Abstract
Purpose and context. Angiotensin-converting enzyme 2 is the entry receptor for SARS-CoV and SARS-CoV-2. Variations in ACE2 expression might explain age-related symptomatology of COVID-19, that is, more gastro-intestinal symptoms and less pulmonary complaints. This study qualitatively investigated ACE2 protein expression in various organs from the fetal to the young adolescent stage. Method. Autopsy samples from lung, heart, liver, stomach, small intestine, pancreas, kidney, adrenals, and brain (when available) were obtained from twenty subjects aged 24 weeks gestational age through 28 years. Formalin-fixed paraffin-embedded 4-um-thick tissue sections were stained against ACE2. Key results. We showed that the extent of ACE2 expression is age-related. With age, expression increases in lungs and decreases in intestines. In the other examined organs, ACE2 protein expression did not change with age. In brain tissue, ACE2 was expressed in astrocytes and endothelial cells. Conclusions. Age-related ACE2 expression differences could be one substrate of the selective clinical vulnerability of the respiratory and gastro-intestinal system to SARS-CoV-2 infection during infancy.
Keywords: ACE2, children, SARS-CoV-2, immunohistochemistry
Introduction
Coronavirus disease 19 (COVID-19) has been a global pandemic for almost 2 years. During this year, many of its mysteries have been unraveled. Interestingly, children seem less affected than adults and when infected, present with different symptomatology, that is, more gastro-intestinal symptoms and less pulmonary complaints.1,2 Reasons for this are largely unknown, but might be attributable to different expression of angiotensin-converting enzyme 2 (ACE2) between children and adults. ACE2 is a metallopeptidase also functioning as receptor for SARS-CoV and SARS-CoV-2,3,4 responsible for COVID-19.
Although in adults the presence of ACE2 mRNA has been established in virtually all organs, 5 studies on its age-related expression are limited6-11 and mainly used RNA sequencing to assess ACE2 localization. However, mRNA and protein expression patterns do not necessarily correspond due to processes as post-translational modification. Furthermore, the use of immunostaining is capable of showing ACE2 expression in different subsets of cells that make up the organ parenchyma. Insight into the ACE2 protein expression profile during development and childhood is of great importance in understanding SARS-CoV-2 pathogenesis and age-related symptomatology of COVID-19. Therefore, the present study qualitatively investigated ACE2 protein expression in various organs from fetuses, younger and older children, adolescents, and young adults.
Materials and Methods
The cohort consisted of twenty subjects aged 24 weeks gestational age through 28 years, who underwent autopsy for diagnostic reasons (see Table 1 for baseline characteristics). Informed consent for research purposes was obtained by the next of kin of all patients. The study was approved by the Ethical Medical Committee of the VU University Medical Center and conducted according to the Declaration of Helsinki. All cases preceded the COVID-19 pandemic; sepsis cases were excluded to avoid changes in expression of ACE2. 12 Formalin-fixed paraffin-embedded 4-um-thick tissue sections were obtained from the lung, heart, liver, stomach, small intestine, pancreas, kidney, adrenals, and brain when available. Sections were stained against ACE2 (HPA Atlas, HPA000288, 1:500, incubated for 48 minutes) in antibody diluent (Dako, S3022) after retrieval in cell conditioning 1 solution (24 minutes, Ventana Medical Systems, 950-124) using a Ventana Benchmark Ultra machine (48 minutes, Roche) and developed with OptiView DAB IHC detection kit (Roche, 760-700). Cellular localization of ACE2 protein was assessed and immunoreactivity intensity qualitatively appraised by 2 histopathologists (PV and MB).
Table 1.
Age and Cause of Death.
