growth on glucose. As with the wild-type, a small increase in the RQ was observed at the end of growth as a consequence of acetate metabolism.
We measured growth, glucose consumption, and the start of ethanol production by the hxk2 mutant at initial glucose concentrations of between 0.5 and 8%. The exponential growth rates of both the wild-type and the hxk2 mutant strains decreased with increasing initial glucose concentrations. The growth rate of the wild-type decreased from 0.39 h1 at 0.5% initial glucose to 0.34 h−1 at 8% initial glucose, and for the hxk2 mutant it decreased from 0.32 h−1 at 0.5% initial glucose to 0.18 h-1 at 8% initial glucose. The onset of ethanol production was not associated with the residual concentration of glucose (results not shown). Additionally, the onset of ethanol production was not a consequence of an insufficient dissolved oxygen tension during batch growth on 1% glucose. Dissolved O2 did not drop below 90% of air saturation during any stage in growth for either the wild-type or the hxk2 mutant strains (results not shown). The onset of ethanol production was associated with the biomass concentration of the cultures and started at a protein concentration of approximately 0.25 g liter−1 irrespective of the initial glucose concentration.
Intracellular changes.
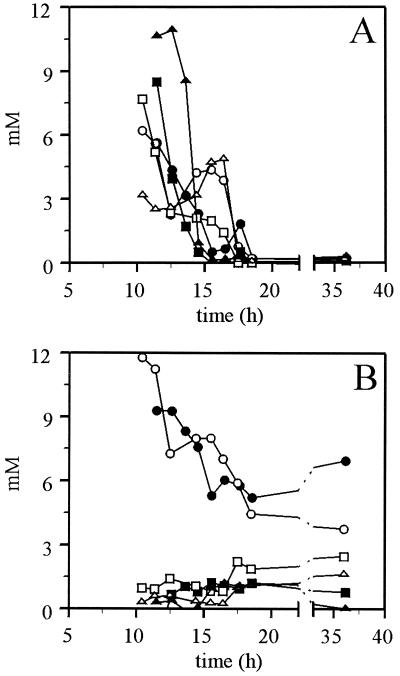
In both the wild type and the hxk2 mutant the metabolite pattern continuously changes during growth on 1% glucose (Fig. 4). The intracellular concentrations of glucose-6-phosphate, fructose-6-phosphate, and ATP decreased during exponential growth on glucose in both strains. The intracellular concentration of fructose-1,6-bisphosphate decreased in the wild type but remained relatively low and constant in the hxk2 mutant. The total concentration of the adenine nucleotides decreased in both the wild-type strain and the hxk2 mutant. The concentrations of ADP and AMP were marginally higher in the hxk2 mutant. The onset of fermentation in the hxk2-null mutant coincided with an intracellular accumulation of pyruvate. From a comparison between the culture supernatant and an extract of the total culture we concluded that pyruvate accumulated intracellularly and was not excreted, whereas the wild type excreted the pyruvate (data not shown and Fig. 2); levels of up to 40 mM intracellular pyruvate were estimated.
FIG. 4.
Internal metabolites during growth on glucose. The wild-type strain (solid symbols) and the hxk2 mutant strain (open symbols) were grown on YNB–1% glucose. The intracellular concentrations of glucose-6-phosphate (circles), fructose-6-phosphate (boxes), and fructose-1,6-bisphosphate (triangles) in panel A, and the adenine nucleotides ATP (circles), ADP (boxes), and AMP (triangles) in panel B are expressed in millimolar concentrations in the cytosol. Errors are <5%. Data are shown from a representative experiment.
