Abstract
Purpose and Context
Streptococcal Infection (SI) is an important cause of pediatric death in children, yet limited reports exist on autopsy findings in fatal SI cases.
Method
Case records (1997–2019) of SI with no pre-existing risk factors were reviewed and selected. Their clinical and pathological findings in the autopsy reports were analyzed.
Results
In our cohort of 38 cases based on bacterial culture results, SI was most commonly caused by Streptococcus pneumoniae (SPn; 45%) and Streptococcus pyogenes (SPy; 37%). 92% of decedents had some prodromal symptoms prior to terminal presentation. The clinical course was often rapid, with 89% found unresponsive, suddenly collapsing, or dying within 24 hours of hospital admission. 64% of deaths were attributed to sepsis, more frequently diagnosed in the SPy group than in the SPn group (71% vs 48%). Pneumonia was found in both SPn and SPy groups, whereas meningitis was exclusively associated with SPn.
Conclusion
Our study shows fatal SI is most commonly caused by either SPn or SPy, both of which are frequently associated with prodromal symptoms, rapid terminal clinical course, and evidence of sepsis. Postmortem diagnosis of sepsis is challenging and should be correlated with clinical features, bacterial culture results, and autopsy findings.
Keywords: Streptococcus, infection, postmortem, microbiology, death, Pediatric
Introduction
Streptococcal infection (SI) remains an important cause of morbidity and mortality in pediatric patients despite the introduction of vaccines and improved antimicrobial treatment. S pneumoniae (SPn) and S pyogenes (SPy) are the two most commonly reported Streptococcus species associated with invasive infection in children. Less common invasive streptococcal pathogens are Streptococcus agalactiae (SAg) and Viridans Streptococci (SVi).
SPn causes meningitis and bacteremia, particularly under 2 years of age, whereas pneumonia is more prevalent in older children. 1 It can also cause otitis media, sinusitis, or arthritis. Since 2000, the global prevalence of invasive pneumococcal disease (IPD) has decreased dramatically due to inclusion of multivalent pneumococcal conjugate vaccines (PCV-7 and PCV-13) in the routine pediatric immunization schedule. Non-vaccine preventable serotypes have been responsible for a more recent increase in IPD cases.2,3
SPy most frequently causes acute pharyngotonsillitis and cutaneous infections in school-aged children, but its pathogenic spectrum includes toxin-mediated disease such as streptococcal toxic shock syndrome (STSS), and immunogenic poststreptococcal glomerulonephritis and rheumatic heart disease. 4 There is currently no licensed vaccine against SPy, although several vaccine candidates are under development. 5
Children are at increased risk for SIs due to a high incidence of nasopharyngeal colonization, frequent exposure to bacteria in day-care centers and schools, and their relatively immature immune system.6,7 Nasopharyngeal carriage in asymptomatic children has been reported to be between 12 and 20% for SPy8,9 and up to 70% for SPn.4,7,10 Early diagnosis of invasive SI in young children can be challenging since clinical signs, such as fever, irritability, rhinitis, and decreased appetite, are often non-specific. 4
Many epidemiological and clinical studies of children with invasive SI exist, but there is limited reported data on autopsy findings in fatal cases. We, therefore, undertook a study to analyze the clinical and pathological features of autopsied cases in which SI was the cause of death in infants and children who were healthy or had comorbidities that did not increase their risk of Streptococcal infection.
Material and Methods
Our database of Coroner-warranted and next-of-kin-consented autopsy cases was searched from January 1997 to December 2019 for pediatric deaths beyond the neonatal period (over the age of 28 days) where SI was documented in the causes of death section. Only decedents who underwent a complete postmortem examination were included in the study. Premortem clinical information was sourced from the medical records and/or Coroner’s warrant. Patients with an underlying medical condition or a risk factor that would have predisposed them to SI were excluded from further analysis.
Postmortem examinations were performed in our autopsy suite by our anatomical pathologists. As per our autopsy standard operating procedure, blood and lung tissue samples at a minimum were taken for microbiology analysis. Blood samples were obtained with a sterile needle from the inferior vena cava or from the heart after opening of the pericardial sac. Lung tissue samples were obtained from the right middle lobe with a sterile scalpel blade. Further samples for microbiology and/or virology testing were decided on a case-by-case basis by the pathologist, and included cerebrospinal fluid (CSF), middle ear swab, nasopharyngeal swab, endotracheal swab, oropharyngeal swab, pleural fluid, pericardial fluid, heart tissue, cerebral swab, peritoneal sample, bowel content, urine, and swabs of infectious sites.
The following data were abstracted from the selected postmortem reports: clinical history, premortem microbiological culture results, administration of antibiotic treatment, postmortem macroscopic and microscopic findings, postmortem culture results, and cause of death.
Culture results were categorized as a) pure growth (positive) when the causative organism was a single isolate, b) sterile when no organisms were detected, and c) contaminated when multiple organisms grew in the culture with or without the causative bacterium. Death was attributed to a particular organism when it was detected in the premortem culture and/or at least 1 postmortem culture as a pure growth.
Postmortem diagnosis of sepsis was based on the combination of premortem clinical symptoms or clinical diagnosis of sepsis, premortem culture results, postmortem stigmata of septic death and postmortem culture results. Premortem clinical signs and symptoms of sepsis included tachycardia, hypotension, hypothermia, tachypnea, shortness of breath, respiratory distress, low O2 saturations, acidosis, and features of liver and renal failure. In those cases, when the decedents were found lifeless at home or collapsed suddenly and only limited premortem information was available, the diagnosis of sepsis was based on the postmortem findings and culture results. Pathologic findings considered as stigmata of sepsis were heavy lungs with pulmonary edema, hemorrhage and acute lung injury, kidneys with fibrin thrombi in glomerular capillaries and acute tubular injury, cerebral edema, acute contraction band necrosis in the heart, marked visceral congestion, stress-related changes in the thymus, fatty change in the adrenal gland, and leukemoid reaction observed in blood vessels. Gram histochemical stain on formalin fixed paraffin embedded sections were performed on cases showing acute inflammation.
This study was approved by The Hospital for Sick Children Research Ethics Board (REB#: 1000045666).
