Abstract
Introduction
We assessed the association between hemoglobin A1c time in range (A1c TIR), based on unique patient-level A1c target ranges, with risks of developing microvascular and macrovascular complications in older adults with diabetes.
Research design and methods
We used a retrospective observational study design and identified patients with diabetes from the Department of Veterans Affairs (n=397 634). Patients were 65 years and older and enrolled in Medicare during the period 2004–2016. Patients were assigned to individualized A1c target ranges based on estimated life expectancy and the presence or absence of diabetes complications. We computed A1c TIR for patients with at least four A1c tests during a 3-year baseline period. The association between A1c TIR and time to incident microvascular and macrovascular complications was studied in models that included A1c mean and A1c SD.
Results
We identified 74 016 patients to assess for incident microvascular complications and 89 625 patients to assess for macrovascular complications during an average follow-up of 5.5 years. Cox proportional hazards models showed lower A1c TIR was associated with higher risk of microvascular (A1c TIR 0% to <20%; HR=1.04; 95%) and macrovascular complications (A1c TIR 0% to <20%; HR=1.07; 95%). A1c mean was associated with increased risk of microvascular and macrovascular complications but A1c SD was not. The association of A1c TIR with incidence and progression of individual diabetes complications within the microvascular and macrovascular composites showed similar trends.
Conclusions
Maintaining stability of A1c levels in unique target ranges was associated with lower likelihood of developing microvascular and macrovascular complications in older adults with diabetes.
Keywords: A1c, diabetes complications
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Intervention studies are needed to determine whether sex-specific physical activity recommendations are needed as although moderate-to-vigorous intensity physical activity is associated with lower risk type 2 diabetes risk markers in men and women, light intensity physical activity seems to be beneficial in women only.
Diabetes increases the risk of microvascular and macrovascular complications and incidence of complications appears to be increasing.
Individualized A1c targets may help management of diabetes.
Considering the amount of time in range (TIR) that an individual’s A1c stays within a targeted range may be an important risk factor.
WHAT THIS STUDY ADDS
Individuals who spent the least amount of time with their A1c values within a targeted range had higher risks of microvascular and macrovascular complications in both unadjusted and adjusted models.
Among individuals with pre-existing complications, lower time in a targeted A1c range was associated with progression or development of greater complications for nephropathy and cardiovascular conditions.
A computed measure of A1c TIR was an independent predictor of diabetes complications accounting for A1c mean levels and variability.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our findings suggest A1c TIR should be considered when thinking about intensification of treatment.
Introduction
Diabetes increases the risk of microvascular and macrovascular complications1 and mortality.2 3 Microvascular and macrovascular complications generate high costs of care for patients with diabetes, with estimates for treating complications ranging between one-third and one-half of direct medical costs.4–6 Development of one diabetes complication increases the risk for additional complications.7 8 Furthermore, the trends for incidence of diabetes complications appears to be increasing.9 Understanding factors that lead to their development may help slow or prevent new complications. Several studies illustrate the complex relationship between glucose control and target organ damage, particularly in type 2 diabetes.10 11 Lowering mean hemoglobin A1c (A1c) reduces microvascular complications but may not consistently affect cardiovascular disease (CVD) or mortality.
Additional risk information may be contained in the variability of A1c over time. A1c variability has emerged as a significant risk factor for microvascular and macrovascular complications and mortality. Visit-to-visit A1c variation is an independent risk factor beyond mean A1c levels for developing microvascular and macrovascular complications12 13 as well as short-term complications, such as hypoglycemia.14 Individual A1c trends over time, particularly declining values, may confer unique risks for mortality.15
Setting and achieving individualized A1c target ranges is a potential pathway for limiting glycemic variability and reducing short-term and long-term risks, particularly for older adults.16 17 Moving beyond measures of average A1c and variability, we operationalized a measure of A1c stability over time, termed A1c time in range (A1c TIR). A1c TIR applies patient-level characteristics and captures A1c variability using individualized target ranges with upper and lower bounds. Unlike A1c variability, which is often reported as SD, A1c TIR is expressed as the percentage of time a person’s A1c levels fall within unique ranges. We recently showed that higher A1c TIR is associated with lower risk of CVD and mortality.18
The current study builds on this prior research by examining the association of A1c TIR with development and progression of microvascular and macrovascular diabetes complications in a large nationwide sample of older Veterans with diabetes.
Methods
Research design and methods
This was an observational cohort study over a multiyear period.
Study population
Nationwide data were obtained from Veterans Affairs (VA) and Medicare between 2004 and 2016. Patients who were aged 65 years or older and enrolled in both VA and Medicare were included to ensure greater confidence in the comprehensiveness of clinical and outcome data. We first identified patients with diabetes using diagnosis codes (eg, two outpatient diagnoses or one inpatient diagnosis) or prescribed antihyperglycemic medications based on VA data.19 Patients meeting these criteria between 2005 and 2012 were eligible for consideration. Each patient had 4 years of data used to establish their A1c TIR values. This comprised a 1-year initial period to determine the presence of diabetes complications and estimate life expectancy and then a 3-year baseline period to establish annual A1c target ranges and A1c TIR (see online supplemental appendix A). Patients were required to have four or more A1c tests during the baseline period. Complications and comorbidities were updated annually using the prior year’s clinical information to establish the subsequent year’s A1c target range and A1c TIR. All patients had at least 12 months of follow-up time before the study period ended on 31 December, 2016. The final sample size was 397 634 patients.
bmjdrc-2021-002738supp002.pdf (369.4KB, pdf)
Measures
The primary measure of interest was the per cent of time during a 3-year baseline period when a patient’s A1c levels fell within their unique target range. We regressed incidence of diabetes complications and progression of diabetes complications on A1c TIR and related patient-level covariates. We controlled for facility variation and calendar quarter effects.