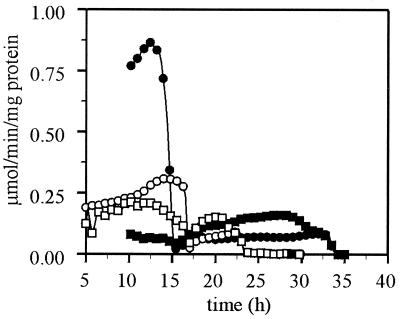
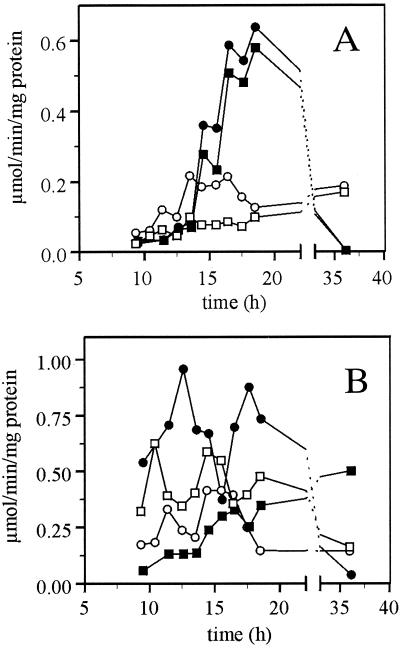
Glucose-phosphorylating activity increased during exponential growth on glucose in the wild type (Fig. 5A). A slightly higher specific phosphorylating activity was measured with fructose than with glucose, which indicates the presence of hexokinase I. In the hxk2 mutant the hexose-phosphorylating capacity increased only slightly during growth on glucose (Fig. 5A). In the hxk2 mutant strain the hexokinase activity was slightly higher with fructose than with glucose early in exponential growth. When growth on glucose continued, fructose clearly became the preferred substrate, with fructose activity being two times higher than glucose activity. After glucose exhaustion the wild type showed a higher glucose- and fructose-phosphorylating activity than the hxk2 mutant.
FIG. 5.
Specific enzyme activities during growth on glucose. The wild-type strain (solid symbols) and the hxk2 mutant strain (open symbols) were grown on YNB–1% glucose. In panel A, the fructose-phosphorylating activity (circles) and the glucose-phosphorylating activity (boxes) (in micromoles of substrate converted per minute per milligram of total cell protein) are depicted. In panel B, the pyruvate decarboxylase activity (circles) and mitochondrial H+-ATPase (boxes) activity are depicted. Errors are <10%. Data are shown from a representative experiment.
Pyruvate decarboxylase converts pyruvate into acetaldehyde en route to ethanol. Both in the hxk2 mutant and in the wild type the pyruvate decarboxylase activity was relatively constant during growth on glucose (Fig. 5B); however, in the hxk2 mutant the pyruvate decarboxylase activity was two to three times lower than in the wild type.
In the wild type, a low, yet increasing, mitochondrial ATPase activity was observed when glucose was consumed during growth on 1% glucose (Fig. 5B). In contrast, in the hxk2 mutant the mitochondrial H+ -ATPase activity was high-throughout exponential growth on glucose (Fig. 5B), a result indicative of a derepressed mitochondrial respiratory capacity.
DISCUSSION
Aerobic batch growth on 1% glucose of wild-type S. cerevisiae is characterized by respirofermentative metabolism. The energy necessary for exponential growth is partly generated by respiration (i.e., the oxidation of glucose to CO2 via the TCA cycle and oxidative phosphorylation) and partly by fermentation (i.e., the conversion of glucose to fermentative products, primarily ethanol). Oxidative growth yields more ATP per glucose molecule than fermentative growth. The production of ethanol from glucose under aerobic growth conditions is referred to as the Crabtree effect. The Crabtree effect has been ascribed to a limited capacity of the mitochondrial respiratory chain or to an overflow of metabolism at the level of pyruvate (29). In the industrial production of yeast biomass the formation of ethanol is undesirable, and industrial fermentations are designed to avoid it. The production of ethanol (or other metabolites) is accompanied by reduction of biomass yield and is related to inhibition of yeast growth and poor performance during subsequent fermentation (43). Furthermore, the production of fermentative products results in a diauxic shift; first, the cells grow on glucose, and then they have to adapt to growth on the fermentative products. Production of fermentative products can be avoided by means of fed batch cultivation of the yeast. The culture is fed below a critical dilution rate and is mixed vigorously by aeration to avoid local high concentrations of glucose and/or low concentrations of dissolved O2.