Results
From January 1997 to December 2019, 4453 autopsy cases were recorded in our database including Coroner and hospital autopsy cases. Of which, 62 (1%) were non-neonatal deaths where SI was documented in the cause of death section. A medical condition or risk factor predisposing a child to SI was identified in the clinical history of 24 decedents (39%) who were thus excluded (Table 1). The remaining 38 cases formed the basis of this study. Thirty-two cases (84%) were performed under Coroner’s warrant and 6 cases (16%) were next-of-kin-consented autopsies. Thirty-four decedents (89%) had no significant past medical history. Four children had a malformation or a syndrome (hydrocephalus, congenital laryngeal cleft, central hypoventilation syndrome and Opitz syndrome) that were not considered to have been a risk factor for acquisition of SI.
Table 1.
List of Decedents with Pre-Existing Condition(s) in the Medical History, Predisposing them to Streptococcal Infection (n = 24).
| Age | Sex | SSs | Immediate COD | Underlying COD/Pre-Existing Medical condition(s) |
|---|---|---|---|---|
| 1m | F | SAg | Sepsis. Meningitis | Ex-preterm infant. Never left hospital |
| 5m | F | SBo | Sepsis | Splenic hypoplasia. s/p cardiac surgery |
| 6m | M | SPn | Sepsis | Volvulus with bowel infarction. s/p partial colectomy |
| 6m | M | SPn | Pneumonia | Ex-preterm infant. Severe brain injury |
| 13 m | F | SPn | Sepsis. Pneumonia | Neuroblastoma. s/p chemotherapy and surgery |
| 13 m | M | SPy | Sepsis. Endocarditis | Congenital heart disease |
| 18 m | M | SPn | Herniation. Meningitis | Ex-preterm child. Spastic diplegia. Recurrent brain infections |
| 2y | M | SPn | Sepsis | Sickle cell disease with multiple crisis episodes |
| 2y | F | SPn | Pneumonia | Down syndrome. Congenital heart disease |
| 3y | F | SPy | STSS | Bilateral congenital chylothoraces |
| 3y | F | SPy | Pneumonia | Glottic/subglottic web. S/p laser surgery |
| 3y | M | SPn | Subacute endocarditis | Supravalvular aortic stenosis. Williams syndrome |
| 4y | M | SVi,SPn | Sepsis. Neutropenic colitis | Chronic constipation. Neurodevelopment disorder (NOS) |
| 5y | F | SPy a | Sepsis. Aspiration pneumonia | Severe central nervous system deficit (NOS). GERD. |
| 5y | M | SVi | Sepsis. Pneumonia | Poor state of hygiene. Prolonged starvation |
| 10y | F | SPy | Sepsis | Klippel-Trenaunay syndrome. Open skin defects overlying vascular malformation |
| 10y | M | SPn | Pneumonia | Progressive neurological deterioration of unknown etiology. Seizures |
| 11y | F | SPy | Meningitis | Systemic juvenile rheumatoid arthritis (on prednisolone) |
| 12y | M | SPy | Sepsis. Pneumonia | Asthma. Eczema |
| 13y | F | SPy | Septic and hemorrhagic shock | Chronic transplant liver failure. Hepatopulmonary syndrome |
| 14y | SVia | Sepsis. Aspiration pneumonia | Cerebral palsy. Spastic quadriplegia | |
| 14y | F | SPn | Sepsis | Down syndrome. Asplenia |
| 14y | M | SAg | Sepsis | Congenital heart disease. s/p cardiac surgeries. Secondary hepatic fibrosis |
| 15y | F | SPy | Sepsis. Suppurative bronchiolitis and bronchitis | Congenital heart disease. s/p cardiac surgeries. Protein losing enteropathy |
COD, cause of death; m, month(s); y, year(s); F, female; M, male; STSS, Streptococcal toxic shock syndrome; NOS, not otherwise specified; GERD, gastro-esophageal reflux disease; s/p, status post; SSp, Streptococcus species; SPn, S. pneumoniae; SPy, S. pyogenes; SAg, S. Agalactiae; SVi, Viridans Str; SBo, S. Bovis.
acontamination.
The decedents’ ages ranged from 5 weeks to 16 years with mean, median, and mode ages of 4.3, 3, and 5 years, respectively. Twenty-six (68%) deaths occurred in children older than 24 months, 7 (18%) under 12 months and 5 (13%) between 13 to 24 months. The overall male to female ratio was 1.05:1 (M: 20, F:19), with no significant age category variation among the sexes.
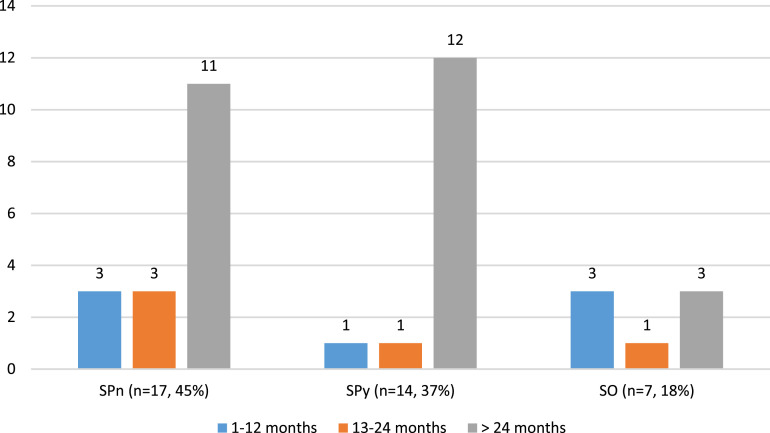
Seventeen (45%) deaths were due to SPn infection, 14 (37%) to SPy, and 7 (18%) to other Streptococcus infective organisms (SO) (Figure 1). SO included S agalactiae (SAg; n = 3) and Viridans streptococci (SVi; n = 3) and S. anginosis (n = 1). In patients younger than 24 months, SPn was responsible for half of the deaths (SPn: 6, SPy: 2, SO:4). For children older than 2 years, the numbers of SPn and SPy deaths were similar (SPn: 11, SPy: 12, SO: 3). In the SO group, SAg caused 2 infant deaths and 1 death at 23 months of age. Sepsis was more frequently associated with SPy than SPn (71% vs 48%) (Table 2). All SAg infection related deaths were due to sepsis. Pneumonia was present in a similar number of cases in SPn and SPy groups (sepsis with pneumonia SPn:3, SPy:2; pneumonia SPn:3, SPy:3). All three SAg deaths were attributed to sepsis. All meningitis-associated deaths were due to SPn infection.