A1c time in range
Patients were assigned to A1c target ranges based on life expectancy and the presence or absence of diabetes complications based on the VA/Department of Defense Diabetes Clinical Practice Guideline.20 For our study, we used a 3×3 table consisting of life expectancy (<5 years, 5–10 years, >10 years) and diabetes complications (absent or mild; moderate or advanced). A patient’s position in the table would determine their A1c target range, with the lowest target range between 6.0% and 7.0% for those with absent or mild complications and life expectancy >10 years and the highest target range between 8% and 9% for those with advanced complications or life expectancy <5 years.
First, to create the A1c TIR variable, we used a multistage process that involved determining estimated life expectancy using a weighted point system for both a 5-year and a 10-year mortality model.21 For baseline years 1, 2, and 3, we used data from the preceding year to assign patients into a unique A1c target range based on their life expectancy and diabetes complications. Patients needed to have at least four A1c tests with none >12 calendar months apart. A1c TIR was calculated as the percentage of days during the 3-year baseline period when the A1c level was within the identified range. We used linear interpolation and extrapolation to establish the daily A1c level, which was summarized as a per cent of time within range over the 3-year baseline period.
Diabetes Complications Severity Index
We used the Diabetes Complications Severity Index (DCSI) to assess the overall severity of diabetes complications22 by combining International Classification of Diseases-9/10 codes and laboratory values to categorize patients based on the prevalence and incidence of specific complications.23 The measure comprised seven categories—retinopathy, nephropathy, neuropathy, cerebrovascular, cardiovascular, peripheral vascular diseases, and metabolic—and scores each between 0 and 2, representing not present, mild, or severe. The neuropathy category is scored as 0–1. When all complication scores are summed, the total DCSI score ranges from 0 to 13. We created six separate and two composite complication measures, namely a microvascular composite comprising retinopathy, nephropathy, and neuropathy and a macrovascular composite consisting of cerebrovascular, cardiovascular, and peripheral vascular diseases.
We examined the commonly used incidence model24 as well as a novel progressive or incremental model. In the incidence models, we excluded patients who had pre-existing complications (ie, score of 1 or 2) during the baseline, and also excluded patients with conditions overlapping with Elixhauser comorbidity categories. Only patients without such complications at baseline (ie, score of 0) were followed for the development of a complication in the outcome period. In the composite measures, patients who had no existing complications during baseline for any of the three underlying measures were followed until they developed a complication in any of the three conditions during the outcome period. In the progressive models, patients with a score of 1 (ie, mild complications) for eligible complications during baseline were followed to examine if and when they developed more severe complications (ie, score of 2).
Covariates
We accounted for several patient characteristics during the baseline period that could influence the development of diabetes complications. These included demographics, measures of ability to obtain VA services, calendar quarter when the patient entered the study cohort to examine time trends, and the VA medical center where care was delivered to account for facility-specific factors. We included Elixhauser comorbidities,25 the baseline DCSI score, diabetes medications, medication adherence (proportion of days covered ≥80%), and several lab measures and clinical provider characteristics to control for quality of care differences. We also accounted for average A1c level, A1c SD, and number of A1c tests during the baseline period to determine the independent predictive role of A1c TIR.
Analysis
We estimated the effect of A1c TIR on developing each of six specific diabetes complications and the microvascular and macrovascular composites using Cox proportional hazard regression models. A1c TIR was separated into five categories of 0% to <20%, 20% to <40%, 40% to <60%, 60% to <80%, and 80% to 100%. Patients were followed from the end of their baseline period until they experienced a censoring event (ie, development of a diabetes complication or mortality) or through the end of the study. We modeled A1c TIR and outcomes in both unadjusted and adjusted models. As a sensitivity test, we divided the study sample into quintiles with similar proportions of patients based on A1c TIR. We also examined categorical values of mean A1c to assess for non-linear effects on diabetes complications.26 We also examined progression of complications among patients with existing complications (ie, whether they developed a more severe complication). We examined incremental risk discrimination statistics of models. Analyses were conducted using STATA V.17 software.
Results
Patients included in the analysis were 76.9 years of age on average at the beginning of follow-up period, predominantly white (86.3%) and male (98.7%) (table 1). The average follow-up period was 5.5 years (range 1–9 years). At the beginning of follow-up, 81% of patients had an existing microvascular complication and 77% of patients had an existing macrovascular complication. Among the remaining patients, 71.4% of 74 016 patients developed a new microvascular complication and 63.9% of 89 625 patients developed a new macrovascular complication during the outcome period. The average baseline A1c was 7.0% (SD=0.98), patients had an average of 6 A1c tests during the 3-year baseline period, 9.1% had an A1c TIR between 80% and 100%, and 48.7% had an A1c TIR between 0% and 20%.