| N | Age at Death | Cause of Death | Hardy Scale | Time to Autopsy (in days) | Ventilation |
|---|---|---|---|---|---|
| 1 | 24+3 weeks GA | Intrauterine fetal demise and growth retardation, placental insufficiency | 3 | 3, 2 | No |
| 2 | 30+2 weeks GA | Intrauterine fetal demise, placental infarction | 3 | 3, 2 | No |
| 3 | 39+3 weeks GA | Intrauterine fetal demise of unknown cause | 3 | 0, 8 | No |
| 4 | 1 day | Pulmonary hypertension with alveolar-capillary dysplasia and large-vessel misalignment | 0 | 0, 9 | Yes |
| 5 | 9 days | Alveolar-capillary dysplasia without misalignment | 0 | 0, 7 | Yes |
| 6 | 2 months | Intracerebral hemorrhage | 0 | 0, 8 | Yes |
| 7 | 5 months | Positional asphyxia | 2 | 0, 8 | No |
| 8 | 7 months | Hypoxic ischemic encephalopathy | 3 | 0, 5 | No |
| 9 | 22 months | Myocarditis | 0 | 0, 7 | Yes |
| 10 | 2 years | Intracerebral hemorrhage | 0 | 1, 6 | Yes |
| 11 | 2 years | Myocarditis with gastroenteritis | 3 | 3, 1 | No |
| 12 | 5 years | Rhabdomyolysis (LPIN1 mutation) | 0 | 0, 5 | Yes |
| 13 | 11 years | Osteosarcoma | 4 | 0, 6 | No |
| 14 | 16 years | Myocarditis | 3 | 0, 1 | No |
| 15 | 18 years | Myocarditis | 0 | 0, 5 | Yes |
| 16 | 23 years | Metastasized testicular seminoma | 0 | 2, 3 | Yes |
| 17 | 24 years | Acute myeloid leukemia | 0 | 0, 1 | Yes |
| 18 | 26 years | Drug overdose | 3 | 1, 8 | No |
| 19 | 26 years | Myocarditis | 3 | 2, 5 | No |
| 20 | 28 years | Drug overdose | 3 | 1, 8 | No |
Abbreviation: GA: gestational age.
Hardy scale: (1) Violent and fast death (terminal phase < 10min); (2) fast death of natural causes (terminal phase estimated at < 1 hr); (3) intermediate death after a terminal phase of 1 to 24; (4) slow death after a long illness, with a terminal phase longer than 1 day; (0) ventilator case, all cases on a ventilator preceding death. (GTEx Consortium. Hardy scale description. [cited 6 Dec 2021]. Available: https://www.ncbi.nlm.nih.gov/projects/gap/cgi-in/variable.cgi?study_id=phs000424.v4.p1&phv=169092#:∼:text=Deathclassificationbasedonthe,phaseestimatedat%3C10min).
Results
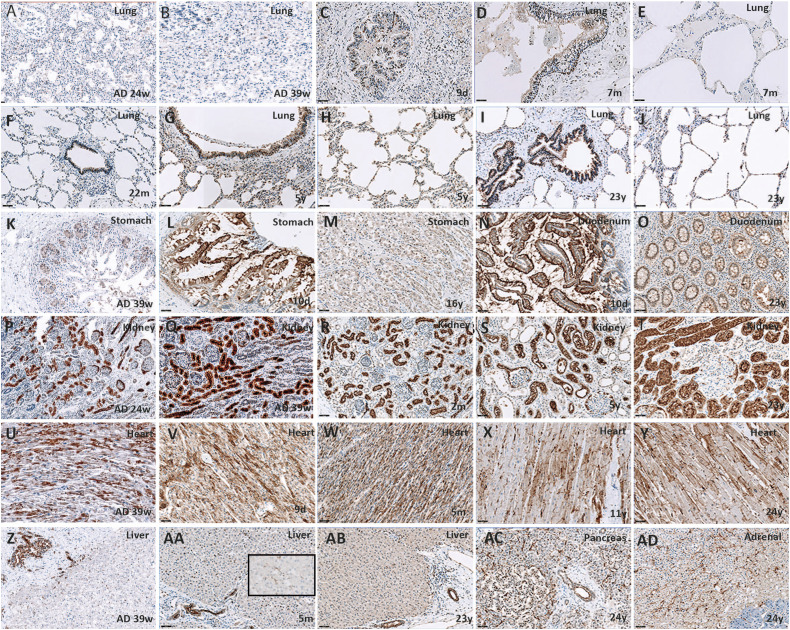
ACE2 showed various subcellular staining patterns (Table 2) and was expressed in various organs. The figure illustrates the results of ACE2 protein expression in different organs (Figure 1). Endothelium of blood vessels was ACE2 protein-immunoreactive in all organs at all ages. ACE2 protein expression in the lungs was detected in type I and II pneumocytes, intra-alveolar macrophages, and respiratory epithelium of large and small bronchioles. Expression increased with age to stabilize from 5 years on. ACE2 protein expression in the stomach was confined to epithelial and ganglion cells and strongly decreased with age. The same ACE2 protein spatial and temporal expression pattern was found in the small intestine. In the other examined organs, ACE2 protein expression did not change with age (cardiomyocytes, liver Kupffer cells and bile duct epithelium, kidney tubular and Bowman capsule epithelium, adrenal cortex, pancreatic acini and ductular epithelial cells, and endocrine cells with capillary endothelium; not shown, but available from authors upon request).