Yeast strains with (null) mutations in HXK2 have a reduced growth rate and reduced glucose repression at higher glucose concentrations (this study and reference 12). This reduction suggests that hexokinase II and/or glucose repression are necessary to regulate glucose metabolism for fast growth at high external glucose concentrations. However, elimination of hexokinase II, an enzyme central in glucose repression, does not result in severe growth defects or apparent changes in cell morphology (results presented in this study and results not shown). Instead, a yeast strain with a null mutation in HXK2 displays fully oxidative growth at high glucose concentrations in early exponential batch cultures, resulting in an initial absence of fermentative products such as ethanol, a postponed and shortened diauxic shift, and higher biomass yields.
Changes in intracellular properties resulting from the absence of the hexokinase II protein are apparent (this study and references 11, 12, 20, 26–28, and 37). Hexokinase II is the dominant hexose-phosphorylating enzyme during exponential growth on glucose. Only when glucose declines do the other hexose-phosphorylating enzymes, hexokinase I and glucokinase, appear (14, 16). Mutations in the genes encoding hexokinase I (HXK1) and glucokinase (GLK1) do not have significant effects on glucose repression. However, overexpression of HXK1 can restore glucose repression in an hxk2 mutant (35). Overproduction of glucokinase on the other hand was not sufficient to restore glucose repression. Hexokinase I, like hexokinase II but in contrast to glucokinase, exists in both monomeric and dimeric states (25). This similarity suggests that the hexose-phosphorylating activity itself is not directly related to glucose repression, yet there might be a relation between the presence of the different oligomers and glucose repression. In this study we show that the hxk2 mutant and the wild type have comparable hexose-phosphorylating activities during exponential growth on glucose. This finding contradicts the hypothesized relationship between glucose repression and hexose-phosphorylating capacity. However, the in vitro phosphorylating capacity might be similar in the different strains, yet the affinities of the glucose-phosphorylating enzymes differ strongly (21), thus the in vivo phosphorylating activity or the resulting metabolite pools cannot be excluded as direct regulators of glucose repression.
During growth on glucose, the fructose-phosphorylating activity of the hxk2 mutant increases, while the glucose-phosphorylating activity remains constant. This result suggests an increase in hexokinase I, since fructose is not phosphorylated by glucokinase and the ratio of fructose over glucose phosphorylation is ca. 1.3 for hexokinase II and ca. 3 for hexokinase I (16).
High-affinity glucose transport is subject to glucose repression (3, 4, 33). In wild-type yeast the kinetics of glucose transport are determined predominantly by low-affinity transporters at high concentrations of glucose. When the glucose concentration declines, glucose is transported primarily by high-affinity transporters (e.g., references 10 and 46). In a strain in which HXK2 is deleted, high levels of high-affinity glucose transporters are found even at high concentrations of glucose (3, 28). Steady-state intracellular metabolite levels are determined by the transport step and the subsequent metabolic machinery. The changes in inhibition of hexokinase activity by trehalose-6-phosphate or in the interaction with trehalose-6-phosphate synthase and the presence of high-affinity glucose transport activity in the hxk2-null mutant during growth at high glucose concentrations may change the concentration of intracellular metabolites (e.g., intracellular glucose or glucose-6-phosphate) and thereby relieve glucose repression.
Both intracellular glucose and glucose-6-phosphate might act as signal molecules for glucose repression. Entian et al. (12) showed that intracellular concentrations of glucose-6-phosphate, fructose-6-phosphate, and fructose-1,6-bisphosphate are comparable in the wild-type and the hxk2 mutant grown on different carbon sources. However, intracellular metabolites were not monitored during growth but were measured in cells harvested at an arbitrary point (after 36 h of growth). In our experiments, both glucose and ethanol metabolism were finished by 36 h, which may indicate that in the experiment described by Entian et al. the cells were no longer exponentially growing on glucose. Here we show that the concentrations of glucose-6-phosphate, fructose-6-phosphate, and ATP change during growth on 1% glucose and yet are comparable in the wild type and in the hxk2-null mutant.