Figure 1.
Distribution of Streptococcal infection cases by decedent’s age and Streptococcus species (n = 38). SPn: S. pneumoniae, SPy: S. pyogenes, SO: S. other, includes S. agalactiae (n = 3), Viridans Streptococci (n = 3), and S. anginosus (n = 1).
Table 2.
Immediate Cause of Death (COD) by Streptococcus species.
| Immediate COD | SPn: 17 (45%) | SPy: 14 (37%) | SO: 7 (18%) | Total: 38 |
|---|---|---|---|---|
| Sepsis a | 8 (48%) | 10 (71%) | 6 (86%) b | 24 (64%) |
| Non-septic causes | ||||
| Pneumonia | 3 (18%) | 3 (21%) | 1 (14%) | 7 (18%) |
| Meningitis | 6 (35%) | 0 | 0 | 6 (16%) |
| Peritonitis | 0 | 1 (7%) | 0 | 1 (3%) |
Abbreviations: SPn, S. pneumoniae; SPy, S. pyogenes; SO, S. other.
aIncluded sepsis (SPn: 3, SPy: 6, SO: 3), and sepsis with underlying pneumonia (SPn 3, SPy 2, SO:1), meningitis (SPn: 2), lung abscess (SPy:1), ruptured retrocecal appendicitis with abscess formation (SO:1), large cavitating abscess of the right side of the neck with septic emboli in both lungs (SO) and acute purulent peritonitis (SPy:1).
bIncluded cases of S. agalactiae (n = 3).
Seventy-six percent (n = 29) of cases occurred between 1997 and 2010 (Table 3). (The decrease in number of Coroner-warranted autopsies from 25 to 7 after 2010 is at least in part attributable to a departmental reduction in service provision to the Coroner’s Office.) Before 2010, the majority of deaths were above the age 24 months (SPn:6, SPy: 9, SO:3), 4 were between 13 to 24 months (SPn: 3; SO:1), and 7 were under 12 months (SPn: 3, SPy: 1, SO: 3). Between 2011 and 2019, there were only 2 cases below the age of 24 months with no infant deaths, and one death in a 16-month-old child due to SPy. The remaining 8 cases were above the age of 2 years (SPn: 5, SPy: 3).
Table 3.
Distribution of Cases by Study Period, Decedent’s Could Age, C and Type of Autopsy Examination.
| Hospital | Coroner | 1–12m | 13–24m | >24m | SPn | SPy | SO | Total: 38 | |
|---|---|---|---|---|---|---|---|---|---|
| 1997–2010 | 4 | 25 | 7 | 4 | 18 | 12 | 10 | 7 | 29 (76%) |
| 2011–2019 | 2 | 7 | 0 | 1 | 8 | 5 | 4 | 0 | 9 (24%) |
Abbreviations: m, months; SPn, Streptococcus pneumoniae; SPy, Streptococcus pyogenes; SO, Streptococcus other.
Circumstances Surrounding Death
The vast majority (92%) of children were reported as being unwell before their terminal presentation. Only 3 children were reportedly asymptomatic. Most (79%) had a history of prodromal symptoms or signs of an illness for >1 day (Table 4). Fever was the most common sign recorded in the clinical history (80%). Gastrointestinal and respiratory symptoms were frequently present at 60% and 51%, respectively. Skin rash was more often seen in the setting of SPy infection (Table 5).
Table 4.
Circumstances of Death by Streptococcus Species.
| Circumstances of Death | SPn: 17 (45%) | SPy: 14 (37%) | SO: 7 (18%) | Total: 38 |
|---|---|---|---|---|
| Prodromal symptoms | ||||
| None | 0 | 1 (7%) | 2 (29%) | 3 (8%) |
| ≤1 day | 2 (12%) | 3 (21%) | 0 | 5 (13%) |
| >1 day | 15 (88%) | 10 (71%) | 5 (71%) | 30 (79%) |
| Medical attention prior to death | 7 (41%) | 3 (21%) | 3 (43%) | 13 (34%) |
| Terminal events | ||||
| Found unresponsive at home | 0 | 2 (14%) | 3 (43%) | 5 (13%) |
| Witnessed collapse at home or in ambulance | 3 (18%) | 4 (28%) | 1 (14%) | 8 (21%) |
| Died <1 day of admission | 12 (71%) | 7 (50%) | 2 (29%) | 21 (55%) |
| Died ≥1 day of admission | 2 (12%) | 1 (7%) | 1 (14%) | 4 (11%) |
Abbreviations: SPn, S. pneumoniae; SPy, S. pyogenes; SO, S. other.
Table 5.
Prodromal Signs and Symptoms by Streptococcus Species.
| Prodromal Signs and Symptoms | SPn: 17 (49%) | SPy: 13 (37%) | SO: 5 (14%) | Total: 35 |
|---|---|---|---|---|
| Fever | 15 (88%) | 11 (85%) | 2 (40%) | 28 (80%) |
| Gastrointestinal a | 10 (59%) | 9 (69%) | 2 (40%) | 21 (60%) |
| Respiratory b | 9 (53%) | 6 (46%) | 3 (60%) | 18 (51%) |
| Rash | 1 (6%) | 5 (38%) | 1 (20%) | 7 (20%) |
| Neurological c | 2 (12%) | 1 (8%) | 0 | 3 (9%) |
| Pain d | 5 (29%) | 1 (8%) | 0 | 6 (17%) |
Abbreviations: SPn, S. pneumoniae; SPy, S. pyogenes; SO: S. other.
aIncluded vomiting, diarrhea, nausea, abdominal pain, and difficulty drinking and feeding.
bIncluded cough, shortness of breath, wheezing, runny nose, grunting, sore throat, chest pain, and tachypnea.
cIncluded seizures, confusion, and slurred speech.
dIncluded headache and neck and knee pain.