Table 1.
Selected descriptive demographic and comorbidity statistics at baseline (n=397 634)
| Mean (SD) or n (%) | |
| Demographics | |
| Age (years) at start of the follow-up period | 76.93 (5.69) |
| 68–72 | 81 816 (20.6%) |
| 73–76 | 97 149 (24.4%) |
| 77–81 | 108 398 (27.3%) |
| 82–105 | 110 271 (27.7%) |
| Sex: male | 392 643 (98.7%) |
| Race/Ethnicity | |
| White | 342 201 (86.3%) |
| Black | 42 476 (10.7%) |
| Hispanic | 6119 (1.5%) |
| Asian | 1422 (0.4%) |
| Other | 4416 (1.1%) |
| Marital status | |
| Married | 271 364 (68.4%) |
| Divorced/Separated | 53 210 (13.4%) |
| Widowed | 54 007 (13.6%) |
| Other | 19 053 (4.8%) |
| HbA1c time in range | |
| 80% to 100% | 36 138 (9.1%) |
| 60% to <80% | 40 622 (10.2%) |
| 40% to <60% | 51 179 (12.9%) |
| 20% to <40% | 76 081 (19.1%) |
| 0% to <20% | 193 614 (48.7%) |
| HbA1c (%) average of all tests during baseline | 7.00 (0.98) |
| HbA1c SD of all tests during baseline | 0.56 (0.44) |
| Diabetes Complications Severity Index mean (highest score during baseline) | 3.92 (2.50) |
| Albumin/Creatinine ratio (urine) | |
| <30 | 105 213 (26.5%) |
| 30–300 | 53 022 (13.3%) |
| >300 | 8828 (2.2%) |
| Missing | 230 571 (58.0%) |
| Creatinine | |
| <0.6 | 167 (0.1%) |
| 0.6–1.2 | 231 647 (58.3%) |
| >1.2 | 154 933 (39%) |
| Missing | 10 887 (2.7%) |
| Albumin | |
| <3.5 | 25 375 (6.4%) |
| >3.5 | 331 233 (83.3%) |
| Missing | 41 026 (10.3%) |
| HDL | |
| <40 | 213 737 (53.8%) |
| 40–60 | 153 134 (41.0%) |
| ≥60 | 19 028 (4.8%) |
| Missing | 1735 (0.4%) |
| LDL | |
| <100 | 295 312 (74.3%) |
| 100–160 | 95 932 (24.1%) |
| ≥160 | 2742 (0.7%) |
| Missing | 3648 (0.9%) |
| Triglycerides | |
| <200 | 313 318 (78.8%) |
| ≥200 | 83 300 (20.9%) |
| Missing | 1016 (0.3%) |
| BMI (kg/m2) during baseline | 30.2 (5.2) |
| <18.5 | 635 (0.2%) |
| 18.5–24.9 | 50 899 (12.8%) |
| 25–29.9 | 151 038 (38.0%) |
| 30–39.9 | 160 211 (40.3%) |
| ≥40 | 17 730 (4.5%) |
| Missing | 17 121 (4.3%) |
| Medications* | |
| Sulfonylurea | 213 116 (53.6%) |
| Biguanide | 196 329 (49.4%) |
| Insulin | 97 183 (24.4%) |
| Thiazolidinedione | 63 084 (15.9%) |
| Alpha-glucosidase inhibitors | 7672 (1.9%) |
| Other† | 6370 (1.6%) |
| Medication adherence—proportion of days covered ≥80% | 226 599 (57.0%) |
| Select comorbidities observed during baseline period | |
| Diabetes: type 1 | 4439 (1.12%) |
| Tobacco use | 85 943 (21.6%) |
| Cardiovascular disease | 274 554 (69.1%) |
| Cerebrovascular disease | 120 400 (30.3%) |
| Congestive heart failure | 119 723 (30.1%) |
| Hypertension | 381 357 (95.9%) |
*Medications report all medications taken by a patient, hence the percentage represents prevalence within each medication category.
†Other medications: amylin analog, bile acid sequestrants, dipeptidyl peptidase inhibitor, dopamine receptor agonist, glucagon-like peptide, meglitinides, sodium-glucose cotransporter inhibitor.
BMI, body mass index; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
In unadjusted Cox proportional hazard models, there was a graded relationship between lower A1c TIR with incident microvascular (between 1.17 and 1.30) and macrovascular complications (HR between 1.18 and 1.34) (online supplemental appendix B1 and B2). Individual complication measures followed a similar pattern.