The ATP/AMP ratio might act as a signal for glucose repression by modulating the Snf1 kinase activity (47). The Snf1 kinase is required for the transcription of glucose-repressed genes. However, the role of the ATP/AMP ratio in glucose repression has been called into question (13). In our experiments, the ATP/AMP ratios are similar in the wild type and in the hxk2 mutant, in spite of the dramatically different states of glucose repression, which suggests that the hypothesis that the ATP/AMP ratio triggers glucose repression is incorrect.
The concentration of fructose-1,6-bisphosphate decreased in wild-type yeast during growth on glucose. In the hxk2 mutant the concentration of fructose-1,6-bisphosphate is lower than in the wild type during growth in glucose medium. Fructose-1,6-bisphosphate is an allosteric activator of pyruvate kinase. Pyruvate kinase is the enzyme that converts phosphenolpyruvate into pyruvate in the last step of glycolysis. The reduced levels of pyruvate decarboxylase and the decreased activation of pyruvate kinase in the hxk2 mutant might be directly responsible for the redirection of carbon flux from the production of ethanol to the production of biomass.
In the wild type the culture density and culture protein concentration remained constant for approximately 5 h after glucose exhaustion. During this diauxic shift the O2 consumption and CO2 evolution decreased strongly; however, they increased again soon, a finding indicative of the reinitiation of metabolism, yet the arrest in growth persisted (Fig. 1 and 3). This lag indicates that the adaptation, involving the conversion of enzymes of the metabolic machinery, is an energy-consuming process. In the hxk2 mutant the adaptation period seemed to be ca. 1 hour (Fig. 1 to 3), which suggests that at least some of the mRNA or enzymes necessary for growth on ethanol were already present. This hypothesis is confirmed by the depressed mitochondrial H+-ATPase activity.
The hxk2 mutant began fermentation after the glucose had been partially consumed. This delay was unexpected, since as the concentration of glucose declines during growth we expected glucose repression and flux through glycolysis to decline as well. Possible explanations for these results include: (i) the dissolved O2 concentration may have been too low, resulting in a limited supply of O2 at elevated concentrations of biomass; (ii) a vitamin or nutrient other than glucose may have been depleted after a certain amount of biomass was produced (30); or (iii) intracellular accumulation of metabolites may have caused an overflow of metabolism into fermentation or else affected the expression of particular genes, e.g., the accumulation of intracellular glucose may result in glucose repression.
For the oxidative degradation of glucose to CO2 and biomass, O2 must be present. Oxygen limitation forces yeast to fermentative glucose metabolism to acquire energy for growth. However, in our experiments, the dissolved O2 levels never dropped below 90% (both in the wild type and in the hxk2 mutant strains); therefore, we conclude that the O2 supply was not limiting.
The intracellular accumulation of pyruvate in the hxk2 mutant during growth on glucose was unexpected. Apparently, the hxk2 mutant either actively transports pyruvate back into the cell or is unable to excrete pyruvate effectively (see reference 30). The ongoing accumulation of pyruvate might result in the overflow of metabolism to the production of ethanol, which is initially not present as a result of the low pyruvate decarboxylase activity.
Furthermore, the increase in hexokinase I activity during growth on glucose in the hxk2 mutant may be responsible for glucose repression at the onset of ethanol production.
ACKNOWLEDGMENTS
We thank Arthur Kuiper for technical assistance and acknowledge Joost Teixeira de Mattos for critically reading the manuscript.
This work was supported by the Foundation for Chemical Research (SON), which is subsidized by The Netherlands Organization for Scientific Research (NWO), The Netherlands Association for Biotechnological Research Centers (ABON), and the European Union through grant no. BIO4-CT95-0107 of the BIOTECH program.