About a third of decedents were seen by a physician shortly prior to the terminal events (n = 13), including 2 who were discharged from hospital a day before death (Table 4). One of the two discharged children was admitted for respiratory distress and died from SAg sepsis with no anatomical focus of infection found at autopsy. The other child who was admitted with fever, cough and runny nose for overnight observation, died of SPn sepsis. Autopsy revealed severe splenic hypoplasia.
Five decedents were found unresponsive at home with subsequent resuscitation being unsuccessful. Of these two children had a history of prodromal symptoms, one was reportedly previously healthy, and in two infant deaths there was an associated unsafe sleeping environment. 6 children collapsed suddenly at home and 2 became unresponsive en route to the hospital in an ambulance. Twenty-one died within 24 hours of hospital presentation or in-transit, and 4 died after at least 1 day in the hospital (Table 4).
Clinical Assessment, Premortem Results, and Antibiotic Treatment
Fourteen children, who later died from sepsis, were either taken to a walk-in-clinic and then subsequently to an emergency room (ER), or directly to an ER where they were resuscitated or admitted to a ward shortly before death. The following clinical signs and symptoms of sepsis were recorded in the notes in these instances: tachycardia, hypotension, hypothermia, tachypnea, shortness of breath, respiratory distress, low O2 saturations, acidosis, and features of liver and renal failure. In three cases, only the clinical diagnosis of sepsis or septic shock was documented in the provided clinical history. Eight decedents had positive culture results (blood n = 7, pleural fluid n = 1) of which 5 received antibiotic treatment (Table 6). In one additional case antibiotic therapy was initiated although the blood culture was sterile. In the remaining 5 cases there was no information provided by the Coroner about premortem culture results or antibiotic treatment.
Table 6.
Summary Table of Findings by Cause of Death (COD).
| Septic COD 24 (64%) | Non-septic COD 14 (37%) | |
|---|---|---|
| Type of autopsy examination | ||
| Hospital (pre-/post-2010) | 2/0 | 2/2 |
| Coroner (pre-/post-2010) | 19/3 | 6/4 |
| Terminal events | ||
| Found unresponsive at home | 3 | 2 |
| Witnessed sudden collapse at home or in ambulance | 7 | 1 |
| Reached hospital | 14 | 11 |
| Died ≤1 day of admission | 13 | 8 |
| Died >1 day of admission | 1 | 3 |
| Terminal clinical assessment— | ||
| Signs or diagnosis of sepsis | 14 | |
| Seizure and other CNS symptoms | 6 | |
| Respiratory distress | 4 | |
| Features suggestive of viral gastroenteritis | 1 | |
| Premortem cultures | ||
| Positive | 11 a | 10 |
| Negative | 1 | |
| Premortem antibiotic therapy | 6 | 9 |
| Postmortem findings | ||
| Stigmata of sepsis | 24 | |
| Tissue or organ inflammation | 12 | 14 |
| Postmortem cultures | ||
| PMBC (n = 35) | ||
| Pure growth | 15 | 2 |
| Sterile | 6 | 5 |
| Contamination | 3 | 4 |
| PMLC (n = 34) | ||
| Pure growth | 9 | 4 d |
| Sterile | 1 | 2 |
| Contamination | 13 b | 5 e |
| Postmortem CSF (n = 15) | ||
| Pure growth | 2 | 2 f |
| Sterile | 7 c | 3 f |
| Contamination | 1 | 0 |
Septic category included deaths due to sepsis (n = 12) and sepsis with underlying pneumonia (n = 6), meningitis (n = 2), lung abscess (n = 1), ruptured retrocecal appendicitis with abscess formation (n = 1), large cavitating abscess of the right side of the neck with septic emboli in both lungs (n = 1) and acute purulent peritonitis (n = 1). Non-septic category included deaths due to pneumonia (n = 7), meningitis (n = 6), and diffuse purulent peritonitis (n = 1).
Abbreviations: PMBC, postmortem blood culture; PMLC, postmortem lung culture; CSF, Cerebrospinal fluid.
aIncluded three blood samples taken at time of resuscitation.
bIncluded deaths due to sepsis with underlying pneumonia (n = 2).
csepsis with underlying meningitis (n = 1).
dpneumonia (n = 4).
epneumonia (n = 3).
fmeningitis (n = 2).
Eleven children were admitted to the hospital and their deaths were due to sequelae of localized tissue or organ infection. Of these, ten had positive premortem culture results (blood n = 7, CSF n = 2, pleural fluid n = 1) and 9 received antibiotic treatment. One child admitted with a clinical diagnosis of viral gastroenteritis and rapidly deteriorated, with no blood culture taken prior to death.
Thirteen decedents were found lifeless (n = 5) or collapsed suddenly at home or in the ambulance (n = 8). In three cases blood taken at time of resuscitation isolated the pathogenic organism.
In 6 cases, there was a clinical history of recent non-Streptococcal infection. Two children had completed antibiotic treatment for otitis media. One child was admitted with respiratory distress and wheezing and was diagnosed with Bordetella pertussis infection. One deceased child had chickenpox diagnosed 3 days prior to death. One infant was admitted with bronchiolitis due to respiratory syncytial viral infection and was discharged 1 day prior to sudden collapse. One decedent had a positive rapid test for Influenza A at the time of hospital admission.
Postmortem Findings, Including Ancillary Investigations
The immediate cause of death was concluded to be sepsis in 64% of the cases (n = 24) (Table 6) based on combination of above listed premortem clinical symptoms, premortem culture results, postmortem stigmata of septic death, and postmortem culture results. Twelve cases showed pathologic findings consistent with septic deaths including heavy lungs with pulmonary edema, hemorrhage and acute lung injury, kidneys with fibrin thrombi in glomerular capillaries and acute tubular injury, cerebral edema, acute contraction band necrosis in the heart, marked visceral congestion, stress-related changes in the thymus, fatty change in the adrenal gland, and leukemoid reaction observed in blood vessels. In the remaining twelve septic deaths, postmortem examination demonstrated additional features of organ or tissue inflammation indicating the source of infection, which included pneumonia (n = 6), meningitis (n = 2), lung abscess (n = 1), ruptured retrocecal appendicitis with abscess formation (n = 1), large cavitating abscess of the right side of the neck with septic emboli in both lungs (n = 1) and acute purulent peritonitis (n = 1) (Table 2). Fifteen postmortem blood cultures (PMBC) taken from septic death cases isolated the infective organism in pure growth. Six decedents had sterile PMBC, of which 5 had positive premortem culture results and 3 received antibiotic treatment. The sixth decedent with a sterile PMBC had also received antibiotic treatment. Three PMBC’s grew contaminants: one decedent had a positive premortem culture, one had acute peritonitis with a positive postmortem peritoneal swab culture, and one had pneumonia with a postmortem lung culture (PMLC) which isolated the infective bacterium. PMLC’s were also positive for the infective organism in 9 septic deaths, of which 4 had underlying pneumonia (Table 6). Two CSF cultures isolated the pathogenic organism, but there was no evidence of meningitis on histologic examination in these cases.