Table 2.
ACE 2 Location and Subcellular Staining Patterns.
| Organ | Cellular Location | Stain Location | ||
|---|---|---|---|---|
| Nuclear | Cytoplasmatic | Membranous | ||
| Lung | Pneumocytes | + | + | - |
| Macrophages | - | + | - | |
| Respiratory epithelium | + | + | + | |
| Heart | Cardiomyocytes | - | + | + (also Z-line) |
| Stomach | Follicular and glandular epithelium | - | + | + |
| Ganglion cells | + | - | - | |
| Small intestine | Epithelium | - | + | + |
| Lymphoid tissue | + | - | - | |
| Ganglion cells | + | - | - | |
| Liver | Kupffer cells | + | + | - |
| Bile ducts | - | + | + | |
| Hepatocytes | Weak, negative | Weak, negative | - | |
| Pancreas | Acini (sporadically) | - | - | + |
| Ducts | - | - | + | |
| Kidneys | Cortical tubuli a | - | + | + |
| Bowman’s capsule | + (endothelium) | + (epithelium, endothelium) | - | |
| Adrenal glands | Zona glomerulosa | - | + | - |
| Zona fasciculate | + | + | - | |
| Zona reticularis | - | + | - | |
| Brain | Astrocytes | - | - | + |
Endothelium of blood vessels was ACE2 protein-immunoreactive in all organs.
aDifference in expression between distal (overt expression of luminal surface and cytoplasm basal and luminal surface) and proximal (much less, cytoplasmatic and nuclear) tubuli.
Figure 1.
ACE2 protein expression through ages. All magnification ×200 (A-J). In the lungs, ACE2 protein expression increases with age in both bronchiolar epithelium and alveolar pneumocytes. (K-M) In stomach mucosal epithelial cells, ACE2 protein expression is enriched at the luminal surface and decreases with age. (N and O) The same occurs in the duodenum. (P-T) In the kidney, ACE2 protein expression is found in tubuli and epithelial blade of the Bowman’s capsule, and does not change with age. (U-Y) ACE2 protein expression in the heart is found in cardiomyocytes with enrichment at the Z-line. It remains stable through ages. (Z-AB) In the liver, ACE2 protein expression in the bile duct and Kupffer cells (insert) does not change with age. The same occurs in the pancreas (AC) and adrenal gland (AD). Note the nuclear localization in many cell types. ACE2 protein may modulate reactive oxygen species formation in the nucleus, providing a protective mechanism against oxidative stress and cell damage. 7
In brain tissue, ACE2 was expressed in astrocytes and endothelial cells; no expression changes were detected amongst ages.
Discussion
Our study confirms that ACE2 is widely expressed during development and across all ages, paralleling findings of mRNA studies in adults. 5 Interestingly, we found previously unreported age-related differences in ACE2 expression in the lungs and gastro-intestinal tract, mainly present in cells in contact with the external environment (bronchiolar epithelial cells, enterocytes). ACE2 expression in the lungs increased during the first years of life, whereas ACE2 expression in the stomach and small intestine decreased with age. These age-related expression differences could be a substrate of the selective clinical vulnerability of the respiratory and gastro-intestinal system to SARS-CoV-2 infection during infancy.1,2 In other organs, expression of ACE2 protein did not change with age.
The majority of studies that evaluate ACE2 expression across ages were limited to the lungs. An age-dependent increase in ACE2 expression in the human lung was further found in this study, with absent staining during later gestational ages and an increase during the first years of life. This has also been reported by others6-8,11 and might partly explain the predominance of respiratory symptoms in older subjects infected with COVID-19. Although results are conflicting, it is important to take note of ventilation as a possible confounding factor11,13 in pulmonary ACE2 expression.