REFERENCES
- 1.Behlke J, Heidrich K, Naumann M, Muller E C, Otto A, Reuter R, Kriegel T. Hexokinase 2 from Saccharomyces cerevisiae: regulation of oligomeric structure by in vivo phosphorylation at serine-14. Biochemistry. 1998;37:11989–11995. doi: 10.1021/bi980914m. [DOI] [PubMed] [Google Scholar]
- 2.Bergmeyer H U. Methods of enzymatic analysis. Weinheim, Germany: Verlag Chemie GmbH; 1974. [Google Scholar]
- 3.Bisson L F. High-affinity glucose transport in Saccharomyces cerevisiae is under general glucose repression control. J Bacteriol. 1988;170:4838–4845. doi: 10.1128/jb.170.10.4838-4845.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bisson L F, Fraenkel D G. Expression of kinase-dependent glucose uptake in Saccharomyces cerevisiae. J Bacteriol. 1984;159:1013–1017. doi: 10.1128/jb.159.3.1013-1017.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blázquez M A, Lagunas R, Gancedo C, Gancedo J M. Trehalose-6-phosphate, a new regulator of yeast glycolysis that inhibits hexokinases. FEBS Lett. 1993;329:51–54. doi: 10.1016/0014-5793(93)80191-v. [DOI] [PubMed] [Google Scholar]
- 6.Boles E, Hollenberg C P. The molecular genetics of hexose transport in yeasts. FEMS Microbiol Rev. 1997;21:85–111. doi: 10.1111/j.1574-6976.1997.tb00346.x. [DOI] [PubMed] [Google Scholar]
- 7.Cárdenas M L, Cornish-Bowden A, Ureta T. Evolution and regulatory role of the hexokinases. Biochim Biophys Acta. 1998;1401:242–264. doi: 10.1016/s0167-4889(97)00150-x. [DOI] [PubMed] [Google Scholar]
- 8.Carlson M. Regulation of glucose utilization in yeast. Curr Opin Genet Dev. 1998;8:560–564. doi: 10.1016/s0959-437x(98)80011-7. [DOI] [PubMed] [Google Scholar]
- 9.De Koning W, Van Dam K. A method for the determination of changes of glycolytic metabolites in yeast on a subsecond time scale using extraction at neutral pH. Anal Biochem. 1992;204:118–123. doi: 10.1016/0003-2697(92)90149-2. [DOI] [PubMed] [Google Scholar]
- 10.Diderich J A, Schepper M, Van Hoek P, Luttik M A, Van Dijken J P, Pronk J T, Klaassen P, Boelens H F, Teixeira de Mattos M J, Van Dam K, Kruckeberg A L. Glucose uptake kinetics and transcription of HXT genes in chemostat cultures of Saccharomyces cerevisiae. J Biol Chem. 1999;274:15350–15359. doi: 10.1074/jbc.274.22.15350. [DOI] [PubMed] [Google Scholar]
- 11.Entian K D, Zimmermann F K. Glycolytic enzymes and intermediates in carbon catabolite repression mutants of Saccharomyces cerevisiae. Mol Gen Genet. 1980;177:345–350. doi: 10.1007/BF00267449. [DOI] [PubMed] [Google Scholar]
- 12.Entian K D, Zimmermann F K, Scheel I. A partial defect in carbon catabolite repression in mutants of Saccharomyces cerevisiae with reduced hexose phosphorylation. Mol Gen Genet. 1977;156:99–105. doi: 10.1007/BF00272258. [DOI] [PubMed] [Google Scholar]
- 13.Gancedo J M. Yeast carbon catabolite repression. Microbiol Mol Biol Rev. 1998;62:334–361. doi: 10.1128/mmbr.62.2.334-361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gancedo J M, Clifton D, Fraenkel D G. Yeast hexokinase mutants. J Biol Chem. 1977;252:4443–4444. [PubMed] [Google Scholar]
- 15.Gietz R D, Schiestl R H. Transforming yeast with DNA. Methods Mol Cell Biol. 1995;5:255–269. [Google Scholar]
- 16.Herrero P, Galindez J, Ruiz N, Martinez-Campa C, Moreno F. Transcriptional regulation of the Saccharomyces cerevisiae HXK1, HXK2 and GLK1 genes. Yeast. 1995;11:137–144. doi: 10.1002/yea.320110205. [DOI] [PubMed] [Google Scholar]