Fourteen deaths (37%) were associated with localized organ or tissue inflammation such as necrotizing pneumonia (n = 7), purulent meningitis (n = 4), acute meningoencephalitis (n = 2), and diffuse purulent peritonitis (n = 1) (Table 2). In the cases of meningitis/meningoencephalitis, the immediate cause of death was neurological complications of the inflammatory process such as hypoxic—ischemic brain injury, cerebral edema leading to tonsillar and uncal herniation, or vascular thrombosis. All decedents with meningitis had premortem positive cultures (CSF n = 2, blood n = 4) and received antibiotic treatment. Postmortem CSF cultures remained positive in 2 cases, and were negative in two of the cases who had positive premortem blood culture results. In the remaining two cases, no samples for culture were taken at autopsy. In two cases of meningitis, the likely origin of infection was the middle ear. In one, there was a positive middle ear swab culture, and in another the middle ear section taken for histology examination showed inflammation. In the case of diffuse purulent peritonitis, PMBC, and PMLC results indicated postmortem contamination, and there was no premortem culture result available. Four decedents with pneumonia had positive premortem cultures (blood n = 3, pleural fluid n = 1). PMBC following positive premortem blood cultures were sterile in all 3 cases where antibiotics were administered.
Postmortem virology testing was positive in 7 cases (by PCR testing). One decedent had a rapid test for Influenza A virus preformed at hospital admission, and postmortem lung tissue detected Respiratory Syncytial Virus. Enterovirus/Rhinovirus was detected in a nasopharyngeal swab of a child found unresponsive at home. Human Herpesvirus 6 was isolated in 3 lung samples and Epstein-Barr virus in an ileal and a lung sample. One decedent had chickenpox 3 days prior to death and varicella-like viral particles were seen in the skin sample by electron microscopy (EM).
Severe splenic hypoplasia was found at autopsy in 2 children who died from SPn sepsis. Splenic weights were 0.6 g and 0.5 g with expected weights for age being 30–33 g and 39 g, respectively. Neither were known to have sickle cell disease and autopsy examination did not reveal any other etiology for the reduced splenic weights.
Discussion
SI causes a variety of pediatric diseases, yet only rarely does it invade tissues and cause significant morbidity and death. Even though there are many clinical studies analyzing SI among hospitalized children, there are limited published data on the terminal clinical course and postmortem findings among infants and children who die following invasive SI. To our knowledge, this is the first in-depth clinicopathologic autopsy-based study that focuses on fatal SI in the post-neonatal pediatric age group.
Although invasive SI is reportable in most countries, a paucity of comprehensive invasive Streptococcal diseases registries and underreporting of the diseases limit accurate estimates of the global disease burden. 11 In Canada, incidence rates of invasive Groups A Streptococcal disease (iGAS) due to SPy in the general population have continuously increased in the last 2 decades, as have incidence rates in infants.12,13 Reported incidence rates for iGAS in the USA were similar to those in Canada in the general population but were lower among infants. 14 A European study involving eleven countries found that 14% of iGAS of cases were under the age of 17 years. 15 The mortality rate of iGAS varies greatly in the literature, from 0% to 19%.15-17 In the USA, mortality rates for iGAS in Active Bacterial Core surveillance (ABCs, collaboration between the Centers for Disease Control and Prevention and several state health departments and universities participating in the Emerging Infections Program Network) areas were fairly constant between 2000 and 2018, including among infants. 18
Overall, SPn causes invasive disease more often than SPy. In Canada, the incidence rate of invasive pneumococcal disease (IPD) has increased from 7.1 per 100,000 in 2000 to 10.9 in 2018. 12 At the same time, the incidence rate for the infants steadily decreased from 53.07 to 11.9. 13 In the US, incidence and mortality rates of IPD in ABCs areas decreased for all ages, including infants.19,20 A study from Spain on pediatric patients admitted to emergency departments between 2008 and 2015 reported that 30.5% of admitted children had an SPn infection and 4% had SPy with mortality rates of 2.9% and 1%, respectively. 1
In the USA, the mortality rates for invasive SAg infection in infants decreased from 5.5 per 100,000 in 2000 to 3.06 per 100,000 in 2018, with only occasional deaths reported above the age of 1 year. 21
In our study, the largest number of SI deaths were due to SPn (45%), followed by SPy (37%). Most deaths from SPy and SPn occurred in children over 2 years old. SPy and SPn caused a similar number of deaths in this age category. Under the age of 24 months, more deaths were due to SPn infection. Additionally, there were 3 deaths due to SAg in this age group. Our results align with the age-related prevalence of SI found in literature. SPn causes more infections under the age of 5 years, particularly in those younger than 2, whereas the majority of SPy infections occur in school-aged children, between 5-17 years of age. 22 SAg usually causes severe disease in neonates (this age group was excluded from our study), but only rarely above the age of 1 year.