ACE2 expression in other organs in early life, childhood, and adolescence has not been studied before. Expression during fetal life has been investigated by few, and results are conflicting.7,9 This might be explained by the differences in techniques used (immunohistochemistry vs single-cell RNA sequencing) and the aforementioned difference between RNA and mRNA/protein expression. Therefore, although our sample size was limited, we are the first to present a comprehensive view on ACE2 expression during the different stages of life and illustrate that not only does ACE2 expression in the lung increase with age, ACE2 expression in the gut decreases with age. Our results could be one substrate of the selective clinical vulnerability of the respiratory and gastro-intestinal system to SARS-CoV-2 infection during infancy in comparison with adulthood.
Acknowledgments
We thank the Mortuary personnel of the Expert Centrum for Post-mortem Diagnostics of the Amsterdam UMC for their assistance during autopsies and O. Bugiani for critically revising the manuscript.
Footnotes
Author Contributions: MB had full access to all of the data in the study and takes responsibility for the integrity and the accuracy of the data analysis.
BS, ER, and MB designed the study.
BS and ER performed the literature search. All authors acquired, analyzed, or interpreted the data. BS and MB drafted the manuscript. All authors critically revised the manuscript for important intellectual content and had final approval of the submitted and revised versions of it. MB supervised the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Amsterdam UMC Corona Research Funds (project number 2007794). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
ORCID iD
Bernadette Schurink https://orcid.org/0000-0002-6523-2094
References
- 1.Mayor S. Covid-19 : UK studies find gastrointestinal symptoms are common in children BMJ. 2020;370:m3484. doi: 10.1136/bmj.m1263. [DOI] [PubMed] [Google Scholar]
- 2.Waterfield T, Watson C, Moore R, et al. Seroprevalence of SARS-CoV-2 antibodies in children: A prospective multicentre cohort study. Arch Dis Child. 2021;106(7):680-686. doi: 10.1136/archdischild-2020-320558. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann M, Kleine-weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salamanna F, Maglio M, Landini MP, Fini M. Body localization of ACE-2: On the trail of the keyhole of SARS-CoV-2. Front Med. 2020;7:1-24. doi: 10.3389/fmed.2020.594495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saheb Sharif-Askari N, Saheb Sharif-Askari F, Alabed M, Temsah M-H, Al Heialy S, Hamid Q, et al. Airways expression of SARS-CoV-2 receptor, ACE2, and TMPRSS2 Is lower in children than adults and increases with smoking and COPD. Molecular Therapy - Methods & Clinical Development. 2020;18:1-6. doi: 10.1016/j.omtm.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faure‐Bardon V, Isnard P, Roux N, et al. Protein expression of angiotensin‐converting enzyme 2, a SARS‐CoV ‐2‐specific receptor, in fetal and placental tissues throughout gestation: new insight for perinatal counseling. Ultrasound Obstet Gynecol. 2021;57(2):242-247. doi: 10.1002/uog.22178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inde Z, Croker BA, Yapp C, et al. Age-dependent regulation of SARS-CoV-2 cell entry genes and cell death programs correlates with COVID-19 severity. Sci Adv. 2021;7(34):1-18. doi: 10.1126/sciadv.abf8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li M, Chen L, Zhang J, Xiong C, Li X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One. 2020;15(4):e0230295-12. doi: 10.1371/journal.pone.0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Li L, Zhang Y, Wang X. An investigation of the expression of 2019 novel coronavirus cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis poverty. 2020:1-7. doi: 10.21203/rs.2.24751/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow RD, Majety M, Chen S. The aging transcriptome and cellular landscape of the human lung in relation to SARS-CoV-2. Nat Commun. 2021;12(1). doi: 10.1038/s41467-020-20323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourgonje AR, Abdulle AE, Timens W, et al. Angiotensin-converting Enzyme 2 ( ACE2 ), SARS-CoV-2 and the Pathophysiology of Coronavirus Disease 2019 ( COVID-19 ) J Pathol. 2020;251(3):228–248. 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker SA, Kwok S, Berry GJ, Montine TJ. Angiotensin-converting enzyme 2 (ACE2) expression increases with age in patients requiring mechanical ventilation. PLoS One. 2021;16:e0247060-17. doi: 10.1371/journal.pone.0247060. [DOI] [PMC free article] [PubMed] [Google Scholar]