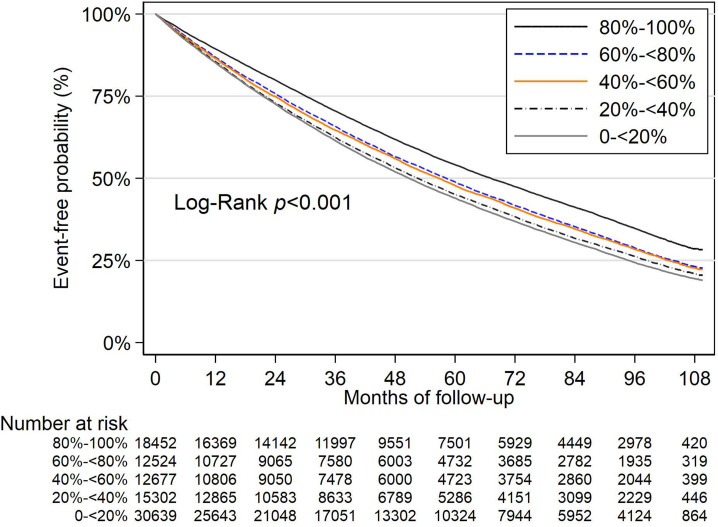
After controlling for all covariates, A1c TIR <80% was associated with greater risks of incident microvascular and macrovascular complications. For risk of developing new microvascular complications during the follow-up, the HR was 1.04 (95% CI 1.02 to 1.07) for patients with A1c TIR 0% to <20% when compared with patients with A1c TIR 80%–100%. HRs for each individual complication were similar (table 2). Survival probability curves for new microvascular complications based on A1c TIR (figure 1) confirmed the significant risk associated with lower A1c TIR. A1c mean was also significantly associated with the composite of microvascular complications and individual complications. A1c SD was not associated with the microvascular composite but had variable higher and lower risks associated with individual components. Other significant associations with incidence of new microvascular complication included age, elevated BMI, and insulin use (online supplemental appendix C1).
Table 2.
Adjusted models HRs of A1c TIR predicting incident microvascular complications
| Main predictor | Microvascular (n=74 016) |
Retinopathy (n=235 580) |
Neuropathy (n=222 274) |
Nephropathy (n=168 616) |
| A1c TIR | ||||
| 80% to 100% | 1.00 | 1.00 | 1.00 | 1.00 |
| 60% to <80% | 1.03* (1.00–1.07) | 1.03 (1.00–1.06) | 1.07* (1.04–1.10) | 1.03 (1.00–1.05) |
| 40% to <60% | 1.02 (0.99–1.05) | 1.04* (1.01–1.07) | 1.05* (1.02–1.08) | 1.05* (1.02–1.08) |
| 20% to <40% | 1.03* (1.00–1.06) | 1.04* (1.01–1.07) | 1.05* (1.02–1.08) | 1.04* (1.02–1.07) |
| 0% to <20% | 1.04* (1.02–1.07) | 1.05* (1.02–1.08) | 1.04* (1.01–1.06) | 1.06* (1.04–1.09) |
| A1c SD | 1.01 (0.98–1.04) | 0.92* (0.90–0.94) | 0.95* (0.93–0.97) | 1.08* (1.05–1.10) |
| A1c mean | 1.09* (1.08–1.11) | 1.17* (1.15–1.18) | 1.10* (1.09–1.11) | 1.08* (1.07–1.09) |
*P<0.05.
A1c, hemoglobin A1c; TIR, time in range.
Figure 1.
Kaplan-Meier estimates for time to incident microvascular events by hemoglobin A1c time in range. A1c, hemoglobin A1c.
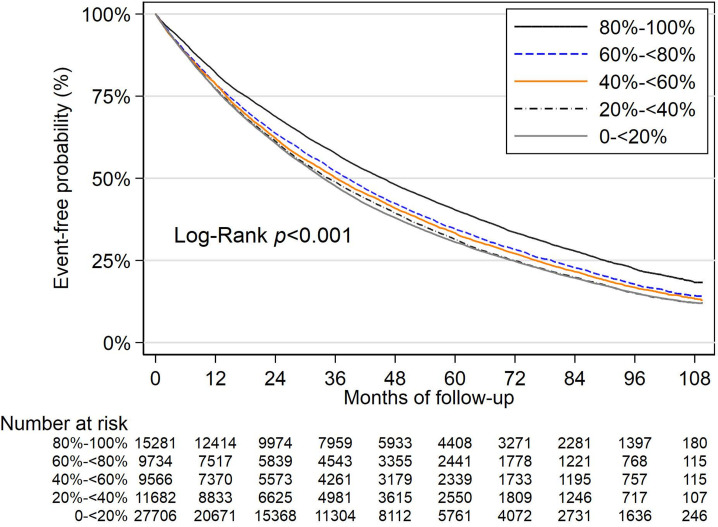
Similar results were seen for incident macrovascular complications. Patients with A1c TIR <80% had greater risk of macrovascular complications. For patients with A1c TIR 0% to <20%, the HR was 1.08 (95% CI 1.05 to 1.10) for incidence of macrovascular complications (table 3). A1c mean was significantly associated (1.05; 95% CI 1.04 to 1.06) whereas A1c SD was not. Survival probability curves (figure 2) again illustrated the increased risk associated with lower A1c TIR. Other measures that were significant in the incident macrovascular complication model included age, increased BMI, elevated LDL, urine albumin-to-creatinine ratio, and insulin use (online supplemental appendix table C2). Risk discrimination model results for the microvascular and macrovascular composites are presented in online supplemental appendix table C3.