Our data showed that almost all children (92%) had a history of prodromal symptoms or signs of an illness prior to death, with a majority of them (79%) unwell for at least a day. Furthermore, a third of the children (34%) were seen by a doctor in the days prior leading up to their terminal presentation. The most common clinical sign was fever (80%). For both SPn and SPy groups, the time course to death was notably brief. Nearly 90% of decedents had a rapid terminal course, including about a third of whom were either found unresponsive at home, or collapsed prior to hospital presentation, the remaining dying within 24 hours of hospital admission. Only 11% survived beyond 1-day post-admission. In a study from England, analyzing SPn infection related pediatric deaths, the terminal event findings were similar to our own with 13.3% dying at home, 5.5% while en route to hospital, and 16.7% in the emergency department. Another 64% died in hospital, though, there was no specification on the length of hospital admission. 23
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. 24 Septic shock is a subset of sepsis in which underlying circulatory and cellular/metabolic abnormalities are profound enough to substantially increase mortality. 24 While clinical guidelines exist for evaluating and diagnosing sepsis in living patients (e.g., Sequential Organ Failure Assessment SOFA, originally Sepsis-related Organ Failure Assessment, scoring system) 24 the postmortem diagnosis of sepsis is far more difficult than in living patients as macroscopic and histological findings are known to be non-specific, positive culture results can be difficult to interpret and information about the circumstances of death is often unavailable (e.g., forensic autopsy cases).
Autopsy diagnosis of sepsis in the reviewed autopsy reports was based on the combination of premortem clinical symptoms or clinical diagnosis of sepsis, premortem culture results, postmortem stigmata of septic death and postmortem culture results. In cases when no premortem assessment and testing were possible, diagnosis of sepsis was based on the postmortem findings and culture results. Morphological features observed at autopsy in septic deaths are discussed and listed in two recent publications.25,26
In our cohort, 64% of the deaths were attributed to sepsis. The diagnosis of sepsis was more frequently made in the SPy group (71%) than in the SPn group (48%). Only rarely do reported studies in fatal pediatric SI cases provide any postmortem pathological correlation. Oligbu et al reported that in England, SPn-related deaths were due to meningitis (47%), lower respiratory tract infection (29%), and septicemia (24%); however, only 33% of these cases had a postmortem examination. 23 In an Australian study, the SPy mortality rate in hospitalized children was 2.8% with 3 deaths due to pneumonia and 2 to bacteremia without a focus of infection. 27 In a similar American study, the rate was 2.3% with 2 children dying from STSS. 28
Microbiological studies are important in the postmortem evaluation of invasive bacterial infection. Interpretation of postmortem culture results, however, has many challenges including determining if the isolate(s) caused death, reflected a terminal event (e.g., agonal spread) or arose during the postmortem interval (e.g., postmortem translocation and contamination). 29 Positive culture is not synonymous with infection and can be also due to contamination and colonization. 29 Diagnosis of infection requires both the invasion of a pathogenic microorganism into the tissues or body fluid and induction of immunologic and/or inflammatory response with variable degree of tissue injury. 29 It is worth noting that certain bacteria can produce toxin (e.g., SPy) which causes tissue injury without direct tissue invasion, and highly virulent microorganisms may overwhelm the host immune response. Contamination means that an isolated or additional growth of microorganisms within the tissue is not pathogenic for that location. Colonization means presence of microorganisms within a tissue which do not elicit an inflammatory response or tissue damage. Interpretation of postmortem microbiology requires clinicopathological correlation, including the patient’s clinical course, administration and duration of antibiotic therapy and autopsy findings.
Types and number of postmortem sampling should be related to the clinical scenario. For example, in cases of clinical suspected infections, the target site(s) should be sampled, whereas in cases of sudden death sampling of random sites (e.g., spleen, lung) may be warranted.29,30 Traditionally, heart blood is a standard site for culture. Due to high contamination rate of PMBC, isolated PMBC result should be interpreted cautiously. Moreover, infective organism detection in PMBC can be influenced by many factors, such as antibiotic administration, low circulating microbial loads 31 and small or insufficient amounts of blood sampled for microbiology. The recommended selection of microbiologic samples for clinical suspicion of sepsis are heart blood and spleen.29,32 A single isolate cultured from spleen and heart blood can be considered as significant as an antemortem positive blood culture. 33 Additionally, lung, liver, and kidney might be considered in order to establish multilocus growth. Histologic sampling of the tissue sites from which specimens are collected for culture can optimize interpretation of any positive culture results, that is, histologic inflammation would corroborate that an isolated organism is pathologic. Lung cultures are difficult to interpret because of frequent false-positive culture results. This can be due to colonization of respiratory tract by the patient’s oropharyngeal flora, especially in hospitalized patients, 32 postmortem growth, or postmortem migration of bacteria from the oral cavity to lungs.29,34
In our cohort, blood (n = 35), lung (n = 34), and CSF (n = 15) were the most commonly sampled sites at autopsy. Thirty-four deceased had both lungs and heart blood sampled, though, none of the cases had a solid organ sample such as spleen, kidney, and liver, submitted for microbiologic analysis. In septic deaths, 15 PMBCs detected the organism in pure growth (total number of sepsis cases: 24). PMLC were positive in 4 pneumonia cases (total number of pneumonia cases: 7) and in 4 cases of sepsis complicating pneumonia (total number of sepsis complicating pneumonia: 6). Postmortem CSF was positive in 2 cases (total number of sampled meningitis cases: 4). Due to the low number of cases, it is not possible to make reliable conclusion on how antibiotic treatment had affected postmortem culture results. However, positive premortem culture results were valuable in those cases, when the postmortem cultures were either sterile (n = 5) or contaminated (n = 1), to support the diagnosis of sepsis.
There were false-positive results. Lung and CSF postmortem cultures detected the organisms in 5 and 2 septic deaths, respectively, when histologic examination did not show inflammation in the lungs or brain sections. High number of PMLC grew contaminants in both septic and non-septic death groups.
We also recommend examination and sampling middle ear in young children. In two cases of meningitis, the likely origin of infection was otitis media, confirmed by microbiologic and histologic examination of the middle ear. Sampling the center and edges of any abnormal skin lesion for microbiology and histological assessment is also recommended 35 as skin is the most common portal of entry in iGAS.4,22
Virology testing is advised in sudden death in infants and children. 30 Indeed, Varicella and influenza viral infections in particular, have been identified as SI risk factors.36-39 A study from Finland reported that 20% of hospitalized children with invasive SPy infection had a Varicella infection 1 month before being diagnosed with invasive SPy disease. 16 In our study only 1 child had a history of chickenpox 3 days prior death who died from SPy infection. One deceased had a positive test result for Influenza A virus at hospital admission and died from pneumonia. PMLC, though, detected RSV.
In this study, we analyzed cases with no known pre-existing factors predisposing for SI. In two deaths, autopsy examination revealed severe splenic hypoplasia, which is a well-known risk factor for fatal SPn infection.