- 17.Herrero P, Martinez-Campa C, Moreno F. The hexokinase 2 protein participates in regulatory DNA-protein complexes necessary for glucose repression of the SUC2 gene in Saccharomyces cerevisiae. FEBS Lett. 1998;434:71–76. doi: 10.1016/s0014-5793(98)00872-2. [DOI] [PubMed] [Google Scholar]
- 18.Kriegel T M, Rush J, Vojtek A B, Clifton D, Fraenkel D G. In vivo phosphorylation site of hexokinase 2 in Saccharomyces cerevisiae. Biochemistry. 1994;33:148–152. doi: 10.1021/bi00167a019. [DOI] [PubMed] [Google Scholar]
- 19.Kruckeberg A L. The hexose transporter family of Saccharomyces cerevisiae. Arch Microbiol. 1996;166:283–292. doi: 10.1007/s002030050385. [DOI] [PubMed] [Google Scholar]
- 20.Liang H, Gaber R F. A novel signal transduction pathway in Saccharomyces cerevisiae defined by Snf3-regulated expression of HXT6. Mol Biol Cell. 1996;7:1953–1966. doi: 10.1091/mbc.7.12.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lobo Z, Maitra P K. Physiological role of glucose-phosphorylating enzymes in Saccharomyces cerevisiae. Arch Biochem Biophys. 1977;182:639–645. doi: 10.1016/0003-9861(77)90544-6. [DOI] [PubMed] [Google Scholar]
- 22.Locher G, Hahnemann U, Sonnleitner B, Fiechter A. Automatic bioprocess control. 4. A prototype batch of Saccharomyces cerevisiae. J Biotechnol. 1993;29:57–74. doi: 10.1016/0168-1656(93)90040-t. [DOI] [PubMed] [Google Scholar]
- 23.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.Ma H, Bloom L M, Walsh C T, Botstein D. The residual enzymatic phosphorylation activity of hexokinase II mutants is correlated with glucose repression in Saccharomyces cerevisiae. Mol Cell Biol. 1989;9:5643–5649. doi: 10.1128/mcb.9.12.5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayes E L, Hoggett J G, Kellett G L. The binding of glucose to native and proteolytically modified yeast hexokinase PI. Eur J Biochem. 1983;133:127–134. doi: 10.1111/j.1432-1033.1983.tb07437.x. [DOI] [PubMed] [Google Scholar]
- 26.McClellan C J, Bisson L F. Glucose uptake in Saccharomyces cerevisiae grown under anaerobic conditions: effect of null mutations in the hexokinase and glucokinase structural genes. J Bacteriol. 1988;170:5396–5400. doi: 10.1128/jb.170.11.5396-5400.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michels C A, Hahnenberger K M, Sylvestre Y. Pleiotropic mutations regulating resistance to glucose repression in Saccharomyces carlsbergensis are allelic to the structural gene for hexokinase B. J Bacteriol. 1983;153:574–578. doi: 10.1128/jb.153.1.574-578.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petit T, Diderich J A, Kruckeberg A L, Gancedo C, Van Dam K. Hexokinase regulates kinetics of glucose transport and expression of genes encoding hexose transporters in Saccharomyces cerevisiae. J Bacteriol. 2000;182:6815–6818. doi: 10.1128/jb.182.23.6815-6818.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pronk J T, Steensma H Y, Van Dijken J P. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast. 1996;12:1607–1633. doi: 10.1002/(sici)1097-0061(199612)12:16<1607::aid-yea70>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Raamsdonk L. Physiological responses of carbon fluxes to deletion of specific genes in Saccharomyces cerevisiae. Ph.D. thesis. Amsterdam, The Netherlands: University of Amsterdam; 2000. [Google Scholar]
- 31.Randez-Gil F, Herrero P, Sanz P, Prieto J A, Moreno F. Hexokinase PII has a double cytosolic-nuclear localisation in Saccharomyces cerevisiae. FEBS Lett. 1998;425:475–478. doi: 10.1016/s0014-5793(98)00289-0. [DOI] [PubMed] [Google Scholar]