Table 3.
Adjusted HRs of A1c TIR predicting incident macrovascular complications
| Main predictor | Macrovascular (n=89 625) |
Cardiovascular (n=122 135) |
Cerebrovascular (n=277 234) |
Peripheral vascular (n=228 583) |
| A1c TIR | * | * | * | * |
| 80% to 100% | 1.00 | 1.00 | 1.00 | 1.00 |
| 60% to <80% | 1.05* (1.02–1.08) | 1.02 (0.99–1.05) | 1.05* (1.02–1.08) | 1.09* (1.06–1.13) |
| 40% to <60% | 1.03* (1.00–1.06) | 1.01 (0.99–1.04) | 1.08* (1.05–1.11) | 1.09* (1.05–1.12) |
| 20% to <40% | 1.05* (1.02–1.08) | 1.05* (1.02–1.08) | 1.07* (1.04–1.10) | 1.09* (1.06–1.12) |
| 0% to <20% | 1.08* (1.05–1.11) | 1.08* (1.05–1.11) | 1.09* (1.07–1.12) | 1.12* (1.09–1.15) |
| A1c SD | 1.00 (0.98–1.03) | 1.00 (0.98–1.02) | 0.98 (0.97–1.00) | 0.99 (0.97–1.01) |
| A1c mean | 1.05* (1.04–1.06) | 1.05* (1.04–1.06) | 1.08* (1.07–1.09) | 1.11* (1.10–1.12) |
*P<0.05.
A1c, hemoglobin A1c; TIR, time in range.
Figure 2.
Kaplan-Meier estimates for time to incident macrovascular events by hemoglobin A1c time in range. A1c, hemoglobin A1c.
In sensitivity models, we examined associations of A1c TIR and diabetes complications by quintiles of the study population and found similar effects (online supplemental appendix table B3). For the microvascular complications, the HR was 1.05 (95% CI 1.02 to 1.08) for the lowest A1c TIR quintile (0%–2.54%) when compared with patients in the highest A1c TIR quintile (80.64%–100%). For macrovascular complications, the HR was 1.10 (95% CI 1.06 to 1.13) for the lowest A1c TIR quintile (0%–2.74%) when compared with patients with the highest A1c TIR quintile (80.55%–100%). In models assessing categorical A1c values, lower A1c TIR had increased risks. We also observed a stepped increase in risk as A1c values increased for both microvascular and macrovascular complications, while A1c below 6.5% was associated with lower microvascular risks (online supplemental appendix table B4).
Among patients with a pre-existing diabetes complication during the baseline period, we modeled the extent to which A1c TIR associated with ‘progression’ or developing more severe complications in each category (ie, severity score increased from 1 to 2) (online supplemental appendix table D). The HR coefficients were 1.08 (95% CI 1.04 to 1.12) for nephropathy; 1.07 (95% CI 1.04 to 1.11) for cardiovascular; and 1.07 (95% CI 1.00 to 1.14) for peripheral vascular when A1c TIR <20%. HRs showed similar trends as with incident complications. A1c TIR was non-significant in models for retinopathy and cerebrovascular. A1c mean was associated with worsening of each of the complications.
Discussion
We evaluated the association between A1c TIR and incidence and progression of microvascular and macrovascular complications among older Veterans with diabetes. A1c TIR is derived from A1c levels and individualized A1c target ranges that incorporate comorbidities, complications, and life expectancy. In models that controlled for patient characteristics, average A1c levels, and A1c SD, we found that lower A1c TIR is associated with greater risks of developing new microvascular and macrovascular complications and progression to more severe complications. Our prior studies showed that lower A1c TIR is also associated with increased risk of mortality and stroke/myocardial infarction.18 These findings suggest that A1c TIR is a potential marker of risk for major complications and mortality among older adults with diabetes. Collectively, these studies highlight that A1c stability within individualized target ranges may convey important and independent risk information beyond average A1c levels alone, and further exploration of this line of investigation is warranted.
Major clinical trials in type 2 diabetes emphasize the need to move beyond A1c levels alone as a means of assessing risk for major diabetes complications and mortality. Lower A1c per se may improve some microvascular complications but does not significantly affect macrovascular outcomes.27 28 A meta-analysis of several large trials reported that renal and retinal events may be improved with intensive control but neuropathic complications are unaffected.29 The UK Prospective Diabetes Study examined intensive treatment in younger patients with newly diagnosed diabetes and showed reductions in microvascular but not macrovascular events during the initial trial, although reduced cardiovascular events in the intensive treatment group were later observed after 10 years of post-trial follow-up.30 31 Despite these findings, many clinical practice guidelines still set A1c treatment goals with only upper limits,32–36 implying that a wide range of levels below that threshold are acceptable. This may expose older adults to risks of polypharmacy and potential overtreatment, such as hospitalization for hypoglycemia and falls,37 38 with uncertain benefits on risk of diabetes complications or mortality.