Study Limitations
This study had several limitations. The data represents a single institution’s experience with a relatively small cohort of patients. The majority of autopsies were performed under a Coroner’s warrant, therefore, biasing our study population towards sudden unexpected death cases. Furthermore, after 2010, the number of Coroner-warranted autopsies performed in our department decreased significantly as our department reduced the service to the Coroner’s office. This may have resulted in a reduction of the number of autopsy-confirmed fatal streptococcal infections in the pediatric age group in our cohort. Publicly-funded 7, 10, and 13-valent pneumococcal vaccines for children were introduced in Ontario in 2005, 2009, and 2010, respectively. The relative role of vaccination in the reduced number of cases post-2010 cannot be evaluated. Our protocol for microbiology analysis included blood and lung tissue samples at a minimum, and no solid organ cultures such as spleen or liver were obtained. Solid organs are known to be more resistant than blood and lungs to agonal or postmortem colonization, contamination or translocation, and assessment of these might have strengthened or eliminated some diagnostic dilemmas of postmortem microbiology interpretation.
In conclusion, our autopsy data showed that fatal Streptococcal deaths in the non-neonatal pediatric setting were most commonly due to SPn and SPy. Almost all children had some prodromal symptoms prior to their terminal presentation, the course of which was very rapid in majority of the cases. In a third of the cases, medical attention was sought prior to death. Sixty-four percent of deaths were due to sepsis or septic complications of an organ infection. Postmortem diagnosis of sepsis is very challenging and should be based on combination of premortem and postmortem findings. Ancillary investigations of multiple sites, including at least one solid organ (e.g., spleen, kidney, and liver), skin lesions if present, and middle ear are helpful to identify portal of entry of the infectious organism and the causal relationship among source of infection and fatal outcome. Further studies are needed to correlate clinical and pathological findings in pediatric septic deaths. Data from the identification of more virulent Streptococcal strains with molecular serotyping and/or genotyping of the bacteria that are associated with a high risk for sudden death could be of great value in future studies of pediatric deaths due to SI and vaccine development.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Anita Nagy https://orcid.org/0000-0002-2904-0618
Jeanette A Reyes https://orcid.org/0000-0002-5136-7000
References
- 1.Gangoiti I, Valle JR, Sota M, Martinez-Indart L, Benito J, Mintegi S. Characteristics of children with microbiologically confirmed invasive bacterial infections in the emergency department. Eur J Emerg Med. 2018;25(4):274-280. doi: 10.1097/MEJ.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 2.Levy C, Ouldali N, Caeymaex L, Angoulvant F, Varon E, Cohen R. Diversity of Serotype Replacement After Pneumococcal Conjugate Vaccine Implementation in Europe. J Pediatr. 2019;213:252-253. e3. doi: 10.1016/j.jpeds.2019.07.057. [DOI] [PubMed] [Google Scholar]
- 3.Wijayasri S, Hillier K, Lim GH, Harris TM, Wilson SE, Deeks SL. The shifting epidemiology and serotype distribution of invasive pneumococcal disease in Ontario, Canada, 2007-2017. PLoS One. 2019;14(12):e0226353. doi: 10.1371/journal.pone.0226353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimberlin DW, Long SS, Brady MT, Jackson MA. Red Book 2015: Report of the Committee on Infectious Diseases. Itasca, IL: American Academy of Pediatrics; 2015. http://ebookcentral.proquest.com/lib/utoronto/detail.action?docID=4443922. Accessed May 8, 2021. [Google Scholar]
- 5.Vekemans J, Gouvea-Reis F, Kim JH, et al. The path to group a streptococcus vaccines: world health organization research and development technology roadmap and preferred product characteristics. Clin Infect Dis. 2019;69(5):877-883. doi: 10.1093/cid/ciy1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Efstratiou A, Lamagni T. Epidemiology of Streptococcus pyogenes. In: Ferretti JJ, Stevens DL, Fischetti VA, eds Streptococcus Pyogenes : Basic Biology to Clinical Manifestations. University of Oklahoma Health Sciences Center; 2016. http://www.ncbi.nlm.nih.gov/books/NBK343616/. Accessed May 8, 2021. [PubMed] [Google Scholar]
- 7.Bogaert D, de Groot R, Hermans P. Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144-154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 8.Shaikh N, Leonard E, Martin JM. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics. 2010;126(3):e557-e564. doi: 10.1542/peds.2009-2648. [DOI] [PubMed] [Google Scholar]
- 9.Martin J. The Streptococcus pyogenes Carrier State. In: Ferretti JJ, Stevens DL, Fischetti VA, eds. Streptococcus Pyogenes : Basic Biology to Clinical Manifestations. Oklahoma, OK: University of Oklahoma Health Sciences Center; 2016. http://www.ncbi.nlm.nih.gov/books/NBK374206/. Accessed May 8, 2021. [PubMed] [Google Scholar]
- 10.Regev‐Yochay G, Raz M, Dagan R, et al. Nasopharyngeal Carriage ofStreptococcus pneumoniaeby Adults and Children in Community and Family Settings. Clin Infect Dis. 2004;38(5):632-639. doi: 10.1086/381547. [DOI] [PubMed] [Google Scholar]
- 11.Sims Sanyahumbi A, Colquhoun S, Wyber R, Carapetis JR. Global Disease Burden of Group A Streptococcus. In: Ferretti JJ, Stevens DL, Fischetti VA, eds. Streptococcus Pyogenes : Basic Biology to Clinical Manifestations. Oklahoma, OK: University of Oklahoma Health Sciences Center; 2016. http://www.ncbi.nlm.nih.gov/books/NBK333415/. Accessed May 8, 2021. [PubMed] [Google Scholar]
- 12.Government of Canada PHA of C . Rates of reported invasive Streptococcal cases in Canada. 2000-2018. Published January 14, 2021. https://dsol-smed.phac-aspc.gc.ca/notifiable/charts?c=pl. Accessed June 7, 2021.
- 13.Government of Canada PHA of C. Rates of reported Streptococcal cases over time in Canada, grouped by disease, ages <1, all sexes, 2000-20018. https://dsol-smed.phac-aspc.gc.ca/notifiable/charts?c=yl. Accessed June 7, 2021. Published January 14, 2021.