- 32.Randez-Gil F, Sanz P, Entian K D, Prieto J A. Carbon source-dependent phosphorylation of hexokinase PII and its role in the glucose-signaling response in yeast. Mol Cell Biol. 1998;18:2940–2948. doi: 10.1128/mcb.18.5.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reifenberger E, Boles E, Ciriacy M. Kinetic characterization of individual hexose transporters of Saccharomyces cerevisiae and their relation to the triggering mechanisms of glucose repression. Eur J Biochem. 1997;245:324–333. doi: 10.1111/j.1432-1033.1997.00324.x. [DOI] [PubMed] [Google Scholar]
- 34.Richard P, Teusink B, Hemker M B, Van Dam K, Westerhoff H V. Sustained oscillations in free-energy state and hexose phosphates in yeast. Yeast. 1996;12:731–740. doi: 10.1002/(SICI)1097-0061(19960630)12:8%3C731::AID-YEA961%3E3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 35.Rose M, Albig W, Entian K D. Glucose repression in Saccharomyces cerevisiae is directly associated with hexose phosphorylation by hexokinases PI and PII. Eur J Biochem. 1991;199:511–518. doi: 10.1111/j.1432-1033.1991.tb16149.x. [DOI] [PubMed] [Google Scholar]
- 36.Schaaff I, Heinisch J, Zimmermann F K. Overproduction of glycolytic enzymes in yeast. Yeast. 1989;5:285–290. doi: 10.1002/yea.320050408. [DOI] [PubMed] [Google Scholar]
- 37.Smits H P. Mechanism and regulation of glucose transport in Saccharomyces cerevisiae. Ph.D. thesis. Amsterdam, The Netherlands: University of Amsterdam; 1996. [Google Scholar]
- 38.Teusink B. Exposing a complex metabolic system: glycolysis in Saccharomyces cerevisiae. Ph.D. thesis. Amsterdam, The Netherlands: University of Amsterdam; 1999. [Google Scholar]
- 39.Teusink B, Diderich J A, Westerhoff H V, Van Dam K, Walsh M C. Intracellular glucose concentration in depressed yeast cells consuming glucose is high enough to reduce the glucose transport rate by 50% J Bacteriol. 1998;180:556–562. doi: 10.1128/jb.180.3.556-562.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teusink B, Walsh M C, Van Dam K, Westerhoff H V. The danger of metabolic pathways with turbo design. Trends Biochem Sci. 1998;23:162–169. doi: 10.1016/s0968-0004(98)01205-5. [DOI] [PubMed] [Google Scholar]
- 41.Thevelein J M, Hohmann S. Trehalose synthase: guard to the gate of glycolysis in yeast? Trends Biochem Sci. 1995;20:3–10. doi: 10.1016/s0968-0004(00)88938-0. [DOI] [PubMed] [Google Scholar]
- 42.Van der Bend R L. Studies on the functional coupling of bacteriorhodopsin and mitochondrial ATP synthase. Ph.D. thesis. Amsterdam, The Netherlands: University of Amsterdam; 1985. [Google Scholar]
- 43.Verduyn C. Physiology of yeasts in relation to biomass yields. Antonie Leeuwenhoek. 1991;60:325–353. doi: 10.1007/BF00430373. [DOI] [PubMed] [Google Scholar]
- 44.Vojtek A B, Fraenkel D G. Phosphorylation of yeast hexokinases. Eur J Biochem. 1990;190:371–375. doi: 10.1111/j.1432-1033.1990.tb15585.x. [DOI] [PubMed] [Google Scholar]
- 45.Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 46.Walsh M C, Smits H P, Scholte M, Van Dam K. Affinity of glucose transport in Saccharomyces cerevisiae is modulated during growth on glucose. J Bacteriol. 1994;176:953–958. doi: 10.1128/jb.176.4.953-958.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson W A, Hawley S A, Hardie D G. Glucose repression/derepression in budding yeast: SNF1 protein kinase is activated by phosphorylation under derepressing conditions, and this correlates with a high AMP:ATP ratio. Curr Biol. 1996;6:1426–1434. doi: 10.1016/s0960-9822(96)00747-6. [DOI] [PubMed] [Google Scholar]