We focused on A1c TIR as a key risk predictor after adjusting for the average A1c level. Unlike traditional measures of A1c variability, such as A1c SD, which are heavily influenced by study population and sample characteristics, A1c TIR is a novel construct that incorporates both guideline-directed A1c levels and A1c stability within those ranges. Our current study shows that A1c TIR helps quantitate the risks associated with both increased A1c mean as well as A1c variability with diabetes complications and mortality. Although the additional information provided by A1c TIR beyond average A1c in risk discrimination is minor, understanding the clinical implications of this measure are important next steps to test in clinical practice. It may be informative for future studies to separate A1c TIR values into time spent primarily above or below A1c target ranges. For example, moving from higher to lower A1c TIR in which levels are predominantly above or below the target range may reflect changes in health conditions, lifestyle or behavior changes, medication intensification, or medication non-adherence. Whether low A1c TIR as a result of these and other factors is associated with risk of complications will be instructive. In addition, overtreatment or undertreatment with a low A1c TIR may be due to clinician practice behavior39 and unique factors related to the practice environment.40 Efforts to provide clinicians with audit and feedback on patient-level A1c TIR may be a strategy to enhance A1c stability over time among older adults.41 A1c TIR may also be useful to consider in understanding the benefits of new antihyperglycemic medications,42 such as sodium-glucose cotransporter-2,43 glucagon-like peptide-1,44 or tirzepatide.45
Strengths and limitations
Strengths of this study include adopting the large nationwide sample of older Veterans and an extended follow-up period. Our use of both VA and Medicare claims data provided a more comprehensive assessment of diagnoses and outcomes. We developed a conceptually novel construct of A1c TIR during a 3-year baseline period. We then adjusted for traditional indicators of A1c variability (including mean and SD) and comprehensive sets of covariates related to demographic, clinical markers, comorbidities, and medications and evaluated its association with subsequent diabetes complications during a follow-up period to minimize the risks of reverse causation. Specifically, study models included A1c TIR and patient characteristics, A1c mean, and A1c SD, with the latter helping to isolate A1c TIR as a unique risk predictor beyond other markers of glucose control.
Nonetheless, the study has limitations. The sample was predominantly male, white, and over 65 years of age. Determining how well A1c TIR differentiates outcomes among a more diverse sample would help in understanding the robustness of A1c TIR as a risk predictor. Furthermore, we studied military Veterans, who have higher comorbidities than non-Veteran peers46 47 and were selected because of regular use of VA healthcare services. Greater reliance on VA could influence complications through differences in treatment practices by setting.48 Complications were assessed using coded data and reflect how clinicians code for different conditions. VA clinicians may be less focused on exact coding because only a minority of patients have external billing. Finally, several factors may also affect A1c stability over time, such as diabetes duration, engagement with diabetes self-management, nutrition, and financial and social stability. These are not typically captured in coded electronic health record data. A limitation of the A1c TIR measure is that A1c test results are obtained at a non-standardized frequency or number. Study models included the number of A1c tests, and this was not associated with outcomes. A prospective study in newly diagnosed patients and those with established diabetes, with A1c testing at 3–4 months intervals and attention to treatment transitions when new medications are begun, would be informative to understand the trajectory of A1c TIR and its relation to clinical outcomes.49
In summary, lower A1c TIR during a 3-year baseline was associated with an increased risk of incidence and progression of microvascular and macrovascular complications independent of average A1c and categorical levels of A1c and A1c SD. These results suggest that A1c stability over time within unique target ranges is associated with a reduced risk of major diabetes complications in older adults with diabetes.
bmjdrc-2021-002738supp001.pdf (369.4KB, pdf)
Acknowledgments
The authors are indebted to Rebecca Lamkin for invaluable administrative support.
Footnotes
Contributors: DCM wrote the first draft and is acting as guarantor. DCM and PRC designed the present study. LZ performed its statistical analysis and coordinated data analysis with DL and EP. JCP and REN reviewed and provided critical comments on all drafts. All authors provided comments on drafts of the manuscript.
Funding: This material is based on work supported by the Department of Veterans Affairs, Health Services Research and Development (IIR 15-116), and the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK114098).
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government, Boston University or University of Utah.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. There would be need to submit to IRB for permission to release de-identified dataset.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was reviewed and approved by the Institutional Review Board at the Veterans Affairs (VA) Boston Healthcare System (1584905).