- 14.Rates of iGAS. ABCs areas by age, Bact Facts interactive. 2000-2018. https://wwwn.cdc.gov/BactFacts/index.html?dl=GAS_CaseRates. Accessed June 7, 2021.
- 15.Lamagni TL, Darenberg J, Luca-Harari B, et al. Epidemiology of Severe Streptococcus pyogenes Disease in Europe. J Clin Microbiol. 2008;46(7):2359-2367. doi: 10.1128/JCM.00422-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tapiainen T, Launonen S, Renko M, et al. Invasive Group A Streptococcal Infections in Children. Pediatr Infect Dis J. 2016;35(2):123-128. doi: 10.1097/INF.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 17.Nelson GE, Pondo T, Toews K-A, et al. Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005-2012. Clin Infect Dis. 2016;63(4):478-486. doi: 10.1093/cid/ciw248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rates of death following iGAS, by age, in ABCs areas, 2000-2018 Bact Facts Interactive . Published June 7, 2021. https://wwwn.cdc.gov/BactFacts/index.html?dl=GAS_DeathRates. Accessed June 7, 2021
- 19.Rates of invasive Streptococcal pneumoniae infections, by age, in ABCs (Active Bacterial Core surveillance) , 2000-2018. Published June 7, 2021. https://wwwn.cdc.gov/BactFacts/index.html?dl=SPN_CaseRates. Accessed June 7, 2021
- 20.Rates of death following invasive Streptococcal infection, by age, in ABCs areas , 2000-2018. Published June 7, 2021. https://wwwn.cdc.gov/BactFacts/index.html?dl=SPN_DeathRates. Accessed June 7, 2021
- 21.Rates of invasive group B Streptococcus infection, by age in ABCs areas 2000-2018. Published June 7, 2021. https://wwwn.cdc.gov/BactFacts/index.html?dl=GBS_CaseRates. Accessed June 7, 2021
- 22.Avire NJ, Whiley H, Ross K. A Review of Streptococcus pyogenes: Public Health Risk Factors, Prevention and Control. Pathogens. 2021;10(2):248. doi: 10.3390/pathogens10020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oligbu G, Collins S, Sheppard CL, et al. Childhood deaths attributable to invasive pneumococcal disease in england and wales, 2006-2014. Clin Infect Dis. 2017;65(2):308-314. doi: 10.1093/cid/cix310. [DOI] [PubMed] [Google Scholar]
- 24.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burton JL, Saegeman V, Arribi A, et al. Postmortem microbiology sampling following death in hospital: an ESGFOR task force consensus statement. J Clin Pathol. 2019;72(5):329-336. doi: 10.1136/jclinpath-2018-205365. [DOI] [PubMed] [Google Scholar]
- 26.Stassi C, Mondello C, Baldino G, Ventura Spagnolo E. Post-Mortem Investigations for the Diagnosis of Sepsis: A Review of Literature. Diagnostics. 2020;10(10):849. doi: 10.3390/diagnostics10100849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thielemans E, Oliver J, McMinn A, et al. Clinical description and outcomes of Australian children with invasive group a streptococcal disease. Pediatr Infect Dis J. 2020;39(5):379-384. doi: 10.1097/INF.0000000000002596. [DOI] [PubMed] [Google Scholar]
- 28.Gauguet S, Ahmed AA, Zhou J, et al. Group a streptococcal bacteremia without a source is associated with less severe disease in children. Pediatr Infect Dis J. 2015;34(4):447-449. doi: 10.1097/INF.0000000000000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caplan M, Kontz F. In: McCurdy B, ed. Cumitech 35 Postmortem Microbiology; 2001. Published online. [Google Scholar]
- 30.Fernández-Rodríguez A, Burton JL, Andreoletti L, et al. Post-mortem microbiology in sudden death: Sampling protocols proposed in different clinical settings. Clin Microbiol Infect. 2019;25(5):570-579. doi: 10.1016/j.cmi.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Boeddha NP, Schlapbach LJ, Schlapbach LJ, et al. Mortality and morbidity in community-acquired sepsis in European pediatric intensive care units: A prospective cohort study from the European Childhood Life-threatening Infectious Disease Study (EUCLIDS). Crit Care. 2018;22(1):143. doi: 10.1186/s13054-018-2052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsokos M. Pathology of Sepsis. Essent Autopsy Pract; 2006:39-85. Published online. 10.1007/1-84628-026-5_3. [DOI] [Google Scholar]
- 33.Roberts FJ. Procurement, interpretation, and value of postmortem cultures. Eur J Clin Microbiol Infect Dis. 1998;17(12):821-827. doi: 10.1007/s100960050200. [DOI] [PubMed] [Google Scholar]
- 34.Riedel S. The value of postmortem microbiology cultures. J Clin Microbiol. 2014;52(4):1028-1033. doi: 10.1128/JCM.03102-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson KM, Sterkel AK, McBride JA, Corliss RF. The shock of strep: rapid deaths due to group a streptococcus. Academic Forensic Pathology. 2018;8(1):136-149. doi: 10.23907/2018.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel RA, Binns HJ, Shulman ST. Reduction in pediatric hospitalizations for varicella-related invasive group a streptococcal infections in the varicella vaccine era. J Pediatr. 2004;144(1):68-74. doi: 10.1016/j.jpeds.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 37.Herrera AL, Huber VC, Chaussee MS. The association between invasive group a streptococcal diseases and viral respiratory tract infections. Front Microbiol. 2016;7. doi: 10.3389/fmicb.2016.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ampofo K, Herbener A, Blaschke AJ, et al. Association of 2009 pandemic influenza A (H1N1) infection and increased hospitalization with parapneumonic empyema in children in Utah. Pediatr Infect Dis J. 2010;29(10):905-909. doi: 10.1097/INF.0b013e3181df2c70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scaber J, Saeed S, Ihekweazu C, Efstratiou A, McCarthy N, O’Moore É. Group A streptococcal infections during the seasonal influenza outbreak 2010/11 in South East England. Euro Surveill. 2011;16(5):19780. doi: 10.2807/ese.16.05.19780-en. [DOI] [PubMed] [Google Scholar]