References
- 1.Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther 2008;88:1254–64. 10.2522/ptj.20080020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goff DC, Gerstein HC, Ginsberg HN, et al. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the action to control cardiovascular risk in diabetes (ACCORD) trial. Am J Cardiol 2007;99:S4–20. 10.1016/j.amjcard.2007.03.002 [DOI] [PubMed] [Google Scholar]
- 3.Forbes A. Reducing the burden of mortality in older people with diabetes: a review of current research. Front Endocrinol 2020;11:133. 10.3389/fendo.2020.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Ferber L, Köster I, Hauner H. Medical costs of diabetic complications total costs and excess costs by age and type of treatment results of the German CoDiM study. Exp Clin Endocrinol Diabetes 2007;115:97–104. 10.1055/s-2007-949152 [DOI] [PubMed] [Google Scholar]
- 5.Hogan P, Dall T, Nikolov P, et al. Economic costs of diabetes in the US in 2002. Diabetes Care 2003;26:917–32. 10.2337/diacare.26.3.917 [DOI] [PubMed] [Google Scholar]
- 6.Yang W, Cintina I, Hoerger T, et al. Estimating costs of diabetes complications in people <65 years in the U.S. using panel data. J Diabetes Complications 2020;34:107735. 10.1016/j.jdiacomp.2020.107735 [DOI] [PubMed] [Google Scholar]
- 7.Park HC, Lee Y-K, Cho AJin, et al. Diabetic retinopathy is a prognostic factor for progression of chronic kidney disease in the patients with type 2 diabetes mellitus. PLoS One 2019;14:e0220506. 10.1371/journal.pone.0220506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamanouchi M, Mori M, Hoshino J, et al. Retinopathy progression and the risk of end-stage kidney disease: results from a longitudinal Japanese cohort of 232 patients with type 2 diabetes and biopsy-proven diabetic kidney disease. BMJ Open Diabetes Res Care 2019;7:e000726. 10.1136/bmjdrc-2019-000726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregg EW, Hora I, Benoit SR. Resurgence in diabetes-related complications. JAMA 2019;321:1867–8. 10.1001/jama.2019.3471 [DOI] [PubMed] [Google Scholar]
- 10.Control Group, Turnbull FM, Abraira C, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288–98. 10.1007/s00125-009-1470-0 [DOI] [PubMed] [Google Scholar]
- 11.Coca SG, Ismail-Beigi F, Haq N, et al. Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch Intern Med 2012;172:761–9. 10.1001/archinternmed.2011.2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Nemeth I, Donnelly L, et al. Visit-to-Visit HbA1c Variability Is Associated With Cardiovascular Disease and Microvascular Complications in Patients With Newly Diagnosed Type 2 Diabetes. Diabetes Care 2020;43:426–32. 10.2337/dc19-0823 [DOI] [PubMed] [Google Scholar]
- 13.Yang C-Y, Su P-F, Hung J-Y, et al. Comparative predictive ability of visit-to-visit HbA1c variability measures for microvascular disease risk in type 2 diabetes. Cardiovasc Diabetol 2020;19:105. 10.1186/s12933-020-01082-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermanns N, Heinemann L, Freckmann G, et al. Impact of CGM on the management of hypoglycemia problems: overview and secondary analysis of the HypoDE study. J Diabetes Sci Technol 2019;13:636–44. 10.1177/1932296819831695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cahn A, Zuker I, Eilenberg R, et al. Machine learning based study of longitudinal HbA1c trends and their association with all‐cause mortality: analyses from a national diabetes registry. Diabetes Metab Res Rev 2022;38:e3485. 10.1002/dmrr.3485 [DOI] [PubMed] [Google Scholar]
- 16.Anderson TS, Lee S, Jing B, et al. Prevalence of diabetes medication Intensifications in older adults discharged from US veterans health administration hospitals. JAMA Netw Open 2020;3:e201511. 10.1001/jamanetworkopen.2020.1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotfredsen DR, Vinther S, Petersen TS, et al. Glycemic control and use of glucose-lowering medications in hospital-admitted type 2 diabetes patients over 80 years. Sci Rep 2020;10:4095. 10.1038/s41598-020-60818-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prentice JC, Mohr DC, Zhang L, et al. Increased Hemoglobin A1c Time in Range Reduces Adverse Health Outcomes in Older Adults With Diabetes. Diabetes Care 2021;44:1750–6. 10.2337/dc21-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller DR, Safford MM, Pogach LM. Who has diabetes? best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care 2004;27 Suppl 2:b10–21. 10.2337/diacare.27.suppl_2.B10 [DOI] [PubMed] [Google Scholar]
- 20.VA/DoD Clinical Practice Guidelines . Management of diabetes mellitus in primary care, 2017. Available: https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDDMCPGFinal508.pdf [Accessed 6 Aug 2021].
- 21.Griffith KN, Prentice JC, Mohr DC, et al. Predicting 5- and 10-year mortality risk in older adults with diabetes. Diabetes Care 2020;43:1724–31. 10.2337/dc19-1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care 2008;14:15–23. [PMC free article] [PubMed] [Google Scholar]
- 23.Glasheen WP, Renda A, Dong Y. Diabetes Complications Severity Index (DCSI)-Update and ICD-10 translation. J Diabetes Complications 2017;31:1007–13. 10.1016/j.jdiacomp.2017.02.018 [DOI] [PubMed] [Google Scholar]
- 24.Pantalone KM, Misra-Hebert AD, Hobbs TM, et al. Effect of glycemic control on the diabetes complications severity index score and development of complications in people with newly diagnosed type 2 diabetes. J Diabetes 2018;10:192–9. 10.1111/1753-0407.12613 [DOI] [PubMed] [Google Scholar]
- 25.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 26.Ghouse J, Isaksen JL, Skov MW, et al. Effect of diabetes duration on the relationship between glycaemic control and risk of death in older adults with type 2 diabetes. Diabetes Obes Metab 2020;22:231–42. 10.1111/dom.13891 [DOI] [PubMed] [Google Scholar]
- 27.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–39. 10.1056/NEJMoa0808431 [DOI] [PubMed] [Google Scholar]
- 28.Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59. 10.1056/NEJMoa0802743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zoungas S, Arima H, Gerstein HC, et al. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:431–7. 10.1016/S2213-8587(17)30104-3 [DOI] [PubMed] [Google Scholar]
- 30.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK prospective diabetes study (UKPDS) group. Lancet 1998;352:837–53. 10.1016/S0140-6736(98)07019-6 [DOI] [PubMed] [Google Scholar]
- 31.Holman RR, Paul SK, Bethel MA, et al. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89. 10.1056/NEJMoa0806470 [DOI] [PubMed] [Google Scholar]
- 32.Glycemic Targets . Standards of medical care in Diabetes-2021. Diabetes Care 2021;44:S73–84. 10.2337/dc21-S006 [DOI] [PubMed] [Google Scholar]
- 33.Garber AJ, Handelsman Y, Grunberger G, et al. CONSENSUS STATEMENT BY THE AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY ON THE COMPREHENSIVE TYPE 2 DIABETES MANAGEMENT ALGORITHM - 2020 EXECUTIVE SUMMARY. Endocr Pract 2020;26:107–39. 10.4158/CS-2019-0472 [DOI] [PubMed] [Google Scholar]
- 34.LeRoith D, Biessels GJ, Braithwaite SS, et al. Treatment of diabetes in older adults: an endocrine Society* clinical practice guideline. J Clin Endocrinol Metab 2019;104:1520–74. 10.1210/jc.2019-00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conlin PR, Colburn J, Aron D, et al. Synopsis of the 2017 U.S. department of Veterans Affairs/U.S. Department of defense clinical practice guideline: management of type 2 diabetes mellitus. Ann Intern Med 2017;167:655–63. 10.7326/M17-1362 [DOI] [PubMed] [Google Scholar]
- 36.Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of physicians. Ann Intern Med 2018;168:569–76. 10.7326/M17-0939 [DOI] [PubMed] [Google Scholar]
- 37.Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015;175:356–62. 10.1001/jamainternmed.2014.7345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hart HE, Rutten GE, Bontje KN, et al. Overtreatment of older patients with type 2 diabetes mellitus in primary care. Diabetes Obes Metab 2018;20:1066–9. 10.1111/dom.13174 [DOI] [PubMed] [Google Scholar]
- 39.Rowe TA, Brown T, Lee JY, et al. Clinician-Level variation in three measures representing overuse based on the American geriatrics Society choosing wisely statement. J Gen Intern Med 2020;35:1797–802. 10.1007/s11606-020-05748-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aron DC, Tseng C-L, Soroka O, et al. Balancing measures: identifying unintended consequences of diabetes quality performance measures in patients at high risk for hypoglycemia. Int J Qual Health Care 2019;31:246–51. 10.1093/intqhc/mzy151 [DOI] [PubMed] [Google Scholar]
- 41.Vecchi S, Agabiti N, Mitrova S, et al. [Audit and feedback, and continuous quality improvement strategies to improve the quality of care for type 2 diabetes: a systematic review of literature]. Epidemiol Prev 2016;40:215–23. 10.19191/EP16.3-4.AD05.079 [DOI] [PubMed] [Google Scholar]
- 42.Guo Q, Zang P, Xu S, et al. Time in range, as a novel metric of glycemic control, is reversely associated with presence of diabetic cardiovascular autonomic neuropathy independent of HbA1c in Chinese type 2 diabetes. J Diabetes Res 2020;202011. 10.1155/2020/5817074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexander JT, Staab EM, Wan W, et al. Longer-Term benefits and risks of sodium-glucose cotransporter-2 inhibitors in type 2 diabetes: a systematic review and meta-analysis. J Gen Intern Med 2022;37:439–48. 10.1007/s11606-021-07227-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alexander JT, Staab EM, Wan W, et al. The longer-term benefits and harms of glucagon-like peptide-1 receptor agonists: a systematic review and meta-analysis. J Gen Intern Med 2022;37:415–38. 10.1007/s11606-021-07105-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dahl D, Onishi Y, Norwood P, et al. Effect of subcutaneous Tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA 2022;327:534–45. 10.1001/jama.2022.0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Betancourt JA, Granados PS, Pacheco GJ, et al. Exploring health outcomes for U.S. veterans compared to non-veterans from 2003 to 2019. Health Care 2021;9:604. 10.3390/healthcare9050604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bloeser K, Lipkowitz-Eaton J. Disproportionate multimorbidity among veterans in middle age. J Public Health 2021. 10.1093/pubmed/fdab149 [DOI] [PubMed] [Google Scholar]
- 48.Cashion W, Gellad WF, Sileanu FE, et al. Source of post-transplant care and mortality among kidney transplant recipients dually enrolled in Va and Medicare. Clin J Am Soc Nephrol 2021;16:437–45. 10.2215/CJN.10020620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 2019;42:400–5. 10.2337/dc18-1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2021-002738supp002.pdf (369.4KB, pdf)
bmjdrc-2021-002738supp001.pdf (369.4KB, pdf)
Data Availability Statement
Data are available on reasonable request. There would be need to submit to IRB for permission to release de-identified dataset.